The Effects of Drought and Re-Watering on Non-Structural Carbohydrates of Pinus tabulaeformis Seedlings
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Drought Treatments
2.2. The Soil Water Content and Physiological Measurements
2.3. Non-Structural Carbohydrates Concentrations
2.4. Statistical Analyses
3. Results
3.1. Photosynthetic Characteristics and Biomass under Different Drought Treatments
3.2. The NSCs Concentrations in Different Organs during Drought and Re-Watering
3.2.1. The NSCs Concentrations in the Leaves
3.2.2. The NSCs Concentrations in the Twigs
3.2.3. The NSCs Concentrations in the Stems
3.2.4. The NSCs Concentrations in the Roots
4. Discussion
4.1. NSC Dynamics under Drought Conditions
4.2. NSCs Dynamics during Re-Watering
4.3. Limitations of the Experimental Methods
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kannenberg, S.A.; Schwalm, C.R.; Anderegg, W.R.L. Ghosts of the past: How drought legacy effects shape forest functioning and carbon cycling. Ecol. Lett. 2020, 23, 891–901. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Liu, H.; Qi, Y.; Liu, F.; Zhu, X. Patterns in nonstructural carbohydrate contents at the tree organ level in response to drought duration. Glob. Chang. Biol. 2020, 26, 3627–3638. [Google Scholar] [CrossRef]
- Sala, A.; Piper, F.; Hoch, G. Physiological mechanisms of drought-induced tree mortality are far from being resolved. New Phytol. 2010, 186, 274–281. [Google Scholar] [CrossRef]
- Mencuccini, M.; Holtta, T. The significance of phloem transport for the speed with which canopy photosynthesis and belowground respiration are linked. New Phytol. 2010, 185, 189–203. [Google Scholar] [CrossRef]
- Deng, X.; Joly, R.J.; Hahn, D.T. The influence of plant water deficit on photosynthesis and translocation of 14C-labeled assimilates in cacao seedlings. Physiol. Plantarum 1990, 78, 623–627. [Google Scholar] [CrossRef]
