Repurposing of Antibiotic Sulfisoxazole Inhibits Lipolysis in Pre-Clinical Model of Cancer-Associated Cachexia
Abstract
Simple Summary
Abstract
1. Background
2. Methods
2.1. Cell Culture
2.2. Chemicals and Materials
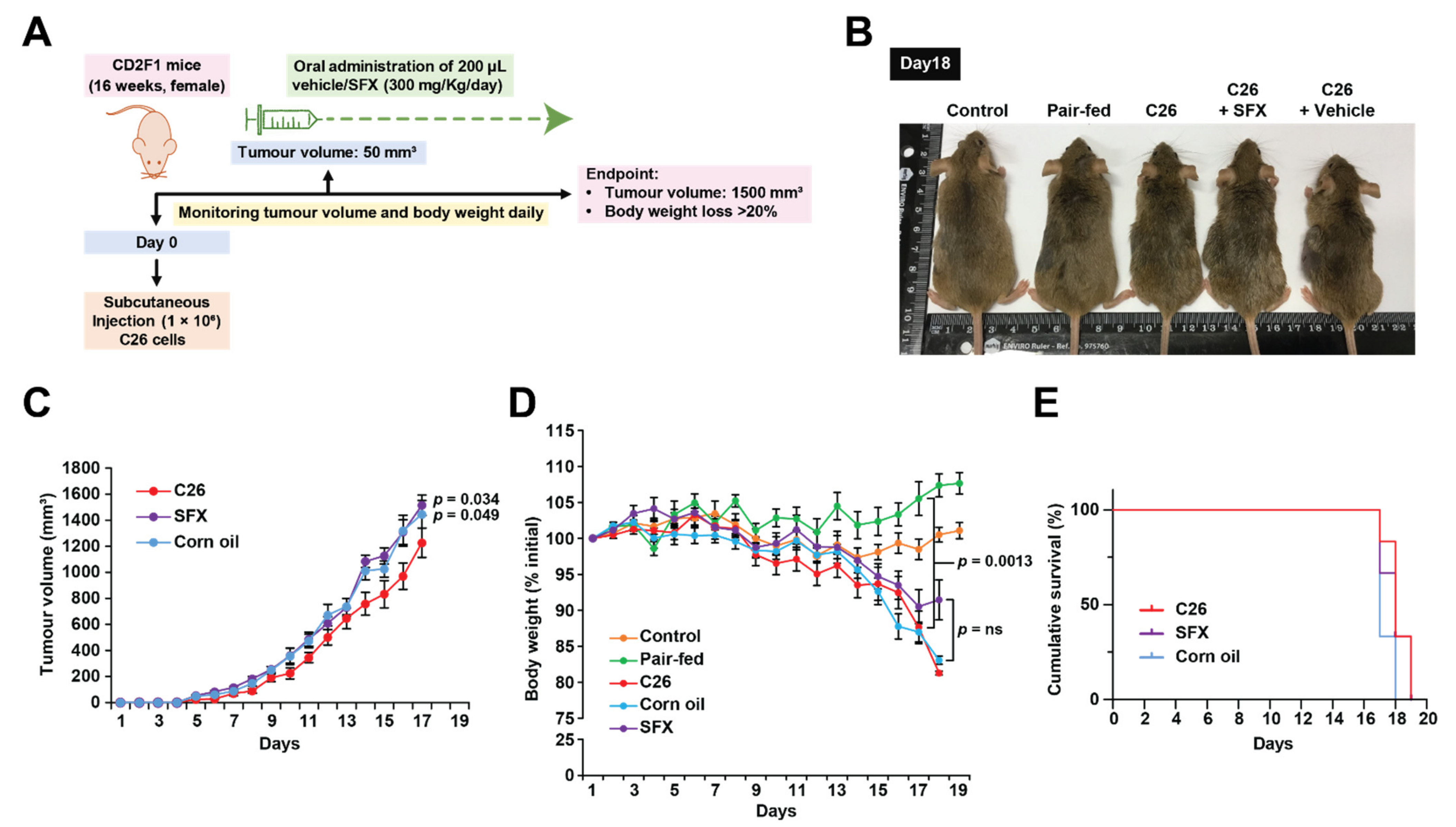
2.3. CD2F1 Mice Model for Cancer-Associated Cachexia
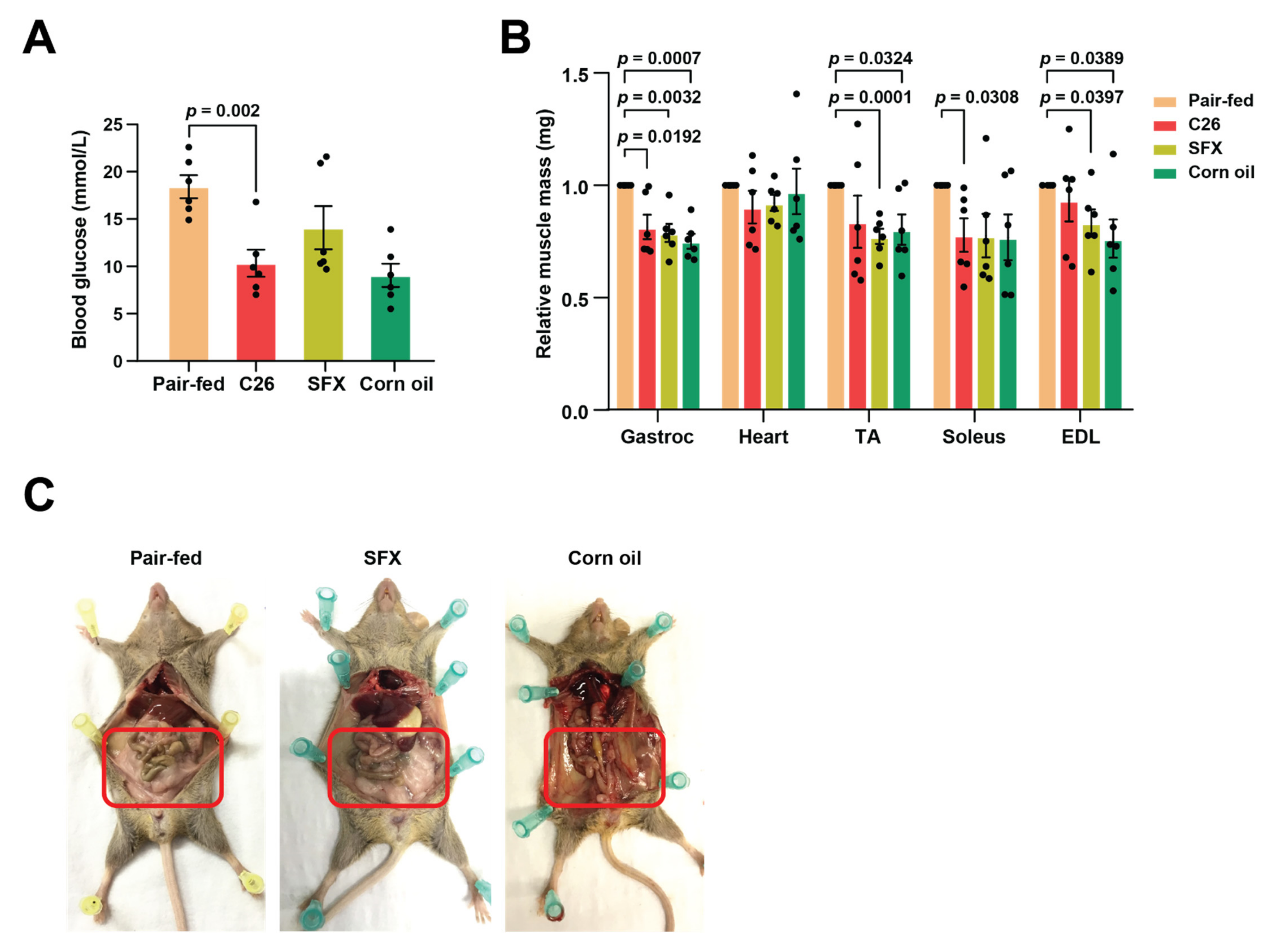
2.4. Murine Blood Glucose Measurements
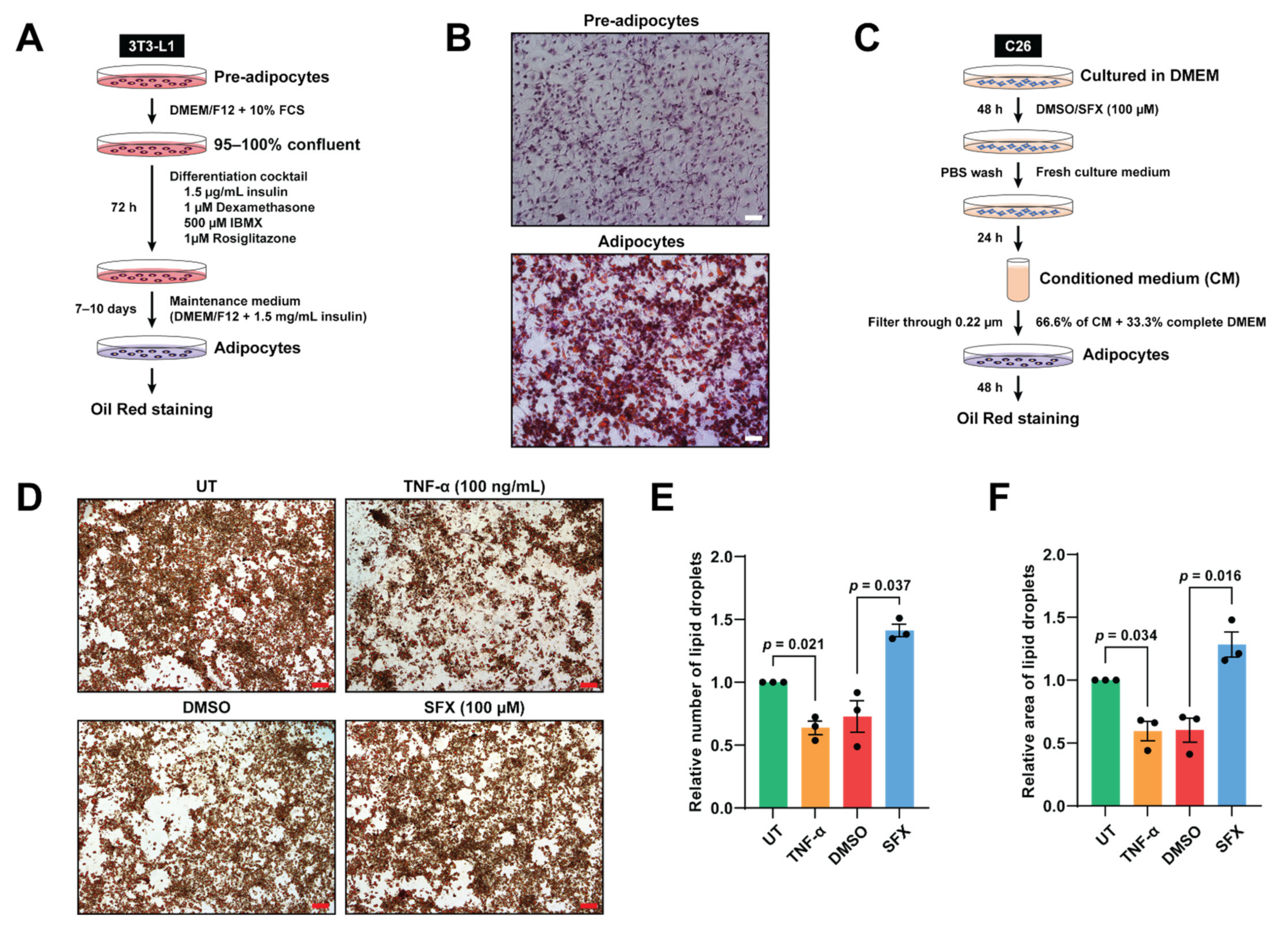
2.5. Oil Red O Staining
2.6. Analysis of Oil Red O Staining
3. Results
3.1. SFX Partially Inhibits Cancer-Induced Weight Loss in C26 Tumour-Bearing Mice
3.2. SFX Rescues Lipolytic Loss in C26 Tumour-Bearing Mice
3.3. SFX Reduces Lipolysis Mediated by C26 Conditioned Media In Vitro
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SFX | Sulfisoxazole |
| FDA | Food and Drug Administration |
| FCS | Fetal Calf Serum |
| BCS | Body Condition Score |
| TA | Tibialis Anterior |
| EDL | Extensor Digitorum Longus |
References
- Fearon, K.; Strasser, F.; Anker, S.D.; Bosaeus, I.; Bruera, E.; Fainsinger, R.L.; Jatoi, A.; Loprinzi, C.; MacDonald, N.; Mantovani, G.; et al. Definition and classification of cancer cachexia: An international consensus. Lancet Oncol. 2011, 12, 489–495. [Google Scholar] [CrossRef]
- Tang, A.M.; Jacobson, D.L.; Spiegelman, D.; Knox, T.A.; Wanke, C. Increasing risk of 5% or greater unintentional weight loss in a cohort of HIV-infected patients, 1995 to 2003. J. Acquir. Immune Defic. Syndr. 2005, 40, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Wallner, C.; Huber, J.; Drysch, M.; Schmidt, S.V.; Wagner, J.M.; Dadras, M.; Lehnhardt, M.; Behr, B. Myostatin Upregulation in Patients in the Chronic Phase of Severe Burn Injury Leads to Muscle Cell Catabolism. Eur. Surg. Res. 2019, 60, 86–96. [Google Scholar] [CrossRef] [PubMed]
- Bruera, E. Clinical management of anorexia and cachexia in patients with advanced cancer. Oncology 1992, 49 (Suppl. S2), 35–42. [Google Scholar] [CrossRef]
