Extracellular Matrix Components as Diagnostic Tools in Inflammatory Bowel Disease
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
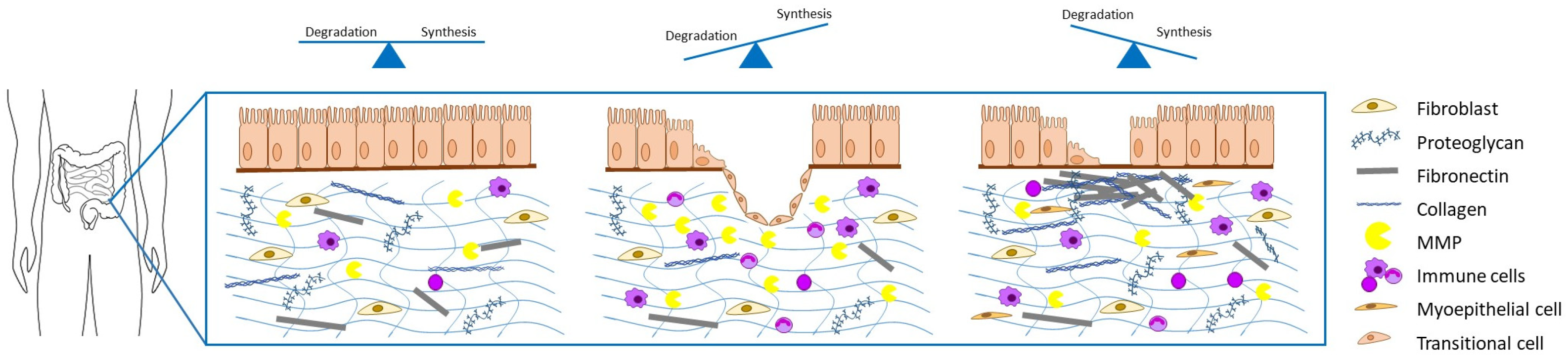
3. Results
3.1. In Vivo Imaging
| Method | Diagnosis | References |
|---|---|---|
| Ultrasound | Bowel wall thickening, lesions, hypervascularity, strictures, dilatation | [37,38,39] |
| Computed tomography | Mesenteric hypervascularity, fistulae, abscesses | [15,37] |
| PET/CT | Strictures, bowel wall thickening, inflammatory activity | [16,40] |
| Magnetic resonance imaging | Strictures, fistulae, abscesses, dilatations, edema, muscular hypertrophy | [19,36] |
| SICUS | Strictures, fistulae, abscesses | [18,19] |
3.2. Histopathology
3.3. Serological Markers
| Serological Marker Group | Detected Component | References |
|---|---|---|
| ECM components | Hyaluronan, C3M, C4M, VICM, COMP, TIMP-1, ECM1 | [69,71,72,73,74,76] |
| Microbial substances | Bacteria, bacterial membrane components | [78,79,80] |
| Growth factors | VEGF, PDGF, bFGF, human-chitinase-3-like 1, HGFA | [74,88,89,90] |
| microRNA | miR-200b, miR-19, miR29b | [92,93,94,95] |
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alatab, S.; Sepanlou, S.G.; Ikuta, K.; Vahedi, H.; Bisignano, C.; Safiri, S.; Naghavi, M. The global, regional, and national burden of inflammatory bowel disease in 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol. Hepatol. 2020, 5, 17–30. [Google Scholar] [CrossRef]
- Zhao, M.; Gonczi, L.; Lakatos, P.L.; Burisch, J. The burden of inflammatory bowel disease in Europe in 2020. J. Crohns Colitis 2021, 15, 1573–1587. [Google Scholar] [CrossRef] [PubMed]
- Bemelman, W.A.; Warusavitarne, J.; Sampietro, G.M.; Serclova, Z.; Zmora, O.; Luglio, G.; Overstraeten, A.D.B.V.; Burke, J.P.; Buskens, C.J.; Francesco, C.; et al. ECCO-ESCP Consensus on Surgery for Crohn’s Disease. J. Crohns Colitis 2018, 12, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Rieder, F.; Fiocchi, C.; Rogler, G. Mechanisms, Management, and Treatment of Fibrosis in Patients With Inflammatory Bowel Diseases. Gastroenterology 2017, 152, 340–350. [Google Scholar] [CrossRef] [PubMed]
- Maaser, C.; Sturm, A.; Vavricka, S.R.; Kucharzik, T.; Fiorino, G.; Annese, V.; Calabrese, E.; Baumgart, D.C.; Bettenworth, D.; Borralho Nunes, P.; et al. ECCO-ESGAR Guideline for Diagnostic Assessment in IBD Part 1: Initial diagnosis, monitoring of known IBD, detection of complications. J. Crohns Colitis 2019, 13, 144–164. [Google Scholar] [CrossRef] [PubMed]
- Sturm, A.; Maaser, C.; Calabrese, E.; Annese, V.; Fiorino, G.; Kucharzik, T.; Vavricka, S.R.; Verstockt, B.; van Rheenen, P.; Tolan, D.; et al. ECCO-ESGAR Guideline for Diagnostic Assessment in IBD Part 2: IBD scores and general principles and technical aspects. J. Crohns Colitis 2019, 13, 273–284. [Google Scholar] [CrossRef] [PubMed]
- Biasci, D.; Lee, J.C.; Noor, N.M.; Pombal, D.R.; Hou, M.; Lewis, N.; Ahmad, T.; Hart, A.; Parkes, M.; McKinney, E.F.; et al. A blood-based prognostic biomarker in IBD. Gut 2019, 68, 1386–1395. [Google Scholar] [CrossRef]
- Casale, J.; Crane, J.S. Biochemistry, Glycosaminoglycans; StatPearls: Treasure Island, FL, USA, 2021. [Google Scholar]
- Morla, S. Glycosaminoglycans and Glycosaminoglycan Mimetics in Cancer and Inflammation. Int. J. Mol. Sci. 2019, 20, 1963. [Google Scholar] [CrossRef]
- Murch, S.H.; MacDonald, T.T.; Walker-Smith, J.A.; Levin, M.; Lionetti, P.; Klein, N.J. Disruption of sulphated glycosaminoglycans in intestinal inflammation. Lancet 1993, 341, 711–714. [Google Scholar] [CrossRef]
- Naba, A.; Clauser, K.R.; Ding, H.; Whittaker, C.A.; Carr, S.A.; Hynes, R.O. The extracellular matrix: Tools and insights for the “omics” era. Matrix Biol. 2016, 49, 10–24. [Google Scholar] [CrossRef]
- Naba, A.; Clauser, K.R.; Hoersch, S.; Liu, H.; Carr, S.A.; Hynes, R.O. The matrisome: In silico definition and in vivo characterization by proteomics of normal and tumor extracellular matrices. Mol. Cell. Proteom. 2012, 11, M111.014647. [Google Scholar] [CrossRef] [PubMed]
