A Review on Biopolymer-Based Fibers via Electrospinning and Solution Blowing and Their Applications
Abstract
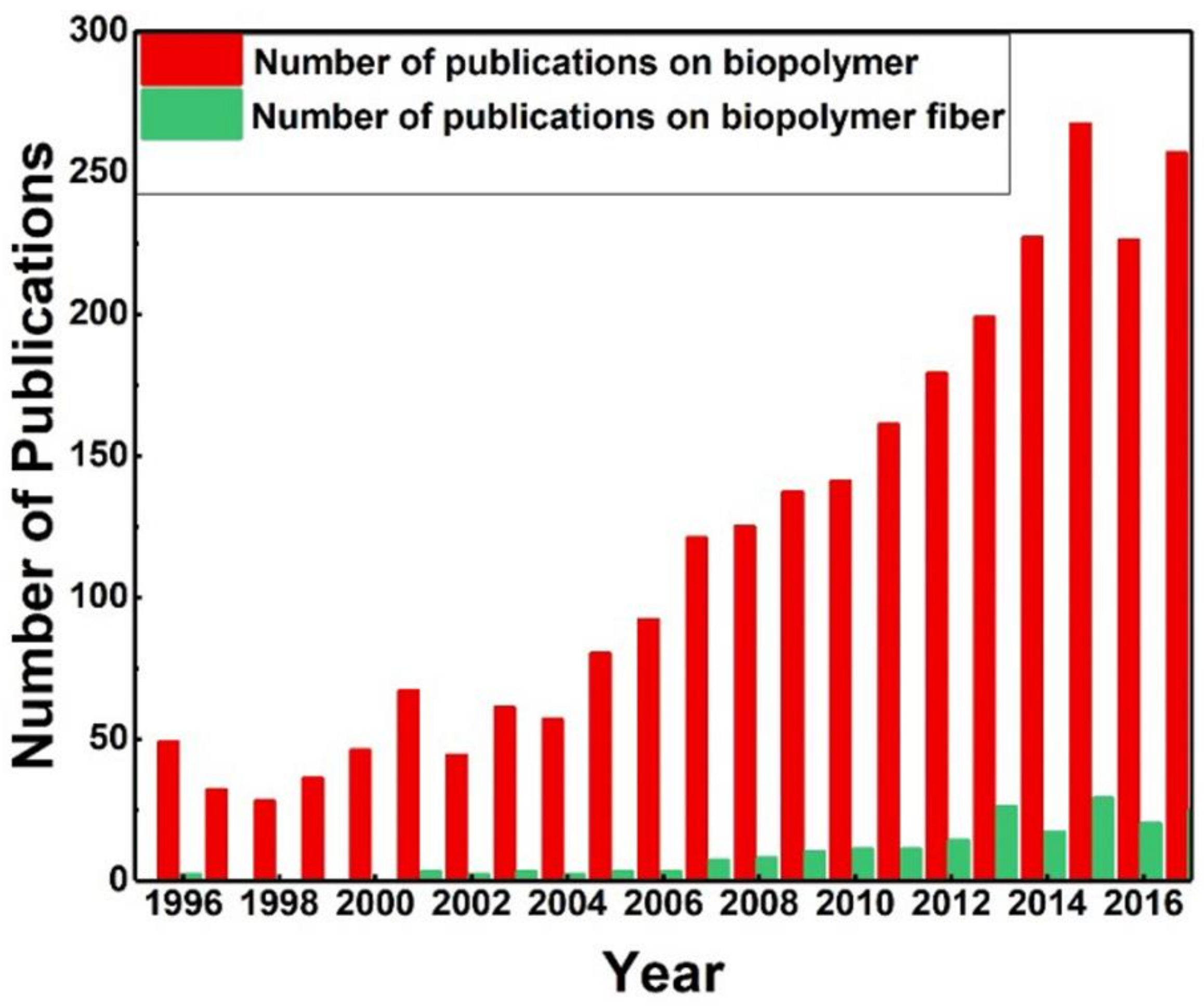
1. Introduction
2. Fabrication of Polymer Fibers: Electrospinning and Solution Blowing
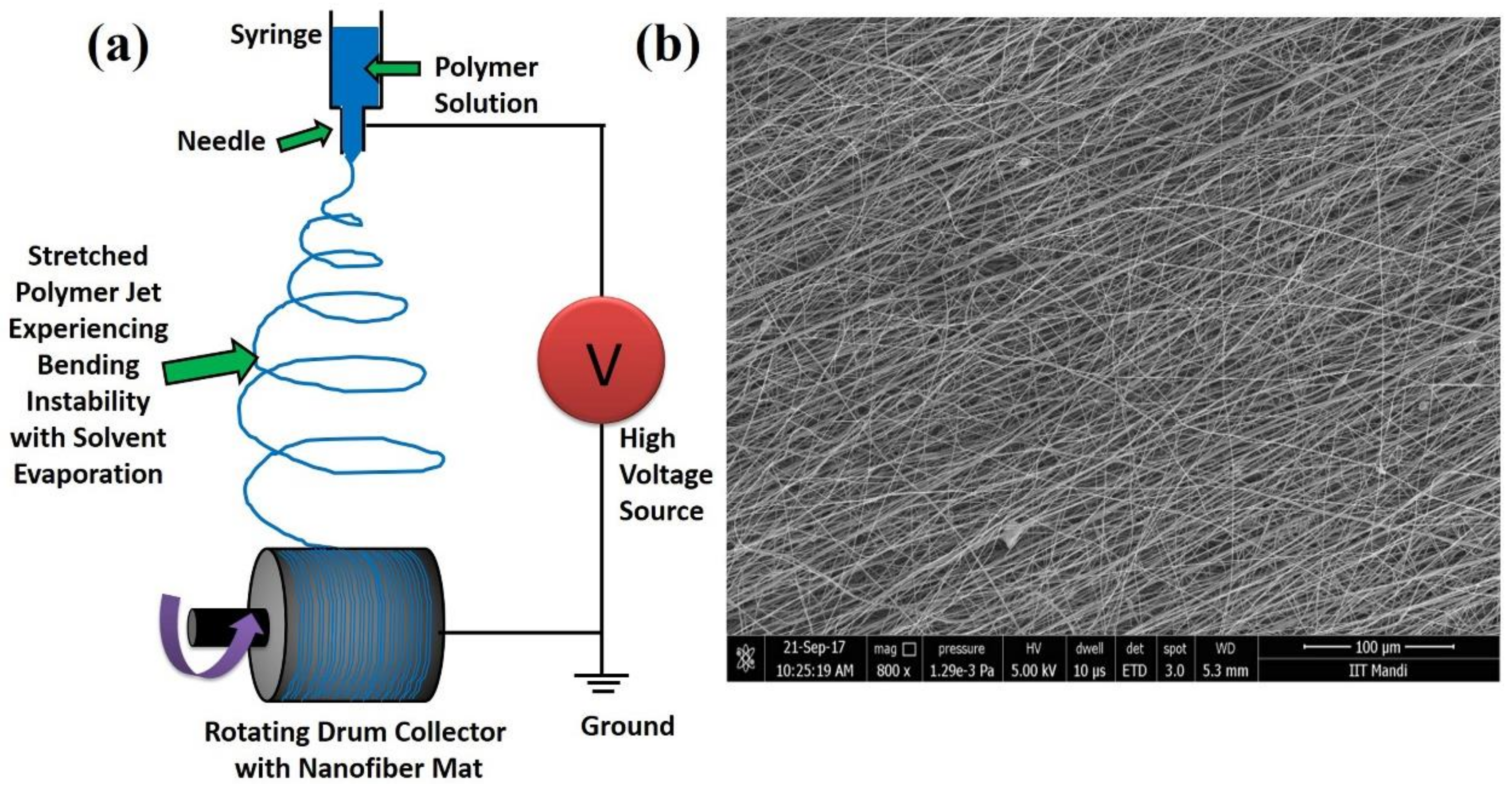
2.1. Electrospinning
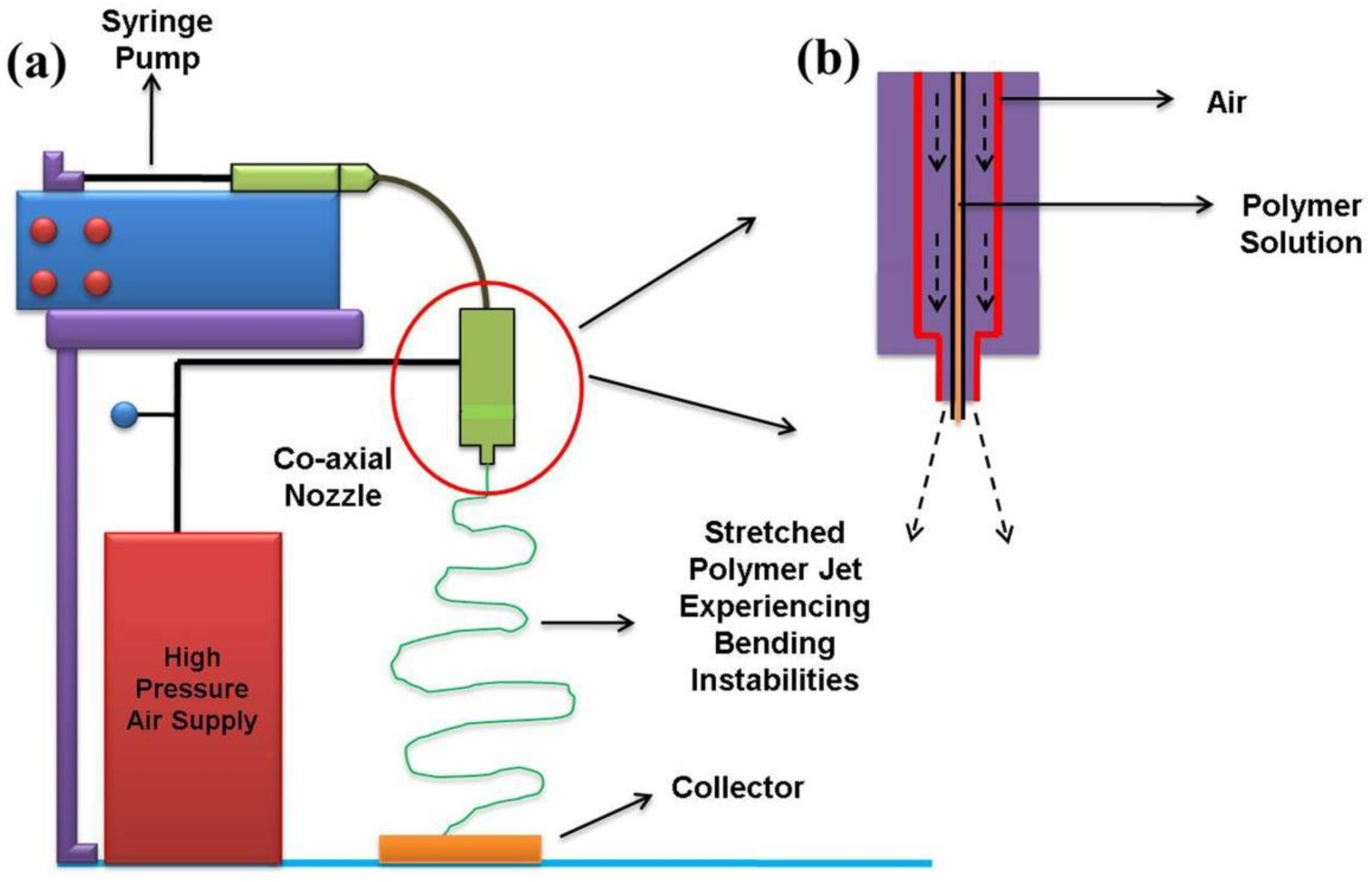
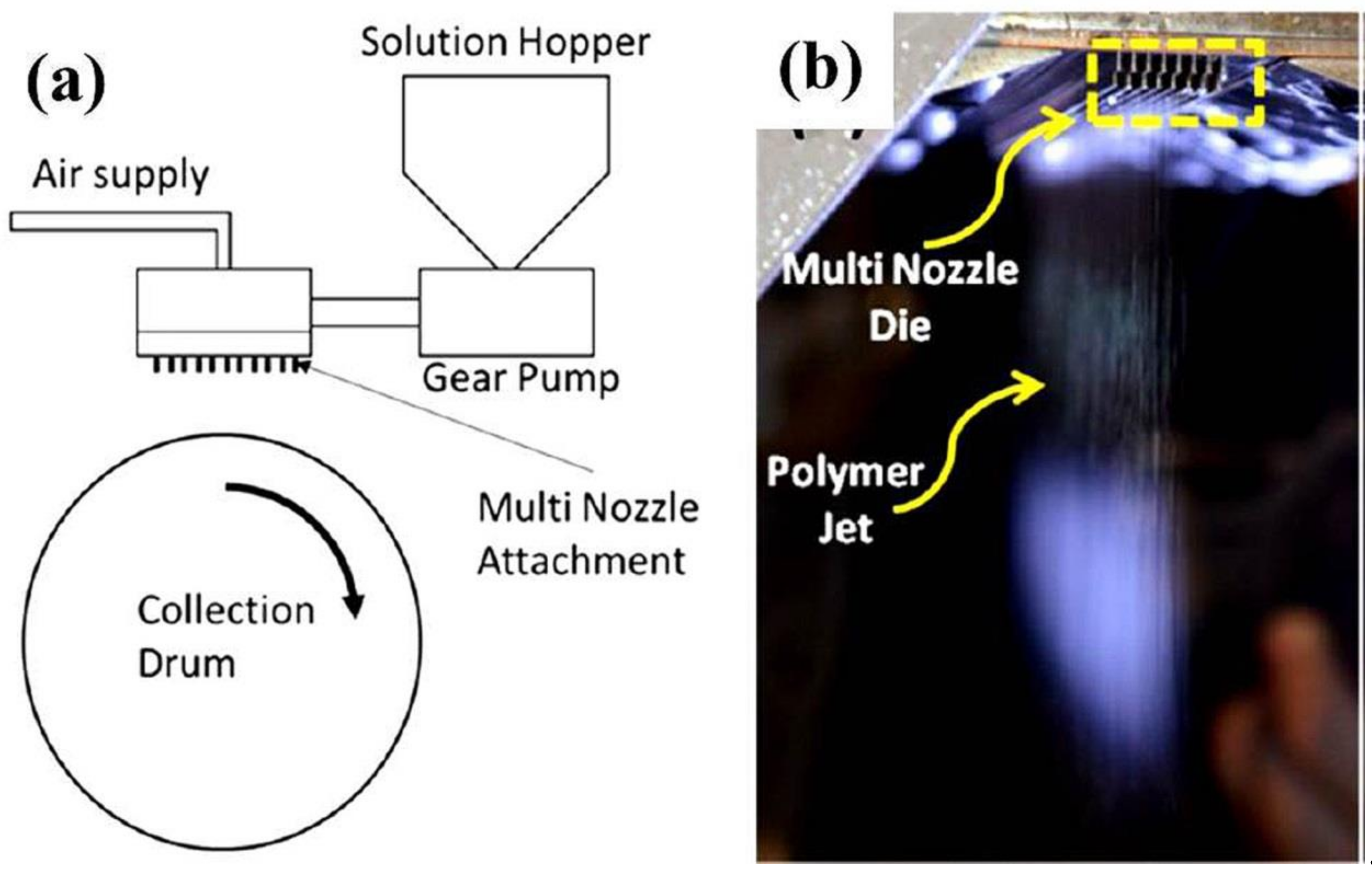
2.2. Solution Blowing
3. Fabrication of Biopolymer Fibers via Electrospinning
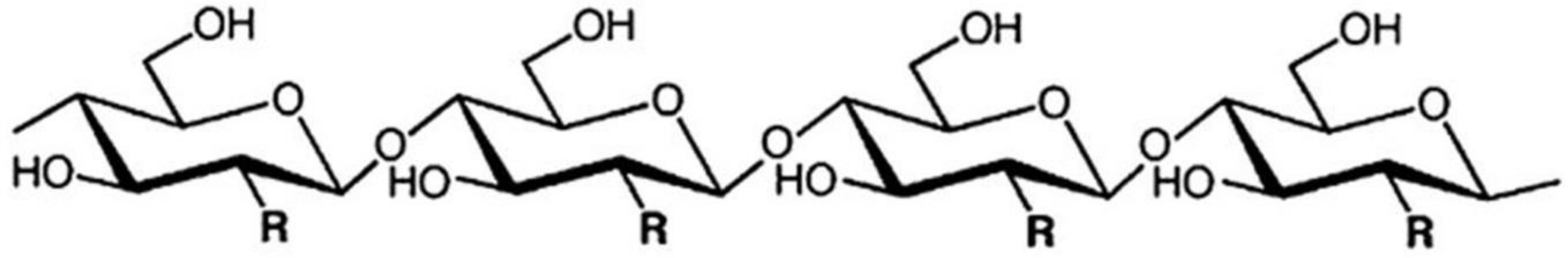
3.1. Cellulose
3.1.1. Derivatives of Cellulose: Cellulose Acetate
3.1.2. Derivatives of Cellulose: Ethyl Cellulose, Hydroxypropyl Cellulose etc.
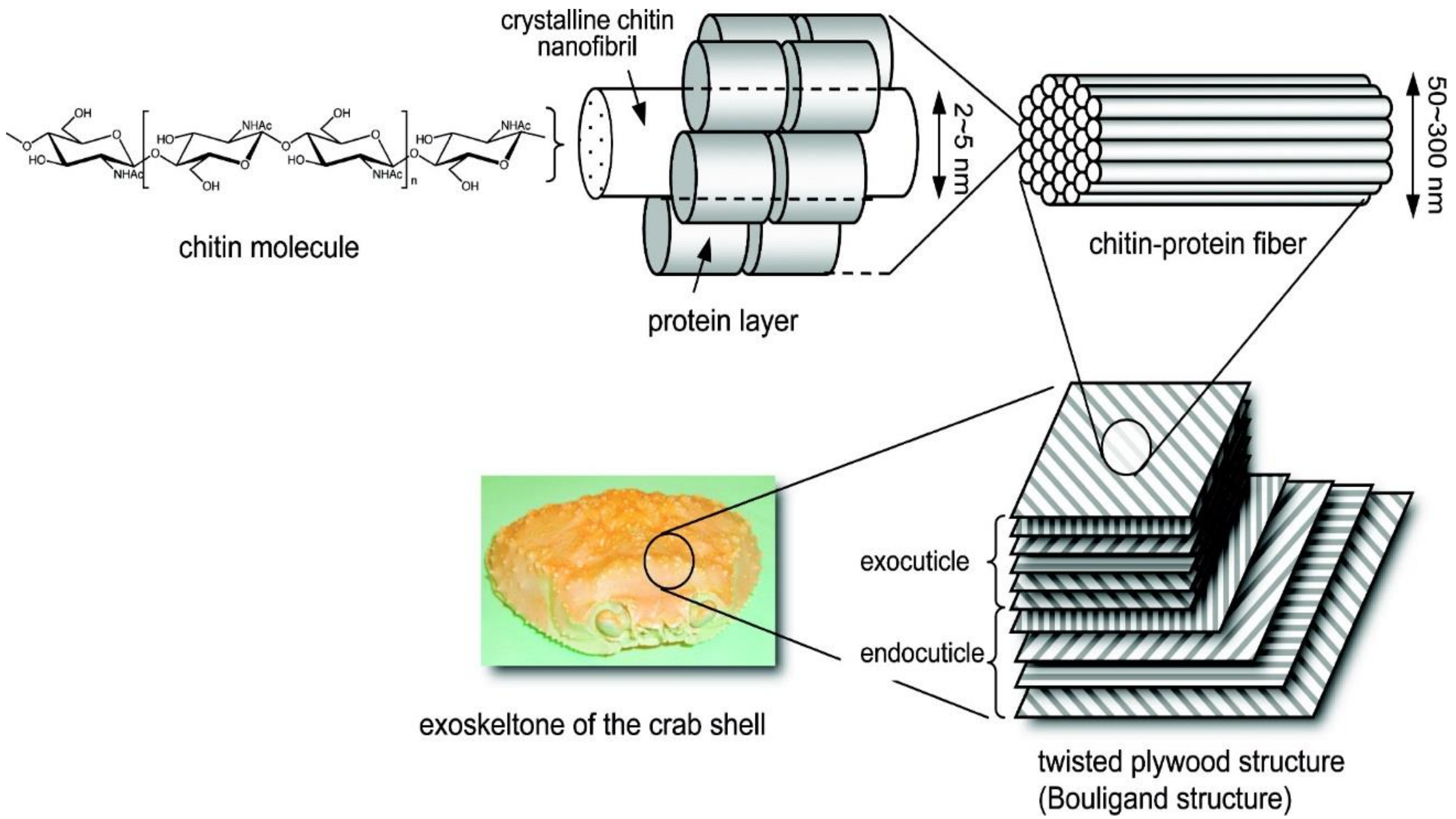
3.2. Chitin and Chitosan
3.2.1. Chitin
Chitin and Other Polymer Blends
Carboxymethyl Chitin
Chitin Whiskers
3.2.2. Chitosan
Chitosan Derivatives
Chitosan/PEO Blends
Chitosan/PVA Blends
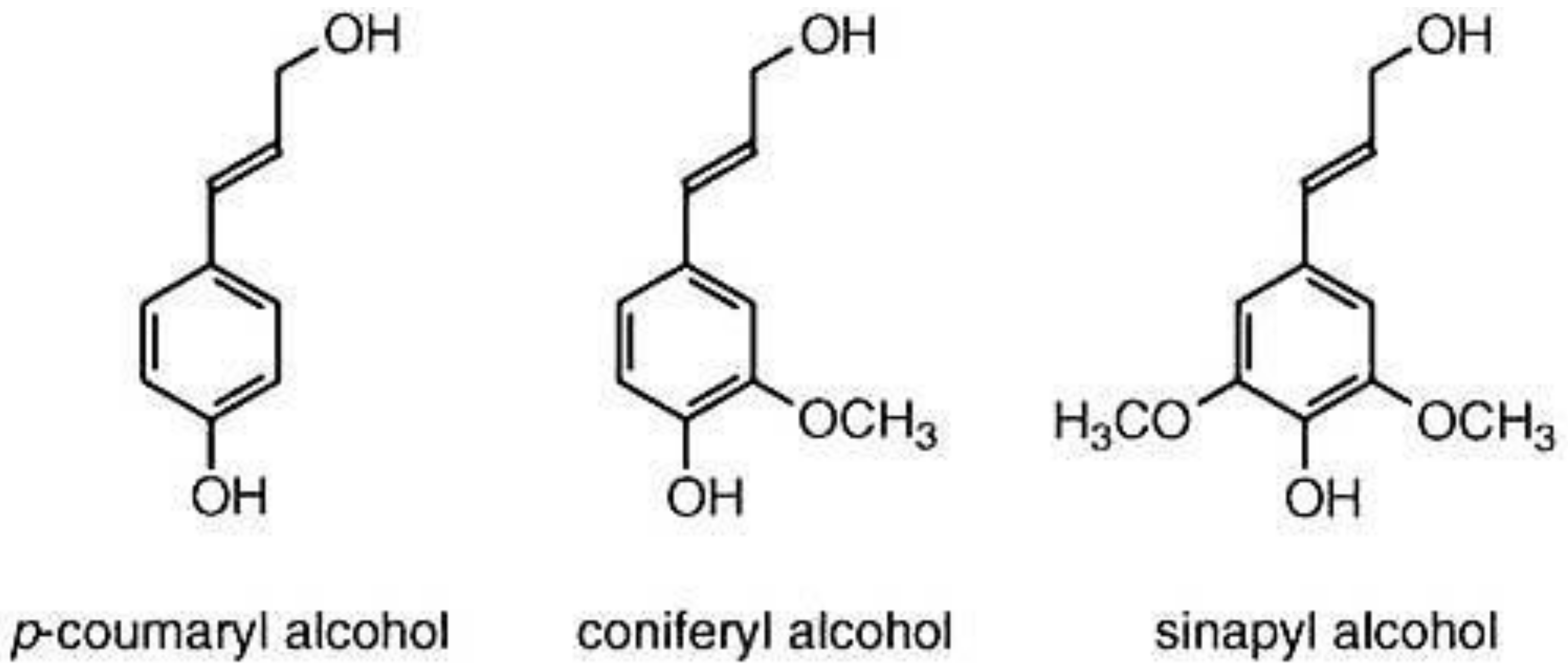
3.3. Lignin
3.3.1. Pure Lignin
3.3.2. Lignin/PEO Blend
3.3.3. Lignin/PAN Blend
3.3.4. Lignin/PVA Blend
3.4. Proteins
3.4.1. Bovine Serum Albumin (BSA)
3.4.2. Collagen
3.4.3. Silk
3.4.4. Soy Protein and Its Isolate
3.4.5. Whey Protein & Its Isolates
3.5. Others: Dextran and Hyaluronic Acid
3.5.1. Dextran
3.5.2. Hyaluronic Acid
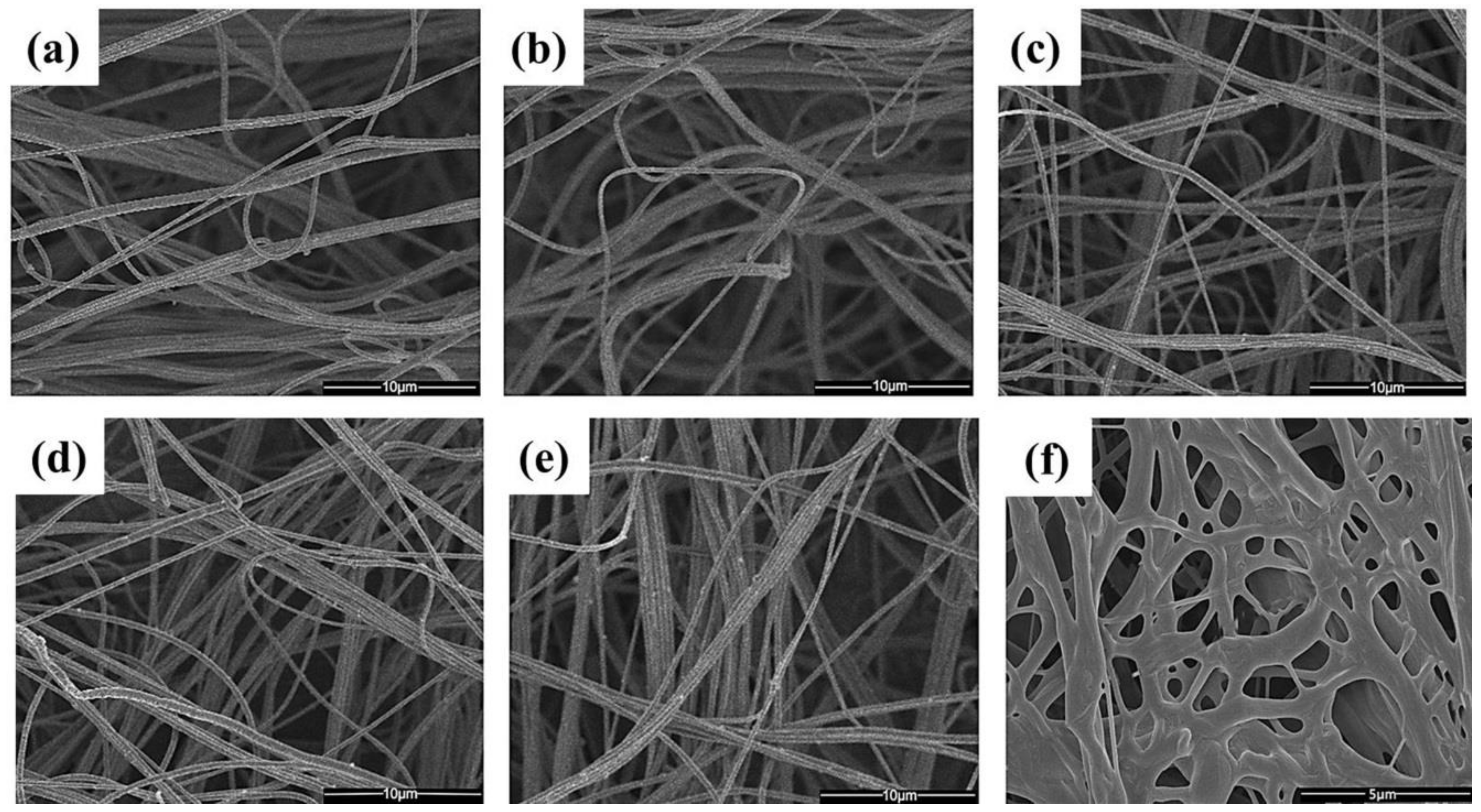
4. Fabrication of Biopolymer Fibers via Solution Blowing
5. Applications of Biopolymer Fibers
5.1. Tissue Engineering
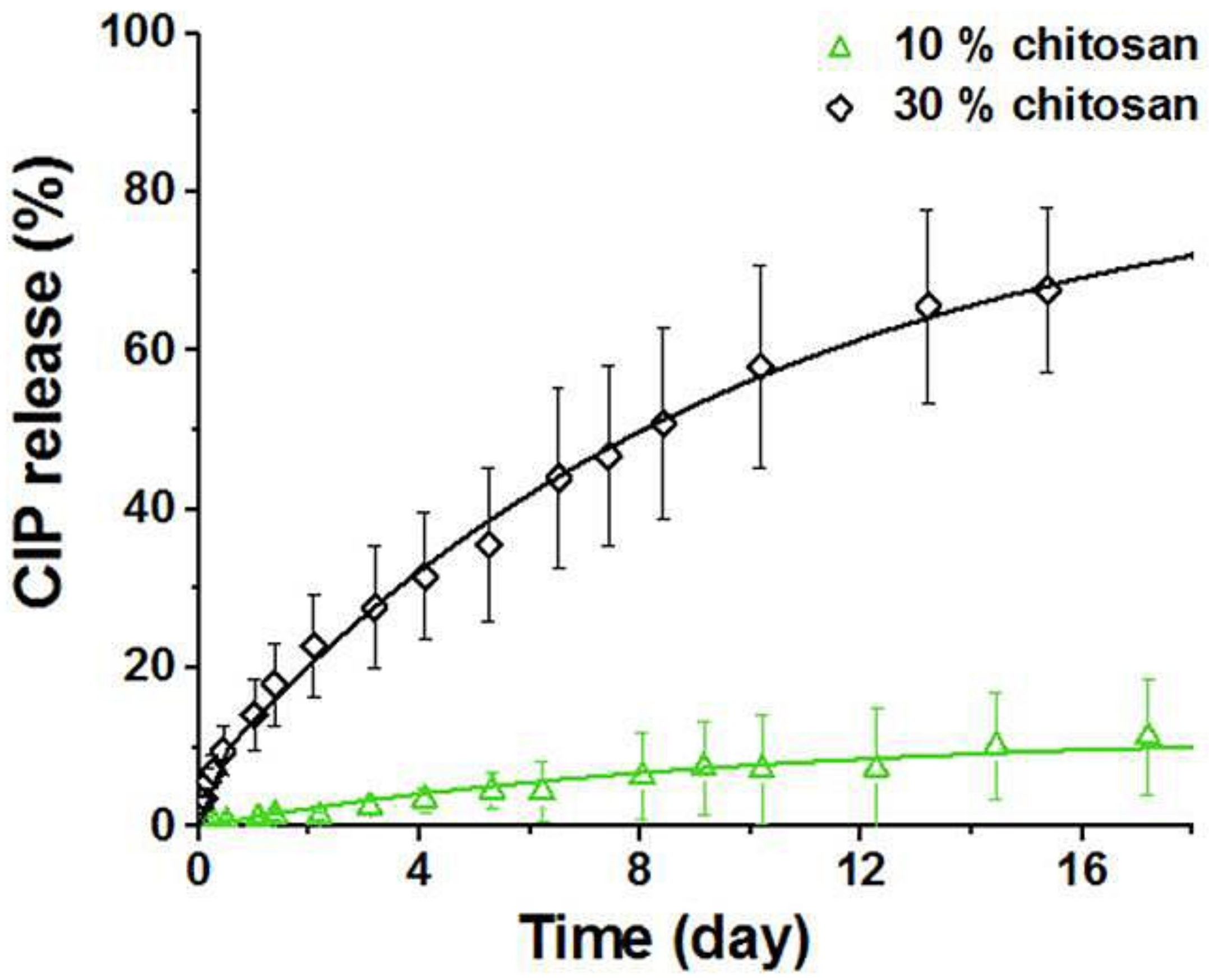
5.2. Drug Delivery
5.3. Filtration and Waste Water Treatment
5.4. Fuel Cells
5.5. Packaging
5.6. Biosensors
6. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Mohanty, A.K.; Misra, M.; Drzal, L.T. Sustainable bio-composites from renewable resources: Opportunities and challenges in the green materials world. J. Polym. Environ. 2002, 10, 19–26. [Google Scholar] [CrossRef]
- Sabater, M.J.; Rodenas, T.; Heredia, A. Biopolymers from Plants (Ch. 3). In Handbook of Biopolymer Based Materials: From Blends and Composites to Gels and Complex Networks; Thomas, S., Durand, D., Chassenieux, C., Jyotishkumar, P., Eds.; Wiley-VCH: Weinheim, Germany, 2013. [Google Scholar]
- Averous, L.; Fringant, C.; Moro, L. Plasticized starch–cellulose interactions in polysaccharide composites. Polymer 2001, 42, 6565–6572. [Google Scholar] [CrossRef]
- Angellier, H.; Molina-Boisseau, S.; Dole, P.; Dufresne, A. Thermoplastic starch–waxy maize starch nanocrystals nanocomposites. Biomacromolecules 2006, 7, 531–539. [Google Scholar] [CrossRef] [PubMed]
- Arroyo, O.H.; Huneault, M.A.; Favis, B.D.; Bureau, M.N. Processing and properties of PLA/thermoplastic starch/montmorillonite nanocomposites. Polym. Compos. 2010, 31, 114–127. [Google Scholar] [CrossRef]
- Krebs, M.D.; Jeon, O.; Alsberg, E. Localized and sustained delivery of silencing RNA from macroscopic biopolymer hydrogels. J. Am. Chem. Soc. 2009, 131, 9204–9206. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Ramay, H.R.; Hauch, K.D.; Xiao, D.; Zhang, M. Chitosan–alginate hybrid scaffolds for bone tissue engineering. Biomaterials 2005, 26, 3919–3928. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Xia, Y. Electrospinning of nanofibers: Reinventing the wheel? Adv. Mater. 2004, 16, 1151–1170. [Google Scholar] [CrossRef]
- Chen, X.; Mao, S.S. Titanium dioxide nano-materials: Synthesis, properties, modifications, and applications. Chem. Rev. 2007, 107, 2891–2959. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Um, I.C.; Fang, D.; Okamoto, A.; Hsiao, B.S.; Chu, B. Formation of water-resistant hyaluronic acid nanofibers by blowing-assisted electro-spinning and non-toxic post treatments. Polymer 2005, 46, 4853–4867. [Google Scholar] [CrossRef]
- Homayoni, H.; Ravandi, S.A.; Valizadeh, M. Electrospinning of chitosan nanofibers: Processing optimization. Carbohydr. Polym. 2009, 77, 656–661. [Google Scholar] [CrossRef]
- Bognitzki, M.; Czado, W.; Frese, T.; Schaper, A.; Hellwig, M.; Steinhart, M.; Greiner, A.; Wendorff, J.H. Nanostructured fibers via electrospinning. Adv. Mater. 2001, 13, 70–72. [Google Scholar] [CrossRef]
- Hassan, M.A.; Yeom, B.Y.; Wilkie, A.; Pourdeyhimi, B.; Khan, S.A. Fabrication of nanofiber meltblown membranes and their filtration properties. J. Membr. Sci. 2013, 427, 336–344. [Google Scholar] [CrossRef]
- Greenhalgh, R.D.; Ambler, W.S.; Quinn, S.J.; Medeiros, E.S.; Anderson, M.; Gore, B.; Menner, A.; Bismarck, A.; Li, X.; Tirelli, N.; et al. Hybrid sol–gel inorganic/gelatin porous fibres via solution blow spinning. J. Mater. Sci. 2017, 52, 9066–9081. [Google Scholar] [CrossRef]
- Gosline, J.M.; DeMont, M.E.; Denny, M.W. The structure and properties of spider silk. Endeavour 1986, 10, 37–43. [Google Scholar] [CrossRef]
- You, Y.; Min, B.M.; Lee, S.J.; Lee, T.S.; Park, W.H. In vitro degradation behavior of electrospun polyglycolide, polylactide, and poly(lactideco-glycolide). J. Appl. Polym. Sci. 2005, 95, 193–200. [Google Scholar] [CrossRef]
- Podgorski, A.; Bałazy, A.; Gradon, L. Application of nanofibers to improve the filtration efficiency of the most penetrating aerosol particles in fibrous filters. Chem. Eng. Sci. 2006, 61, 6804–6815. [Google Scholar] [CrossRef]
- Oliveira, J.E.; Moraes, E.A.; Costa, R.G.; Afonso, A.S.; Mattoso, L.H.; Orts, W.J.; Medeiros, E.S. Nano and submicrometric fibers of poly (D, L-lactide) obtained by solution blow spinning: Process and solution variables. J. Appl. Polym. Sci. 2011, 122, 3396–3405. [Google Scholar] [CrossRef]
- Tseng, R.J.; Huang, J.; Ouyang, J.; Kaner, R.B.; Yang, Y. Polyaniline nanofiber/gold nanoparticle nonvolatile memory. Nano Lett. 2005, 5, 1077–1080. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.R.; Muzzarelli, R.; Muzzarelli, C.; Sashiwa, H.; Domb, A.J. Chitosan chemistry and pharmaceutical perspectives. Chem. Rev. 2004, 104, 6017–6084. [Google Scholar] [CrossRef] [PubMed]
- Yarin, A.L.; Pourdeyhimi, B.; Ramakrishna, S. Fundamentals and Applications of Micro-and Nanofibers; Cambridge University Press: Cambridge, UK, 2014. [Google Scholar]
- Tolaimate, A.; Desbrieres, J.; Rhazi, M.; Alagui, A.; Vincendon, M.; Vottero, P. On the influence of deacetylation process on the physicochemical characteristics of chitosan from squid chitin. Polymer 2000, 41, 2463–2469. [Google Scholar] [CrossRef]
- Ji, W.; Yang, F.; Van den Beucken, J.J.; Bian, Z.; Fan, M.; Chen, Z.; Jansen, J.A. Fibrous scaffolds loaded with protein prepared by blend or coaxial electrospinning. Acta Biomater. 2010, 6, 4199–4207. [Google Scholar] [CrossRef] [PubMed]
- Fang, W.; Yang, S.; Yuan, T.Q.; Charlton, A.; Sun, R.C. Effects of various surfactants on alkali lignin electrospinning ability and spun fibers. Ind. Eng. Chem. Res. 2017, 56, 9551–9559. [Google Scholar] [CrossRef]
- He, J.H.; Wan, Y.Q.; Xu, L. Nano-effects, quantum-like properties in electrospun nanofibers. Chaos Solitons Fract. 2007, 33, 26–37. [Google Scholar] [CrossRef]
- Drosou, C.; Krokida, M.; Biliaderis, C.G. Composite pullulan-whey protein nanofibers made by electrospinning: Impact of process parameters on fiber morphology and physical properties. Food Hydrocoll. 2017, 77, 726–735. [Google Scholar] [CrossRef]
- Reneker, D.H.; Chun, I. Nanometre diameter fibres of polymer, produced by electrospinning. Nanotechnology 1996, 7, 216. [Google Scholar] [CrossRef]
- Guan, K.T.; Zhuang, X.P.; Yan, G.L.; Cheng, B.W. Fabrication and properties of polyurethane nanofibers nonwoven by solution blowing. Adv. Mater. Res. 2011, 32, 1339–1342. [Google Scholar] [CrossRef]
- Al-Itry, R.; Lamnawar, K.; Maazouz, A. Biopolymer blends based on poly (lactic acid): Shear and elongation rheology/structure/blowing process relationships. Polymers 2015, 7, 939–962. [Google Scholar] [CrossRef]
- Briggs, T.; Arinzeh, T.L. Examining the formulation of emulsion electrospinning for improving the release of bioactive proteins from electrospun fibers. J. Biomed. Mater. Res. Part A 2014, 102, 674–684. [Google Scholar] [CrossRef] [PubMed]
- Subbiah, T.; Bhat, G.S.; Tock, R.W.; Parameswaran, S.; Ramkumar, S.S. Electrospinning of nanofibers. J. Appl. Polym. Sci. 2005, 96, 557–569. [Google Scholar] [CrossRef]