- Mantovani, G.; Maccio, A.; Madeddu, C.; Serpe, R.; Antoni, G.; Massa, E.; Dessi, M.; Panzone, F. Phase II nonrandomized study of the efficacy and safety of COX-2 inhibitor celecoxib on patients with cancer cachexia. J. Mol. Med. 2010, 88, 85–92. [Google Scholar] [CrossRef]
- Casaburi, R.; Nakata, J.; Bistrong, L.; Torres, E.; Rambod, M.; Porszasz, J. Effect of Megestrol Acetate and Testosterone on Body Composition and Hormonal Responses in COPD Cachexia. Chronic Obstr. Pulm. Dis. 2015, 3, 389–397. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Szabo, T.; Scherbakov, N.; Sandek, A.; Kung, T.; von Haehling, S.; Lainscak, M.; Jankowska, E.A.; Rudovich, N.; Anker, S.D.; Frystyk, J.; et al. Plasma adiponectin in heart failure with and without cachexia: Catabolic signal linking catabolism, symptomatic status, and prognosis. Nutr. Metab. Cardiovasc. Dis. 2014, 24, 50–56. [Google Scholar] [CrossRef]
- Kir, S.; Komaba, H.; Garcia, A.P.; Economopoulos, K.P.; Liu, W.; Lanske, B.; Hodin, R.A.; Spiegelman, B.M. PTH/PTHrP Receptor Mediates Cachexia in Models of Kidney Failure and Cancer. Cell Metab. 2016, 23, 315–323. [Google Scholar] [CrossRef]
- Archer, A.G.; Watkins, P.J.; Thomas, P.K.; Sharma, A.K.; Payan, J. The natural history of acute painful neuropathy in diabetes mellitus. J. Neurol. Neurosurg. Psychiatry 1983, 46, 491–499. [Google Scholar] [CrossRef]
- Klaude, M.; Fredriksson, K.; Tjader, I.; Hammarqvist, F.; Ahlman, B.; Rooyackers, O.; Wernerman, J. Proteasome proteolytic activity in skeletal muscle is increased in patients with sepsis. Clin. Sci. 2007, 112, 499–506. [Google Scholar] [CrossRef]
- Dodson, S.; Baracos, V.E.; Jatoi, A.; Evans, W.J.; Cella, D.; Dalton, J.T.; Steiner, M.S. Muscle wasting in cancer cachexia: Clinical implications, diagnosis, and emerging treatment strategies. Annu. Rev. Med. 2011, 62, 265–279. [Google Scholar] [CrossRef]
- Tan, B.H.; Fearon, K.C. Cachexia: Prevalence and impact in medicine. Curr. Opin. Clin. Nutr. Metab. Care 2008, 11, 400–407. [Google Scholar] [CrossRef]
