Effect of Physical Exercise Program Based on Active Breaks on Physical Fitness and Vigilance Performance
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
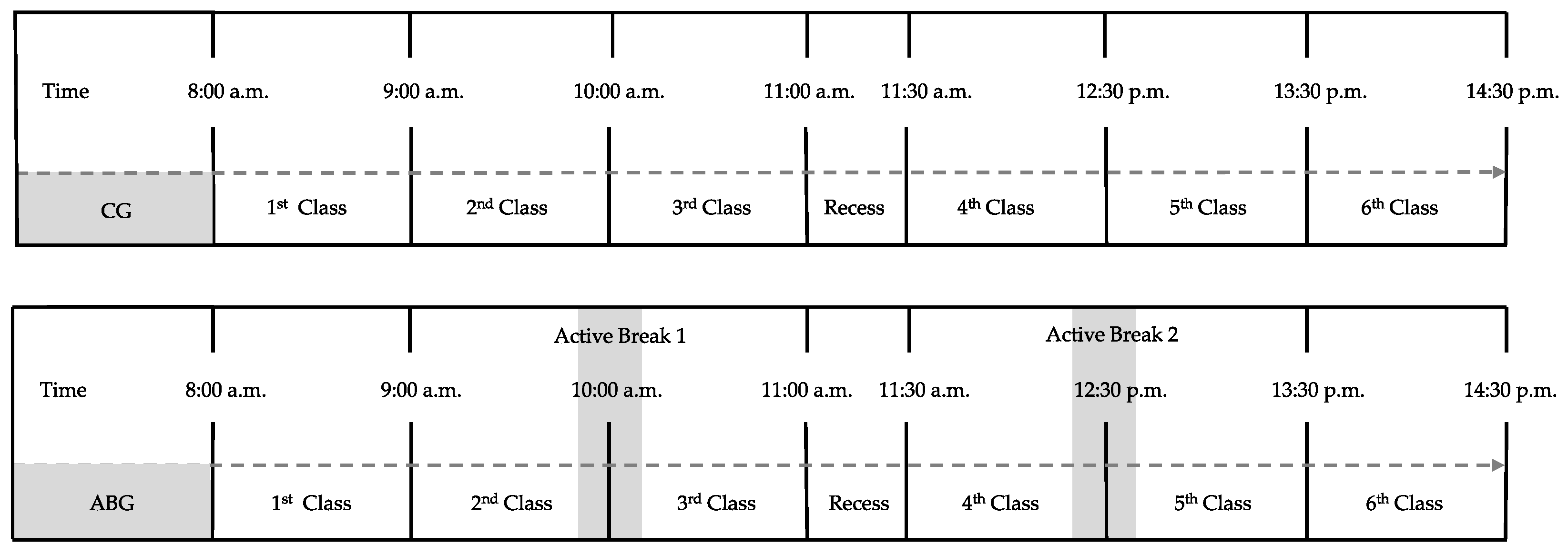
2.1. Study Design
2.2. Participants
2.3. Procedure
2.3.1. Preintervention
2.3.2. Intervention
2.3.3. Post-Intervention
2.4. Measures
2.4.1. Anthropometry
2.4.2. Physical Fitness Assessment
20-m Shuttle Run Test
4 × 10 m Speed-Agility Test
Standing Broad Jump
2.4.3. Rating of Perceived Exertion (RPE)
2.4.4. Cognitive Measurement: Psychomotor Vigilance Task
2.5. Data Analysis
3. Results
3.1. Anthropometrical Characteristics
3.2. Physical Fitness Assessment
3.3. Rating of Perceived Exertion (RPE)
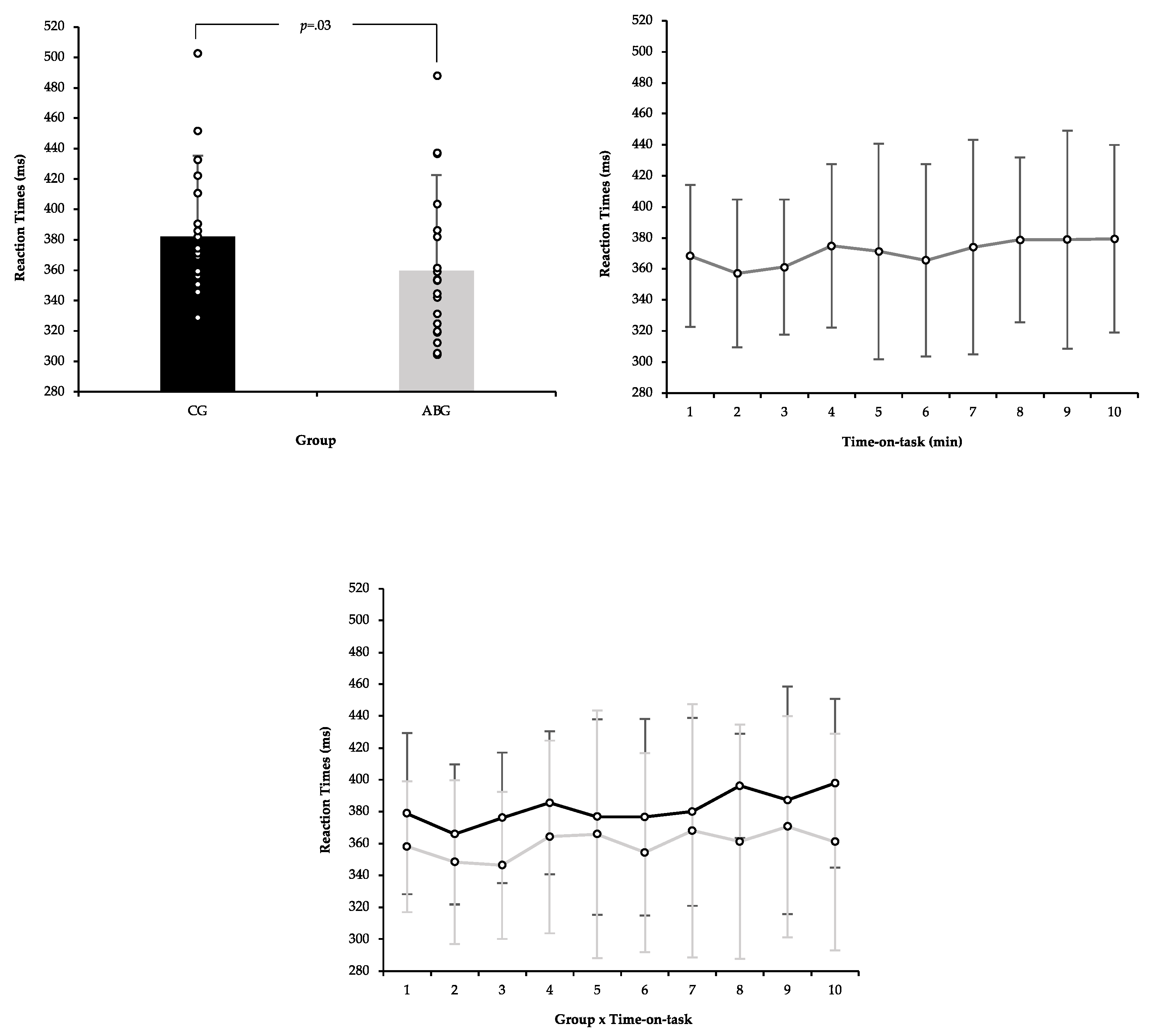
3.4. Psychomotor Vigilance Task
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Etnier, J.L.; Chang, Y.K. Exercise, cognitive function, and the brain: Advancing our understanding of complex relationships. J. Sport Health Sci. 2019, 8, 299–300. [Google Scholar] [CrossRef] [PubMed]
- Guiney, H.; Machado, L. Benefits of regular aerobic exercise for executive functioning in healthy populations. Psychon. Bull. 2013, 20, 73–86. [Google Scholar] [CrossRef] [PubMed]
- Hillman, C.H.; Erickson, K.I.; Kramer, A.F. Be smart, exercise your heart: Exercise effects on brain and cognition. Nat. Rev. Neurosci. 2008, 9, 58–65. [Google Scholar] [CrossRef]
- Mandolesi, L.; Polverino, A.; Montuori, S.; Foti, F.; Ferraioli, G.; Sorrentino, P.; Sorrentino, G. Effects of Physical Exercise on Cognitive Functioning and Wellbeing: Biological and Psychological Benefits. Front. Psychol. 2018, 9, 509. [Google Scholar] [CrossRef]
- Tomporowski, P.D.; Lambourne, K.; Okumura, M.S. Physical activity interventions and children’s mental function: An introduction and overview. Prev. Med. 2011, 52, S3–S9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, P.J.; Blumenthal, J.A.; Hoffman, B.M.; Cooper, H.; Strauman, T.A.; Welsh-Bohmer, K.; Browndyke, J.N.; Sherwood, A. Aerobic exercise and neurocognitive performance: A meta-analytic review of randomized controlled trials. Psychosom Med. 2010, 72, 239–252. [Google Scholar] [CrossRef] [PubMed]
- Chaddock, L.; Erickson, K.I.; Prakash, R.S.; Voss, M.W.; VanPatter, M.; Pontifex, M.B.; Hillman, C.H.; Kramer, A.F. A functional MRI investigation of the association between childhood aerobic fitness and neurocognitive control. Biol. Psychol. 2012, 89, 260–268. [Google Scholar] [CrossRef]
- Best, J.R. Effects of Physical Activity on Children’s Executive Function: Contributions of Experimental Research on Aerobic Exercise. Dev. Rev. 2010, 30, 331–551. [Google Scholar] [CrossRef]
