Beneficial Role of Carica papaya Extracts and Phytochemicals on Oxidative Stress and Related Diseases: A Mini Review
Abstract
Simple Summary
Abstract
1. Introduction
2. Methods
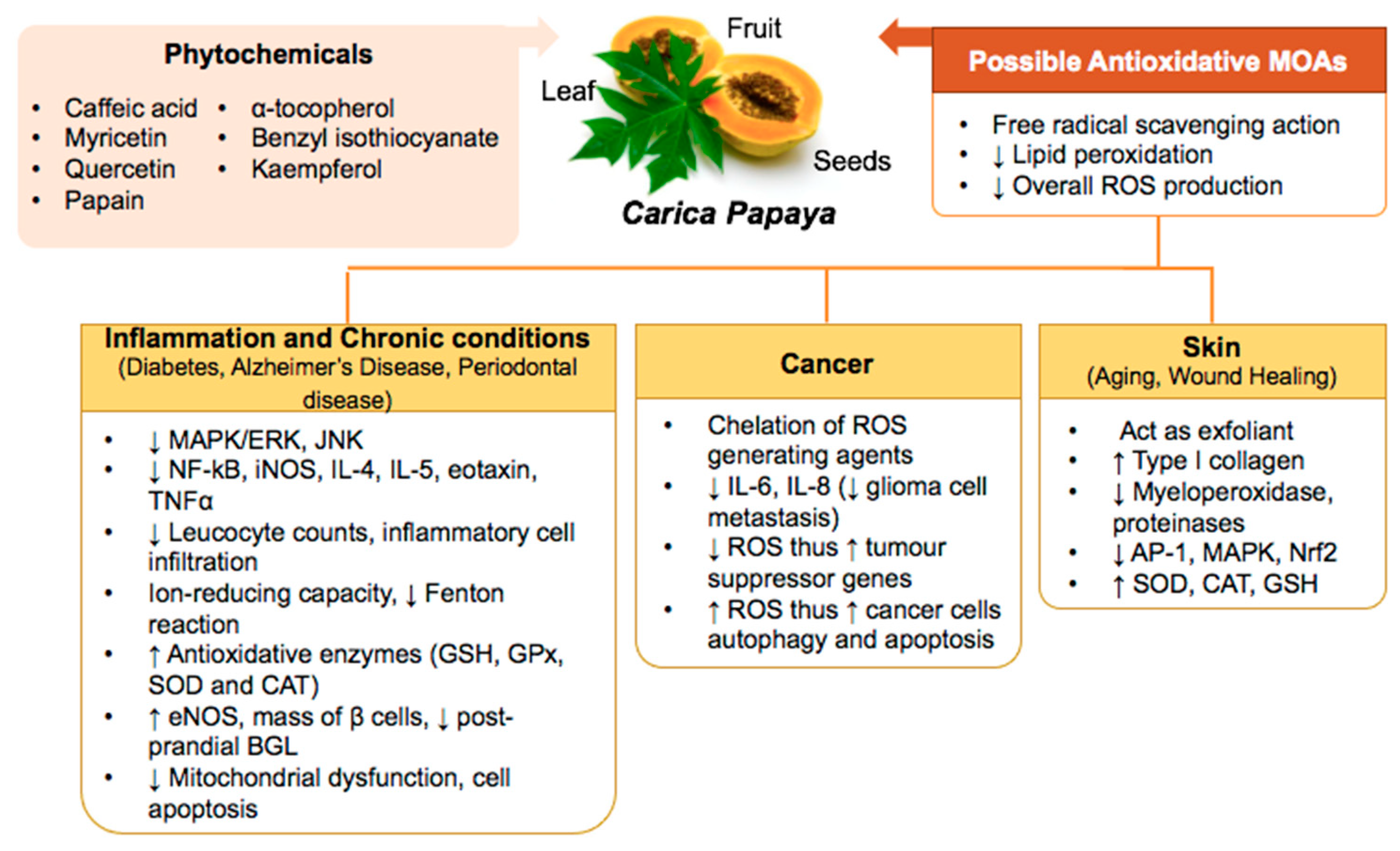
3. Carica papaya Counteracts Oxidative Stress in Inflammation, Skin Aging, and Healing, Chronic Diseases, and Cancers
3.1. Inflammation
3.2. Diabetes
3.3. Alzheimer’s Disease (AD)
3.4. Periodontal Disease
3.5. Skin Aging
3.6. Wound Healing
| Part of the Plant | Extract | Type of Experiment | Results | Reference |
|---|---|---|---|---|
| Seed | Aqueous extract | In vitro cytoprotective assay | Aqueous extract of papaya seeds at 1mg/mL showed cytoprotective against H2O2 induced cell toxicity. | [61] |
| Cell apoptosis assay | Aqueous extract of papaya seeds at a concentration of 1 mg/mL inhibited H2O2 induced apoptosis by approximately 30%. | |||
| MMP and Cytochrome C assay | Seed extract at 1 mg/mL inhibited oxidative stress-induced cell apoptosis, reduced mitochondrial dysfunction and impeded release of cytochrome C. | |||
| Western blot analysis | 1 mg/mL of seed extract decreased overexpression of HSP-70 in fibroblasts. | |||
| - | Fermented papaya (Biorex) | In vitro HRBC model | Biorex inhibited superoxide (IC50 = 5 mg/mL), hydroxyl radicals (IC50 = 1.1 mg/mL), and total ROS (IC50 = 2 mg/mL) in human red blood cells. | [62] |
| In vitro animal model | Biorex (1–5 mg/mL) decreased the elevated radical generation in rats with burn trauma. Biorex reduced local inflammation and catalase activity. | |||
| Unripe pulp | Papaya extract +/− Selenium | In vitro animal model | Papaya extract alone (PE) or with selenium (PES) enhanced wound closure in rats. Both PE and PES augmented SOD, CAT, and GPx activities. PE with selenium ameliorated oxidative damage at the wound site. PE enhanced wound healing via attenuating excessive inflammation, reduced COX-2, and MPO enzyme activity. PE and PES increased NO content by increasing iNOS, stimulating collagen deposition and angiogenesis. PE suppressed arginase activity during wound healing as indicated by decreased wound urea content. | [63] |
| Unripe papaya pulp | Papaya aqueous extract. Or Papaya PBS extract + Selenium | In vitro animal model | Total protein content (95.14 ± 1.15 mg/g tissue) in wound tissue was significantly higher in rats treated with PES at a dose 5 mg/mL twice daily for papaya and 0.5 μg/20 mL for selenium. Rats treated with PES demonstrated elevation in wound hydroxyproline (*55.15 ± 1.06 μg/mg), hexuronic acid (*60.84 ± 6.08 mg/g), and hexosamine (*35.23 ± 4.95 mg/g) contents. Overall reduced in migration of polymorphonuclear monocytes and increased fibroblast recruitment at wound sites. PE enhanced collagen synthesis and vascularization. Time required for wound closure was shortened, indicated by earlier increment in VEGFA and TGF-β1 expression. | [64] |
| - | FPP | In vitro animal model | FPP at a dose of 200 mg/kg s improved wound closure via increasing ROS (superoxides) production by macrophages at wound site and promoting NO production at ~60%. Increased NO and ROS to support redox signaling and angiogenesis. FPP increased CD68, VEGF transcription, macrophages recruitment to wound site and promoted optimal angiogenesis environment. | [65] |
| Leaf | Aqueous extract | In vitro animal model | Aqueous extract of papaya leaves at a dose of 500 mg/kg protected the stomach from absolute ethanol induced injury. Aqueous extract decreased MDA levels by 0.031 μmol/L and increased GPx by 0.246 U/mg protein. | [68] |
| Fruit | Aqueous extract | In vitro animal model | Aqueous extract of papaya fruit significantly shrank the wound area at 100 mg/kg by 77% by increasing epithelialization rate, weight of dry and wet granulation tissues and promoting enzymatic debridement of wound. Aqueous extract-treated wound showed rapid collagen turnover and accumulation that enhanced wound healing. | [69] |
| Tree | Dried latex incorporated into hydrogel | In vitro animal model | Topical application of the dried latex-containing hydrogel (1–2.5%) increased hydroxyproline content. Significant wound contraction after application of this hydrogel day 12 at concentration of 2.5% and on day 20 at both concentrations of 1.0% and 2.5%. | [70] |