- Gomollon, F.; Dignass, A.; Annese, V.; Tilg, H.; Van Assche, G.; Lindsay, J.O.; Peyrin-Biroulet, L.; Cullen, G.J.; Daperno, M.; Kucharzik, T.; et al. Ecco, 3rd European Evidence-based Consensus on the Diagnosis and Management of Crohn’s Disease 2016: Part 1: Diagnosis and Medical Management. J. Crohns Colitis 2017, 11, 3–25. [Google Scholar] [CrossRef] [PubMed]
- Magro, F.; Gionchetti, P.; Eliakim, R.; Ardizzone, S.; Armuzzi, A.; Barreiro-de Acosta, M.; Burisch, J.; Gecse, K.B.; Hart, A.L.; Hindryckx, P.; et al. Third European Evidence-based Consensus on Diagnosis and Management of Ulcerative Colitis. Part 1: Definitions, Diagnosis, Extra-intestinal Manifestations, Pregnancy, Cancer Surveillance, Surgery, and Ileo-anal Pouch Disorders. J. Crohns Colitis 2017, 11, 649–670. [Google Scholar] [CrossRef] [PubMed]
- Adler, J.; Punglia, D.R.; Dillman, J.R.; Polydorides, A.D.; Dave, M.; Al-Hawary, M.M.; Platt, J.F.; McKenna, B.J.; Zimmermann, E.M. Computed tomography enterography findings correlate with tissue inflammation, not fibrosis in resected small bowel Crohn’s disease. Inflamm. Bowel. Dis. 2012, 18, 849–856. [Google Scholar] [CrossRef] [PubMed]
- Pellino, G.; Nicolai, E.; Catalano, O.A.; Campione, S.; D’Armiento, F.P.; Salvatore, M.; Cuocolo, A.; Selvaggi, F. PET/MR Versus PET/CT Imaging: Impact on the Clinical Management of Small-Bowel Crohn’s Disease. J. Crohns Colitis 2016, 10, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Onali, S.; Calabrese, E.; Petruzziello, C.; Zorzi, F.; Sica, G.; Fiori, R.; Ascolani, M.; Lolli, E.; Condino, G.; Palmieri, G.; et al. Small intestine contrast ultrasonography vs computed tomography enteroclysis for assessing ileal Crohn’s disease. World J. Gastroenterol. 2012, 18, 6088–6095. [Google Scholar] [CrossRef][Green Version]
- Pallotta, N.; Vincoli, G.; Montesani, C.; Chirletti, P.; Pronio, A.; Caronna, R.; Ciccantelli, B.; Romeo, E.; Marcheggiano, A.; Corazziari, E. Small intestine contrast ultrasonography (SICUS) for the detection of small bowel complications in crohn’s disease: A prospective comparative study versus intraoperative findings. Inflamm. Bowel. Dis. 2012, 18, 74–84. [Google Scholar] [CrossRef]
- Kumar, S.; Hakim, A.; Alexakis, C.; Chhaya, V.; Tzias, D.; Pilcher, J.; Vlahos, J.; Pollok, R. Small intestinal contrast ultrasonography for the detection of small bowel complications in Crohn’s disease: Correlation with intraoperative findings and magnetic resonance enterography. J. Gastroenterol. Hepatol. 2015, 30, 86–91. [Google Scholar] [CrossRef]
- Ripolles, T.; Paredes, J.M.; Martinez-Perez, M.J.; Rimola, J.; Jauregui-Amezaga, A.; Bouzas, R.; Martin, G.; Moreno-Osset, E. Ultrasonographic Changes at 12 Weeks of Anti-TNF Drugs Predict 1-year Sonographic Response and Clinical Outcome in Crohn’s Disease: A Multicenter Study. Inflamm. Bowel. Dis. 2016, 22, 2465–2473. [Google Scholar] [CrossRef]
- Novak, K.L.; Kaplan, G.G.; Panaccione, R.; Afshar, E.E.; Tanyingoh, D.; Swain, M.; Kellar, A.; Wilson, S. A Simple Ultrasound Score for the Accurate Detection of Inflammatory Activity in Crohn’s Disease. Inflamm. Bowel. Dis. 2017, 23, 2001–2010. [Google Scholar] [CrossRef]
- Limberg, B. Diagnosis of chronic inflammatory bowel disease by ultrasonography. Z. Für Gastroenterol. 1999, 37, 495–508. [Google Scholar]
- Jing, J.; Wu, Y.; Zhang, H.; Zhang, Y.; Mu, J.; Luo, Y.; Zhuang, H. The establishment of a regression model from four modes of ultrasound to predict the activity of Crohn’s disease. Sci. Rep. 2021, 11, 77. [Google Scholar] [CrossRef]
- Bhatnagar, G.; Rodriguez-Justo, M.; Higginson, A.; Bassett, P.; Windsor, A.; Cohen, R.; Halligan, S.; Taylor, S.A. Inflammation and fibrosis in Crohn’s disease: Location-matched histological correlation of small bowel ultrasound features. Abdom. Radiol. 2021, 46, 144–155. [Google Scholar] [CrossRef]
