Toward the Enhancement of Microalgal Metabolite Production through Microalgae–Bacteria Consortia †
Abstract
Simple Summary
Abstract
1. Introduction
2. Mutualistic Interaction in Microalgae–Bacteria Consortia
2.1. The Concept of Phycosphere
2.2. Mechanisms Acting in Mutualistic Co-Cultures
3. Enhancement of Biomass and Metabolite Production
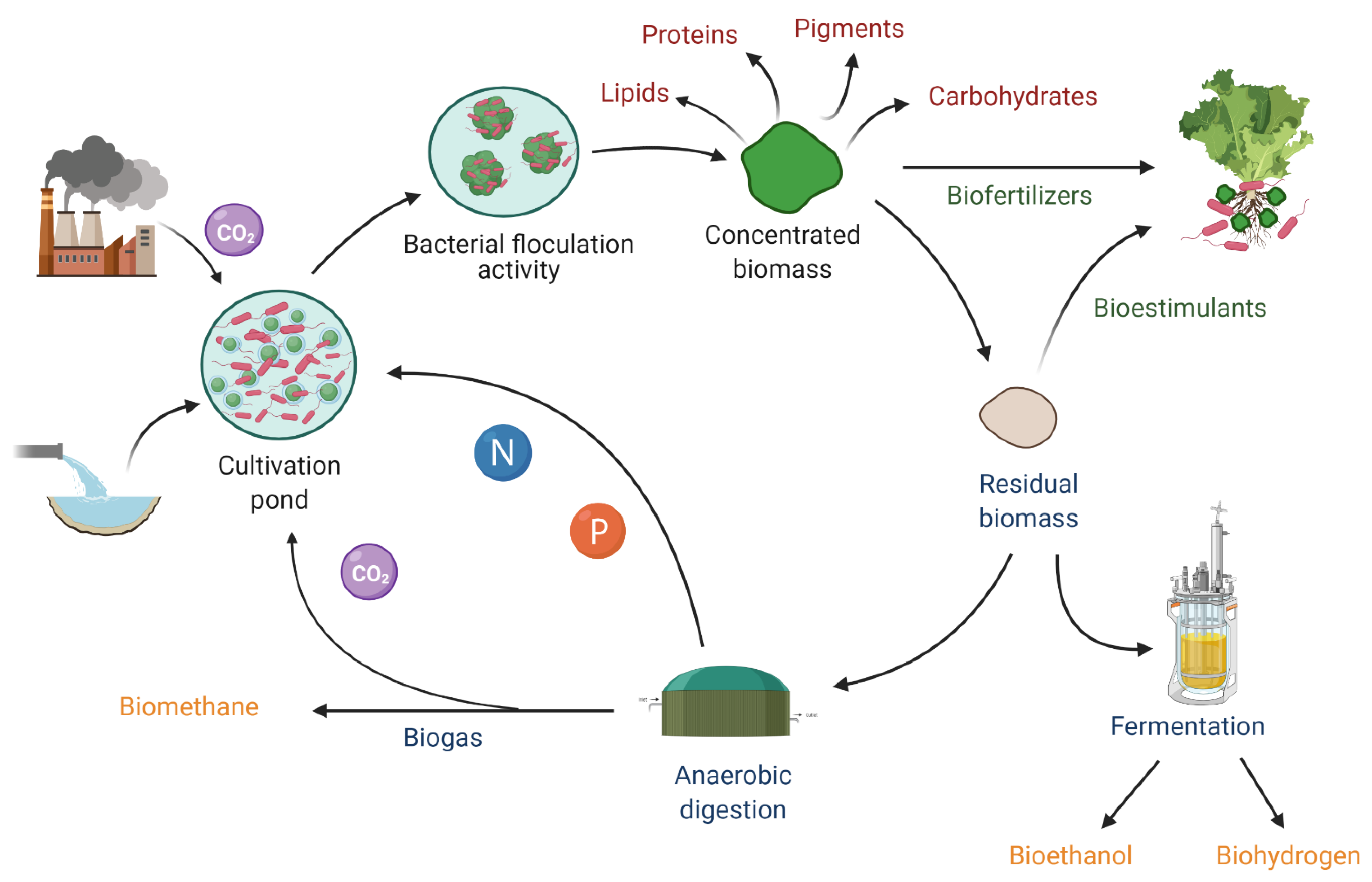
4. Green Chemistry Projections and Circular Bioeconomy
5. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vuppaladadiyam, A.K.; Prinsen, P.; Raheem, A.; Luque, R.; Zhao, M. Microalgae cultivation and metabolites production: A comprehensive review. Biofuels Bioprod. Biorefining 2018, 12, 304–324. [Google Scholar] [CrossRef]
- Khan, M.I.; Shin, J.H.; Kim, J.D. The promising future of microalgae: Current status, challenges, and optimization of a sustainable and renewable industry for biofuels, feed, and other products. Microb. Cell Factories 2018, 17, 1–21. [Google Scholar] [CrossRef]
- Lauersen, K.J. Eukaryotic microalgae as hosts for light-driven heterologous isoprenoid production. Planta 2019, 249, 155–180. [Google Scholar] [CrossRef] [PubMed]
- Fabris, M.; Abbriano, R.M.; Pernice, M.; Sutherland, D.L.; Commault, A.S.; Hall, C.C.; Labeeuw, L.; Mccauley, J.I.; Kuzhiuparambil, U.; Ray, P. Emerging technologies in algal biotechnology: Toward the establishment of a sustainable, algae-based bioeconomy. Front. Plant Sci. 2020, 11, 279. [Google Scholar] [CrossRef] [PubMed]
- Vavitsas, K.; Fabris, M.; Vickers, C.E. Terpenoid metabolic engineering in photosynthetic microorganisms. Genes 2018, 9, 520. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Chen, L.; Zhang, W. Chemicals to enhance microalgal growth and accumulation of high-value bioproducts. Front. Microbiol. 2015, 6, 56. [Google Scholar] [CrossRef]
- Commault, A.S.; Fabris, M.; Kuzhiumparambil, U.; Adriaans, J.; Pernice, M.; Ralph, P.J. Methyl jasmonate treatment affects the regulation of the 2-C-methyl-D-erythritol 4-phosphate pathway and early steps of the triterpenoid biosynthesis in Chlamydomonas reinhardtii. Algal Res. 2019, 39, 101462. [Google Scholar] [CrossRef]
- Commault, A.S.; Kuzhiumparambil, U.; Herdean, A.; Fabris, M.; Jaramillo-Madrid, A.C.; Abbriano, R.M.; Ralph, P.J.; Pernice, M. Methyl jasmonate and methyl-β-cyclodextrin individually boost triterpenoid biosynthesis in Chlamydomonas Reinhardtii UVM4. Pharmaceuticals 2021, 14, 125. [Google Scholar] [CrossRef] [PubMed]
- Yao, S.; Lyu, S.; An, Y.; Lu, J.; Gjermansen, C.; Schramm, A. Microalgae–bacteria symbiosis in microalgal growth and biofuel production: A review. J. Appl. Microbiol. 2019, 126, 359–368. [Google Scholar] [CrossRef]
- Fuentes, J.L.; Garbayo, I.; Cuaresma, M.; Montero, Z.; Gonzalez-Del-Valle, M.; Vilchez, C. Impact of microalgae-bacteria interactions on the production of algal biomass and associated compounds. Mar. Drugs 2016, 14, 100. [Google Scholar] [CrossRef]
- Ramanan, R.; Kim, B.H.; Cho, D.H.; Oh, H.M.; Kim, H.S. Algae-bacteria interactions: Evolution, ecology and emerging applications. Biotechnol. Adv. 2016, 34, 14–29. [Google Scholar] [CrossRef]
- Bronstein, J.L. Our current understanding of mutualism. Q. Rev. Biol. 1994, 69, 31–51. [Google Scholar] [CrossRef]
- Doebeli, M.; Knowlton, N. The evolution of interspecific mutualisms. Proc. Natl. Acad. Sci. USA 1998, 95, 8676–8680. [Google Scholar] [CrossRef]
- Wang, H.; Hill, R.T.; Zheng, T.; Hu, X.; Wang, B. Effects of bacterial communities on biofuel-producing microalgae: Stimulation, inhibition and harvesting. Crit. Rev. Biotechnol. 2016, 36, 341–352. [Google Scholar] [CrossRef] [PubMed]
- Croft, M.T.; Lawrence, A.D.; Raux-Deery, E.; Warren, M.J.; Smith, A.G. Algae acquire vitamin B12 through a symbiotic relationship with bacteria. Nature 2005, 438, 90–93. [Google Scholar] [CrossRef] [PubMed]
- Subashchandrabose, S.R.; Ramakrishnan, B.; Megharaj, M.; Venkateswarlu, K.; Naidu, R. Consortia of cyanobacteria/microalgae and bacteria: Biotechnological potential. Biotechnol. Adv. 2011, 29, 896–907. [Google Scholar] [CrossRef] [PubMed]
- Samo, T.J.; Kimbrel, J.A.; Nilson, D.J.; Pett-Ridge, J.; Weber, P.K.; Mayali, X. Attachment between heterotrophic bacteria and microalgae influences symbiotic microscale interactions. Environ. Microbiol. 2018, 20, 4385–4400. [Google Scholar] [CrossRef] [PubMed]
- Seymour, J.R.; Amin, S.A.; Raina, J.-B.; Stocker, R. Zooming in on the phycosphere: The ecological interface for phytoplankton–bacteria relationships. Nat. Microbiol. 2017, 2, 1–12. [Google Scholar] [CrossRef]
- Fouilland, E.; Galès, A.; Beaugelin, I.; Lanouguère, E.; Pringault, O.; Leboulanger, C. Influence of bacteria on the response of microalgae to contaminant mixtures. Chemosphere 2018, 211, 449–455. [Google Scholar] [CrossRef]
- Kouzuma, A.; Watanabe, K. Exploring the potential of algae/bacteria interactions. Curr. Opin. Biotechnol. 2015, 33, 125–129. [Google Scholar] [CrossRef]
- Sapp, M.; Schwaderer, A.S.; Wiltshire, K.H.; Hoppe, H.-G.; Gerdts, G.; Wichels, A. Species-specific bacterial communities in the phycosphere of microalgae? Microb. Ecol. 2007, 53, 683–699. [Google Scholar] [CrossRef] [PubMed]
- Ramanan, R.; Kang, Z.; Kim, B.-H.; Cho, D.-H.; Jin, L.; Oh, H.-M.; Kim, H.-S. Phycosphere bacterial diversity in green algae reveals an apparent similarity across habitats. Algal Res. 2015, 8, 140–144. [Google Scholar] [CrossRef]
- Cooper, M.B.; Smith, A.G. Exploring mutualistic interactions between microalgae and bacteria in the omics age. Curr. Opin. Plant Biol. 2015, 26, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Dunham, M.J. Synthetic ecology: A model system for cooperation. Proc. Natl. Acad. Sci. USA 2007, 104, 1741–1742. [Google Scholar] [CrossRef]