- Matthews, J.A.; Wnek, G.E.; Simpson, D.G.; Bowlin, G.L. Electrospinning of collagen nanofibers. Biomacromolecules 2002, 3, 232–238. [Google Scholar] [CrossRef] [PubMed]
- Sinha-Ray, S.; Yarin, A.L.; Pourdeyhimi, B. The production of 100/400 nm inner/outer diameter carbon tubes by solution blowing and carbonization of core–shell nanofibers. Carbon 2010, 48, 3575–3578. [Google Scholar] [CrossRef]
- Amna, T.; Yang, J.; Ryu, K.S.; Hwang, I.H. Electrospun antimicrobial hybrid mats: Innovative packaging material for meat and meat-products. J. Food Sci. Technol. 2015, 52, 4600–4606. [Google Scholar] [CrossRef] [PubMed]
- Sinha-Ray, S.; Khansari, S.; Yarin, A.L.; Pourdeyhimi, B. Effect of chemical and physical cross-linking on tensile characteristics of solution-blown soy protein nanofiber mats. Ind. Eng. Chem. Res. 2012, 51, 15109–15121. [Google Scholar] [CrossRef]
- Sinha-Ray, S.; Pelot, D.D.; Zhou, Z.P.; Rahman, A.; Wu, X.F.; Yarin, A.L. Encapsulation of self-healing materials by co-electrospinning, emulsion electrospinning, solution blowing and intercalation. J. Mater. Chem. 2012, 22, 9138–9146. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, X.; Duan, B.; Wu, L.; Li, S.; Yuan, X. Preparation of electrospun chitosan/poly (vinyl alcohol) membranes. Colloid Polym. Sci. 2007, 285, 855–863. [Google Scholar] [CrossRef]
- Banker, G.S.; Kumar, V.; Biocontrol Inc. Microfibrillated Oxycellulose. U.S. Patent US 5,405,953, 11 April 1995. [Google Scholar]
- Ma, P.X.; Zhang, R. Synthetic nano-scale fibrous extracellular matrix. J. Biomed. Mater. Res. 1999, 46, 60–72. [Google Scholar] [CrossRef]
- Nakata, K.; Fujii, K.; Ohkoshi, Y.; Gotoh, Y.; Nagura, M.; Numata, M.; Kamiyama, M. Poly (ethylene terephthalate) Nanofibers Made by Sea–Island Type Conjugated Melt Spinning and Laser Heated Flow Drawing. Macromol. Rapid Commun. 2007, 28, 792–795. [Google Scholar] [CrossRef]
- Liu, R.; Cai, N.; Yang, W.; Chen, W.; Liu, H. Sea-island polyurethane/polycarbonate composite nanofiber fabricated through electrospinning. J. Appl. Polym. Sci. 2010, 116, 1313–1321. [Google Scholar] [CrossRef]
- Ondarcuhu, T.; Joachim, C. Drawing a single nanofibre over hundreds of microns. EPL (Europhys. Lett.) 1998, 42, 215. [Google Scholar] [CrossRef]
- Liu, D.; Zhang, H.; Grim, P.C.; De Feyter, S.; Wiesler, U.M.; Berresheim, A.J.; Müllen, K.; De Schryver, F.C. Self-assembly of polyphenylene dendrimers into micrometer long nanofibers: An atomic force microscopy study. Langmuir 2002, 18, 2385–2391. [Google Scholar] [CrossRef]
- Baumgarten, P.K. Electrostatic spinning of acrylic microfibers. J. Colloid Interface Sci. 1971, 36, 71–79. [Google Scholar] [CrossRef]
- Berth, G.; Dautzenberg, H.; Peter, M.G. Physico-chemical characterization of chitosans varying in degree of acetylation. Carbohydr. Polym. 1998, 36, 205–216. [Google Scholar] [CrossRef]
- Zupančič, S.; Sinha-Ray, S.; Sinha-Ray, S.; Kristl, J.; Yarin, A.L. Long-term sustained ciprofloxacin release from pmma and hydrophilic polymer blended nanofibers. Mol. Pharm. 2016, 13, 295–305. [Google Scholar] [CrossRef] [PubMed]
- Kolbasov, A.; Sinha-Ray, S.; Joijode, A.; Hassan, M.A.; Brown, D.; Maze, B.; Pourdeyhimi, B.; Yarin, A.L. Industrial-scale solution blowing of soy protein nanofibers. Ind. Eng. Chem. Res. 2015, 55, 323–333. [Google Scholar] [CrossRef]
- Ifuku, S.; Nogi, M.; Abe, K.; Yoshioka, M.; Morimoto, M.; Saimoto, H.; Yano, H. Preparation of chitin nanofibers with a uniform width as α-chitin from crab shells. Biomacromolecules 2009, 10, 1584–1588. [Google Scholar] [CrossRef] [PubMed]
- Bhattarai, N.; Ramay, H.R.; Gunn, J.; Matsen, F.A.; Zhang, M. PEG-grafted chitosan as an injectable thermosensitive hydrogel for sustained protein release. J. Control. Release 2005, 103, 609–624. [Google Scholar] [CrossRef] [PubMed]
- Tucker, N.; Stanger, J.; Staiger, M.; Razzaq, H.; Hofman, K. The history of the science and technology of electrospinning from 1600 to 1995. J. Eng. Fibers Fabr. 2012, 7, 63–73. [Google Scholar]
- Li, D.; Xia, Y. Fabrication of titania nanofibers by electrospinning. Nano Lett. 2003, 3, 555–560. [Google Scholar] [CrossRef]
- Torres-Giner, S.; Ocio, M.J.; Lagaron, J.M. Development of Active Antimicrobial Fiber-Based Chitosan Polysaccharide Nanostructures using Electrospinning. Eng. Life Sci. 2008, 8, 303–314. [Google Scholar] [CrossRef]
- Bhumkar, D.R.; Pokharkar, V.B. Studies on effect of pH on cross-linking of chitosan with sodium tripolyphosphate: A technical note. Aaps Pharmscitech. 2006, 7, E138–E143. [Google Scholar] [CrossRef] [PubMed]
- Bishop, A.; Balazsi, C.; Yang, J.H.; Gouma, P.I. Biopolymer-hydroxyapatite composite coatings prepared by electrospinning. Polym. Adv. Technol. 2006, 17, 902–926. [Google Scholar] [CrossRef]
- Xu, X.; Yang, Q.; Wang, Y.; Yu, H.; Chen, X.; Jing, X. Biodegradable electrospun poly (L-lactide) fibers containing antibacterial silver nanoparticles. Eur. Polym. J. 2006, 42, 2081–2087. [Google Scholar] [CrossRef]
- Sinha-Ray, S.; Sinha-Ray, S.; Yarin, A.L.; Pourdeyhimi, B. Application of solution-blown 20–50 nm nanofibers infiltration of nanoparticles: The efficient van der Waals collectors. J. Membr. Sci. 2015, 485, 132–150. [Google Scholar] [CrossRef]
- Neo, Y.P.; Ray, S.; Jin, J.; Gizdavic-Nikolaidis, M.; Nieuwoudt, M.K.; Liu, D.; Quek, S.Y. Encapsulation of food grade antioxidant in natural biopolymer by electrospinning technique: A physicochemical study based on zein–gallic acid system. Food Chem. 2013, 136, 1013–1021. [Google Scholar] [CrossRef] [PubMed]
- Teo, W.E.; Ramakrishna, S. A review on electrospinning design and nanofibre assemblies. Nanotechnology. 2006, 17, R89–R106. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, T.; Taniguchi, M.; Kawai, T. Fabrication of DNA nanofibers on a planar surface by electrospinning. Jpn. J. Appl. Phys. 2005, 44, L860. [Google Scholar] [CrossRef]
- Chew, S.Y.; Mi, R.; Hoke, A.; Leong, K.W. The effect of the alignment of electrospun fibrous scaffolds on Schwann cell maturation. Biomaterials 2008, 29, 653–661. [Google Scholar] [CrossRef] [PubMed]
- Taylor, G. Electrically driven jets. Proc. R. Soc. Lond. Ser. A 1969, 313, 453–475. [Google Scholar] [CrossRef]
- Yarin, A.L.; Koombhongse, S.; Reneker, D.H. Taylor cone and jetting from liquid droplets in electrospinning of nanofibers. J. Appl. Phys. 2001, 90, 4836–4846. [Google Scholar] [CrossRef]
- Reneker, D.H.; Yarin, A.L. Electrospinning jets and polymer nanofibers. Polymer 2008, 49, 2387–2425. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, J.; Misoska, V.; Wallace, G.G. Preparation of novel ultrafine fibers based on DNA and poly (ethylene oxide) by electrospinning from aqueous solutions. React. Funct. Polymers 2007, 67, 461–467. [Google Scholar] [CrossRef]
- Fong, H.; Chun, I.; Reneker, D.H. Beaded nanofibers formed during electrospinning. Polymer 1999, 40, 4585–4592. [Google Scholar] [CrossRef]
- Bhardwaj, N.; Kundu, S.C. Electrospinning: A fascinating fiber fabrication technique. Biotechnol. Adv. 2010, 28, 325–347. [Google Scholar] [CrossRef] [PubMed]
- Luu, Y.K.; Kim, K.; Hsiao, B.S.; Chu, B.; Hadjiargyrou, M. Development of a nanostructured DNA delivery scaffold via electrospinning of PLGA and PLA–PEG block copolymers. J. Control. Release 2003, 89, 341–353. [Google Scholar] [CrossRef]
- Lee, K.H.; Kim, H.Y.; Bang, H.J.; Jung, Y.H.; Lee, S.G. The change of bead morphology formed on electrospun polystyrene fibers. Polymer 2003, 44, 4029–4034. [Google Scholar] [CrossRef]
- Fu, W.; Liu, Z.; Feng, B.; Hu, R.; He, X.; Wang, H.; Yin, M.; Huang, H.; Zhang, H.; Wang, W. Electrospun gelatin/PCL and collagen/PLCL scaffolds for vascular tissue engineering. Int. J. Nanomed. 2014, 9, 2335–2344. [Google Scholar] [CrossRef] [PubMed]
- Hartman, R.P.; Brunner, D.J.; Camelot, D.M.; Marijnissen, J.C.; Scarlett, B. Jet break-up in electrohydrodynamic atomization in the cone-jet mode. J. Aerosol Sci. 2000, 31, 65–95. [Google Scholar] [CrossRef]