- Porporato, P.E. Understanding cachexia as a cancer metabolism syndrome. Oncogenesis 2016, 5, e200. [Google Scholar] [CrossRef] [PubMed]
- Argiles, J.M.; Busquets, S.; Stemmler, B.; Lopez-Soriano, F.J. Cancer cachexia: Understanding the molecular basis. Nat. Rev. Cancer 2014, 14, 754–762. [Google Scholar] [CrossRef]
- Lee, D.E.; Brown, J.L.; Rosa-Caldwell, M.E.; Blackwell, T.A.; Perry, R.A., Jr.; Brown, L.A.; Khatri, B.; Seo, D.; Bottje, W.G.; Washington, T.A.; et al. Cancer cachexia-induced muscle atrophy: Evidence for alterations in microRNAs important for muscle size. Physiol. Genom. 2017, 49, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Prado, C.M.; Sawyer, M.B.; Ghosh, S.; Lieffers, J.R.; Esfandiari, N.; Antoun, S.; Baracos, V.E. Central tenet of cancer cachexia therapy: Do patients with advanced cancer have exploitable anabolic potential? Am. J. Clin. Nutr. 2013, 98, 1012–1019. [Google Scholar] [CrossRef] [PubMed]
- Biswas, A.K.; Acharyya, S. Understanding cachexia in the context of metastatic progression. Nat. Rev. Cancer 2020, 20, 274–284. [Google Scholar] [CrossRef]
- Argiles, J.M. The 2015 ESPEN Sir David Cuthbertson lecture: Inflammation as the driving force of muscle wasting in cancer. Clin. Nutr. 2017, 36, 798–803. [Google Scholar] [CrossRef]
- Bonetto, A.; Aydogdu, T.; Jin, X.; Zhang, Z.; Zhan, R.; Puzis, L.; Koniaris, L.G.; Zimmers, T.A. JAK/STAT3 pathway inhibition blocks skeletal muscle wasting downstream of IL-6 and in experimental cancer cachexia. Am. J. Physiol. Endocrinol. Metab. 2012, 303, E410–E421. [Google Scholar] [CrossRef]
- Chitti, S.V.; Fonseka, P.; Mathivanan, S. Emerging role of extracellular vesicles in mediating cancer cachexia. Biochem. Soc. Trans. 2018, 46, 1129–1136. [Google Scholar] [CrossRef]
- Jatoi, A.; Ritter, H.L.; Dueck, A.; Nguyen, P.L.; Nikcevich, D.A.; Luyun, R.F.; Mattar, B.I.; Loprinzi, C.L. A placebo-controlled, double-blind trial of infliximab for cancer-associated weight loss in elderly and/or poor performance non-small cell lung cancer patients (N01C9). Lung Cancer 2010, 68, 234–239. [Google Scholar] [CrossRef]