- Kubo, I.; Hosoda, K.; Suzuki, S.; Yamamoto, K.; Kihara, K.; Mori, K.; Yomo, T. Construction of bacteria–eukaryote synthetic mutualism. Biosystems 2013, 113, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Goers, L.; Freemont, P.; Polizzi, K.M. Co-culture systems and technologies: Taking synthetic biology to the next level. J. R. Soc. Interface 2014, 11, 20140065. [Google Scholar] [CrossRef]
- de-Bashan, L.E.; Mayali, X.; Bebout, B.M.; Weber, P.K.; Detweiler, A.M.; Hernandez, J.-P.; Prufert-Bebout, L.; Bashan, Y. Establishment of stable synthetic mutualism without co-evolution between microalgae and bacteria demonstrated by mutual transfer of metabolites (NanoSIMS isotopic imaging) and persistent physical association (Fluorescent in situ hybridization). Algal Res. 2016, 15, 179–186. [Google Scholar] [CrossRef]
- Nguyen, T.-T.; Binh, Q.A.; Bui, X.-T.; Ngo, H.H.; Vo, H.N.P.; Lin, K.-Y.A.; Guo, W.; Lin, C.; Breider, F. Co-culture of microalgae-activated sludge for wastewater treatment and biomass production: Exploring their role under different inoculation ratios. Bioresour. Technol. 2020, 314, 123754. [Google Scholar] [CrossRef]
- Su, Y.; Mennerich, A.; Urban, B. Synergistic cooperation between wastewater-born algae and activated sludge for wastewater treatment: Influence of algae and sludge inoculation ratios. Bioresour. Technol. 2012, 105, 67–73. [Google Scholar] [CrossRef]
- Rajapitamahuni, S.; Bachani, P.; Sardar, R.K.; Mishra, S. Co-cultivation of siderophore-producing bacteria Idiomarina loihiensis RS14 with Chlorella variabilis ATCC 12198, evaluation of micro-algal growth, lipid, and protein content under iron starvation. J. Appl. Phycol. 2019, 31, 29–39. [Google Scholar] [CrossRef]
- Subasankari, K.; Thanappan, V.; Jeyapragash, D.; Anantharaman, P.; Sarangi, R. Growth promoting studies on co-culturing Nannochloropsis oceanica with Halomonas aquamarina actively enhance the algal biomass and lipid production. Biocatal. Agric. Biotechnol. 2020, 29, 101790. [Google Scholar]
- Toyama, T.; Kasuya, M.; Hanaoka, T.; Kobayashi, N.; Tanaka, Y.; Inoue, D.; Sei, K.; Morikawa, M.; Mori, K. Growth promotion of three microalgae, Chlamydomonas reinhardtii, Chlorella vulgaris and Euglena gracilis, by in situ indigenous bacteria in wastewater effluent. Biotechnol. Biofuels 2018, 11, 176. [Google Scholar] [CrossRef] [PubMed]
- Rivas, M.O.; Vargas, P.; Riquelme, C.E. Interactions of Botryococcus braunii cultures with bacterial biofilms. Microb. Ecol. 2010, 60, 628–635. [Google Scholar] [CrossRef]
- Zhuang, L.-L.; Yu, H.; Yang, T.; Sun, S.; Wang, J. A novel light source provided by photobacteria to improve the growth of microalgal biofilm. Bioresour. Technol. Rep. 2019, 6, 138–144. [Google Scholar] [CrossRef]
- Abinandan, S.; Subashchandrabose, S.R.; Venkateswarlu, K.; Megharaj, M. Microalgae–bacteria biofilms: A sustainable synergistic approach in remediation of acid mine drainage. Appl. Microbiol. Biotechnol. 2018, 102, 1131–1144. [Google Scholar] [CrossRef] [PubMed]
- Lubarsky, H.V.; Hubas, C.; Chocholek, M.; Larson, F.; Manz, W.; Paterson, D.M.; Gerbersdorf, S.U. The stabilisation potential of individual and mixed assemblages of natural bacteria and microalgae. PLoS ONE 2010, 5, e13794. [Google Scholar] [CrossRef]
- de-Bashan, L.E.; Bashan, Y. Joint immobilization of plant growth-promoting bacteria and green microalgae in alginate beads as an experimental model for studying plant-bacterium interactions. Appl. Environ. Microbiol. 2008, 74, 6797–6802. [Google Scholar] [CrossRef]
- Nagy, K.; Sipos, O.; Gombai, É.; Kerényi, Á.; Valkai, S.; Ormos, P.; Galajda, P. Interaction of bacterial populations in coupled microchambers. Chem. Biochem. Eng. Q. 2014, 28, 225–231. [Google Scholar] [CrossRef]
- Burmeister, A.; Hilgers, F.; Langner, A.; Westerwalbesloh, C.; Kerkhoff, Y.; Tenhaef, N.; Drepper, T.; Kohlheyer, D.; Von Lieres, E.; Noack, S. A microfluidic co-cultivation platform to investigate microbial interactions at defined microenvironments. Lab Chip 2019, 19, 98–110. [Google Scholar] [CrossRef]
- Berthold, D.E.; Shetty, K.G.; Jayachandran, K.; Laughinghouse, H.D.; Gantar, M. Enhancing algal biomass and lipid production through bacterial co-culture. Biomass Bioenergy 2019, 122, 280–289. [Google Scholar] [CrossRef]
- Lopez, B.R.; Palacios, O.A.; Bashan, Y.; Hernández-Sandoval, F.E.; de-Bashan, L.E. Riboflavin and lumichrome exuded by the bacterium Azospirillum brasilense promote growth and changes in metabolites in Chlorella sorokiniana under autotrophic conditions. Algal Res. 2019, 44, 101696. [Google Scholar] [CrossRef]
- Amavizca, E.; Bashan, Y.; Ryu, C.-M.; Farag, M.A.; Bebout, B.M.; de-Bashan, L.E. Enhanced performance of the microalga Chlorella sorokiniana remotely induced by the plant growth-promoting bacteria Azospirillum brasilense and Bacillus pumilus. Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Bruckner, C.G.; Rehm, C.; Grossart, H.P.; Kroth, P.G. Growth and release of extracellular organic compounds by benthic diatoms depend on interactions with bacteria. Environ. Microbiol. 2011, 13, 1052–1063. [Google Scholar] [CrossRef] [PubMed]
- Krohn-Molt, I.; Wemheuer, B.; Alawi, M.; Poehlein, A.; Güllert, S.; Schmeisser, C.; Pommerening-Röser, A.; Grundhoff, A.; Daniel, R.; Hanelt, D. Metagenome survey of a multispecies and alga-associated biofilm revealed key elements of bacterial-algal interactions in photobioreactors. Appl. Environ. Microbiol. 2013, 79, 6196–6206. [Google Scholar] [CrossRef] [PubMed]
