Abstract
The aim of this study is to synthesize an organic core-shell co-polymer with a different glass transition temperature (Tg) between the core and the shell that can be used for several applications such as the selective debonding of coatings or the release of encapsulated materials. The co-polymer was synthesized using free radical polymerization and was characterized with respect to its morphology, composition and thermal behavior. The obtained results confirmed the successful synthesis of the co-polymer copolymer poly(methyl methacrylate)@poly(methacrylic acid-co-ethylene glycol dimethacrylate), PMMA@P(MAA-co-EGDMA), which can be used along with water-based solvents. Furthermore, the Tg of the polymer’s core PMMA was 104 °C, while the Tg of the shell P(MAA-co-EGDMA) was 228 °C, making it appropriate for a wide variety of applications. It is worth mentioning that by following this specific experimental procedure, methacrylic acid was copolymerized in water, as the shell of the copolymer, without forming a gel-like structure (hydrogel), as happens when a monomer is polymerized in aqueous media, such as in the case of super-absorbent polymers. Moreover, the addition and subsequent polymerization of the monomer methyl methacrylate (MAA) into the mixture of the already polymerized PMMA resulted in a material that was uniform in size, without any agglomerations or sediments.
1. Introduction
In recent years, the need to synthesize new core-shell polymers has increased as chemical procedures have required accuracy and effectiveness more than ever. Micro- and nanostructured core-shell copolymers solve many problems of materials industry, medicine and infrastructure, while remaining practically easy to synthesize and apply [1].
Core-shell polymers can be manufactured through a vast number of experimental means, including various methods such as electrospinning [2] and solvent-free and large scale processes [3], as well as ultrasound-assisted methods [4]. Furthermore, material properties have been studied under several circumstances and environmental conditions, such as laser fields, mini-emulsions, pre-emulsions and acid media [5,6].
Many attempts have been made to synthesize a novel core-shell in the last five years. In order to cover the continuously increasing number of modern needs for core-shells, scientists have tried many new chemistry-based approaches, while attempting to keep the total cost relatively low. A few novel approaches from the literature include Hu et al., who wrote a review about a single-component core-shell [7]; Han et al., who developed a core-shell magnetic material with applications in food chemistry [8]; Gul et al., who managed to imprint a polymer onto a core-shell material with magnetic properties [9]; and Farboudi et al., who synthesized core-shell nanofibers grafted with chitosan for the controlled release of drugs [10]. Furthermore, several studies were conducted by our group based on the production of core-shell particles and microcapsules that can be used either for the removal of chlorides from water or as additives into coatings that can protect metal alloys from corrosion [11].
In recent years, research focusing on this type of polymer has increased. Core-shell polymers have been used for numerous biological and medical applications, such as controlled calcium and drug delivery, antibacterial applications, stem cells, wound healing, virus chromatography, cancer and hyperthermia therapy and glaucoma treatment [12,13,14]. They have also been used for electronics and energy applications such as solar cells, energy storage, batteries, photovoltaics, fuel cells and sensors, as they enhance electrical conductivity and electrical properties in general [15,16,17,18,19,20]. However, the majority of core-shell materials concern engineering applications such as mechanical reinforcements, where they are used as fillers for their high strength and tensile performance [21] as well as for their high interfacial strength between the cell and the core [22]. They are also used for toughening epoxy resins [23].
Additionally, lots of synthetic processes and characterization techniques have been deployed in order to investigate core-shell materials more accurately and fully understand their effects on other compounds. Various methods are already known, with in situ synthesis, emulsion polymerization, catalysis, use of particles as sacrificial templates, and use of reactive surfactants being some of the most popular among them research in this field remains ongoing [24,25,26,27]. Moreover, acidic media, transmission electron microscopy (TEM) and other numerous techniques have shed light on the structure, morphology and characteristics of these materials [28,29,30,31].
In this study, the concept was to synthesize core-shell polymers that could be triggered by heat. Therefore, the shell can be selectively weakened by an external trigger (heat), while leaving the core exposed, to release its encapsulations at a specific moment in a given targeted area. For this to happen, it is necessary that the polymers that form the core and the cell have different glass transition temperatures (Tg), meaning that the Tg of the shell should be higher than the Tg of the core [32]. According to this idea, a core-shell polymer (core@shell) was produced—poly(methyl methacrylate)@poly(methacrylic acid), PMMA@P(MAA-co-EGDMA)—in which the Tg of the core and the shell were 104 and 228 °C, respectively. This organic polymer was characterized via scanning electron microscopy (SEM), Fourier-transform infrared spectroscopy (FT-IR), TEM, thermogravimetric analysis (TGA) and differential scanning calorimetry (DSC). It should be mentioned, that, following this experimental path, MAA was polymerized via classic radical polymerization using water as solvent, without forming a hydrogel. The produced material was uniform in size, without any agglomerations or sediments.
Finally, it may be remarked that the added value of our work resides in the fact that this copolymer combined hydrophilic and lipophilic parts and different glass transition temperatures, while it was synthesized in a one-pot synthetic process.
2. Materials and Methods
2.1. Materials
Analytical reagent grade chemicals were used. Methacrylic acid (MAA, Fisher Chemicals, Waltham, MA, USA), methyl methacrylate (MMA, Sigma Aldrich, St. Louis, Missouri, USA), ethylene glycol dimethacrylate (EGDMA, Sigma Aldrich, St. Louis, MO, USA) and potassium peroxodisulphate (KPS, Acros Organics, Geel, Belgium) were used as received without further purification. Also, distilled water was used. Methacrylic acid and methyl methacrylate were distilled under vacuum prior to use.
