Pattern of Urban Flora in Intra-City Railway Habitats (Alexandria, Egypt): A Conservation Perspective
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
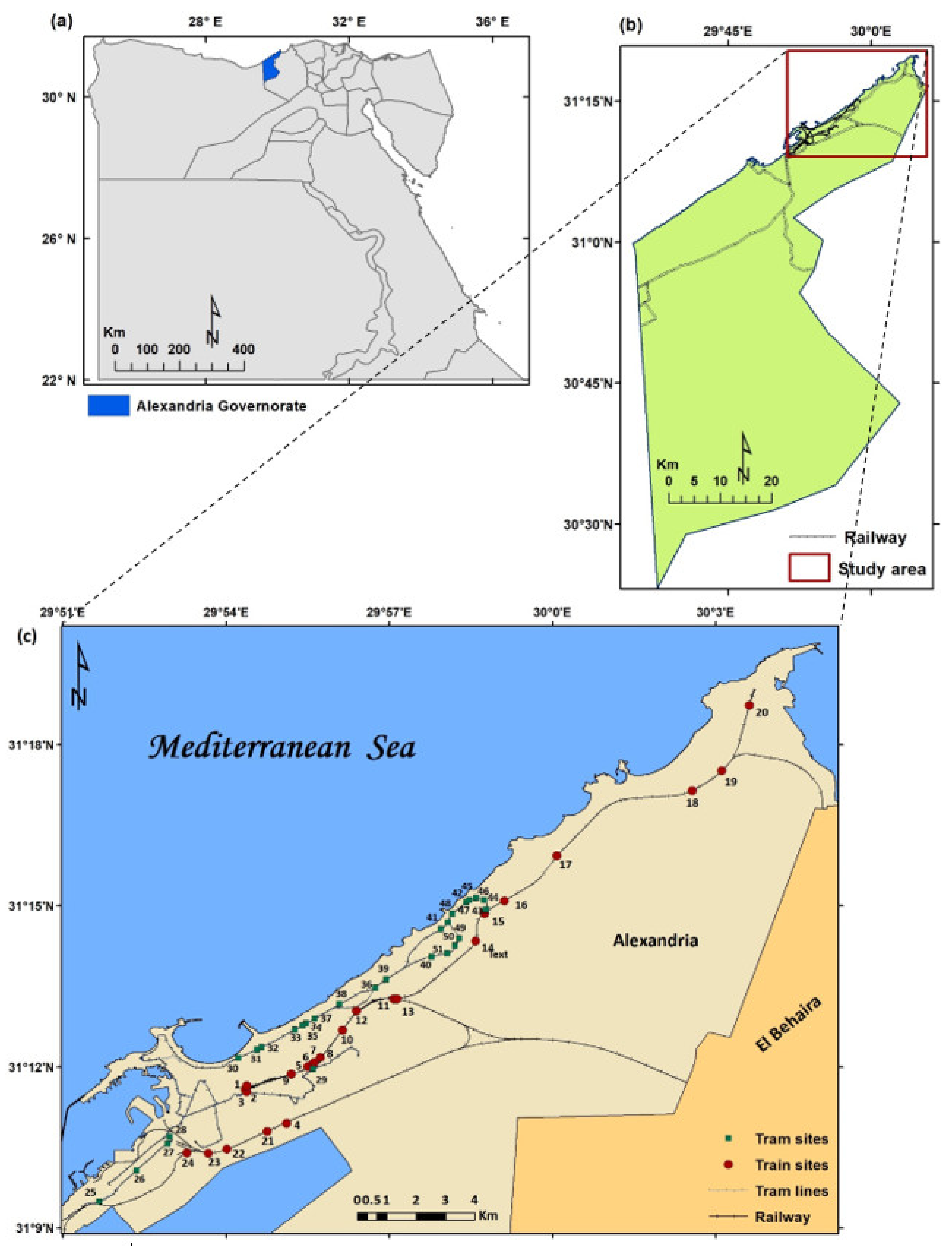
2.1. Study Area
2.2. Sampling and Data Collection
2.3. Vegetation Measurements
2.4. Environmental Variables and Measures of Disturbance
2.5. Assessment of Human Impact
2.6. Diversity Indices
2.7. Data Analysis
3. Results
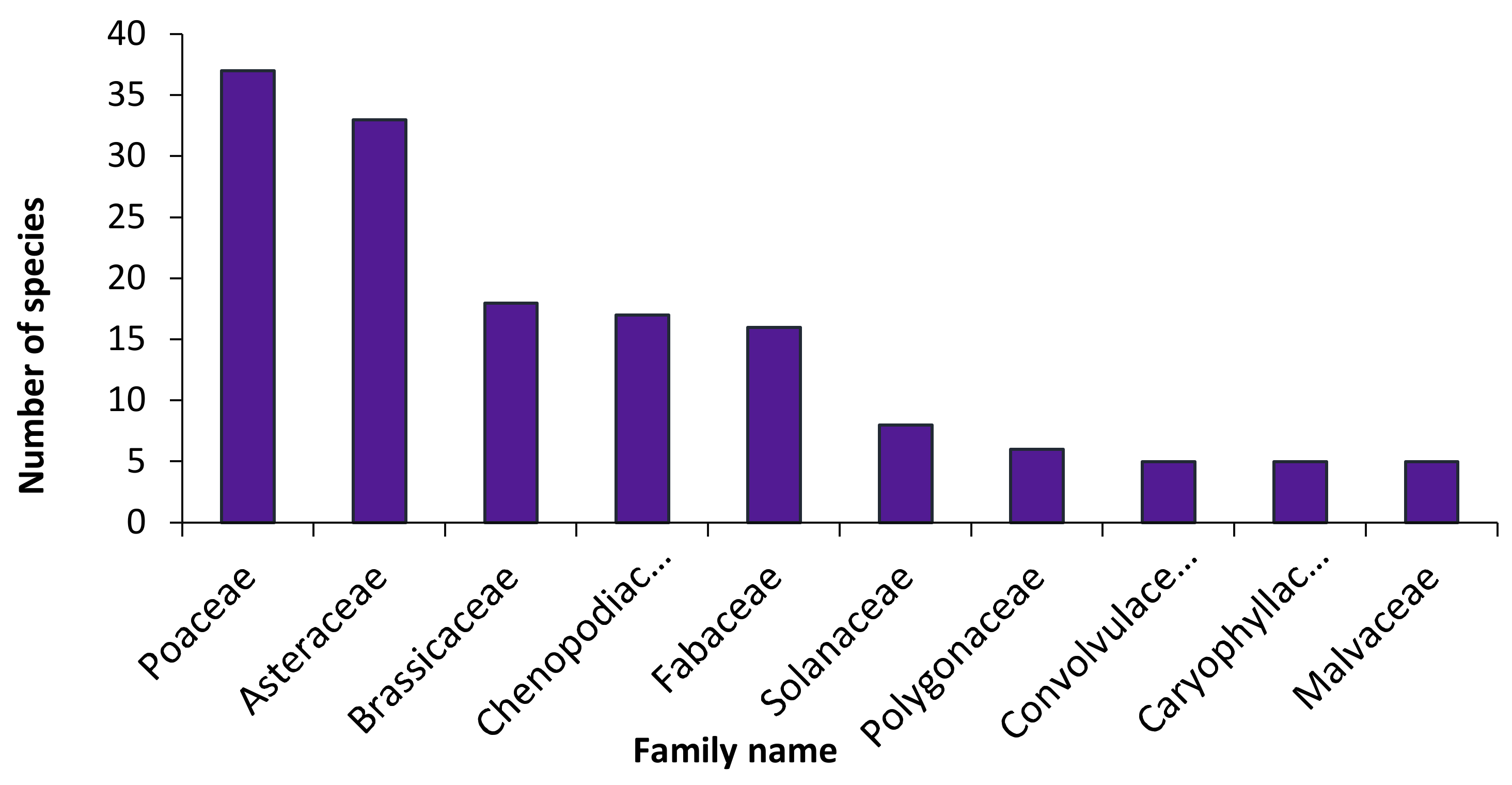
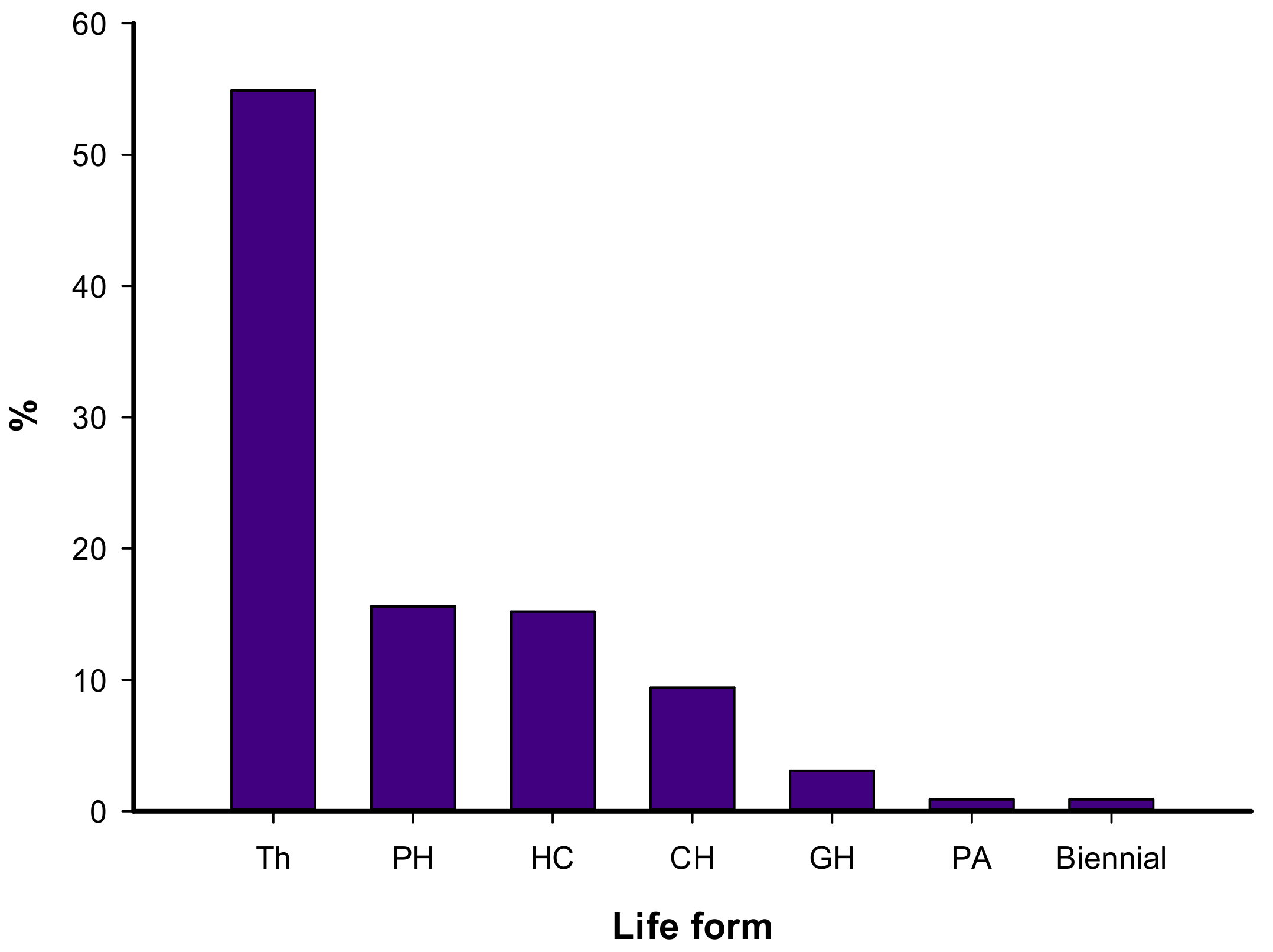
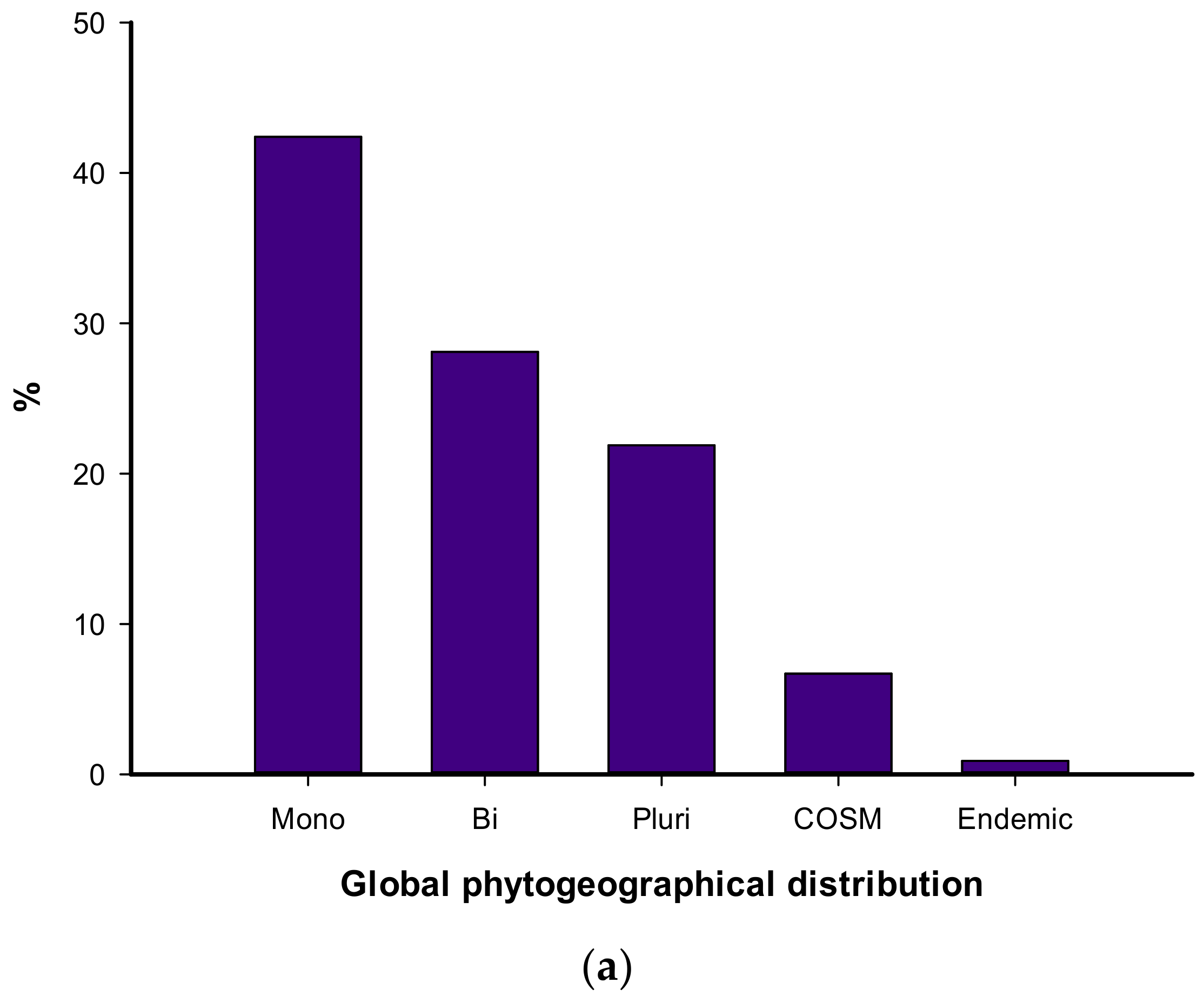
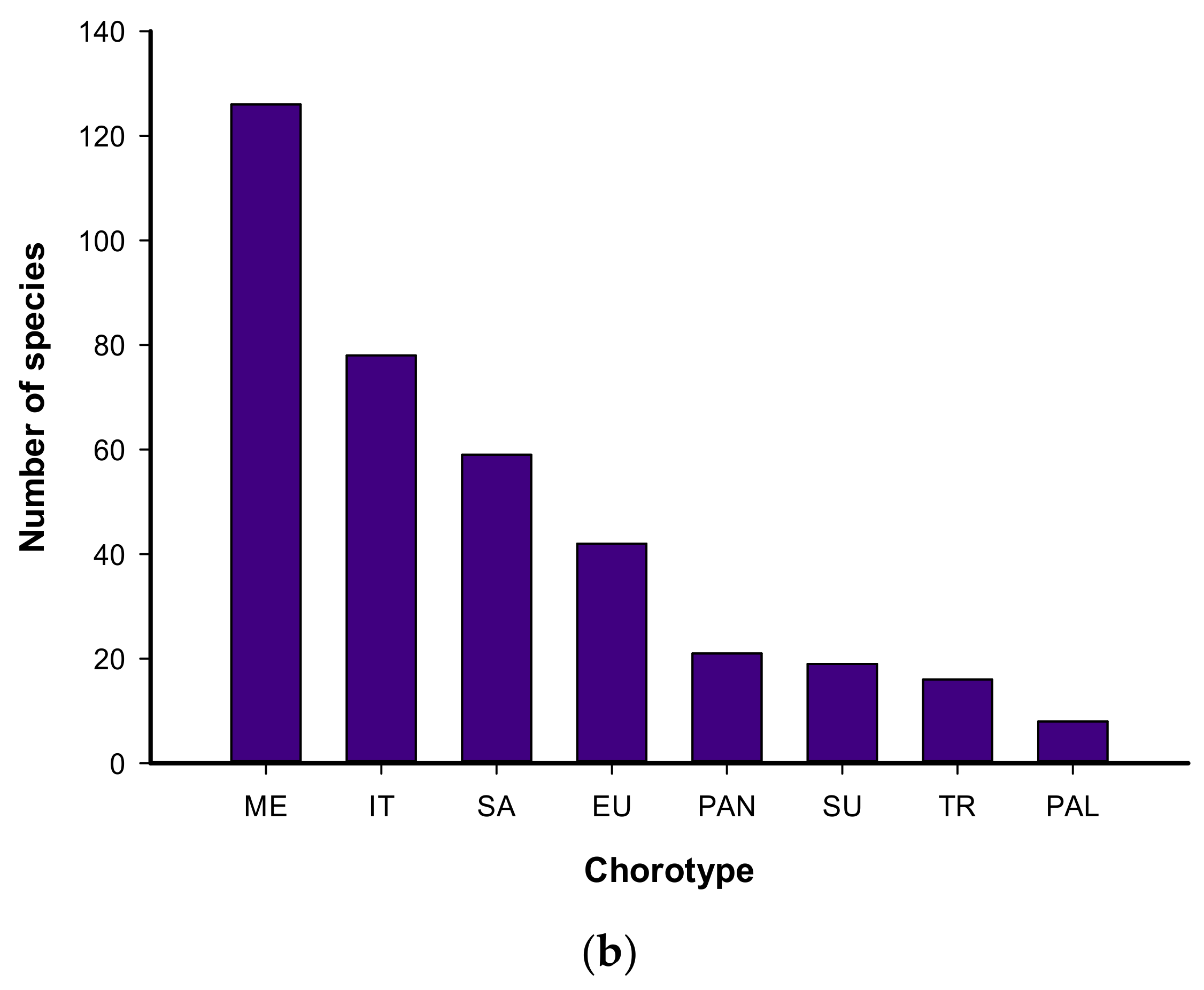
3.1. Floristic Composition
3.2. Plant Communities and Diversity Indices
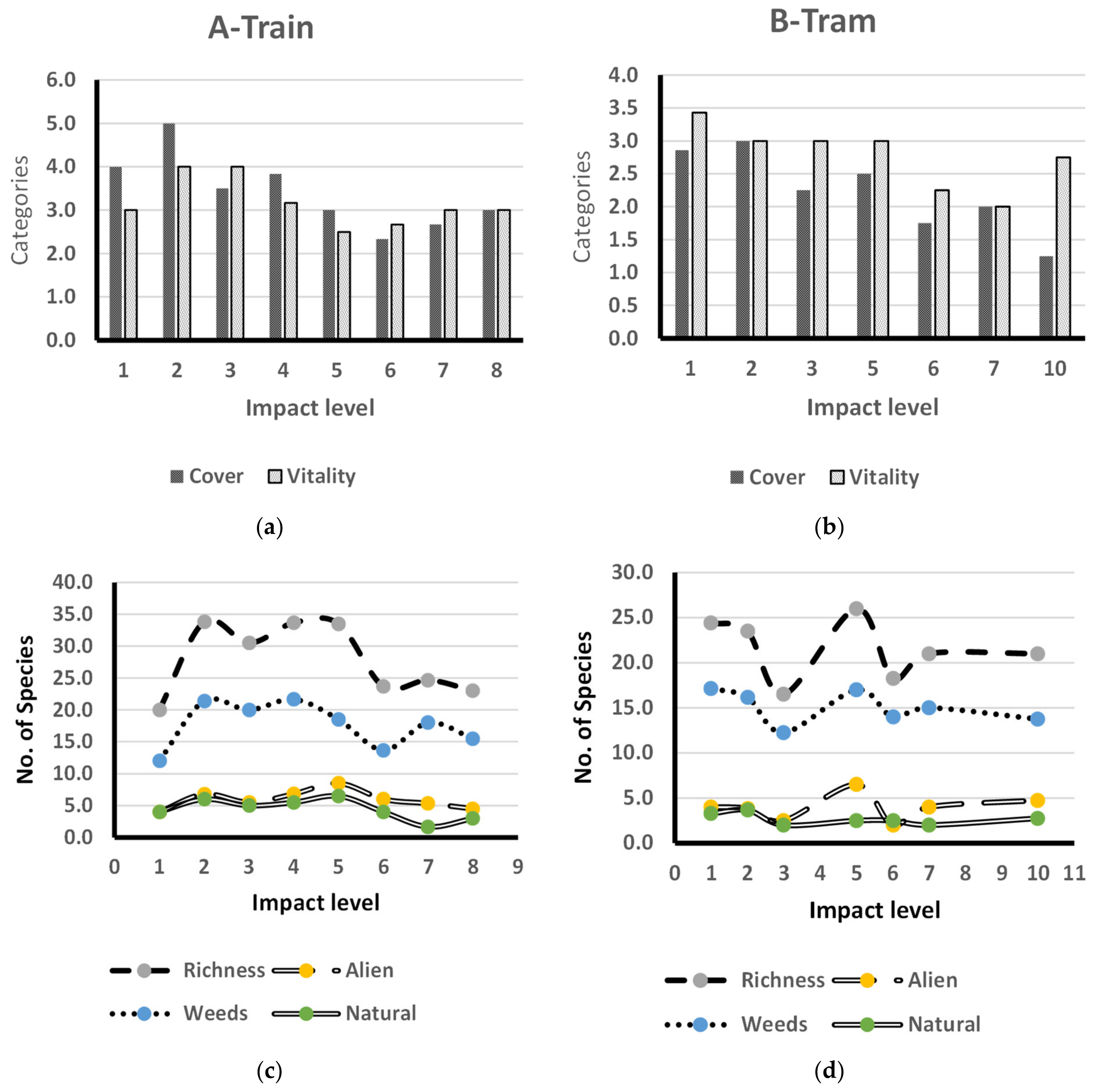
3.3. The Impact of Human Activities
4. Discussion
4.1. Floristic Composition
4.2. Plant Communities and Diversity Indices
4.3. The Impact of Human Activities
5. Conclusions and Recommendations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kowarik, I. Some responses of flora and vegetation to urbanization in Central Europe. In Urban Ecology; Sukopp, H., Hejný, S., Kowarik, I., Eds.; SPB Academic Publishing: The Hague, The Netherlands, 1990; pp. 45–74. [Google Scholar]
- Wrzesień, M.; Denisow, B.; Mamchur, Z.; Chuba, M.; Resler, I. Composition and structure of the flora in intra-urban railway areas. Acta Agrobot. 2016, 69, 1–14. [Google Scholar] [CrossRef]
- Wrzesień, M.; Denisow, B. Factors responsible for the distribution of invasive plant species in the surroundings of railway areas. A case study from SE Poland. Biologia 2017, 72, 1275–1284. [Google Scholar] [CrossRef]
- Májeková, J.; Zaliberová, M.; Andrik, E.J.; Protopopova, V.V.; Shevera, M.V.; Ikhardt, P. A comparison of the flora of the Chop (Ukraine) and Čierna nad Tisou (Slovakia) border railway stations. Biologia 2020. [Google Scholar] [CrossRef]
- Sukopp, H.; Numata, M.; Huber, A. (Eds.) Urban Ecology as the Basis of Urban Planning; SPB Academic Publishing: Amsterdam, The Netherlands, 1995. [Google Scholar]
- Pyšek, P. Alien and native species in Central European urban floras: A quantitative comparison. J. Biogeogr. 1998, 25, 155–163. [Google Scholar] [CrossRef]
