Projecting the Potential Distribution of Glossina morsitans (Diptera: Glossinidae) under Climate Change Using the MaxEnt Model
Abstract
Simple Summary
Abstract
1. Materials and Methods
1.1. Occurrence Data
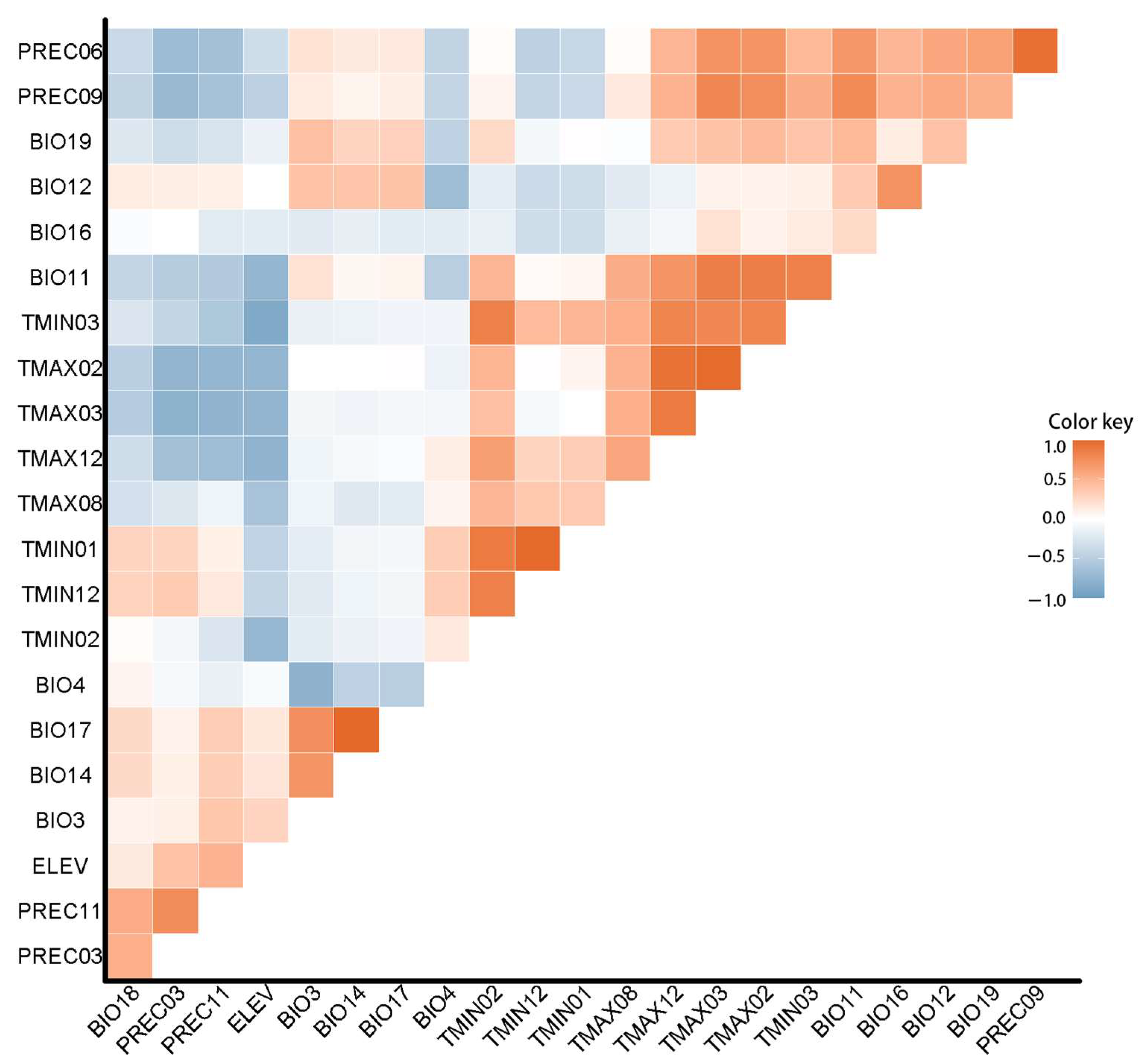
1.2. Environmental Variable and Processing
1.3. Model Process
1.4. Pre-Experiment
1.5. Formal Experiments
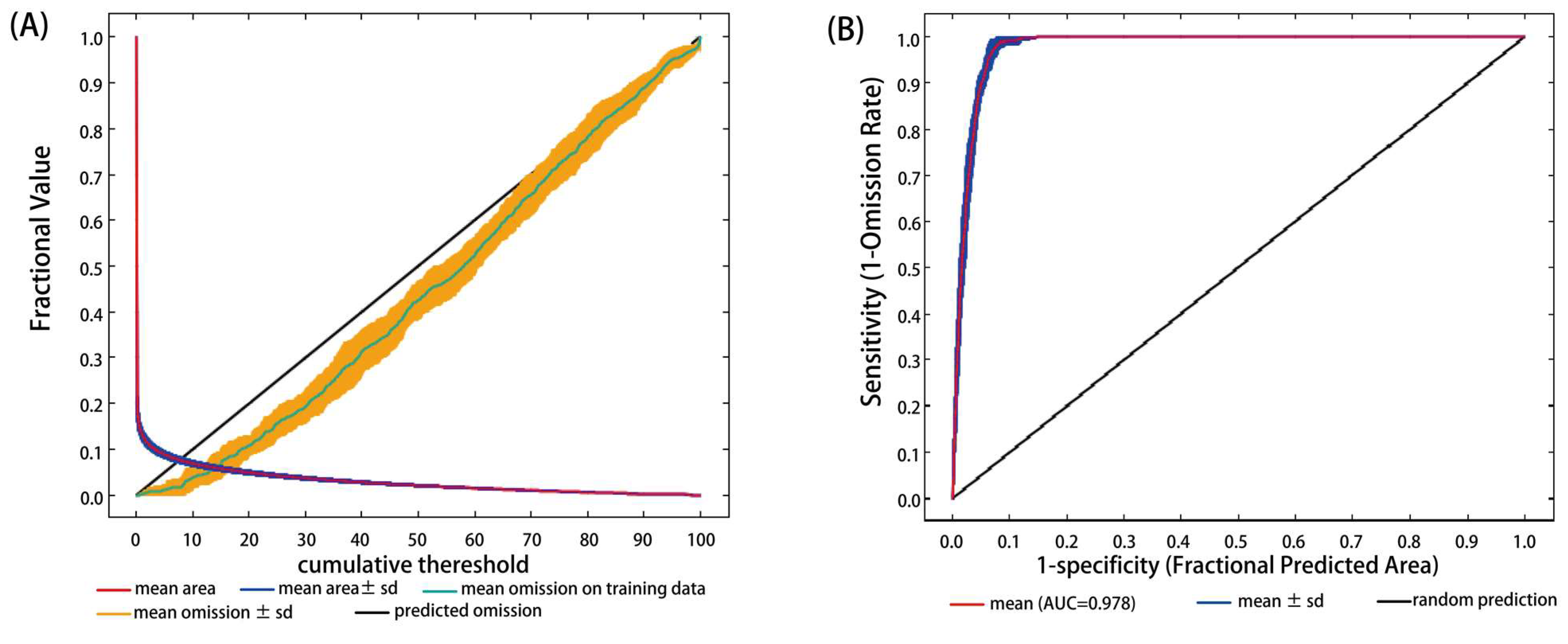
1.6. Model Performance
2. Results
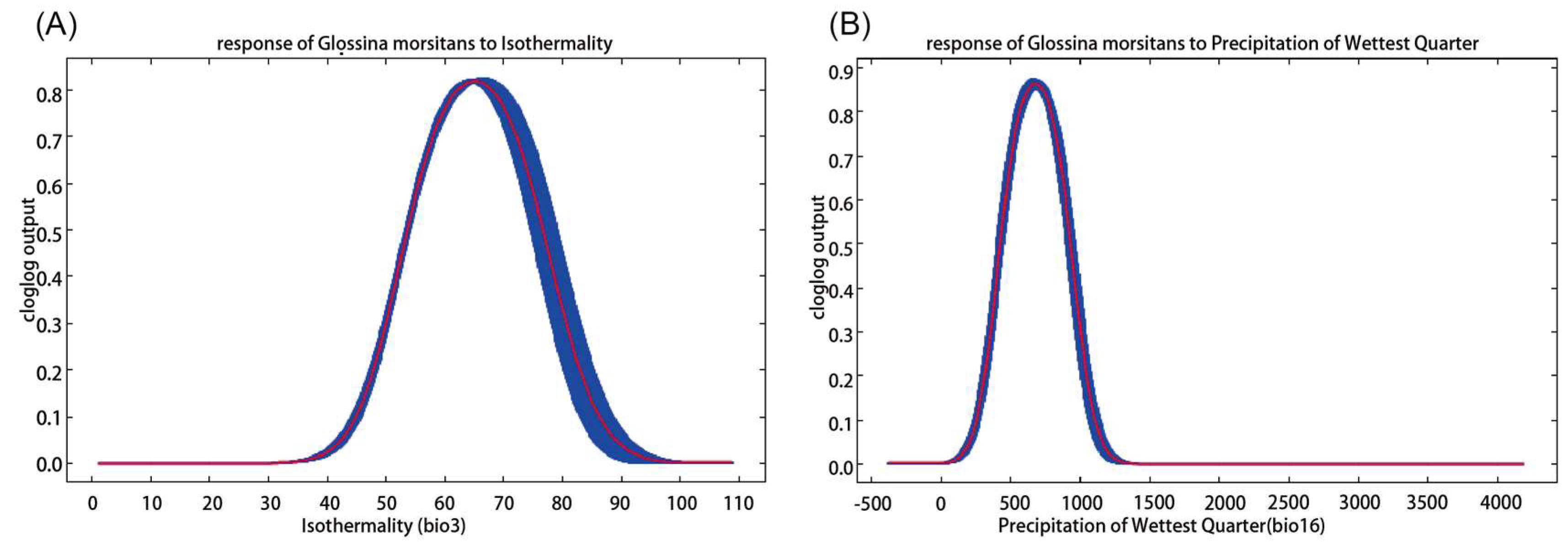
2.1. Environmental Variable Contribution
2.2. Potential Distribution of G. morsitans under Historical Conditions
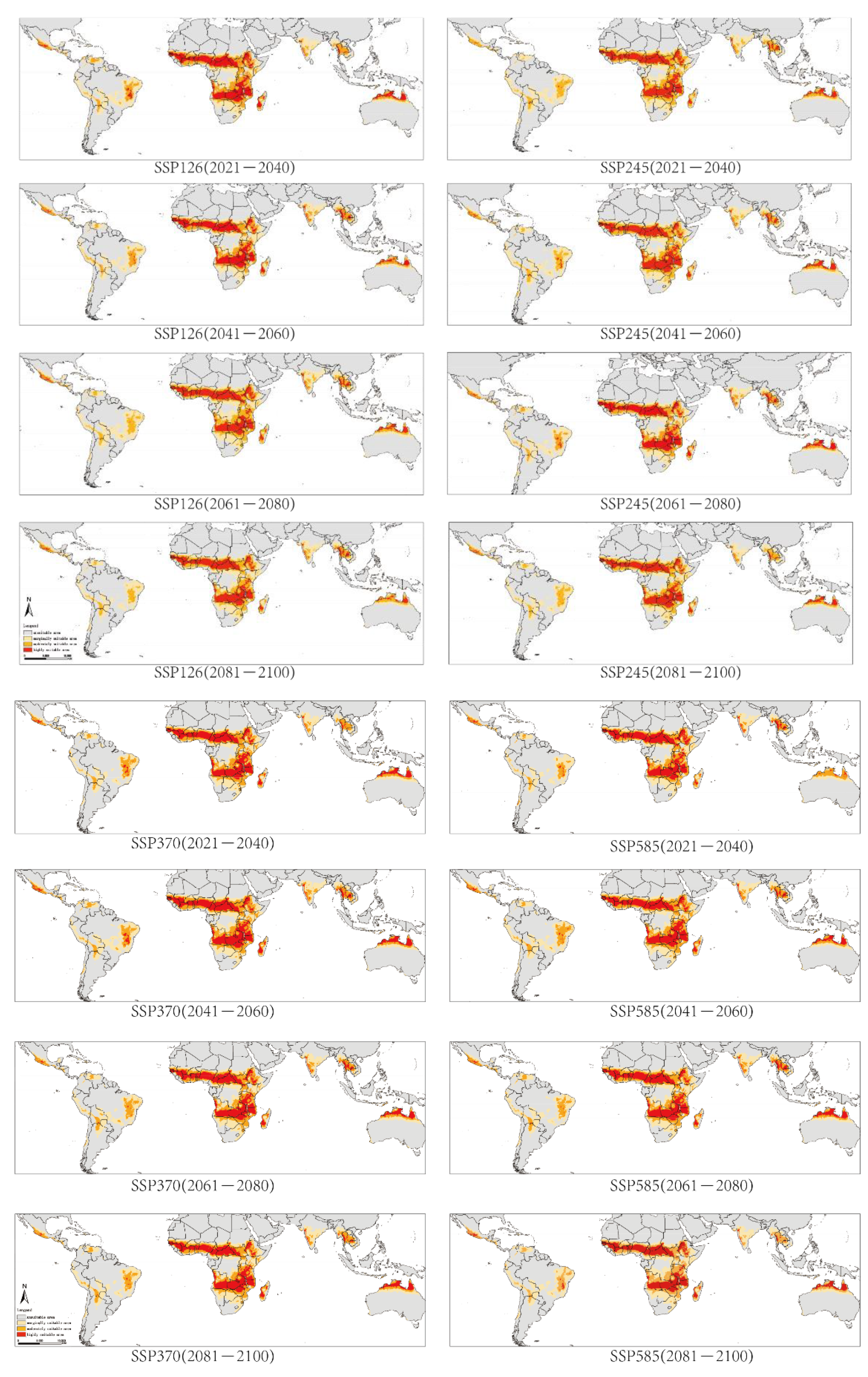
