Salvage Boron Neutron Capture Therapy for Malignant Brain Tumor Patients in Compliance with Emergency and Compassionate Use: Evaluation of 34 Cases in Taiwan
Abstract
:Simple Summary
Abstract
1. Introduction
2. Methods
3. Results
3.1. Patient Characteristics and BNCT Parameters
3.2. The Association between Tumor Response and Survival Outcomes
3.3. Association between BNCT Parameters and Survival Outcomes
3.4. Adverse Reactions
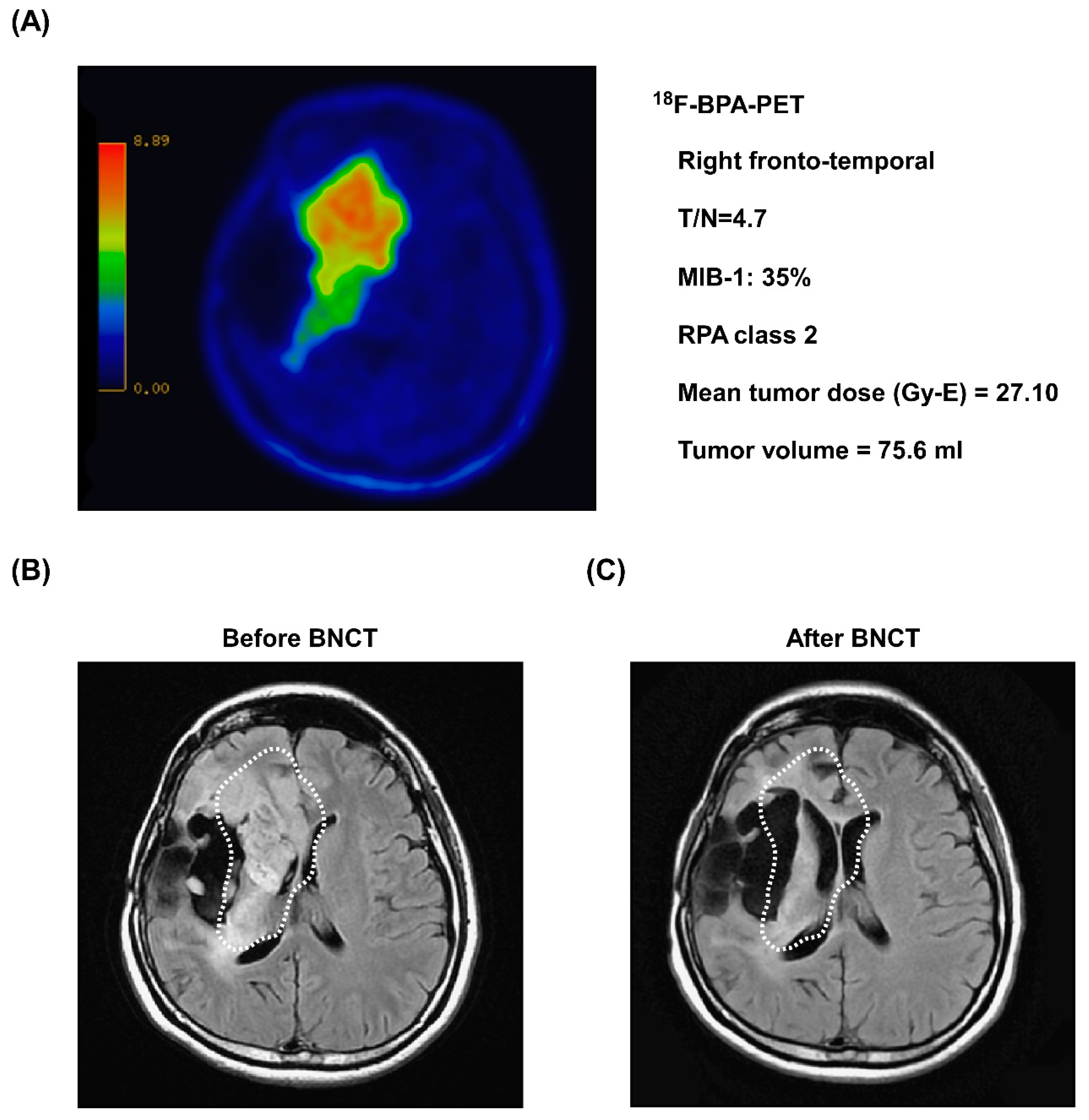
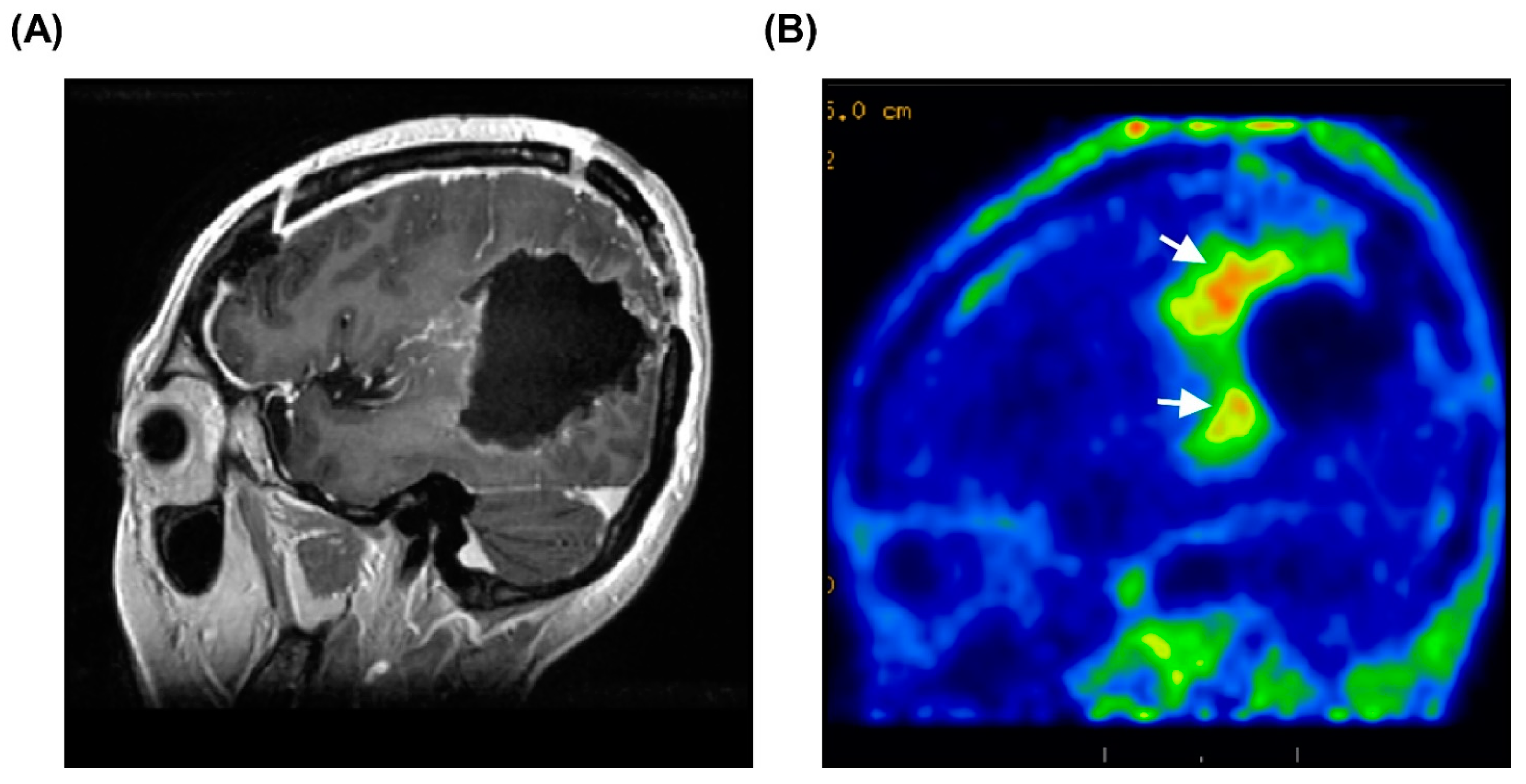
3.5. Representative Case
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Johnson, D.R.; Guerin, J.B.; Giannini, C.; Morris, J.M.; Eckel, L.J.; Kaufmann, T.J. 2016 Updates to the WHO Brain Tumor Classification System: What the Radiologist Needs to Know. Radiographics 2017, 37, 2164–2180. [Google Scholar] [CrossRef] [Green Version]
- Batash, R.; Asna, N.; Schaffer, P.; Francis, N.; Schaffer, M. Glioblastoma Multiforme, Diagnosis and Treatment; Recent Literature Review. Curr. Med. Chem. 2017, 24, 3002–3009. [Google Scholar] [CrossRef]
- Cohen, M.H.; Shen, Y.L.; Keegan, P.; Pazdur, R. FDA drug approval summary: Bevacizumab (Avastin) as treatment of recurrent glioblastoma multiforme. Oncologist 2009, 14, 1131–1138. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Xue, Y.Q.; Zhao, M.M.; Xu, P. Effectiveness of lomustine and bevacizumab in progressive glioblastoma: A meta-analysis. Onco Targets Ther. 2018, 11, 3435–3439. [Google Scholar] [CrossRef]
- Wick, W.; Gorlia, T.; Bendszus, M.; Taphoorn, M.; Sahm, F.; Harting, I.; Brandes, A.A.; Taal, W.; Domont, J.; Idbaih, A.; et al. Lomustine and Bevacizumab in Progressive Glioblastoma. N. Engl. J. Med. 2017, 377, 1954–1963. [Google Scholar] [CrossRef]
- Li, Y.; Ali, S.; Clarke, J.; Cha, S. Bevacizumab in Recurrent Glioma: Patterns of Treatment Failure and Implications. Brain Tumor Res. Treat. 2017, 5, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Sauerwein, W.; Wittig, A.; Moss, R.; Nakagawa, Y. Neutron Capture Therapy—Principles and Applications; Springer: Berlin, Germany, 2012. [Google Scholar]
- Dymova, M.A.; Taskaev, S.Y.; Richter, V.A.; Kuligina, E.V. Boron neutron capture therapy: Current status and future perspectives. Cancer Commun. (Lond. Engl.) 2020, 40, 406–421. [Google Scholar] [CrossRef] [PubMed]