2.2. Synthesis of Core-Shell Copolymer
Radical polymerization was performed for the synthesis of the thermo-responsive core-shell co-polymer PMMA@(PMAA-co-EGDMA). First, 20 mL of methyl methacrylate was dissolved in 500 mL of water and nitrogen gas was flushed into a round, three-neck, 1 L glass reaction flask. Then, 0.2 g potassium persulfate (KPS-initiator) was added and the temperature was adjusted to 90 °C for the initiation of the polymerization. A glass reflux condenser was used to recycle the solvent during the polymerization reaction. After 4 h, when the polymerization of methyl methacrylate was completed, 20 mL of distilled methacrylic acid was added to the flask together with 2 mL of crosslinker (EGDMA) and 0.2 g of initiator (KPS). The reaction was left to react for 4 h under vigorous stirring at 90 °C. After this, the contents of the flask were centrifuged and gently dried in an oven at 40 °C for 24 h. Figure 1 illustrates the schema of the produced core-shell copolymer PMMA@P(MAA-co-EGDMA). The produced thermo-responsive core-shell nanostructured co-polymer was characterized in respect to its morphology, composition and structure.
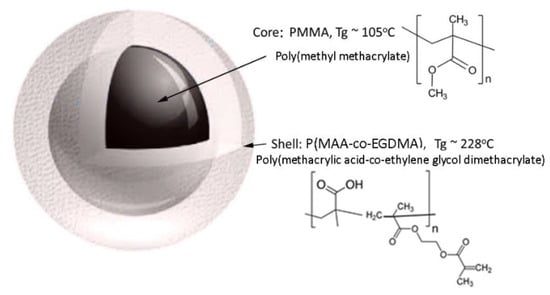
Figure 1.
Schematic representation of the produced core-shell copolymer PMMA@P(MAA-co-EGDMA).
2.3. Characterization
The morphology and composition of the produced materials were estimated with an ultra-high resolution scanning electron microscope (UHR-SEM) using NOVA NANOSEM 230 (FEI Company) coupled with a Hitachi electron microscope TM3030 and an energy dispersive X-ray spectrophotometer (EDS) (QUANTAX 70) [33]. In addition, the morphology of the core-shell copolymers was also characterized by TEM (model FEI CM20), operated at an accelerating voltage of 200 kV [34,35,36].
The FT-IR spectra of the produced polymers was analyzed via an attenuated total reflectance FT-IR instrument, the Cary 630 spectrometer (Agilent), with a resolution of 4 cm−1 and an operating wavelength range of 4000–400 cm−1 [34]. Thermal analysis of the produced polymers was conducted via a TGA/DSC instrument, the STA 449 F5 Jupiter. The system consists of an SiC furnace with an operation temperature varying between 25 and 1550 °C. The gas used was nitrogen at a volumetric rate of 50 mL/min and at a heating rate of 20 °C/min [34]. The size distribution of the produced polymers was estimated via dynamic light scattering (DLS) measurements using a Zetasizer Nano ZS.
Gel permeated chromatography (GPC) analysis was performed using a modular instrument consisting of a waters model 510 pump, a U6K sample injector, a 401 refractometer and a set of four l µm-Styragel columns with a continuous porosity range of 106–103 Å. The carrier solvent was CHCl3 at a flow rate of 1 mL/min. The system was calibrated with seven polystyrene (PS) standards having molecular weights (MWs) between 1000 and 900,000 g/mol. The system was operated at 25 °C.
3. Results and Discussion
3.1. Morphological Characterization (SEM and TEM)
Figure 2a illustrates the SEM image of the produced core-shell copolymer PMMA@P(MAA-co-EGDMA). It can be seen that the synthetic process of producing the material resulted in a copolymer with a spherical morphology. Taking into account Figure 2b, which depicts a corresponding particle size distribution histogram with values from 63 core-shell copolymer particles, it can be seen that the diameter of these spheres was in the nanoscale, ranging from approximately 160 to 210 nm [37]. These properties indicate that this material’s volume was appropriate for its use in several applications that would require the capsules to take up as little space as possible (e.g., incorporation into coatings or layers). It can be seen from the transmission electron microscopy images (Figure 3) that there was a thin shell on the outside of the polymeric core [38,39].
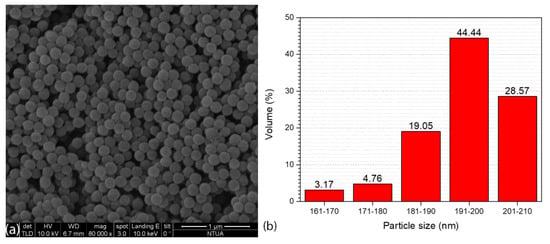
Figure 2.
SEM image (a) and particle size distribution histogram (b) of the PMMA@P(MAA-co-EGDMA) core-shell copolymer.
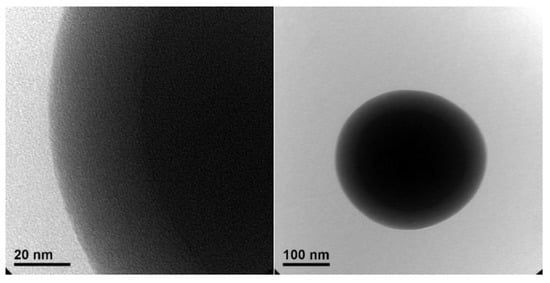
Figure 3.
TEM images of the core-shell copolymer PMMA@P(MAA-co-EGDMA).
The thin shell of P(MAA-co-EGDMA) around the core of PMMA protects and surrounds the core, while on the other hand it can easily be weakened and removed without the use of a great amount of energy, if an application requires the core to be exposed relatively easy for the selective release of ingredients encapsulated in the core (e.g., targeted delivery or coatings).
3.2. Thermal Analysis (TGA and DSC)
Thermogravimetric Analysis was performed in inert atmosphere with a gas flow of 50 mL/min and a heating rate of 5 °C/min. Figure 4 illustrates the TGA/DSC graphs of the produced (a) PMMA core and (b) PMMA@P(MAA-co-EGDMA) core-shell copolymer. From the TGA curve of Figure 4a, it can be seen that the weight loss of the PMMA (core) was much more abrupt than in the case of the core-shell copolymer. This was expected, because the presence of the shell in the second case allowed a slower degradation, which was also smoother. The slight increase in mass at the start of the measurement is attributable to the scale drift of the instrument. This effect occurs when the measurement starts after the temperature has been increased from room temperature to 100 °C at an accelerating rate.