2.3. Potential Distribution of G. morsitans under Future Conditions
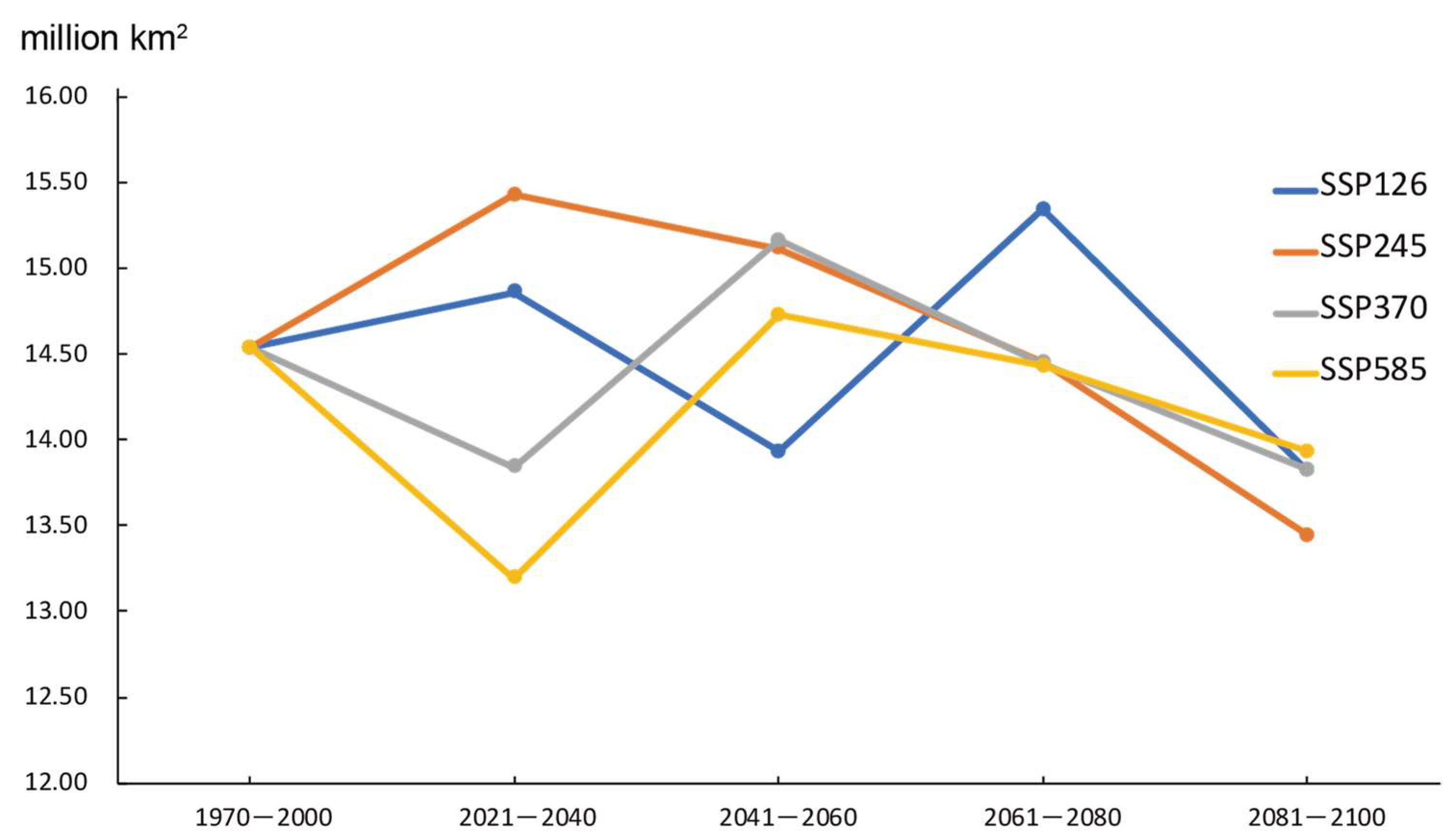
2.4. The Trend of Prediction Area of G. morsitans
2.4.1. The Overall Trend of Potentially Suitable Areas
2.4.2. Secular Trend of Suitable Area for Different Periods
3. Discussion
3.1. Discussion of Major Environmental Factors and Prediction Results
3.2. Discussion of Potential Distribution of G.morsitans under Historical Conditions
3.3. Discussion of Potential Distribution of G.morsitans under Future Conditions
3.4. Advantages and Disadvantages
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| BIO1 = Annual Mean Temperature |