- Li, X.; Liu, H.; Wang, J.; Cui, H.; Zhang, X.; Han, F. Preparation of YAG: Nd nano-sized powder by co-precipitation method. Mater. Sci. Eng. A 2004, 379, 347–350. [Google Scholar] [CrossRef]
- Chase, G.G.; Reneker, D.H. Nanofibers in filter media. Fluid/Part. Sep. J. 2004, 16, 105–117. [Google Scholar]
- Jaworska, M.; Sakurai, K.; Gaudon, P.; Guibal, E. Influence of chitosan characteristics on polymer properties. I: Crystallographic properties. Poly. Int. 2003, 52, 198–205. [Google Scholar] [CrossRef]
- Yoo, H.S.; Kim, T.G.; Park, T.G. Surface-functionalized electrospun nanofibers for tissue engineering and drug delivery. Adv. Drug Deliv. Rev. 2009, 61, 1033–1042. [Google Scholar] [CrossRef] [PubMed]
- Medeiros, E.S.; Glenn, G.M.; Klamczynski, A.P.; Orts, W.J.; Mattoso, L.H. Solution blow spinning: A new method to produce micro-and nanofibers from polymer solutions. J. Appl. Polym. Sci. 2009, 113, 2322–2330. [Google Scholar] [CrossRef]
- Hsiao, H.Y.; Huang, C.M.; Hsu, M.Y.; Chen, H. Preparation of high-surface-area PAN-based activated carbon by solution-blowing process for CO2 adsorption. Sep. Purif. Technol. 2011, 82, 19–27. [Google Scholar] [CrossRef]
- Ghosal, A.; Sinha-Ray, S.; Sinha-Ray, S.; Yarin, A.L.; Pourdeyhimi, B. Numerical modeling and experimental study of solution-blown nonwovens formed on a rotating drum. Polymer 2016, 105, 255–263. [Google Scholar] [CrossRef]
- Khansari, S.; Duzyer, S.; Sinha-Ray, S.; Hockenberger, A.; Yarin, A.L.; Pourdeyhimi, B. Two-stage desorption-controlled release of fluorescent dye and vitamin from solution-blown and electrospun nanofiber mats containing porogens. Mol. Pharm. 2013, 10, 4509–4526. [Google Scholar] [CrossRef] [PubMed]
- Sinha-Ray, S.; Sinha-Ray, S.; Yarin, A.L.; Pourdeyhimi, B. Theoretical and experimental investigation of physical mechanisms responsible for polymer nanofiber formation in solution blowing. Polymer 2015, 56, 452–463. [Google Scholar] [CrossRef]
- Li, J.; Song, G.; Yu, J.; Wang, Y.; Zhu, J.; Hu, Z. Preparation of Solution Blown Polyamic Acid Nanofibers and Their Imidization into Polyimide Nanofiber Mats. Nanomaterials 2017, 7, 395. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Hou, H.; Harnisch, F.; Patil, S.A.; Carmona-Martinez, A.A.; Agarwal, S.; Zhang, Y.; Sinha-Ray, S.; Yarin, A.L.; Greiner, A.; et al. Electrospun and solution blown three-dimensional carbon fiber nonwovens for application as electrodes in microbial fuel cells. Energy Environ. Sci. 2011, 4, 1417–1421. [Google Scholar] [CrossRef]
- Oliveira, J.E.; Moraes, E.A.; Marconcini, J.M.; Mattoso, C.; Luiz, H.; Glenn, G.M.; Medeiros, E.S. Properties of poly (lactic acid) and poly (ethylene oxide) solvent polymer mixtures and nanofibers made by solution blow spinning. J. Appl. Polym. Sci. 2013, 129, 3672–3681. [Google Scholar] [CrossRef]
- Khansari, S.; Sinha-Ray, S.; Yarin, A.L.; Pourdeyhimi, B. Biopolymer-based nanofiber mats and their mechanical characterization. Ind. Eng. Chem. Res. 2013, 52, 15104–15113. [Google Scholar] [CrossRef]
- Greiner, A.; Wendorff, J.H. Electrospinning: A fascinating method for the preparation of ultrathin fibers. Angew. Chem. Int. Ed. 2007, 46, 5670–5703. [Google Scholar] [CrossRef] [PubMed]
- Thomas, V.; Jose, M.V.; Chowdhury, S.; Sullivan, J.F.; Dean, D.R.; Vohra, Y.K. Mechano-morphological studies of aligned nanofibrous scaffolds of polycaprolactone fabricated by electrospinning. J. Biomater. Sci. Polym. Ed. 2006, 17, 969–984. [Google Scholar] [CrossRef] [PubMed]
- Gorji, M.; Jeddi, A.; Gharehaghaji, A.A. Fabrication and characterization of polyurethane electrospun nanofiber membranes for protective clothing applications. J. Appl. Polym. Sci. 2012, 125, 4135–4141. [Google Scholar] [CrossRef]
- Stevens, M.J.; Plimpton, S.J. The effect of added salt on polyelectrolyte structure. Eur. Phys. J. B-Condens. Matter Complex Syst. 1998, 2, 341–345. [Google Scholar] [CrossRef]
- De Oliveira Santos, R.P.; Rodrigues, B.V.; dos Santos, D.M.; Campana-Filho, S.P.; Ruvolo-Filho, A.C.; Frollini, E. Electrospun recycled PET-based mats: Tuning the properties by addition of cellulose and/or lignin. Polym. Test. 2017, 60, 422–431. [Google Scholar] [CrossRef]
- Mann, B.; Everaers, R.; Holm, C.; Kremer, K. Scaling in polyelectrolyte networks. EPL (Europhys. Lett.) 2004, 67. [Google Scholar] [CrossRef]
- Lee, C.H.; Lim, H.S.; Kim, J.; Cho, J.H. Counterion-induced reversibly switchable transparency in smart windows. ACS Nano 2011, 5, 7397–7403. [Google Scholar] [CrossRef] [PubMed]
- Chae, D.W.; Chae, H.G.; Kim, B.C.; Oh, Y.S.; Jo, S.M.; Lee, W.S. Physical properties of lyocell fibers spun from isotropic cellulose dope in NMMO monohydrate. Text. Res. J. 2002, 72, 335–340. [Google Scholar] [CrossRef]
- Plank, J. Applications of biopolymers and other biotechnological products in building materials. Appl. Microbiol. Biotechnol. 2004, 66, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Kulpinski, P. Cellulose nanofibers prepared by the N-methylmorpholine N-oxide method. J. Appl. Polym. Sci. 2005, 98, 1855–1859. [Google Scholar] [CrossRef]
- Edgar, K.J.; Pecorini, T.J.; Glasser, W.G. Long-chain cellulose esters: Preparation, properties, and perspective. ACS Symp. Ser. 1998, 688, 38–60. [Google Scholar]
- Zhao, S.; Wu, X.; Wang, L.; Huang, Y. Electrospinning of ethyl–cyanoethyl cellulose/tetrahydrofuran solutions. J. Appl. Polym. Sci. 2004, 91, 242–246. [Google Scholar] [CrossRef]
- Son, W.K.; Youk, J.H.; Lee, T.S.; Park, W.H. Electrospinning of ultrafine cellulose acetate fibers: Studies of a new solvent system and deacetylation of ultrafine cellulose acetate fibers. J. Polym. Sci. Part B Polym. Phys. 2004, 42, 5–11. [Google Scholar] [CrossRef]
- Blades, H.; EI, du Pont de Nemours. Dry Jet Wet Spinning Process. U.S. Patent US 3,767,756, 3 June 1972. [Google Scholar]
- Son, W.K.; Youk, J.H.; Park, W.H. Preparation of ultrafine oxidized cellulose mats via electrospinning. Biomacromolecules 2004, 5, 197–201. [Google Scholar] [CrossRef] [PubMed]
- Roder, T.; Morgenstern, B.; Schelosky, N.; Glatter, O. Solutions of cellulose in N, N-dimethylacetamide/lithium chloride studied by light scattering methods. Polymer 2001, 42, 6765–6773. [Google Scholar] [CrossRef]
- Anton, F.; Richard Schreiber Gastell. Process and Apparatus for Preparing Artificial Threads. U.S. Patent US 1,975,504, 2 October 1934. [Google Scholar]
- Zhuang, X.; Yang, X.; Shi, L.; Cheng, B.; Guan, K.; Kang, W. Solution blowing of submicron-scale cellulose fibers. Carbohydr. Polym. 2012, 90, 982–987. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, T.; Tatsumi, D.; Tamai, N.; Takaki, T. Solution properties of celluloses from different biological origins in LiCl· DMAc. Cellulose 2001, 8, 275–282. [Google Scholar] [CrossRef]
- Ellison, C.J.; Phatak, A.; Giles, D.W.; Macosko, C.W.; Bates, F.S. Melt blown nanofibers: Fiber diameter distributions and onset of fiber breakup. Polymer 2007, 48, 3306–3316. [Google Scholar] [CrossRef]
- Kim, D.B.; Lee, W.S.; Jo, S.M.; Lee, Y.M.; Kim, B.C. Physical properties of lyocell fibers spun from different solution dope phases. J. Appl. Polym. Sci. 2002, 83, 981–989. [Google Scholar] [CrossRef]
- Ishii, D.; Tatsumi, D.; Matsumoto, T. Effect of solvent exchange on the supramolecular structure, the molecular mobility and the dissolution behavior of cellulose in LiCl/DMAc. Carbohydr. Res. 2008, 343, 919–928. [Google Scholar] [CrossRef] [PubMed]
- Biganska, O.; Navard, P. Phase diagram of a cellulose solvent: N-methylmorpholine–N-oxide–water mixtures. Polymer 2003, 44, 1035–1039. [Google Scholar] [CrossRef]
- Kim, C.W.; Kim, D.; Kang, S.Y.; Marquez, M.; Joo, Y.L. Structural studies of electrospun cellulose nanofibers. Polymer 2006, 47, 5097–5107. [Google Scholar] [CrossRef]
- Garcia-Mateos, F.J.; Berenguer, R.; Valero-Romero, M.J.; Rodriguez-Mirasol, J.; Cordero, T. Phosphorus functionalization for the rapid preparation of highly nanoporous submicron-diameter carbon fibers by electrospinning of lignin solutions. J. Mater. Chem. A 2018. [Google Scholar] [CrossRef]