- Young, I.R.; Clarke, G.J.; Bailes, D.R.; Pennock, J.M.; Doyle, F.H.; Bydder, G.M. Enhancement of relaxation rate with paramagnetic contrast agents in NMR imaging. J. Comput. Tomogr. 1981, 5, 543–547. [Google Scholar] [CrossRef]
- Niendorf, H.P.; Haustein, J.; Cornelius, I.; Alhassan, A.; Clauss, W. Safety of gadolinium-DTPA: Extended clinical experience. Magn. Reson. Med. 1991, 22, 222–228, discussion 229–322. [Google Scholar] [CrossRef]
- Rollandi, G.A.; Martinoli, C.; Conzi, R.; Cittadini, G.; Molinari, F.; Bertolotto, M.; Talenti, A.; Curone, P. Magnetic resonance imaging of the small intestine and colon in Crohn’s disease. Radiol. Med. 1996, 91, 81–85. [Google Scholar]
- Aime, S.; Caravan, P. Biodistribution of gadolinium-based contrast agents, including gadolinium deposition. J. Magn. Reson. Imaging 2009, 30, 1259–1267. [Google Scholar] [CrossRef] [PubMed]
- Holm Nielsen, S.; Jonasson, L.; Kalogeropoulos, K.; Karsdal, M.A.; Reese-Petersen, A.L.; Auf dem Keller, U.; Genovese, F.; Nilsson, J.; Goncalves, I. Exploring the role of extracellular matrix proteins to develop biomarkers of plaque vulnerability and outcome. J. Intern. Med. 2020, 287, 493–513. [Google Scholar] [CrossRef] [PubMed]
- Uca, Y.O.; Hallmann, D.; Hesse, B.; Seim, C.; Stolzenburg, N.; Pietsch, H.; Schnorr, J.; Taupitz, M. Microdistribution of Magnetic Resonance Imaging Contrast Agents in Atherosclerotic Plaques Determined by LA-ICP-MS and SR-muXRF Imaging. Mol. Imaging Biol. 2021, 23, 382–393. [Google Scholar] [CrossRef] [PubMed]
- Chow, A.M.; Tan, M.; Gao, D.S.; Fan, S.J.; Cheung, J.S.; Man, K.; Lu, Z.R.; Wu, E.X. Molecular MRI of liver fibrosis by a peptide-targeted contrast agent in an experimental mouse model. Investig. Radiol. 2013, 48, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Ye, F.; Wu, X.; Jeong, E.K.; Jia, Z.; Yang, T.; Parker, D.; Lu, Z.R. A peptide targeted contrast agent specific to fibrin-fibronectin complexes for cancer molecular imaging with MRI. Bioconjug. Chem. 2008, 19, 2300–2303. [Google Scholar] [CrossRef] [PubMed]
- Taylor, S.A.; Mallett, S.; Bhatnagar, G.; Baldwin-Cleland, R.; Bloom, S.; Gupta, A.; Hamlin, P.J.; Hart, A.L.; Higginson, A.; Jacobs, I.; et al. Diagnostic accuracy of magnetic resonance enterography and small bowel ultrasound for the extent and activity of newly diagnosed and relapsed Crohn’s disease (METRIC): A multicentre trial. Lancet Gastroenterol. Hepatol. 2018, 3, 548–558. [Google Scholar] [CrossRef]
- Quaia, E.; Cabibbo, B.; Sozzi, M.; Gennari, A.G.; Pontello, M.; Degrassi, F.; Cova, M.A. Biochemical markers and MR imaging findings as predictors of crohn disease activity in patients scanned by contrast-enhanced MR enterography. Acad. Radiol. 2014, 21, 1225–1232. [Google Scholar] [CrossRef] [PubMed]
- Li, X.H.; Mao, R.; Huang, S.Y.; Sun, C.H.; Cao, Q.H.; Fang, Z.N.; Zhang, Z.W.; Huang, L.; Lin, J.J.; Chen, Y.J.; et al. Characterization of Degree of Intestinal Fibrosis in Patients with Crohn Disease by Using Magnetization Transfer MR Imaging. Radiology 2018, 287, 494–503. [Google Scholar] [CrossRef] [PubMed]
- Wagner, M.; Ko, H.M.; Chatterji, M.; Besa, C.; Torres, J.; Zhang, X.; Panchal, H.; Hectors, S.; Cho, J.; Colombel, J.F.; et al. Magnetic Resonance Imaging Predicts Histopathological Composition of Ileal Crohn’s Disease. J. Crohns Colitis 2018, 12, 718–729. [Google Scholar] [CrossRef] [PubMed]
- Biernacka, K.B.; Baranska, D.; Grzelak, P.; Czkwianianc, E.; Szabelska-Zakrzewska, K. Up-to-date overview of imaging techniques in the diagnosis and management of inflammatory bowel diseases. Prz Gastroenterol. 2019, 14, 19–25. [Google Scholar] [CrossRef]
- Pallotta, N.; Tomei, E.; Viscido, A.; Calabrese, E.; Marcheggiano, A.; Caprilli, R.; Corazziari, E. Small intestine contrast ultrasonography: An alternative to radiology in the assessment of small bowel disease. Inflamm. Bowel. Dis. 2005, 11, 146–153. [Google Scholar] [CrossRef]