| BIO2 = Mean Diurnal Range [Mean of monthly (max temp − min temp)] |
| BIO3 = Isothermality [(BIO2/BIO7) × 100] |
| BIO4 = Temperature Seasonality (standard deviation ×100) |
| BIO5 = Max Temperature of Warmest Month |
| BIO6 = Min Temperature of Coldest Month |
| BIO7 = Temperature Annual Range (BIO5 − BIO6) |
| BIO8 = Mean Temperature of Wettest Quarter |
| BIO9 = Mean Temperature of Driest Quarter |
| BIO10 = Mean Temperature of Warmest Quarter |
| BIO11 = Mean Temperature of Coldest Quarter |
| BIO12 = Annual Precipitation |
| BIO13 = Precipitation of Wettest Month |
| BIO14 = Precipitation of Driest Month |
| BIO15 = Precipitation Seasonality (Coefficient of Variation) |
| BIO16 = Precipitation of Wettest Quarter |
| BIO17 = Precipitation of Driest Quarter |
| BIO18 = Precipitation of Warmest Quarter |
| BIO19 = Precipitation of Coldest Quarter |
References
- Spath, J. Feeding patterns of three sympatric tsetse species (Glossina spp.) (Diptera: Glossinidae) in the preforest zone of Cte d’Ivoire. Acta Trop. 2000, 75, 109–118. [Google Scholar] [CrossRef]
- Clausen, P.H.; Adeyemi, I.; Bauer, B.; Breloeer, M.; Staak, C. Host preferences of tsetse (Diptera: Glossinidae) based on bloodmeal identifications. Med. Vet. Entomol. 2010, 12, 169–180. [Google Scholar] [CrossRef] [PubMed]
- Malele, I.I.; Parker, A.G. Mating age of Glossina austeni Newstead. Acta Trop. 1999, 72, 319–324. [Google Scholar] [CrossRef]
- Büscher, P.G.C.; Jamonneau, V.; Priotto, G. Human African Trypanosomiasis. Lancet 2017, 390, 2397–2409. [Google Scholar] [CrossRef]
- Wamwiri, F.N.; Changasi, R.E. Tsetse Flies (Glossina) as Vectors of Human African Trypanosomiasis: A Review. BioMed Res. Int. 2016, 2016, 6201350. [Google Scholar] [CrossRef]
- Human African Trypanosomiasis (Sleeping Sickness). Available online: https://www.who.int/health-topics/human-african-trypanosomiasis#tab=tab_1 (accessed on 7 April 2021).