- Miyatake, S.I.; Wanibuchi, M.; Hu, N.; Ono, K. Boron neutron capture therapy for malignant brain tumors. J. Neuro Oncol. 2020, 149, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Izadi Vasafi, G.; Firoozabadi, M.M. (10)B Concentration, Phantom Size and Tumor Location Dependent Dose Enhancement and Neutron Spectra in Boron Neutron Capture Therapy. J. Biomed. Phys. Eng. 2019, 9, 653–660. [Google Scholar] [CrossRef]
- Miyatake, S.; Kawabata, S.; Hiramatsu, R.; Kuroiwa, T.; Suzuki, M.; Kondo, N.; Ono, K. Boron Neutron Capture Therapy for Malignant Brain Tumors. Neurol. Med. Chir. (Tokyo) 2016, 56, 361–371. [Google Scholar] [CrossRef] [Green Version]
- Takeuchi, K.; Kawabata, S.; Hiramatsu, R.; Matsushita, Y.; Tanaka, H.; Sakurai, Y.; Suzuki, M.; Ono, K.; Miyatake, S.I.; Kuroiwa, T. Boron Neutron Capture Therapy for High-Grade Skull-Base Meningioma. J. Neurol. Surg. B Skull Base 2018, 79, S322–S327. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.C.; Chuang, K.S.; Chen, Y.W.; Hsu, F.Y.; Chou, F.I.; Yen, S.H.; Wu, Y.H. Preliminary dosimetric study on feasibility of multi-beam boron neutron capture therapy in patients with diffuse intrinsic pontine glioma without craniotomy. PLoS ONE 2017, 12, e0180461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lan, T.L.; Chou, F.I.; Lin, K.H.; Pan, P.S.; Lee, J.C.; Huang, W.S.; Liu, Y.M.; Chao, Y.; Chen, Y.W. Using salvage Boron Neutron Capture Therapy (BNCT) for recurrent malignant brain tumors in Taiwan. Appl. Radiat. Isot. 2020, 160, 109105. [Google Scholar] [CrossRef]
- Miyatake, S.; Kawabata, S.; Yokoyama, K.; Kuroiwa, T.; Michiue, H.; Sakurai, Y.; Kumada, H.; Suzuki, M.; Maruhashi, A.; Kirihata, M.; et al. Survival benefit of Boron neutron capture therapy for recurrent malignant gliomas. J. Neuro Oncol. 2009, 91, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, M.; Kato, I.; Aihara, T.; Hiratsuka, J.; Yoshimura, K.; Niimi, M.; Kimura, Y.; Ariyoshi, Y.; Haginomori, S.; Sakurai, Y.; et al. Boron neutron capture therapy outcomes for advanced or recurrent head and neck cancer. J. Radiat. Res. 2014, 55, 146–153. [Google Scholar] [CrossRef]