- Novak, K.L.; Nylund, K.; Maaser, C.; Petersen, F.; Kucharzik, T.; Lu, C.; Allocca, M.; Maconi, G.; de Voogd, F.; Christensen, B.; et al. Expert Consensus on Optimal Acquisition and Development of the International Bowel Ultrasound Segmental Activity Score [IBUS-SAS]: A Reliability and Inter-rater Variability Study on Intestinal Ultrasonography in Crohn’s Disease. J. Crohns Colitis 2021, 15, 609–616. [Google Scholar] [CrossRef]
- Basu, S.; Zhuang, H.; Torigian, D.A.; Rosenbaum, J.; Chen, W.; Alavi, A. Functional imaging of inflammatory diseases using nuclear medicine techniques. Semin. Nucl. Med. 2009, 39, 124–145. [Google Scholar] [CrossRef]
- Stidham, R.W.; Xu, J.; Johnson, L.A.; Kim, K.; Moons, D.S.; McKenna, B.J.; Rubin, J.M.; Higgins, P.D. Ultrasound elasticity imaging for detecting intestinal fibrosis and inflammation in rats and humans with Crohn’s disease. Gastroenterology 2011, 141, 819–826. [Google Scholar] [CrossRef]
- Fraquelli, M.; Branchi, F.; Cribiu, F.M.; Orlando, S.; Casazza, G.; Magarotto, A.; Massironi, S.; Botti, F.; Contessini-Avesani, E.; Conte, D.; et al. The Role of Ultrasound Elasticity Imaging in Predicting Ileal Fibrosis in Crohn’s Disease Patients. Inflamm. Bowel. Dis. 2015, 21, 2605–2612. [Google Scholar] [CrossRef]
- Baumgart, D.C.; Muller, H.P.; Grittner, U.; Metzke, D.; Fischer, A.; Guckelberger, O.; Pascher, A.; Sack, I.; Vieth, M.; Rudolph, B. US-based Real-time Elastography for the Detection of Fibrotic Gut Tissue in Patients with Stricturing Crohn Disease. Radiology 2015, 275, 889–899. [Google Scholar] [CrossRef]
- Serra, C.; Rizzello, F.; Pratico, C.; Felicani, C.; Fiorini, E.; Brugnera, R.; Mazzotta, E.; Giunchi, F.; Fiorentino, M.; D’Errico, A.; et al. Real-time elastography for the detection of fibrotic and inflammatory tissue in patients with stricturing Crohn’s disease. J. Ultrasound 2017, 20, 273–284. [Google Scholar] [CrossRef]
- Wilkens, R.; Hagemann-Madsen, R.H.; Peters, D.A.; Nielsen, A.H.; Norager, C.B.; Glerup, H.; Krogh, K. Validity of Contrast-enhanced Ultrasonography and Dynamic Contrast-enhanced MR Enterography in the Assessment of Transmural Activity and Fibrosis in Crohn’s Disease. J. Crohns Colitis 2018, 12, 48–56. [Google Scholar] [CrossRef]
- Dillman, J.R.; Stidham, R.W.; Higgins, P.D.; Moons, D.S.; Johnson, L.A.; Keshavarzi, N.R.; Rubin, J.M. Ultrasound shear wave elastography helps discriminate low-grade from high-grade bowel wall fibrosis in ex vivo human intestinal specimens. J. Ultrasound Med. 2014, 33, 2115–2123. [Google Scholar] [CrossRef]
- Lu, C.; Gui, X.; Chen, W.; Fung, T.; Novak, K.; Wilson, S.R. Ultrasound Shear Wave Elastography and Contrast Enhancement: Effective Biomarkers in Crohn’s Disease Strictures. Inflamm. Bowel. Dis. 2017, 23, 421–430. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.J.; Mao, R.; Li, X.H.; Cao, Q.H.; Chen, Z.H.; Liu, B.X.; Chen, S.L.; Chen, B.L.; He, Y.; Zeng, Z.R.; et al. Real-Time Shear Wave Ultrasound Elastography Differentiates Fibrotic from Inflammatory Strictures in Patients with Crohn’s Disease. Inflamm. Bowel. Dis. 2018, 24, 2183–2190. [Google Scholar] [CrossRef] [PubMed]
- Goertz, R.S.; Lueke, C.; Schellhaas, B.; Pfeifer, L.; Wildner, D.; Neurath, M.F.; Strobel, D. Acoustic radiation force impulse(ARFI) shear wave elastography of the bowel wall in healthy volunteers and in ulcerative colitis. Acta Radiol. Open. 2019, 8, 2058460119840969. [Google Scholar] [CrossRef] [PubMed]
- Venkatesh, S.K.; Ehman, R.L. Magnetic resonance elastography of abdomen. Abdom. Imaging 2015, 40, 745–759. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.; Loch, F.N.; Kamphues, C.; Bayerl, C.; Marticorena Garcia, S.R.; Siegmund, B.; Kuhl, A.A.; Hamm, B.; Braun, J.; Sack, I.; et al. Feasibility of Intestinal MR Elastography in Inflammatory Bowel Disease. J. Magn. Reson. Imaging 2021. [Google Scholar] [CrossRef] [PubMed]