- IPCC. The IPCC Fifth Assessment Report Climate Change 2013; IPCC: Geneva, Switzerland, 2013. [Google Scholar]
- Okiwelu, S.N. Host preference and trypanosome infection rates of Glossina morsitans morsitans Westwood in the Republic of Zambia. Ann. Trop. Med. Parasitol. 1977, 71, 101–107. [Google Scholar] [CrossRef]
- Hargrove, J.W.; Vale, G.A. Models for the rates of pupal development, fat consumption and mortality in tsetse (Glossina spp.). Bull. Entomol. Res. 2019, 110, 44–56. [Google Scholar] [CrossRef]
- Lehane, M.J. Biology of Blood-Sucking Insects. Q. Rev. Biol. 1991, 4, 45. [Google Scholar]
- Moore, S.; Shrestha, S.; Tomlinson, K.W.; Vuong, H. Predicting the effect of climate change on African trypanosomiasis: Integrating epidemiology with parasite and vector biology. J. R. Soc. Interface 2012, 9, 817–830. [Google Scholar] [CrossRef] [PubMed]
- Food and Agriculture Organization. Chapter 5 Distribution Of Glossina; FAO: Rome, Italy, 1992. [Google Scholar]
- Perkins-Taylor, I.E.; Frey, J.K. Predicting the distribution of a rare chipmunk (Neotamias quadrivittatus oscuraensis): Comparing MaxEnt and occupancy models. J. Mammal. 2020, 101, 1035–1048. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.Z.; Gen, Q.F.; Xiao, M.Y.; Zhang, M.Y.; Zhang, Y.Y.; Wang, Z.S. Predicting Pseudolarix amabilis potential habitat based on four Niche models. Acta Ecol. Sin. 2020, 40, 6096–6104. [Google Scholar]
- Baptista-Rosas, R.C.; Hinojosa, A.; Riquelme, M. Ecological Niche Modeling of Coccidioides Spp. In Western North American Deserts. Ann. N. Y. Acad. Sci. 2007, 1111, 35–46. [Google Scholar] [CrossRef]
- Dicko, A.H.; Lancelot, R.; Seck, M.T.; Guerrini, L.; Sall, B.; Lo, M.; Vreysen, M.; Lefrancois, T.; Fonta, W.M.; Peck, S.L. Using species distribution models to optimize vector control in the framework of the tsetse eradication campaign in Senegal. Proc. Natl. Acad. Sci. USA 2014, 111, 10149–10154. [Google Scholar] [CrossRef]
- Sallam, M.F.; Xue, R.D.; Pereira, R.M.; Koehler, P.G. Ecological niche modeling of mosquito vectors of West Nile virus in St. John’s County, Florida, USA. Parasit Vectors 2016, 9, 371. [Google Scholar] [CrossRef]
- Cardoso-Leite, R.; Vilarinho, A.C.; Novaes, M.C.; Tonetto, A.F.; Vilardi, G.C.; Guillermo-Ferreira, R. Recent and future environmental suitability to dengue fever in Brazil using species distribution model. Trans. R. Soc. Trop. Med. Hyg. 2014, 108, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Longbottom, J.; Caminade, C.; Gibson, H.S.; Weiss, D.J.; Torr, S.; Lord, J.S. Modelling the impact of climate change on the distribution and abundance of tsetse in Northern Zimbabwe. Parasit Vectors 2020, 13, 526. [Google Scholar] [CrossRef] [PubMed]
- Moore, N.; Messina, J. A landscape and climate data logistic model of tsetse distribution in Kenya. PLoS ONE 2010, 5, e11809. [Google Scholar] [CrossRef] [PubMed]
- Kariithi, H.M.; Boeren, S.; Murungi, E.K.; Vlak, J.M.; Abd-Alla, A.M.M. A proteomics approach reveals molecular manipulators of distinct cellular processes in the salivary glands of Glossina m. morsitans in response to Trypanosoma b. brucei infections. Parasites Vectors 2016, 9, 424. [Google Scholar] [CrossRef]
- Gbif.Org (1 April 2021) Gbif Occurrence Download. Available online: https://doi.org/10.15468/dl.kjpwk2 (accessed on 1 April 2021).