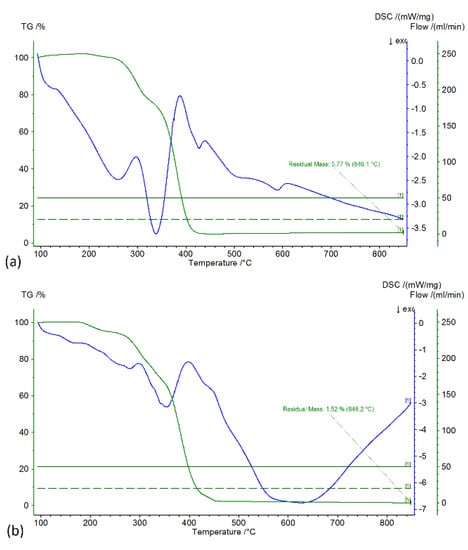
Figure 4.
TGA/DSC graphs of the produced PMMA core (a) and PMMA@P(MAA-co-EGDMA) core-shell copolymer (b).
The DSC endothermic curve at 400 °C on the same graph corresponds to the polymer’s degradation. The exothermic curve at 340 °C can be attributed to the C-OCH3 bond rupture, which enriched the nitrogen atmosphere with oxygen and led to partial oxidation of the polymer [40]. From the TGA curve of the Figure 4b, it can be readily observed that there was a first weight loss between 200 and 290 °C that can be attributed to the degradation of PMMA, and the second sharp weight loss between 290 and 440 °C can be attributed to the degradation of P(MAA-co-EGDMA). Furthermore, the DSC curve (blue) demonstrates two endothermic curves, the first small one at 280 °C and the second big one at 360 °C, which correspond to the two thermal decompositions of PMMA and P(MAA-co-EGDMA), respectively. The residual mass at 850 °C is the remaining carbon, approximately 1.5% of the initial mass [41].
Finally, it should be mentioned that an exothermic DSC curve can be observed in the TGA/DSC graphs of Figure 4a at about 105 °C, where the Tg of the PMMA is 104 °C. Additionally, in the TGA/DSC graphs of Figure 4b, there is an exothermic DSC curve at about 230 °C where the Tg of the PMAA is 228 °C. These two curves correspond to the respective Tg of the two polymers, although their intensity was low, due to the slight decrease in bond strength after the Tg point. On the other hand, the DSC exothermic curves corresponding to the decomposition had high intensity.
3.3. Attenuated Total Reflectance Fourier-Transform Infrared Spectroscopy (FT-IR)
The FT-IR graphs concerning the PMMA core as well as the PMMA@P(MAA-co-EGDMA) core-shell copolymer are illustrated in Figure 5 and the corresponding peaks are tabulated in Table 1. The peak at 1156 cm−1 corresponds to the -CH2- bond wagging of the aliphatic chains of both PMMA and PMAA. Moreover, the peak at 475 cm−1 corresponds to the C-C=O in the plane deformation vibration of the carboxyls of the two polymers, while the peaks at 747 and 840 cm−1 can be ascribed to the C-H deformation vibration and the peak at 963 cm−1 is related to the C-O stretching vibration. The peak at 1187 cm−1 can be attributed to the -OCH3 vibration (methyl methacrylate) [42]. Furthermore, the peaks at 1237, 1384 and 1435 cm−1 can be ascribed to the -CH- bending of the methyl groups, which were present in both compounds, as well as to the -CH3 deformation of the branches of the main polymer chain [43]. A C-H scissor vibration can be seen at 1474 cm−1, while the C=C stretching vibration can be observed at 1636 cm−1. Additionally, the -C=O stretch can be noticed at 1716 cm−1. The stretching of -CH3 is visible at 2925 cm−1, while the stretching of -CH2 can be seen at 2996 cm−1 [44]. Finally, the -O-H stretches of methacrylic acid can be seen in the region of 3400 cm−1 [45].
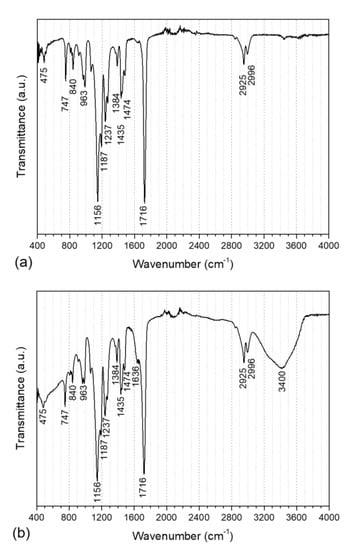
Figure 5.
FT-IR spectra of the PMMA core polymer (a) and PMMA@P(MAA-co-EGDMA) core-shell copolymer (b).

Table 1.
FT-IR peak analysis.
3.4. Dynamic Light Scattering (DLS)
Dynamic light scattering (DLS) analysis is very useful for polymer characterization, because it can offer important information about organic compounds. DLS can be used to monitor polymer formation processes, in diffusion analysis and to characterize polymer solutions and surfactants, as well as to investigate natural polymers [46]. Moreover, a lot of properties can be determined via DLS analysis such as viscosity, polymerization mechanisms, molecular weight and molecular size, even particle size and particle size distribution. The study of reaction kinetics during polymerization and the detection of polymeric structures in emulsions are also feasible using DLS [47]. In our case, about 10 mg of the solid polymer was dispersed in 5 mL of water in a small vial. The vial was left under supersonic vibrations for 10 min so that the polymer would be sufficiently dispersed for DLS characterization. The Z-Average diameter was 446 nm, while the polydispersity index (PDI) was 0.077 (Figure 6). These facts denote that the sample was uniform in size distribution, without any agglomerations or sediments [48]. It should be mentioned that the average diameter of the produced PMMA@P(MAA-co-EGDMA) core-shell copolymer determined by DLS was higher than the values illustrated in the particle size distribution histogram of Figure 2b. The reason for this is that the hydrodynamic volume of the materials was measured as part of the DLS method. In our case, the produced PMMA@P(MAA-co-EGDMA) core-shell copolymer interacted with the solvent that was used for the measurement (water), resulting in an expansion of its hydrodynamic volume.