- Miya, M.I.; Wamoto, R.; Mima, S. FTIR study of intermolecular interactions in polymer blends. J. Polym. Sci. Part B Polym. Phys. 1984, 22, 1149–1151. [Google Scholar] [CrossRef]
- El-Kafrawy, A. Investigation of the cellulose/LiCl/dimethylacetamide and cellulose/LiCl/N-methyl-2-pyrrolidinone solutions by 13C NMR spectroscopy. J. Appl. Polym. Sci. 1982, 27, 2435–2443. [Google Scholar] [CrossRef]
- Jaeger, R.; Bergshoef, M.M.; Batlle, C.M.; Schonherr, H.; Julius Vancso, G. Electrospinning of ultra-thin polymer fibers. Macromole. Symp. 1998, 127, 141–150. [Google Scholar] [CrossRef]
- Liu, H.; Hsieh, Y.L. Ultrafine fibrous cellulose membranes from electrospinning of cellulose acetate. J. Polym. Sci. Part B Polym. Phys. 2002, 40, 2119–2129. [Google Scholar] [CrossRef]
- Son, W.K.; Youk, J.H.; Lee, T.S.; Park, W.H. Preparation of antimicrobial ultrafine cellulose acetate fibers with silver nanoparticles. Macromol. Rapid Commun. 2004, 25, 1632–1637. [Google Scholar] [CrossRef]
- Galgut, P.N. Oxidized cellulose mesh: I. Biodegradable membrane in periodontal surgery. Biomaterials 1990, 11, 561–564. [Google Scholar] [CrossRef]
- Han, S.O.; Youk, J.H.; Min, K.D.; Kang, Y.O.; Park, W.H. Electrospinning of cellulose acetate nanofibers using a mixed solvent of acetic acid/water: Effects of solvent composition on the fiber diameter. Mater. Lett. 2008, 62, 759–762. [Google Scholar] [CrossRef]
- Tungprapa, S.; Puangparn, T.; Weerasombut, M.; Jangchud, I.; Fakum, P.; Semongkhol, S.; Meechaisue, C.; Supaphol, P. Electrospun cellulose acetate fibers: Effect of solvent system on morphology and fiber diameter. Cellulose 2007, 14, 563–575. [Google Scholar] [CrossRef]
- Harris, J.M.; Struck, E.C.; Case, M.G.; Paley, M.S.; Yalpani, M.; Van Alstine, J.M.; Brooks, D.E. Synthesis and characterization of poly (ethylene glycol) derivatives. J. Polym. Sci. Part A Polym. Chem. 1984, 22, 341–352. [Google Scholar] [CrossRef]
- Zhang, L.; Kopperstad, P.; West, M.; Hedin, N.; Fong, H. Generation of polymer ultrafine fibers through solution (air) blowing. J. Appl. Polym. Sci. 2009, 114, 3479–3486. [Google Scholar] [CrossRef]
- Taepaiboon, P.; Rungsardthong, U.; Supaphol, P. Vitamin-loaded electrospun cellulose acetate nanofiber mats as transdermal and dermal therapeutic agents of vitamin A acid and vitamin E. Eur. J. Pharm. Biopharm. 2007, 67, 387–397. [Google Scholar] [CrossRef] [PubMed]
- Jarusuwannapoom, T.; Hongrojjanawiwat, W.; Jitjaicham, S.; Wannatong, L.; Nithitanakul, M.; Pattamaprom, C.; Koombhongse, P.; Rangkupan, R.; Supaphol, P. Effect of solvents on electro-spinnability of polystyrene solutions and morphological appearance of resulting electrospun polystyrene fibers. Eur. Polym. J. 2005, 41, 409–421. [Google Scholar] [CrossRef]
- Ma, Z.; Kotaki, M.; Ramakrishna, S. Electrospun cellulose nanofiber as affinity membrane. J. Membr. Sci. 2005, 265, 115–123. [Google Scholar] [CrossRef]
- Tungprapa, S.; Jangchud, I.; Supaphol, P. Release characteristics of four model drugs from drug-loaded electrospun cellulose acetate fiber mats. Polymer 2007, 48, 5030–5041. [Google Scholar] [CrossRef]
- Xiang, C.; Frey, M.W.; Taylor, A.; Rebovich, M.E. Selective chemical absorbance in electrospun nonwovens. J. Appl. Polym. Sci. 2007, 106, 363–370. [Google Scholar] [CrossRef]
- Xu, W.; Yang, Y. Drug release and its relationship with kinetic and thermodynamic parameters of drug sorption onto starch acetate fibers. Biotechnol. Bioeng. 2010, 105, 814–822. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Fan, Z.; Shen, M.; Shi, X. Hyaluronic Acid-Functionalized Electrospun Polyvinyl Alcohol/Polyethyleneimine Nanofibers for Cancer Cell Capture Applications. Adv. Mater. Interfaces 2015, 2. [Google Scholar] [CrossRef]
- Shukla, S.; Brinley, E.; Cho, H.J.; Seal, S. Electrospinning of hydroxypropyl cellulose fibers and their application in synthesis of nano and submicron tin oxide fibers. Polymer 2005, 46, 12130–12145. [Google Scholar] [CrossRef]
- Jin, Y.; Yang, D.; Zhou, Y.; Ma, G.; Nie, J. Photocrosslinked electrospun chitosan-based biocompatible nanofibers. J. Appl. Polym. Sci. 2008, 109, 3337–3343. [Google Scholar] [CrossRef]
- Matthews, J.; Gustafsson, J.A. Estrogen signaling: A subtle balance between ERα and ERβ. Mol. Interv. 2003, 3, 281–292. [Google Scholar] [CrossRef] [PubMed]
- Wiseman, D.M.; Saferstein, L.; Wolf, S.; Ethicon Inc. Bioresorbable Oxidized Cellulose Composite Material for Prevention of Postsurgical Adhesions. U.S. Patent US 6,500,777, 31 December 2002. [Google Scholar]
- Faraji, S.; Danesh, E.; Tate, D.J.; Turner, M.L.; Majewski, L.A. Cyanoethyl cellulose-based nanocomposite dielectric for low-voltage, solution-processed organic field-effect transistors (OFETs). J. Phys. D Appl. Phys. 2016, 49, 185102. [Google Scholar] [CrossRef]
- Feng, L.; Li, S.; Li, H.; Zhai, J.; Song, Y.; Jiang, L.; Zhu, D. Super-hydrophobic surface of aligned polyacrylonitrile nanofibers. Angew. Chem. 2002, 114, 1269–1271. [Google Scholar] [CrossRef]
- Wu, X.; Wang, L.; Yu, H.; Huang, Y. Effect of solvent on morphology of electrospinning ethyl cellulose fibers. J. Appl. Polym. Sci. 2005, 97, 1292–1297. [Google Scholar] [CrossRef]
- Li, X.Y.; Yu, D.G.; Fu, C.T.; Wang, R.; Wang, X. Ketoprofen/ethyl Cellulose Nanofibers Fabricated Using an Epoxy-coated Spinneret. Model. Numer. Simul. Mater. Sci. 2013, 3, 6–10. [Google Scholar] [CrossRef]
- Shinsuke, I. Chitin Nanofibers: Preparations, Modifications, and Applications (ch. 10). In Handbook of Polymer Nanocomposites. Processing, Performance and Application; Pandey, J.K., Takagi, H., Nakagaito, A.N., Kim, H., Eds.; Springer: Berlin, Germany, 2015. [Google Scholar]
- Kumar, M.N. A review of chitin and chitosan applications. React. Funct. Polym. 2000, 46, 1–27. [Google Scholar] [CrossRef]
- Schiffman, J.D.; Stulga, L.A.; Schauer, C.L. Chitin and chitosan: Transformations due to the electrospinning process. Polym. Eng. Sci. 2009, 49, 1918–1928. [Google Scholar] [CrossRef]
- Min, B.M.; Lee, G.; Kim, S.H.; Nam, Y.S.; Lee, T.S.; Park, W.H. Electrospinning of silk fibroin nanofibers and its effect on the adhesion and spreading of normal human keratinocytes and fibroblasts in vitro. Biomaterials 2004, 25, 1289–1297. [Google Scholar] [CrossRef] [PubMed]
- Min, B.M.; Lee, S.W.; Lim, J.N.; You, Y.; Lee, T.S.; Kang, P.H.; Park, W.H. Chitin and chitosan nanofibers: Electrospinning of chitin and deacetylation of chitin nanofibers. Polymer 2004, 45, 7137–7142. [Google Scholar] [CrossRef]
- Park, K.E.; Jung, S.Y.; Lee, S.J.; Min, B.M.; Park, W.H. Biomimetic nanofibrous scaffolds: Preparation and characterization of chitin/silk fibroin blend nanofibers. Int. J. Biol. Macromol. 2006, 38, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Park, K.E.; Kang, H.K.; Lee, S.J.; Min, B.M.; Park, W.H. Biomimetic nanofibrous scaffolds: Preparation and characterization of PGA/chitin blend nanofibers. Biomacromolecules 2006, 7, 635–643. [Google Scholar] [CrossRef] [PubMed]
- Shalumon, K.T.; Binulal, N.S.; Selvamurugan, N.; Nair, S.V.; Menon, D.; Furuike, T.; Tamura, H.; Jayakuma, R.R. Electrospinning of carboxymethyl chitin/poly (vinyl alcohol) nanofibrous scaffolds for tissue engineering applications. Carbohydr. Polym. 2009, 77, 863–869. [Google Scholar] [CrossRef]
- Li, M.C.; Wu, Q.; Song, K.; Cheng, H.N.; Suzuki, S.; Lei, T. Chitin nanofibers as reinforcing and antimicrobial agents in carboxymethyl cellulose films: Influence of partial deacetylation. ACS Sustain. Chem. Eng. 2016, 4, 4385–4395. [Google Scholar] [CrossRef]
- Junkasem, J.; Rujiravanit, R.; Supaphol, P. Fabrication of α-chitin whisker-reinforced poly (vinyl alcohol) nanocomposite nanofibres by electrospinning. Nanotechnology 2006, 17, 4519. [Google Scholar] [CrossRef]
- Sorlier, P.; Denuziere, A.; Viton, C.; Domard, A. Relation between the degree of acetylation and the electrostatic properties of chitin and chitosan. Biomacromolecules 2001, 2, 765–772. [Google Scholar] [CrossRef] [PubMed]
