Alteration of Metabolites Accumulation in Maize Inbreds Leaf Tissue under Long-Term Water Deficit
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Field Trial
2.3. Growth Conditions
2.4. Physiological Parameters (Nitrogen Balance Index, Chlorophyll, Flavonols and Anthocyanins Indices)
2.5. Samples Preparation and Extraction of Free Phenolic Acids (PA)
Quantification of PA
2.6. Statistical Analyses
3. Results
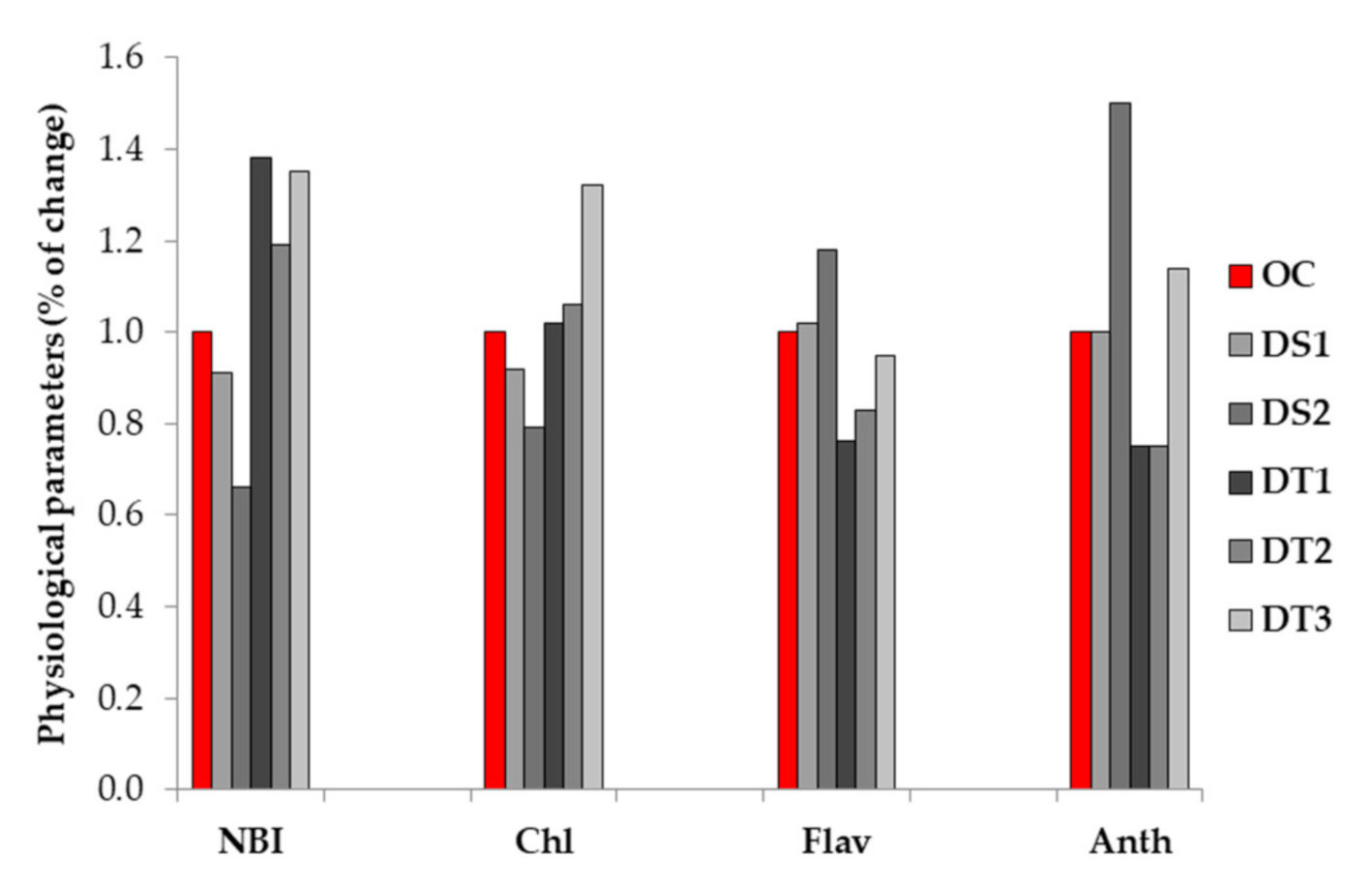
3.1. Physiological Parameters (NBI, Chlorophyll, Flavonols and Anthocyanins Indices)
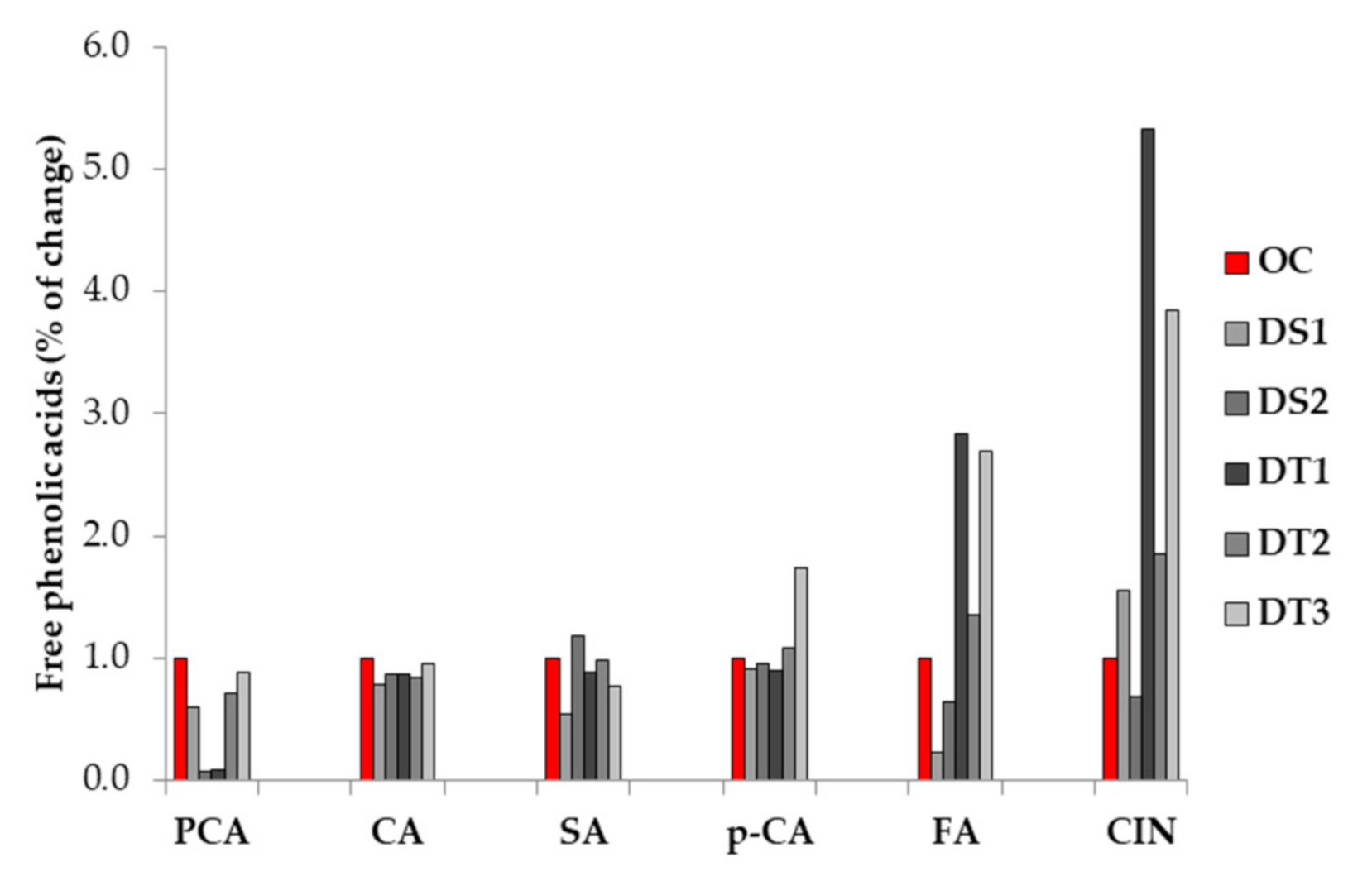
3.2. Free Phenolic Acids (PA) Content
3.3. Grain Yield (GY)
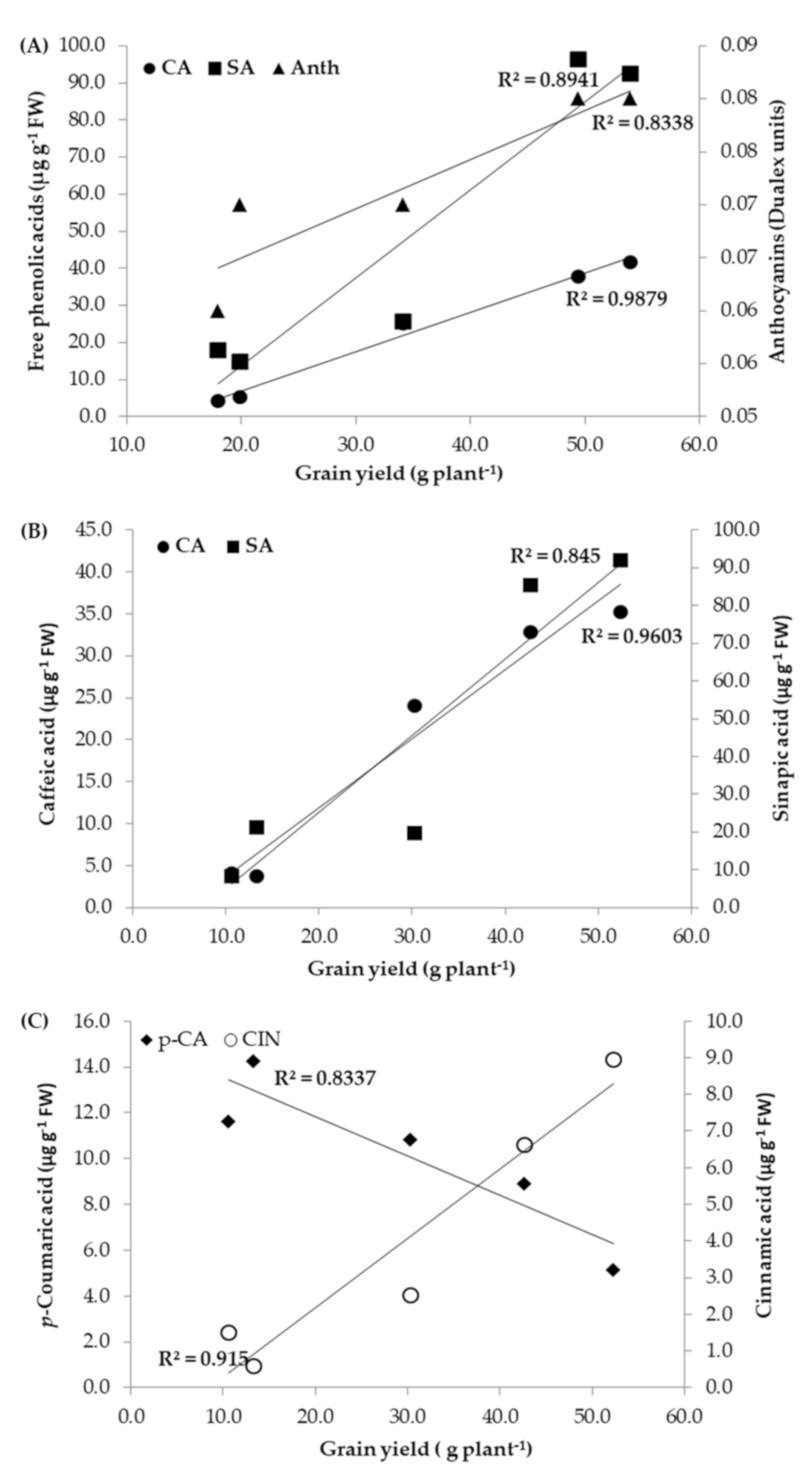
3.4. Correlations
4. Discussion
4.1. Physiological Parameters (NBI, Chlorophyll, Flavonols and Anthocyanins Indices)
4.2. Free Phenolic Acids (PA) Content
4.3. Grain Yield (GY)
4.4. Correlations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mittler, R.; Blumwald, E. Genetic engineering for modern agriculture: Challenges and perspectives. Annu. Rev. Plant Biol. 2010, 61, 443–462. [Google Scholar] [CrossRef] [Green Version]
- Sun, C.X.; Li, M.Q.; Gao, X.X.; Liu, L.N.; Wu, X.F.; Zhou, J.H. Metabolic response of maize plants to multi-factorial abiotic stresses. Plant Biol. 2016, 18, 120–129. [Google Scholar] [CrossRef]
- Piasecka, A.; Kachlicki, P.; Stobiecki, M. Analytical methods for detection of plant metabolomes changes in response to biotic and abiotic stresses. Int. J. Mol. Sci. 2019, 20, 379. [Google Scholar] [CrossRef] [Green Version]
- Deng, Y.; Lu, S. Biosynthesis and regulation of phenylpropanoids in plants. Crit. Rev. Plant Sci. 2017, 36, 257–290. [Google Scholar] [CrossRef]
- Arbona, V.; Manzi, M.; de Ollas, C.; Gómez-Cadenas, A. Metabolomics as a tool to investigate abiotic stress tolerance in plants. Int. J. Mol. Sci. 2013, 14, 4885–4911. [Google Scholar] [CrossRef] [PubMed]
- Urban, L.; Aarrouf, J.; Bidel, L.P.R. Assessing the effects of water deficit on photosynthesis using parameters derived from measurements of leaf gas exchange and of chlorophyll a fluorescence. Front. Plant Sci. 2017, 8, 2068. [Google Scholar] [CrossRef] [Green Version]