- Kazamia, E.; Czesnick, H.; Nguyen, T.T.V.; Croft, M.T.; Sherwood, E.; Sasso, S.; Hodson, S.J.; Warren, M.J.; Smith, A.G. Mutualistic interactions between vitamin B12-dependent algae and heterotrophic bacteria exhibit regulation. Environ. Microbiol. 2012, 14, 1466–1476. [Google Scholar] [CrossRef] [PubMed]
- Grant, M.A.; Kazamia, E.; Cicuta, P.; Smith, A.G. Direct exchange of vitamin B 12 is demonstrated by modelling the growth dynamics of algal–bacterial cocultures. ISME J. 2014, 8, 1418–1427. [Google Scholar] [CrossRef]
- Helliwell, K.E.; Pandhal, J.; Cooper, M.B.; Longworth, J.; Kudahl, U.J.; Russo, D.A.; Tomsett, E.V.; Bunbury, F.; Salmon, D.L.; Smirnoff, N.; et al. Quantitative proteomics of a B12 -dependent alga grown in coculture with bacteria reveals metabolic tradeoffs required for mutualism. New Phytol. 2018, 217, 599–612. [Google Scholar] [CrossRef]
- Paerl, R.; Bertrand, E.; Allen, A.; Palenik, B.; Azam, F. Vitamin B1 ecophysiology of marine picoeukaryotic algae: Strain-specific differences and a new role for bacteria in vitamin cycling. Limnol. Oceanogr. 2015, 60, 215–228. [Google Scholar] [CrossRef]
- Wagner-Döbler, I.; Ballhausen, B.; Berger, M.; Brinkhoff, T.; Buchholz, I.; Bunk, B.; Cypionka, H.; Daniel, R.; Drepper, T.; Gerdts, G. The complete genome sequence of the algal symbiont Dinoroseobacter shibae: A hitchhiker’s guide to life in the sea. ISME J. 2010, 4, 61–77. [Google Scholar] [CrossRef]
- Higgins, B.T.; Gennity, I.; Samra, S.; Kind, T.; Fiehn, O.; Vandergheynst, J.S. Cofactor symbiosis for enhanced algal growth, biofuel production, and wastewater treatment. Algal Res. 2016, 17, 308–315. [Google Scholar] [CrossRef]
- Palacios, O.A.; Bashan, Y.; Schmid, M.; Hartmann, A.; de-Bashan, L.E. Enhancement of thiamine release during synthetic mutualism between Chlorella sorokiniana and Azospirillum brasilense growing under stress conditions. J. Appl. Phycol. 2016, 28, 1521–1531. [Google Scholar] [CrossRef]
- Kazamia, E.; Helliwell, K.E.; Purton, S.; Smith, A.G. How mutualisms arise in phytoplankton communities: Building eco-evolutionary principles for aquatic microbes. Ecol. Lett. 2016, 19, 810–822. [Google Scholar] [CrossRef]
- Wan, M.; Jin, X.; Xia, J.; Rosenberg, J.N.; Yu, G.; Nie, Z.; Oyler, G.A.; Betenbaugh, M.J. The effect of iron on growth, lipid accumulation, and gene expression profile of the freshwater microalga Chlorella sorokiniana. Appl. Microbiol. Biotechnol. 2014, 98, 9473–9481. [Google Scholar] [CrossRef]
- Kramer, J.; Özkaya, Ö.; Kümmerli, R. Bacterial siderophores in community and host interactions. Nature Rev. Microbiol. 2020, 18, 152–163. [Google Scholar] [CrossRef]
- D’onofrio, A.; Crawford, J.M.; Stewart, E.J.; Witt, K.; Gavrish, E.; Epstein, S.; Clardy, J.; Lewis, K. Siderophores from neighboring organisms promote the growth of uncultured bacteria. Chem. Biol. 2010, 17, 254–264. [Google Scholar]
- Keshtacher-Liebso, E.; Hadar, Y.; Chen, Y. Oligotrophic bacteria enhance algal growth under iron-deficient conditions. Appl. Environ. Microbiol. 1995, 61, 2439–2441. [Google Scholar] [CrossRef] [PubMed]
- Le Chevanton, M.; Garnier, M.; Bougaran, G.; Schreiber, N.; Lukomska, E.; Bérard, J.B.; Fouilland, E.; Bernard, O.; Cadoret, J.P. Screening and selection of growth-promoting bacteria for Dunaliella cultures. Algal Res. 2013, 2, 212–222. [Google Scholar] [CrossRef]
- Amin, S.A.; Green, D.H.; Hart, M.C.; Küpper, F.C.; Sunda, W.G.; Carrano, C.J. Photolysis of iron–siderophore chelates promotes bacterial–algal mutualism. Proc. Natl. Acad. Sci. USA 2009, 106, 17071–17076. [Google Scholar] [CrossRef]
- Amin, S.; Hmelo, L.; Van Tol, H.; Durham, B.; Carlson, L.; Heal, K.; Morales, R.; Berthiaume, C.; Parke, R.M.; Djunaedi, B. Interaction and signaling between a cosmopolitan phytoplankton and associated bacteria. Nature 2015, 522, 98–101. [Google Scholar] [CrossRef] [PubMed]
- Palacios, O.A.; Gomez-Anduro, G.; Bashan, Y.; de-Bashan, L.E. Tryptophan, thiamine and indole-3-acetic acid exchange between Chlorella sorokiniana and the plant growth-promoting bacterium Azospirillum brasilense. FEMS Microbiol. Ecol. 2016, 92, fiw077. [Google Scholar] [CrossRef]
- Pagnussat, L.A.; Maroniche, G.; Curatti, L.; Creus, C. Auxin-dependent alleviation of oxidative stress and growth promotion of Scenedesmus obliquus C1S by Azospirillum brasilense. Algal Res. 2020, 47, 101839. [Google Scholar] [CrossRef]
- Palacios, O.A.; Lopez, B.R.; Bashan, Y.; de-Bashan, L.E. Early changes in nutritional conditions affect formation of synthetic mutualism between Chlorella sorokiniana and the bacterium Azospirillum brasilense. Microb. Ecol. 2019, 77, 980–992. [Google Scholar] [CrossRef] [PubMed]
- Choix, F.J.; López-Cisneros, C.G.; Méndez-Acosta, H.O. Azospirillum brasilense increases CO2 fixation on microalgae Scenedesmus obliquus, Chlorella vulgaris, and Chlamydomonas reinhardtii cultured on high CO2 concentrations. Microb. Ecol. 2018, 76, 430–442. [Google Scholar] [CrossRef] [PubMed]
- Meza, B.; de-Bashan, L.E.; Bashan, Y. Involvement of indole-3-acetic acid produced by Azospirillum brasilense in accumulating intracellular ammonium in Chlorella vulgaris. Res. Microbiol. 2015, 166, 72–83. [Google Scholar] [CrossRef]
- Choix, F.J.; Bashan, Y.; Mendoza, A.; de-Bashan, L.E. Enhanced activity of ADP glucose pyrophosphorylase and formation of starch induced by Azospirillum brasilense in Chlorella vulgaris. J. Biotechnol. 2014, 177, 22–34. [Google Scholar] [CrossRef]