- Sukopp, H. On the early history of urban ecology in Europe. Preslia 2002, 74, 373–393. [Google Scholar]
- McDonnell, M.J.; Niemelä, J. The history of urban ecology. Urban Ecol. 2011, 9, 34–49. [Google Scholar]
- Chocholoušková, Z.; Pyšek, P. Changes in composition and structure of urban flora over120 years: A case study of the city of Plzeň. Flora 2003, 198, 366–376. [Google Scholar] [CrossRef]
- Kühn, I.; Brandl, R.; Klotz, S. The flora of German cities is naturally species rich. Evol. Ecol. Res. 2004, 6, 749–764. [Google Scholar]
- Wittig, R. The origin and development of the urban flora of Central Europe. Urban Ecosyst. 2004, 7, 323–329. [Google Scholar]
- Wittig, R.; Becker, U. The spontaneous flora around street trees in cities—A striking example for the worldwide homogenization of the flora of urban habitats. Flora-Morphol. Distrib. Funct. Ecol. Plants 2010, 205, 704–709. [Google Scholar] [CrossRef]
- Gilbert, O.L. Ecology of Urban Environment; Chapman & Hall: London, UK, 1989. [Google Scholar]
- Lousley, J.E. The Influence of transport on a changing flora. In The Flora of a Changing Britain; Perring, F., Ed.; E.W. Classey, Ltd.: London, UK, 1970; pp. 73–83. [Google Scholar]
- Niemelä, J.; Breuste, J.H.; Guntenspergen, G.; McIntyre, N.E.; Elmqvist, T.; James, P. (Eds.) URBAN Ecology: Patterns, Processes, and Applications; OUP: Oxford, UK, 2011. [Google Scholar]
- Cai, D.; Fraedrich, K.; Guan, Y.; Guo, S.; Zhang, C.; Carvalho, L.M.V.; Zhu, X. Causality of Biodiversity Loss: Climate, Vegetation, and Urbanization in China and America. Sensors 2019, 19, 4499. [Google Scholar] [CrossRef]
- Shaltout, K.H.; Sharaf El-Din, A. Habitat Types and Plant Communities along A Transect in the Nile Delta Region. Feddes Repert. 1988, 99, 153–162. [Google Scholar] [CrossRef]
- Shaltout, K.H.; El-Sheikh, M.A. Vegetation of the Urban Habitats in the Nile Delta Region, Egypt. Urban Ecosyst. 2003, 6, 205–221. [Google Scholar] [CrossRef]
- Aberg, G.; Charalampides, G.; Fosse, G.; Hjelmseth, H. The use of Pb isotopes to differentiate between contemporary and ancient sources of pollution in Greece. Atmos. Environ. 2001, 35, 4609–4615. [Google Scholar] [CrossRef]