- Zlatev, Z. Drought-induced changes in chlorophyll fluorescence of young wheat plants. Biotechnol. Equip. 2009, 23, 438–441. [Google Scholar] [CrossRef] [Green Version]
- Noctor, G.; Mhamdi, A.; Foyer, C.H. The roles of reactive oxygen metabolism in drought: Not so cut and dried. Plant Physiol. 2014, 164, 1636–1648. [Google Scholar] [CrossRef] [Green Version]
- Riedelsheimer, C.; Lisec, J.; Czedik-Eysenberg, A.; Sulpice, R.; Flis, A.; Grieder, C.; Altmann, T.; Stitt, M.; Willmitzer, L.; Melchinger, A.E. Genome-wide association mapping of leaf metabolic profiles for dissecting complex traits in maize. Proc. Natl. Acad. Sci. USA 2012, 109, 8872–8877. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hill, C.B.; Taylor, J.D.; Edwards, J.; Mather, D.; Langridge, P.; Bacic, A. Roessner, U. Detection of QTL for metabolic and agronomic traits in wheat with adjustments for variation at genetic loci that affect plant phenology. Plant Sci. 2015, 233, 143–154. [Google Scholar] [CrossRef] [Green Version]
- Shi, T.; Zhu, A.; Jia, J.; Hu, X.; Chen, J.; Liu, W.; Ren, X.; Sun, D.; Fernie, A.R.; Cui, F.; et al. Metabolomics analysis and metabolite-agronomic trait associations using kernels of wheat (Triticum aestivum) recombinant inbred lines. Plant J. 2020, 103, 279–292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khodarahmpour, Z.; Hamidi, J. Evaluation of drought tolerance in different growth stages of maize (Zea mays L.) inbred lines using tolerance indices. Afr. J. Biotechnol. 2011, 10, 13482–13490. [Google Scholar] [CrossRef]
- Witt, S.; Galicia, L.; Lisec, J.; Cairns, J.; Tiessen, A.; Araus, J.L.; Palacios-Rojas, N.; Fernie, A.R. Metabolic and phenotypic responses of greenhouse-grown maize hybrids to experimentally controlled drought stress. Mol. Plant 2012, 5, 401–417. [Google Scholar] [CrossRef] [Green Version]
- Chaves, M.M.; Flexas, J.; Pinheiro, C. Photosynthesis under drought and salt stress: Regulation mechanisms from whole plant to cell. Ann. Bot. 2009, 103, 551–560. [Google Scholar] [CrossRef] [Green Version]
- Lawlor, D.W.; Tezara, W. Causes of decreased photosynthetic rate and metabolic capacity in water-deficient leaf cells: A critical evaluation of mechanisms and integration of processes. Ann. Bot. 2009, 103, 561–579. [Google Scholar] [CrossRef] [Green Version]