- Gordon, I.O.; Bettenworth, D.; Bokemeyer, A.; Srivastava, A.; Rosty, C.; de Hertogh, G.; Robert, M.E.; Valasek, M.A.; Mao, R.; Li, J.; et al. International consensus to standardise histopathological scoring for small bowel strictures in Crohn’s disease. Gut 2021. [Google Scholar] [CrossRef]
- Movat, H.Z. Demonstration of all connective tissue elements in a single section; pentachrome stains. AMA Arch. Pathol. 1955, 60, 289–295. [Google Scholar] [PubMed]
- Goldner, J. A modification of the masson trichrome technique for routine laboratory purposes. Am. J. Pathol. 1938, 14, 237–243. [Google Scholar] [PubMed]
- Schmidt Rodney, W.J. Modification of Movat Pentachrome Stain with Improved Reliability of Elastin Staining. J. Histotechnol. 1996, 19, 325–327. [Google Scholar] [CrossRef]
- Lakatos, G.; Sipos, F.; Miheller, P.; Hritz, I.; Varga, M.Z.; Juhasz, M.; Molnar, B.; Tulassay, Z.; Herszenyi, L. The behavior of matrix metalloproteinase-9 in lymphocytic colitis, collagenous colitis and ulcerative colitis. Pathol. Oncol. Res. 2012, 18, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Fonseca-Camarillo, G.; Furuzawa-Carballeda, J.; Martinez-Benitez, B.; Barreto-Zuniga, R.; Yamamoto-Furusho, J.K. Increased expression of extracellular matrix metalloproteinase inducer(EMMPRIN) and MMP10, MMP23 in inflammatory bowel disease: Cross-sectional study. Scand. J. Immunol. 2021, 93, e12962. [Google Scholar] [CrossRef]
- De Bruyn, J.R.; van den Brink, G.R.; Steenkamer, J.; Buskens, C.J.; Bemelman, W.A.; Meisner, S.; Muncan, V.; Te Velde, A.A.; D’Haens, G.R.; Wildenberg, M.E. Fibrostenotic Phenotype of Myofibroblasts in Crohn’s Disease is Dependent on Tissue Stiffness and Reversed by LOX Inhibition. J. Crohns Colitis 2018, 12, 849–859. [Google Scholar] [CrossRef]
- Ning, L.; Li, S.; Gao, J.; Ding, L.; Wang, C.; Chen, W.; Shan, G.; Zhang, F.; Yu, J.; Xu, G. Tenascin-C Is Increased in Inflammatory Bowel Disease and Is Associated with response to Infliximab Therapy. Biomed. Res. Int. 2019, 2019, 1475705. [Google Scholar] [CrossRef]
- Li, J.; Mao, R.; Kurada, S.; Wang, J.; Lin, S.; Chandra, J.; Rieder, F. Pathogenesis of fibrostenosing Crohn’s disease. Transl. Res. 2019, 209, 39–54. [Google Scholar] [CrossRef]
- Thomson, C.A.; Nibbs, R.J.; McCoy, K.D.; Mowat, A.M. Immunological roles of intestinal mesenchymal cells. Immunology 2020, 160, 313–324. [Google Scholar] [CrossRef]
- Alfredsson, J.; Wick, M.J. Mechanism of fibrosis and stricture formation in Crohn’s disease. Scand. J. Immunol. 2020, 92, e12990. [Google Scholar] [CrossRef]
- Roulis, M.; Flavell, R.A. Fibroblasts and myofibroblasts of the intestinal lamina propria in physiology and disease. Differentiation 2016, 92, 116–131. [Google Scholar] [CrossRef]
- Wynn, T.A. Cellular and molecular mechanisms of fibrosis. J. Pathol. 2008, 214, 199–210. [Google Scholar] [CrossRef]
- Rieder, F.; Brenmoehl, J.; Leeb, S.; Scholmerich, J.; Rogler, G. Wound healing and fibrosis in intestinal disease. Gut 2007, 56, 130–139. [Google Scholar] [CrossRef]
- Rieder, F.; Fiocchi, C. Intestinal fibrosis in inflammatory bowel disease—Current knowledge and future perspectives. J. Crohns Colitis 2008, 2, 279–290. [Google Scholar] [CrossRef]
- Rieder, F.; Kessler, S.P.; West, G.A.; Bhilocha, S.; de la Motte, C.; Sadler, T.M.; Gopalan, B.; Stylianou, E.; Fiocchi, C. Inflammation-induced endothelial-to-mesenchymal transition: A novel mechanism of intestinal fibrosis. Am. J. Pathol. 2011, 179, 2660–2673. [Google Scholar] [CrossRef]
- Lovisa, S.; Genovese, G.; Danese, S. Role of Epithelial-to-Mesenchymal Transition in Inflammatory Bowel Disease. J. Crohns Colitis 2019, 13, 659–668. [Google Scholar] [CrossRef] [PubMed]
- Derkacz, A.; Olczyk, P.; Jura-Poltorak, A.; Olczyk, K.; Komosinska-Vassev, K. The Diagnostic Usefulness of Circulating Profile of Extracellular Matrix Components: Sulfated Glycosaminoglycans(sGAG), Hyaluronan(HA) and Extracellular Part of Syndecan-1(sCD138) in Patients with Crohn’s Disease and Ulcerative Colitis. J. Clin. Med. 2021, 10, 1722. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, Y.; Noda, H.; Okaniwa, N.; Adachi, K.; Shinmura, T.; Nakagawa, S.; Ebi, M.; Ogasawara, N.; Funaki, Y.; Zhuo, L.; et al. Serum-Derived Hyaluronan-Associated Protein Is a Novel Biomarker for Inflammatory Bowel Diseases. Digestion 2017, 95, 146–155. [Google Scholar] [CrossRef] [PubMed]