- Abdel-Rahman, N.H. Alexandria’s cultural landscapes: Historical parks between originality and deterioration. WIT Trans. Built Environ. 2017, 170, 73–83. [Google Scholar]
- Saad El Din, M.; Steen, G.L.; De Luca, A. Alexandria: The Site and the History; New York University Press: New York, NY, USA, 1993. [Google Scholar]
- Naiman, R.J.; Décamps, H. The ecology of interfaces: Riparian zones. Annu. Rev. Ecol. Syst. 1997, 28, 621–658. [Google Scholar] [CrossRef]
- Mohamed, S.A. Coastal vulnerability assessment using GIS-Based multicriteria analysis of Alexandria-northwestern Nile Delta, Egypt. J. Afr. Earth Sci. 2020, 163, 103751. [Google Scholar] [CrossRef]
- Barakat, A.O. PAHs and petroleum markers in the atmospheric environment of Alexandria City, Egypt. Water Air Soil Pollut. 2002, 139, 289–310. [Google Scholar] [CrossRef]
- Abd El-Ghani, M.; Bornkamm, R.; El-Sawaf, N.; Turky, H. Plant species distribution and spatial habitat heterogeneity in the landscape of urbanizing desert ecosystems in Egypt. Urban Ecosyst. 2011, 14, 585–616. [Google Scholar] [CrossRef]
- Grime, J.P. Plant Strategies and Vegetation Processes; John Wiley & Sons: Hoboken, NJ, USA, 1979. [Google Scholar]
- Holzapfel, C.; Schmidt, W. Roadside vegetation along transects in the Judean desert. Isr. J Bot. 1990, 39, 263–270. [Google Scholar]
- Brandes, D. Flora and vegatation of railway stations in Central Europe. Phytocoenologia 1983, 11, 31–115. [Google Scholar] [CrossRef]
- Plakhotnik, V.N.; Onyshchenko, J.V.; Yaryshkina, L.A. The environmental impacts of railway transportation in the Ukraine. Transp. Res. Part D Transp. Environ. 2005, 10, 263–268. [Google Scholar] [CrossRef]
- Penone, C.; Kerbiriou, C.; Julien, J.F.; Julliard, R.; Machon, N.; Viol, I. Urbanization effect on orthoptera: Which scale matters? Insect Conserv. Divers. 2013, 6, 319–327. [Google Scholar] [CrossRef]
- Clauzel, C.; Girardet, X.; Foltête, J.-C. Impact assessment of a high-speed railway line on species distribution: Application to the European tree frog (Hyla arborea) in Franche-Comté. J. Environ. Manag. 2013, 127, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Woźnica, P.; Urbisz, A.; Franiel, I. Tram tracks as specific anthropogenic habitats for the growth of plants. Peer J. Prepr. 2016, 4, e2606v1. [Google Scholar]