| Seed | Ethanol extract | In vitro animal model | Ethanol extract of papaya seeds at a dose of 50 mg/kg significantly reduced wound area by 88.96%. Ethanol extract produces well-organized collagen deposition and significant fibroblast activity. | [71] |
3.7. Cancers
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sies, H. Oxidative stress: A concept in redox biology and medicine. Redox Biol. 2015, 4, 180–183. [Google Scholar] [CrossRef]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxidative Med. Cell. Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef] [PubMed]
- Aradhya, M.K.; Manshardt, R.M.; Zee, F.; Morden, C.W. A phylogenetic analysis of the genus Carica, L. (Caricaceae) based on restriction fragment length variation in a cpDNA intergenic spacer region. Genet. Resour. Crop Evol. 1999, 46, 579–586. [Google Scholar] [CrossRef]
- Yogiraj, V.; Goyal, P.; Chauhan, C.S.; Goyal, A.; Vyas, B. Carica papaya Linn: An overview. Int. J. Herb. Med. 2014, 2, 1–8. [Google Scholar]
- Yap, J.Y.; Hii, C.L.; Ong, S.P.; Lim, K.H.; Abas, F.; Pin, K.Y. Effects of drying on total polyphenols content and antioxidant properties of Carica papaya leaves. J. Sci. Food Agric. 2020, 100, 2932–2937. [Google Scholar] [CrossRef]
- Fei, X.; Yuan, W.; Zhao, Y.; Wang, H.; Bai, S.; Huang, Q. Papain Ameliorates the MPAs Formation-Mediated Activation of Monocytes by Inhibiting Cox-2 Expression via Regulating the MAPKs and PI3K/Akt Signal Pathway. BioMed Res. Int. 2018, 2018, 3632084. [Google Scholar] [CrossRef]
- Silva, C.R.d.; Oliveira, M.B.N.; Motta, E.S.; Almeida, G.S.d.; Varanda, L.L.; Pádula, M.d.; Leitão, A.C.; Caldeira-de-Araújo, A. Genotoxic and Cytotoxic Safety Evaluation of Papain (Carica papaya L.) Using In Vitro Assays. J. Biomed. Biotechnol. 2010, 2010, 197898. [Google Scholar] [CrossRef]
- Park, M.J.; Bae, Y.S. Fermented Acanthopanax koreanum Root Extract Reduces UVB- and H2O2-Induced Senescence in Human Skin Fibroblast Cells. J. Microbiol. Biotechnol. 2016, 26, 1224–1233. [Google Scholar] [CrossRef]
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget 2017, 9, 7204–7218. [Google Scholar] [CrossRef] [PubMed]
- Luster, A.D. The role of chemokines in linking innate and adaptive immunity. Curr. Opin. Immunol. 2002, 14, 129–135. [Google Scholar] [CrossRef]
- Morgan, M.J.; Liu, Z.-g. Crosstalk of reactive oxygen species and NF-κB signaling. Cell Res. 2011, 21, 103–115. [Google Scholar] [CrossRef]
- Hussain, T.; Tan, B.; Yin, Y.; Blachier, F.; Tossou, M.C.B.; Rahu, N. Oxidative Stress and Inflammation: What Polyphenols Can Do for Us? Oxidative Med. Cell. Longev. 2016, 2016, 7432797. [Google Scholar] [CrossRef] [PubMed]
- Kanda, Y.; Osaki, M.; Okada, F. Chemopreventive Strategies for Inflammation-Related Carcinogenesis: Current Status and Future Direction. Int. J. Mol. Sci. 2017, 18, 867. [Google Scholar] [CrossRef]
- Chatterjee, S. Chapter Two—Oxidative Stress, Inflammation, and Disease. In Oxidative Stress and Biomaterials; Dziubla, T., Butterfield, D.A., Eds.; Academic Press: Cambridge, MA, USA, 2016; pp. 35–58. [Google Scholar] [CrossRef]
- Wijesooriya, A.; Deraniyagala, S.; Hettiarachchi, C. Antioxidant, Anti-Inflammatory and Antibacterial Activities of the Seeds of A Sri Lankan Variety of Carica papaya. Biomed. Pharmacol. J. 2019, 12, 539–547. [Google Scholar] [CrossRef]
- Aruoma, O.I.; Colognato, R.; Fontana, I.; Gartlon, J.; Migliore, L.; Koike, K.; Coecke, S.; Lamy, E.; Mersch-Sundermann, V.; Laurenza, I.; et al. Molecular effects of fermented papaya preparation on oxidative damage, MAP Kinase activation and modulation of the benzo[a]pyrene mediated genotoxicity. Biofactors 2006, 26, 147–159. [Google Scholar] [CrossRef] [PubMed]
- Zuhrotun Nisa, F.; Astuti, M.; Mubarika Haryana, S.; Murdiati, A. Effect of Papaya Leaves (Carica papaya L.) Extract on Immune Response (TLR-7, TLR-9) and Inflammation (COX-2) in Rats Induces DMBA (7,12-Dimethylbenz[a]antrasen). Pak. J. Biol. Sci. 2020, 23, 1450–1455. [Google Scholar] [CrossRef] [PubMed]
- Somanah, J.; Bourdon, E.; Bahorun, T. Extracts of Mauritian Carica papaya (var. solo) protect SW872 and HepG2 cells against hydrogen peroxide induced oxidative stress. J. Food Sci. Technol. 2017, 54, 1917–1927. [Google Scholar] [CrossRef] [PubMed]