- Ono, K.; Masunaga, S.-i.; Kinashi, Y.; Nagata, K.; Suzuki, M.; Sakurai, Y.; Maruhashi, A.; Kato, I.; Nakazawa, M.; Ariyoshi, Y.; et al. Neutron irradiation under continuous BPA injection for solving the problem of heterogeneous distribution of BPA. In Proceedings of the 12th International Congress on Neutron Capture Therapy—From the Past to the Future, Takamatsu, Japan, 9–13 October 2006. [Google Scholar]
- Liu, H.-M. Beam Collimator Design for the Epithermal Neutron Beam at the Tsing Hua Open-Pool Reactor (THOR). In Frontiers in Neutron Capture Therapy: Volume 1; Hawthorne, M.F., Shelly, K., Wiersema, R.J., Eds.; Springer: Boston, MA, USA, 2001; pp. 325–329. [Google Scholar]
- Common Terminology Criteria for Adverse Events (CTCAE) Version 5.0. Available online: https://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_8.5x11.pdf. (accessed on 9 April 2021).
- Shiba, H.; Takeuchi, K.; Hiramatsu, R.; Furuse, M.; Nonoguchi, N.; Kawabata, S.; Kuroiwa, T.; Kondo, N.; Sakurai, Y.; Suzuki, M.; et al. Boron Neutron Capture Therapy Combined with Early Successive Bevacizumab Treatments for Recurrent Malignant Gliomas—A Pilot Study. Neurol. Med. Chir. (Tokyo) 2018, 58, 487–494. [Google Scholar] [CrossRef] [Green Version]
- Hopewell, J.W.; Gorlia, T.; Pellettieri, L.; Giusti, V.; H-Stenstam, B.; Skold, K. Boron neutron capture therapy for newly diagnosed glioblastoma multiforme: An assessment of clinical potential. Appl. Radiat. Isot. 2011, 69, 1737–1740. [Google Scholar] [CrossRef]
- Kawabata, S.; Miyatake, S.; Kuroiwa, T.; Yokoyama, K.; Doi, A.; Iida, K.; Miyata, S.; Nonoguchi, N.; Michiue, H.; Takahashi, M.; et al. Boron neutron capture therapy for newly diagnosed glioblastoma. J. Radiat. Res. 2009, 50, 51–60. [Google Scholar] [CrossRef] [Green Version]
- Kawabata, S.; Miyatake, S.; Nonoguchi, N.; Hiramatsu, R.; Iida, K.; Miyata, S.; Yokoyama, K.; Doi, A.; Kuroda, Y.; Kuroiwa, T.; et al. Survival benefit from boron neutron capture therapy for the newly diagnosed glioblastoma patients. Appl. Radiat. Isot. 2009, 67, S15–S18. [Google Scholar] [CrossRef]
- Yamamoto, T.; Nakai, K.; Kageji, T.; Kumada, H.; Endo, K.; Matsuda, M.; Shibata, Y.; Matsumura, A. Boron neutron capture therapy for newly diagnosed glioblastoma. Radiother Oncol. 2009, 91, 80–84. [Google Scholar] [CrossRef] [Green Version]