- Májeková, J.; Jehlík, V.; Zaliberová, M. Railway stations vs. Thermophilous species (example from Eastern Slovakia). Thaiszia J. Bot. 2016, 26, 173–188. [Google Scholar]
- Sudnik-Wójcikowska, B.; Galera, H. Floristic differences in some anthropogenic habitats in Warsaw. Ann. Bot. Fenn. 2005, 42, 185–193. [Google Scholar]
- Galera, H.; Sudnik-Wójcikowska, B.; Wierzbicka, M.; Jarzyna, I.; Wiłkomirski, B. Structure of the flora of railway areas under various kinds of anthropopression. Pol. Bot. J. 2014, 59, 1–10. [Google Scholar] [CrossRef]
- Altay, V.; Ozviqit, I.I.; Osma, E.; Bakir, Y.; Demir, G.; Severoglu, Z.; Yarci, C. Environmental relationships of the vascular flora alongside the railway tracks between Haydarpaşa and Gebze (Istanbul- Kocaeli/Turkey). J. Environ. Biol. 2015, 36, 153–162. [Google Scholar]
- Denisow, B.; Wrzesień, M.; Mamchur, Z.; Chuba, M. Invasive flora within urban railway areas: A case study from Lublin (Poland) and Lviv (Ukraine). Acta Agrobot. 2017, 70, 1727. [Google Scholar] [CrossRef]
- Wilkomirski, B.; Galera, H.; Sudnik-Wojcikowska, B.; Staszewski, T.; Malawska, M. Railway Tracks-Habitat Conditions, Contamination, Floristic Settlement-A Review. Environ. Nat. Resour. Res. 2012, 2, 86–95. [Google Scholar] [CrossRef]
- Liu, H.; Chen, L.P.; Ai, Y.W.; Yang, X.; Yu, Y.H.; Zuo, Y.B.; Fu, G.Y. Heavy metal contamination in soil alongside mountain railway in Sichuan, China. Environ. Monit. Assess. 2009, 152, 25–33. [Google Scholar] [CrossRef]
- Májeková, J.; Letz, D.R.; Slezák, M.; Zaliberová, M.; Hrivnák, R. Rare and threatened vascular plants of the railways in Slovakia. Biodivers. Res. Conserv. 2015, 35, 75–85. [Google Scholar] [CrossRef][Green Version]
- Sikorski, P.; Wińska-Krysiak, M.; Chormański, J.; Krauze, K.; Kubacka, K.; Sikorska, D. Low-maintenance green tram tracks as a socially acceptable solution to greening a city. Urban For. Urban Green. 2018, 35, 148–164. [Google Scholar] [CrossRef]