- Od-Ek, P.; Deenin, W.; Malakul, W.; Phoungpetchara, I.; Tunsophon, S. Anti-obesity effect of Carica papaya in high-fat diet fed rats. Biomed. Rep. 2020, 13, 30. [Google Scholar] [CrossRef]
- Owoyele, B.V.; Adebukola, O.M.; Funmilayo, A.A.; Soladoye, A.O. Anti-inflammatory activities of ethanolic extract of Carica papaya leaves. Inflammopharmacology 2008, 16, 168–173. [Google Scholar] [CrossRef] [PubMed]
- Amazu, L.U.; Azikiwe, C.C.A.; Njoku, C.J.; Osuala, F.N.; Nwosu, P.J.C.; Ajugwo, A.O.; Enye, J.C. Antiinflammatory activity of the methanolic extract of the seeds of Carica papaya in experimental animals. Asian Pac. J. Trop. Med. 2010, 3, 884–886. [Google Scholar] [CrossRef]
- Ahmed, M.; Ramabhimalah, S. Anti-Inflammatory Activity of Aqueous Extract of Carica papaya Seeds in Albino Rats. Biomed. Pharmacol. J. 2012, 5, 173–177. [Google Scholar] [CrossRef]
- Skyler, J.S.; Bakris, G.L.; Bonifacio, E.; Darsow, T.; Eckel, R.H.; Groop, L.; Groop, P.-H.; Handelsman, Y.; Insel, R.A.; Mathieu, C.; et al. Differentiation of Diabetes by Pathophysiology, Natural History, and Prognosis. Diabetes 2017, 66, 241–255. [Google Scholar] [CrossRef]
- Maritim, A.C.; Sanders, R.A.; Watkins Iii, J.B. Diabetes, oxidative stress, and antioxidants: A review. J. Biochem. Mol. Toxicol. 2003, 17, 24–38. [Google Scholar] [CrossRef] [PubMed]
- King, G.L.; Loeken, M.R. Hyperglycemia-induced oxidative stress in diabetic complications. Histochem. Cell Biol. 2004, 122, 333–338. [Google Scholar] [CrossRef] [PubMed]
- Rolo, A.P.; Palmeira, C.M. Diabetes and mitochondrial function: Role of hyperglycemia and oxidative stress. Toxicol. Appl. Pharmacol. 2006, 212, 167–178. [Google Scholar] [CrossRef]
- Agada, R.; Usman, W.A.; Shehu, S.; Thagariki, D. In vitro and in vivo inhibitory effects of Carica papaya seed on α-amylase and α-glucosidase enzymes. Heliyon 2020, 6, e03618. [Google Scholar] [CrossRef] [PubMed]
- Raffaelli, F.; Nanetti, L.; Montecchiani, G.; Borroni, F.; Salvolini, E.; Faloia, E.; Ferretti, G.; Mazzanti, L.; Vignini, A. In vitro effects of fermented papaya (Carica papaya, L.) on platelets obtained from patients with type 2 diabetes. Nutr. Metab. Cardiovasc. Dis. 2015, 25, 224–229. [Google Scholar] [CrossRef]
- Somanah, J.; Aruoma, O.I.; Gunness, T.K.; Kowelssur, S.; Dambala, V.; Murad, F.; Googoolye, K.; Daus, D.; Indelicato, J.; Bourdon, E.; et al. Effects of a short term supplementation of a fermented papaya preparation on biomarkers of diabetes mellitus in a randomized Mauritian population. Prev. Med. 2012, 54, S90–S97. [Google Scholar] [CrossRef]
- Somanah, J.; Bourdon, E.; Rondeau, P.; Bahorun, T.; Aruoma, O.I. Relationship between fermented papaya preparation supplementation, erythrocyte integrity and antioxidant status in pre-diabetics. Food Chem. Toxicol. 2014, 65, 12–17. [Google Scholar] [CrossRef]
- Miranda-Osorio, P.H.; Castell-Rodríguez, A.E.; Vargas-Mancilla, J.; Tovilla-Zárate, C.A.; Ble-Castillo, J.L.; Aguilar-Domínguez, D.E.; Juárez-Rojop, I.E.; Díaz-Zagoya, J.C. Protective Action of Carica papaya on β-Cells in Streptozotocin-Induced Diabetic Rats. Int. J. Environ. Res. Public Health 2016, 13, 446. [Google Scholar] [CrossRef]
- Juárez-Rojop, I.E.; Díaz-Zagoya, J.C.; Ble-Castillo, J.L.; Miranda-Osorio, P.H.; Castell-Rodríguez, A.E.; Tovilla-Zárate, C.A.; Rodríguez-Hernández, A.; Aguilar-Mariscal, H.; Ramón-Frías, T.; Bermúdez-Ocaña, D.Y. Hypoglycemic effect of Carica papaya leaves in streptozotocin-induced diabetic rats. BMC Complement. Altern. Med. 2012, 12, 236. [Google Scholar] [CrossRef] [PubMed]
- Oboh, G.; Olabiyi, A.A.; Akinyemi, A.J.; Ademiluyi, A.O. Inhibition of key enzymes linked to type 2 diabetes and sodium nitroprusside-induced lipid peroxidation in rat pancreas by water-extractable phytochemicals from unripe pawpaw fruit (Carica papaya). J. Basic Clin. Physiol. Pharmacol. 2014, 25, 21–34. [Google Scholar] [CrossRef]
- Gella, A.; Durany, N. Oxidative stress in Alzheimer disease. Cell Adh. Migr. 2009, 3, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhao, B. Oxidative stress and the pathogenesis of Alzheimer’s disease. Oxid. Med. Cell Longev. 2013, 2013, 316523. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.-J.; Zhang, X.; Chen, W.-W. Role of oxidative stress in Alzheimer’s disease. Biomed. Rep. 2016, 4, 519–522. [Google Scholar] [CrossRef]
