Biological Impact of Photoperiod on Fairy Shrimp (Branchinecta orientalis): Life History and Biochemical Composition
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Hatching Experiment
2.2. Breeding Conditions
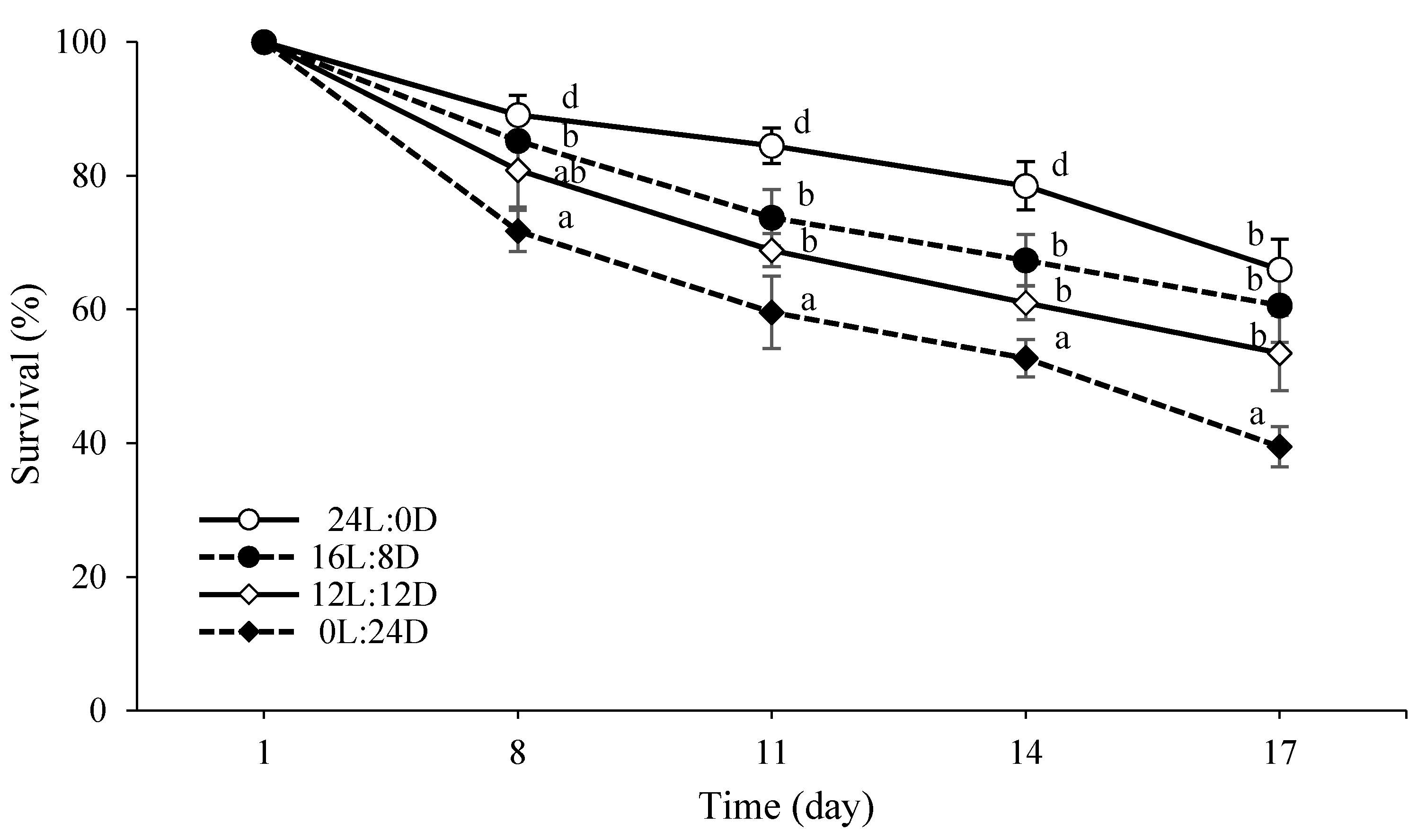
2.3. Survival and Growth
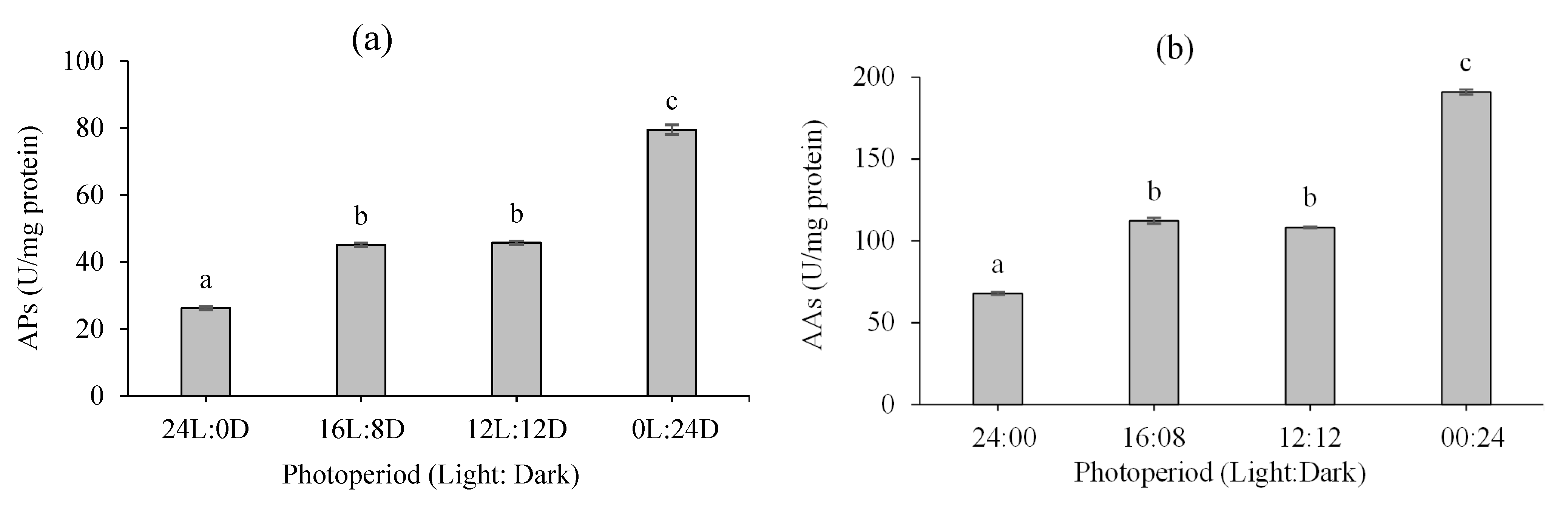
2.4. Digestive Enzyme Activity
2.4.1. Sample Preparation
2.4.2. Amylase Activity
2.4.3. Lipase Activity
2.4.4. Alkaline Protease Activity
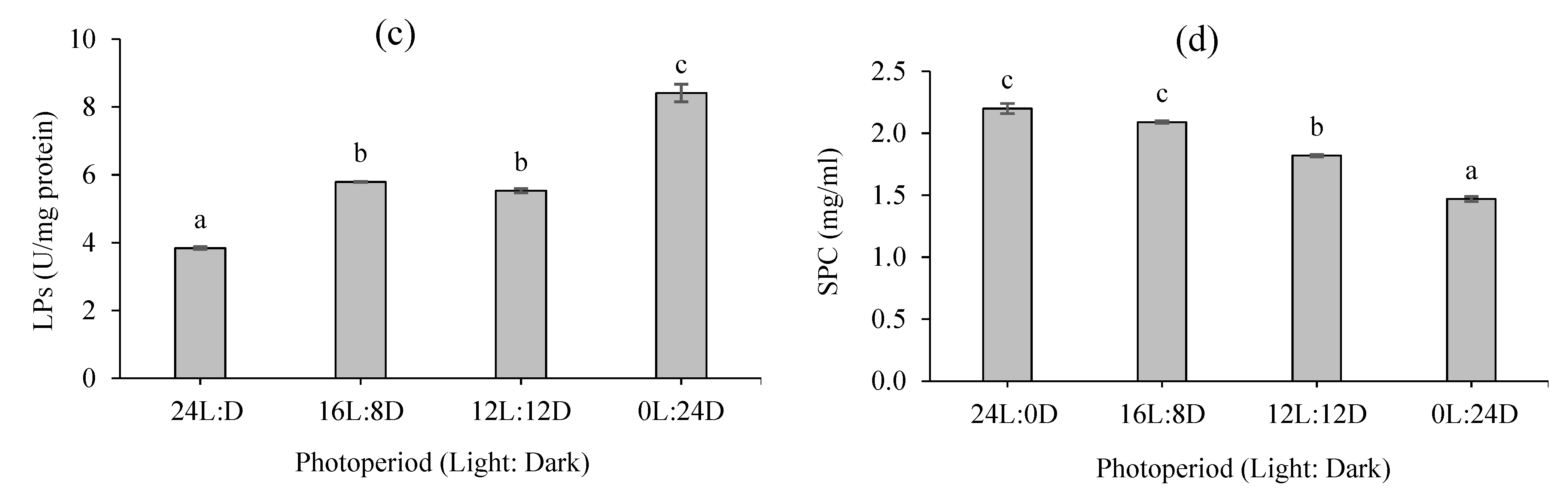
2.5. Soluble Protein Quantification
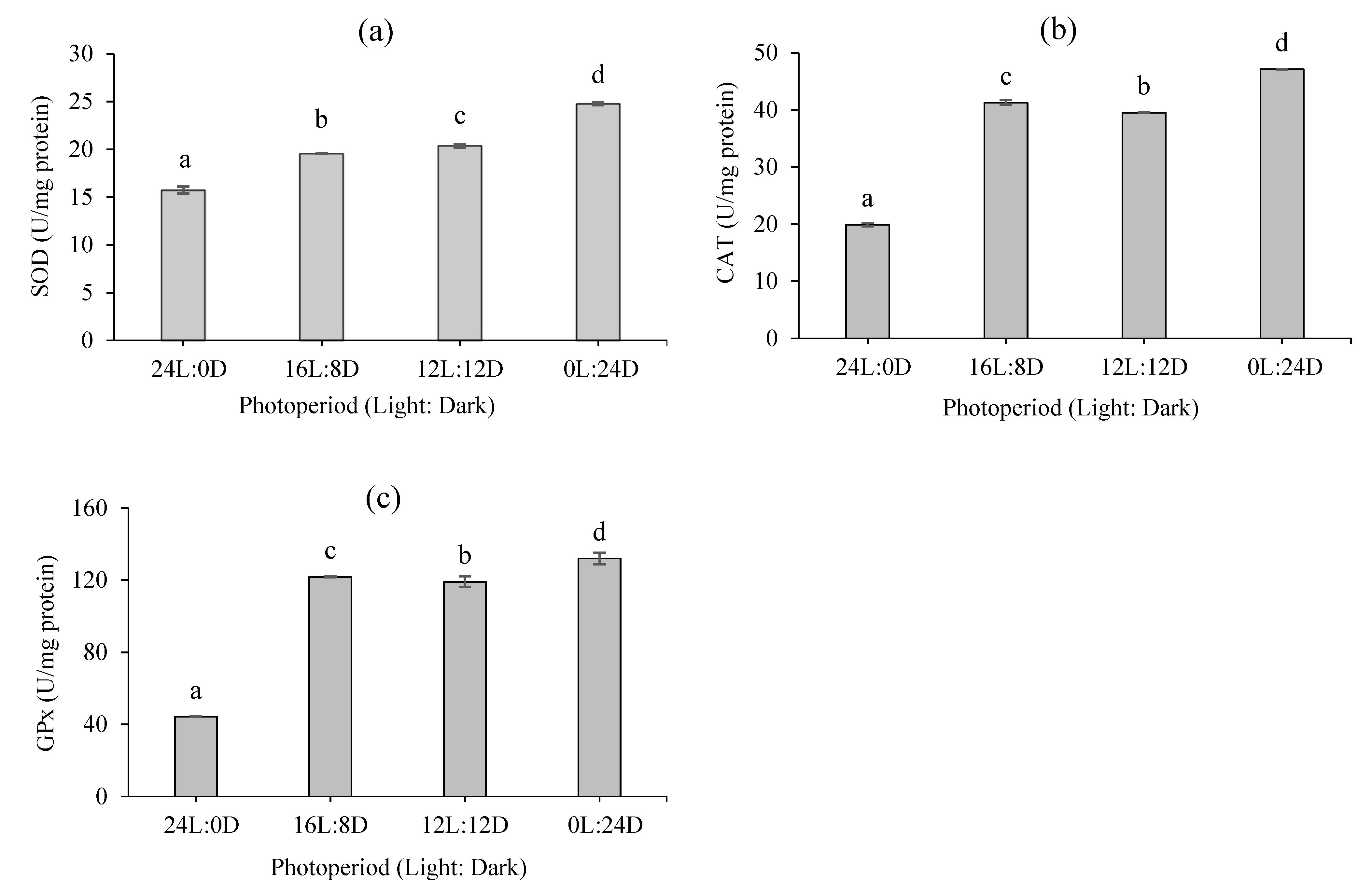
2.6. Antioxidant Status
2.7. Proximate Composition
2.8. Fatty-Acid Profile
2.9. Amino-Acid Analysis
2.10. Data Analysis
3. Results
3.1. Hatching Rate
3.2. Growth and Survival
3.3. Digestive Enzyme Activity
3.4. Antioxidant Status
3.5. Proximate Body Composition
3.6. Fatty-Acid Profile
3.7. Amino-Acid Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Eng, L.L.; Belk, D.; Eriksen, C.H. Californian Anostraca: Distribution, habitat and status. J. Crustac. Biol. 1990, 10, 247–277. [Google Scholar] [CrossRef]
- Eriksen, C.; Belk, D. Fairy Shrimps of California’s Puddles, Pools, and Playas; Mad River Press: Eureka, CA, USA, 1999. [Google Scholar]
- Manca, M.; Mura, G. On Branchinecta orientalis Sars, 1901 (Anostraca) in the Himalayas. Hydrobiologia 1997, 356, 111–116. [Google Scholar] [CrossRef]
- Horváth, Z.; Vad, C.F.; Vörös, L.; Boros, E. Distribution and conservation status of fairy shrimps (Crustacea: Anostraca) in the astatic soda pans of the Carpathian basin: The role of local and spatial factors. J. Limnol. 2013, 72, 103–116. [Google Scholar] [CrossRef]
- Petkovski, S. On the presence of the genus Branchinecta Verril, 1869 (Crustacea, Anostraca) in Yugoslavia. Hydrobiologia 1991, 226, 261–266. [Google Scholar] [CrossRef]
- Atashbar, B.; Agh, N.; Van Stappen, G.; Beladjal, L. Diversity and distribution patterns of large branchiopods (Crustacea: Branchiopoda) in temporary pools (Iran). J. Arid Environ. 2014, 111, 27–34. [Google Scholar] [CrossRef]
- Mura, G. The life history of Chirocephalus kerkyrensis Pesta (Crustacea: Anostraca) in temporary water of Circeo National Park (Latium, Italy). Hydrobiologia 1997, 346, 11–23. [Google Scholar] [CrossRef]
- Hulsmans, A.; Bracke, S.; Moreau, K.; Riddoch, B.J.; De Meester, L.; Brendoch, L. Dormant egg bank characteristics and hatching pattern of the Phallocryptus spinosa (Anostraca) population in the Makgadikgadi Pans (Botswana). Hydrobiologia 2006, 571, 123–132. [Google Scholar] [CrossRef]
- Atashbar, A.; Agh, N.; Beladjal, L.; Jalili, R.; Mertens, J. Effects of temperature on Survival, Growth, Reproductive and life span characteristics of Branchinecta orientalis (Branchiopoda: Anostraca) from Iran. Crustaceana 2012, 85, 1099–1114. [Google Scholar]
- Pormehr Yabandeh, N.; Beladjal, L.; Agh, N.; Atashbar, B.; Van Stappen, G. Mass culture of fairy shrimp Branchinecta orientalis (G. O. Sars 1901) (Crustacea: Anostraca) using effluent of rainbow trout Oncorhynchus mykiss (Walbaum 1792) ponds. Aquac. Res. 2017, 48, 5455–5462. [Google Scholar] [CrossRef]