- Masuda, M.; Hanada, T.; Okada, Y.; Yase, K.; Shimizu, T. Polymerization in nanometer-sized fibers: Molecular packing order and polymerizability. Macromolecules 2000, 33, 9233–9238. [Google Scholar] [CrossRef]
- Han, J.; Zhang, J.; Yin, R.; Ma, G.; Yang, D.; Nie, J. Electrospinning of methoxy poly (ethylene glycol)-grafted chitosan and poly (ethylene oxide) blend aqueous solution. Carbohydr. Polym. 2011, 83, 270–276. [Google Scholar] [CrossRef]
- Yang, D.; Jin, Y.; Zhou, Y.; Ma, G.; Chen, X.; Lu, F.; Nie, J. In situ mineralization of hydroxyapatite on electrospun chitosan based nanofibrous scaffolds. Macromol. Biosci. 2008, 8, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Rinaudo, M. Chitin and chitosan: Properties and applications. Prog. Polym. Sci. 2006, 31, 603–632. [Google Scholar] [CrossRef]
- Coseri, S.; Biliuta, G.; Simionescu, B.C.; Stana-Kleinschek, K.; Ribitsch, V.; Harabagiu, V. Oxidized cellulose—Survey of the most recent achievements. Carbohydr. Polym. 2013, 93, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Helander, I.M.; Nurmiaho-Lassila, E.L.; Ahvenainen, R.; Rhoades, J.; Roller, S. Chitosan disrupts the barrier properties of the outer membrane of Gram-negative bacteria. Int. J. Food Microbiol. 2001, 71, 235–244. [Google Scholar] [CrossRef]
- Zheng, L.Y.; Zhu, J.F. Study on antimicrobial activity of chitosan with different molecular weights. Carbohydr. Polym. 2003, 54, 527–530. [Google Scholar] [CrossRef]
- Cao, H.; Jiang, X.; Chai, C.; Chew, S.Y. RNA interference by nanofiber-based siRNA delivery system. J. Control. Release 2010, 144, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Claesson, P.M.; Ninham, B.W. pH-dependent interactions between adsorbed chitosan layers. Langmuir 1992, 8, 1406–1412. [Google Scholar] [CrossRef]
- Qin, C.; Li, H.; Xiao, Q.; Liu, Y.; Zhu, J.; Du, Y. Water-solubility of chitosan and its antimicrobial activity. Carbohydr. Polym. 2006, 63, 367–374. [Google Scholar] [CrossRef]
- Rhoades, J.; Roller, S. Antimicrobial actions of degraded and native chitosan against spoilage organisms in laboratory media and foods. Appl. Environ. Microbiol. 2000, 66, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Lehr, C.M.; Bouwstra, J.A.; Schach, T.E.H.; Junginger, H.E. In vitro evaluation of mucoadhesive properties of chitosan and some other natural polymers. Int. J. Pharm. 1992, 78, 43–48. [Google Scholar] [CrossRef]
- Holopainen, J.; Ritala, M. Rapid production of bioactive hydroxyapatite fibers via electroblowing. J. Eur. Ceram. Soc. 2016, 36, 3219–3224. [Google Scholar] [CrossRef]
- Geng, X.; Kwon, O.H.; Jang, J. Electrospinning of chitosan dissolved in concentrated acetic acid solution. Biomaterials 2005, 26, 5427–5432. [Google Scholar] [CrossRef] [PubMed]
- Ohkawa, K.; Cha, D.; Kim, H.; Nishida, A. Yamamoto H. Electrospinning of chitosan. Macromol. Rapid Commun. 2004, 25, 1600–1605. [Google Scholar] [CrossRef]
- Schiffman, J.D.; Schauer, C.L. A review: Electrospinning of biopolymer nanofibers and their applications. Polym. Rev. 2008, 48, 317–352. [Google Scholar] [CrossRef]
- Neamnark, A.; Rujiravanit, R.; Supaphol, P. Electrospinning of hexanoyl chitosan. Carbohydr. Polym. 2006, 66, 298–305. [Google Scholar] [CrossRef]
- Du, J.; Hsieh, Y.L. PEGylation of chitosan for improved solubility and fiber formation via electrospinning. Cellulose 2007, 14, 543–552. [Google Scholar] [CrossRef]
- Duan, B.; Dong, C.; Yuan, X.; Yao, K. Electrospinning of chitosan solutions in acetic acid with poly (ethylene oxide). J. Biomater. Sci. Polym. Ed. 2004, 15, 797–811. [Google Scholar] [CrossRef] [PubMed]
- Spasova, M.; Manolova, N.; Paneva, D.; Rashkov, I. Preparation of chitosan-containing nanofibres by electrospinning of chitosan/poly (ethylene oxide) blend solutions. e-Polymers 2014. [Google Scholar] [CrossRef]
- Bhattarai, N.; Edmondson, D.; Veiseh, O.; Matsen, F.A.; Zhang, M. Electrospun chitosan-based nanofibers and their cellular compatibility. Biomaterials 2005, 26, 6176–6184. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.M.; Zhang, Y.Z.; Kotaki, M.; Ramakrishna, S. A review on polymer nanofibers by electrospinning and their applications in nanocomposites. Compos. Sci. Technol. 2003, 63, 2223–2253. [Google Scholar] [CrossRef]
- Jia, Y.T.; Gong, J.; Gu, X.H.; Kim, H.Y.; Dong, J.; Shen, X.Y. Fabrication and characterization of poly (vinyl alcohol)/chitosan blend nanofibers produced by electrospinning method. Carbohydr. Polym. 2007, 67, 403–409. [Google Scholar] [CrossRef]
- Jiang, Z.; Zhang, H.; Zhu, M.; Lv, D.; Yao, J.; Xiong, R.; Huang, C. Electrospun soy protein based nanofibrous membranes for effective antimicrobial air filtration. J. Appl. Polym. Sci. 2018, 135. [Google Scholar] [CrossRef]
- Das, P.; Ojah, N.; Kandimalla, R.; Mohan, K.; Gogo, I.D.; Dolui, S.K.; Choudhury, A.J. Surface modification of electrospun PVA/chitosan nanofibers by dielectric barrier discharge plasma at atmospheric pressure and studies of their mechanical properties and biocompatibility. Int. J. Biol. Macromol. 2018, 114, 1026–1032. [Google Scholar] [CrossRef] [PubMed]
- Habiba, U.; Siddique, T.A.; Lee, J.J.; Joo, T.C.; Ang, B.C.; Afifi, A.M. Adsorption study of methyl orange by chitosan/polyvinyl alcohol/zeolite electrospun composite nanofibrous membrane. Carbohydr. Polym. 2018, 191, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Li, K. Synthesis of Lignin-Carbohydrate Model Compounds and Neolignans. Ph.D. Thesis, Virginia Tech, Blacksburg, VA, USA, 1996. [Google Scholar]
- Lewis, N.G.; Lantzy, T.R. Lignin in adhesives: Introduction and historical perspective. ACS Symp. Ser. 1989, 385, 13–26. [Google Scholar]
- Ruiz-Rosas, R.; Bedia, J.; Lallave, M.; Loscertales, I.G.; Barrero, A.; Rodriguez-Mirasol, J.; Cordero, T. The production of submicron diameter carbon fibers by the electrospinning of lignin. Carbon 2010, 48, 696–705. [Google Scholar] [CrossRef]
- Garcia-Mateos, F.J.; Cordero-Lanzac, T.; Berenguer, R.; Morallon, E.; Cazorla-Amoros, D.; Rodriguez-Mirasol, J.; Cordero, T. Lignin-derived Pt supported carbon (submicron) fiber electrocatalysts for alcohol electro-oxidation. Appl. Catal. B Environ. 2017, 211, 18–30. [Google Scholar] [CrossRef]
- Ahn, Y.; Lee, S.H.; Kim, H.J.; Yang, Y.H.; Hong, J.H.; Kim, Y.H.; Kim, H. Electrospinning of lignocellulosic biomass using ionic liquid. Carbohydr. Polym. 2012, 88, 395–398. [Google Scholar] [CrossRef]
- Pouteau, C.; Dole, P.; Cathala Averous, L.; Boquillon, N. Antioxidant properties of lignin in polypropylene. Polym. Degrad. Stab. 2003, 81, 9–18. [Google Scholar] [CrossRef]
- Dallmeyer, I.; Ko, F.; Kadla, J.F. Correlation of elongational fluid properties to fiber diameter in electrospinning of softwood Kraft lignin solutions. Ind. Eng. Chem. Res. 2014, 53, 2697–2705. [Google Scholar] [CrossRef]
- Seo, D.K.; Jeun, J.P.; Kim, H.B.; Kang, P.H. Preparation and characterization of the carbon nanofiber mat produced from electrospun PAN/lignin precursors by electron beam irradiation. Rev. Adv. Mater. Sci. 2011, 28, 31–34. [Google Scholar]
- Jonoobi, M.; Harun, J.; Mathew, A.P.; Oksman, K. Mechanical properties of cellulose nanofiber (CNF) reinforced polylactic acid (PLA) prepared by twin screw extrusion. Compos. Sci. Technol. 2010, 70, 1742–1747. [Google Scholar] [CrossRef]
- Beck, R.J.; Zhao, Y.; Fong, H.; Menkhaus, T.J. Electrospun lignin carbon nanofiber membranes with large pores for highly efficient adsorptive water treatment applications. J. Water Process Eng. 2017, 16, 240–248. [Google Scholar] [CrossRef]
- Park, C.W.; Youe, W.J.; Han, S.Y.; Kim, Y.S.; Lee, S.H. Characteristics of carbon nanofibers produced from lignin/(PAN)/kraft lignin-g-PAN copolymer blends electrospun nanofibers. Holzforschung 2017, 71, 743–750. [Google Scholar] [CrossRef]
- Schreiber, M.; Vivekanandhan, S.; Mohanty, A.K.; Misra, M. A study on the electrospinning behaviour and nanofibre morphology of anionically charged lignin. Adv. Mater. Lett. 2012, 3, 476. [Google Scholar] [CrossRef]