- Chaddock, L.; Erickson, K.I.; Prakash, R.S.; Kim, J.S.; Voss, M.W.; Vanpatter, M.; Pontifex, M.B.; Raine, L.B.; Konkel, A.; Hillman, C.H.; et al. A neuroimaging investigation of the association between aerobic fitness, hippocampal volume, and memory performance in preadolescent children. Brain Res. 2010, 1358, 172–183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaddock, L.; Hillman, C.H.; Buck, S.M.; Cohen, N.J. Aerobic fitness and executive control of relational memory in preadolescent children. Med. Sci. Sports Exec. 2011, 43, 344–349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trudeau, F.; Shephard, R.J. Physical education, school physical activity, school sports and academic performance. Int. J. Beh. Nut. Phys. Act. 2008, 5, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scudder, M.R.; Federmeier, K.D.; Raine, L.B.; Direito, A.; Boyd, J.K.; Hillman, C.H. The association between aerobic fitness and language processing in children: Implications for academic achievement. Brain Cogn. 2014, 87, 140–152. [Google Scholar] [CrossRef] [Green Version]
- Erickson, K.I.; Gildengers, A.G.; Butters, M.A. Physical activity and brain plasticity in late adulthood. Dialogues Clin. Neurosci. 2013, 15, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Pereira, A.C.; Huddleston, D.E.; Brickman, A.M.; Sosunov, A.A.; Hen, R.; McKhann, G.M.; Sloan, R.; Gage, F.H.; Brown, T.R.; Small, S.A. An in vivo correlate of exercise-induced neurogenesis in the adult dentate gyrus. Proc. Natl. Acad. Sci. USA 2007, 104, 5638–5643. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cotman, C.W.; Berchtold, N.C.; Christie, L.A. Exercise builds brain health: Key roles of growth factor cascades and inflammation. Trends Neurosci. 2007, 30, 464–472. [Google Scholar] [CrossRef]
- Sarter, M.; Givens, B.; Bruno, J.P. The cognitive neuroscience of sustained attention: Where top-down meets bottom-up. Brain Res. Rev. 2001, 35, 146–160. [Google Scholar] [CrossRef]
- Parasuraman, R. Human-Computer Monitoring. Hum. Factors 1987, 29, 695–706. [Google Scholar] [CrossRef]
- Basner, M.; Dinges, D.F. Maximizing sensitivity of the psychomotor vigilance test (PVT) to sleep loss. Sleep 2011, 34, 581–591. [Google Scholar] [CrossRef]
- Davies, D.R.; Parasuraman, R. The Psychology of Vigilance; Academic Press: Cambridge, MA, USA, 1982. [Google Scholar]
- Helton, W.S.; Warm, J.S. Signal salience and the mindlessness theory of vigilance. Acta Psychol. 2008, 129, 18–25. [Google Scholar] [CrossRef]
- Warm, J.S.; Parasuraman, R.; Matthews, G. Vigilance requires hard mental work and is stressful. Hum. Factors 2008, 50, 433–441. [Google Scholar] [CrossRef]