- Galiano, L.; Martínez-Vilalta, J.; Lloret, F. Carbon reserves and canopy defoliation determine the recovery of Scots pine 4 yr after a drought episode. New Phytol. 2011, 190, 750–759. [Google Scholar] [CrossRef]
- Mitchell, P.J.; O’Grady, A.P.; Tissue, D.T.; White, D.A.; Ottenschlaeger, M.L.; Pinkard, E.A. Drought response strategies define the relative contributions of hydraulic dysfunction and carbohydrate depletion during tree mortality. New Phytol. 2013, 197, 862–872. [Google Scholar] [CrossRef]
- Dietze, M.C.; Sala, A.; Carbone, M.S.; Czimczik, C.I.; Mantooth, J.A.; Richardson, A.D.; Vargas, R. Nonstructural carbon in woody plants. Annu. Rev. Plant Biol. 2014, 65, 667–687. [Google Scholar] [CrossRef]
- McDowell, N.G. Mechanisms linking drought, hydraulics, carbon metabolism, and vegetation mortality. Plant Physiol. 2011, 155, 1051–1059. [Google Scholar] [CrossRef] [PubMed]
- Dichio, B.; Margiotta, G.; Xiloyannis, C.; Bufo, S.A.; Sofo, A.; Cataldi, T.R.I. Changes in water status and osmolyte contents in leaves and roots of olive plants (Olea europaea L.) subjected to water deficit. Trees 2008, 23, 247–256. [Google Scholar] [CrossRef]
- Wang, Z.; Stutte, G.W. The Role of Carbohydrates in Active Osmotic Adjustment in Apple Under Water Stress. J. Am. Soc. Hortic. Sci. 1992, 117, 816–823. [Google Scholar] [CrossRef]
- Koppenaal, R.S.; Tschaplinski, T.J.; Colombo, S.J. Carbohydrate accumulation and turgor maintenance in seedling shoots and roots of two boreal conifers subjected to water stress. Can. J. Bot. 1991, 69, 2522–2528. [Google Scholar] [CrossRef]
- Kannenberg, S.A.; Phillips, R.P. Non-structural carbohydrate pools not linked to hydraulic strategies or carbon supply in tree saplings during severe drought and subsequent recovery. Tree Physiol. 2019, 40, 259–271. [Google Scholar] [CrossRef] [PubMed]
- Chapin, F.; Schulze, E.D.; Mooney, H.A. The ecology and economics of storage in plants. Annu. Rev. Ecol. Syst. 1990, 21, 423–447. [Google Scholar] [CrossRef]
- Lacointe, A. Carbon allocation among tree organs: A review of basic processes and representation infunctional-structural tree models. Ann. For. Sci. 2000, 57, 521–533. [Google Scholar] [CrossRef]