- Goffin, L.; Fagagnini, S.; Vicari, A.; Mamie, C.; Melhem, H.; Weder, B.; Lutz, C.; Lang, S.; Scharl, M.; Rogler, G.; et al. Anti-MMP-9 Antibody: A Promising Therapeutic Strategy for Treatment of Inflammatory Bowel Disease Complications with Fibrosis. Inflamm. Bowel. Dis. 2016, 22, 2041–2057. [Google Scholar] [CrossRef] [PubMed]
- Mortensen, J.H.; Godskesen, L.E.; Jensen, M.D.; Van Haaften, W.T.; Klinge, L.G.; Olinga, P.; Dijkstra, G.; Kjeldsen, J.; Karsdal, M.A.; Bay-Jensen, A.C.; et al. Fragments of Citrullinated and MMP-degraded Vimentin and MMP-degraded Type III Collagen Are Novel Serological Biomarkers to Differentiate Crohn’s Disease from Ulcerative Colitis. J. Crohns Colitis 2015, 9, 863–872. [Google Scholar] [CrossRef]
- Kapsoritakis, A.N.; Kapsoritaki, A.I.; Davidi, I.P.; Lotis, V.D.; Manolakis, A.C.; Mylonis, P.I.; Theodoridou, A.T.; Germenis, A.E.; Potamianos, S.P. Imbalance of tissue inhibitors of metalloproteinases(TIMP)—1 and—4 serum levels, in patients with inflammatory bowel disease. BMC Gastroenterol. 2008, 8, 55. [Google Scholar] [CrossRef]
- Stidham, R.W.; Wu, J.; Shi, J.; Lubman, D.M.; Higgins, P.D. Serum Glycoproteome Profiles for Distinguishing Intestinal Fibrosis from Inflammation in Crohn’s Disease. PLoS ONE 2017, 12, e0170506. [Google Scholar] [CrossRef]
- Van Haaften, W.T.; Mortensen, J.H.; Karsdal, M.A.; Bay-Jensen, A.C.; Dijkstra, G.; Olinga, P. Misbalance in type III collagen formation/degradation as a novel serological biomarker for penetrating(Montreal B3) Crohn’s disease. Aliment. Pharmacol. Ther. 2017, 46, 26–39. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Lubman, D.M.; Kugathasan, S.; Denson, L.A.; Hyams, J.S.; Dubinsky, M.C.; Griffiths, A.M.; Baldassano, R.N.; Noe, J.D.; Rabizadeh, S.; et al. Serum Protein Biomarkers of Fibrosis Aid in Risk Stratification of Future Stricturing Complications in Pediatric Crohn’s Disease. Am. J. Gastroenterol. 2019, 114, 777–785. [Google Scholar] [CrossRef]
- Alfano, M.; Canducci, F.; Nebuloni, M.; Clementi, M.; Montorsi, F.; Salonia, A. The interplay of extracellular matrix and microbiome in urothelial bladder cancer. Nat. Rev. Urol. 2016, 13, 77–90. [Google Scholar] [CrossRef] [PubMed]
- Targan, S.R.; Landers, C.J.; Yang, H.; Lodes, M.J.; Cong, Y.; Papadakis, K.A.; Vasiliauskas, E.; Elson, C.O.; Hershberg, R.M. Antibodies to CBir1 flagellin define a unique response that is associated independently with complicated Crohn’s disease. Gastroenterology 2005, 128, 2020–2028. [Google Scholar] [CrossRef] [PubMed]
- Mow, W.S.; Vasiliauskas, E.A.; Lin, Y.C.; Fleshner, P.R.; Papadakis, K.A.; Taylor, K.D.; Landers, C.J.; Abreu-Martin, M.T.; Rotter, J.I.; Yang, H.; et al. Association of antibody responses to microbial antigens and complications of small bowel Crohn’s disease. Gastroenterology 2004, 126, 414–424. [Google Scholar] [CrossRef]
- Pellino, G.; Pallante, P.; Selvaggi, F. Novel biomarkers of fibrosis in Crohn’s disease. World J. Gastrointest. Pathophysiol. 2016, 7, 266–275. [Google Scholar] [CrossRef] [PubMed]
- Degenhardt, F.; Dirmeier, A.; Lopez, R.; Lang, S.; Kunst, C.; Roggenbuck, D.; Reinhold, D.; Szymczak, S.; Rogler, G.; Klebl, F.; et al. Serologic Anti-GP2 Antibodies Are Associated with Genetic Polymorphisms, Fibrostenosis, and Need for Surgical Resection in Crohn’s Disease. Inflamm. Bowel. Dis. 2016, 22, 2648–2657. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Li, C.; Zhao, X.; Lv, C.; He, Q.; Lei, S.; Guo, Y.; Zhi, F. Anti-Saccharomyces cerevisiae antibodies associate with phenotypes and higher risk for surgery in Crohn’s disease: A meta-analysis. Dig. Dis. Sci. 2012, 57, 2944–2954. [Google Scholar] [CrossRef]