- Formentin, C.; De Rui, M.; Zoncapè, M.; Ceccato, S.; Zarantonello, L.; Senzolo, M.; Burra, P.; Angeli, P.; Amodio, P.; Montagnese, S. The psychomotor vigilance task: Role in the diagnosis of hepatic encephalopathy and relationship with driving ability. J. Hepatol. 2019, 70, 648–657. [Google Scholar] [CrossRef]
- Weinberg, R.S.; Gould, D. Foundations of Sport and Exercise Psychology; Human Kinetics: Melbourne, Australia, 1995. [Google Scholar]
- Hillman, C.H.; Belopolsky, A.V.; Snook, E.M.; Kramer, A.F.; McAuley, E. Physical activity and executive control: Implications for increased cognitive health during older adulthood. RQES 2004, 75, 176–185. [Google Scholar] [CrossRef]
- Kamijo, K.; Pontifex, M.B.; O’Leary, K.C.; Scudder, M.R.; Wu, C.T.; Castelli, D.M.; Hillman, C.H. The effects of an afterschool physical activity program on working memory in preadolescent children. Dev. Sci. 2011, 14, 1046–1058. [Google Scholar] [CrossRef] [Green Version]
- Predovan, D.; Fraser, S.A.; Renaud, M.; Bherer, L. The effect of three months of aerobic training on stroop performance in older adults. J. Aging Res. 2012, 2012, 269815. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steinmayr, R.; Ziegler, M.; Träuble, B. Do intelligence and sustained attention interact in predicting academic achievement? Learn. Individ. Differ. 2010, 20, 14–18. [Google Scholar] [CrossRef]
- Bunce, D. Age differences in vigilance as a function of health-related physical fitness and task demands. Neuropsychology 2001, 39, 787–797. [Google Scholar] [CrossRef]
- Hillman, C.H.; Castelli, D.M.; Buck, S.M. Aerobic fitness and neurocognitive function in healthy preadolescent children. Med. Sci. Sports Exerc. 2005, 37, 1967–1974. [Google Scholar] [CrossRef] [Green Version]
- Pontifex, M.B.; Scudder, M.R.; Brown, M.L.; O’Leary, K.C.; Wu, C.T.; Themanson, J.R.; Hillman, C.H. On the number of trials necessary for stabilization of error-related brain activity across the life span. Psychophysiology 2010, 47, 767–773. [Google Scholar] [CrossRef] [Green Version]
- Pontifex, M.B.; Scudder, M.R.; Drollette, E.S.; Hillman, C.H. Fit and vigilant: The relationship between poorer aerobic fitness and failures in sustained attention during preadolescence. Neuropsychology 2012, 26, 407–413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sibley, B.A.; Etnier, J.L.; Le Masurier, G.C. Effects of an Acute Bout of Exercise on Cognitive Aspects of Stroop Performance. J. Sport Exerc. Psychol. 2006, 28, 285–299. [Google Scholar] [CrossRef]