- Brett, J.R. Environmental factors and growth. In Fish Physiology: Bioenergetics and Growth; Hoar, W.S., Randall, D.J., Brett, J.R., Eds.; Academic Press: New York, NY, USA, 1979; Volume 8, pp. 599–677. [Google Scholar]
- Biswas, A.K.; Seoka, M.; Ueno, K.; Yong, A.S.K.; Biswas, B.K.; Kim, Y.S.; Takii, K.; Kumaia, H. Growth performance and physiological responses in striped knifejaw, Oplegnathus fasciatus, held under different photoperiods. Aquaculture 2008, 279, 42–46. [Google Scholar] [CrossRef]
- Masoudi Asil, S.; Fereidouni, A.E.; Ouraji, H.; Khalili, K.J. Effects of different light intensities on growth, survival, reproductive and life span characteristics of Artemia urmiana Günther 1890. Aquac. Res. 2013, 44, 554–566. [Google Scholar] [CrossRef]
- Morales, M.I.; Barba, R.B., Jr. Effects of photoperiod, water levels and sex on the feeding efficiency and weight increment of mudcrabs (Scylla serrata Forskall) in a crab-fattening culture system. Int. J. Fish Aquat. Stud. 2015, 3, 320–324. [Google Scholar]
- Hou, Z.S.; Wen, H.S.; Li, J.F.; He, F.; Li, Y.; Qi, X.; Tao, Y.X. Effects of photoperiod and light spectrum on growth performance, digestive enzymes, hepatic biochemistry and peripheral hormones in spotted sea bass (Lateolabrax maculatus). Aquaculture 2019, 507, 419–427. [Google Scholar] [CrossRef]
- Moreno-Reyes, J.; Méndez-Ruiz, C.A.; Díaz, G.X.; Meruane, J.A.; Toledo, P.H. Chemical composition of the freshwater prawn Cryphiops caementarius (Molina, 1782) (Decapoda: Palaemonidae) in two populations in northern Chile: Reproductive and environmental considerations. Lat. Am. J. Aquat. Res. 2015, 43, 745–754. [Google Scholar] [CrossRef]
- Ueberschär, B. Measurement of proteolytic enzyme activity: Significance and application in larval fish research. In Physiological and Biochemical Aspects of Fish Development; Walther, B.T., Fyhn, H.J., Eds.; University of Bergen: Bergen, Norway, 1993; pp. 233–239. [Google Scholar]
- Herzig, A. Some Population Characteristics of Planktonic Crustaceans in Neusidler Sea. Oecologia 1974, 15, 127–141. [Google Scholar] [CrossRef] [PubMed]
- Hairston, N.G.; Hansen, A.M.; Schaffner, W.R. The Effect of Diapause Emergence on the Sysonal Dynamics of a Zooplankton Assemblage. Freshw. Biol. 2000, 45, 133–145. [Google Scholar] [CrossRef]
- Vandekerkhove, J.; Declerck, S.; Brendonck, L.; Conde-Porcuna, J.M.; Jeppesen, E.; Meester, L.D. Hatching of Cladoceran Resting Eggs: Temperature and Photoperiod. Freshw. Biol. 2005, 50, 96–104. [Google Scholar] [CrossRef]
- Sorgeloos, P. First report on the triggering effect of light on the hatching mechanism of Artemia salina dry cysts. Mar. Biol. 1973, 22, 75–76. [Google Scholar] [CrossRef]
- Takahashi, F. Effect of light on the hatching of eggs in Triops granarius. (Notostraca: Triopsidae). Environ. Control Biol. 1975, 13, 29–33. [Google Scholar] [CrossRef][Green Version]