- Del Fabbro, E.; Dev, R.; Hui, D.; Palmer, L.; Bruera, E. Effects of melatonin on appetite and other symptoms in patients with advanced cancer and cachexia: A double-blind placebo-controlled trial. J. Clin. Oncol. 2013, 31, 1271–1276. [Google Scholar] [CrossRef]
- Jatoi, A.; Dakhil, S.R.; Nguyen, P.L.; Sloan, J.A.; Kugler, J.W.; Rowland, K.M., Jr.; Soori, G.S.; Wender, D.B.; Fitch, T.R.; Novotny, P.J.; et al. A placebo-controlled double blind trial of etanercept for the cancer anorexia/weight loss syndrome: Results from N00C1 from the North Central Cancer Treatment Group. Cancer 2007, 110, 1396–1403. [Google Scholar] [CrossRef]
- Mehrzad, V.; Afshar, R.; Akbari, M. Pentoxifylline treatment in patients with cancer cachexia: A double-blind, randomized, placebo-controlled clinical trial. Adv. Biomed. Res. 2016, 5, 60. [Google Scholar] [CrossRef] [PubMed]
- Simons, J.P.; Schols, A.M.; Hoefnagels, J.M.; Westerterp, K.R.; ten Velde, G.P.; Wouters, E.F. Effects of medroxyprogesterone acetate on food intake, body composition, and resting energy expenditure in patients with advanced, nonhormone-sensitive cancer: A randomized, placebo-controlled trial. Cancer 1998, 82, 553–560. [Google Scholar] [CrossRef]
- Bosaeus, I.; Daneryd, P.; Svanberg, E.; Lundholm, K. Dietary intake and resting energy expenditure in relation to weight loss in unselected cancer patients. Int. J. Cancer 2001, 93, 380–383. [Google Scholar] [CrossRef]
- Boddaert, M.S.; Gerritsen, W.R.; Pinedo, H.M. On our way to targeted therapy for cachexia in cancer? Curr. Opin. Oncol. 2006, 18, 335–340. [Google Scholar] [CrossRef] [PubMed]
- Crawford, J. What are the criteria for response to cachexia treatment? Ann. Palliat. Med. 2019, 8, 43–49. [Google Scholar] [CrossRef]
