Assessment of Albumin ECM Accumulation and Inflammation as Novel In Vivo Diagnostic Targets for Multi-Target MR Imaging
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Gadofosveset Trisodium as a Gd-Based Albumin-Specific Contrast Agent
2.3. Iron Oxide Particles as a Macrophage-Specific MRI Probe
2.4. In Vivo MRI
2.4.1. Mouse MRI Protocol
2.4.2. Instrumental MRI Setup
2.4.3. Assessment of T1-Weighted MRI with Gadofosveset
2.4.4. Assessment of T2*-Weighted MRI with Ferumoxytol
2.4.5. Evaluation of T1-Weighted Sequences for Gadofosveset Enhancement
2.4.6. Evaluation of the T2*-Weighted Sequences for Ferumoxytol
2.5. Ex Vivo Examinations
2.5.1. Histology of the Brachiocephalic Artery and Plaque Morphometry
2.5.2. Immunofluorescence Staining
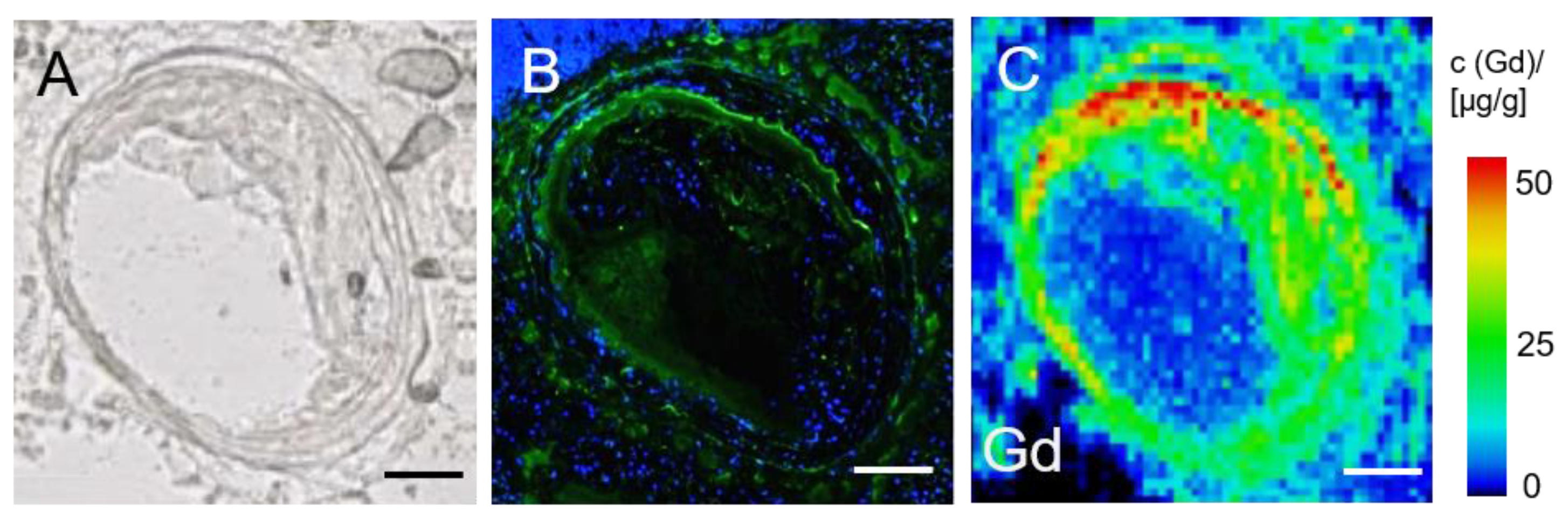
2.5.3. Gadolinium Localization Using Laser Ablation Inductively Coupled Plasma Mass Spectrometry (LA-ICP-MS)
2.6. Statistical Methods
3. Results
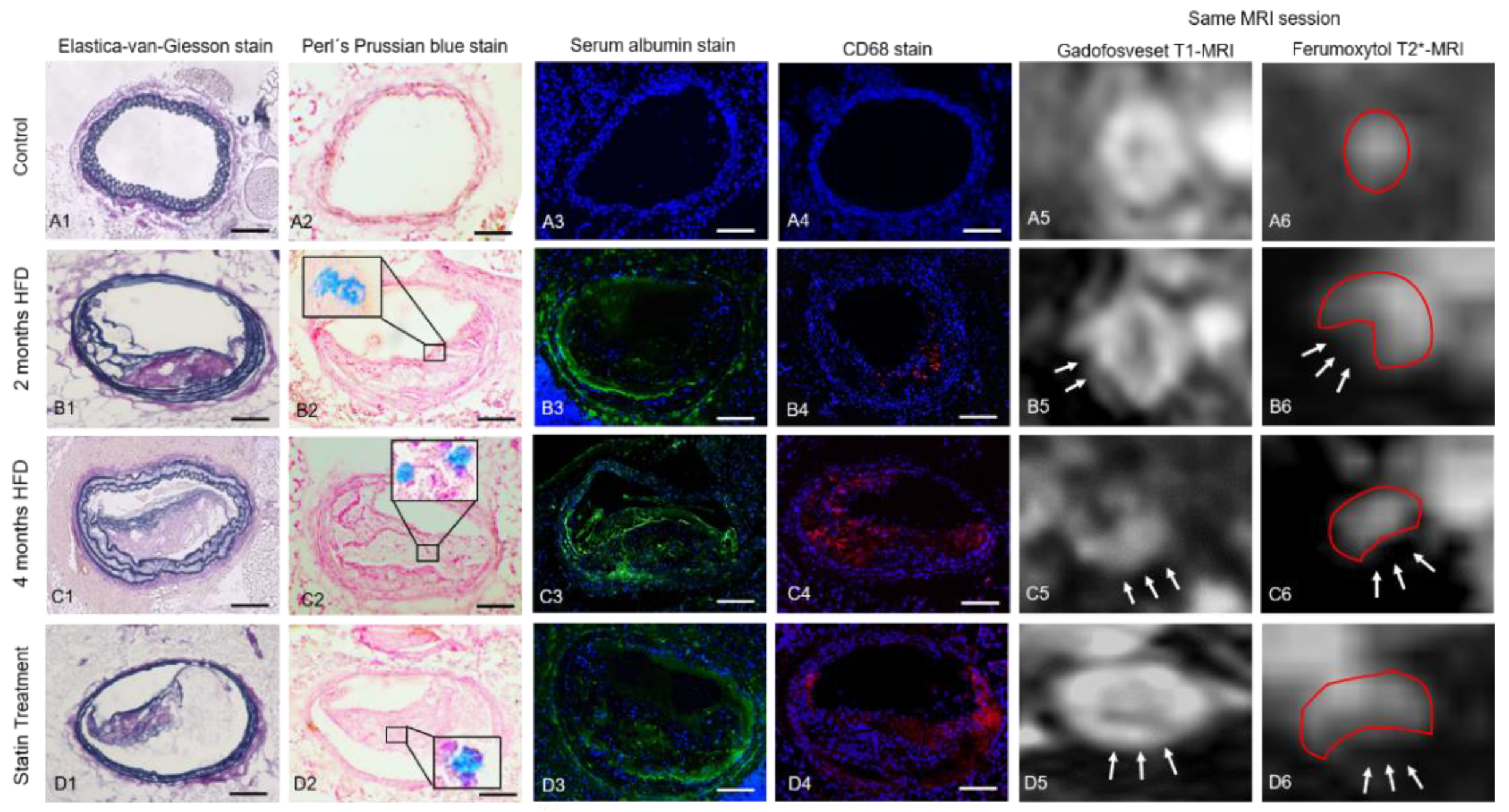
3.1. Assessment of Vessel Wall Inflammation and Permeability by Multi-Target MRI
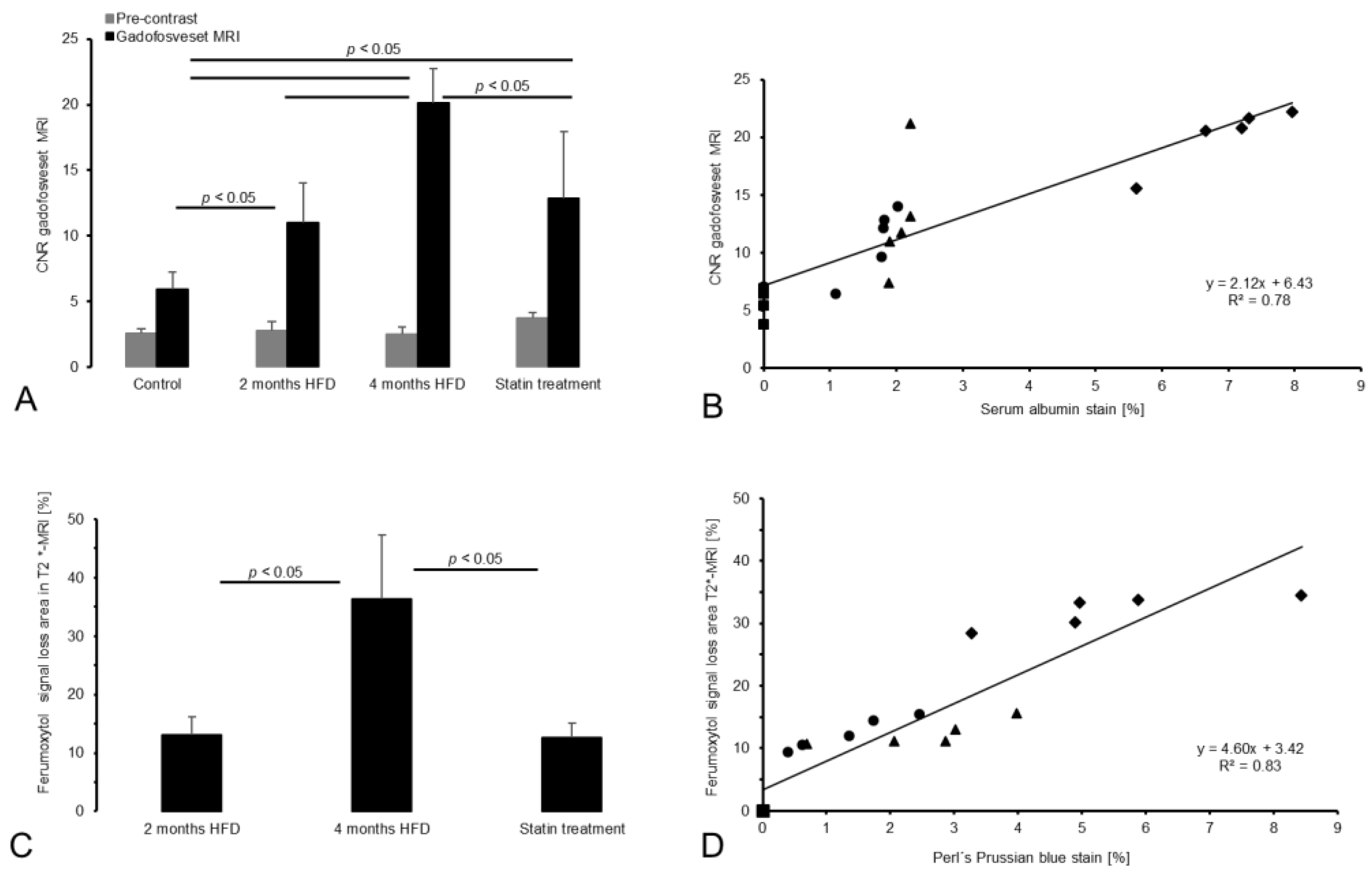
3.2. Gadofosveset-Enhanced T1-Weighted MRI
3.3. T2*-Weighted MRI for Assessment of Inflammatory Processes
3.4. Histology and Immunofluorescence
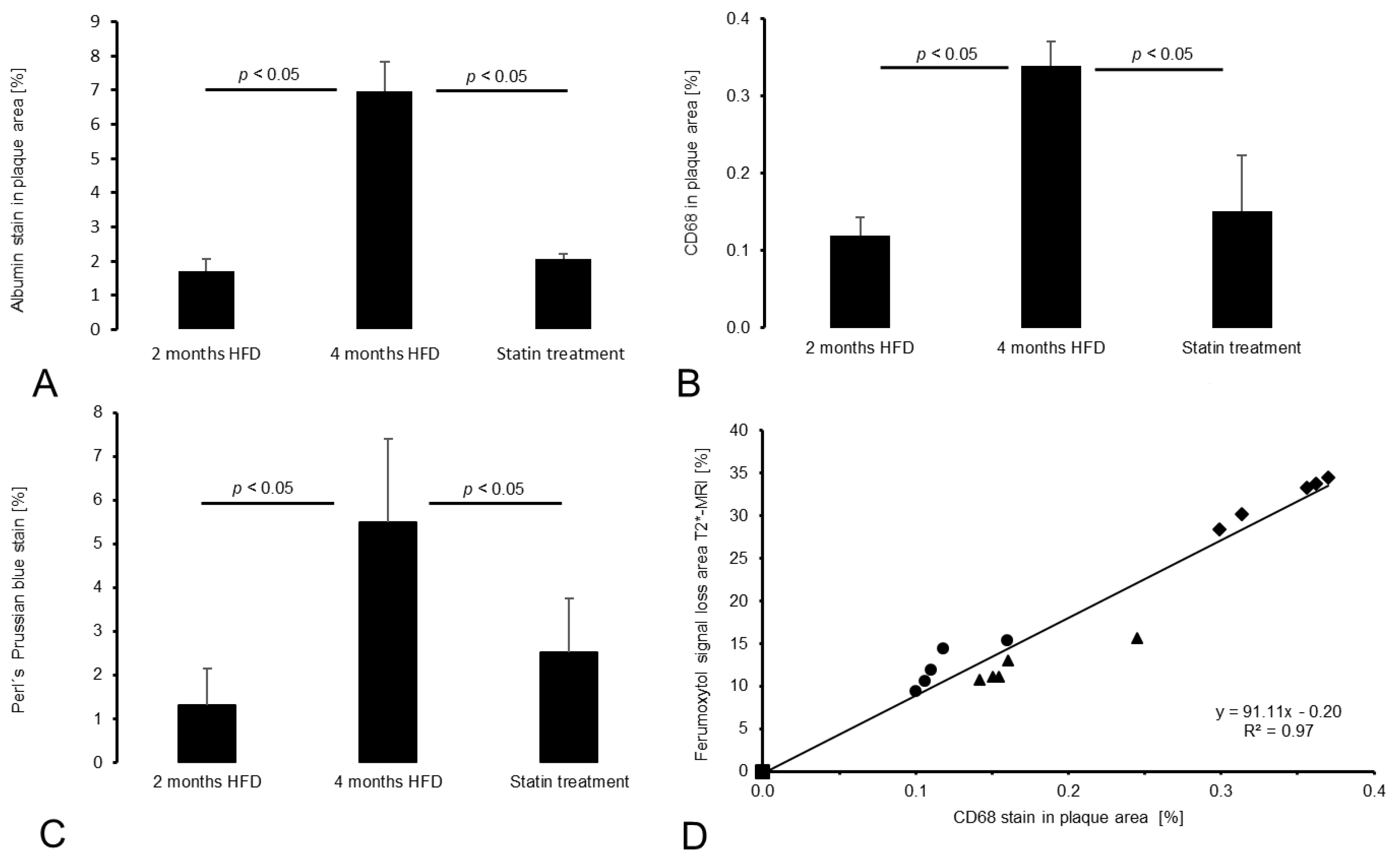
3.4.1. Intraplaque Albumin as Biomarker for Vessel Wall Permeability
3.4.2. CD68 and Perls’ Prussian Blue Staining for Macrophage and Iron Oxide Particle Detection
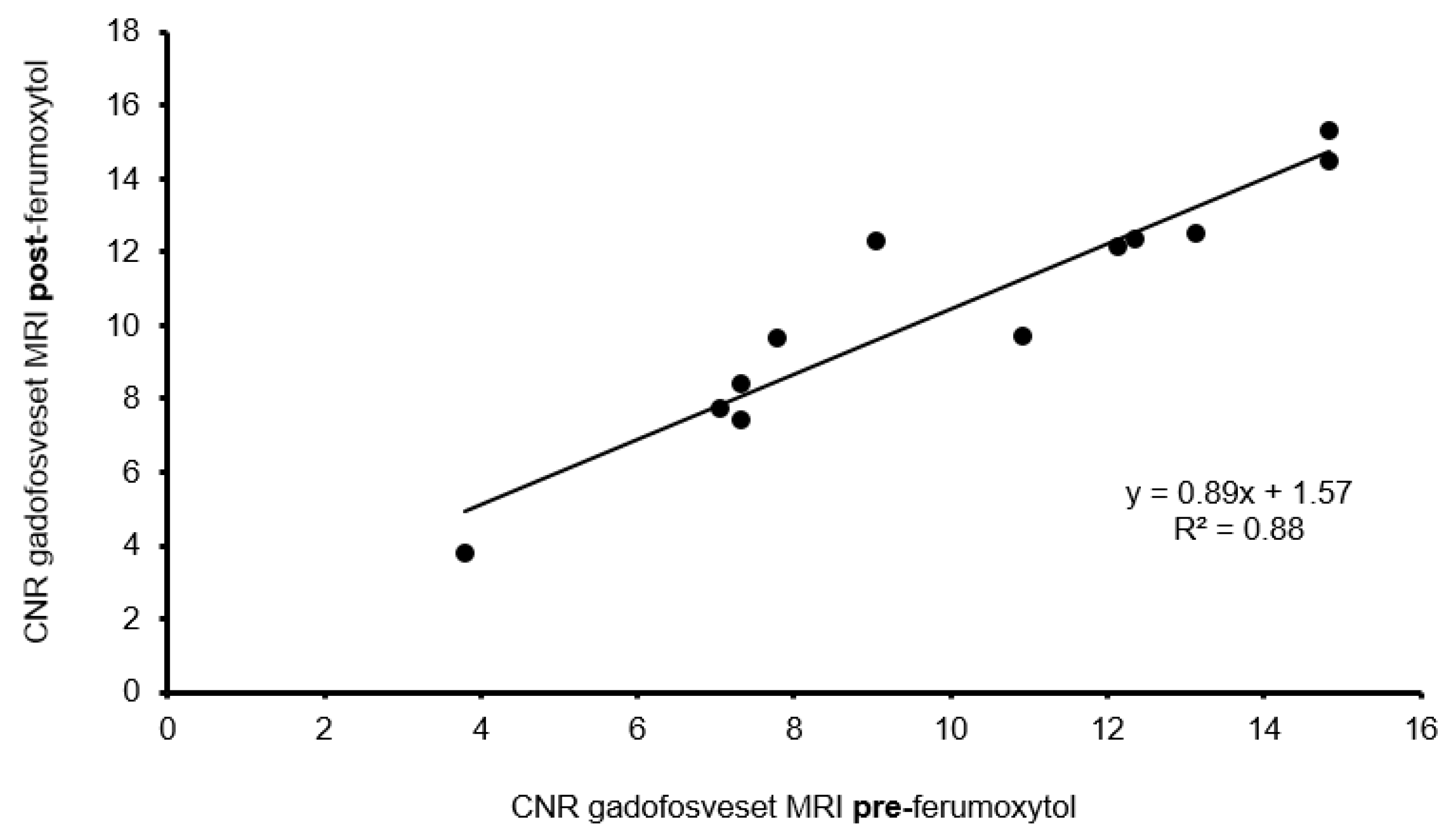
3.4.3. Influence of Ferumoxytol on Gadofosveset Signal Enhancement
3.5. Correlation between In Vivo and Ex Vivo Analysis
3.6. LA-ICP-MS for Analysis of Gadolinium Distribution in the Brachiocephalic Artery
4. Discussion
4.1. Limitations
4.2. Translational Study Design
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AAA | abdominal aortic aneurysm |
| ApoE-/- | apolipoprotein E-deficient |
| CNR | contrast-to-noise-ratio |
| ECM | extracellular matrix |
| EvG | Elastica-van-Gieson |