- Empereur, J.Y. Alexandrie Redécouverte; Fayard/Stock: Paris, French, 1998; p. 253. [Google Scholar]
- Yoyotte, J.; Charvet, P.; Gompertz, S. Strabon: Le Voyage en Egypte, un Regard Romain; Nil: Paris, French, 1998; p. 313. [Google Scholar]
- Véron, A.; Goiran, J.P.; Morhange, C.; Marriner, N.; Empereur, J.Y. Pollutant lead reveals the pre-Hellenistic occupation and ancient growth of Alexandria, Egypt. Geophys. Res. Lett. 2006, 33, L06409. [Google Scholar] [CrossRef]
- UNDP (United Nations Development Program). Egypt Human Development Report 2003; Al-Ahram Commercial Press: Cairo, Egypt, 2003. [Google Scholar]
- CAPMAS. Central Agency for Public Mobilization and Statistics. 2021. Available online: www.capmas.gov.eg (accessed on 4 January 2021).
- Mahfouz, B.M.; Osman, A.G.; Saber, S.A.; KanhalafAllah, H.M. Assessment of weather and climate variability over Western Harbor of Alexandria, Egypt. Egypt. J. Aquat. Biol. Fish. 2020, 24, 323–339. [Google Scholar] [CrossRef]
- Müller-Dombois, D.; Ellenberg, H. Aims and Methods of Vegetation Ecology; Wiley: New York, NY, USA, 1974. [Google Scholar]
- Boulos, L. Flora of Egypt; Al Hadara Publishing: Cairo, Egypt, 1999; Volumes 1–4, (updated in 2000 2005). [Google Scholar]
- Boulos, L. Flora of Egypt Checklist; Al-Hadara Publishing: Cairo, Egypt, 2009; p. 410. [Google Scholar]
- Raunkiaer, C. Plant Life Forms; Clarendon: Oxford, UK, 1937; p. 104. [Google Scholar]
- Zohary, M. Flora Palaestina, Parts I and II; The Israel Academy of Science and Humanities: Jerusalem, Israel, 1966; (updated in 1972). [Google Scholar]
- Feinbrun-Dothan, N. Flora Palaestina: Parts III and IV; The Israel Academy of Science and Humanities: Jerusalem, Israel, 1978; (updated in 1986). [Google Scholar]
- Täckholm, V. Student’s Flora of Egypt; Cairo University Press: Cairo, Egypt, 1974; p. 888. [Google Scholar]
- El-Hadidi, N.; Hosni, H.A. Flora Aegyptiaca. Part 1; Cairo University Herbarium 6-The Palm Press: Cairo, Egypt, 2000; Volume 1, p. 187. [Google Scholar]
- OpenStreetMap. Planet Dump: OpenStreetMap.2015. Available online: https://planet.openstreetmap.org (accessed on 19 May 2021).