- Wang, S.X.; Yang, L.; Stubbs, L.P.; Li, X.; He, C. Lignin-derived fused electrospun carbon fibrous mats as high-performance anode materials for lithium ion batteries. ACS Appl. Mater. Interfaces 2013, 5, 12275–12282. [Google Scholar] [CrossRef] [PubMed]
- Dallmeyer, I.; Ko, F.; Kadla, J.F. Electrospinning of technical lignins for the production of fibrous networks. J. Wood Chem. Technol. 2010, 30, 315–329. [Google Scholar] [CrossRef]
- Poursorkhabi, V.; Mohanty, A.K.; Misra, M. Electrospinning of aqueous lignin/poly (ethylene oxide) complexes. J. Appl. Polym. Sci. 2015, 132. [Google Scholar] [CrossRef]
- Aslanzadeh, S.; Ahvazi, B.; Boluk, Y.; Ayranci, C. Carbon Fiber Production from Electrospun Sulfur Free Softwood Lignin Precursors. J. Eng. Fabr. Fibers (JEFF) 2017, 12, 33–43. [Google Scholar]
- Shi, X.; Wang, X.; Tang, B.; Dai, Z.; Chen, K.; Zhou, J. Impact of lignin extraction methods on microstructure and mechanical properties of lignin-based carbon fibers. J. Appl. Polym. Sci. 2018, 135. [Google Scholar] [CrossRef]
- Luo, Y.; Nartker, S.; Miller, H.; Hochhalter, D.; Wiederoder, M.; Wiederoder, S.; Setterington, E.; Drzal, L.T.; Alocilja, E.C. Surface functionalization of electrospun nanofibers for detecting E. coli O157: H7 and BVDV cells in a direct-charge transfer biosensor. Biosens. Bioelectron. 2010, 26, 1612–1617. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.; Zhou, Z.; Zhang, L.; Wang, X.; Zhou, Q.; Zhao, Y.; Wang, Y.; Wu, X.F.; Zhu, Z.; Fong, H. Free-standing and mechanically flexible mats consisting of electrospun carbon nanofibers made from a natural product of alkali lignin as binder-free electrodes for high-performance supercapacitors. J. Power Sources 2014, 247, 134–141. [Google Scholar] [CrossRef]
- Lai, C.; Kolla, P.; Zhao, Y.; Fong, H.; Smirnova, A.L. Lignin-derived electrospun carbon nanofiber mats with supercritically deposited Ag nanoparticles for oxygen reduction reaction in alkaline fuel cells. Electrochim. Acta 2014, 130, 431–438. [Google Scholar] [CrossRef]
- Fang, X.D.; Reneke, D.H. DNA fibers by electrospinning. J. Macromol. Sci. Part B Phys. 1997, 36, 169–173. [Google Scholar] [CrossRef]
- Ruiz-Rosas, R.; Bedia, J.; Lallave, M.; Barrero, A.; Loscertales, I.G.; Rodiíguez-Mirasol, J.; Cordero, T. Preparation and characterization of co-electrospun lignin/alumina microfibers and tubes. In Proceedings of the International Carbon Conference, Clemson, SC, USA, 11–16 July 2010. [Google Scholar]
- Ago, M.; Jakes, J.E.; Johansson, L.S.; Park, S.; Rojas, O.J. Interfacial properties of lignin-based electrospun nanofibers and films reinforced with cellulose nano-crystals. ACS Appl. Mater. Interfaces 2012, 4, 6849–6856. [Google Scholar] [CrossRef] [PubMed]
- Ago, M.; Jakes, J.E.; Rojas, O.J. Thermo-mechanical properties of lignin-based electrospun nanofibers and films reinforced with cellulose nano-crystals. A dynamic mechanical and nanoindentation study. ACS Appl. Mater. Interfaces 2013, 5, 11768–11776. [Google Scholar] [CrossRef] [PubMed]
- Ago, M.; Okajima, K.; Jakes, J.E.; Park, S.; Rojas, O.J. Lignin-based electrospun nanofibers reinforced with cellulose nano-crystals. Bio-Macromolecules 2012, 13, 918–926. [Google Scholar] [CrossRef] [PubMed]
- Doshi, J.; Reneker, D.H. Electrospinning process and applications of electrospun fibers. J. Electrostat. 1995, 35, 151–160. [Google Scholar] [CrossRef]
- Schreiber, M.; Vivekanandhan, S.; Cooke, P.; Mohanty, A.K.; Misra, M. Electrospun green fibres from lignin and chitosan: A novel polycomplexation process for the production of lignin-based fibres. J. Mater. Sci. 2014, 49, 7949–7958. [Google Scholar] [CrossRef]
- Garvey, M.; Klose, H.; Fischer, R.; Lambertz, C.; Commandeur, U. Cellulases for biomass degradation: Comparing recombinant cellulase expression platforms. Trends Biotechnol. 2013, 31, 581–593. [Google Scholar] [CrossRef] [PubMed]
- Dallmeyer, I.; Lin, L.T.; Li, Y.; Ko, F.; Kadla, J.F. Preparation and Characterization of Interconnected, Kraft Lignin-Based Carbon Fibrous Materials by Electrospinning. Macromol. Mater. Eng. 2014, 299, 540–551. [Google Scholar] [CrossRef]
- Bahi, A.; Shao, J.; Mohseni, M.; Ko, F.K. Membranes based on electrospun lignin-zeolite composite nanofibers. Sep. Purif. Technol. 2017, 187, 207–213. [Google Scholar] [CrossRef]
- Jin, J.; Yu, B.J.; Shi, Z.Q.; Wang, C.Y.; Chong, C.B. Lignin-based electrospun carbon nanofibrous webs as free-standing and binder-free electrodes for sodium ion batteries. J. Power Sources 2014, 272, 800–807. [Google Scholar] [CrossRef]
- Khadka, D.B.; Haynie, D.T. Protein-and peptide-based electrospun nanofibers in medical biomaterials. Nanomed. Nanotechnol. Biol. Med. 2012, 8, 1242–1262. [Google Scholar] [CrossRef] [PubMed]
- Gelse, K.; Poschl, E.; Aigne, T. Collagens—Structure, function, and biosynthesis. Adv. Drug Deliv. Rev. 2003, 55, 1531–1546. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Li, Q.; Fan, H.; Li, S.; Su, Y.; Zhao, L.; Huang, Y.; Wang, D.; Zhang, Z.; Su, Z.; et al. Multi-layer dextran-decorated poly (glycidyl methacrylate)-co-divinyl benzene copolymer matrices enabling efficient protein chromatographic separation. React. Funct. Polym. 2017, 112, 45–52. [Google Scholar] [CrossRef]
- Zeng, J.; Aigner, A.; Czubayko, F.; Kissel, T.; Wendorff, J.H.; Greiner, A. Poly (vinyl alcohol) nanofibers by electrospinning as a protein delivery system and the retardation of enzyme release by additional polymer coatings. Biomacromolecules 2005, 6, 1484–1488. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Li, X.; Qi, M.; Zhou, S.; Weng, J. Release pattern and structural integrity of lysozyme encapsulated in core–sheath structured poly (DL-lactide) ultrafine fibers prepared by emulsion electrospinning. Eur. J. Pharm. Biopharm. 2008, 69, 106–116. [Google Scholar] [CrossRef] [PubMed]
- Kowalczyk, T.; Nowicka, A.; Elbaum, D.; Kowalewski, T.A. Electrospinning of bovine serum albumin. Optimization and the use for production of biosensors. Biomacromolecules 2008, 9, 2087–2090. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Su, Y.; Liu, S.; Tan, L.; Mo, X.; Ramakrishna, S. Encapsulation of proteins in poly (L-lactide-co-caprolactone) fibers by emulsion electrospinning. Colloids Surfaces B Biointerfaces 2010, 75, 418–424. [Google Scholar] [CrossRef] [PubMed]
- Moradzadegan, A.; Ranaei-Siadat, S.O.; Ebrahim-Habibi, A.; Barshan-Tashnizi, M.; Jalili, R.; Torabi, S.F.; Khajeh, K. Immobilization of acetylcholinesterase in nanofibrous PVA/BSA membranes by electrospinning. Eng. Life Sci. 2010, 10, 57–64. [Google Scholar] [CrossRef]
- Ravichandran, R.; Sridhar, R.; Venugopal, J.R.; Sundarrajan, S.; Mukherjee, S.; Ramakrishna, S. Gold nanoparticle loaded hybrid nanofibers for cardiogenic differentiation of stem cells for infarcted myocardium regeneration. Macromol. Biosci. 2014, 14, 515–525. [Google Scholar] [CrossRef] [PubMed]
- Fratz, L.P. Collagen: Structure and mechanics, an introduction. In Collagen; Springer: Boston, MA, USA, 2008; pp. 1–13. [Google Scholar]
- How, T.V.; Guidoin, R.; Young, S.K. Engineering design of vascular prostheses. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 1992, 206, 61–71. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Nagapudi, K.P.; Apkarian, R.; Chaikof, E.L. Engineered collagen–PEO nanofibers and fabrics. J. Biomater. Sci. Polym. Ed. 2001, 12, 979–993. [Google Scholar] [CrossRef] [PubMed]
- Matthews, J.A.; Boland, E.D.; Wnek, G.E.; Simpson, D.G.; Bowlin, G.L. Electrospinning of collagen type II: A feasibility study. J. Bioact. Compat. Polym. 2003, 18, 125–134. [Google Scholar] [CrossRef]
- Tillman, B.W.; Yazdani, S.K.; Lee, S.J.; Geary, R.L.; Atala, A.; Yoo, J.J. The in vivo stability of electrospun polycaprolactone-collagen scaffolds in vascular reconstruction. Biomaterials 2009, 30, 583–588. [Google Scholar] [CrossRef] [PubMed]
- Altman, G.H.; Diaz, F.; Jakuba, C.; Calabro, T.; Horan, R.L.; Chen, J.; Lu, H.; Richmond, J.; Kaplan, D.L. Silk-based biomaterials. Biomaterials 2003, 24, 401–416. [Google Scholar] [CrossRef]