- Leyva, L.A.; Bashan, Y.; de-Bashan, L.E. Activity of acetyl-CoA carboxylase is not directly linked to accumulation of lipids when Chlorella vulgaris is co-immobilised with Azospirillum brasilense in alginate under autotrophic and heterotrophic conditions. Ann. Microbiol. 2015, 65, 339–349. [Google Scholar] [CrossRef]
- Peng, H.; de-Bashan, L.E.; Bashan, Y.; Higgins, B.T. Indole-3-acetic acid from Azosprillum brasilense promotes growth in green algae at the expense of energy storage products. Algal Res. 2020, 47, 101845. [Google Scholar] [CrossRef]
- Park, Y.; Je, K.-W.; Lee, K.; Jung, S.-E.; Choi, T.-J. Growth promotion of Chlorella ellipsoidea by co-inoculation with Brevundimonas sp. isolated from the microalga. Hydrobiologia 2008, 598, 219–228. [Google Scholar] [CrossRef]
- Park, J.; Park, B.S.; Wang, P.; Patidar, S.K.; Kim, J.H.; Kim, S.-H.; Han, M.-S. Phycospheric native bacteria Pelagibaca bermudensis and Stappia sp. ameliorate biomass productivity of Tetraselmis striata (KCTC1432BP) in co-cultivation system through mutualistic interaction. Front. Plant Sci. 2017, 8, 289. [Google Scholar] [CrossRef]
- Choix, F.J.; de-Bashan, L.E.; Bashan, Y. Enhanced accumulation of starch and total carbohydrates in alginate-immobilized Chlorella spp. induced by Azospirillum brasilense: I. Autotrophic conditions. Enzym. Microb. Technol. 2012, 51, 294–299. [Google Scholar] [CrossRef]
- Hernandez, J.-P.; de-Bashan, L.E.; Rodriguez, D.J.; Rodriguez, Y.; Bashan, Y. Growth promotion of the freshwater microalga Chlorella vulgaris by the nitrogen-fixing, plant growth-promoting bacterium Bacillus pumilus from arid zone soils. Eur. J. Soil Biol. 2009, 45, 88–93. [Google Scholar] [CrossRef]
- Higgins, B.T.; Vandergheynst, J.S. Effects of Escherichia coli on mixotrophic growth of Chlorella minutissima and production of biofuel precursors. PLoS ONE 2014, 9, e96807. [Google Scholar] [CrossRef] [PubMed]
- Do Nascimento, M.; De Los Angele, S.; Dublan, M.; Ortiz-Marquez, J.C.F.; Curatti, L. High lipid productivity of an Ankistrodesmus–Rhizobium artificial consortium. Bioresour. Technol. 2013, 146, 400–407. [Google Scholar] [CrossRef]
- Xue, L.; Shang, H.; Ma, P.; Wang, X.; He, X.; Niu, J.; Wu, J. Analysis of growth and lipid production characteristics of Chlorella vulgaris in artificially constructed consortia with symbiotic bacteria. J. Basic Microbiol. 2018, 58, 358–367. [Google Scholar] [CrossRef] [PubMed]
- de-Bashan, L.E.; Bashan, Y.; Moreno, M.; Lebsky, V.K.; Bustillos, J.J. Increased pigment and lipid content, lipid variety, and cell and population size of the microalgae Chlorella spp. when co-immobilized in alginate beads with the microalgae-growth-promoting bacterium Azospirillum brasilense. Can. J. Microbiol. 2002, 48, 514–521. [Google Scholar] [CrossRef] [PubMed]
- Paul, C.; Mausz, M.A.; Pohnert, G. A co-culturing/metabolomics approach to investigate chemically mediated interactions of planktonic organisms reveals influence of bacteria on diatom metabolism. Metabolomics 2013, 9, 349–359. [Google Scholar] [CrossRef]
- Vuong, T.T.; Kwon, B.-R.; Eom, J.-I.; Shin, B.-K.; Kim, S.M. Interaction between marine bacterium Stappia sp. K01 and diatom Phaeodactylum tricornutum through extracellular fatty acids. J. Appl. Phycol. 2019, 32, 71–82. [Google Scholar] [CrossRef]
- Sandhya, S.; Vijayan, K. Symbiotic association among marine microalgae and bacterial flora: A study with special reference to commercially important Isochrysis galbana culture. J. Appl. Phycol. 2019, 31, 2259–2266. [Google Scholar] [CrossRef]
- Choix, F.J.; de-Bashan, L.E.; Bashan, Y. Enhanced accumulation of starch and total carbohydrates in alginate-immobilized Chlorella spp. induced by Azospirillum brasilense: II. Heterotrophic conditions. Enzym. Microb. Technol. 2012, 51, 300–309. [Google Scholar] [CrossRef]
- Lee, C.; Jeon, M.S.; Kim, J.Y.; Lee, S.H.; Kim, D.G.; Roh, S.W.; Choi, Y.-E. Effects of an auxin-producing symbiotic bacterium on cell growth of the microalga Haematococcus pluvialis: Elevation of cell density and prolongation of exponential stage. Algal Res. 2019, 41, 101547. [Google Scholar] [CrossRef]
- Cho, D.H.; Ramanan, R.; Heo, J.; Lee, J.; Kim, B.H.; OH, H.M.; Kim, H.S. Enhancing microalgal biomass productivity by engineering a microalgal-bacterial community. Bioresour. Technol. 2015, 175, 578–585. [Google Scholar] [CrossRef] [PubMed]
- Hirayama, K. Application of a growth-promoting bacteria for stable mass culture of three marine microalgae. In Live Food in Aquaculture; Springer: Dordrecht, The Netherlands, 1997; pp. 223–230. [Google Scholar]
- Xu, L.; Cheng, X.; Wang, Q. Enhanced lipid production in Chlamydomonas reinhardtii by co-culturing with Azotobacter chroococcum. Front Plant Sci. 2018, 9, 741. [Google Scholar] [CrossRef] [PubMed]
- de-Bashan, L.E.; Schmid, M.; Rothballer, M.; Hartmann, A.; Bashan, Y. Cell-cell interaction in the eukaryote-prokaryote model of the microalgae Chlorella vulgaris and the bacterium Azospirillum brasilense immobilized in polymer beads. J. Phycol. 2011, 47, 1350–1359. [Google Scholar] [CrossRef] [PubMed]
- Chorazyczewski, A.M.; Huang, I.S.; Abdulla, H.; Mayali, X.; Zimba, P.V. The influence of bacteria on the growth, lipid production, and extracellular metabolite accumulation by Phaeodactylum tricornutum (Bacillariophyceae). J. Phycol. 2021. [Google Scholar] [CrossRef]
- Rasouli, Z.; Valverde-Pérez, B.; D’este, M.; De Francisci, D.; Angelidaki, I. Nutrient recovery from industrial wastewater as single cell protein by a co-culture of green microalgae and methanotrophs. Biochem. Eng. J. 2018, 134, 129–135. [Google Scholar] [CrossRef]