- ESRI. ArcGIS Desktop: Release 10.1; Environmental Systems Research Institute: Redlands, CA, USA, 2012. [Google Scholar]
- Fick, S.E.; Hijmans, R.J. WorldClim 2: New 1-km spatial resolution climate surfaces for global land areas. Int. J. Climatol. 2017, 37, 4302–4315. [Google Scholar] [CrossRef]
- Ayyad, M.A.; Le Floch, E. An Ecological Assessment of Renewable Resources for Rural Agricultural Development in the Western Mediterranean Coastal Region of Egypt; Case Study: El-Omayed Test Area. In Ministère des Affaires Étrangères and C.N.R.S./C.E.P.E.L; Emberger: Montpellier, France, 1983. [Google Scholar]
- Stirling, G.; Wilsey, B. Empirical relationships between species richness, evenness, and proportional diversity. Am. Nat. 2001, 158, 286–299. [Google Scholar] [CrossRef]
- Magurran, A.E. Measuring Biological Diversity; Blackwell Publishing: Oxford, UK, 2004; p. 256. [Google Scholar]
- Hill, M.O. DECORANA A Fortran Program for Detrended Correspondence Analysis and Reciprocal Averaging; Cornell University: Ithaca, NY, USA, 1979. [Google Scholar]
- Hill, M.O. TWINSPAN A Fortran Program for Arranging Multivariate Data in An Ordered Two-Way Table by Classification the Individuals and The Attributes; Cornell University: Ithaca, NY, USA, 1979. [Google Scholar]
- Gauch, H.G.; Whittaker, R.H. Hierarchical classification of community data. J. Ecol. 1981, 69, 537–558. [Google Scholar] [CrossRef]
- SPSS. IBM SPSS Statistics for Windows, Version 21.0; IBM Corp: Armonk, NY, USA, 2012. [Google Scholar]
- TerBraak, C.J.F.; Looman, C.W.N. Regression. In Data Analysis in Community and Landscape Ecology; Jongman, R.H.G., TerBraak, C.J.F., van Tongeren, O.F.R., Eds.; Pudoc: Wageningen, The Netherlands, 1987. [Google Scholar]
- Palmer, M.W. Putting things in even better order: The advantages of canonical correspondence analysis. Ecology 1993, 74, 2215–2230. [Google Scholar] [CrossRef]
- Shaltout, K.H.; Al-Sodany, Y.M.; Eid, E.M.; Heneidy, S.Z.; Taher, M.A. Vegetation diversity along the altitudinal and environmental gradients in the main wadi beds in the mountainous region of South Sinai, Egypt. J. Mt. Sci. 2020, 17, 2447–2458. [Google Scholar] [CrossRef]
- Heneidy, S.Z.; Bidak, L.M. Multipurpose plant species in Bisha, Asir region, southwestern Saudi Arabia. J. King Saud Univ. Sci. 2001, 13, 11–26. [Google Scholar]
- Shaltout, K.H.; Hosni, H.A.; El-Kady, H.F.; El-Beheiry, M.A.; Shaltout, S.K. Composition and pattern of alien species in the Egyptian flora. Flora 2016, 222, 104–110. [Google Scholar] [CrossRef]
- Täckholm, V. Students’ Flora of Egypt; Anglo—Egyptian Bookshop: Cairo, Egypt, 1956. [Google Scholar]
- El-Hadidi, M.N.; Fayed, A. Material for excursion flora of Egypt. Taeckholmia 1995, 15, 1–233. [Google Scholar]
- Vilà, M.; Meggaro, Y.; Weber, E. Preliminary analysis of the naturalized flora of northern Africa. Orsis 1999, 14, 009–020. [Google Scholar]
- El-Bana, M.I. Effect of invasion by exotic Acacia saligna (Labill.) H. Wendl. On native species diversity across an aridity gradient along the coastal Mediterranean dunes of Sinai Peninsula. Catrina 2008, 3, 41–48. [Google Scholar]
- Draz, C.O. Some Desert Plants and Their Uses in Animal Feeding (Kochia Indica and Prosopis Juliflora); Publication de l’Institut du Desert d’Égypte No. 2: Cairo, Egypt, 1954. [Google Scholar]
- Austin, D.F. Realignment of the species placed in Exogonium (Convolvulaceae). Ann. Mo. Bot. Gard. 1977, 64, 330–339. [Google Scholar] [CrossRef]
- Shaltout, K.H.; Al-Sodany, Y.M.; Eid, E.M. Growth behavior of the invasive species Ipomoea carnea in the Nile Delta, Egypt. Hydrobiologia 2010, 656, 187–197. [Google Scholar] [CrossRef]
- Keshta, A.E. Population ecology of Dalbergia sisso Roxb.ex DC. In Nile Delta. In Dissertation; Tanta University: Tanta, Egypt, 2010. [Google Scholar]
- Sheded, M.G.; Shaltout, K.H. Weed flora in plantations of recently established tourist resorts along Red Sea coast. J. Union Arab. Biol. 1998, 5, 109–119. [Google Scholar]