- Verburgh, L.; Königs, M.; Scherder, E.J.; Oosterlaan, J. Physical exercise and executive functions in preadolescent children, adolescents and young adults: A meta-analysis. Brit. J. Sports Med. 2014, 48, 973–979. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Léger, L.A.; Lambert, J.A. maximal multistage 20-m shuttle run test to predict VO2 max. Eur J. Appl. Physiol. Occup. Physiol. 1982, 49, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.H.; Macfarlane, D.J.; Lam, T.H.; Stewart, S.M. Validity of the International Physical Activity Questionnaire Short Form (IPAQ-SF): A systematic review. Int. J. Behav. Nut. Phys. Act. 2011, 8, 115. [Google Scholar] [CrossRef] [Green Version]
- American College of Sports Medicine. ACSM’s Guidelines for Exercise Testing and Prescription, 9th ed.; Lippincott Williams: Philadelphia, PA, USA, 2010. [Google Scholar]
- Ruiz, J.R.; España Romero, V.; Castro Piñero, J.; Artero, E.G.; Ortega, F.B.; Cuenca García, M.; Jiménez Pavón, D.; Chillón, P.; Girela Rejón, M.J.; Mora, J.; et al. Batería ALPHA-Fitness: Test de campo para la evaluación de la condición física relacionada con la salud en niños y adolescentes [ALPHA-fitness test battery: Health-related field-based fitness tests assessment in children and adolescents]. Nutr. Hosp. 2011, 26, 1210–1214. [Google Scholar] [CrossRef]
- Ortega, F.B.; Ruiz, J.R.; Castillo, M.J.; Sjöström, M. Physical fitness in childhood and adolescence: A powerful marker of health. Int. J. Obes. Lond. 2008, 32, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Borg, G. Borg’s Perceived Exertion and Pain Scales; Human Kinetics: Melburne, Australia, 1998. [Google Scholar]
- Drummond, S.P.; Bischoff-Grethe, A.; Dinges, D.; Ayalon, L.; Mednick, S.C.; Meloy, M.J. The Neural Basis of the Psychomotor Vigilance Task. Sleep 2005, 28, 1059–1068. [Google Scholar]
- Luque-Casado, A.; Perakakis, P.; Hillman, C.H.; Kao, S.C.; Llorens, F.; Guerra, P.; Sanabria, D. Differences in Sustained Attention Capacity as a Function of Aerobic Fitness. Med. Sci. Sports Exerc. 2015, 48, 887–895. [Google Scholar] [CrossRef] [Green Version]
- Loh, S.; Lamond, N.; Dorrian, J.; Roach, G.; Dawson, D. The validity of psychomotor vigilance tasks of less than 10-minute duration. Behav. Res. Methods 2004, 36, 339–346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lambourne, K.; Tomporowski, P. The effect of exercise-induced arousal on cognitive task performance: A meta-regression analysis. Brain Res. 2010, 1341, 12–24. [Google Scholar] [CrossRef] [PubMed]