- Galvez, D.A.; Landhausser, S.M.; Tyree, M.T. Root carbon reserve dynamics in aspen seedlings: Does simulated drought induce reserve limitation? Tree Physiol. 2011, 31, 250–257. [Google Scholar] [CrossRef]
- Adams, H.D.; Germino, M.J.; Breshears, D.D.; Barron-Gafford, G.A.; Guardiola-Claramonte, M.; Zou, C.B.; Huxman, T.E. Nonstructural leaf carbohydrate dynamics of Pinus edulis during drought-induced tree mortality reveal role for carbon metabolism in mortality mechanism. New Phytol. 2013, 197, 1142–1151. [Google Scholar] [CrossRef]
- Gruber, A.; Pirkebner, D.; Florian, C.; Oberhuber, W. No evidence for depletion of carbohydrate pools in Scots pine (Pinus sylvestris L.) under drought stress. Plant Biol. 2012, 14, 142–148. [Google Scholar] [CrossRef]
- O’Brien, M.J.; Burslem, D.F.R.P.; Caduff, A.; Tay, J.; Hector, A. Contrasting nonstructural carbohydrate dynamics of tropical tree seedlings under water deficit and variability. New Phytol. 2015, 205, 1083–1094. [Google Scholar] [CrossRef]
- Woodruff, D.R. The impacts of water stress on phloem transport in Douglas-fir trees. Tree Physiol. 2013, 34, 5–14. [Google Scholar] [CrossRef]
- Klein, T.; Hoch, G.; Yakir, D.; Korner, C. Drought stress, growth and nonstructural carbohydrate dynamics of pine trees in a semi-arid forest. Tree Physiol. 2014, 34, 981–992. [Google Scholar] [CrossRef]
- McDowell, N.; Pockman, W.T.; Allen, C.D.; Breshears, D.D.; Cobb, N.; Kolb, T.; Plaut, J.; Sperry, J.; West, A.; Williams, D.G.; et al. Mechanisms of plant survival and mortality during drought: Why do some plants survive while others succumb to drought? New Phytol. 2008, 178, 719–739. [Google Scholar] [CrossRef]
- Hartmann, H.; Trumbore, S. Understanding the roles of nonstructural carbohydrates in forest trees—From what we can measure to what we want to know. New Phytol. 2016, 211, 386–403. [Google Scholar] [CrossRef] [PubMed]