- Zhang, J.; Mori, A.; Chen, Q.; Zhao, B. Fermented papaya preparation attenuates beta-amyloid precursor protein: Beta-amyloid-mediated copper neurotoxicity in beta-amyloid precursor protein and beta-amyloid precursor protein Swedish mutation overexpressing SH-SY5Y cells. Neuroscience 2006, 143, 63–72. [Google Scholar] [CrossRef]
- Barbagallo, M.; Marotta, F.; Dominguez, L.J. Oxidative stress in patients with Alzheimer’s disease: Effect of extracts of fermented papaya powder. Mediat. Inflamm. 2015, 2015, 624801. [Google Scholar] [CrossRef]
- Highfield, J. Diagnosis and classification of periodontal disease. Aust. Dent. J. 2009, 54 (Suppl. S1), S11–S26. [Google Scholar] [CrossRef]
- Liu, C.; Mo, L.; Niu, Y.; Li, X.; Zhou, X.; Xu, X. The Role of Reactive Oxygen Species and Autophagy in Periodontitis and Their Potential Linkage. Front. Physiol. 2017, 8, 439. [Google Scholar] [CrossRef]
- Saliasi, I.; Llodra, J.C.; Bravo, M.; Tramini, P.; Dussart, C.; Viennot, S.; Carrouel, F. Effect of a Toothpaste/Mouthwash Containing Carica papaya Leaf Extract on Interdental Gingival Bleeding: A Randomized Controlled Trial. Int. J. Environ. Res. Public Health 2018, 15, 2660. [Google Scholar] [CrossRef]
- Kharaeva, Z.F.; Zhanimova, L.R.; Mustafaev, M.; De Luca, C.; Mayer, W.; Chung Sheun Thai, J.; Tiew Siok Tuan, R.; Korkina, L.G. Effects of Standardised Fermented Papaya Gel on Clinical Symptoms, Inflammatory Cytokines, and Nitric Oxide Metabolites in Patients with Chronic Periodontitis: An Open Randomised Clinical Study. Mediat. Inflamm. 2016, 2016, 9379840. [Google Scholar] [CrossRef]
- Rinnerthaler, M.; Bischof, J.; Streubel, M.K.; Trost, A.; Richter, K. Oxidative stress in aging human skin. Biomolecules 2015, 5, 545–589. [Google Scholar] [CrossRef]
- Masaki, H. Role of antioxidants in the skin: Anti-aging effects. J. Dermatol. Sci. 2010, 58, 85–90. [Google Scholar] [CrossRef]
- Jarisarapurin, W.; Sanrattana, W.; Chularojmontri, L.; Kunchana, K.; Wattanapitayakul, S. Antioxidant Properties of Unripe Carica papaya Fruit Extract and Its Protective Effects against Endothelial Oxidative Stress. Evid. Based Complement. Altern. Med. 2019, 2019, 4912631. [Google Scholar] [CrossRef]
- Sanchez, B.; Li, L.; Dulong, J.; Aimond, G.; Lamartine, J.; Liu, G.; Sigaudo-Roussel, D. Impact of Human Dermal Microvascular Endothelial Cells on Primary Dermal Fibroblasts in Response to Inflammatory Stress. Front. Cell Dev. Biol. 2019, 7, 44. [Google Scholar] [CrossRef]
- Seo, S.A.; Ngo, H.T.T.; Hwang, E.; Park, B.; Yi, T.-H. Protective effects of Carica papaya leaf against skin photodamage by blocking production of matrix metalloproteinases and collagen degradation in UVB-irradiated normal human dermal fibroblasts. S. Afr. J. Bot. 2020, 131, 398–405. [Google Scholar] [CrossRef]
- Bertuccelli, G.; Zerbinati, N.; Marcellino, M.; Nanda Kumar, N.S.; He, F.; Tsepakolenko, V.; Cervi, J.; Lorenzetti, A.; Marotta, F. Effect of a quality-controlled fermented nutraceutical on skin aging markers: An antioxidant-control, double-blind study. Exp. Ther. Med. 2016, 11, 909–916. [Google Scholar] [CrossRef] [PubMed]
- Saini, R.; Mittal, A.; Rathi, V. Formulation & in vitro antioxidant analysis of anti-ageing cream of Carica papaya fruit extract. IJOD 2016, 4, 8–14. [Google Scholar]
- Magnani, C.; Isaac, V.; Corrêa, M.; Salgado, H. Caffeic acid: A review of its potential use in medications and cosmetics. Anal. Methods 2014, 6, 3203. [Google Scholar] [CrossRef]
- Gomes, W.F.; França, F.R.M.; Denadai, M.; Andrade, J.K.S.; da Silva Oliveira, E.M.; de Brito, E.S.; Rodrigues, S.; Narain, N. Effect of freeze- and spray-drying on physico-chemical characteristics, phenolic compounds and antioxidant activity of papaya pulp. J. Food Sci. Technol. 2018, 55, 2095–2102. [Google Scholar] [CrossRef] [PubMed]
- Nugroho, A.; Heryani, H.; Choi, J.S.; Park, H.-J. Identification and quantification of flavonoids in Carica papaya leaf and peroxynitrite-scavenging activity. Asian Pac. J. Trop. Biomed. 2017, 7, 208–213. [Google Scholar] [CrossRef]