- Gonzalez, L.E.; Bashan, Y. Increased growth of the microalga Chlorella vulgaris when coimmobilized and cocultured in alginate beads with the plant-growth-promoting bacterium Azospirillum brasilense. Appl. Environ. Microbiol. 2000, 66, 1527–1531. [Google Scholar] [CrossRef]
- Gonzalez-Bashan, L.E.; Lebsky, V.K.; Hernandez, J.P.; Bustillos, J.J.; Bashan, Y. Changes in the metabolism of the microalga Chlorella vulgaris when coimmobilized in alginate with the nitrogen-fixing Phyllobacterium myrsinacearum. Can. J. Microbiol. 2000, 46, 653–659. [Google Scholar] [CrossRef]
- Sen, T.; Barrow, C.J.; Deshmukh, S.K. Microbial Pigments in the Food Industry—Challenges and the Way Forward. Front. Nutr. 2019, 6, 7. [Google Scholar] [CrossRef]
- Da Silva, T.L.; Gouveia, L.; Reis, A. Integrated microbial processes for biofuels and high value-added products: The way to improve the cost effectiveness of biofuel production. Appl. Microbiol. Biotechnol. 2014, 98, 1043–1053. [Google Scholar] [CrossRef]
- Kulkarni, S.; Nikolov, Z. Process for selective extraction of pigments and functional proteins from Chlorella vulgaris. Algal Res. 2018, 35, 185–193. [Google Scholar] [CrossRef]
- Monte, J.; Ribeiro, C.; Parreira, C.; Costa, L.; Brive, L.; Casal, S.; Brazinha, C.; Crespo, J.G. Biorefinery of Dunaliella salina: Sustainable recovery of carotenoids, polar lipids and glycerol. Bioresour. Technol. 2020, 297, 122509. [Google Scholar] [CrossRef] [PubMed]
- Gifuni, I.; Pollio, A.; Safi, C.; Marzocchella, A.; Olivieri, G. Current bottlenecks and challenges of the microalgal biorefinery. Trends Biotechnol. 2019, 37, 242–252. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ho, S.-H.; Cheng, C.-L.; Guo, W.-Q.; Nagarajan, D.; Ren, N.-Q.; Lee, D.-J.; Chang, J.-S. Perspectives on the feasibility of using microalgae for industrial wastewater treatment. Bioresour. Technol. 2016, 222, 485–497. [Google Scholar] [CrossRef]
- Safafar, H.; Van Wagenen, J.; Møller, P.; Jacobsen, C. Carotenoids, phenolic compounds and tocopherols contribute to the antioxidative properties of some microalgae species grown on industrial wastewater. Mar. Drugs 2015, 13, 7339–7356. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Zhang, P.; Zhang, G.; Peng, M. Biomass and pigments production in photosynthetic bacteria wastewater treatment: Effects of photoperiod. Bioresour. Technol. 2015, 190, 196–200. [Google Scholar] [CrossRef]
- Zhou, Q.; Zhang, P.; Zhang, G. Biomass and carotenoid production in photosynthetic bacteria wastewater treatment: Effects of light intensity. Bioresour. Technol. 2014, 171, 330–335. [Google Scholar] [CrossRef]
- Paddock, M.B.; Fernández-Bayo, J.D.; Vandergheynst, J.S. The effect of the microalgae-bacteria microbiome on wastewater treatment and biomass production. Appl. Microbiol. Biotechnol. 2020, 104, 893–905. [Google Scholar] [CrossRef]
- Solimeno, A.; García, J. Microalgae-bacteria models evolution: From microalgae steady-state to integrated microalgae-bacteria wastewater treatment models–a comparative review. Sci. Total Environ. 2017, 607, 1136–1150. [Google Scholar] [CrossRef]
- Jia, H.; Yuan, Q. Removal of nitrogen from wastewater using microalgae and microalgae–bacteria consortia. Cogent Environ. Sci. 2016, 2, 1275089. [Google Scholar] [CrossRef]
- Makut, B.B.; Das, D.; Goswami, G. Production of microbial biomass feedstock via co-cultivation of microalgae-bacteria consortium coupled with effective wastewater treatment: A sustainable approach. Algal Res. 2019, 37, 228–239. [Google Scholar] [CrossRef]
- Tighiri, H.O.; Erkurt, E.A. Biotreatment of landfill leachate by microalgae-bacteria consortium in sequencing batch mode and product utilization. Bioresour. Technol. 2019, 286, 121396. [Google Scholar] [CrossRef] [PubMed]
- Bélanger-Lépine, F.; Tremblay, A.; Huot, Y.; Barnabé, S. Cultivation of an algae-bacteria consortium in wastewater from an industrial park: Effect of environmental stress and nutrient deficiency on lipid production. Bioresour. Technol. 2018, 267, 657–665. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Hu, Z.; Qi, Y.; Song, C.; Chen, G. The interactions of algae-activated sludge symbiotic system and its effects on wastewater treatment and lipid accumulation. Bioresour. Technol. 2019, 292, 122017. [Google Scholar] [CrossRef] [PubMed]
- González-González, L.M.; Astals, S.; Pratt, S.; Jensen, P.D.; Schenk, P.M. Osmotic shock pre-treatment of Chaetoceros muelleri wet biomass enhanced solvent-free lipid extraction and biogas production. Algal Res. 2021, 54, 102177. [Google Scholar] [CrossRef]
- Sánchez-Bayo, A.; López-Chicharro, D.; Morales, V.; Espada, J.J.; Puyol, D.; Martínez, F.; Astals, S.; Vicente, G.; Bautista, L.F.; Rodríguez, R. Biodiesel and biogas production from Isochrysis galbana using dry and wet lipid extraction: A biorefinery approach. Renew. Energy 2020, 146, 188–195. [Google Scholar] [CrossRef]
- Sposob, M.; Kim, D.-H.; Yun, G.-S.; Yun, Y.-M. Assessment of the relationship between solubilization and biogas production on anaerobic digestion of pretreated lipid-extracted microalgae waste. Biomass Bioenergy 2020, 141, 105702. [Google Scholar] [CrossRef]
- Kim, E.J.; Kim, S.; Choi, H.-G.; Han, S.J. Co-production of biodiesel and bioethanol using psychrophilic microalga Chlamydomonas sp. KNM0029C isolated from Arctic sea ice. Biotechnol. Biofuels 2020, 13, 1–13. [Google Scholar] [CrossRef]
- Özçimen, D.; Koçer, A.T.; İnan, B.; Özer, T. Bioethanol production from microalgae. In Handbook of Microalgae-Based Processes and Products; Elsevier: Amsterdam, The Netherlands, 2020. [Google Scholar]