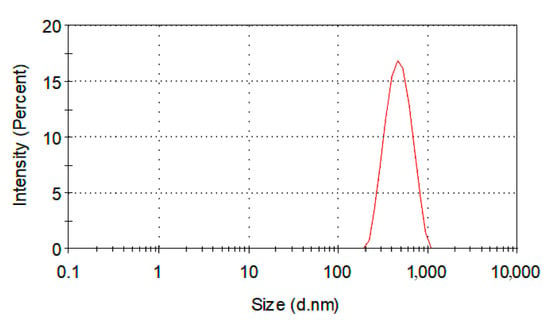
Figure 6.
Particle size distribution by intensity of PMMA@P(MAA-co-EGDMA) core-shell copolymer.
3.5. Gel Permeated Chromatography
GPC was used to estimate the molecular weights of the produced materials. Unfortunately, it was not feasible to determine the molecular weight of the PMMA@P(MAA-co-EGDMA) core-shell copolymer due to solubility difficulties. In detail, GPC measurements were conducted using four different chromatography solvents including water with acetonitrile, chloroform, tetrahydrofuran, and dimethylformamide (DMF). The PMMA (core) was fully soluble in DMF, allowing a GPC graph to be obtained. On the other hand, the PMMA@P(MAA-co-EGDMA) core-shell copolymer was not soluble in any of these four solvents because it formed a gel-like two-phase structure that looked like an emulsion or suspension in the solvents. It should be mentioned that this final remark underlines the successful synthesis of the core-shell copolymer between the lipophilic PMMA and the hydrophilic PMAA, although it was not possible to obtain a molecular weight measurement. The distinctive ability of the instrument was up to a million Daltons. Judging from the GPC graph (Figure 7), it can be observed that the polydispersity index of the PMMA was relatively high, as there was a very wide distribution of molecular weights after the elution of the polymer (Table 2). The molecular weight ranged from one million to a few thousand Daltons. It should be noted that the first inverse peak (at an approximate elution time of 5 min) is attributable to the solvent and was not taken under consideration.
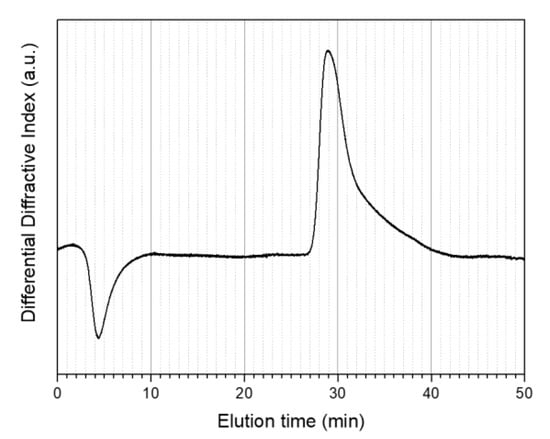
Figure 7.
Gel permeated chromatography (GPC) traces of the PMMA (core) polymer.

Table 2.
Tabulated values of the PMMA core polymer GPC measurement.
3.6. Outcome Analysis
The Yield(%) of the polymerization reaction for the synthesis of PMMA@P(MAA-co-EGDMA) core-shell copolymer can be calculated from the following Equations (1)–(3):
where mMMA is the theoretical mass of the MMA, mMAA is the theoretical mass of the MAA, dMMA is the density of the MMA, dMAA is the density of the MAA, VMMA is the volume of the MMA, VMAA is the volume of the MAA, and mexpe is the mass of the copolymer after the termination of the experiment.
We considered the initiator and crosslinker to have no contribution to the yield of the reaction. After drying, we obtained mexpe = 32.1 g of the PMMA@P(MAA-co-EGDMA) core-shell copolymer. Theoretically, 18.8 g of MMA and 20.3 g of MAA react, resulting in 39.1 g of product. The surplus amount of MAA (20.3 g − 18.8 g = 1.5 g) was added just to make sure that there would be enough MAA to form the P(MAA-co-EGDMA) shell. According to Equation (3), the Yield(%) of the polymerization reaction for the synthesis of the PMMA@P(MAA-co-EGDMA) core-shell copolymer was 78.1(%). Concerning the kinetics of the reaction, free radical polymerization started at a slow rate, but quickly accelerated, forming the polymer. Temperature plays a significant role in the polymerization reaction, as the initiator is activated at about 90 °C, producing the free radicals that polymerize the monomers [49,50,51,52].
Judging from the results of the different characterization techniques, it can be concluded that the product had a spherical shape at a nanoscale, with a very thin shell of P(MAA-co-EGDMA) around the core of the PMMA. FT-IR analysis showed peaks in the polymer’s graph corresponding both to PMMA and PMAA bonds, while the SEM and TEM images confirmed its morphological properties. The morphological characterization also provided information about the composition of the product in regards to the two polymers, which was further demonstrated by the thermal analysis. The larger size of the core and the thinness of the shell exhibit advantages for potential applications, as the core could encapsulate larger amounts of other nanocompounds while the shell breaks smoothly to release the encapsulations. This is the case of delivery of antioxidants and other substances as well as adsorption and catalysis [53,54].