| FELASA | Federation of Laboratory Animal Science Associations |
| FEM | Forschungseinrichtung für experimentelle Medizin |
| FDA | Food and Drug Administration |
| FOV | field of view |
| Gd | Gadolinium |
| HE- | hematoxylin and eosin |
| HFD | high fat diet |
| LA-ICP-MS | laser coupled mass spectrometry |
| LDL | low-density lipoprotein |
| MI | myocardial infarction |
| MIP | maximum intensity projection |
| MR | magnetic resonance |
| MRI | magnetic resonance imaging |
| Ox-LDL | oxidized low-density lipoproteins |
| ROI | region of interest |
| SMCs | smooth muscle cell |
| TE | echo time |
| TI | inversions time |
| TIMP | tissue inhibitor of metalloproteinase |
| TOF | time of flight |
| TR | repetitions time |
| USPIO | ultrasmall superparamagnetic iron oxide |
| VSCM | vascular smooth muscle cell |
| 3D | three-dimensional |
| 2D | two-dimensional |
References
- Calcagno, C.; Ramachandran, S.; Millon, A.; Robson, P.M.; Mani, V.; Fayad, Z. Gadolinium-Based Contrast Agents for Vessel Wall Magnetic Resonance Imaging (MRI) of Atherosclerosis. Curr. Cardiovasc. Imaging Rep. 2013, 6, 11–24. [Google Scholar] [CrossRef] [Green Version]
- Qi, C.; Deng, L.; Li, D.; Wu, W.; Gong, L.; Li, Y.; Zhang, Q.; Zhang, T.; Zhang, C.; Zhang, Y. Identifying Vulnerable Atherosclerotic Plaque in Rabbits Using DMSA-USPIO Enhanced Magnetic Resonance Imaging to Investigate the Effect of Atorvastatin. PLoS ONE 2015, 10, e0125677. [Google Scholar] [CrossRef]
- Rafieian-Kopaei, M.; Setorki, M.; Doudi, M.; Baradaran, A.; Nasri, H. Atherosclerosis: Process, indicators, risk factors and new hopes. Int. J. Prev. Med. 2014, 5, 927–946. [Google Scholar] [PubMed]
- Reimann, C.; Brangsch, J.; Colletini, F.; Walter, T.; Hamm, B.; Botnar, R.M.; Makowski, M.R. Molecular imaging of the extracellular matrix in the context of atherosclerosis. Adv. Drug Deliv. Rev. 2017, 113, 49–60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Londoño, I.; Leclerc, Y.; Bendayan, M. Ultrastructural localization of endogenous albumin in human aortic tissue by protein A-gold immunocytochemistry. Am. J. Pathol. 1992, 140, 179–191. [Google Scholar] [PubMed]