- Colcombe, S.; Kramer, A.F. Fitness effects on the cognitive function of older adults: A meta-analytic study. Psychol. Sci. 2003, 14, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Erickson, K.I.; Voss, M.W.; Prakash, R.S.; Basak, C.; Szabo, A.; Chaddock, L.; Kim, J.S.; Heo, S.; Alves, H.; White, S.M.; et al. Exercise training increases size of hippocampus and improves memory. Proc. Natl. Acad. Sci. USA 2011, 108, 3017–3022. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kenney, W.L.; Willmore, J.H.; Costill, D.L. Physiology of Sport and Exercise, 5th ed.; Human Kinetics: Melbourne, Australia, 2013. [Google Scholar]
- Colcombe, S.J.; Erickson, K.I.; Scalf, P.E.; Kim, J.S.; Prakash, R.; McAuley, E.; Elavsky, S.; Marquez, D.X.; Hu, L.; Kramer, A.F. Aerobic exercise training increases brain volume in aging humans. J. Ger. Ser. A Biol. Sci. Med. Sci. 2006, 61, 1166–1170. [Google Scholar] [CrossRef] [Green Version]
- Colcombe, S.J.; Kramer, A.F.; Erickson, K.I.; Scalf, P.; McAuley, E.; Cohen, N.J.; Webb, A.; Jerome, G.J.; Marquez, D.X.; Elavsky, S. Cardiovascular fitness, cortical plasticity, and aging. Proc. Natl. Acad. Sci. USA 2004, 101, 3316–3321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomas, A.G.; Dennis, A.; Bandettini, P.A.; Johansen-Berg, H. The effects of aerobic activity on brain structure. Front. Psychol. 2012, 3, 86. [Google Scholar] [CrossRef] [Green Version]
- Acevedo, E.O.; Ekkekakis, P. Psychobiology of Physical Activity; Human Kinetics: Melbourne, Australia, 2006. [Google Scholar]
- McMorris, T.; Tomporowski, P.; Audiffren, M. Exercise and Cognitive Function; Wiley-Blackwell: Hoboken, NJ, USA, 2009. [Google Scholar]
- Voss, M.W.; Nagamatsu, L.S.; Liu-Ambrose, T.; Kramer, A.F. Exercise, brain, and cognition across the life span. J. Appl. Phys. 2011, 111, 1505–1513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferris, L.T.; Williams, J.S.; Shen, C.L. The effect of acute exercise on serum brain-derived neurotrophic factor levels and cognitive function. Med. Sci. Sport Exerc. 2007, 39, 728–734. [Google Scholar] [CrossRef]
- Gillen, J.B.; Gibala, M.J. Is high-intensity interval training a time-efficient exercise strategy to improve health and fitness? Appl. Phys. Nutr. Metab. 2014, 39, 409–412. [Google Scholar] [CrossRef] [PubMed]
- Lee, I. Dose-Response Relation Between Physical Activity and Fitness: Even a Little Is Good; More Is Better. JAMA 2007, 297, 2137–2139. [Google Scholar] [CrossRef]
- de Geus, B.; Joncheere, J.; Meeusen, R. Commuter cycling: Effect on physical performance in untrained men and women in Flanders: Minimum dose to improve indexes of fitness. Scand. J. Med. Sci. Sports 2009, 19, 179–187. [Google Scholar] [CrossRef]