- Tollenaar, M.; Lee, E.A. Dissection of physiological processes underlying grain yield in maize by examining genetic improvement and heterosis. Maydica 2006, 51, 399–408. [Google Scholar]
- Kravić, N.; Marković, K.; Anđelković, V.; Hadži-Tašković Šukalović, V.; Babić, V.; Vuletić, M. Growth, proline accumulation and peroxidase activity in maize seedlings under osmotic stress. Acta Physiol. Plant. 2013, 35, 233–239. [Google Scholar] [CrossRef]
- Dewanto, V.; Wu, X.; Liu, R.H. Processed sweet corn has higher antioxidant activity. J. Agric. Food Chem. 2002, 50, 4959–4964. [Google Scholar] [CrossRef]
- Farooq, M.; Wahid, A.; Kobayashi, N.; Fujita, D.; Basra, S.M.A. Plant drought stress: Effects, mechanisms and management. Agron. Sustain. Dev. 2009, 29, 185–212. [Google Scholar] [CrossRef] [Green Version]
- Sunil, B.; Talla, S.K.; Aswani, V.; Raghavendra, A.S. Optimization of photosynthesis by multiple metabolic pathways involving interorganelle interactions: Resource sharing and ROS maintenance as the bases. Photosynth. Res. 2013, 117, 61–71. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Dugo, V.; Durand, J.L.; Gastal, F. Water deficit and nitrogen nutrition of crops. A review. Agron. Sustain. Dev. 2010, 30, 529–544. [Google Scholar] [CrossRef] [Green Version]
- Yi, X.P.; Zhang, Y.L.; Yao, H.S.; Zhang, X.J.; Luo, H.H.; Gou, L.; Zang, W. Alternative electron sinks are crucial for conferring photoprotection in field grown cotton under water deficit during flowering and boll setting stages. Funct. Plant Biol. 2014, 41, 737–747. [Google Scholar] [CrossRef]
- Tremblay, N.; Wang, Z.; Belec, C. Performance of Dualex in spring wheat for crop nitrogen status assessment, yield prediction and estimation of soil nitrate content. J. Plant Nutr. 2010, 33, 57–70. [Google Scholar] [CrossRef]
- Cartelat, A.; Cerovic, Z.G.; Goulas, Y.; Meyer, S.; Lelarge, C.; Prioul, J.-L.; Barbottin, A.; Jeuffroy, M.-H.; Gate, P.; Agati, G.; et al. Optically assessed contents of leaf polyphenolics and chlorophyll as indicators of nitrogen deficiency in wheat (Triticum aestivum L.). Field Crop. Res. 2005, 91, 35–49. [Google Scholar] [CrossRef]