- van Oostrom, O.; Velema, E.; Schoneveld, A.H.; de Vries, J.P.; de Bruin, P.; Seldenrijk, C.A.; de Kleijn, D.P.; Busser, E.; Moll, F.L.; Verheijen, J.H.; et al. Age-related changes in plaque composition: A study in patients suffering from carotid artery stenosis. Cardiovasc. Pathol. 2005, 14, 126–134. [Google Scholar] [CrossRef]
- Kong, C.H.; Lin, X.Y.; Woo, C.C.; Wong, H.C.; Lee, C.N.; Richards, A.M.; Sorokin, V.A. Characteristics of aortic wall extracellular matrix in patients with acute myocardial infarction: Tissue microarray detection of collagen I, collagen III and elastin levels. Interact. Cardiovasc. Thorac. Surg. 2013, 16, 11–15. [Google Scholar] [CrossRef] [PubMed]
- Phinikaridou, A.; Andia, M.E.; Protti, A.; Indermuehle, A.; Shah, A.; Smith, A.; Warley, A.; Botnar, R.M. Noninvasive magnetic resonance imaging evaluation of endothelial permeability in murine atherosclerosis using an albumin-binding contrast agent. Circulation 2012, 126, 707–719. [Google Scholar] [CrossRef] [Green Version]
- Aldecoa, C.; Llau, J.V.; Nuvials, X.; Artigas, A. Role of albumin in the preservation of endothelial glycocalyx integrity and the microcirculation: A review. Ann. Intensive Care 2020, 10, 85. [Google Scholar] [CrossRef]
- Phinikaridou, A.; Andia, M.E.; Lavin, B.; Smith, A.; Saha, P.; Botnar, R.M. Increased Vascular Permeability Measured With an Albumin-Binding Magnetic Resonance Contrast Agent Is a Surrogate Marker of Rupture-Prone Atherosclerotic Plaque. Circ. Cardiovasc. Imaging 2016, 9, e004910. [Google Scholar] [CrossRef] [Green Version]
- Doyle, B.; Caplice, N. Plaque Neovascularization and Antiangiogenic Therapy for Atherosclerosis. J. Am. Coll. Cardiol. 2007, 49, 2073–2080. [Google Scholar] [CrossRef] [Green Version]