- Alekseev, V.R.; Hwang, J.S.; Tseng, M.H. Diapause in aquatic invertebrates: What’s known and what’s next in research and medical application. J. Mar. Sci. Technol. 2006, 14, 269–286. [Google Scholar]
- Wang, Z.C.; Asem, A.; Sun, S.C. Coupled effects of photoperiod, temperature and salinity on diapause induction of the parthenogenetic Artemia (Crustacea: Anostraca) from Barkol Lake, China. North–West. J. Zool. 2017, 13, 12–17. [Google Scholar]
- Sorgeloos, P.; Leger, P. Improved larviculture outputs of marine fish; shrimp and prawn. J. World Aquacult. Soc. 1992, 23, 251–264. [Google Scholar] [CrossRef]
- Lim, L.C.; Soh, A.; Dhert, P.; Sorgeloos, P. Production and application of on-grown Artemia in freshwater ornamental fish farm. Aquac. Econ. Manag. 2001, 5, 3–4. [Google Scholar] [CrossRef]
- Velu, C.S.; Munuswamy, N. Composition and nutritional efficacy of adult fairy shrimp Streptocephalus dichotomus as live feed. Food Chem. 2007, 100, 1435–1442. [Google Scholar] [CrossRef]
- Sornsupharp, S.; Dahms, H.U.; Sanoamuang, L. Nutrient composition of fairy shrimp Streptocephalus sirindhornae nauplii as live food and growth performance of giant freshwater prawn postlarvae. Aquac. Nutr. 2013, 19, 349–359. [Google Scholar] [CrossRef]
- Sornsupharp, B.; Lomthaisong, K.; Dahms, H.U.; Sanoamuang, L. Effects of dried fairy shrimp Streptocephalus sirindhornae meal on pigmentation and carotenoid deposition in flowerhorn cichlid; Amphilophus citrinellus (Günther, 1864) × Cichlasoma trimaculatum (Günther 1867). Aquac. Res. 2015, 46, 173–184. [Google Scholar] [CrossRef]
- Dararat, W.; Starkweather, P.L.; Sanoamuang, L. Life history of three fairy shrimps (Branchiopoda: Anostraca) from Thailand. J. Crustac. Biol. 2011, 31, 623–629. [Google Scholar] [CrossRef]
- Coutteau, P.; Brendonck, L.; Lavens, P.; Sorgeloos, P. The use of manipulated baker’s yeast as an algal substitute for the laboratory culture of Anostraca. Hydrobiologia 1992, 234, 25–32. [Google Scholar] [CrossRef]
- Chong, A.S.C.; Hashim, R.H.; Yang, L.C.; Ali, A.B. Partial characterization and activities of protease from the digestive tract of discus fish (Symphysodon aequifasciata). Aquaculture 2002, 203, 321–333. [Google Scholar] [CrossRef]
- Bernfeld, P. α and β amylases. In Methods in enzymology; Colowick, S.P., Kaplan, N.O., Eds.; Academic Press: New York, NY, USA, 1955; Volume 1, pp. 149–158. [Google Scholar]
- Iijima, N.; Tanaka, S.; Ota, Y. Purification and characterization of bile salt-activated lipase from the hepatopancreas of red sea bream, Pagrus major. Fish Physiol. Biochem. 1998, 18, 59–69. [Google Scholar] [CrossRef]
- Garcıa-Carreno, F.L.; Haard, N.F. Characterization of proteinase classes in langostilla (Pleuroncodes planipes) and crayfish (Pacifastacus astacus) extracts. J. Food Biochem. 1993, 17, 97–113. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the estimation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Barata, C.; Varo, I.; Navarro, J.C.; Arun, S.; Porte, C. Antioxidant enzyme activities and lipid peroxidation in the freshwater cladoceran Daphnia magna exposed to redox cycling compounds. Comp. Biochem. Physiol. 2005, 140, 175–186. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of the Association of Official Analytical Chemistry; Association of Official Analytical Chemists: Arlington, VA, USA, 1995. [Google Scholar]