- Rong-hua, L.; Pei-pol, G.; Baumz, M.; Grand, S.; Ceccarelli, S. Evaluation of chlorophyll content and fluorescence parameters as indicators of drought tolerance in barley. Agric. Sci. China 2006, 5, 751–757. [Google Scholar]
- Agati, G.; Azzarello, E.; Pollastri, S.; Tattini, M. Flavonoids as antioxidants in plants: Location and functional significance. Plant Sci. 2012, 196, 67–76. [Google Scholar] [CrossRef]
- Selmar, D. Potential of salt and drought stress to increase pharmaceutical significant secondary compounds in plants. Landbauforsch. Volkenrode 2008, 58, 139–144. [Google Scholar]
- Nakabayashi, R.; Yonekura-Sakakibara, K.; Urano, K.; Suzuki, M.; Yamada, Y.; Nishizawa, T.; Matsuda, F.; Kojima, M.; Sakakibara, H.; Shinozaki, K.; et al. Enhancement of oxidative and drought tolerance in Arabidopsis by overaccumulation of antioxidant flavonoids. Plant J. 2014, 77, 367–379. [Google Scholar] [CrossRef] [PubMed]
- Peng, M.; Hudson, D.; Schofield, A.; Tsao, R.; Yang, R.; Gu, H.; Bi, Y.M.; Rothstein, S.J. Adaptation of Arabidopsis to nitrogen limitation involves induction of anthocyanin synthesis which is controlled by the NLA gene. J. Exp. Bot. 2008, 59, 2933–2944. [Google Scholar] [CrossRef]
- Steyn, W.J.; Wand, S.J.E.; Holcroftm, D.M.; Jacobs, G. Anthocyanins in vegetative tissues: A proposed unified function in photoprotection. New Phytol. 2002, 155, 349–361. [Google Scholar] [CrossRef]
- Sharma, A.; Shahzad, B.; Rehman, A.; Bhardwaj, R.; Landi, M.; Zheng, B. Response of phenylpropanoid pathway and the role of polyphenols in pants under abiotic stress. Molecules 2019, 24, 2452. [Google Scholar] [CrossRef] [Green Version]
- Marchiosi, R.; dos Santos, W.D.; Constantin, R.P.; de Lima, R.B.; Soares, A.R.; Finger-Teixeira, A.; Rodrigues Mota, T.; de Oliveira, D.M.; de Paiva Foletto-Felipe, M.; Abrahão, J.; et al. Biosynthesis and metabolic actions of simple phenolic acids in plants. Phytochem. Rev. 2020, 19, 865–906. [Google Scholar] [CrossRef]