- Ingrisch, J.; Bahn, M. Towards a Comparable Quantification of Resilience. Trends Ecol. Evol. 2018, 33, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Ruehr, N.K.; Grote, R.; Mayr, S.; Arneth, A. Beyond the extreme: Recovery of carbon and water relations in woody plants following heat and drought stress. Tree Physiol. 2019, 39, 1285–1299. [Google Scholar] [CrossRef] [PubMed]
- Chinese Academy of Sciences Editorial Board of Chinese Ethnography. Flora of China-Volume 7: Gymnospermae; Science Press: Beijing, China, 1978; p. 251. [Google Scholar]
- Qiao, W.; Xiumei, L.; Huatian, W.; Xiaopeng, M.; Guiping, C.; Jian, L.; Changbao, H. Effects of drought and waterlogging on growth and photosynthesis of potted young Pinus tabulaeformis Carr. Sci. Soil Water Conserv. 2015, 13, 40–47. (In Chinese) [Google Scholar]
- Na, L.; Guang, B.; Ming, B. Response Characteristics of Chinese Pine (Pinus tabulaeformis Carr.) Radial Growth to Climate and Drought Variability Reconstruction in Western Liaoning, Northeast China. Forests 2019, 10, 752–767. [Google Scholar]
- Hua, H.; Zongsuo, L.; Ruilian, H.; Peizhen, W. Growth and drought tolerance of Pinus tabulaeformis under water deficit. J. Northwest. For. Univ. 2004, 19, 1–4. (In Chinese) [Google Scholar]
- Wurth, M.K.; Pelaez-Riedl, S.; Wright, S.J.; Korner, C. Non-structural carbohydrate pools in a tropical forest. Oecologia 2005, 143, 11–24. [Google Scholar] [CrossRef]
- Myers, J.A.; Kitajima, K. Carbohydrate storage enhances seedlings shade and stress tolerance in a neotropical forest. J. Ecol. 2007, 95, 383–395. [Google Scholar] [CrossRef]
- Wilson, R.; Cataldo, A.; Andersen, C.P. Determination of total nonstructural carbohydrates in tree species by high-performance anion-exchange chromatography with pulsed amperometric detection. Can. J. For. Res. 1995, 25, 2022–2028. [Google Scholar] [CrossRef]
- Raessler, M.; Wissuwa, B.; Breul, A.; Unger, W.; Grimm, T. Chromatographic analysis of major nonstructural carbohydrates in several wood species—An analytical approach for higher accuracy of data. Anal. Methods 2010, 2, 532–538. [Google Scholar] [CrossRef]