- Spagnol, C.M.; Di Filippo, L.D.; Isaac, V.L.B.; Correa, M.A.; Salgado, H.R.N. Caffeic Acid in Dermatological Formulations: In Vitro Release Profile and Skin Absorption. Comb. Chem. High Throughput Screen. 2017, 20, 675–681. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.J.; Lee, S.N.; Kim, K.; Joo da, H.; Shin, S.; Lee, J.; Lee, H.K.; Kim, J.; Kwon, S.B.; Kim, M.J.; et al. Biological effects of rutin on skin aging. Int. J. Mol. Med. 2016, 38, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Midwood, K.S.; Williams, L.V.; Schwarzbauer, J.E. Tissue repair and the dynamics of the extracellular matrix. Int. J. Biochem. Cell Biol. 2004, 36, 1031–1037. [Google Scholar] [CrossRef]
- Gonzalez, A.C.; Costa, T.F.; Andrade, Z.A.; Medrado, A.R. Wound healing—A literature review. Bras. Dermatol. 2016, 91, 614–620. [Google Scholar] [CrossRef] [PubMed]
- Lephart, E.D. Skin aging and oxidative stress: Equol’s anti-aging effects via biochemical and molecular mechanisms. Ageing Res. Rev. 2016, 31, 36–54. [Google Scholar] [CrossRef]
- Süntar, I.; Akkol, E.K.; Nahar, L.; Sarker, S.D. Wound healing and antioxidant properties: Do they coexist in plants? Free Radic. Antioxid. 2012, 2, 1–7. [Google Scholar] [CrossRef]
- Cano Sanchez, M.; Lancel, S.; Boulanger, E.; Neviere, R. Targeting Oxidative Stress and Mitochondrial Dysfunction in the Treatment of Impaired Wound Healing: A Systematic Review. Antioxidants 2018, 7, 98. [Google Scholar] [CrossRef]
- Singh, S.; Young, A.; McNaught, C.-E. The physiology of wound healing. Surg. Oxf. Int. Ed. 2017, 35, 473–477. [Google Scholar] [CrossRef]
- Panzarini, E.; Dwikat, M.; Mariano, S.; Vergallo, C.; Dini, L. Administration Dependent Antioxidant Effect of Carica papaya Seeds Water Extract. Evid. Based Complement. Alternat. Med. 2014, 2014, 281508. [Google Scholar] [CrossRef]
- Mikhal’chik, E.V.; Ivanova, A.V.; Anurov, M.V.; Titkova, S.M.; Pen’kov, L.Y.; Kharaeva, Z.F.; Korkina, L.G. Wound-healing effect of papaya-based preparation in experimental thermal trauma. Bull. Exp. Biol. Med. 2004, 137, 560–562. [Google Scholar] [CrossRef]
- Nafiu, A.B.; Rahman, M.T. Anti-inflammatory and antioxidant properties of unripe papaya extract in an excision wound model. Pharm. Biol. 2015, 53, 662–671. [Google Scholar] [CrossRef]
- Nafiu, A.B.; Rahman, M.T. Selenium added unripe Carica papaya pulp extracts enhance wound repair through TGF-β1 and VEGF-a signalling pathway. BMC Complement. Altern. Med. 2015, 15, 369. [Google Scholar] [CrossRef]
- Collard, E.; Roy, S. Improved function of diabetic wound-site macrophages and accelerated wound closure in response to oral supplementation of a fermented papaya preparation. Antioxid. Redox Signal. 2010, 13, 599–606. [Google Scholar] [CrossRef]
- Dickerson, R.; Deshpande, B.; Gnyawali, U.; Lynch, D.; Gordillo, G.M.; Schuster, D.; Osei, K.; Roy, S. Correction of aberrant NADPH oxidase activity in blood-derived mononuclear cells from type II diabetes mellitus patients by a naturally fermented papaya preparation. Antioxid. Redox Signal. 2012, 17, 485–491. [Google Scholar] [CrossRef] [PubMed]
- Dickerson, R.; Banerjee, J.; Rauckhorst, A.; Pfeiffer, D.R.; Gordillo, G.M.; Khanna, S.; Osei, K.; Roy, S. Does oral supplementation of a fermented papaya preparation correct respiratory burst function of innate immune cells in type 2 diabetes mellitus patients? Antioxid. Redox Signal. 2015, 22, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Indran, M.; Mahmood, A.A.; Kuppusamy, U.R. Protective effect of Carica papaya L leaf extract against alcohol induced acute gastric damage and blood oxidative stress in rats. West Indian Med. J. 2008, 57, 323–326. [Google Scholar]
- Nayak, S.B.; Pinto Pereira, L.; Maharaj, D. Wound healing activity of Carica papaya L. in experimentally induced diabetic rats. Indian J. Exp. Biol. 2007, 45, 739–743. [Google Scholar] [PubMed]
- Gurung, S.; Skalko-Basnet, N. Wound healing properties of Carica papaya latex: In vitro evaluation in mice burn model. J. Ethnopharmacol 2009, 121, 338–341. [Google Scholar] [CrossRef] [PubMed]
- Nayak, B.S.; Ramdeen, R.; Adogwa, A.; Ramsubhag, A.; Marshall, J.R. Wound-healing potential of an ethanol extract of Carica papaya (Caricaceae) seeds. Int. Wound J. 2012, 9, 650–655. [Google Scholar] [CrossRef]
- Hakim, R.F.; Fakhrurrazi; Dinni. Effect of Carica papaya Extract toward Incised Wound Healing Process in Mice (Mus musculus) Clinically and Histologically. Evid. Based Complement. Alternat. Med. 2019, 2019, 8306519. [Google Scholar] [CrossRef] [PubMed]