- Pushpakom, S.; Iorio, F.; Eyers, P.A.; Escott, K.J.; Hopper, S.; Wells, A.; Doig, A.; Guilliams, T.; Latimer, J.; McNamee, C.; et al. Drug repurposing: Progress, challenges and recommendations. Nat. Rev. Drug Discov. 2019, 18, 41–58. [Google Scholar] [CrossRef] [PubMed]
- Im, E.J.; Lee, C.H.; Moon, P.G.; Rangaswamy, G.G.; Lee, B.; Lee, J.M.; Lee, J.C.; Jee, J.G.; Bae, J.S.; Kwon, T.K.; et al. Sulfisoxazole inhibits the secretion of small extracellular vesicles by targeting the endothelin receptor A. Nat. Commun. 2019, 10, 1387. [Google Scholar] [CrossRef]
- Samuel, M.; Fonseka, P.; Sanwlani, R.; Gangoda, L.; Chee, S.H.; Keerthikumar, S.; Spurling, A.; Chitti, S.V.; Zanker, D.; Ang, C.S.; et al. Oral administration of bovine milk-derived extracellular vesicles induces senescence in the primary tumor but accelerates cancer metastasis. Nat. Commun. 2021, 12, 3950. [Google Scholar] [CrossRef]
- Fonseka, P.; Kang, T.; Chee, S.; Chitti, S.V.; Sanwlani, R.; Ang, C.S.; Mathivanan, S. Temporal Quantitative Proteomics Analysis of Neuroblastoma Cells Treated with Bovine Milk-Derived Extracellular Vesicles Highlights the Anti-Proliferative Properties of Milk-Derived Extracellular Vesicles. Cells 2021, 10, 750. [Google Scholar] [CrossRef] [PubMed]
- Fonseka, P.; Chitti, S.V.; Sanwlani, R.; Mathivanan, S. Sulfisoxazole does not inhibit the secretion of small extracellular vesicles. Nat. Commun. 2021, 12, 977. [Google Scholar] [CrossRef]
- Fearon, K.C. The Sir David Cuthbertson Medal Lecture 1991. The mechanisms and treatment of weight loss in cancer. Proc. Nutr Soc. 1992, 51, 251–265. [Google Scholar] [CrossRef]
- Fouladiun, M.; Korner, U.; Bosaeus, I.; Daneryd, P.; Hyltander, A.; Lundholm, K.G. Body composition and time course changes in regional distribution of fat and lean tissue in unselected cancer patients on palliative care—correlations with food intake, metabolism, exercise capacity, and hormones. Cancer 2005, 103, 2189–2198. [Google Scholar] [CrossRef]