- Folch, J.; Lees, M.; Sloane Stanley, G. A simple method for the isolation and purification of total lipides from animal tissues. Inter. J. Biol. Chem. 1975, 226, 497–509. [Google Scholar] [CrossRef]
- Ichihara, K.I.; Shibahara, A.; Yamamoto, K.; Nakayama, T. An improved method for rapid analysis of the fatty acids of glycerolipids. Lipids 1996, 31, 535–539. [Google Scholar] [CrossRef] [PubMed]
- Lepage, G.; Roy, C.C. Improved recovery of fatty acid through direct transesterification without prior extraction or purification. J. Lipid Res. 1984, 25, 1391–1396. [Google Scholar] [CrossRef]
- Lee, K.S.; Drescher, D.G. Fluorometric amino acid analysis with O-phthaldialdehyde (OPA). Int. J. Biochem. 1978, 9, 457–467. [Google Scholar] [CrossRef]
- NRC. Nutrient Requirements of Fish and Shrimp; The National Academies Press: Washington, DC, USA, 2011. [Google Scholar]
- Fiogbé, E.D.; Kestemont, P. An assessment of the protein and amino acid requirements in goldfish (Carassius auratus) larvae. J. Appl. Ichthyol. 1995, 11, 282–289. [Google Scholar] [CrossRef]
- Takahashi, A.; Kasagi, S.; Murakami, N.; Furufuji, S.; Kikuchi, S.; Mizusawa, K.; Andoh, T. Chronic effects of light irradiated from LED on the growth performance and endocrine properties of barfin flounder Verasper moseri. Gen. Comp. Endocrinol. 2016, 232, 101–108. [Google Scholar] [CrossRef]
- Santos Romero, R.; García Guerrero, M.; Vega Villasante, F.; Cortés Jacinto, E.; Nolasco Soria, H. Effect of photoperiod and temperature on growth and activity of digestive enzymes in juveniles of the long-arm river shrimp Macrobrachium tenellum (Smith, 1871) (Caridea: Palaemonidae). J. Crustac. Biol. 2017, 37, 445–452. [Google Scholar] [CrossRef]
- Bermudes, M.; Ritar, A.J. Response of early-stage spiny lobster Jasus edwardsii phyllosoma larvae to changes in temperature and photoperiod. Aquaculture 2008, 281, 63–69. [Google Scholar] [CrossRef]
- Dawirs, R.R. Methodological aspects of rearing decapod larvae Pagurus bernhardus (Paguridae) and Carcinus maenas (Portunidae). Helgol. Meeresunters. 1982, 35, 439–464. [Google Scholar] [CrossRef]
- Mikami, S.; Greenwood, J.G. Influence of light regimes on phyllosomal growth and timing of moulting in Thenus orientalis (Lund) (Decapoda: Scyllaridae). Mar. Freshw. Res. 1997, 48, 777–782. [Google Scholar] [CrossRef]
- Petit, G.; Beauchaud, M.; Attia, J.; Buisson, B. Food intake and growth of largemouth bass (Micropterus salmoides) held under alternated light/dark cycle (12L:12D) or exposed to continuous light. Aquaculture 2013, 228, 397–401. [Google Scholar] [CrossRef]