- Hura, T.; Grzesiak, S.; Hura, K.; Thiemt, E.; Tokarz, K.; Wedzony, M. Physiological and biochemical tools useful in drought-tolerance detection in genotypes of winter triticale: Accumulation of ferulic acid correlates with drought tolerance. Ann. Bot. 2007, 100, 767–775. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hura, T.; Hura, K.; Grzesiak, S. Contents of total phenolics and ferulic acid, and PAL activity during water potential changes in leaves of maize single-cross hybrids of different drought tolerance. J. Agron. Crop Sci. 2008, 194, 104–112. [Google Scholar] [CrossRef]
- Raval, S.S.; Mahatma, M.K.; Chakraborty, K.; Bishi, S.K.; Singh, A.L.; Rathod, K.J.; Jadav, J.K.; Sanghani, J.M.; Mandavia, M.K.; Gajera, H.P.; et al. Metabolomics of groundnut (Arachis hypogaea L.) genotypes under varying temperature regimes. Plant Growth Regul. 2017, 84, 493–505. [Google Scholar] [CrossRef]
- Gundaraniya, S.A.; Ambalam, P.S.; Tomar, R.S. Metabolomic profiling of drought-tolerant and susceptible peanut (Arachis hypogaea L.) genotypes in response to drought stress. ACS Omega 2020, 5, 31209–31219. [Google Scholar] [CrossRef]
- Yang, H.; Zhao, L.; Zhao, S.; Wang, J.; Shi, H. Biochemical and transcriptomic analyses of drought stress responses of LY1306 tobacco strain. Sci. Rep. 2017, 7, 17442. [Google Scholar] [CrossRef] [Green Version]
- Singh, P.K.; Singh, R.; Singh, S. Cinnamic acid induced changes in reactive oxygen species scavenging enzymes and protein profile in maize (Zea mays L.) plants grown under salt stress. Physiol. Mol. Biol. Plants 2013, 19, 53–59. [Google Scholar] [CrossRef]
- Sun, W.-J.; Nie, Y.-X.; Gao, Y.; Dai, A.-H.; Bai, J.-G. Exogenous cinnamic acid regulates antioxidant enzyme activity and reduces lipid peroxidation in drought-stressed cucumber leaves. Acta Physiol. Plant. 2012, 34, 641–655. [Google Scholar] [CrossRef]
- Chung, I.M.; Kim, J.J.; Lim, J.D.; Yu, C.Y.; Kim, S.H.; Hahn, S.J. Comparison of resveratrol, SOD activity, phenolic compounds and free amino acid in Rehmannia glutinose under temperature and water stress. Environ. Exp. Bot. 2006, 56, 44–53. [Google Scholar] [CrossRef]
- Król, A.; Amarowicz, R.; Weidner, S. Changes in the composition of phenolic compounds and antioxidant properties of grapevine roots and leaves (Vitis vinifera L.) under continuous of long-term drought stress. Acta Physiol. Plant. 2014, 36, 1491–1499. [Google Scholar] [CrossRef] [Green Version]