- Ajlia, S.A.; Majid, F.A.; Suvik, A.; Effendy, M.A.; Nouri, H.S. Efficacy of papain-based wound cleanser in promoting wound regeneration. Pak. J. Biol. Sci. 2010, 13, 596–603. [Google Scholar] [CrossRef][Green Version]
- Aravind, G.; Bhowmik, D.; S, D.; Harish, G. Traditional and medicinal uses of Carica papaya. J. Med. Plants Stud. 2013, 1, 7–15. [Google Scholar]
- Murthy, M.B.; Murthy, B.K.; Bhave, S. Comparison of safety and efficacy of papaya dressing with hydrogen peroxide solution on wound bed preparation in patients with wound gape. Indian J. Pharm. 2012, 44, 784–787. [Google Scholar] [CrossRef]
- Saha, S.K.; Lee, S.B.; Won, J.; Choi, H.Y.; Kim, K.; Yang, G.-M.; Dayem, A.A.; Cho, S.-G. Correlation between Oxidative Stress, Nutrition, and Cancer Initiation. Int. J. Mol. Sci. 2017, 18, 1544. [Google Scholar] [CrossRef] [PubMed]
- Sosa, V.; Moliné, T.; Somoza, R.; Paciucci, R.; Kondoh, H.; Lleonart, M.E. Oxidative stress and cancer: An overview. Ageing Res. Rev. 2013, 12, 376–390. [Google Scholar] [CrossRef] [PubMed]
- García-Solís, P.; Yahia, E.M.; Morales-Tlalpan, V.; Díaz-Muñoz, M. Screening of antiproliferative effect of aqueous extracts of plant foods consumed in México on the breast cancer cell line MCF-7. Int. J. Food Sci. Nutr. 2009, 60 (Suppl. S6), 32–46. [Google Scholar] [CrossRef]
- Zuhrotun Nisa, F.; Astuti, M.; Murdiati, A.; Mubarika Haryana, S. Anti-proliferation and Apoptosis Induction of Aqueous Leaf Extract of Carica papaya L. on Human Breast Cancer Cells MCF-7. Pak. J. Biol. Sci. 2017, 20, 36–41. [Google Scholar] [CrossRef]
- Pathak, N.; Khan, S.; Bhargava, A.; Raghuram, G.V.; Jain, D.; Panwar, H.; Samarth, R.M.; Jain, S.K.; Maudar, K.K.; Mishra, D.K.; et al. Cancer chemopreventive effects of the flavonoid-rich fraction isolated from papaya seeds. Nutr. Cancer 2014, 66, 857–871. [Google Scholar] [CrossRef] [PubMed]
- Somanah, J.; Ramsaha, S.; Verma, S.; Kumar, A.; Sharma, P.; Singh, R.K.; Aruoma, O.I.; Bourdon, E.; Bahorun, T. Fermented papaya preparation modulates the progression of N-methyl-N-nitrosourea induced hepatocellular carcinoma in Balb/c mice. Life Sci. 2016, 151, 330–338. [Google Scholar] [CrossRef]
- Waly, M.I.; Al-Rawahi, A.S.; Al Riyami, M.; Al-Kindi, M.A.; Al-Issaei, H.K.; Farooq, S.A.; Al-Alawi, A.; Rahman, M.S. Amelioration of azoxymethane induced-carcinogenesis by reducing oxidative stress in rat colon by natural extracts. BMC Complement. Altern. Med. 2014, 14, 60. [Google Scholar] [CrossRef] [PubMed]
- Murakami, S.; Eikawa, S.; Kaya, S.; Imao, M.; Aji, T. AntiTumor and Immunoregulatory Effects of Fermented Papaya Preparation (FPP: SAIDOPS501). Asian Pac. J. Cancer Prev. 2016, 17, 3077–3084. [Google Scholar] [PubMed]
- Bussmann, R.W.; Malca, G.; Glenn, A.; Sharon, D.; Nilsen, B.; Parris, B.; Dubose, D.; Ruiz, D.; Saleda, J.; Martinez, M.; et al. Toxicity of medicinal plants used in traditional medicine in Northern Peru. J. Ethnopharmacol. 2011, 137, 121–140. [Google Scholar] [CrossRef] [PubMed]
- Afzan, A.; Abdullah, N.R.; Halim, S.Z.; Rashid, B.A.; Semail, R.H.R.; Abdullah, N.; Jantan, I.; Muhammad, H.; Ismail, Z. Repeated dose 28-days oral toxicity study of Carica papaya L. leaf extract in Sprague Dawley rats. Molecules 2012, 17, 4326–4342. [Google Scholar] [CrossRef] [PubMed]
- Halim, S.Z.; Abdullah, N.; Afzan, A.; Abd Rashid, B.; Jantan, I.; Ismail, Z. Acute toxicity study of Carica papaya leaf extract in Sprague Dawley rats. J. Med. Plants Res. 2011, 5, 1867–1872. [Google Scholar]
- Tarkang, P.; Agbor, G.; Armelle, T.; Tchokouaha, L.R.; David, K.; Ngadena, Y. Acute and Chronic Toxicity Studies of the aqueous and ethanol leaf extracts of Carica papaya Linn in Wistar rats. J. Nat. Prod. Plant Resour. 2012, 2, 617–627. [Google Scholar]
| Part of the Plant | Extract | Type of Experiment | Results | Reference |
|---|---|---|---|---|
| Seed | Aqueous extract | In vitro cell free model In vitro HRBC assay | Aqueous extract of papaya seeds at 20 μg/mL decreased NO radical by 69.4%, comparable to ascorbic acid. | [15] |
| Aqueous extract of papaya seeds at 150 μg/mL inhibited the release of lysosomal enzyme by 22.7%. | ||||
| Leaf | Leaf extract | In vitro animal model | Leaf extract at 1.32 μg/mL enhanced adaptive immune response by upregulated TLR-7 and TLR-9 expressions. | [17] |