- Coderre, J.A.; Hopewell, J.W.; Turcotte, J.C.; Riley, K.J.; Binns, P.J.; Kiger, W.S., 3rd; Harling, O.K. Tolerance of normal human brain to boron neutron capture therapy. Appl. Radiat. Isot. 2004, 61, 1083–1087. [Google Scholar] [CrossRef]
- Kageji, T.; Nakagawa, Y.; Kumada, H. Clinical Results of Sodium Borocaptate (BSH)—Based Intraoperative Boron Neutron Capture Therapy (IO-BNCT); Springer: Berlin, Heidelberg, 2012. [Google Scholar]
- Ono, K. An analysis of the structure of the compound biological effectiveness factor. J. Radiat. Res. 2016, 57 (Suppl. 1), i83–i89. [Google Scholar] [CrossRef] [Green Version]
- Ono, K.; Tanaka, H.; Tamari, Y.; Watanabe, T.; Suzuki, M.; Masunaga, S.I. Proposal for determining absolute biological effectiveness of boron neutron capture therapy-the effect of 10B(n,alpha)7Li dose can be predicted from the nucleocytoplasmic ratio or the cell size. J. Radiat. Res. 2019, 60, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Naito, F. Introduction to accelerators for boron neutron capture therapy. Ther. Radiol. Oncol. 2018, 2, 54. [Google Scholar] [CrossRef]
- Kiyanagi, Y.; Sakurai, Y.; Kumada, H.; Tanaka, H. Status of accelerator-based BNCT projects worldwide. AIP Conf. Proc. 2019, 2160, 050012. [Google Scholar]
- Kreiner, A.J.; Bergueiro, J.; Cartelli, D.; Baldo, M.; Castell, W.; Asoia, J.G.; Padulo, J.; Suarez Sandin, J.C.; Igarzabal, M.; Erhardt, J.; et al. Present status of Accelerator-Based BNCT. Rep. Pract. Oncol. Radiother. 2016, 21, 95–101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ishiwata, K. 4-Borono-2-(18)F-fluoro-L-phenylalanine PET for boron neutron capture therapy-oriented diagnosis: Overview of a quarter century of research. Ann. Nucl. Med. 2019, 33, 223–236. [Google Scholar] [CrossRef]
- Lo, Y.-W.; Lee, J.-C.; Hu, Y.-S.; Li, C.-Y.; Chen, Y.-L.; Lin, C.-S.; Huang, W.-S.; Lin, K.-H.; Chen, Y.-W. The importance of optimal ROIs delineation for FBPA-PET before BNCT. Appl. Radiat. Isotopes 2020, 163, 109219. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.H.; Chen, Y.W.; Lee, R.C.; Wang, L.W.; Chou, F.I.; Chang, C.W.; Yen, S.H.; Huang, W.S. Nuclear Theranostics in Taiwan. Nucl. Med. Mol. Imag. 2019, 53, 86–91. [Google Scholar] [CrossRef]