- Cairns, J.E.; Sanchez, C.; Vargas, M.; Ordoñez, R.; Araus, J.L. Dissecting maize productivity: Ideotypes associated with grain yield under drought stress and well-watered conditions. J. Integr. Plant Biol. 2012, 54, 1007–1020. [Google Scholar] [CrossRef]
- Messina, C.D.; Podlich, D.; Dong, Z.; Samples, M.; Cooper, M. Yield-trait performance landscapes: From theory to application in breeding maize for drought tolerance. J. Exp. Bot. 2011, 62, 855–868. [Google Scholar] [CrossRef]
- Adebayo, M.A.; Menkir, A.; Blay, E.; Gracen, V.; Danquah, E.; Hearne, S. Genetic analysis of drought tolerance in adapted x exotic crosses of maize inbred lines under managed stress conditions. Euphytica 2014, 196, 261–270. [Google Scholar] [CrossRef]
- Blum, A. Crop responces to drought and the interpretation of adaptation. Plant Growth Regul. 1996, 20, 135–148. [Google Scholar] [CrossRef]
- Zhong, C.; Cao, X.; Hu, J.; Zhu, L.; Zhang, J.; Huang, J.; Jin, Q. Nitrogen metabolism in adaptation of photosynthesis to water stress in rice grown under different nitrogen levels. Front. Plant Sci. 2017, 8, 1079. [Google Scholar] [CrossRef] [Green Version]
- Nunes-Nesi, A.; Fernie, A.R.; Stitt, M. Metabolic and signaling aspects underpinning the regulation of plant carbon nitrogen interactions. Mol. Plant 2010, 3, 973–996. [Google Scholar] [CrossRef]
- Makino, A.; Sakuma, H.; Sudo, E.; Mae, T. Differences between maize and rice in N-use efficiency for photosynthesis and protein allocation. Plant Cell Physiol. 2003, 44, 952–956. [Google Scholar] [CrossRef] [Green Version]
- Deng, B.; Li, Y.; Xu, D.; Liu, G. Nitrogen availability alters flavonoid accumulation in Cyclocarya paliurus via the effects on the internal carbon/nitrogen balance. Sci. Rep. 2019, 9, 2370. [Google Scholar] [CrossRef]
- Manzoni, S.; Vico, G.; Katul, G.; Fay, P.A.; Polley, W.; Palmroth, S.; Porporato, A. Optimizing stomatal conductance for maximum carbon gain under water stress: A meta-analysis across plant functional types and climates. Funct. Ecol. 2011, 25, 456–467. [Google Scholar] [CrossRef]
- Riaz, U.; Kharal, M.A.; Murtaza, G.; Zaman, Q.; Javaid, S.; Malik, H.A.; Aziz, H.; Abbas, Z. Prospective roles and mechanisms of caffeic acid in counter plant stress: A mini review. Pak. J. Agric. Res. 2019, 32, 8–19. [Google Scholar] [CrossRef]