- Albano, M.; Panarello, G.; Di Paola, D.; Capparucci, F.; Crupi, R.; Gugliandolo, E.; Spanò, N.; Capillo, G.; Savoca, S. The Influence of Polystyrene Microspheres Abundance on Development and Feeding Behavior of Artemia salina (Linnaeus, 1758). Appl. Sci. 2021, 11, 3352. [Google Scholar] [CrossRef]
- Dararat, W.; Lomthaisong, K.; Sanoamuang, L. Biochemical composition of three species of fairy shrimp (Branchiopoda: Anostraca) from Thailand. J. Crustac. Biol. 2012, 32, 81–87. [Google Scholar] [CrossRef]
- Sanoamuang, L.O.; Saengphan, N.; Murugan, G. First record of the family Thamnocephalidae (Crustacea: Anostraca) from Southeast Asia and description of a new species of Branchinella. Hydrobiologia 2002, 486, 63–69. [Google Scholar] [CrossRef]
- Lancia, J.P.; Fernandez Gimenez, A.; Bas, C.; Spivak, E. Adaptive differences in digestive enzyme activity in the crab Neohelice granulata in relation to sex and habitat. J. Crustac. Biol. 2012, 32, 940–948. [Google Scholar] [CrossRef]
- Martínez-Alarcón, D.; Saborowski, R.; Rojo-Arreola, L.; García-Carreño, F. Is digestive cathepsin D the rule in decapod crustaceans? Comp. Biochem. Physiol. 2018, 215, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Krylov, P.I.; Alekseev, V.R.; Frenkel, O.A. Feeding and digestive activity of cyclopoid copepods in active diapause. Hydrobiologia 1996, 320, 71–79. [Google Scholar] [CrossRef]
- Cronin, T.W.; Bok, M.J.; Lin, C. Crustacean larvae—vision in the plankton. Integr. Comp. Biol. 2017, 57, 1139–1150. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B.; Gutteridge, J.M.C. Free Radicals in Biology and Medicine; University Press: Oxford, UK, 1999. [Google Scholar]
- Pandey, S.; Parvez, S.; Sayeed, I.; Haque, R.; BinHafeez, B.; Raisuddin, S. Biomarkers of oxidative stress: A comparative study of river Yamuna fish Wallago attu (Bl. & Schn.). Sci. Total Environ. 2003, 309, 105–115. [Google Scholar] [PubMed]
- Tian, H.Y.; Zhang, D.D.; Xu, C.; Wang, F.; Liu, W.B. Effects of light intensity on growth, immune responses, antioxidant capability and disease resistance of juvenile blunt snout bream Megalobrama amblycephala. Fish Shellfish Immunol. 2015, 47, 674–680. [Google Scholar] [CrossRef] [PubMed]
- Cañavate, J.P.; Prieto, A.; Zerolo, R.; Sole, M.; Sarasquete, C.; Fernandez-Diaz, C. Effects of light intensity and addition of carotene rich Dunaliella salina live cells on growth and antioxidant activity of Solea senegalensis Kaup (1858) larval and metamorphic stages. J. Fish Biol. 2007, 71, 781–794. [Google Scholar] [CrossRef]
- Wang, T.; Yongzhou, C.; Zhaopu, L.; Shaohua, Y.; Xiaohua, L. Effects of light intensity on growth, immune response, plasma cortisol and fatty acid composition of juvenile Epinephelus coioides reared in artificial seawater. Aquaculture 2013, 414–415, 135–139. [Google Scholar] [CrossRef]
- Jung, S.J.; Choi, Y.J.; Kim, N.N.; Choi, J.Y.; Kim, B.S.; Choi, C.Y. Effects of melatonin injection or green-wavelength LED light on the antioxidant system in goldfish (Carassius auratus) during thermal stress. Fish Shellfish Immunol. 2016, 52, 157–166. [Google Scholar] [CrossRef]