- Quentin, A.G.; Pinkard, E.A.; Ryan, M.G.; Tissue, D.T.; Baggett, L.S.; Adams, H.D.; Maillard, P.; Marchand, J.; Landhäusser, S.M.; Lacointe, A.; et al. Non-structural carbohydrates in woody plants compared among laboratories. Tree Physiol. 2015, 35, 1146–1165. [Google Scholar] [CrossRef]
- Mauchly, J.W. Significance Test for Sphericity of a Normal n-Variate Distribution. Ann. Math. Stat. 1940, 11, 204–209. [Google Scholar] [CrossRef]
- Hartmann, H.; Ziegler, W.; Kolle, O.; Trumbore, S. Thirst beats hunger—Declining hydration during drought prevents carbon starvation in Norway spruce saplings. New Phytol. 2013, 200, 340–349. [Google Scholar] [CrossRef]
- Lemoine, R.; La Camera, S.; Atanassova, R.; Dedaldechamp, F.; Allario, T.; Pourtau, N.; Bonnemain, J.L.; Laloi, M.; Coutos-Thévenot, P.; Maurousset, L.; et al. Source-to-sink transport of sugar and regulation by environmental factors. Front. Plant Sci. 2013, 4, 272. [Google Scholar] [CrossRef]
- Brunner, I.; Herzog, C.; Dawes, M.A.; Arend, M.; Sperisen, C. How tree roots respond to drought. Front. Plant Sci. 2015, 6, 547–563. [Google Scholar] [CrossRef]
- Karst, J.; Gaster, J.; Wiley, E.; Landhausser, S.M. Stress differentially causes roots of tree seedlings to exude carbon. Tree Physiol. 2017, 37, 154–164. [Google Scholar] [CrossRef] [PubMed]
- Bin, Y.; Changhui, P.; Harrison, S.P.; Hua, W.; Han, W.; Qiuan, Z.; Meng, W. Allocation Mechanisms of Non-Structural Carbohydrates of Robinia pseudoacacia L. Seedlings in Response to Drought and Waterlogging. Forests 2018, 9, 754–773. [Google Scholar]
- Meir, P.; Metcalfe, D.B.; Costa, A.C.; Fisher, R.A. The fate of assimilated carbon during drought: Impacts on respiration in Amazon rainforests. Philos. Trans. R. Soc. B Biol. Sci. 2008, 363, 1849–1855. [Google Scholar] [CrossRef] [PubMed]
- McDowell, N.G.; Sevanto, S. The mechanisms of carbon starvation: How, when, or does it even occur at all? New Phytol. 2010, 186, 264–266. [Google Scholar] [CrossRef]