- Das, S.K.; Eder, S.; Schauer, S.; Diwoky, C.; Temmel, H.; Guertl, B.; Gorkiewicz, G.; Tamilarasan, K.P.; Kumari, P.; Trauner, M.; et al. Adipose triglyceride lipase contributes to cancer-associated cachexia. Science 2011, 333, 233–238. [Google Scholar] [CrossRef]
- Moley, J.F.; Aamodt, R.; Rumble, W.; Kaye, W.; Norton, J.A. Body cell mass in cancer-bearing and anorexic patients. JPEN J. Parenter. Enter. Nutr. 1987, 11, 219–222. [Google Scholar] [CrossRef]
- Tisdale, M.J. Biology of Cachexia. JNCI J. Natl. Cancer Inst. 1997, 89, 1763–1773. [Google Scholar] [CrossRef] [PubMed]
- Dewys, W.D.; Begg, C.; Lavin, P.T.; Band, P.R.; Bennett, J.M.; Bertino, J.R.; Cohen, M.H.; Douglass, H.O., Jr.; Engstrom, P.F.; Ezdinli, E.Z.; et al. Prognostic effect of weight loss prior to chemotherapy in cancer patients. Am. J. Med. 1980, 69, 491–497. [Google Scholar] [CrossRef]
- Murphy, R.A.; Yeung, E.; Mazurak, V.C.; Mourtzakis, M. Influence of eicosapentaenoic acid supplementation on lean body mass in cancer cachexia. Br. J. Cancer 2011, 105, 1469–1473. [Google Scholar] [CrossRef] [PubMed]
- Penna, F.; Busquets, S.; Pin, F.; Toledo, M.; Baccino, F.M.; Lopez-Soriano, F.J.; Costelli, P.; Argiles, J.M. Combined approach to counteract experimental cancer cachexia: Eicosapentaenoic acid and training exercise. J. Cachexia Sarcopenia Muscle 2011, 2, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Wyke, S.M.; Russell, S.T.; Tisdale, M.J. Induction of proteasome expression in skeletal muscle is attenuated by inhibitors of NF-kappaB activation. Br. J. Cancer 2004, 91, 1742–1750. [Google Scholar] [CrossRef]
- Shadfar, S.; Couch, M.E.; McKinney, K.A.; Weinstein, L.J.; Yin, X.; Rodriguez, J.E.; Guttridge, D.C.; Willis, M. Oral resveratrol therapy inhibits cancer-induced skeletal muscle and cardiac atrophy in vivo. Nutr. Cancer 2011, 63, 749–762. [Google Scholar] [CrossRef]
- Tsubouchi, H.; Yanagi, S.; Miura, A.; Matsumoto, N.; Kangawa, K.; Nakazato, M. Ghrelin relieves cancer cachexia associated with the development of lung adenocarcinoma in mice. Eur. J. Pharmacol. 2014, 743, 1–10. [Google Scholar] [CrossRef]