| Fruit (ripe, unripe fruit, peel, seed, pulp) | Aqueous extract | In vitro ROS assay | Unripe peel (69.7%) and seed (79.1%) extract at 2mg/mL showed ROS scavenging activity of 69.7 and 79.1% at 2 mg dry weight/mL. | [18] |
| In vitro antioxidant enzyme assay | Aqueous extract of papaya unripe peel at 2 mg dry weight/mL increased SOD activity by 21.9%. | |||
| In vitro protein carbonyl assay | Aqueous extract reduced oxidative damage by lowered protein carbonyl production for ripe seed (60.4%) and ripe peel (57.6%) extract at 0.1 and 2 mg/mL. | |||
| In vitro inflammatory cytokines assay | The extracts augmented IL-10 levels at a low concentration of 0.1 mg/mL. Seed extracts exerted highest increment in IL-10 secretory level (+140.1%), followed by peel and pulp extracts. Seed extracts at 0.1 mg dry weight/mL exerted increment in IL-10 secretory level (+140.1%). | |||
| Aqueous extract of papaya seeds at 2 mg dry weight/mL down regulated IL-6 by 37.8%. | ||||
| Unripe extracts at 2 mg/mL showed inhibitory activity against TNF-α with 71.2% for pulp extract, 62.7% for peel and 65.3% for seed extract. | ||||
| Fruit (Flesh) | Juice | In vitro animal model | Papaya juice downregulated the elevated serum IL-6 (217.6 vs. 28.3 pg/dL) and MDA (3.2 vs.1.4 pg/dL) in high fat diets treated rats. Papaya juice affected serum SOD of the high fat treated rat by increased serum SOD (30.41 U/L). | [19] |
| Leaf | Ethanol extract | In vitro animal model | Ethanol extract of papaya leaves at 200 mg/kg reduced paw edema (2.6 mm) and inhibited granuloma formation (0.2 g). | [20] |
| Part of the Plant | Extract | Type of Experiment | Results | Reference |
|---|---|---|---|---|
| Seed | Hexane extract & ethyl acetate extract | In vitro DPPH radical scavenging assay | Hexane extract possessed DPPH radical scavenging activity with IC50 = 41.5 mg/mL. | [27] |
| In vitro TBA method | Hexane extract demonstrated TBA scavenging activity with IC50 = 38.2 mg/mL. | |||
| In vitro α-glucosidase inhibition | Hexane extract displayed α-glucosidase enzyme inhibitory activity with IC50 = 75.78 mg/mL. | |||
| Ethyl acetate extract exhibited α-glucosidase enzyme inhibitory activity with IC50 = 77.41 mg/mL. | ||||
| In vitro α-amylase inhibition | Hexane extract demonstrated α-amylase inhibitory activity with IC50 = 76.96 mg/mL. | |||
| Ethyl acetate extract displayed α-amylase inhibitory activity with IC50 = 79.18 mg/mL. | ||||
| In vitro FRAP assay | Ethyl acetate extract displayed FRAP inhibitory activity with IC50 = 38.75 mg/mL. | |||
| In vitro animal model | Ethyl acetate extract at 500 mg/kg/body weight significantly decreased the blood glucose level of the diabetic rats to approximately 120 mmol/L over 120 min comparable with standard drug, acarbose. | |||
| - | FPP | In vitro analysis | FPP at concentration 50 μg/mL increased inner and outer platelet membrane fluidity, displayed by a decrease of ~0.015 r in DPH anisotropy and ~0.02 r in TMA-DPH anisotropy. FPP increased Naþ/Kþ-ATPase activity by ~0.5 μmol Pi/mg prot/h.-FPP improved platelet function in vitro and this might help preventing diabetic complications. FPP also slightly increased TAC by ~5 nmol/μL and SOD activity by ~0.5 units/μL. FPP at 50 μg/mL lowered lipid peroxidation. | [28] |
| - | FPP® | Human trial | FPP significantly improved liver sensitivity to insulin, which was indicated by decreased circulating AST and ALT. FPP scavenged NO and hydroxyl radicals and displayed an increased in total antioxidant status. | [29] |
| - | FPP | In vitro DPPH radical scavenging assay | FPP displayed DPPH scavenging with AA50 = 55.69 mg/mL. | [30] |
| In vitro ABTS+ scavenging assay | FPP demonstrated ABTS+ scavenging action with AA50 = 14.56 mg/mL. | |||
| In vitro AAPH-induced lipid oxidation inhibition | FPP inhibited AAPH-induced lipid oxidation with AA50 = 68.06 mg/mL. | |||
| In vitro O2− scavenging assay | FPP showed O2− scavenging action with AA50 = 88.70 mg/mL. | |||
| In vitro •OH scavenging assay | FPP showed hydroxyl radical scavenging activity with AA50 = 4.13 mg/mL. | |||
| Human trial | FPP at a dose of 6g/day showed an increase of 4.9% and 5.7% in TAS for male and female respectively after 14-week consumption at 6g/day. FPP decreased protein carbonyl level by 1.9% in males and 9.7% in females after a 14-week FPP ingestion. FPP delayed red blood cell hemolysis. | |||