- Gregorová, Z.; Kováčik, J.; Klejdus, B.; Maglovski, M.; Kuna, R.; Hauptvogel, P.; Matušíková, I. Drought-induced responses of physiology, metabolites, and PR proteins in Triticum aestivum. J. Agric. Food Chem. 2015, 63, 8125–8133. [Google Scholar] [CrossRef]
- Oveysi, M.; Mirhadi, M.J.; Madani, H.; Nourmohammadi, G.; Zarghami, R.; Madani, A. The impact of source restriction on yield formation of corn (Zea mays L.) due to water deficiency. Plant Soil Environ. 2010, 56, 476–481. [Google Scholar] [CrossRef] [Green Version]
- Shao, R.; Xin, L.; Zheng, H.; Li, L.; Ran, W.; Mao, J.; Yang, Q.H. Changes in chloroplast ultrastructure in leaves of drought-stressed maize inbred lines. Photosynthetica 2016, 54, 74–80. [Google Scholar] [CrossRef]

| Month | Temperature [°C] | Precipitation [L m−2] | ||
|---|---|---|---|---|
| 20-yr Avg. | 2017 | 20-yr Avg. | 2017 | |
| April | 13.5 | −0.8 | 46.1 | 5.7 |
| May | 18.3 | 0.1 | 63.4 | 21.7 |
| June | 21.7 | 2.6 | 86.0 | −33.0 |
| July | 23.6 | 2.3 | 77.6 | −51.2 |
| August | 23.3 | 2.8 | 62.6 | −43.1 |
| September | 18.1 | 0.3 | 66.1 | −20.3 |
| April–Sept. | 19.8 | 1.2 | 67.0 | −20.0 |
| Trait | SV | df | MS | p |
|---|---|---|---|---|
| NBI | IL | 4 | 27.247 | ≤0.01 |
| T | 1 | 39.931 | ≤0.001 | |
| IL × T | 4 | 145.139 | ≤0.001 | |
| Chl | IL | 4 | 18.564 | ≤0.001 |
| T | 1 | 0.169 | ≤0.001 | |
| IL × T | 4 | 71.678 | ≤0.001 | |
| Flav | IL | 4 | 0.020 | ≤0.001 |
| T | 1 | 0.023 | ≤0.001 | |
| IL × T | 4 | 0.042 | ≤0.001 | |
| Anth | IL | 4 | 0.000 | ns |
| T | 1 | 0.000 | ns | |
| IL × T | 4 | 0.000 | ≤0.05 |
| Trait | SV | df | MS | p |
|---|---|---|---|---|
| PCA | IL | 4 | 1650.324 | ≤0.001 |
| T | 1 | 425.872 | ≤0.001 | |
| IL × T | 4 | 30.262 | ≤0.01 | |
| CA | IL | 4 | 1076.958 | ≤0.001 |
| T | 1 | 40.442 | ≤0.001 | |
| IL × T | 4 | 0.988 | ≤0.01 | |
| SA | IL | 4 | 6565.992 | ≤0.001 |
| T | 1 | 89.465 | ≤0.01 | |
| IL × T | 4 | 31.525 | ≤0.01 | |
| p-CA | IL | 4 | 52.949 | ≤0.001 |
| T | 1 | 1.260 | ≤0.05 | |
| IL × T | 4 | 5.526 | ≤0.001 | |
| FA | IL | 4 | 0.352 | ≤0.001 |
| T | 1 | 0.255 | ≤0.001 | |
| IL × T | 4 | 0.875 | ≤0.001 | |
| CIN | IL | 4 | 26.190 | ≤0.001 |
| T | 1 | 27.028 | ≤0.001 | |
| IL × T | 4 | 5.636 | ≤0.001 |