Recent literature on this subject by Peralta et al. [55] demonstrated the grafting of thermo-responsive polymers on inorganic compounds. However, these materials were activated at a temperature of 40 °C for drug delivery, while the copolymer in this study had a shell with a Tg of 228 °C, enabling its use at elevated temperatures for different applications such as incorporation in coatings. To that end, Wang et al. [56] synthesized lipophilic core-shell microspheres with inorganic shells. Nonetheless, the goal in the present research was to have both a polymeric core and a polymeric shell, and preferably for them to be hydrophilic. This aim was achieved using the PMAA as a compound of the shell. While Huang et al. have shown that the size of a core-shell copolymer can be tuned by following a specific method [37], that method requires an emulsion polymerization that is substantially more complex than the conventional radical polymerization proposed in the present work. Additionally, other researchers have synthesized core-shell compounds using specialized laboratory equipment and elaborate experimental paths [57,58,59,60] using catalysts [24] or by performing in situ processes [61].
Core-shell copolymers, both organic and inorganic, are very promising materials for several applications in the near future. Specifically, they can enhance the stability of crystal structures [62] and the responsiveness of sensors [63]. In addition, they increase the reaction activity of composite catalysts [64], have contributed to greener syntheses using natural plant products [65] and can also be very useful in magnetic applications [66]. It should be mentioned that core-shell polymers could play a significant role in effectively designing new multifunctional active materials for battery cells [67].
These applications notwithstanding, the simple synthesis discussed in this study was designed to be able to be performed in vitro, employing common experimental techniques and laboratory instruments without using intricate means for the production of the final product. Most importantly, the use of distilled water as the solvent/medium of the polymerization reaction is worth mentioning, due to the fact that most chemical routes nowadays continue to use expensive organic solvents to produce these or similar types of materials.
4. Conclusions
Following a simple experimental process of a two-step radical polymerization, a novel core-shell polymer was formed with a uniform size distribution, homogeneous spherical shape and thin shell around the polymeric core, without any admixtures. The novelty of this study is the relatively easy experimental setup and the fact that the copolymer exhibited different glass transition temperatures between the core and the shell, rendering the compound suitable for several applications. In addition, methacrylic acid was successfully polymerized as the shell in water, without forming a gel. This phenomenon was possible due to the already polymerized methyl methacrylate in the mixtures, which allowed the second monomer to be polymerized around the core. The produced core-shell copolymer was thoroughly characterized in respect of its morphology, structure, composition and thermal behavior. The results confirmed the successful synthesis of the material.
Author Contributions
Conceptualization, P.G. and I.A.K.; methodology, P.G. and I.A.K.; software, P.G. and I.A.K.; validation, P.G., I.A.K. and C.A.C.; formal analysis, P.G. and I.A.K.; investigation, P.G. and I.A.K.; resources, P.G. and I.A.K.; data curation, P.G.; writing—original draft preparation, P.G. and I.A.K.; writing—review and editing, I.A.K. and C.A.C.; visualization, P.G. and I.A.K.; supervision, C.A.C.; project administration, C.A.C.; funding acquisition, C.A.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the HORIZON 2020 Collaborative project “DECOAT” (Recycling of coated and painted textile and plastic materials, Grant agreement no.: 814505).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ramli, R.A.; Laftah, W.A.; Hashim, S. Core–shell polymers: A review. RSC Adv. 2013, 3, 15543. [Google Scholar] [CrossRef]
- Afshar, S.; Rashedi, S.; Nazockdast, H.; Ghazalian, M. Preparation and characterization of electrospun poly(lactic acid)-chitosan core-shell nanofibers with a new solvent system. Int. J. Biol. Macromol. 2019, 138, 1130–1137. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.S.M.; Eisa, W.H.; Abouelsayed, A. Solvent-free and large-scale preparation of silver@polypyrrole core@shell nanocomposites; structural properties and terahertz spectroscopic studies. Compos. Part. B Eng. 2019, 176, 107289. [Google Scholar] [CrossRef]
- Balakumar, V.; Kim, H.; Manivannan, R.; Kim, H.; Ryu, J.W.; Heo, G.; Son, Y.A. Ultrasound-assisted method to improve the structure of CeO2@polyprrole core-shell nanosphere and its photocatalytic reduction of hazardous Cr(6). Ultrason. Sonochem. 2019, 59, 104738. [Google Scholar] [CrossRef]
- Anitha, B.; Nithiananthi, P. Oscillator strength and carrier dynamics in type I and inverted type I spherical core/ shell nanostructures under external laser field. Superlattices Microstruct. 2019, 135, 106288. [Google Scholar] [CrossRef]