| Seed, flesh and peel of unripe fruit | Aqueous extract | In vitro α-amylase inhibition In vitro α-glucosidase inhibition In vitro lipid peroxidation assay In vitro NO scavenging assay | Aqueous extract inhibited α-glucosidase and α-amylase activities with IC50 of 1.76 mg/mL and IC50: 0.87 mg/mL. At a concentration of 7.5 mg/mL, the extract also displayed the highest NO radical scavenging activity (52.5%). | [31] |
| Leaf | Chloroform extract | In vitro animal model | The chloroform extract at a dose of 31 mg/kg/day significantly increased islet area by 16,842.2 μm2 by stimulating regeneration of β-cells of islet of Langerhans. | [32] |
| The extract successfully decreased fasting glucose levels by 222.3 mg/dL in diabetic group in vivo. | ||||
| Leaf | Aqueous extract | In vitro animal model | Aqueous extract at a dose of 3 g/100 mL decreased blood glucose levels in diabetic rats by 184 mg/dL. Aqueous extract at a dose of 1.5 g/100 mL preserved Islet cell size in diabetic rats. Aqueous extract increased NO levels by 17.39 μM and hence reduced ROS production. | [33] |
| Part of the Plant | Extract | Type of Experiment | Results | Reference |
|---|---|---|---|---|
| Unripe papaya juice | In vitro antioxidant enzyme assays | Papaya juice at 1 mg/mL enhanced SOD (49%) and CAT (40.5%) activities. | [45] | |
| Western blot analysis | Papaya juice at 1 mg/mL restrained NF-κB translocation to nuclei and downregulated Nrf2 levels. | |||
| Leaf | Ethanol extract | In vitro DPPH assay | Ethanol extract at 250 μg/mL showed ROS scavenging effect at 60%. | [47] |
| In vitro DCFH-DA assay | Ethanol extract at 50 μg/mL showed potent suppressing action towards UVB-induced ROS production (60%). | |||
| In vitro MMPs and inflammatory cytokines production | Ethanol extract at 50 μg/mL of Carica papaya leaves enhances synthesis and prevents degradation of type I collagen via upregulating TGF-β1 and down-regulating MMP-1 (34% at 50 μg/mL), MMP-3, and IL-6 generation. Ethanol extract at 50 μg/mL of Carica papaya leaves reduced mRNA level of MMP-1 (56.8%) and type I procollagen (288.8%). | |||
| Western blotting assay | Ethanol extract at 50 µg/mL showed AP-1 activation via down-regulating c-Fos (89%) and c-Jun (44%) phosphorylation. Ethanol extract at 50 µg/mL attenuated MAPK activation, and p38 phosphorylation (82%), followed by ERK, and JNK phosphorylation. | |||
| FPP | Double-blinded RCT | FPP at a dose of 4.5 g showed anti-skin aging by demonstrating overall higher skin moisturization, elasticity, and surface evenness. FPP at a 4.5 g inhibited MDA production and up modulation of AQP-3, enhanced SOD and NO levels in FPP-treated group. FPP at 4.5 g downregulated pro-aging factors (CyPA and CD147 genes) suggesting to reduce risk of skin carcinogenesis. | [48] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kong, Y.R.; Jong, Y.X.; Balakrishnan, M.; Bok, Z.K.; Weng, J.K.K.; Tay, K.C.; Goh, B.H.; Ong, Y.S.; Chan, K.G.; Lee, L.H.; et al. Beneficial Role of Carica papaya Extracts and Phytochemicals on Oxidative Stress and Related Diseases: A Mini Review. Biology 2021, 10, 287. https://doi.org/10.3390/biology10040287
Kong YR, Jong YX, Balakrishnan M, Bok ZK, Weng JKK, Tay KC, Goh BH, Ong YS, Chan KG, Lee LH, et al. Beneficial Role of Carica papaya Extracts and Phytochemicals on Oxidative Stress and Related Diseases: A Mini Review. Biology. 2021; 10(4):287. https://doi.org/10.3390/biology10040287
Chicago/Turabian StyleKong, Yew Rong, Yong Xin Jong, Manisha Balakrishnan, Zhui Ken Bok, Janice Kwan Kah Weng, Kai Ching Tay, Bey Hing Goh, Yong Sze Ong, Kok Gan Chan, Learn Han Lee, and et al. 2021. "Beneficial Role of Carica papaya Extracts and Phytochemicals on Oxidative Stress and Related Diseases: A Mini Review" Biology 10, no. 4: 287. https://doi.org/10.3390/biology10040287
APA StyleKong, Y. R., Jong, Y. X., Balakrishnan, M., Bok, Z. K., Weng, J. K. K., Tay, K. C., Goh, B. H., Ong, Y. S., Chan, K. G., Lee, L. H., & Khaw, K. Y. (2021). Beneficial Role of Carica papaya Extracts and Phytochemicals on Oxidative Stress and Related Diseases: A Mini Review. Biology, 10(4), 287. https://doi.org/10.3390/biology10040287