- Lausi, D.; Nimis, P.L. Roadside vegetation in boreal South Yukon and adjacent Alaska. Phytocoenologia 1985, 13, 103–138. [Google Scholar] [CrossRef]
- Jehlík, V.; Zaliberová, M.; Májeková, J. The influence of the eastern migration route on the Slovak flora–a comparison after 40 years. Tuexenia 2017, 37, 313–332. [Google Scholar]
- Shaltout, K.H.; El-Kady, H.F.; El-Sheikh, M.A. Species diversity of the ruderal habitats in the Nile Delta. Taeckholmia 1999, 19, 203–225. [Google Scholar] [CrossRef]
- El-Ghandour, M. Suitability of ground water in the delta region “Egypt” for irrigation. Middle East Water Sew. 1983, 1, 108–120. [Google Scholar]
- Rendeková, A.; Mičieta, K.; Randáková, Z.; Ballová, D.; Eliašová, M.; Miškovic, J. Flora of the tram tracks of Bratislava. Urban Ecosyst. 2020, 23, 875–891. [Google Scholar] [CrossRef]
- El-Fahar, R.A.; Heneidy, S.Z. Diversity in plant communities of the Mediterranean coastal land, between Abu-Kir and Rosetta, Egypt. Täkholmia 2003, 23, 199–215. [Google Scholar]
- White, M.D.; Greer, K.A. The effects of watershed urbanization on the stream hydrology and riparian vegetation of Los Peñasquitos Creek, California. Landsc. Urban Plan. 2006, 74, 125–138. [Google Scholar] [CrossRef]
- Yuan, Z.Y.; Jiao, F.; Li, Y.H.; Kallenbach, R.L. Anthropogenic disturbances are key to maintaining the biodiversity of grasslands. Sci. Rep. 2016, 6, 22132. [Google Scholar] [CrossRef] [PubMed]
- Cameron, G.N.; Culley, T.M.; Kolbe, S.E.; Miller, A.I.; Matter, S.F. Effects of urbanization on herbaceous forest vegetation: The relative impacts of soil, geography, forest composition, human access, and an invasive shrub. Urban Ecosyst. 2015, 18, 1051–1069. [Google Scholar] [CrossRef]
- Washitani, I. Plant conservation ecology for management and restoration of riparian habitats of lowland Japan. Popul. Ecol. 2001, 43, 189–195. [Google Scholar] [CrossRef]
- Hobbs, R.J.; Huenneke, L.F. Disturbance, Diversity, and Invasion: Implications for Conservation. Conserv. Biol. 1992, 6, 324–337. [Google Scholar] [CrossRef]
- McKinney, M.L. Urbanization, Biodiversity, and Conservation: The impacts of urbanization on native species are poorly studied, but educating a highly urbanized human population about these impacts can greatly improve species conservation in all ecosystems. BioScience 2002, 52, 883–890. [Google Scholar] [CrossRef]
- Zhu, J.; Mao, Z.; Hu, L.; Zhang, J. Plant diversity of secondary forests in response to anthropogenic disturbance levels in montane regions of northeastern China. J. For. Res. 2007, 12, 403–416. [Google Scholar] [CrossRef]
- Perrino, E.V.; Silletti, G.N.; Erben, M.; Wagensommer, R.P. Viola cassinensis lucana (Violaceae), a new subspecies from Lucanian Apennine, southern Italy. Phyton 2018, 58, 109–115. [Google Scholar]
- Wagensommer, R.P.; Venanzoni, R. Geranium lucarinii nov. and re-evaluation of G. kikianum (Geraniaceae). Phytotaxa 2021, 489, 252–262. [Google Scholar] [CrossRef]
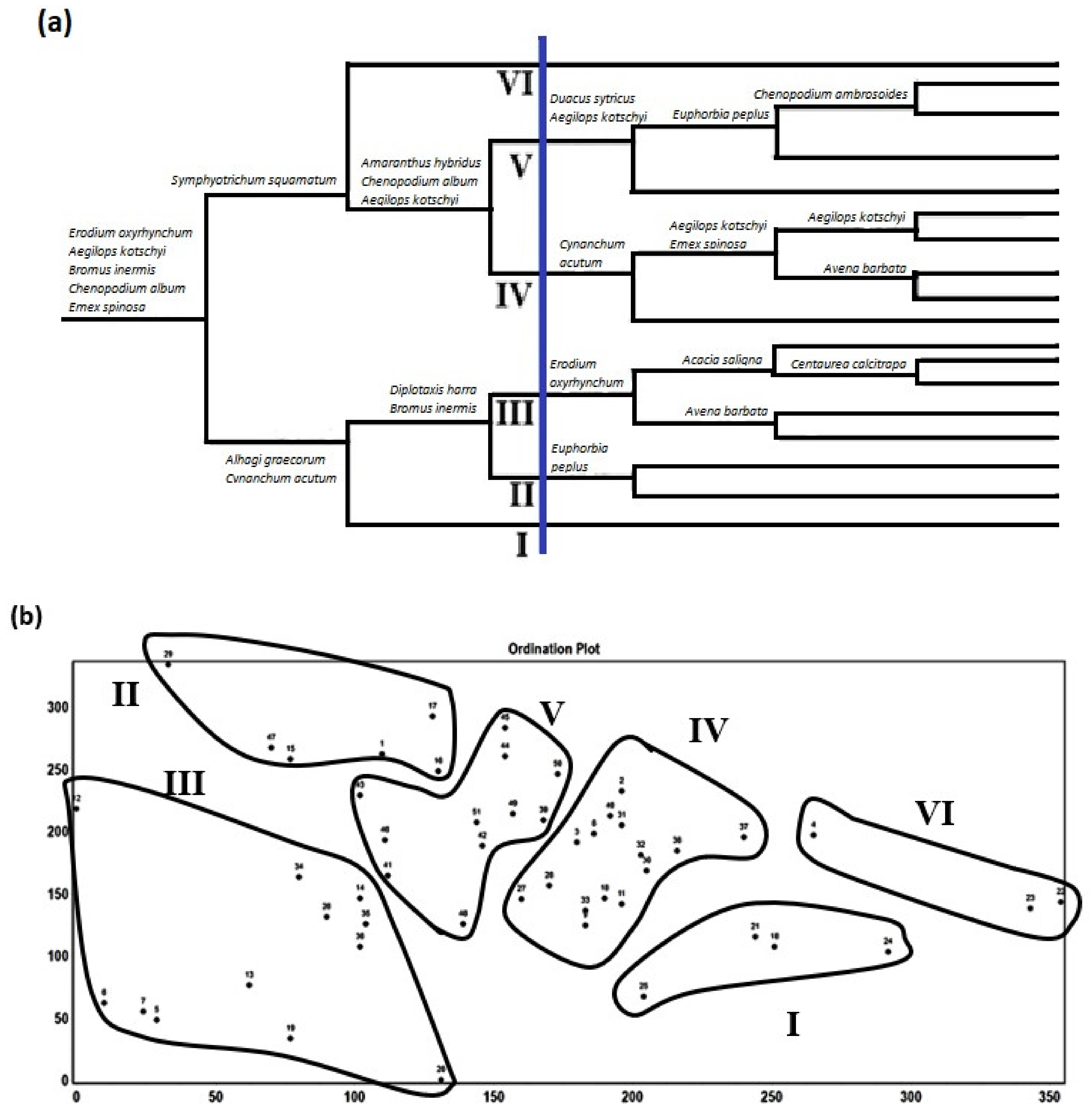
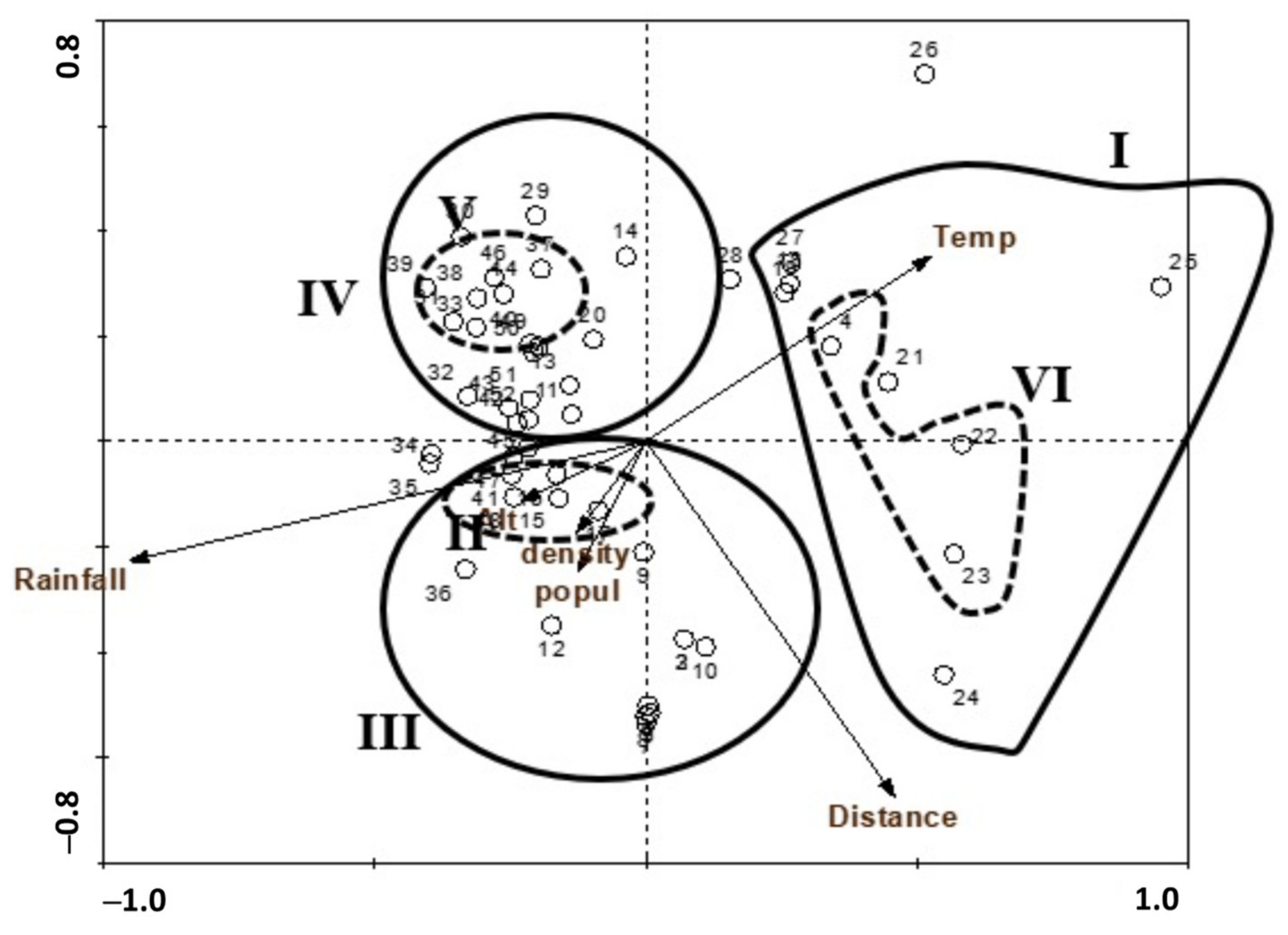
| VG | No. of Stands | Train | Tram | First Dominant | Presence (%) | Second Dominant | Presence (%) |
|---|---|---|---|---|---|---|---|
| I | 4 | 75 | 25 | Urospermum picroides | 83.3 | Sisymbrium irio | 83.3 |
| II | 6 | 66.7 | 33.3 | Chenopodium murale | 100 | Malva parviflora | 100 |
| III | 12 | 66.7 | 33.3 | Malva parviflora | 100 | Lactuca serriola | 100 |
| IV | 15 | 40 | 60 | Cynodon dactylon | 93.3 | Aegilops kotschyi | 93.3 |
| V | 11 | 100 | Hordeum leporinum | 100 | Phoenix dactylifera | 90.9 | |
| VI | 3 | 100 | Sonchus oleraceus | 100 | Cynanchum acutum | 100 |
| Character | Richness | Simpson | Shannon | Sh. Even | Hill | Cover (%) | No. of Species | Natural Species | Native Weeds | Alien Species | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Vegetation groups | I | 20.50 ± 7.34 | 0.04 ± 0.02 | 2.77 ± 0.38 | 0.96 ± 0.01 | 1.80 ± 0.96 | 31.40 ± 25.49 | 58 | 6 | 33 | 19 |