- Ripa, R.S.; Kjær, A. Imaging Atherosclerosis with Hybrid Positron Emission Tomography/Magnetic Resonance Imaging. BioMed Res. Int. 2015, 2015, 914516. [Google Scholar] [CrossRef]
- Suh William, M.; Seto Arnold, H.; Margey Ronan, J.P.; Cruz-Gonzalez, I.; Jang, I.-K. Intravascular Detection of the Vulnerable Plaque. Circ. Cardiovasc. Imaging 2011, 4, 169–178. [Google Scholar] [CrossRef] [Green Version]
- Voros, S.; Rinehart, S.; Qian, Z.; Joshi, P.; Vazquez, G.; Fischer, C.; Belur, P.; Hulten, E.; Villines, T.C. Coronary Atherosclerosis Imaging by Coronary CT Angiography: Current Status, Correlation With Intravascular Interrogation and Meta-Analysis. JACC Cardiovasc. Imaging 2011, 4, 537–548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herborn, C.U.; Vogt, F.M.; Lauenstein, T.C.; Dirsch, O.; Corot, C.; Robert, P.; Ruehm, S.G. Magnetic resonance imaging of experimental atherosclerotic plaque: Comparison of two ultrasmall superparamagnetic particles of iron oxide. J. Magn. Reson. Imaging 2006, 24, 388–393. [Google Scholar] [CrossRef]
- Lepedda, A.J.; Zinellu, A.; Nieddu, G.; De Muro, P.; Carru, C.; Spirito, R.; Guarino, A.; Piredda, F.; Formato, M. Human Serum Albumin Cys34Oxidative Modifications following Infiltration in the Carotid Atherosclerotic Plaque. Oxidative Med. Cell. Longev. 2014, 2014, 690953. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rademakers, T.; Douma, K.; Hackeng, T.M.; Post, M.J.; Sluimer, J.C.; Daemen, M.J.; Biessen, E.A.; Heeneman, S.; van Zandvoort, M.A. Plaque-associated vasa vasorum in aged apolipoprotein E-deficient mice exhibit proatherogenic functional features in vivo. Arter. Thromb. Vasc. Biol. 2013, 33, 249–256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van der Donckt, C.; Van Herck, J.L.; Schrijvers, D.M.; Vanhoutte, G.; Verhoye, M.; Blockx, I.; Van Der Linden, A.; Bauters, D.; Lijnen, H.R.; Sluimer, J.C.; et al. Elastin fragmentation in atherosclerotic mice leads to intraplaque neovascularization, plaque rupture, myocardial infarction, stroke, and sudden death. Eur. Heart J. 2015, 36, 1049–1058. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sadat, U.; Usman, A.; Gillard, J.H. Imaging pathobiology of carotid atherosclerosis with ultrasmall superparamagnetic particles of iron oxide: An update. Curr. Opin. Cardiol. 2017, 32, 437–440. [Google Scholar] [CrossRef] [Green Version]
- Adams, L.C.; Brangsch, J.; Reimann, C.; Kaufmann, J.O.; Nowak, K.; Buchholz, R.; Karst, U.; Botnar, R.M.; Hamm, B.; Makowski, M.R. Noninvasive imaging of vascular permeability to predict the risk of rupture in abdominal aortic aneurysms using an albumin-binding probe. Sci. Rep. 2020, 10, 3231. [Google Scholar] [CrossRef] [PubMed]
- Reimann, C.; Brangsch, J.; Kaufmann, J.O.; Adams, L.C.; Onthank, D.C.; Thöne-Reineke, C.; Robinson, S.P.; Hamm, B.; Botnar, R.M.; Makowski, M.R. Dual-probe molecular MRI for the in vivo characterization of atherosclerosis in a mouse model: Simultaneous assessment of plaque inflammation and extracellular-matrix remodeling. Sci. Rep. 2019, 9, 13827. [Google Scholar] [CrossRef] [Green Version]
- Getz, G.S.; Reardon, C.A. Animal models of atherosclerosis. Arter. Thromb. Vasc. Biol. 2012, 32, 1104–1115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Man, J.J.; Beckman, J.A.; Jaffe, I.Z. Sex as a Biological Variable in Atherosclerosis. Circ. Res. 2020, 126, 1297–1319. [Google Scholar] [CrossRef] [PubMed]
- Cannon, C.P.; Braunwald, E.; McCabe, C.H.; Rader, D.J.; Rouleau, J.L.; Belder, R.; Joyal, S.V.; Hill, K.A.; Pfeffer, M.A.; Skene, A.M. Intensive versus Moderate Lipid Lowering with Statins after Acute Coronary Syndromes. N. Engl. J. Med. 2004, 350, 1495–1504. [Google Scholar] [CrossRef]
- Nissen, S.E.; Tuzcu, E.M.; Schoenhagen, P.; Crowe, T.; Sasiela, W.J.; Tsai, J.; Orazem, J.; Magorien, R.D.; O’Shaughnessy, C.; Ganz, P. Statin therapy, LDL cholesterol, C-reactive protein, and coronary artery disease. N. Engl. J. Med. 2005, 352, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Lavin, B.; Protti, A.; Lorrio, S.; Dong, X.; Phinikaridou, A.; Botnar, R.M.; Shah, A. MRI with gadofosveset: A potential marker for permeability in myocardial infarction. Atherosclerosis 2018, 275, 400–408. [Google Scholar] [CrossRef] [Green Version]