- Izu, N.; Uchida, T.; Itoh, T.; Shin, W. Decreasing the shell ratio of core-shell type nanoparticles with a ceria core and polymer shell by acid treatment. Solid State Sci. 2018, 85, 32–37. [Google Scholar] [CrossRef]
- Hu, H.; Lin, Y.; Hu, Y.H. Synthesis, structures and applications of single component core-shell structured TiO2: A review. Chem. Eng. J. 2019, 375, 122029. [Google Scholar] [CrossRef]
- Han, J.; Wang, L.; Wang, L.; Li, C.; Mao, Y.; Wang, Y. Fabrication of a core-shell-shell magnetic polymeric microsphere with excellent performance for separation and purification of bromelain. Food Chem. 2019, 283, 1–10. [Google Scholar] [CrossRef]
- Gul, S.; Shah, N.; Arain, M.B.; Rahman, N.; Rehan, T.; Ul-Islam, M.; Ullah, M.W.; Yang, G. Fabrication of magnetic core shell particles coated with phenylalanine imprinted polymer. Polym. Test. 2019, 75, 262–269. [Google Scholar] [CrossRef]
- Farboudi, A.; Nouri, A.; Shirinzad, S.; Sojoudi, P.; Davaran, S.; Akrami, M.; Irani, M. Synthesis of magnetic gold coated poly (epsilon-caprolactonediol) based polyurethane/poly(N-isopropylacrylamide)-grafted-chitosan core-shell nanofibers for controlled release of paclitaxel and 5-FU. Int. J. Biol. Macromol. 2019, 150, 1130–1140. [Google Scholar] [CrossRef]
- Karaxi, E.K.; Kartsonakis, I.A.; Charitidis, C.A. Assessment of Self-Healing Epoxy-Based Coatings Containing Microcapsules Applied on Hot Dipped Galvanized Steel. Front. Mater. 2019, 6, 222. [Google Scholar] [CrossRef]
- Jabbar, T.A.; Ammar, S.H. Core/shell phosphomolybdic acid-supported magnetic silica nanocomposite (Ni@SiO2-PMo): Synthesis, characterization and its application as a recyclable antibacterial agent. Colloid Interface Sci. Commun. 2019, 33, 100214. [Google Scholar] [CrossRef]
- Amagat Molas, J.; Chen, M. Injectable PLCL/gelatin core-shell nanofibers support noninvasive 3D delivery of stem cells. Int. J. Pharm. 2019, 568, 118566. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Fang, A.; Wang, F.; Li, H.; Jin, Q.; Huang, L.; Fu, C.; Zeng, J.; Jin, Z.; Song, X. Core-shell lipid-polymer nanoparticles as a promising ocular drug delivery system to treat glaucoma. Chin. Chem. Lett. 2019, 31, 494–500. [Google Scholar] [CrossRef]
- Wang, P.; Ji, Y.; Shao, Q.; Li, Y.; Huang, X. Core@shell structured Au@SnO2 nanoparticles with improved N2 adsorption/activation and electrical conductivity for efficient N2 fixation. Sci. Bull. 2019, 65, 350–358. [Google Scholar] [CrossRef]
- Shabzendedar, S.; Modarresi-Alam, A.R.; Noroozifar, M.; Kerman, K. Core-shell nanocomposite of superparamagnetic Fe3O4 nanoparticles with poly(m-aminobenzenesulfonic acid) for polymer solar cells. Org. Electron. 2019, 77, 105462. [Google Scholar] [CrossRef]
- Pavlenko, M.; Siuzdak, K.; Coy, E.; Załęski, K.; Jancelewicz, M.; Iatsunskyi, I. Enhanced solar-driven water splitting of 1D core-shell Si/TiO2/ZnO nanopillars. Int. J. Hydrogen Energy 2019, 45, 26426–26433. [Google Scholar] [CrossRef]
- Wang, D.; An, Y.; Gao, S. Synthesis and characterization of urchin-like CuO nanorod/TiCu-based metallic glass core-shell powders with surface photovoltage performance. Appl. Surf. Sci. 2019, 506, 144871. [Google Scholar] [CrossRef]
- Kawasaki, D.; Maeno, K.; Yamada, H.; Sueyoshi, K.; Hisamoto, H.; Endo, T. TiN-contained polymer-metal core-shell structured nanocone array: Engineering of sensor performance by controlling plasmonic properties. Sens. Actuators B Chem. 2019, 299, 126932. [Google Scholar] [CrossRef]
- Hu, Y.; Wang, H.; Liu, D.; Lin, G.; Wan, J.; Jiang, H.; Lai, X.; Hao, S.; Liu, X. Lychee-like ZnO/ZnFe2O4 core-shell hollow microsphere for improving acetone gas sensing performance. Ceram. Int. 2019, 46, 5960–5967. [Google Scholar] [CrossRef]
- Cheng, J.; Wang, J.; Yun, Y.; Rui, J.; Zhao, W.; Li, F. A novel core-shell structure reinforced Zr-based metallic glass composite with combined high strength and good tensile ductility. J. Alloy. Compd. 2019, 803, 413–416. [Google Scholar] [CrossRef]
- Jia, E.; Zhao, S.; Shangguan, Y.; Zheng, Q. Toughening mechanism of polypropylene bends with polymer particles in core-shell structure: Equivalent rubber content effect related to core-shell interfacial strength. Polymer 2019, 178, 121602. [Google Scholar] [CrossRef]
- Wang, J.; Xue, Z.; Li, Y.; Li, G.; Wang, Y.; Zhong, W.-H.; Yang, X. Synergistically effects of copolymer and core-shell particles for toughening epoxy. Polymer 2018, 140, 39–46. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, Y.; Lv, X.; Mao, C.; Zhou, Y.; Wu, W.; Zhang, H.; Huang, Z. Synthesis of polymeric ionic liquids mircrospheres/Pd nanoparticles/CeO2 core-shell structure catalyst for catalytic oxidation of benzyl alcohol. J. Taiwan Inst. Chem. Eng. 2019, 107, 161–170. [Google Scholar] [CrossRef]
- Lynch, D.E.; Fellows, A.C.; Wilcock, R.; Sethi, S.; Armour, S.C.; Conteh, L. The use of poly(1-methylpyrrol-2-ylsquaraine) particles as a sacrificial template for the preparation of core-shell materials. Mater. Chem. Phys. 2019, 227, 163–169. [Google Scholar] [CrossRef]