- Loturco, I.; Contreras, B.; Kobal, R.; Fernandes, V.; Moura, N.; Siqueira, F.; Winckler, C.; Suchomel, T.; Pereira, L.A. Vertically and horizontally directed muscle power exercises: Relationships with top-level sprint performance. PLoS ONE 2018, 13, e0201475. [Google Scholar] [CrossRef]
- Feeney, D.; Stanhope, S.J.; Kaminski, T.W.; Machi, A.; Jaric, S. Loaded Vertical Jumping: Force-Velocity Relationship, Work, and Power. J. Appl. Biomech. 2016, 32, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Behringer, M.; Vom Heede, A.; Matthews, M.; Mester, J. Effects of strength training on motor performance skills in children and adolescents: A meta-analysis. Pediatr. Exerc. Sci. 2011, 23, 186–206. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Participants | |||
|---|---|---|---|
| CG n = 21 | ABG n = 21 | Total n = 42 | |
| Age (years) | 16.52 ± 0.60 | 16.48 ± 0.60 | 16.50 ± 0.59 |
| Height (cm) | 170.32 ± 7.94 | 171.80 ± 8.34 | 171.08 ± 8.07 |
| Weight (kg) | 66.79 ± 9.59 | 67.40 ± 17.07 | 67.10 ± 13.76 |
| BMI (kg·m−2) | 22.90 ± 2.44 | 22.41 ± 3.74 | 22.64 ± 3.16 |
| Agility test (s) | 10.98 ± 1.23 | 10.55 ± 2.21 | 10.77 ± 1.78 |
| Standing broad jump (cm) | 177.00 ± 0.44 | 173.00 ±0.36 | 175.00 ± 0.40 |
| 20-m shuttle run test [V02max (mL/kg/min)] | 43.08 ± 7.25 | 43.91 ± 6.75 | 43.50 ± 6.94 |
| IPAQ-SF (Mets) | 712.85 ± 435.40 | 686.85 ± 402.99 | 699.85 ± 419.28 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-Fernández, F.T.; González-Víllora, S.; Baena-Morales, S.; Pastor-Vicedo, J.C.; Clemente, F.M.; Badicu, G.; Murawska-Ciałowicz, E. Effect of Physical Exercise Program Based on Active Breaks on Physical Fitness and Vigilance Performance. Biology 2021, 10, 1151. https://doi.org/10.3390/biology10111151
González-Fernández FT, González-Víllora S, Baena-Morales S, Pastor-Vicedo JC, Clemente FM, Badicu G, Murawska-Ciałowicz E. Effect of Physical Exercise Program Based on Active Breaks on Physical Fitness and Vigilance Performance. Biology. 2021; 10(11):1151. https://doi.org/10.3390/biology10111151
Chicago/Turabian StyleGonzález-Fernández, Francisco Tomás, Sixto González-Víllora, Salvador Baena-Morales, Juan Carlos Pastor-Vicedo, Filipe Manuel Clemente, Georgian Badicu, and Eugenia Murawska-Ciałowicz. 2021. "Effect of Physical Exercise Program Based on Active Breaks on Physical Fitness and Vigilance Performance" Biology 10, no. 11: 1151. https://doi.org/10.3390/biology10111151
APA StyleGonzález-Fernández, F. T., González-Víllora, S., Baena-Morales, S., Pastor-Vicedo, J. C., Clemente, F. M., Badicu, G., & Murawska-Ciałowicz, E. (2021). Effect of Physical Exercise Program Based on Active Breaks on Physical Fitness and Vigilance Performance. Biology, 10(11), 1151. https://doi.org/10.3390/biology10111151