- Stitt, M.; Zeeman, S.C. Starch turnover: Pathways, regulation and role in growth. Curr. Opin. Plant Biol. 2012, 15, 282–292. [Google Scholar] [CrossRef]
- Pelleschi, S.; Rocher, J.P.; Prioul, J.L. Effect of water restriction on carbohydrate metabolism and photosynthesis in mature maize leaves. Plant Cell Environ. 1997, 20, 493–503. [Google Scholar] [CrossRef]
- Olesinski, J.; Lavigne, M.B.; Krasowski, M.J. Effects of soil moisture manipulations on fine root dynamics in a mature balsam fir (Abies balsamea L. Mill.) forest. Tree Physiol. 2011, 31, 339–348. [Google Scholar] [CrossRef]
- Montwé, D.; Spiecker, H.; Hamann, A. An experimentally controlled extreme drought in a Norway spruce forest reveals fast hydraulic response and subsequent recovery of growth rates. Trees 2014, 28, 891–900. [Google Scholar] [CrossRef]
- Sergent, A.S.; Rozenberg, P.; Bréda, N. Douglas-fir is vulnerable to exceptional and recurrent drought episodes and recovers less well on less fertile sites. Ann. For. Sci. 2012, 71, 697–708. [Google Scholar] [CrossRef]
- Anderegg, W.R.L.; Schwalm, C.; Biondi, F.; Camarero, J.J.; Koch, G.; Litvak, M.; Ogle, K.; Shaw, J.D.; Shevliakova, E.; Williams, A.P.; et al. Pervasive drought legacies in forest ecosystems and their implications for carbon cycle models. Science 2015, 349, 528–532. [Google Scholar] [CrossRef] [PubMed]
- Galiano, L.; Timofeeva, G.; Saurer, M.; Siegwolf, R.; Martinez-Vilalta, J.; Hommel, R.; Gessler, A. The fate of recently fixed carbon after drought release: Towards unravelling C storage regulation in Tilia platyphyllos and Pinus sylvestris. Plant Cell Environ. 2017, 40, 1711–1724. [Google Scholar] [CrossRef]
- Hagedorn, F.; Joseph, J.; Peter, M.; Luster, J.; Pritsch, K.; Geppert, U.; Kerner, R.; Molinier, V.; Egli, S.; Schaub, M.; et al. Recovery of trees from drought depends on belowground sink control. Nat. Plants 2016, 2, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Anderegg, W.R.L.; Berry, J.A.; Field, C.B. Linking definitions, mechanisms, and modeling of drought-induced tree death. Trends Plant Sci. 2012, 17, 693–700. [Google Scholar] [CrossRef]