- Fayad, Z.A.; Swirski, F.K.; Calcagno, C.; Robbins, C.S.; Mulder, W.; Kovacic, J.C. Monocyte and Macrophage Dynamics in the Cardiovascular System: JACC Macrophage in CVD Series (Part 3). J. Am. Coll. Cardiol. 2018, 72, 2198–2212. [Google Scholar] [CrossRef]
- Knobloch, G.; Colgan, T.; Wiens, C.N.; Wang, X.; Schubert, T.; Hernando, D.; Sharma, S.D.; Reeder, S.B. Relaxivity of Ferumoxytol at 1.5 T and 3.0 T. Investig. Radiol. 2018, 53, 257–263. [Google Scholar] [CrossRef]
- Rong, J.X.; Shapiro, M.; Trogan, E.; Fisher, E.A. Transdifferentiation of mouse aortic smooth muscle cells to a macrophage-like state after cholesterol loading. Proc. Natl. Acad. Sci. USA 2003, 100, 13531. [Google Scholar] [CrossRef] [Green Version]
- Phinikaridou, A.; Andia, M.E.; Passacquale, G.; Ferro, A.; Botnar, R.M. Noninvasive MRI monitoring of the effect of interventions on endothelial permeability in murine atherosclerosis using an albumin-binding contrast agent. J. Am. Heart Assoc. 2013, 2, e000402. [Google Scholar] [CrossRef] [Green Version]
- Palombo, C.; Kozakova, M. Arterial stiffness, atherosclerosis and cardiovascular risk: Pathophysiologic mechanisms and emerging clinical indications. Vasc. Pharm. 2016, 77, 1–7. [Google Scholar] [CrossRef]
- Adiguzel, E.; Ahmad, P.J.; Franco, C.; Bendeck, M.P. Collagens in the progression and complications of atherosclerosis. Vasc. Med. 2009, 14, 73–89. [Google Scholar] [CrossRef] [Green Version]
- Anlamlert, W.; Lenbury, Y.; Bell, J. Modeling fibrous cap formation in atherosclerotic plaque development: Stability and oscillatory behavior. Adv. Differ. Equ. 2017, 2017, 195. [Google Scholar] [CrossRef] [Green Version]