- Krafsur, E.S. Tsetse fly population genetics: An indirect approach to dispersal. Trends Parasitol. 2003, 19, 162–166. [Google Scholar] [CrossRef]
- Pollock, J.N. Training Manual for Testse Control Personnel. pp. 48–49. Available online: https://www.fao.org/3/P5444E/p5444e.pdf (accessed on 15 May 2021).
- Chagas, C. Nova tripanozomiaze humana: Estudos sobre a morfolojia e o ciclo evolutivo do Schizotrypanum cruzi n. gen, n sp, ajente etiolojico de nova entidade morbida do homem. Mem. Inst. Oswaldo Cruz. 1909, 1, 159–218. [Google Scholar] [CrossRef]
- Zhang, J.J.; Jiang, F.; Li, G.Y.; Qin, W.; Zhang, T.Z. Maxent modeling for predicting the spatial distribution of three raptors in the Sanjiangyuan National Park, China. Ecol. Evol. 2019, 9, 6643–6654. [Google Scholar] [CrossRef] [PubMed]
- Molloy, S.W.; Davis, R.A.; Van Etten, E.J.B. Species distribution modelling using bioclimatic variables to determine the impacts of a changing climate on the western ringtail possum (Pseudocheirus occidentals; Pseudocheiridae). Environ. Conserv. 2014, 41, 176–186. [Google Scholar] [CrossRef]
- Alcala-Canto, Y.; Alberti-Navarro, A.; Figueroa-Castillo, J.A.; Ibarra-Velarde, F.; Cervantes-Valencia, M.E. Maximum Entropy Ecological Niche Prediction of the Current Potential Geographical Distribution of Eimeria Species of Cattle, Sheep and Goats in Mexico. Open J. Anim. Sci. 2019, 9, 234–248. [Google Scholar] [CrossRef][Green Version]
- Gebrewahid, Y.; Abrehe, S.; Meresa, E.; Eyasu, G.; Abay, K.; Gebreab, G.; Kidanemariam, K.; Adissu, G.; Abreha, G.; Darcha, G. Current and future predicting potential areas of Oxytenanthera abyssinica (A. Richard) using MaxEnt model under climate change in Northern Ethiopia. Ecol. Process. 2020, 9, 6. [Google Scholar] [CrossRef]
- Swets, J.A. Measuring the Accuracy of Diagnostic Systems. Science 1998, 240, 1285–1293. [Google Scholar] [CrossRef]
- Leak, S.G.A. Chapter 258-Tsetse Fly. In Encyclopedia of Insects, 2nd ed.; Resh, V.H., Cardé, R.T., Eds.; Academic Press: San Diego, CA, USA, 2009; pp. 1020–1024. [Google Scholar]
- Jordan, A. Tsetse Biology and Ecology: Their Role in the Epidemiology and Control of Trypanosomosis by S.G.A. Leak. Parasitol. Today 1999, 77, 450. [Google Scholar]
- Alderton, S.; Macleod, E.T.; Anderson, N.E.; Palmer, G.; Machila, N.; Simuunza, M.; Welburn, S.C.; Atkinson, P.M. An agent-based model of tsetse fly response to seasonal climatic drivers: Assessing the impact on sleeping sickness transmission rates. PLoS Negl. Trop. Dis. 2018, 12, 2. [Google Scholar] [CrossRef]
- Bursell, E. The effect of temperature on the consumption of fat during pupal development in Glossina. Bull. Entomol. Res. 1960, 51, 583–598. [Google Scholar] [CrossRef]
- Nnko, H.J.; Gwakisa, P.S.; Ngonyoka, A.; Sindato, C.; Estes, A.B. Potential impacts of climate change on geographical distribution of three primary vectors of African Trypanosomiasis in Tanzania’s Maasai Steppe: G. m. morsitans, G. pallidipes and G. swynnertoni. PLoS Negl. Trop. Dis. 2021, 15, e0009081. [Google Scholar] [CrossRef]