- Funakoshi, Y.; Hata, N.; Kuga, D.; Hatae, R.; Sangatsuda, Y.; Fujioka, Y.; Takigawa, K.; Mizoguchi, M. Update on Chemotherapeutic Approaches and Management of Bevacizumab Usage for Glioblastoma. Pharmaceuticals 2020, 13, 470. [Google Scholar] [CrossRef]
- Yang, S.B.; Gao, K.D.; Jiang, T.; Cheng, S.J.; Li, W.B. Bevacizumab combined with chemotherapy for glioblastoma: A meta-analysis of randomized controlled trials. Oncotarget 2017, 8, 57337–57344. [Google Scholar] [CrossRef] [Green Version]
- Narita, Y. Bevacizumab for glioblastoma. Ther. Clin. Risk Manag. 2015, 11, 1759–1765. [Google Scholar] [CrossRef] [Green Version]
- Furuse, M.; Kawabata, S.; Kuroiwa, T.; Miyatake, S. Repeated treatments with bevacizumab for recurrent radiation necrosis in patients with malignant brain tumors: A report of 2 cases. J. Neuro Oncol. 2011, 102, 471–475. [Google Scholar] [CrossRef] [PubMed]
- Levin, V.A.; Bidaut, L.; Hou, P.; Kumar, A.J.; Wefel, J.S.; Bekele, B.N.; Grewal, J.; Prabhu, S.; Loghin, M.; Gilbert, M.R.; et al. Randomized double-blind placebo-controlled trial of bevacizumab therapy for radiation necrosis of the central nervous system. Int. J. Radiat. Oncol. Biol. Phys. 2011, 79, 1487–1495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miyatake, S.; Furuse, M.; Kawabata, S.; Maruyama, T.; Kumabe, T.; Kuroiwa, T.; Ono, K. Bevacizumab treatment of symptomatic pseudoprogression after boron neutron capture therapy for recurrent malignant gliomas. Report of 2 cases. Neuro Oncol. 2013, 15, 650–655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miyatake, S.; Kawabata, S.; Hiramatsu, R.; Furuse, M.; Kuroiwa, T.; Suzuki, M. Boron neutron capture therapy with bevacizumab may prolong the survival of recurrent malignant glioma patients: Four cases. Radiat. Oncol. 2014, 9, 6. [Google Scholar] [CrossRef] [Green Version]
| Characteristics | ||
|---|---|---|
| Age (years) | 37.50 ± 20.51 | |
| Gender | ||
| Female | 19 | (55.9%) |
| Male | 15 | (44.1%) |
| Tumor type | ||
| Glioblastoma | 15 | (44.1%) |
| Astrocytoma | 11 | (32.4%) |
| Oligoastrocytoma | 2 | (5.9%) |
| Medulloblastoma | 2 | (5.9%) |
| Brainstem glioma | 4 | (11.7%) |
| Tumor location | ||
| Frontal | 7 | (20.6%) |
| Non-Frontal in cerebrum | 21 | (61.8%) |
| Posterior fossa | 6 | (17.7%) |