- Stephan, J.P.; Mao, W.; Filvaroff, E.; Cai, L.; Rabkin, R.; Pan, G. Albumin Stimulates the Accumulation of Extracellular Matrix in Renal Tubular Epithelial Cells. Am. J. Nephrol. 2004, 24, 14–19. [Google Scholar] [CrossRef]
- Parmar, K.M.; Nambudiri, V.; Dai, G.; Larman, H.B.; Gimbrone, M.A., Jr.; García-Cardeña, G. Statins exert endothelial atheroprotective effects via the KLF2 transcription factor. J. Biol. Chem. 2005, 280, 26714–26719. [Google Scholar] [CrossRef] [Green Version]
- Usman, A.; Patterson, A.J.; Yuan, J.; Cluroe, A.; Patterson, I.; Graves, M.J.; Gillard, J.H.; Sadat, U. Ferumoxytol-enhanced three-dimensional magnetic resonance imaging of carotid atheroma- a feasibility and temporal dependence study. Sci. Rep. 2020, 10, 1808. [Google Scholar] [CrossRef]
- Smits, L.P.; Tiessens, F.; Zheng, K.H.; Stroes, E.S.; Nederveen, A.J.; Coolen, B.F. Evaluation of ultrasmall superparamagnetic iron-oxide (USPIO) enhanced MRI with ferumoxytol to quantify arterial wall inflammation. Atherosclerosis 2017, 263, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Alam, S.R.; Stirrat, C.; Richards, J.; Mirsadraee, S.; Semple, S.I.K.; Tse, G.; Henriksen, P.; Newby, D.E. Vascular and plaque imaging with ultrasmall superparamagnetic particles of iron oxide. J. Cardiovasc. Magn. Reson. 2015, 17, 83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sorokin, V.; Vickneson, K.; Kofidis, T.; Woo, C.C.; Lin, X.Y.; Foo, R.; Shanahan, C.M. Role of Vascular Smooth Muscle Cell Plasticity and Interactions in Vessel Wall Inflammation. Front. Immunol. 2020, 11, 599415. [Google Scholar] [CrossRef] [PubMed]
- de Carvalho, L.P.; Tan, S.H.; Ow, G.S.; Tang, Z.; Ching, J.; Kovalik, J.P.; Poh, S.C.; Chin, C.T.; Richards, A.M.; Martinez, E.C.; et al. Plasma Ceramides as Prognostic Biomarkers and Their Arterial and Myocardial Tissue Correlates in Acute Myocardial Infarction. JACC Basic Transl. Sci. 2018, 3, 163–175. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhang, H.; Hu, L.; Shi, H.; Liu, X.; Jia, J.; Sun, S.; Ou, Y.; Luo, X.; Zhou, G.; et al. Pravastatin attenuates atherosclerosis after myocardial infarction by inhibiting inflammatory Ly6Chigh monocytosis in apolipoprotein E knockout mice. J. Int. Med. Res. 2020, 48, 0300060520932816. [Google Scholar] [CrossRef] [PubMed]