- Hartmann, H.; Moura, C.F.; Anderegg, W.R.L.; Ruehr, N.K.; Salmon, Y.; Allen, C.D.; Arndt, S.K.; Breshears, D.D.; Davi, H.; Galbraith, D.; et al. Research frontiers for improving our understanding of drought-induced tree and forest mortality. New Phytol. 2018, 218, 15–28. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, H.; Ziegler, W.; Trumbore, S. Lethal drought leads to reduction in nonstructural carbohydrates in Norway spruce tree roots but not in the canopy. Funct. Ecol. 2013, 27, 413–427. [Google Scholar] [CrossRef]
- Qingpeng, Y.; Weidong, Z.; Renshan, L.; Ming, X.; Silong, W. Different responses of non-structural carbohydrates in above-ground tissues/organs and root to extreme drought and re-watering in Chinese fir (Cunninghamia lanceolata) saplings. Trees 2016, 30, 1863–1871. [Google Scholar]
- Wiley, E.; Casper, B.B.; Helliker, B.R. Recovery following defoliation involves shifts in allocation that favour storage and reproduction over radial growth in black oak. J. Ecol. 2017, 105, 412–424. [Google Scholar] [CrossRef]
- Wiley, E.; Helliker, B. A re-evaluation of carbon storage in trees lends greater support for carbon limitation to growth. New Phytol. 2012, 195, 285–289. [Google Scholar] [CrossRef]
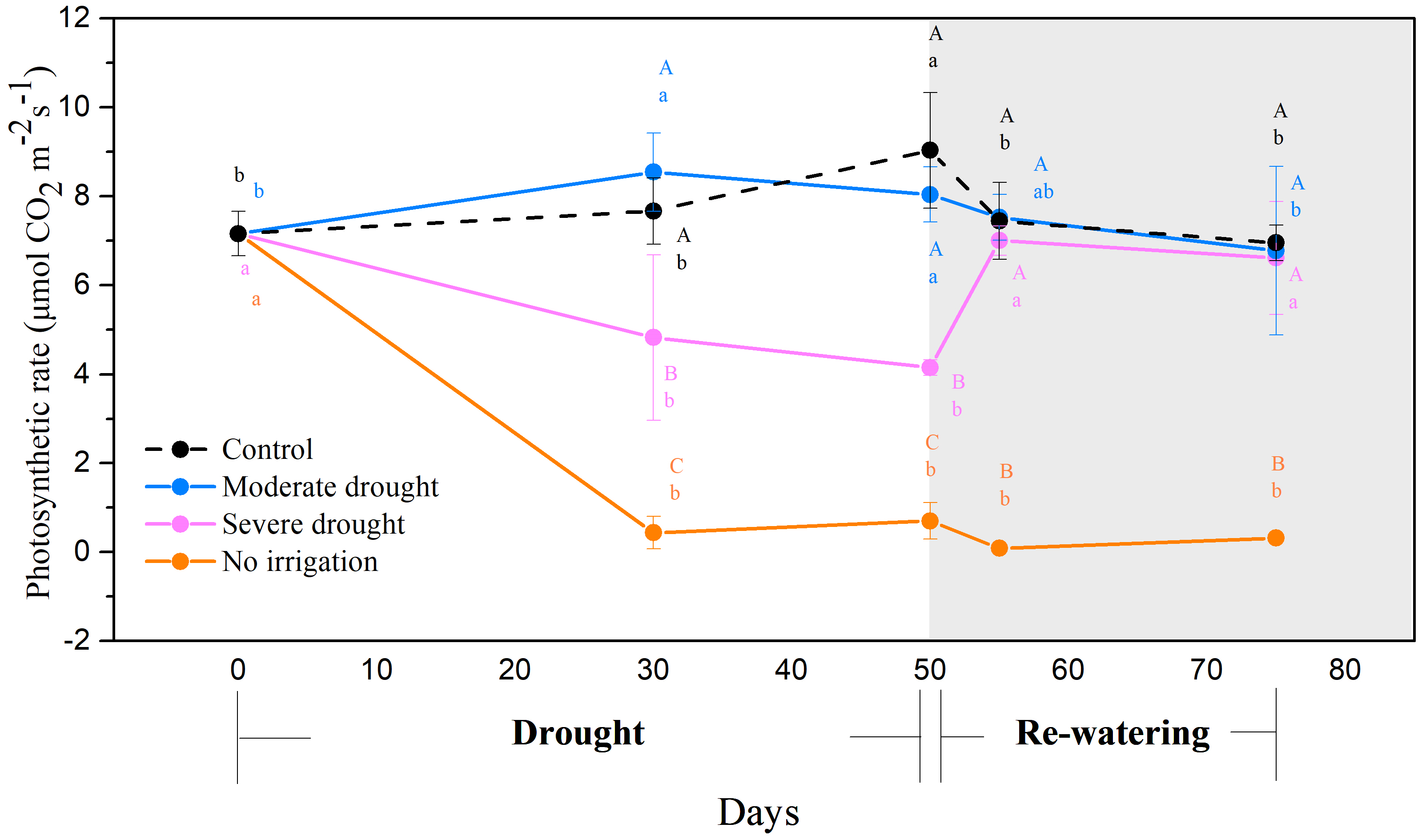
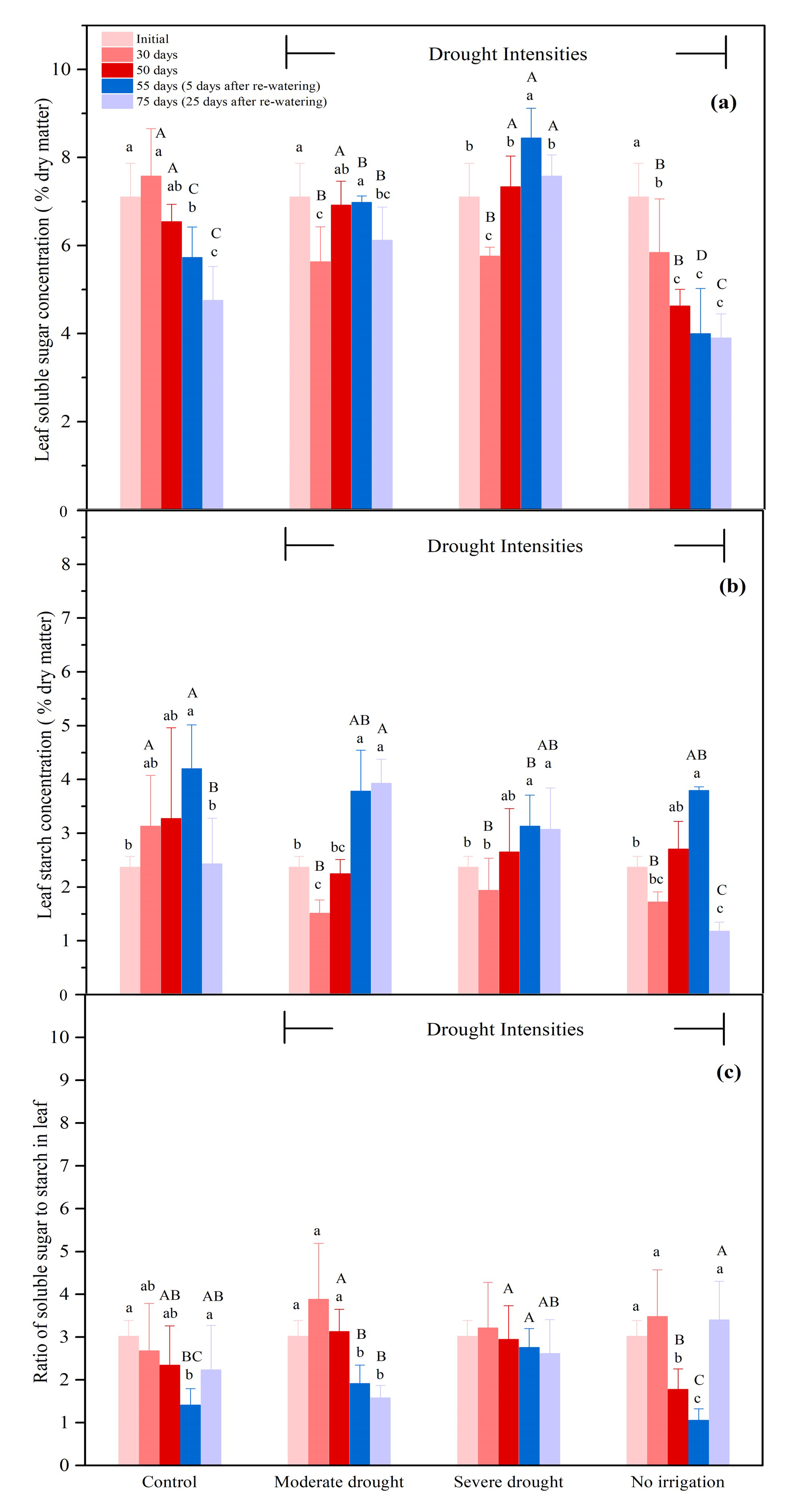

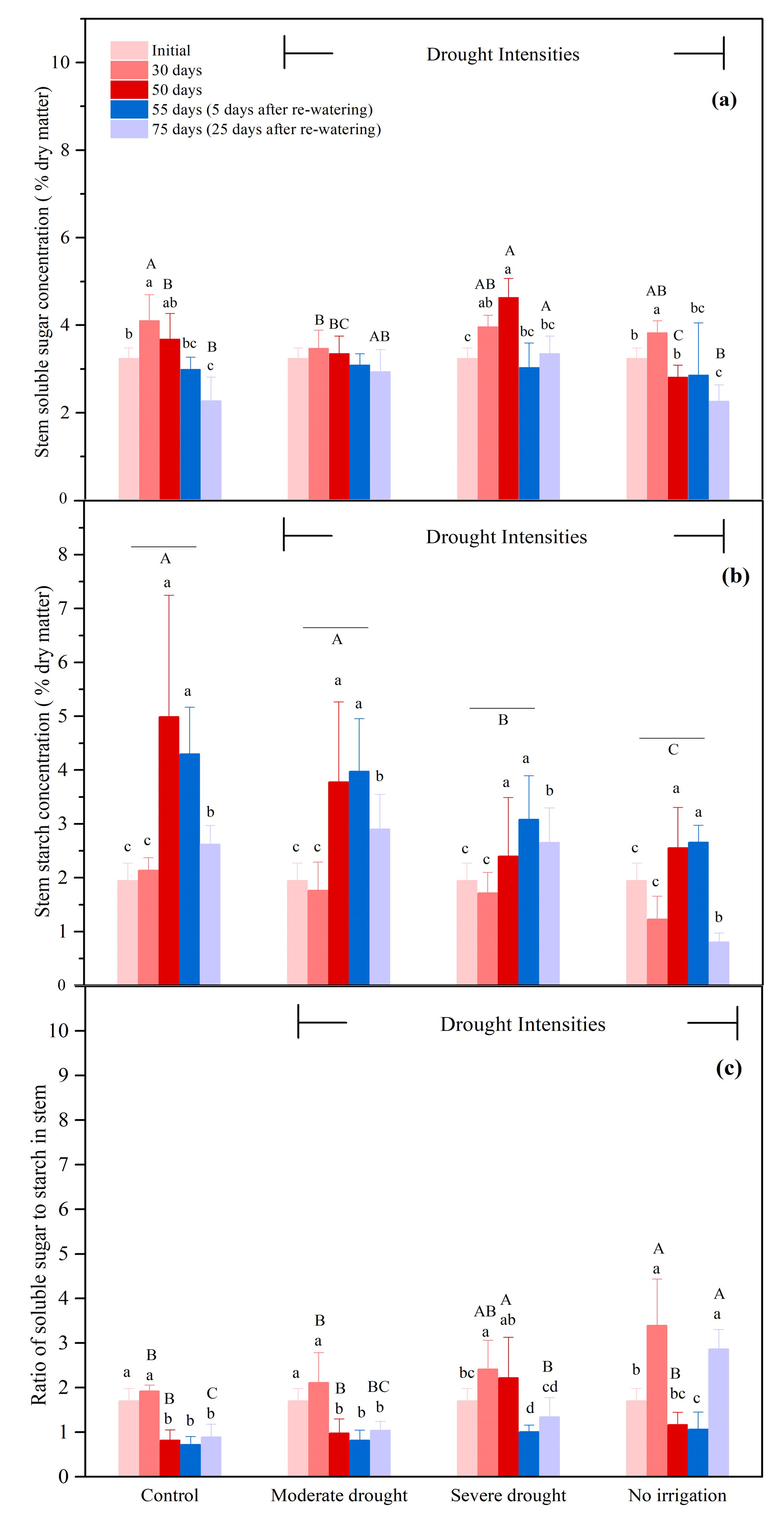
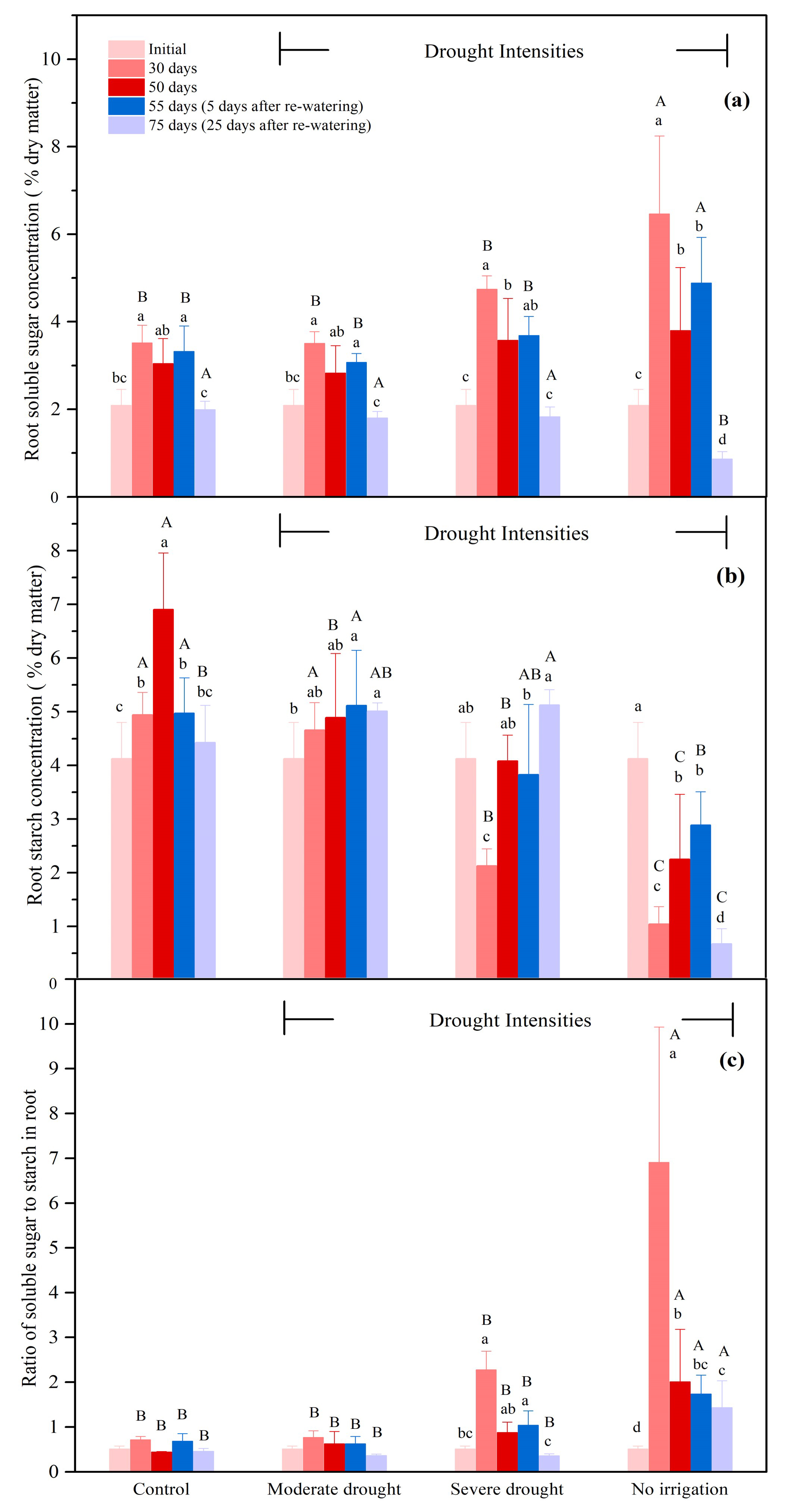
| Organ | Treatment | 0 Day (Initial) | 50 Days | 75 Days (25 Days after Re-Watering) |
|---|---|---|---|---|
| Leaf | Control | 13.898(3.538) A c | 17.653(1.867) AB b | 23.620(3.108) A a |
| Moderate drought | 13.898(3.538) A b | 12.994(1.459) BC b | 24.151(4.801) A a | |
| Severe drought | 13.898(3.538) A b | 20.978(5.717) A a | 16.579(1.837) B ab | |
| No irrigation | 13.898(3.538) A a | 12.194(2.672) C a | 12.271(2.022) B a | |