- Klemm, D.; Heublein, B.; Fink, H.P.; Bohn, A. Cellulose: Fascinating biopolymer and sustainable raw material. Angew. Chem. Int. Ed. 2005, 44, 3358–3393. [Google Scholar] [CrossRef] [PubMed]
- Graham, K.; Ouyang, M.; Raether, T.; Grafe, T.; McDonald, B.; Knauf, P. Polymeric nanofibers in air filtration applications. In Proceedings of the Fifteenth Annual Technical Conference & Expo of the American Filtration & Separations Society, Galveston, TX, USA, 19–21 May 2002; pp. 9–12. [Google Scholar]
- Kadakia, P.U.; Growney Kalaf, E.A.; Dunn, A.J.; Shornick, L.P.; Sell, S.A. Comparison of silk fibroin electrospun scaffolds with poloxamer and honey additives for burn wound applications. J. Bioact. Compat. Polym. 2018, 33, 79–94. [Google Scholar] [CrossRef]
- Cappello, J.; McGrath, K.P. Spinning of protein polymer fibers. ACS Symp. Ser. 1994, 26, 311–327. [Google Scholar]
- Jin, H.J.; Fridrikh, S.V.; Rutledge, G.C.; Kaplan, D.L. Electrospinning Bombyx mori silk with poly (ethylene oxide). Biomacromolecules 2002, 3, 1233–1239. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Vepari, C.; Jin, H.J.; Kim, H.J.; Kaplan, D.L. Electrospun silk-BMP-2 scaffolds for bone tissue engineering. Biomaterials 2006, 27, 3115–3124. [Google Scholar] [CrossRef] [PubMed]
- Sukigara, S.; Gandhi, M.; Ayutsede, J.; Micklus, M.; Ko, F. Regeneration of Bombyx mori silk by electrospinning—Part 1: Processing parameters and geometric properties. Polymer 2003, 44, 5721–5727. [Google Scholar] [CrossRef]
- Zhang, F.; Zhang, H.; Zuo, B.; Zhang, X. Preparation and characterization of electrospun silk fibroin nanofiber with addition of 1-ethyl-3-(3-dimethylarainopropyl) carbodiimide. Polym. Sci. Ser. A 2011, 53, 397–402. [Google Scholar] [CrossRef]
- López-Rubio, A.; Lagaron, J.M. Whey protein capsules obtained through electrospraying for the encapsulation of bioactives. Innov. Food Sci. Emerg. Technol. 2012, 13, 200–206. [Google Scholar] [CrossRef]
- Turan, D.; Gibis, M.; Gunes, G.; Baier, S.K.; Weiss, J. The impact of the molecular weight of dextran on formation of whey protein isolate (WPI)–dextran conjugates in fibers produced by needleless electrospinning after annealing. Food Funct. 2018. [Google Scholar] [CrossRef] [PubMed]
- Zhong, J.; Mohan, S.D.; Bell, A.; Terry, A.; Mitchell, G.R.; Davis, F.J. Electrospinning of food-grade nanofibres from whey protein. Int. J. Biol. Macromol. 2018, 113, 764–773. [Google Scholar] [CrossRef] [PubMed]
- Vega-Lugo, A.C.; Lim, L.T. Electrospinning of soy protein isolate nanofibers. J. Biobased Mater. Bioenergy 2008, 2, 223–230. [Google Scholar] [CrossRef]
- Vega-Lugo, A.C.; Lim, L.T. Controlled release of allyl isothiocyanate using soy protein and poly (lactic acid) electrospun fibers. Food Res. Int. 2009, 42, 933–940. [Google Scholar] [CrossRef]
- Xu, X.; Jiang, L.; Zhou, Z.; Wu, X.; Wang, Y. Preparation and properties of electrospun soy protein isolate/polyethylene oxide nanofiber membranes. ACS Appl. Mater. Interfaces 2012, 4, 4331–4337. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Li, X.; Cui, W.; Zhou, S.; Tan, R.; Wang, C. Structural stability and release profiles of proteins from core-shell poly (DL-lactide) ultrafine fibers prepared by emulsion electrospinning. J. Biomed. Mater. Res. A 2008, 86, 374–385. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.; Zhao, C.; Li, P.; Zhang, H.; Huang, Y.; Li, H.; Fan, J.; Feng, W.; Yuan, X.; Fan, Y. Sustained release of VEGF by coaxial electrospun dextran/PLGA fibrous membranes in vascular tissue engineering. J. Biomater. Sci. Polym. Ed. 2011, 22, 1811–1827. [Google Scholar] [CrossRef] [PubMed]
- Nieuwland, M.; Geerdink, P.; Brier, P.; Van Den Eijnden, P.; Henket, J.T.; Langelaan, M.L.; Stroeks, N.; van Deventer, H.C.; Martin, A.H. Food-grade electrospinning of proteins. Innov. Food Sci. Emerg. Technol. 2013, 20, 269–275. [Google Scholar] [CrossRef]
- Kim, T.G.; Lee, D.S.; Park, T.G. Controlled protein release from electrospun biodegradable fiber mesh composed of poly (ɛ-caprolactone) and poly (ethylene oxide). Int. J. Pharm. 2007, 338, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Da Silva Parize, D.D.; de Oliveira, J.E.; Foschini, M.M.; Marconcini, J.M.; Mattoso, L.H. Poly (lactic acid) fibers obtained by solution blow spinning: Effect of a greener solvent on the fiber diameter. J. Appl. Polym. Sci. 2016, 133. [Google Scholar] [CrossRef]
- Remondetto, G.; Anon, M.C.; Gonzalez, R.J. Hydration properties of soybean protein isolates. Brazil. Arc. Biol. Technol. 2001, 44, 425–431. [Google Scholar] [CrossRef]
- Coussy, H.A.; Chalier, P.; Gastaldi, E.; Guillard, V.; Guillaume, C.; Gontard, N.; Peyron, S. Protein-Based Nanocomposites for Food Packaging (Chapter 25), Biopolymer Nanocomposites Processing, Properties, And Applications; Dufresne, A., Thomas, S., Pothan, L.A., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2013. [Google Scholar]
- Hong, H.; Tronstad, Z.C.; Yang, Y.; Green, M.D. Characterization of PVC-soy protein nonwoven mats prepared by electrospinning. AIChE J. 2018. [Google Scholar] [CrossRef]
- Morr, C.V.; Swenson, P.E.; Richter, R.L. Functional characteristics of whey protein concentrates. J. Food Sci. 1973, 38, 324–330. [Google Scholar] [CrossRef]
- Ribadeau-Dumas, B.; Grappin, R. Milk protein analysis. Le Lait. 1989, 69, 357–416. [Google Scholar] [CrossRef]
- De Geest, B.G.; Van Camp, W.; Du Prez, F.E.; De Smedt, S.C.; Demeester, J.; Hennink, W.E. Biodegradable microcapsules designed via ‘click’ chemistry. Chem. Commun. 2008, 190–192. [Google Scholar] [CrossRef] [PubMed]
- Unnithan, A.R.; Barakat, N.A.; Pichiah, P.T.; Gnanasekaran, G.; Nirmala, R.; Cha, Y.S.; Jung, C.H.; El-Newehy, M.; Kim, H.Y. Wound-dressing materials with antibacterial activity from electrospun polyurethane–dextran nanofiber mats containing ciprofloxacin HCl. Carbohydr. Polym. 2012, 90, 1786–1793. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Zhou, F.; Liu, B.; Zhao, Y.; Yuan, X. Functional electrospun fibrous scaffolds with dextran-g-poly (L-lysine)-VAPG/microRNA-145 to specially modulate vascular SMCs. J. Mater. Chem. B 2017, 5, 9312–9325. [Google Scholar] [CrossRef]
- Papakonstantinou, E.; Roth, M.; Karakiulakis, G. Hyaluronic acid: A key molecule in skin aging. Dermato Endocrinol. 2012, 4, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Akasaka, T.; Nishida, J.; Araki, S.; Shimamura, T.; Amadio, P.C.; An, K.N. Hyaluronic acid diminishes the resistance to excursion after flexor tendon repair: An in vitro biomechanical study. J. Biomech. 2005, 38, 503–507. [Google Scholar] [CrossRef] [PubMed]
- Ahire, J.J.; Robertson, D.D.; van Reenen, A.J.; Dicks, L.M. Polyethylene oxide (PEO)-hyaluronic acid (HA) nanofibers with kanamycin inhibits the growth of listeria monocytogenes. Biomed. Pharmacother. 2017, 86, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Kolbasov, A.; Sinha-Ray, S.; Yarin, A.L.; Pourdeyhimi, B. Heavy metal adsorption on solution-blown biopolymer nanofiber membranes. J. Membr. Sci. 2017, 530, 250–263. [Google Scholar] [CrossRef]
- Sinha-Ray, S.; Zhang, Y.; Yarin, A.L.; Davis, S.C.; Pourdeyhimi, B. Solution blowing of soy protein fibers. Biomacromolecules 2011, 12, 2357–2363. [Google Scholar] [CrossRef] [PubMed]
- Sinha-Ray, S.; Lee, M.W.; Sinha-Ray, S.; An, S.; Pourdeyhimi, B.; Yoon, S.S.; Yarin, A.L. Supersonic nanoblowing: A new ultra-stiff phase of nylon 6 in 20–50 nm confinement. J. Mater. Chem. C 2013, 1, 3491–3498. [Google Scholar] [CrossRef]
- Sett, S.; Stephansen, K.; Yarin, A.L. Solution-blown nanofiber mats from fish sarcoplasmic protein. Polymer 2016, 93, 78–87. [Google Scholar] [CrossRef]
- Polat, Y.; Pampal, E.S.; Stojanovska, E.; Simsek, R.; Hassanin, A.; Kilic, A.; Demir, A.; Yilmaz, S. Solution blowing of thermoplastic polyurethane nanofibers: A facile method to produce flexible porous materials. J. Appl. Polym. Sci. 2016, 133. [Google Scholar] [CrossRef]
- Klemm, D.; Heinze, T.; Philipp, B.; Wagenknecht, W. New approaches to advanced polymers by selective cellulose functionalization. Acta Polym. 1997, 48, 277–297. [Google Scholar] [CrossRef]