- Cao-Luu, N.-H.; Pham, Q.-T.; Yao, Z.-H.; Wang, F.-M.; Chern, C.-S. Synthesis and characterization of poly(N-isopropylacrylamide-co-N,N′-methylenebisacrylamide-co-acrylamide) core—Silica shell nanoparticles by using reactive surfactant polyoxyethylene alkylphenyl ether ammonium sulfate. Eur. Polym. J. 2019, 120, 109263. [Google Scholar] [CrossRef]
- Gasaymeh, S.S.; Almansoori, N.N. Novel Formation Mechanism of Ag/PANI/PVP Core-Shell Nanocomposites. Results Phys. 2019, 16, 102882. [Google Scholar] [CrossRef]
- Park, S. Complex core-shell morphologies of block copolymers revealed beneath the surface. Appl. Surf. Sci. 2019, 494, 309–314. [Google Scholar] [CrossRef]
- Navin, K.; Kurchania, R. Structural, magnetic and electrochemical properties of LSMO-ZnO core-shell nanostructure. Mater. Chem. Phys. 2019, 234, 25–31. [Google Scholar] [CrossRef]
- Khanal, A.; Inoue, Y.; Yada, M.; Nakashima, K. Synthesis of silica hollow nanoparticles templated by polymeric micelle with core-shell-corona structure. J. Am. Chem Soc. 2007, 129, 1534–1535. [Google Scholar] [CrossRef]
- Wang, Y.; Rong, Z.; Tang, X.; Cao, S.; Chen, X.; Dang, W.; Wu, L. The design of scorodite@FeOOH core-shell materials and its stability treatment for arsenide. Appl. Surf. Sci. 2019, 496, 143719. [Google Scholar] [CrossRef]
- Schroffenegger, M.; Reimhult, E. Thermoresponsive Core-Shell Nanoparticles and Their Potential Applications. Compr. Nanosci. Nanotechnol. 2019, 2, 145–170. [Google Scholar] [CrossRef]
- Balaskas, A.C.; Kartsonakis, I.A.; Kordas, G.; Cabral, A.M.; Morais, P.J. Influence of the doping agent on the corrosion protection properties of polypyrrole grown on aluminium alloy 2024-T3. Prog. Org. Coat. 2011, 71, 181–187. [Google Scholar] [CrossRef]
- Goulis, P.; Kartsonakis, I.; Konstantopoulos, G.; Charitidis, C. Synthesis and Processing of Melt Spun Materials from Esterified Lignin with Lactic Acid. Appl. Sci. 2019, 9, 5361. [Google Scholar] [CrossRef]
- Goulis, P.; Kartsonakis, I.A.; Mpalias, K.; Charitidis, C. Combined effects of multi-walled carbon nanotubes and lignin on polymer fiber-reinforced epoxy composites. Mater. Chem. Phys. 2018, 218, 18–27. [Google Scholar] [CrossRef]
- Goulis, P.; Konstantopoulos, G.; Kartsonakis, I.A.; Mpalias, K.; Anagnou, S.; Dragatogiannis, D.; Charitidis, C. Thermal Treatment of Melt-Spun Fibers Based on High Density PolyEthylene and Lignin. C J. Carbon Res. 2017, 3, 35. [Google Scholar] [CrossRef]
- Huang, W.; Mao, Z.; Xu, Z.; Xiang, B.; Zhang, J. Synthesis and characterization of size-tunable core-shell structural polyacrylate-graft-poly(acrylonitrile-ran-styrene) (ASA) by pre-emulsion semi-continuous polymerization. Eur. Polym. J. 2019, 120, 109247. [Google Scholar] [CrossRef]
- Weiss, A.V.; Koch, M.; Schneider, M. Combining cryo-TEM and energy-filtered TEM for imaging organic core-shell nanoparticles and defining the polymer distribution. Int. J. Pharm. 2019, 570, 118650. [Google Scholar] [CrossRef]
- Dinc, M.; Esen, C.; Mizaikoff, B. Recent advances on core–shell magnetic molecularly imprinted polymers for biomacromolecules. Trac. Trends Anal. Chem. 2019, 114, 202–217. [Google Scholar] [CrossRef]
- Ravindar Reddy, M.; Subrahmanyam, A.R.; Maheshwar Reddy, M.; Siva Kumar, J.; Kamalaker, V.; Jaipal Reddy, M. X-RD, SEM, FT-IR, DSC Studies of Polymer Blend Films of PMMA and PEO. Mater. Today Proc. 2016, 3, 3713–3718. [Google Scholar] [CrossRef]
- Mekuria, T.D.; Zhang, C.; Fouad, D.E. The effect of thermally developed SiC@SiO2 core-shell structured nanoparticles on the mechanical, thermal and UV-shielding properties of polyimide composites. Compos. Part. B Eng. 2019, 173, 106917. [Google Scholar] [CrossRef]
- Tommasini, F.J.; Ferreira, L.d.C.; Tienne, L.G.P.; Aguiar, V.d.O.; Silva, M.H.P.d.; Rocha, L.F.d.M.; Marques, M.d.F.V. Poly (Methyl Methacrylate)-SiC Nanocomposites Prepared Through in Situ Polymerization. Mater. Res. 2018, 21, 1–7. [Google Scholar] [CrossRef]
- Yuksel, N.; Baykara, M.; Shirinzade, H.; Suzen, S. Investigation of triacetin effect on indomethacin release from poly(methyl methacrylate) microspheres: Evaluation of interactions using FT-IR and NMR spectroscopies. Int J. Pharm. 2011, 404, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Sivy, G.T.; Coleman, M.M. Fourier transform ir studies of the degradation of polyacrylonitrile copolymers—II. Carbon 1981, 19, 127–131. [Google Scholar] [CrossRef]
- Kartsonakis, I.A.; Danilidis, I.L.; Pappas, G.S.; Kordas, G.C. Encapsulation and release of corrosion inhibitors into titania nanocontainers. J. Nanosci. Nanotechnol. 2010, 10, 5912–5920. [Google Scholar] [CrossRef]
- Eissa, A.S. Effect of SDS on whey protein polymers. Molecular investigation via dilute solution viscometry and dynamic light scattering. Food Hydrocoll. 2019, 87, 97–100. [Google Scholar] [CrossRef]
- Makan, A.C.; Spallek, M.J.; du Toit, M.; Klein, T.; Pasch, H. Advanced analysis of polymer emulsions: Particle size and particle size distribution by field-flow fractionation and dynamic light scattering. J. Chromatogr. A 2016, 1442, 94–106. [Google Scholar] [CrossRef]