- Raheem, A.; Cui, X.; Mangi, F.H.; Memon, A.A.; Ji, G.; Cheng, B.; Dong, W.; Zhao, M. Hydrogen-rich energy recovery from microalgae (lipid-extracted) via steam catalytic gasification. Algal Res. 2020, 52, 102102. [Google Scholar] [CrossRef]
- Kumar, A.N.; Min, B.; Mohan, S.V. Defatted algal biomass as feedstock for short chain carboxylic acids and biohydrogen production in the biorefinery format. Bioresour. Technol. 2018, 269, 408–416. [Google Scholar] [CrossRef]
- Nobre, B.P.; Villalobos, F.; Barragan, B.E.; Oliveira, A.; Batista, A.P.; Marques, P.; Mendes, R.L.; Sovová, H.; Palavra, A.F.; Gouveia, L. A biorefinery from Nannochloropsis sp. microalga–extraction of oils and pigments. Production of biohydrogen from the leftover biomass. Bioresour. Technol. 2013, 135, 128–136. [Google Scholar] [CrossRef]
- Lakatos, G.; Deák, Z.; Vass, I.; Rétfalvi, T.; Rozgonyi, S.; Rákhely, G.; Ördög, V.; Kondorosi, É.; Maróti, G. Bacterial symbionts enhance photo-fermentative hydrogen evolution of Chlamydomonas algae. Green Chem. 2014, 16, 4716–4727. [Google Scholar] [CrossRef]
- Ferreira, G.; Pinto, L.R.; Maciel Filho, R.; Fregolente, L. A review on lipid production from microalgae: Association between cultivation using waste streams and fatty acid profiles. Renew. Sustain. Energy Rev. 2019, 109, 448–466. [Google Scholar] [CrossRef]
- Aketo, T.; Hoshikawa, Y.; Nojima, D.; Yabu, Y.; Maeda, Y.; Yoshino, T.; Takano, H.; Tanaka, T. Selection and characterization of microalgae with potential for nutrient removal from municipal wastewater and simultaneous lipid production. J. Biosci. Bioeng. 2020, 129, 565–572. [Google Scholar] [CrossRef]
- Molinuevo-Salces, B.; Mahdy, A.; Ballesteros, M.; González-Fernández, C. From piggery wastewater nutrients to biogas: Microalgae biomass revalorization through anaerobic digestion. Renew. Energy 2016, 96, 1103–1110. [Google Scholar] [CrossRef]
- González-González, L.M.; Astals, S.; Pratt, S.; Jensen, P.D.; Schenk, P.M. Impact of osmotic shock pre-treatment on microalgae lipid extraction and subsequent methane production. Bioresour. Technol. Rep. 2019, 7, 100214. [Google Scholar] [CrossRef]
- Bohutskyi, P.; Phan, D.; Spierling, R.E.; Kopachevsky, A.M.; Bouwer, E.J.; Lundquist, T.J.; Betenbaugh, M.J. Production of lipid-containing algal-bacterial polyculture in wastewater and biomethanation of lipid extracted residues: Enhancing methane yield through hydrothermal pretreatment and relieving solvent toxicity through co-digestion. Sci. Total Environ. 2019, 653, 1377–1394. [Google Scholar] [CrossRef] [PubMed]
- González-González, L.M.; Correa, D.F.; Ryan, S.; Jensen, P.D.; Pratt, S.; Schenk, P.M. Integrated biodiesel and biogas production from microalgae: Towards a sustainable closed loop through nutrient recycling. Renew. Sustain. Energy Rev. 2018, 82, 1137–1148. [Google Scholar] [CrossRef]
- Koutra, E.; Economou, C.N.; Tsafrakidou, P.; Kornaros, M. Bio-based products from microalgae cultivated in digestates. Trends Biotechnol. 2018, 36, 819–833. [Google Scholar] [CrossRef] [PubMed]
- González-Fernández, C.; Sialve, B.; Bernet, N.; Steyer, J.P. Impact of microalgae characteristics on their conversion to biofuel. Part II: Focus on biomethane production. Biofuels Bioprod. Biorefining 2012, 6, 205–218. [Google Scholar] [CrossRef]
- Ghasemi Naghdi, F.; González González, L.M.; Chan, W.; Schenk, P.M. Progress on lipid extraction from wet algal biomass for biodiesel production. Microb. Biotechnol. 2016, 9, 718–726. [Google Scholar] [CrossRef]
- Gerardo, M.L.; Van Den Hende, S.; Vervaeren, H.; Coward, T.; Skill, S.C. Harvesting of microalgae within a biorefinery approach: A review of the developments and case studies from pilot-plants. Algal Res. 2015, 11, 248–262. [Google Scholar] [CrossRef]
- Powell, R.J.; Hill, R.T. Rapid aggregation of biofuel-producing algae by the bacterium Bacillus sp. strain RP1137. Appl. Environ. Microbiol. 2013, 79, 6093–6101. [Google Scholar] [CrossRef]
- Wang, H.; Laughinghouse, H.D.; Anderson, M.A.; Chen, F.; Willliams, E.; Place, A.R.; Zmora, O.; Zohar, Y.; Zheng, T.; Hill, R.T. Novel bacterial isolate from Permian groundwater, capable of aggregating potential biofuel-producing microalga Nannochloropsis oceanica IMET1. Appl. Environ. Microbiol. 2012, 78, 1445–1453. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Cho, D.-H.; Ramanan, R.; Kim, B.-H.; Oh, H.-M.; Kim, H.-S. Microalgae-associated bacteria play a key role in the flocculation of Chlorella vulgaris. Bioresour. Technol. 2013, 131, 195–201. [Google Scholar] [CrossRef]
- Guo, H.; Chen, H.; Fan, L.; Linklater, A.; Zheng, B.; Jiang, D.; Qin, W. Enzymes produced by biomass-degrading bacteria can efficiently hydrolyze algal cell walls and facilitate lipid extraction. Renew. Energy 2017, 109, 195–201. [Google Scholar] [CrossRef]
- Chen, C.-Y.; Bai, M.-D.; Chang, J.-S. Improving microalgal oil collecting efficiency by pretreating the microalgal cell wall with destructive bacteria. Biochem. Eng. J. 2013, 81, 170–176. [Google Scholar] [CrossRef]
- Maurya, R.; Paliwal, C.; Ghosh, T.; Pancha, I.; Chokshi, K.; Mitra, M.; Ghosh, A.; Mishra, S. Applications of de-oiled microalgal biomass towards development of sustainable biorefinery. Bioresour. Technol. 2016, 214, 787–796. [Google Scholar] [CrossRef]
- Ferreira, A.; Ribeiro, B.; Ferreira, A.F.; Tavares, M.L.; Vladic, J.; Vidović, S.; Cvetkovic, D.; Melkonyan, L.; Avetisova, G.; Goginyan, V. Scenedesmus obliquus microalga-based biorefinery–from brewery effluent to bioactive compounds, biofuels and biofertilizers–aiming at a circular bioeconomy. Biofuels Bioprod. Biorefining 2019, 13, 1169–1186. [Google Scholar] [CrossRef]