| Tumor volume (mL) | 58.19 ± 61.14 | |
| MIB-1 (%) | 37.35 ± 16.04 | |
| KPS | 70 | (60, 80) |
| RPA class | ||
| I | 5 | (15.2%) |
| II | 6 | (18.2%) |
| III | 0 | (0.00%) |
| IV | 6 | (18.2%) |
| V | 9 | (27.3%) |
| VI | 7 | (21.2%) |
| Comorbidity | ||
| Epilepsy | 12 | (34.3%) |
| Diabetes mellitus | 4 | (11.4%) |
| Sinus bradycardia | 2 | (5.7%) |
| Panic | 2 | (5.7%) |
| Hyperlipidemia | 2 | (5.7%) |
| Arrhythmia | 2 | (5.7%) |
| Mismatch repair syndrome | 2 | (5.7%) |
| Asthma | 4 | (11.4%) |
| Abducent palsy | 1 | (2.9%) |
| T/N ratio | 2.93 ± 0.98 | |
| T/B ratio | 2.67 ± 0.94 | |
| Blood boron concentration during neutron irradiation (ppm) | 26.75 ± 4.99 | |
| Mean tumor dose (Gy-E) | 17.44 ± 7.50 | |
| Bevacizumab treatment after BNCT | ||
| Yes | 28 | (82.4%) |
| No | 5 | (14.7%) |
| Response | N (%) | 95% CI |
|---|---|---|
| CR | 6 (17.6%) | 0.05–0.30 |
| PR | 11 (32.4%) | 0.17–0.48 |
| SD | 12 (35.3%) | 0.19–0.51 |
| PD | 5 (14.7%) | 0.03–0.27 |
| ORR | 50.0% | 0.33–0.66 |
| DCR | 85.3% | 0.73–0.97 |
| OS | CSS | RFS | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Median Survival (Months) | 6 Months | 12 Months | Median Survival (Months) | 6 Months | 12 Months | Median Survival (Months) | 6 Months | 12 Months | |
| All cases | 7.25 | 52% | 29% | 7.80 | 58% | 38% | 4.18 | 32% | 16% |
| CR | 17.43 | 100% | 64% | 28.00 | 100% | 50% | 9.23 | 65% | 0% |
| PR | 15.47 | 73% | 62% | 15.47 | 73% | 62% | 8.21 | 55% | 33% |
| SD | 6.00 | 14% | 0% | 6.00 | 33% | 0% | 3.00 | 0% | 0% |
| PD | 4.83 | 0% | 0% | 4.83 | 40% | 0% | 1.50 | 0% | 0% |
| ORR | 15.65 | 82% | 61% | 17.11 | 82% | 73% | 8.44 | 59% | 29% |
| DCR | 8.10 | 62% | 35% | 9.07 | 62% | 41% | 4.57 | 39% | 19% |
| OS | CSS | RFS | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean(Month) | 95% CI | p-Value | Mean(Month) | 95% CI | p-Value | Mean(Month) | 95% CI | p-Value | |
| Gender | |||||||||
| Male | 9.67 | 4.87, 14.46 | 0.758 | 11.32 | 5.56, 17.08 | 0.590 | 7.09 | 3.40, 10.78 | 0.930 |
| Female | 9.90 | 7.23, 12.58 | 10.78 | 8.01, 13.54 | 5.72 | 3.16 8.27 | |||
| Tumor type | |||||||||
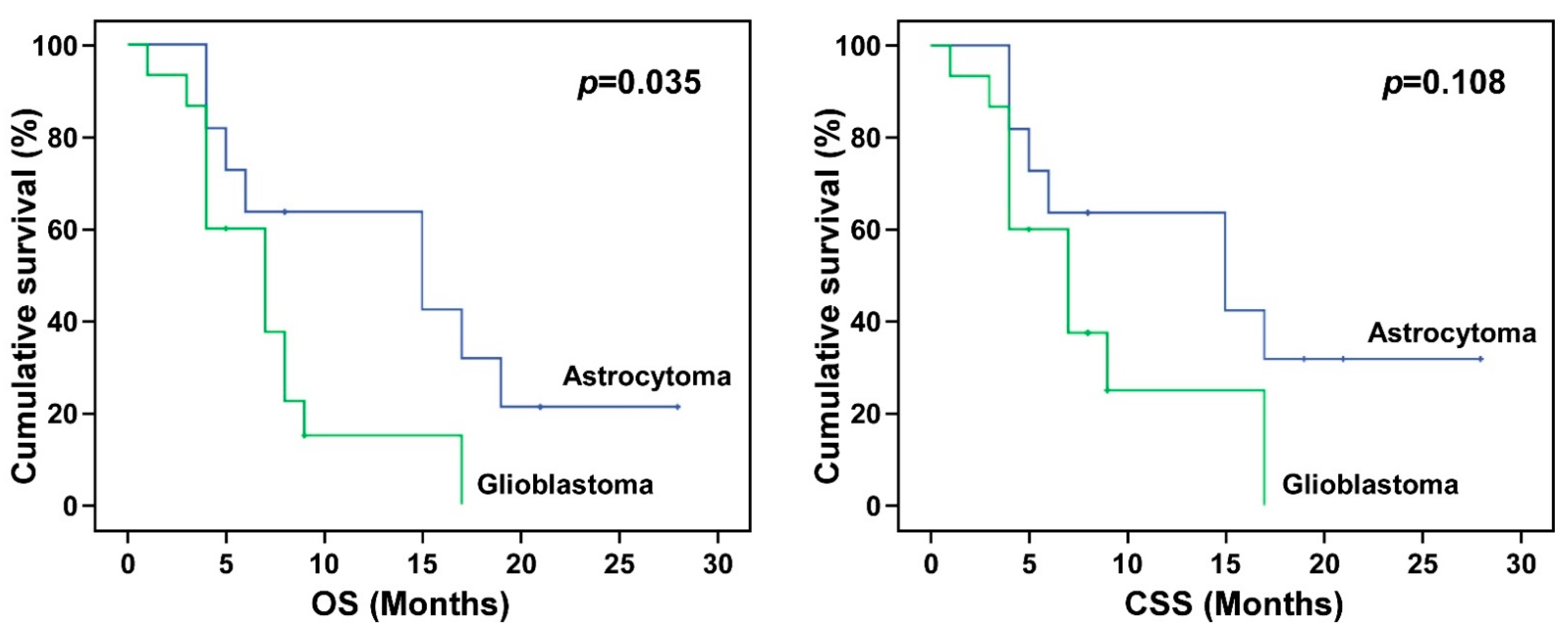
| Astrocytoma | 14.67 | 9.40, 19.94 | 0.003 | 12.91 | 8.15, 17.67 | 0.005 | 9.00 | 4.93, 13.07 | 0.245 |