- Cametti, C.; D’Amato, R.; Furlani, A.; Russo, M.V. Dynamic light scattering and optical absorption study of poly(monosubstituted)acetylene polymers and copolymers. Chem. Phys. Lett. 2003, 370, 602–608. [Google Scholar] [CrossRef]
- Gao, H.; Waechter, A.; Konstantinov, I.A.; Arturo, S.G.; Broadbelt, L.J. Application and comparison of derivative-free optimization algorithms to control and optimize free radical polymerization simulated using the kinetic Monte Carlo method. Comput. Chem. Eng. 2018, 108, 268–275. [Google Scholar] [CrossRef]
- Sankar, K.; Rajendran, V. Ultrasound assisted free radical polymerization of glycidyl methacrylate by a new disite phase-transfer catalyst system: A kinetic study. Ultrason. Sonochem. 2012, 19, 1205–1212. [Google Scholar] [CrossRef]
- Prabha, J.; Susan Jemima, W.; Jayaprada, M.; Umapathy, M.J. Synergistic effect of ultrasonication and phase transfer catalysts in radical polymerization of methyl methacrylate—A kinetic study. Ultrason. Sonochem. 2017, 35, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Marimuthu, E.; Murugesan, V. Influence of ultrasonic condition on phase transfer catalyzed radical polymerization of methyl methacrylate in two phase system—A kinetic study. Ultrason. Sonochem. 2017, 38, 560–569. [Google Scholar] [CrossRef] [PubMed]
- Van Tran, V.; Loi Nguyen, T.; Moon, J.-Y.; Lee, Y.-C. Core-shell materials, lipid particles and nanoemulsions, for delivery of active anti-oxidants in cosmetics applications: Challenges and development strategies. Chem. Eng. J. 2019, 368, 88–114. [Google Scholar] [CrossRef]
- Su, H.; Tian, Q.; Hurd Price, C.-A.; Xu, L.; Qian, K.; Liu, J. Nanoporous core@shell particles: Design, preparation, applications in bioadsorption and biocatalysis. Nano Today 2020, 31, 100834. [Google Scholar] [CrossRef]
- Peralta, M.E.; Jadhav, S.A.; Magnacca, G.; Scalarone, D.; Martire, D.O.; Parolo, M.E.; Carlos, L. Synthesis and in vitro testing of thermoresponsive polymer-grafted core-shell magnetic mesoporous silica nanoparticles for efficient controlled and targeted drug delivery. J. Colloid Interface Sci. 2019, 544, 198–205. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Bao, Y.; Gao, Z.; Wu, Y.; Wu, L. Synthesis of mesoporous silica-shell/oil-core microspheres for common waterborne polymer coatings with robust superhydrophobicity. Prog. Org. Coat. 2019, 132, 275–282. [Google Scholar] [CrossRef]
- Zhang, W.; Kong, Y.; Jin, X.; Yan, B.; Diao, G.; Piao, Y. Supramolecule-assisted synthesis of cyclodextrin polymer functionalized polyaniline/carbon nanotube with core-shell nanostructure as high-performance supercapacitor material. Electrochim. Acta 2020, 331, 135345. [Google Scholar] [CrossRef]
- Yang, X.; Wan, G.; Ma, S.; Xia, H.; Wang, J.; Liu, J.; Liu, Y.; Chen, G.; Bai, Q. Synthesis and optimization of SiO2@SiO2 core-shell microspheres by an improved polymerization-induced colloid aggregation method for fast separation of small solutes and proteins. Talanta 2020, 207, 120310. [Google Scholar] [CrossRef]
- Pourjavadi, A.; Rahemipoor, S.; Kohestanian, M. Synthesis and characterization of multi stimuli-responsive block copolymer-silica hybrid nanocomposite with core-shell structure via RAFT polymerization. Compos. Sci. Technol. 2020, 188, 107951. [Google Scholar] [CrossRef]
- Shevchenko, N.; Pankova, G.; Laishevkina, S.; Iakobson, O.; Koshkin, A.; Shabsels, B. Core-shell polymer particles containing derivatives of 1,3-diphenyl-β-diketonate boron difluoride: Synthesis and spectroscopic investigation of toluene vapor sorption. Colloids Surf. A Physicochem. Eng. Asp. 2019, 562, 310–320. [Google Scholar] [CrossRef]
- Zhang, S.; Wen, Y.; Wang, L.; Yang, Y.; Liu, S. In situ synthesis of a multifunctional polymer with a stable core-shell structure for effective dewatering. Miner. Eng. 2019, 141, 105858. [Google Scholar] [CrossRef]
- Zhang, H.; Jiao, Q.; Zhao, W.; Guo, X.; Li, D.; Sun, X. Enhanced Crystal Stabilities of ε-CL-20 via Core-Shell Structured Energetic Composites. Appl. Sci. 2020, 10, 2663. [Google Scholar] [CrossRef]
- Bui, Q.C.; Largeau, L.; Morassi, M.; Jegenyes, N.; Mauguin, O.; Travers, L.; Lafosse, X.; Dupuis, C.; Harmand, J.-C.; Tchernycheva, M.; et al. GaN/Ga2O3 Core/Shell Nanowires Growth: Towards High Response Gas Sensors. Appl. Sci. 2019, 9, 3528. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Q.; Wang, D.; Yan, X. NO-CH4-SCR Over Core-Shell MnH-Zeolite Composites. Appl. Sci. 2019, 9, 1773. [Google Scholar] [CrossRef]
- Khatami, M.; Alijani, H.; Nejad, M.; Varma, R. Core@shell Nanoparticles: Greener Synthesis Using Natural Plant Products. Appl. Sci. 2018, 8, 411. [Google Scholar] [CrossRef]
- Obaidat, I.; Nayek, C.; Manna, K. Investigating the Role of Shell Thickness and Field Cooling on Saturation Magnetization and Its Temperature Dependence in Fe3O4/γ-Fe2O3 Core/Shell Nanoparticles. Appl. Sci. 2017, 7, 1269. [Google Scholar] [CrossRef]
- Dell’Era, A.; Scaramuzzo, F.A.; Stoller, M.; Lupi, C.; Rossi, M.; Passeri, D.; Pasquali, M. Spinning Disk Reactor Technique for the Synthesis of Nanometric Sulfur TiO2 Core–Shell Powder for Lithium Batteries. Appl. Sci. 2019, 9, 1913. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).