- Silambarasan, S.; Logeswari, P.; Sivaramakrishnan, R.; Incharoensakdi, A.; Cornejo, P.; Kamaraj, B.; Chi, N.T.L. Removal of nutrients from domestic wastewater by microalgae coupled to lipid augmentation for biodiesel production and influence of deoiled algal biomass as biofertilizer for Solanum lycopersicum cultivation. Chemosphere 2020, 268, 129323. [Google Scholar] [CrossRef]
- Ronga, D.; Biazzi, E.; Parati, K.; Carminati, D.; Carminati, E.; Tava, A. Microalgal biostimulants and biofertilisers in crop productions. Agronomy 2019, 9, 192. [Google Scholar] [CrossRef]
- Renuka, N.; Guldhe, A.; Prasanna, R.; Singh, P.; Bux, F. Microalgae as multi-functional options in modern agriculture: Current trends, prospects and challenges. Biotechnol. Adv. 2018, 36, 1255–1273. [Google Scholar] [CrossRef]
- Vessey, J.K. Plant growth promoting rhizobacteria as biofertilizers. Plant Soil 2003, 255, 571–586. [Google Scholar] [CrossRef]
- Trejo, A.; de-Bashan, L.E.; Hartmann, A.; Hernandez, J.-P.; Rothballer, M.; Schmid, M.; Bashan, Y. Recycling waste debris of immobilized microalgae and plant growth-promoting bacteria from wastewater treatment as a resource to improve fertility of eroded desert soil. Environ. Exp. Bot. 2012, 75, 65–73. [Google Scholar] [CrossRef]
- Lopez, B.R.; Bashan, Y.; Trejo, A.; de-Bashan, L.E. Amendment of degraded desert soil with wastewater debris containing immobilized Chlorella sorokiniana and Azospirillum brasilense significantly modifies soil bacterial community structure, diversity, and richness. Biol. Fertil. Soils 2013, 49, 1053–1063. [Google Scholar] [CrossRef]
- Raposo, M.F.D.J.; Morais, R.M.S. Chlorella vulgaris as soil amendment: Influence of encapsulation and enrichment with rhizobacteria. Int. J. Agric. Biol. 2011, 13, 719–724. [Google Scholar]
- Hernández-Calderón, O.M.; Ponce-Ortega, J.M.; Ortiz-Del-Castillo, J.S.R.L.; Cervantes-Gaxiola, M.E.; Milán-Carrillo, J.; Serna-González, M.; Rubio-Castro, E. Optimal design of distributed algae-based biorefineries using CO2 emissions from multiple industrial plants. Ind. Eng. Chem. Res. 2016, 55, 2345–2358. [Google Scholar] [CrossRef]
- Jacob-Lopes, E.; Franco, T.T. Microalgae-based systems for carbon dioxide sequestration and industrial biorefineries. Biomass. Croat. Sciyo 2010, 2, 135–146. [Google Scholar]
- Nagarajan, D.; Lee, D.-J.; Chang, J.-S. Integration of anaerobic digestion and microalgal cultivation for digestate bioremediation and biogas upgrading. Bioresour. Technol. 2019, 290, 121804. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Su, Y. Process effect of microalgal-carbon dioxide fixation and biomass production: A review. Renew. Sustain. Energy Rev. 2014, 31, 121–132. [Google Scholar] [CrossRef]
- Sforza, E.; Barbera, E.; Girotto, F.; Cossu, R.; Bertucco, A. Anaerobic digestion of lipid-extracted microalgae: Enhancing nutrient recovery towards a closed loop recycling. Biochem. Eng. J. 2017, 121, 139–146. [Google Scholar] [CrossRef]
- Del Mar Morales-Amaral, M.; Gómez-Serrano, C.; Acién, F.G.; Fernández-Sevilla, J.M.; Molina-Grima, E. Production of microalgae using centrate from anaerobic digestion as the nutrient source. Algal Res. 2015, 9, 297–305. [Google Scholar] [CrossRef]
- González-González, L.M.; Eltanahy, E.; Schenk, P.M. Assessing the fertilizing potential of microalgal digestates using the marine diatom Chaetoceros muelleri. Algal Res. 2019, 41, 101534. [Google Scholar] [CrossRef]
- Barreiro, D.L.; Bauer, M.; Hornung, U.; Posten, C.; Kruse, A.; Prins, W. Cultivation of microalgae with recovered nutrients after hydrothermal liquefaction. Algal Res. 2015, 9, 99–106. [Google Scholar] [CrossRef]
- Biller, P.; Ross, A.B.; Skill, S.; Lea-Langton, A.; Balasundaram, B.; Hall, C.; Riley, R.; Llewellyn, C. Nutrient recycling of aqueous phase for microalgae cultivation from the hydrothermal liquefaction process. Algal Res. 2012, 1, 70–76. [Google Scholar] [CrossRef]
- Steffen, W.; Richardson, K.; Rockström, J.; Cornell, S.E.; Fetzer, I.; Bennett, E.M.; Biggs, R.; Carpenter, S.R.; De Vries, W.; De Wit, C.A. Planetary boundaries: Guiding human development on a changing planet. Science 2015, 347, 1259855. [Google Scholar] [CrossRef]
- Mccollum, D.L.; Echeverri, L.G.; Busch, S.; Pachauri, S.; Parkinson, S.; Rogelj, J.; Krey, V.; Minx, J.C.; Nilsson, M.; Stevance, A.-S. Connecting the sustainable development goals by their energy inter-linkages. Environ. Res. Lett. 2018, 13, 033006. [Google Scholar] [CrossRef]
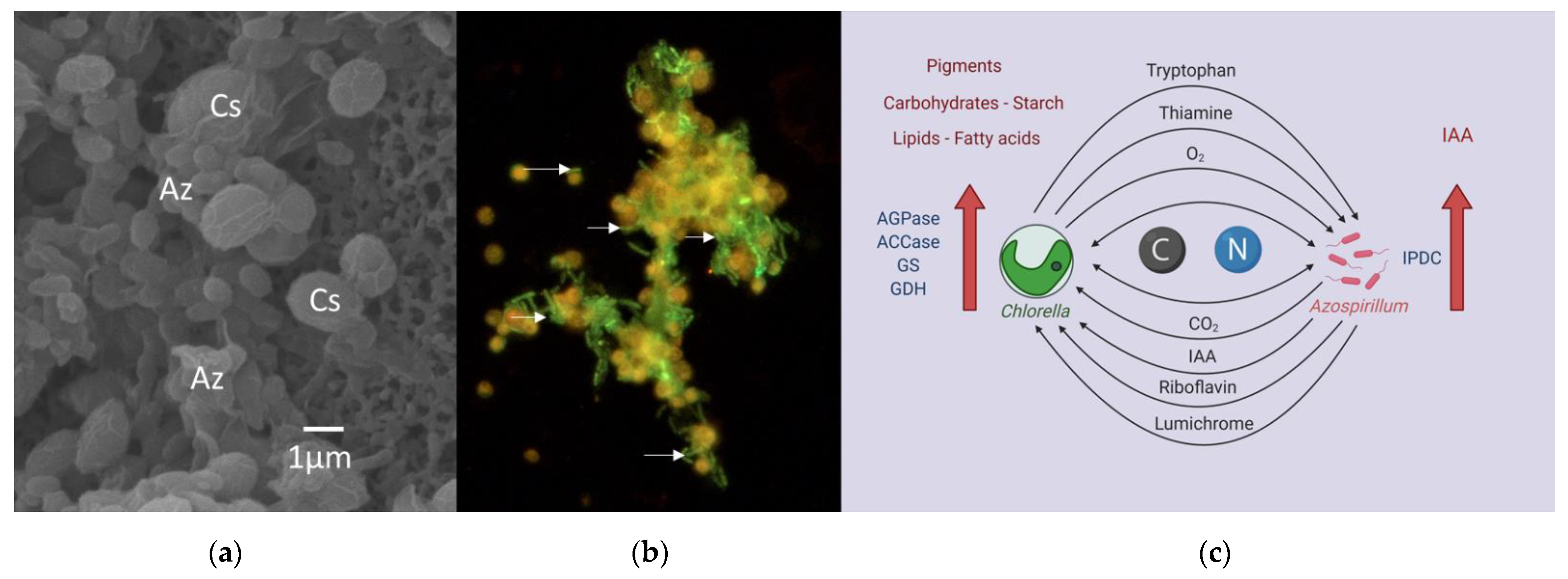
| Bacteria | Microalgae | Growth Promotion Effect | Culture Medium | Reference |
|---|---|---|---|---|
| Azospirillum brasilense Cd | Chlorella sorokiniana UTEX 2714 | 11% increase in cell density (g dw/L) | N8 medium | [67] |
| Azospirillum brasilense Cd | A. protothecoides UTEX 2341 | 90% increase in cell density (g dw/L) | N8-NH4 | |
| Brevundimonas sp. | Chlorella ellipsoidea UTEX 247 | 50-fold increase in cell density (cel/mL), longer exponential phase | Modified BBM | [68] |
| Pelagibaca bermudensis KCTC 13073BP | Tetraselmis striata KCTC1432BP | 2-fold increase in biomass productivity (mg/L/d) | O3 medium | [69] |
| Azospirillum brasilense Cd | Chlorella vulgaris UTEX 2714 | 16 and 11% increase in cell density (cel/mL) and growth rate, respectively | Synthetic growth medium (SGM) | [70] |
| Azospirillum brasilense Cd | Chlorella sorokiniana UTEX 2805 | 40 and 35% increase in cell density (cel/mL) and growth rate, respectively | ||
| Bacillus pumilus ES4 | Chlorella vulgaris UTEX 2714 | 1.5-fold increase in cell density (cel/mL) | N-free SGM | [71] |