| Twig | Control | 1.593(1.009) A b | 2.457(1.070) A a | 3.923(0.568) A a |
| Moderate drought | 1.593(1.009) A b | 2.833(0.676) A a | 3.013(0.693) A a | |
| Severe drought | 1.593(1.009) AB b | 3.037(0.983) AB a | 2.660(0.617) AB a | |
| No irrigation | 1.593(1.009) A b | 1.583(0.406) A a | 1.950(0.310) A a | |
| Stem | Control | 15.777(6.436) A a | 19.243(3.113) A a | 19.523(0.846) A a |
| Moderate drought | 15.777(6.436) A a | 17.837(6.898) A a | 16.780(2.973) A a | |
| Severe drought | 15.777(6.436) A a | 17.260(0.877) A a | 15.477(5.413) A a | |
| No irrigation | 15.777(6.436) A a | 16.467(3.974) A a | 16.167(1.685) A a | |
| Root | Control | 9.349(1.239) A b | 9.342(0.729) A b | 12.926(1.099) A a |
| Moderate drought | 9.349(1.239) A b | 11.204(5.173) A ab | 12.250(1.319) A a | |
| Severe drought | 9.349(1.239) A a | 8.977(1.889) A a | 8.566(0.887) B a | |
| No irrigation | 9.349(1.239) A a | 7.020(1.051) A b | 7.538(1.486) B b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, X.; Peng, C.; Li, T.; Huang, J.; Song, H.; Zhu, Q.; Wang, M. The Effects of Drought and Re-Watering on Non-Structural Carbohydrates of Pinus tabulaeformis Seedlings. Biology 2021, 10, 281. https://doi.org/10.3390/biology10040281
Guo X, Peng C, Li T, Huang J, Song H, Zhu Q, Wang M. The Effects of Drought and Re-Watering on Non-Structural Carbohydrates of Pinus tabulaeformis Seedlings. Biology. 2021; 10(4):281. https://doi.org/10.3390/biology10040281
Chicago/Turabian StyleGuo, Xinyi, Changhui Peng, Tong Li, Jingjing Huang, Hanxiong Song, Qiuan Zhu, and Meng Wang. 2021. "The Effects of Drought and Re-Watering on Non-Structural Carbohydrates of Pinus tabulaeformis Seedlings" Biology 10, no. 4: 281. https://doi.org/10.3390/biology10040281
APA StyleGuo, X., Peng, C., Li, T., Huang, J., Song, H., Zhu, Q., & Wang, M. (2021). The Effects of Drought and Re-Watering on Non-Structural Carbohydrates of Pinus tabulaeformis Seedlings. Biology, 10(4), 281. https://doi.org/10.3390/biology10040281