- Toth, G.B.; Varallyay, C.G.; Horvath, A.; Bashir, M.R.; Choyke, P.L.; Daldrup-Link, H.E.; Dosa, E.; Finn, J.P.; Gahramanov, S.; Harisinghani, M.; et al. Current and potential imaging applications of ferumoxytol for magnetic resonance imaging. Kidney Int. 2017, 92, 47–66. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Möckel, J.; Brangsch, J.; Reimann, C.; Kaufmann, J.O.; Sack, I.; Mangarova, D.B.; Kader, A.; Taupitz, M.; Adams, L.C.; Keller, S.; et al. Assessment of Albumin ECM Accumulation and Inflammation as Novel In Vivo Diagnostic Targets for Multi-Target MR Imaging. Biology 2021, 10, 964. https://doi.org/10.3390/biology10100964
Möckel J, Brangsch J, Reimann C, Kaufmann JO, Sack I, Mangarova DB, Kader A, Taupitz M, Adams LC, Keller S, et al. Assessment of Albumin ECM Accumulation and Inflammation as Novel In Vivo Diagnostic Targets for Multi-Target MR Imaging. Biology. 2021; 10(10):964. https://doi.org/10.3390/biology10100964
Chicago/Turabian StyleMöckel, Jana, Julia Brangsch, Carolin Reimann, Jan O. Kaufmann, Ingolf Sack, Dilyana B. Mangarova, Avan Kader, Matthias Taupitz, Lisa C. Adams, Sarah Keller, and et al. 2021. "Assessment of Albumin ECM Accumulation and Inflammation as Novel In Vivo Diagnostic Targets for Multi-Target MR Imaging" Biology 10, no. 10: 964. https://doi.org/10.3390/biology10100964
APA StyleMöckel, J., Brangsch, J., Reimann, C., Kaufmann, J. O., Sack, I., Mangarova, D. B., Kader, A., Taupitz, M., Adams, L. C., Keller, S., Ludwig, A., Hamm, B., Botnar, R. M., & Makowski, M. R. (2021). Assessment of Albumin ECM Accumulation and Inflammation as Novel In Vivo Diagnostic Targets for Multi-Target MR Imaging. Biology, 10(10), 964. https://doi.org/10.3390/biology10100964