- Wei, H.; Li, H.; Xia, Y.; Liu, H.; Han, D.; Zhu, X.; Yang, Y.; Jin, J.; Xie, S. Effects of light intensity on phototaxis, growth, antioxidant and stress of juvenile gibel carp (Carassius auratus gibelio). Aquaculture 2019, 501, 39–47. [Google Scholar] [CrossRef]
- Sun, X.L.; Yang, S.Y.; Chen, C.X.; Wang, Q.K.; Xue-Quan, Y.U.; Jin-Cheng, H.U.; Xing, K.Z. Effects of photoperiod on growth and antioxidant indices in half-smooth tongue-sole (Cynoglossus semilaevis). Chin. J. Fish. 2012, 25, 23–27. [Google Scholar]
- McGaw, I.J.; Curtis, D.L. A review of gastric processing in decapod crustaceans. J. Comp. Physiol. 2013, 183, 443–465. [Google Scholar] [CrossRef]
- Nemova, N.N.; Murzina, S.A.; Lysenko, L.A. Ecological and Biochemical Status of the Atlantic Salmon Salmo salar L. and the Brown Trout Salmo trutta L. at Early Stages of Development. Biol. Bull. Rev. 2020, 10, 239–249. [Google Scholar] [CrossRef]
- Gharibi, M.R.; Noori, A.; Agh, N.; Atashbar, B. Rainbow trout farm effluent as a potential source of feed and medium for mass culture of Artemia parthenogenetica. Aquaculture 2021, 530, 7–18. [Google Scholar] [CrossRef]
- Richoux, N.B.; Ndhlovu, R.T. Temporal shifts in the fatty acid profiles of rocky intertidal invertebrates. Mar. Biol. 2014, 161, 2199–2211. [Google Scholar] [CrossRef]
- Mitra, G.; Mukhopadhyay, P.K.; Ayyappan, S. Biochemical composition of zooplankton community grown in freshwater earthen ponds: Nutritional implication in nursery rearing of fish larvae and early juveniles. Aquaculture 2007, 272, 346–360. [Google Scholar] [CrossRef]
- McWhinnie, M.A.; Kirchenberg, R.J.; Urbanski, R.J.; Schwarz, J.E. Crustecdysone mediated changes in crayfish. Am. Zool. 1972, 12, 357–372. [Google Scholar] [CrossRef][Green Version]
- Graney, R.L.; Giesy, J.P., Jr. Seasonal changes in the free amino acid pool of the freshwater Amphipod Gammarus pseudolimnaeus bousfield (Crustacea: Amphipoda). Comp. Biochem. Physiol. 1986, 85, 535–543. [Google Scholar] [CrossRef]
- Farhadi, A.; Harlıoğlu, M.M. Photoperiod affects gamete production, and protein and lipid metabolism in male narrow-clawed Crayfish Pontastacus leptodactylus (Eschscholtz, 1823). Anim. Reprod. Sci. 2019, 51, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Saejung, C.; Chaiyarat, A.; Sanoamuang, L.O. Effects of algae, yeast and photosynthetic bacteria diets on survival and growth performance in the fairy shrimp, Streptocephalus sirindhornae (Branchiopoda, Anostraca). Crustaceana 2018, 91, 1505–1522. [Google Scholar] [CrossRef]

| Fatty Acids Profile (% Area of Total Fatty Acids) | |
|---|---|
| C 14 | 3.40 ± 1.14 |
| C 14:1n-5 | 1.84 ± 0.18 |
| C 16 | 2.94 ± 0.04 |
| C 16:1n-7 | 1.56 ± 0.18 |
| C 18 | 14.87 ± 1.15 |
| C 18:1n-9 | 2.37 ± 0.24 |
| C 18:1n-7 | - |
| C 18:2n-6 | 4.03 ± 0.04 |
| C 18:3n-3 | 4.55 ± 0.05 |
| C 20 | 0.55 ± 0.12 |
| C 20:4n-6 | 0.48 ± 0.27 |
| C 20:3n-3 | - |
| C 20:5n-3 | 0.69 ± 0.02 |
| C 22 | 2.91 ± 0.16 |
| C 24 | 0.28 ± 0.01 |
| C 24:1n-9 | 0.47 ± 0.02 |