| Trait | NBI | Chl | Flav | Anth | PCA | CA | SA | p-CA | FA | CIN |
|---|---|---|---|---|---|---|---|---|---|---|
| NBI | – | 0.856 | −0.269 | −0.653 | −0.803 | −0.586 | −0.340 | 0.666 | 0.662 | 0.032 |
| Chl | 0.877 * | – | 0.235 | −0.369 | −0.828 | −0.308 | 0.040 | 0.678 | 0.515 | 0.011 |
| Flav | −0.910 * | −0.621 | – | 0.567 | 0.151 | 0.696 | 0.812 | −0.205 | −0.404 | 0.151 |
| Anth | −0.715 | −0.317 | 0.920 * | – | 0.563 | 0.889 * | 0.879 * | −0.698 | −0.139 | 0.599 |
| PCA | 0.825 | 0.966 ** | −0.595 | −0.247 | – | 0.706 | 0.359 | −0.943 * | −0.668 | 0.329 |
| CA | 0.942 * | 0.849 | −0.839 | −0.712 | 0.773 | – | 0.912 * | −0.825 | −0.399 | 0.637 |
| SA | 0.695 | 0.492 | −0.695 | −0.769 | 0.292 | 0.863 | – | −0.550 | −0.115 | 0.656 |
| p-CA | −0.772 | −0.572 | 0.856 | 0.855 | −0.489 | −0.868 | −0.838 | – | 0.441 | −0.624 |
| FA | 0.846 | 0.746 | −0.684 | −0.593 | 0.573 | 0.906 * | 0.859 | −0.637 | – | 0.333 |
| CIN | 0.773 | 0.561 | −0.817 | −0.852 | 0.415 | 0.906 * | 0.957 * | −0.960 * | 0.783 | – |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kravic, N.; Babic, V.; Vukadinovic, J.; Ristic, D.; Dragicevic, V.; Mladenovic Drinic, S.; Andjelkovic, V. Alteration of Metabolites Accumulation in Maize Inbreds Leaf Tissue under Long-Term Water Deficit. Biology 2021, 10, 694. https://doi.org/10.3390/biology10080694
Kravic N, Babic V, Vukadinovic J, Ristic D, Dragicevic V, Mladenovic Drinic S, Andjelkovic V. Alteration of Metabolites Accumulation in Maize Inbreds Leaf Tissue under Long-Term Water Deficit. Biology. 2021; 10(8):694. https://doi.org/10.3390/biology10080694
Chicago/Turabian StyleKravic, Natalija, Vojka Babic, Jelena Vukadinovic, Danijela Ristic, Vesna Dragicevic, Snezana Mladenovic Drinic, and Violeta Andjelkovic. 2021. "Alteration of Metabolites Accumulation in Maize Inbreds Leaf Tissue under Long-Term Water Deficit" Biology 10, no. 8: 694. https://doi.org/10.3390/biology10080694
APA StyleKravic, N., Babic, V., Vukadinovic, J., Ristic, D., Dragicevic, V., Mladenovic Drinic, S., & Andjelkovic, V. (2021). Alteration of Metabolites Accumulation in Maize Inbreds Leaf Tissue under Long-Term Water Deficit. Biology, 10(8), 694. https://doi.org/10.3390/biology10080694