| Glioblastoma | 7.34 | 4.84 9.83 | 6.47 | 4.56, 8.37 | 4.00 | 2.06, 5.94 | |||
| Oligoastrocytoma | - | - | - | - | 2.00 | 2.00, 2.00 | |||
| Medulloblastoma | - | - | - | - | 4.00 | 4.00, 4.00 | |||
| Brainstem glioma | 6.75 | 3.76, 9.74 | 3.75 | 3.94, 9.56 | 4.50 | 2.86, 6.14 | |||
| Tumor location | |||||||||
| Frontal | 11.29 | 5.64, 16.93 | 0.671 | 13.86 | 7.74, 19.97 | 0.440 | 8.71 | 2.67, 14.76 | 0.225 |
| non-Frontal in cerebrum | 9.08 | 5.70, 12.46 | 10.13 | 6.16, 14.10 | 4.00 | 2.65, 5.35 | |||
| Posterior fossa | 9.34 | 4.90, 13.76 | 10.61 | 5.74, 15.49 | 7.40 | 3.82, 10.98 | |||
| MIB-1 (%) | |||||||||
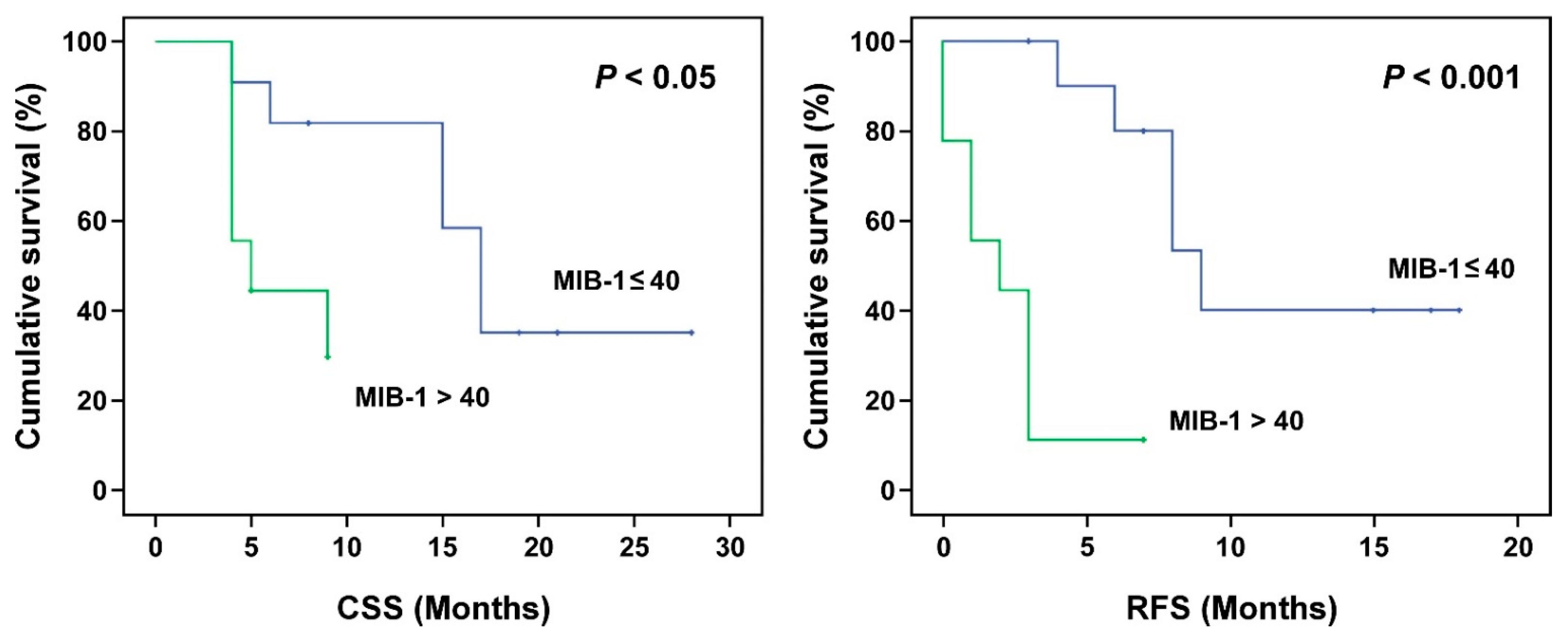
| ≤40 | 15.00 | 10.42, 19.59 | 0.086 | 18.21 | 13.03, 23.38 | 0.022 | 11.53 | 7.82, 15.25 | 0.000 |
| >40 | 6.33 | 4.61, 8.05 | 6.34 | 4.61, 8.05 | 2.22 | 0.89, 3.56 | |||
| RPA class | |||||||||
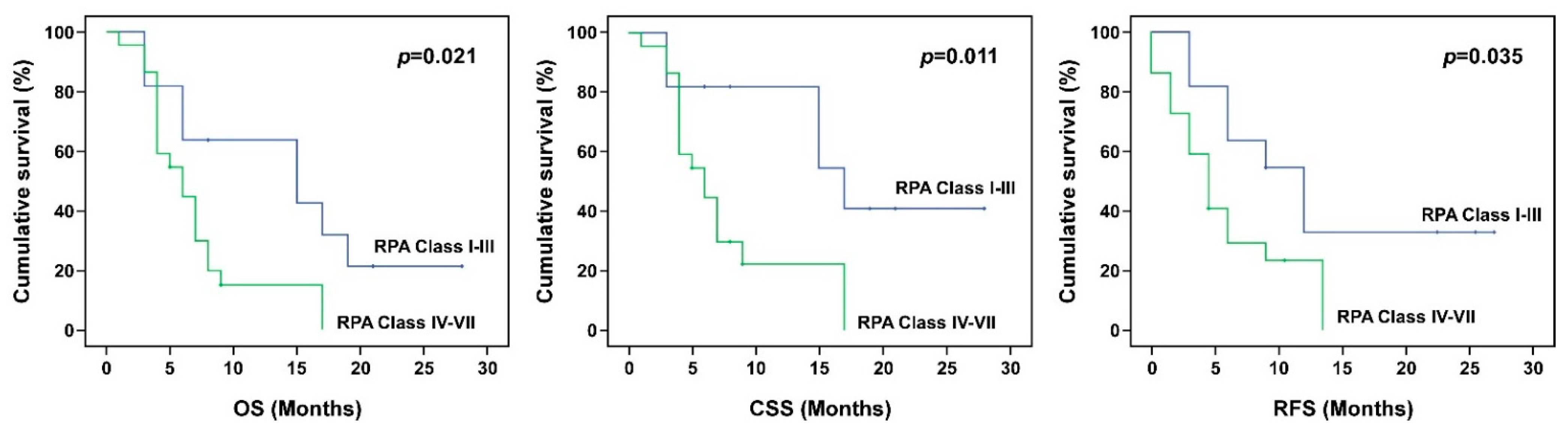
| 1–3 | 14.58 | 9.22, 19.93 | 0.021 | 18.41 | 12.50, 24.32 | 0.011 | 9.27 | 5.37, 13.17 | 0.035 |
| 4–7 | 7.04 | 5.03, 9.05 | 7.74 | 5.34, 10.14 | 3.88 | 2.46, 5.29 | |||
| KPS | |||||||||
| <70 | 8.75 | 3.81, 13.69 | 0.604 | 8.75 | 3.81, 13.69 | 0.182 | 4.85 | 1.56, 8.14 | 0.487 |
| ≥70 | 9.81 | 7.27, 12.34 | 11.30 | 8.57 14.02 | 6.19 | 3.69, 8.69 | |||
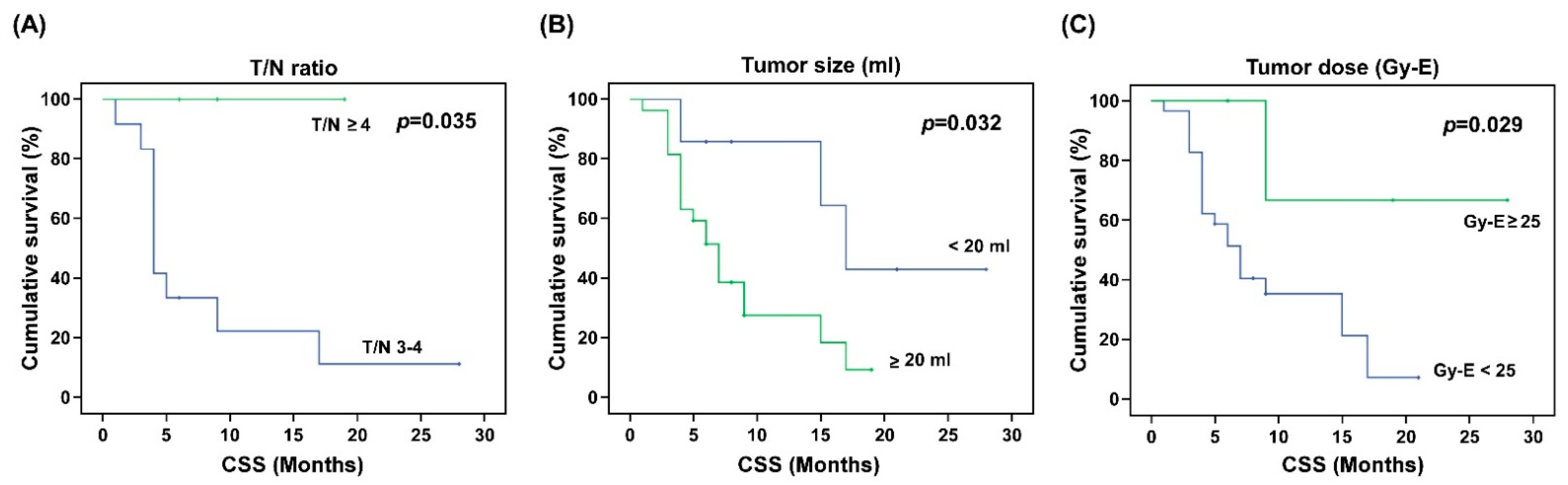
| T/N ratio | |||||||||
| <4 | 9.21 | 6.49, 11.92 | 0.283 | 8.23 | 6.05, 10.42 | 0.050 | 5.71 | 3.50, 7.92 | 0.507 |
| ≥4 | 14.67 | 4.86, 24.47 | - | - | 8.00 | 0.78, 15.22 | |||
| T/B ratio | |||||||||
| <4 | 9.77 | 6.94, 12.61 | 0.921 | 10.77 | 7.55, 13.99 | 0.359 | 5.50 | 3.38, 7.62 | 0.183 |
| ≥4 | 9.33 | 0.00, 18.96 | 13.67 | 5.13, 22.20 | 10.50 | 1.49, 19.51 | |||
| Tumor size (mL) | |||||||||
| <20 | 15.14 | 8.33, 21.96 | 0.056 | 19.43 | 12.54, 26.32 | 0.032 | 9.95 | 4.80, 15.11 | 0.091 |
| ≥20 | 7.92 | 5.78, 10.05 | 8.45 | 6.16, 10.74 | 4.79 | 2.76, 6.81 | |||
| Mean tumor dose (Gy-E) | |||||||||
| <25 | 8.52 | 6.34, 10.70 | 0.215 | 9.11 | 6.75, 11.47 | 0.029 | 5.84 | 3.47, 8.22 | 0.721 |
| ≥25 | 13.6 | 6.03, 21.17 | 21.67 | 11.53, 31.80 | 6.60 | 1.91 11.29 | |||
| Blood boron concentration (ppm) | |||||||||
| <30 | 10.23 | 7.12, 13.34 | 0.631 | 12.70 | 8.75, 16.66 | 0.305 | 7.13 | 4.50 9.75 | 0.008 |
| ≥30 | 6.57 | 4.54, 8.60 | 6.57 | 4.54, 8.60 | 2.14 | 1.24, 3.04 | |||