| SFA | 24.95 ± 2.62 |
| MUFA | 6.24 ± 0.62 |
| PUFA | 9.74 ± 0.38 |
| Proximate composition (% dry matter) | |
| Crude protein | 13.53 ± 1.37 |
| Crude lipid | 43.58 ± 1.77 |
| Ash | 7.18 ± 2.01 |
| Indices | Photoperiod (Light/Dark) | |||
|---|---|---|---|---|
| 24L:0D | 16L:08D | 12L:12D | 0L:24D | |
| TL on day 1 | 0.58 ± 0.04 a | 0.58 ± 0.04 a | 0.58 ± 0.04 a | 0.58 ± 0.04 a |
| TL on day 8 | 4.03 ± 0.20 a | 3.86 ± 0.31 a | 3.59 ± 0.17 a | 4.00 ± 0.11 a |
| TL on day 11 | 7.72 ± 0.23 a | 6.81 ± 0.48 a | 5.82 ± 0.11 a | 6.41 ± 0.28 a |
| TL on day 15 | 11.32 ± 0.50 a | 10.37 ± 0.31 a | 11.31 ± 0.82 a | 11.19 ± 0.81 a |
| TL on day 17 | 11.62 ± 0.57 a | 11.70 ± 0.31 ab | 12.73 ± 0.54 ab | 13.31 ± 0.78 b |
| Daily growth rate from day 1–8 (mm/day) * | 0.43 ± 0.02 a | 0.41 ± 0.03 a | 0.38 ± 0.02 a | 0.43 ± 0.01 a |
| Daily growth rate from day 9–17 (mm/day) | 0.84 ± 0.04 a | 0.87 ± 0.00 ab | 1.02 ± 0.04 b | 1.03 ± 0.07 b |
| SGR (% body weight/day) ** | 16.52 ± 1.01 a | 17.18 ± 0.86 a | 16.63 ± 0.32 a | 16.81 ± 0.85 a |
| Average body weight (mg) | 17.09 ± 2.28 a | 18.65 ± 1.06 a | 17.51 ± 2.87 a | 18.14 ± 2.64 a |
| Sexual maturity on day 17 (%) *** | 25.75 ± 1.23 c | 17.59 ± 0.78 bc | 18.06 ± 2.33 bc | 11.81 ± 1.19 a |
| Proximate Composition (% Dry Matter) | Photoperiod (Light/Dark) | |||
|---|---|---|---|---|
| 24L:0D | 16L: 8D | 12L:12D | 0L:24D | |
| Crude protein | 56.15 ± 1.23 b | 53.87 ± 1.70 b | 53.46 ± 2.62 b | 50.33 ± 0.64 a |
| Crude lipid | 14.51 ± 0.54 c | 6.91 ± 0.66 a | 5.05 ± 0.40 a | 12.61 ± 0.86 b |
| Ash | 12.73 ± 1.40 a | 11.32 ± 1.46 a | 12.86 ± 1.14 a | 11.69 ± 0.87 a |
| Fatty Acids Profile | Photoperiod (Light: Dark) | |||
|---|---|---|---|---|
| (% area of total fatty acids) | 24L:0D | 16L: 8D | 12L:12D | 0L:24D |
| SFA | 24.87 ± 0.23 b | 21.40 ± 0.40 a | 27.84 ± 0.16 d | 24.18 ± 0.19 c |
| MUFA | 28.28 ± 0.27 d | 16.61 ± 0.10 a | 25.06 ± 0.07 c | 18.50 ± 0.08 b |
| PUFA | 46.21 ± 0.22 a | 54.86 ± 0.06 d | 46.81 ± 0.06 b | 52.43 ± 0.13 c |
| PUFA n-3 | 13.91 ± 0.11 a | 18.68 ± 0.33 d | 16.48 ± 0.22 c | 15.22 ± 0.23 b |
| PUFA n-6 | 32.30 ± 0.11 b | 36.19 ± 0.40 c | 30.33 ± 0.28 a | 37.21 ± 0.10 d |
| n-3/n-6 | 0.43 ± 0.00 a | 0.52 ± 0.01 b | 0.54 ± 0.01 b | 0.41 ± 0.01 a |
| EPA | 1.25 ± 0.02 d | 0.72 ± 0.01 b | 1.18 ± 0.03 c | 0.34 ± 0.01 a |
| DHA | 2.29 ± 0.01 c | 0.54 ± 0.00 a | 2.30 ± 0.02 c | 1.61 ± 0.02 b |
| EPA/DHA | 1.84 ± 0.03 b | 0.75 ±0.01 a | 1.95 ± 0.06 b | 4.71 ± 0.17 c |
| Amino Acid | Photoperiod (Light/Dark) | |||
|---|---|---|---|---|
| 24L:0D | 16L:8D | 12L:12D | 0L:24D | |
| Alanine | 33.78 | 40.10 | 40.60 | 36.50 |
| Arginine | 31.86 | 43.60 | 38.30 | 37.00 |
| Aspartic acid | 49.50 | 61.70 | 59.50 | 50.90 |
| Glutamic acid | 70.79 | 89.70 | 85.10 | 77.50 |
| Glycine | 26.79 | 32.30 | 32.20 | 30.70 |
| Histidine | 12.40 | 16.50 | 14.90 | 13.10 |
| Isoleucine | 22.05 | 27.30 | 26.50 | 24.10 |
| Leucine | 38.18 | 47.70 | 45.90 | 42.70 |
| Lysine | 43.84 | 55.20 | 52.70 | 45.60 |
| Methionine | 9.82 | 12.30 | 11.80 | 10.30 |