| II | 29.17 ± 11.70 | 0.03 ± 0.01 | 3.18 ± 0.34 | 0.96 ± 0.01 | 1.71 ± 0.78 | 73.58 ± 23.69 | 142 | 34 | 70 | 37 | |
| III | 29.25 ± 8.22 | 0.03 ± 0.01 | 3.21 ± 0.27 | 0.96 ± 0.01 | 1.43 ± 0.06 | 65.00 ± 7.07 | 72 | 14 | 42 | 16 | |
| IV | 25.80 ± 6.73 | 0.03 ± 0.01 | 3.09 ± 0.26 | 0.96 ± 0.01 | 1.63 ± 0.74 | 38.87 ± 22.20 | 104 | 24 | 58 | 22 | |
| V | 18.09 ± 2.98 | 0.04 ± 0.02 | 2.77 ± 0.18 | 0.88 ± 0.26 | 2.55 ± 3.05 | 16.55 ± 20.83 | 60 | 13 | 32 | 15 | |
| VI | 38.67 ± 15.82 | 0.03 ± 0.01 | 3.44 ± 0.39 | 0.95 ± 0.00 | 1.37 ± 0.03 | 61.67 ± 20.21 | 81 | 14 | 46 | 21 | |
| F-value | 4.19 ** | 1.40 ns | 4.97 *** | 0.67 ns | 0.62 ns | 8.77 *** | -- | -- | -- | -- | |
| Habitat | Train | 29.58 ± 10.91 | 0.03 ± 0.01 | 3.18 ± 0.35 | 0.96 ± 0.01 | 1.52 ± 0.49 | 59.77 ± 27.20 | 197 | 48 | 98 | 51 |
| Tram | 21.56 ± 6.61 | 0.04 ± 0.02 | 2.91 ± 0.29 | 0.93 ± 0.17 | 2.12 ± 2.05 | 30.96 ± 26.26 | 139 | 26 | 72 | 41 | |
| t-value | 10.36 *** | 2.35 ns | 9.24 ** | 0.91 ns | 1.93 ns | 13.88 *** | -- | -- | -- | -- | |
| Total mean | 25.33 ± 9.69 | 0.03 ± 0.01 | 3.04 ± 0.34 | 0.94 ± 0.12 | 1.84 ± 1.55 | 44.17 ± 30.13 | 224 | 56 | 108 | 60 | |
| Alt (m) | Population (km−2) | Rainfall (mm) | Temp. (°C) | Rd. Density (m/m2) | Distance (m) | ||
|---|---|---|---|---|---|---|---|
| VG | I | 12.50 ± 14.38 | 29,736.0 ± 18,931.1 | 183.33 ± 0.82 | 20.33 ± 0.52 | 88.15 ± 42.05 | 8279.77 ± 7241.88 |
| II | 47.83 ± 133.50 | 28,322.5 ± 12,730.0 | 182.67 ± 2.67 | 20.08 ± 0.29 | 99.55 ± 31.14 | 8478.83 ± 8137.16 | |
| III | 1.50 ± 1.91 | 25,715.5 ± 7216.3 | 177.00 ± 3.37 | 20.75 ± 0.50 | 83.42 ± 28.10 | 12,038.66 ± 11,759.29 | |
| IV | 17.33 ± 10.53 | 32,756.0 ± 14,661.1 | 182.67 ± 1.88 | 20.67 ± 0.49 | 125.17 ± 35.88 | 7565.04 ± 9019.00 | |
| V | 40.36 ± 28.88 | 51,816.9 ± 22,813.4 | 184.09 ± 0.30 | 20.00 ± 0.00 | 75.69 ± 17.41 | 630.76 ± 336.78 | |
| VI | 6.33 ± 6.81 | 30,949.0 ± 7175.9 | 178.67 ± 1.15 | 21.00 ± 0.00 | 65.79 ± 17.11 | 21,803.77 ± 1384.22 | |
| Habitat | Train | 12.33 ± 13.03 | 27,263.3 ± 10,507.0 | 181.75 ± 2.13 | 20.38 ± 0.49 | 90.59 ± 36.27 | 15,363.05 ± 6638.39 |
| Tram | 40.07 ± 89.25 | 41,518.6 ± 20,760.9 | 182.93 ± 3.06 | 20.37 ± 0.49 | 103.35 ± 35.24 | 618.20 ± 366.49 | |
| Total | 27.02 ± 66.45 | 34,810.2 ± 18,070.7 | 182.37 ± 2.71 | 20.37 ± 0.49 | 97.35 ± 35.95 | 7556.95 ± 8694.20 | |
| Alt | Popul. | Cover | Rainfall | Temp | Rd. Density | Distance | |
|---|---|---|---|---|---|---|---|
| Richness | 0.112 | –0.277 * | 0.562 *** | –0.196 | 0.189 | 0.007 | 0.535 *** |
| Simpson | –0.225 | 0.335 * | –0.229 | 0.054 | –0.247 | –0.152 | –0.354 ** |
| Shannon | 0.131 | –0.328 * | 0.589 *** | –0.211 | 0.205 | 0.009 | 0.498 *** |
| Sh. Even | –0.001 | 0.061 | 0.154 | –0.075 | 0.111 | 0.083 | 0.123 |
| Hill | 0.089 | –0.074 | –0.290 * | 0.164 | –0.061 | –0.019 | –0.145 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heneidy, S.Z.; Halmy, M.W.A.; Toto, S.M.; Hamouda, S.K.; Fakhry, A.M.; Bidak, L.M.; Eid, E.M.; Al-Sodany, Y.M. Pattern of Urban Flora in Intra-City Railway Habitats (Alexandria, Egypt): A Conservation Perspective. Biology 2021, 10, 698. https://doi.org/10.3390/biology10080698
Heneidy SZ, Halmy MWA, Toto SM, Hamouda SK, Fakhry AM, Bidak LM, Eid EM, Al-Sodany YM. Pattern of Urban Flora in Intra-City Railway Habitats (Alexandria, Egypt): A Conservation Perspective. Biology. 2021; 10(8):698. https://doi.org/10.3390/biology10080698
Chicago/Turabian StyleHeneidy, Selim Z., Marwa W. A. Halmy, Soliman M. Toto, Sania K. Hamouda, Amal M. Fakhry, Laila M. Bidak, Ebrahem M. Eid, and Yassin M. Al-Sodany. 2021. "Pattern of Urban Flora in Intra-City Railway Habitats (Alexandria, Egypt): A Conservation Perspective" Biology 10, no. 8: 698. https://doi.org/10.3390/biology10080698
APA StyleHeneidy, S. Z., Halmy, M. W. A., Toto, S. M., Hamouda, S. K., Fakhry, A. M., Bidak, L. M., Eid, E. M., & Al-Sodany, Y. M. (2021). Pattern of Urban Flora in Intra-City Railway Habitats (Alexandria, Egypt): A Conservation Perspective. Biology, 10(8), 698. https://doi.org/10.3390/biology10080698