- Davis, T.W.; Zweifel, B.S.; O’Neal, J.M.; Heuvelman, D.M.; Abegg, A.L.; Hendrich, T.O.; Masferrer, J.L. Inhibition of cyclooxygenase-2 by celecoxib reverses tumor-induced wasting. J. Pharmacol. Exp. Ther. 2004, 308, 929–934. [Google Scholar] [CrossRef]
- Baumgarten, A.J.; Fiebig, H.H.; Burger, A.M. Molecular analysis of xenograft models of human cancer cachexia--possibilities for therapeutic intervention. Cancer Genom. Proteom. 2007, 4, 223–231. [Google Scholar]
- Hussey, H.J.; Tisdale, M.J. Effect of the specific cyclooxygenase-2 inhibitor meloxicam on tumour growth and cachexia in a murine model. Int. J. Cancer 2000, 87, 95–100. [Google Scholar] [CrossRef]
- Combaret, L.; Ralliere, C.; Taillandier, D.; Tanaka, K.; Attaix, D. Manipulation of the ubiquitin-proteasome pathway in cachexia: Pentoxifylline suppresses the activation of 20S and 26S proteasomes in muscles from tumor-bearing rats. Mol. Biol. Rep. 1999, 26, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Combaret, L.; Tilignac, T.; Claustre, A.; Voisin, L.; Taillandier, D.; Obled, C.; Tanaka, K.; Attaix, D. Torbafylline (HWA 448) inhibits enhanced skeletal muscle ubiquitin-proteasome-dependent proteolysis in cancer and septic rats. Biochem. J. 2002, 361, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Toledo, M.; Springer, J.; Busquets, S.; Tschirner, A.; Lopez-Soriano, F.J.; Anker, S.D.; Argiles, J.M. Formoterol in the treatment of experimental cancer cachexia: Effects on heart function. J. Cachexia Sarcopenia Muscle 2014, 5, 315–320. [Google Scholar] [CrossRef]
- Joassard, O.R.; Amirouche, A.; Gallot, Y.S.; Desgeorges, M.M.; Castells, J.; Durieux, A.C.; Berthon, P.; Freyssenet, D.G. Regulation of Akt-mTOR, ubiquitin-proteasome and autophagy-lysosome pathways in response to formoterol administration in rat skeletal muscle. Int J. Biochem. Cell Biol. 2013, 45, 2444–2455. [Google Scholar] [CrossRef] [PubMed]