| Phenylalanine | 24.62 | 29.30 | 29.60 | 28.30 |
| Serine | 23.96 | 30.60 | 28.80 | 25.20 |
| Threonine | 26.04 | 31.20 | 31.30 | 30.80 |
| Tryptophan | 6.24 | 7.50 | 7.50 | 2.30 |
| Tyrosine | 21.30 | 26.90 | 25.60 | 14.30 |
| Valine | 26.87 | 32.00 | 32.30 | 29.80 |
| TAAs | 468.04 | 583.9 | 562.6 | 499.1 |
| TEAAs * | 241.92 | 302.6 | 290.8 | 264 |
| B. orientalis Reared under Different Photoperiods | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AA | 24l:0D | 16L:8D | 12L:12D | 0L:24D | Artemia * | Blood worm * | Moina * | Rainbow trout ** | Nile tilapia ** | Common carp ** | Catla (Fry) ** | Marigal ** | Channel catfish ** | Goldfish larvae *** |
| Arginine | 3.19 | 4.36 | 3.83 | 3.70 | 6.2 | 2.81 | 5.1 | 1.8 | 1.2 | 1.7 | 1.92 | 1.8 | 1.0 | 0.30 |
| Lysine | 4.38 | 5.52 | 5.27 | 4.56 | 4.0 | 3.05 | 4.31 | 1.9 | 1.4 | 2.2 | 2.49 | 2.3 | 1.2 | 0.36 |
| Histidine | 1.24 | 1.65 | 1.49 | 1.31 | 1.6 | 1.41 | 1.57 | 0.6 | 1.0 | 1.0 | 0.98 | 0.9 | 0.4 | 0.12 |
| Isoleucine | 2.21 | 2.73 | 2.65 | 2.41 | 2.4 | 2.5 | 2.55 | 1.4 | 1.8 | 1.0 | 0.94 | 1.7 | 0.6 | 0.18 |
| Leucine | 3.82 | 4.77 | 4.59 | 4.27 | 5.0 | 4.22 | 5.1 | 3.4 | 1.9 | 1.3 | 1.48 | 1.5 | 0.8 | 0.24 |
| Valine | 2.69 | 3.20 | 3.23 | 2.98 | 3.2 | 3.28 | 3.73 | 1.6 | 1.6 | 1.4 | 1.42 | 1.5 | 0.7 | 0.21 |
| Methionine | 0.98 | 1.23 | 1.18 | 1.03 | 1.6 | 2.19 | 1.96 | 0.8 | 0.8 | 0.8 | 1.42 | 1.2 | 0.6 | 0.18 |
| Phenylalanine | 2.46 | 2.93 | 2.96 | 2.83 | 3.4 | 3.83 | 3.53 | 0.7 | 1.1 | 1.5 | 1.48 | 1.3 | 0.5 | 0.15 |
| Threonine | 2.60 | 3.12 | 3.13 | 3.08 | 3.4 | 2.58 | 3.33 | 1.1 | 1.1 | 1.5 | 1.98 | 1.8 | 0.5 | 0.15 |
| Tryptophan | 0.62 | 0.75 | 0.75 | 0.23 | - | - | - | 0.2 | 0.28 | 0.3 | 0.38 | 0.4 | 0.1 | - |
| TEAA | 24.19 | 30.26 | 29.08 | 26.40 | 30.8 | 25.87 | 31.18 | - | - | - | - | - | - | - |
| TEAA/TAA | 51.69 | 51.82 | 51.69 | 52.90 | 51.00 | 54.84 | 49.58 | - | - | - | - | - | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farhadi, S.; Atashbar Kangarloei, B.; Imani, A.; Sarvi Moghanlou, K. Biological Impact of Photoperiod on Fairy Shrimp (Branchinecta orientalis): Life History and Biochemical Composition. Biology 2021, 10, 695. https://doi.org/10.3390/biology10080695
Farhadi S, Atashbar Kangarloei B, Imani A, Sarvi Moghanlou K. Biological Impact of Photoperiod on Fairy Shrimp (Branchinecta orientalis): Life History and Biochemical Composition. Biology. 2021; 10(8):695. https://doi.org/10.3390/biology10080695
Chicago/Turabian StyleFarhadi, Sara, Behrooz Atashbar Kangarloei, Ahmad Imani, and Kourosh Sarvi Moghanlou. 2021. "Biological Impact of Photoperiod on Fairy Shrimp (Branchinecta orientalis): Life History and Biochemical Composition" Biology 10, no. 8: 695. https://doi.org/10.3390/biology10080695
APA StyleFarhadi, S., Atashbar Kangarloei, B., Imani, A., & Sarvi Moghanlou, K. (2021). Biological Impact of Photoperiod on Fairy Shrimp (Branchinecta orientalis): Life History and Biochemical Composition. Biology, 10(8), 695. https://doi.org/10.3390/biology10080695