- Welburn, S.C.; Molyneux, D.H.; Maudlin, I. Beyond Tsetse—Implications for Research and Control of Human African Trypanosomiasis Epidemics. Trends Parasitol. 2016, 32, 230–241. [Google Scholar] [CrossRef]
- FAO. Intervening against Bovine Trypanosomosis in Eastern Africa: Mapping the Costs and Benefits. In PAAT Technical and Scientific Series; FAO: Rome, Italy, 2017. [Google Scholar]
- Cecchi, G.; Mattioli, R.C.; Slingenbergh, J.; De La Rocque, S. Land cover and tsetse fly distributions in sub-Saharan Africa. Med. Vet. Entomol. 2008, 22, 364–373. [Google Scholar] [CrossRef] [PubMed]
- Rogers, D.J. Satellites, space, time and the African trypanosomiases. In Advances in Parasitology; Elsevier: Amsterdam, The Netherlands, 2000; Volume 47, pp. 129–171. [Google Scholar]


| Option | Parameter | |
|---|---|---|
| Default Value | Setting Value | |
| Randomly selected test set percentage | 0 | 25 |
| Regularization multiplier | 1 | 0.5 |
| Replicated run type | Crossvalidate | Bootstrap |
| Number of iterations repeated | 1 | 20 |
| Maximum number of repetitions | 500 | 5000 |
| Apply threshold rules | None | 10 percentile training presence |
| Features | Auto (hinge, product, quadratic, linear) | Liner, quadratic, product |
| Abbreviation | Definition | Contribution Rate (%) |
|---|---|---|
| bio3 * | Isothermality (Bio2/Bio7 × 100) (%) # | 26.4 |
| bio16 * | Precipitation of Wettest Quarter (mm) | 22.4 |
| bio14 | Precipitation of Driest Month (mm) | 6.8 |
| bio4 | Temperature Seasonality (standard deviation ∗ 100) | 4.9 |
| bio11 | Average Temperature of Coldest Quarter (°C) | 4.6 |
| tmax02 | Maximum Temperature of February (°C) | 4.3 |
| prec11 | Average Precipitation of November (mm) | 3.2 |
| prec03 | Average Precipitation of March (mm) | 2.7 |
| bio12 | Annual Precipitation (mm) | 2.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, R.; Gao, Y.; Chang, N.; Gao, T.; Ma, D.; Li, C.; Liu, Q. Projecting the Potential Distribution of Glossina morsitans (Diptera: Glossinidae) under Climate Change Using the MaxEnt Model. Biology 2021, 10, 1150. https://doi.org/10.3390/biology10111150
Zhou R, Gao Y, Chang N, Gao T, Ma D, Li C, Liu Q. Projecting the Potential Distribution of Glossina morsitans (Diptera: Glossinidae) under Climate Change Using the MaxEnt Model. Biology. 2021; 10(11):1150. https://doi.org/10.3390/biology10111150
Chicago/Turabian StyleZhou, Ruobing, Yuan Gao, Nan Chang, Tai Gao, Delong Ma, Chao Li, and Qiyong Liu. 2021. "Projecting the Potential Distribution of Glossina morsitans (Diptera: Glossinidae) under Climate Change Using the MaxEnt Model" Biology 10, no. 11: 1150. https://doi.org/10.3390/biology10111150
APA StyleZhou, R., Gao, Y., Chang, N., Gao, T., Ma, D., Li, C., & Liu, Q. (2021). Projecting the Potential Distribution of Glossina morsitans (Diptera: Glossinidae) under Climate Change Using the MaxEnt Model. Biology, 10(11), 1150. https://doi.org/10.3390/biology10111150