- Papadakis, K.A.; Yang, H.; Ippoliti, A.; Mei, L.; Elson, C.O.; Hershberg, R.M.; Vasiliauskas, E.A.; Fleshner, P.R.; Abreu, M.T.; Taylor, K.; et al. Anti-flagellin(CBir1) phenotypic and genetic Crohn’s disease associations. Inflamm. Bowel. Dis. 2007, 13, 524–530. [Google Scholar] [CrossRef] [PubMed]
- Dotan, I. Disease behavior in adult patients: Are there predictors for stricture or fistula formation? Dig. Dis. 2009, 27, 206–211. [Google Scholar] [CrossRef] [PubMed]
- Koutroubakis, I.E.; Drygiannakis, D.; Tsirogianni, A.; Oustamanolakis, P.; Karmiris, K.; Papamichael, K.; Mantzaris, G.J.; Kouroumalis, E.A. Antiglycan antibodies in Greek patients with inflammatory bowel disease. Dig. Dis. Sci. 2011, 56, 845–852. [Google Scholar] [CrossRef]
- Smids, C.; Horjus Talabur Horje, C.S.; Groenen, M.J.M.; van Koolwijk, E.H.M.; Wahab, P.J.; van Lochem, E.G. The value of serum antibodies in differentiating inflammatory bowel disease, predicting disease activity and disease course in the newly diagnosed patient. Scand. J. Gastroenterol. 2017, 52, 1104–1112. [Google Scholar] [CrossRef]
- Rieder, F.; Schleder, S.; Wolf, A.; Dirmeier, A.; Strauch, U.; Obermeier, F.; Lopez, R.; Spector, L.; Fire, E.; Yarden, J.; et al. Association of the novel serologic anti-glycan antibodies anti-laminarin and anti-chitin with complicated Crohn’s disease behavior. Inflamm. Bowel. Dis. 2010, 16, 263–274. [Google Scholar] [CrossRef]
- Giuffrida, P.; Pinzani, M.; Corazza, G.R.; Di Sabatino, A. Biomarkers of intestinal fibrosis—One step towards clinical trials for stricturing inflammatory bowel disease. United Eur. Gastroenterol. J. 2016, 4, 523–530. [Google Scholar] [CrossRef]
- Di Sabatino, A.; Ciccocioppo, R.; Armellini, E.; Morera, R.; Ricevuti, L.; Cazzola, P.; Fulle, I.; Corazza, G.R. Serum bFGF and VEGF correlate respectively with bowel wall thickness and intramural blood flow in Crohn’s disease. Inflamm. Bowel. Dis. 2004, 10, 573–577. [Google Scholar] [CrossRef] [PubMed]
- Kumagai, S.; Ohtani, H.; Nagai, T.; Funa, K.; Hiwatashi, N.O.; Shimosegawa, T.; Nagura, H. Platelet-derived growth factor and its receptors are expressed in areas of both active inflammation and active fibrosis in inflammatory bowel disease. Tohoku J. Exp. Med. 2001, 195, 21–33. [Google Scholar] [CrossRef]
- Matusiewicz, M.; Neubauer, K.; Mierzchala-Pasierb, M.; Gamian, A.; Krzystek-Korpacka, M. Matrix metalloproteinase-9: Its interplay with angiogenic factors in inflammatory bowel diseases. Dis. Markers 2014, 2014, 643645. [Google Scholar] [CrossRef]
- Murakami, Y.; Toyoda, H.; Tanaka, M.; Kuroda, M.; Harada, Y.; Matsuda, F.; Tajima, A.; Kosaka, N.; Ochiya, T.; Shimotohno, K. The progression of liver fibrosis is related with overexpression of the miR-199 and 200 families. PLoS ONE 2011, 6, e16081. [Google Scholar] [CrossRef]
- Mehta, S.J.; Lewis, A.; Nijhuis, A.; Jeffery, R.; Biancheri, P.; Di Sabatino, A.; Feakins, R.; Silver, A.; Lindsay, J.O. Epithelial down-regulation of the miR-200 family in fibrostenosing Crohn’s disease is associated with features of epithelial to mesenchymal transition. J. Cell Mol. Med. 2018, 22, 5617–5628. [Google Scholar] [CrossRef]
- Lewis, A.; Mehta, S.; Hanna, L.N.; Rogalski, L.A.; Jeffery, R.; Nijhuis, A.; Kumagai, T.; Biancheri, P.; Bundy, J.G.; Bishop, C.L.; et al. Low Serum Levels of MicroRNA-19 Are Associated with a Stricturing Crohn’s Disease Phenotype. Inflamm. Bowel. Dis. 2015, 21, 1926–1934. [Google Scholar] [CrossRef] [PubMed]
- Nijhuis, A.; Biancheri, P.; Lewis, A.; Bishop, C.L.; Giuffrida, P.; Chan, C.; Feakins, R.; Poulsom, R.; Di Sabatino, A.; Corazza, G.R.; et al. In Crohn’s disease fibrosis-reduced expression of the miR-29 family enhances collagen expression in intestinal fibroblasts. Clin Sci. Lond. 2014, 127, 341–350. [Google Scholar] [CrossRef] [PubMed]