| Post BNCT response | |||||||||
| CR | 17.66 | 10.20, 25.13 | 0.000 | 22.50 | 14.88, 30.12 | 0.000 | 7.50 | 5.26, 9.75 | 0.009 |
| PR | 12.78 | 8.90, 16.66 | 13.03 | 8.98, 17.09 | 8.55 | 4.29, 12.80 | |||
| SD | 5.08 | 3.77, 6.39 | 5.08 | 3.77, 6.39 | 3.03 | 2.01 4.05 | |||
| PD | 4.80 | 3.84, 5.76 | 4.80 | 3.941, 5.66 | 1.80 | 0.00, 3.60 | |||
| 6-Month Survival Rate | 12-Month Survival Rate | |||||
|---|---|---|---|---|---|---|
| OS | CSS | RFS | OS | CSS | RFS | |
| T/N ratio | ||||||
| <3 | 68% | 68% | 45% | 32% | 42% | 11% |
| 3–4 | 25% | 33% | 11% | 17% | 22% | 11% |
| >4 | 67% | 100% | 33% | 67% | 100% | 33% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.-W.; Lee, Y.-Y.; Lin, C.-F.; Pan, P.-S.; Chen, J.-K.; Wang, C.-W.; Hsu, S.-M.; Kuo, Y.-C.; Lan, T.-L.; Hsu, S.P.C.; et al. Salvage Boron Neutron Capture Therapy for Malignant Brain Tumor Patients in Compliance with Emergency and Compassionate Use: Evaluation of 34 Cases in Taiwan. Biology 2021, 10, 334. https://doi.org/10.3390/biology10040334
Chen Y-W, Lee Y-Y, Lin C-F, Pan P-S, Chen J-K, Wang C-W, Hsu S-M, Kuo Y-C, Lan T-L, Hsu SPC, et al. Salvage Boron Neutron Capture Therapy for Malignant Brain Tumor Patients in Compliance with Emergency and Compassionate Use: Evaluation of 34 Cases in Taiwan. Biology. 2021; 10(4):334. https://doi.org/10.3390/biology10040334
Chicago/Turabian StyleChen, Yi-Wei, Yi-Yen Lee, Chun-Fu Lin, Po-Shen Pan, Jen-Kun Chen, Chun-Wei Wang, Shih-Ming Hsu, Yu-Cheng Kuo, Tien-Li Lan, Sanford P. C. Hsu, and et al. 2021. "Salvage Boron Neutron Capture Therapy for Malignant Brain Tumor Patients in Compliance with Emergency and Compassionate Use: Evaluation of 34 Cases in Taiwan" Biology 10, no. 4: 334. https://doi.org/10.3390/biology10040334
APA StyleChen, Y.-W., Lee, Y.-Y., Lin, C.-F., Pan, P.-S., Chen, J.-K., Wang, C.-W., Hsu, S.-M., Kuo, Y.-C., Lan, T.-L., Hsu, S. P. C., Liang, M.-L., Chen, R. H.-H., Chang, F.-C., Wu, C.-C., Lin, S.-C., Liang, H.-K., Lee, J.-C., Chen, S.-K., Liu, H.-M., ... Ono, K. (2021). Salvage Boron Neutron Capture Therapy for Malignant Brain Tumor Patients in Compliance with Emergency and Compassionate Use: Evaluation of 34 Cases in Taiwan. Biology, 10(4), 334. https://doi.org/10.3390/biology10040334