- Steffen, B.T.; Lees, S.J.; Booth, F.W. Anti-TNF treatment reduces rat skeletal muscle wasting in monocrotaline-induced cardiac cachexia. J. Appl. Physiol. 2008, 105, 1950–1958. [Google Scholar] [CrossRef] [PubMed]
- Gordon, J.N.; Trebble, T.M.; Ellis, R.D.; Duncan, H.D.; Johns, T.; Goggin, P.M. Thalidomide in the treatment of cancer cachexia: A randomised placebo controlled trial. Gut 2005, 54, 540–545. [Google Scholar] [CrossRef] [PubMed]
- Kedar, I.; Mermershtain, W.; Ivgi, H. Thalidomide reduces serum C-reactive protein and interleukin-6 and induces response to IL-2 in a fraction of metastatic renal cell cancer patients who failed IL-2-based therapy. Int. J. Cancer 2004, 110, 260–265. [Google Scholar] [CrossRef] [PubMed]
- Reed, D.R.; Bachmanov, A.A.; Tordoff, M.G. Forty mouse strain survey of body composition. Physiol. Behav. 2007, 91, 593–600. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chitti, S.V.; Marzan, A.L.; Shahi, S.; Kang, T.; Fonseka, P.; Mathivanan, S. Repurposing of Antibiotic Sulfisoxazole Inhibits Lipolysis in Pre-Clinical Model of Cancer-Associated Cachexia. Biology 2021, 10, 700. https://doi.org/10.3390/biology10080700
Chitti SV, Marzan AL, Shahi S, Kang T, Fonseka P, Mathivanan S. Repurposing of Antibiotic Sulfisoxazole Inhibits Lipolysis in Pre-Clinical Model of Cancer-Associated Cachexia. Biology. 2021; 10(8):700. https://doi.org/10.3390/biology10080700
Chicago/Turabian StyleChitti, Sai V., Akbar L. Marzan, Sanjay Shahi, Taeyoung Kang, Pamali Fonseka, and Suresh Mathivanan. 2021. "Repurposing of Antibiotic Sulfisoxazole Inhibits Lipolysis in Pre-Clinical Model of Cancer-Associated Cachexia" Biology 10, no. 8: 700. https://doi.org/10.3390/biology10080700
APA StyleChitti, S. V., Marzan, A. L., Shahi, S., Kang, T., Fonseka, P., & Mathivanan, S. (2021). Repurposing of Antibiotic Sulfisoxazole Inhibits Lipolysis in Pre-Clinical Model of Cancer-Associated Cachexia. Biology, 10(8), 700. https://doi.org/10.3390/biology10080700