| Escherichia coli ATCC 25922 | Chlorella minutissima UTEX 2341 | 3.5-fold biomass productivity (mg/L/d) | N8-NH4, 1% Glucose | [72] |
| 3.4-fold biomass productivity (mg/L/d) | N8-NH4, 1% Glycerol | |||
| 7.2-fold biomass productivity (mg/L/d) | N8-NH4, 1% Acetate | |||
| Rhizobium sp. 10II | Ankistrodesmus sp. SP2-15 | 29% increase in dry weight (mg/L) | BG11 medium | [73] |
| Stenotrophomona smaltophilia | Chlorella vulgaris | 22, 20, and 18% increase in biomass (g/L), growth rate and productivity (mg/L/d), respectively | BG11 medium | [74] |
| Azospirillum brasilense Cd | Chlorella vulgaris UTEX 395 | 62% increase in cell size | Synthetic wastewater | [75] |
| Azospirillum brasilense Cd | Chlorella vulgaris UTEX 2714 | 3-fold increase in cell density | ||
| Azospirillum brasilense Cd | Chlorella sorokiniana UTEX 1602 | 2.2-fold increase in cell density | ||
| Rhizobium sp. | Botryococcus braunii | 55% increase in optical density | Modified Jaworski medium | [33] |
| Muricauda sp. | Dunaliella sp. | 7% increase in cell biovolume | Modified Walne’s medium | [57] |
| Dinoroseobacter shibae | Thalassiosira pseudonana | 35% increase in cell density | SW+ medium | [76] |
| Phaeodactylum tricornutum | Stappia sp. | 72% increase in cell density | F/2 medium | [77] |
| Alteromonas sp. | Isochrysis galbana | 52% increase in cell density | Zobell Marine Broth | [78] |
| Labrenzia sp. | Isochrysis galbana | 71% increase in cell density |
| Bacteria | Microalgae | Metabolite Production Enhanced | Culture Medium | Reference |
|---|---|---|---|---|
| Escherichia coli ATCC 25922 | Chlorella minutissima UTEX 2341 | 6.2-fold lipid productivity (mg/L/d) | N8-NH4, 1% Glucose | [72] |
| 18.8-fold starch productivity (mg/L/d) | ||||
| 1.8-fold lipid content (%) | ||||
| 5.4-fold starch content (%) | ||||
| 3.1-fold lipid productivity (mg/L/d) | N8-NH4, 1% Glycerol | |||
| 9.9-fold starch productivity (mg/L/d) | ||||
| 2.9-fold starch content (%) | ||||
| 8.2-fold lipid productivity (mg/L/d) | N8-NH4, 1% Acetate | |||
| 27.1-fold starch productivity (mg/L/d) | ||||
| 3.7-fold starch content (%) | ||||
| Azotobacter chroococcum No 1.0233 | Chlamydomonas reinhardtii cc849 | 2.4-fold lipid content (%) | N-free TAP medium | [83] |
| 5.9-fold lipid production (mg/L) | ||||
| 19.4-fold lipid productivity (mg/L/d) | ||||
| Stenotrophomona smaltophilia | Chlorella vulgaris | Lipid increase by 8–34% | BG11 | [74] |
| Phaeodactylum tricornutum | Stappia sp. | 172% increase in fucoxanthin | F/2 medium | [77] |
| 144% increase in chlorophylls | ||||
| Phaeodactylum tricornutum | Marinobacter sp. | 50% increase in total lipids | F/2 medium | [85] |
| Rhizobium sp. 10II | Ankistrodesmus sp. SP2-15 | 39% increase in chlorophyll a | BG11 | [73] |
| Methylococcus capsulatus | Chlorella sorokiniana | 42% increase in carbohydrates | Industrial wastewater with synthetic biogas as methane source | [86] |
| Methylococcus capsulatus | Chlorella sorokiniana | 15% increase in lipid content | ||
| Azospirillum brasilense Cd | Chlorella sorokiniana UTEX 1602 | 1.6-fold chlorophyll a (µg/g cells) | Synthetic Wastewater | [75] |
| 1.6-fold chlorophyll b (µg/g cells) | ||||
| 1.7-fold lutein (µg/g cells) | ||||
| 2.5-fold violaxanthin (µg/g cells) | ||||
| 5.5-fold lipid content (µg/g dw) | ||||
| Azospirillum brasilense Cd | Chlorella vulgaris UTEX 395 | 1.6-fold chlorophyll a (µg/g cells) | ||
| 1.8-fold chlorophyll b (µg/g cells) | ||||
| 1.8-fold lipid content (µg/g dw) | ||||
| Azospirillum brasilense Cd | Chlorella vulgaris UTEX 2714 | 2.8-fold chlorophyll a (µg/g cells) | ||
| 2.5-fold chlorophyll b (µg/g cells) | ||||
| 2.3-fold lutein (µg/g cells) | ||||
| 1.5-fold violaxanthin (µg/g cells) | ||||
| 3.9-fold lipid content (µg/g dw) | ||||
| Azospirillum brasilense Cd | Chlorella vulgaris UTEX 2714 | 1.4-fold chlorophyll a (µg/g cells) | Synthetic Wastewater | [87] |
| 2.8-fold chlorophyll b (µg/g cells) | ||||
| 2.9-fold ß-carotene (µg/g cells) | ||||
| 2.5-fold lutein (µg/g cells) | ||||
| 2.3-fold violaxanthin (µg/g cells) | ||||
| Phyllobacterium myrsinacearum | Chlorella vulgaris UTEX 2714 | 1.8-fold chlorophyll b (µg/g cells) | Synthetic Wastewater | [88] |
| 1.8-fold ß-carotene (µg/g cells) | ||||
| 2-fold lutein (µg/g cells) | ||||
| 2.2-fold violaxanthin (µg/g cells) | ||||
| Azospirillum brasilense Cd | Chlorella sorokiniana UTEX 2714 | 3-fold chlorophyll a (µg/mg dw) | N8 medium | [67] |
| 5-fold chlorophyll b (µg/mg dw) | ||||
| 2.5-fold soluble protein (%) | ||||
| Azospirillum brasilense Cd | A. protothecoides UTEX 2341 | 40–60% increase in soluble protein | N8-NH4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-González, L.M.; de-Bashan, L.E. Toward the Enhancement of Microalgal Metabolite Production through Microalgae–Bacteria Consortia. Biology 2021, 10, 282. https://doi.org/10.3390/biology10040282
González-González LM, de-Bashan LE. Toward the Enhancement of Microalgal Metabolite Production through Microalgae–Bacteria Consortia. Biology. 2021; 10(4):282. https://doi.org/10.3390/biology10040282
Chicago/Turabian StyleGonzález-González, Lina Maria, and Luz E. de-Bashan. 2021. "Toward the Enhancement of Microalgal Metabolite Production through Microalgae–Bacteria Consortia" Biology 10, no. 4: 282. https://doi.org/10.3390/biology10040282
APA StyleGonzález-González, L. M., & de-Bashan, L. E. (2021). Toward the Enhancement of Microalgal Metabolite Production through Microalgae–Bacteria Consortia. Biology, 10(4), 282. https://doi.org/10.3390/biology10040282