- De Bruyn, J.R.; Becker, M.A.; Steenkamer, J.; Wildenberg, M.E.; Meijer, S.L.; Buskens, C.J.; Bemelman, W.A.; Lowenberg, M.; Ponsioen, C.Y.; van den Brink, G.R.; et al. Intestinal fibrosis is associated with lack of response to Infliximab therapy in Crohn’s disease. PLoS ONE 2018, 13, e0190999. [Google Scholar] [CrossRef]
- Paramsothy, S.; Rosenstein, A.K.; Mehandru, S.; Colombel, J.F. The current state of the art for biological therapies and new small molecules in inflammatory bowel disease. Mucosal. Immunol. 2018, 11, 1558–1570. [Google Scholar] [CrossRef] [PubMed]
- Carlo Cosimo Quattrocchi, A.J.V.D.M. Gadolinium Retention in Brain and Body: Clinical and Preclinical Evidence. In Imaging in Nephrology; Granata, A.B.M., Ed.; Springer: Cham, The Netherlands, 2021. [Google Scholar]
- Vajravelu, R.K.; Copelovitch, L.; Osterman, M.T.; Scott, F.I.; Mamtani, R.; Lewis, J.D.; Denburg, M.R. Inflammatory Bowel Diseases Are Associated With an Increased Risk for Chronic Kidney Disease, Which Decreases With Age. Clin. Gastroenterol. Hepatol. 2020, 18, 2262–2268. [Google Scholar] [CrossRef] [PubMed]
- Wagner, B.; Drel, V.; Gorin, Y. Pathophysiology of gadolinium-associated systemic fibrosis. Am. J. Physiol. Renal. Physiol. 2016, 311, F1–F11. [Google Scholar] [CrossRef] [PubMed]
- Maaser, C.; Petersen, F.; Helwig, U.; Fischer, I.; Roessler, A.; Rath, S.; Lang, D.; Kucharzik, T.; German, I.B.D.S.G.; The, T.; et al. Intestinal ultrasound for monitoring therapeutic response in patients with ulcerative colitis: Results from the TRUST&UC study. Gut 2020, 69, 1629–1636. [Google Scholar]
- Crohn, B.B.; Ginzburg, L.; Oppenheimer, G.D. Landmark article Oct 15, 1932. Regional ileitis. A pathological and clinical entity. By Burril B. Crohn, Leon Ginzburg, and Gordon D. Oppenheimer. JAMA 1984, 251, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Weakley, F.L.; Turnbull, R.B. Recognition of regional ileitis in the operating room. Dis. Colon. Rectum. 1971, 14, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Kredel, L.I.; Siegmund, B. Adipose-tissue and intestinal inflammation—Visceral obesity and creeping fat. Front. Immunol. 2014, 5, 462. [Google Scholar] [CrossRef]
- Branchi, F.; Caprioli, F.; Orlando, S.; Conte, D.; Fraquelli, M. Non-invasive evaluation of intestinal disorders: The role of elastographic techniques. World J. Gastroenterol. 2017, 23, 2832–2840. [Google Scholar] [CrossRef] [PubMed]
- Juillerat, P.; Manz, M.; Sauter, B.; Zeitz, J.; Vavricka, S.R.; IBDnet, S. Therapies in Inflammatory Bowel Disease Patients with Extraintestinal Manifestations. Digestion 2020, 101 (Suppl. S1), 83–97. [Google Scholar] [CrossRef] [PubMed]
| Histological Stainings | Detected Features | References |
|---|---|---|
| H&E staining | Mucosal architecture, lamina propria cellularity, neutrophil infiltration, epithelial abnormality, granuloma | [13,14] |
| Trichrome staining | Muscle fibers, collagen, keratin | [54] |
| Pentachrome staining | Elastin, collagen, reticular fibers, muscle, fibrin | [53] |
| Immunohistochemical antibody staining | Detection of immune cell composition, matrix metalloproteinases, matrix metalloproteinase inducers | [42,56,57,58] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Golusda, L.; Kühl, A.A.; Siegmund, B.; Paclik, D. Extracellular Matrix Components as Diagnostic Tools in Inflammatory Bowel Disease. Biology 2021, 10, 1024. https://doi.org/10.3390/biology10101024
Golusda L, Kühl AA, Siegmund B, Paclik D. Extracellular Matrix Components as Diagnostic Tools in Inflammatory Bowel Disease. Biology. 2021; 10(10):1024. https://doi.org/10.3390/biology10101024
Chicago/Turabian StyleGolusda, Laura, Anja A. Kühl, Britta Siegmund, and Daniela Paclik. 2021. "Extracellular Matrix Components as Diagnostic Tools in Inflammatory Bowel Disease" Biology 10, no. 10: 1024. https://doi.org/10.3390/biology10101024
APA StyleGolusda, L., Kühl, A. A., Siegmund, B., & Paclik, D. (2021). Extracellular Matrix Components as Diagnostic Tools in Inflammatory Bowel Disease. Biology, 10(10), 1024. https://doi.org/10.3390/biology10101024