- Liu, R.; Xu, X.; Zhuang, X.; Cheng, B. Solution blowing of chitosan/PVA hydrogel nanofiber mats. Carbohydr. Polym. 2014, 101, 1116–1121. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Liu, Z.M.; Xu, L.; Lv, X.; Ning, J.; Hou, J.; Ge, G.B.; Cui, J.N.; Yang, L. A highly selective long-wavelength fluorescent probe for the detection of human carboxylesterase 2 and its biomedical applications. Chem. Commun. 2014, 50, 4519–4522. [Google Scholar] [CrossRef] [PubMed]
- Lecommandoux, S.; Garanger, É. Precision polymers with biological activity: Design towards self-assembly and bioactivity. C. R. Chim. 2016, 19, 143–147. [Google Scholar] [CrossRef]
- Li, W.J.; Laurencin, C.T.; Caterson, E.J.; Tuan, R.S.; Ko, F.K. Electrospun nanofibrous structure: A novel scaffold for tissue engineering. J. Biomed. Mater. Res. Part A 2002, 60, 613–621. [Google Scholar] [CrossRef] [PubMed]
- Shankar, A.; Seyam, A.F.; Hudson, S. Electrospinning of soy protein fibers and their compatibility with synthetic polymers. J. Text. Appar. Technol. Manag. 2013, 8, 1–14. [Google Scholar]
- Sankar, S.; Sharma, C.S.; Rath, S.N.; Ramakrishna, S. Electrospun nanofibres to mimic natural hierarchical structure of tissues: Application in musculoskeletal regeneration. J. Tissue Eng. Regener. Med. 2018, 12, e604–e619. [Google Scholar] [CrossRef] [PubMed]
- Noh, H.K.; Lee, S.W.; Kim, J.M.; Oh, J.E.; Kim, K.H.; Chung, C.P.; Choi, S.C.; Park, W.H.; Min, B.M. Electrospinning of chitin nanofibers: Degradation behavior and cellular response to normal human keratinocytes and fibroblasts. Biomaterials 2006, 27, 3934–3944. [Google Scholar] [CrossRef] [PubMed]
- Sisson, K.; Zhang, C.; Farach-Carson, M.C.; Chase, D.B.; Rabolt, J.F. Fiber diameters control osteoblastic cell migration and differentiation in electrospun gelatin. J. Biomed. Mater. Res. A 2010, 94, 1312–1320. [Google Scholar] [CrossRef] [PubMed]
- Verreck, G.; Chun, I.; Peeters, J.; Rosenblatt, J.; Brewster, M.E. Preparation and characterization of nanofibers containing amorphous drug dispersions generated by electrostatic spinning. Pharm. Res. 2003, 20, 810–817. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Liu, S.; Zhou, G.; Huang, Y.; Xie, Z.; Jing, X. Electrospinning of polymeric nanofibers for drug delivery applications. J. Control. Release 2014, 185, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Sill, T.J.; Von Recum, H.A. Electrospinning: Applications in drug delivery and tissue engineering. Biomaterials 2008, 29, 1989–2006. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.; Lowe, L.; Hamilton, T.A.; Fisher, G.J.; Voorhees, J.J.; Kang, S. Long-term treatment of photoaged human skin with topical retinoic acid improves epidermal cell atypia and thickens the collagen band in papillary dermis. J. Am. Acad. Dermatol. 2005, 53, 769–774. [Google Scholar] [CrossRef] [PubMed]
- Srikar, R.; Yarin, A.L.; Megaridis, C.M.; Bazilevsky, A.V.; Kelley, E. Desorption-limited mechanism of release from polymer nanofibers. Langmuir. 2008, 24, 965–974. [Google Scholar] [CrossRef] [PubMed]
- Juang, R.S.; Shiau, R.C. Metal removal from aqueous solutions using chitosan-enhanced membrane filtration. J. Membr. Sci. 2000, 165, 159–167. [Google Scholar] [CrossRef]
- Mondal, K. Recent advances in the synthesis of metal oxide nanofibers and their environmental remediation applications. Inventions 2017, 2, 9. [Google Scholar] [CrossRef]
- Kumar, S.; Lively, B.; Sun, L.L.; Li, B.; Zhong, W.H. Highly dispersed and electrically conductive polycarbonate/oxidized carbon nanofiber composites for electrostatic dissipation applications. Carbon 2010, 48, 3846–3857. [Google Scholar] [CrossRef]
- Appendini, P.; Hotchkiss, J.H. Review of antimicrobial food packaging. Innov. Food Sci. Emerg. Technol. 2002, 3, 113–126. [Google Scholar] [CrossRef]
- Wen, P.; Zhu, D.H.; Feng, K.; Liu, F.J.; Lou, W.Y.; Li, N.; Zong, M.H.; Wu, H. Fabrication of electrospun polylactic acid nanofilm incorporating cinnamon essential oil/β-cyclodextrin inclusion complex for antimicrobial packaging. Food Chem. 2016, 196, 996–1004. [Google Scholar] [CrossRef] [PubMed]
- Mendes, A.C.; Stephansen, K.; Chronakis, I.S. Electrospinning of food proteins and polysaccharides. Food Hydrocolloids 2017, 68, 53–68. [Google Scholar] [CrossRef]
- De Azeredo, H.M. Nanocomposites for food packaging applications. Food Res. Int. 2009, 42, 1240–1253. [Google Scholar] [CrossRef]
- Huang, W.; Xu, H.; Xue, Y.; Huang, R.; Deng, H.; Pan, S. Layer-by-layer immobilization of lysozyme–chitosan–organic rectorite composites on electrospun nanofibrous mats for pork preservation. Food Res. Int. 2012, 48, 784–791. [Google Scholar] [CrossRef]
- Perez-Masia, R.; Lopez-Rubio, A.; Lagaron, J.M. Development of zein-based heat-management structures for smart food packaging. Food Hydrocoll. 2013, 30, 182–191. [Google Scholar] [CrossRef]
- Mondal, K.; Sharma, A. Recent advances in electrospun metal-oxide nanofiber based interfaces for electrochemical biosensing. RSC Adv. 2016, 6, 94595–94616. [Google Scholar] [CrossRef]
- Oliveira, J.E.; Scagion, V.P.; Grassi, V.; Correa, D.S.; Mattoso, L.H. Modification of electrospun nylon nanofibers using layer-by-layer films for application in flow injection electronic tongue: Detection of paraoxon pesticide in corn crop. Sens. Actuators B Chem. 2012, 171, 249–255. [Google Scholar] [CrossRef]
- Marazuela, M.; Moreno-Bondi, M. Fiber-optic biosensors—An overview. Anal. Bioanal. Chem. 2002, 372, 664–682. [Google Scholar] [CrossRef] [PubMed]
- Ren, G.; Xu, X.; Liu, Q.; Cheng, J.; Yuan, X.; Wu, L.; Wan, Y. Electrospun poly (vinyl alcohol)/glucose oxidase biocomposite membranes for biosensor applications. React. Funct. Polym. 2006, 66, 1559–1564. [Google Scholar] [CrossRef]
- Li, D.; Frey, M.W.; Baeumner, A.J. Electrospun polylactic acid nanofiber membranes as substrates for biosensor assemblies. J. Membr. Sci. 2006, 279, 354–363. [Google Scholar] [CrossRef]
- Ahmad, M.; Pan, C.; Luo, Z.; Zhu, J. A single ZnO nanofiber-based highly sensitive amperometric glucose biosensor. J. Phys. Chem. C 2010, 114, 9308–9313. [Google Scholar] [CrossRef]
- Yurova, N.S.; Danchuk, A.; Mobarez, S.N.; Wongkaew, N.; Rusanova, T.; Baeumner, A.J.; Duerkop, A. Functional electrospun nanofibers for multimodal sensitive detection of biogenic amines in food via a simple dipstick assay. Anal. Bioanal. Chem. 2018, 410, 1111–1121. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.; Shao, Y.; Wang, Y.; Wu, T.; Zhang, L.; Huang, Y.; Zhang, F.; Liao, C.; He, J.; Wang, Y. Highly sensitive surface plasmon resonance biosensor based on a low-index polymer optical fiber. Opt. Express 2018, 26, 3988–3994. [Google Scholar] [CrossRef] [PubMed]
- Thiha, A.; Ibrahim, F.; Muniandy, S.; Dinshaw, I.J.; The, S.J.; Thong, K.L.; Leo, B.F.; Madou, M. All-carbon suspended nanowire sensors as a rapid highly-sensitive label-free chemiresistive biosensing platform. Biosens. Bioelectron. 2018, 107, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Muri, H.I.; Bano, A.; Hjelme, D.R. A Single Point, Multi-Parameter, Fiber Optic Sensor Based on a Combination of Interferometry and LSPR. J. Lightw. Technol. 2018, 36, 1159–1167. [Google Scholar] [CrossRef]
- Baker, K.L.; Bolger, F.B.; Lowry, J.P. Development of a microelectrochemical biosensor for the real-time detection of choline. Sens. Actuators B Chem. 2017, 243, 412–420. [Google Scholar] [CrossRef]
- Urrutia, A.; Goicoechea, J.; Rivero, P.J.; Pildain, A.; Arregui, F.J. Optical fiber sensors based on gold nanorods embedded in polymeric thin films. Sens. Actuators B Chem. 2018, 255, 2105–2112. [Google Scholar] [CrossRef]
- Zhong, N.; Wang, Z.; Chen, M.; Xin, X.; Wu, R.; Cen, Y.; Li, Y. Three-layer-structure polymer optical fiber with a rough inter-layer surface as a highly sensitive evanescent wave sensor. Sens. Actuators B Chem. 2018, 254, 133–142. [Google Scholar] [CrossRef]
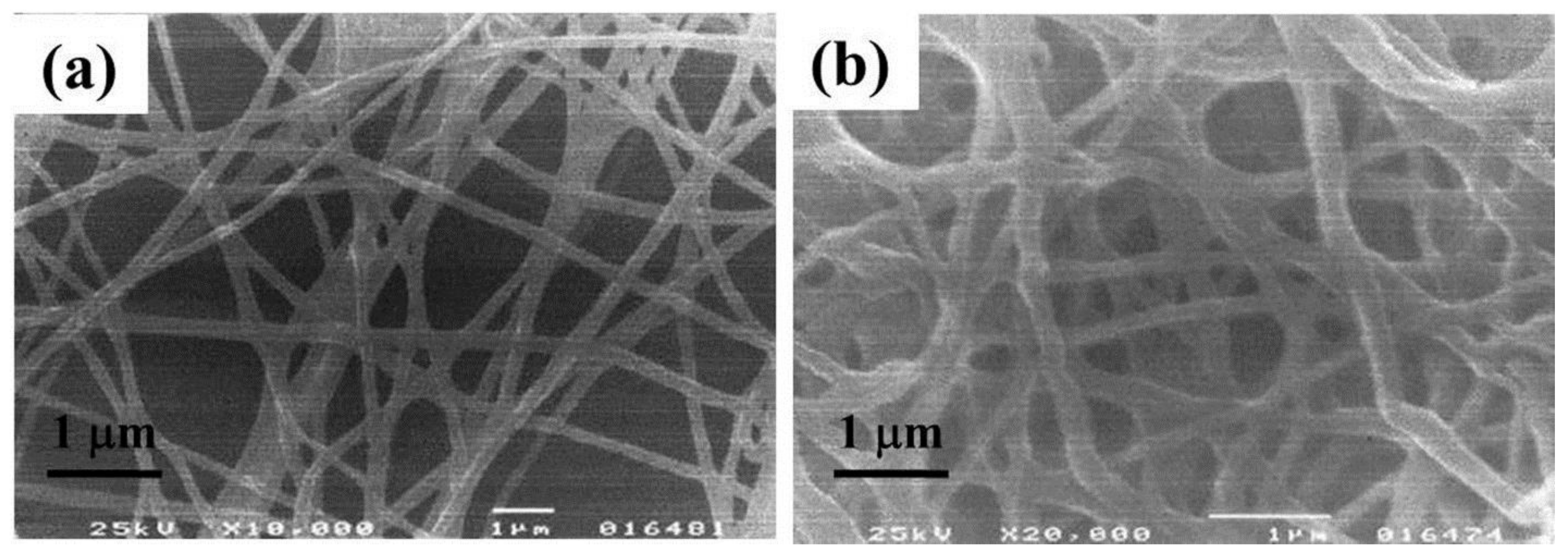
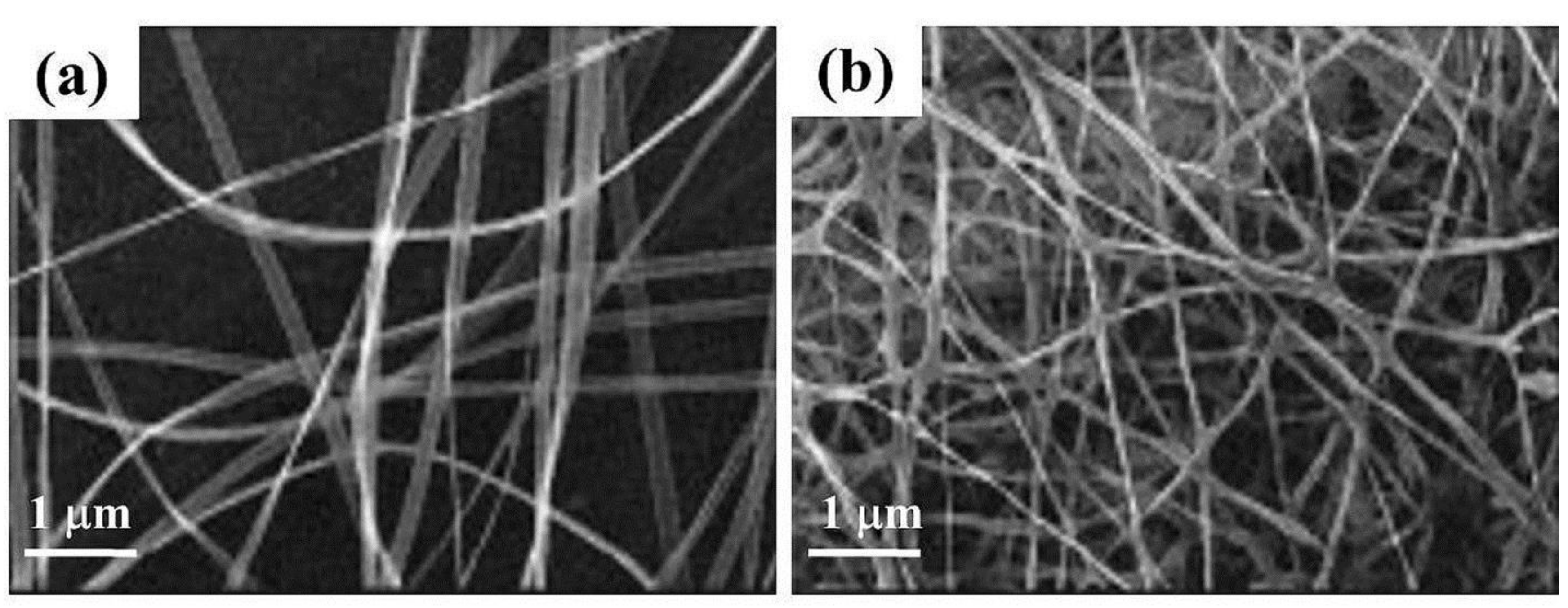
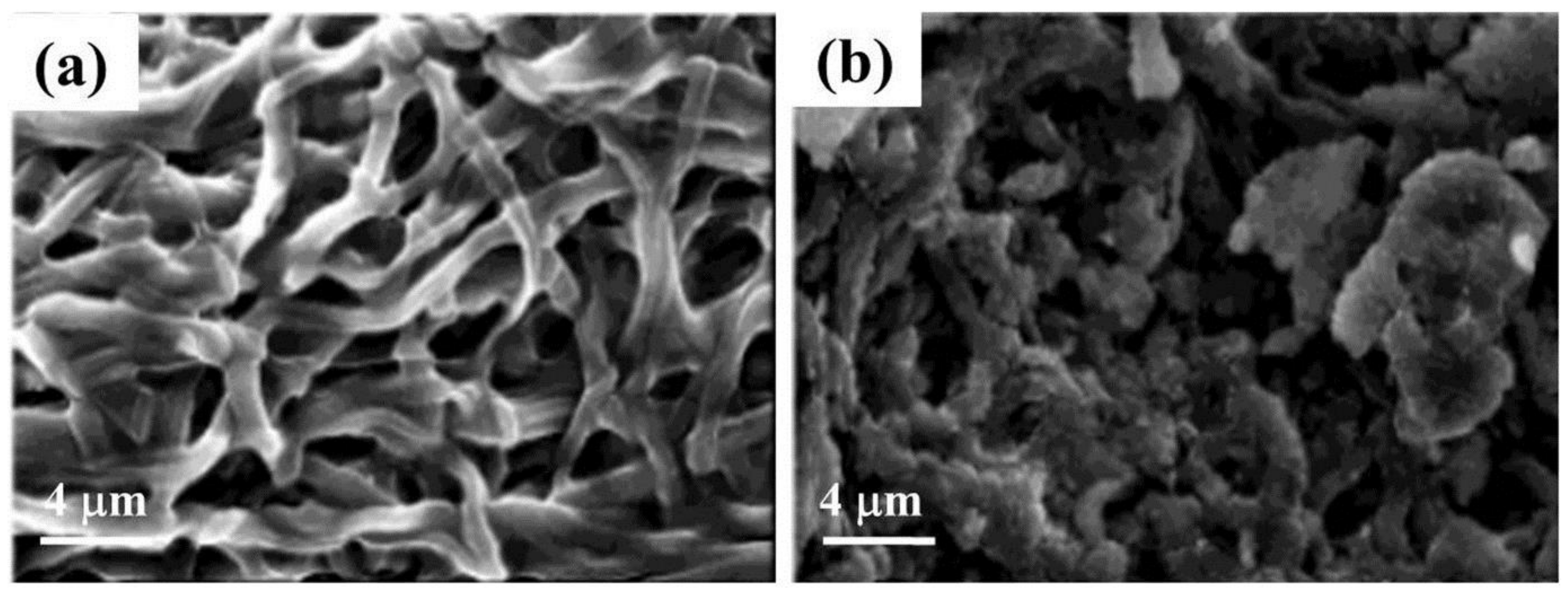
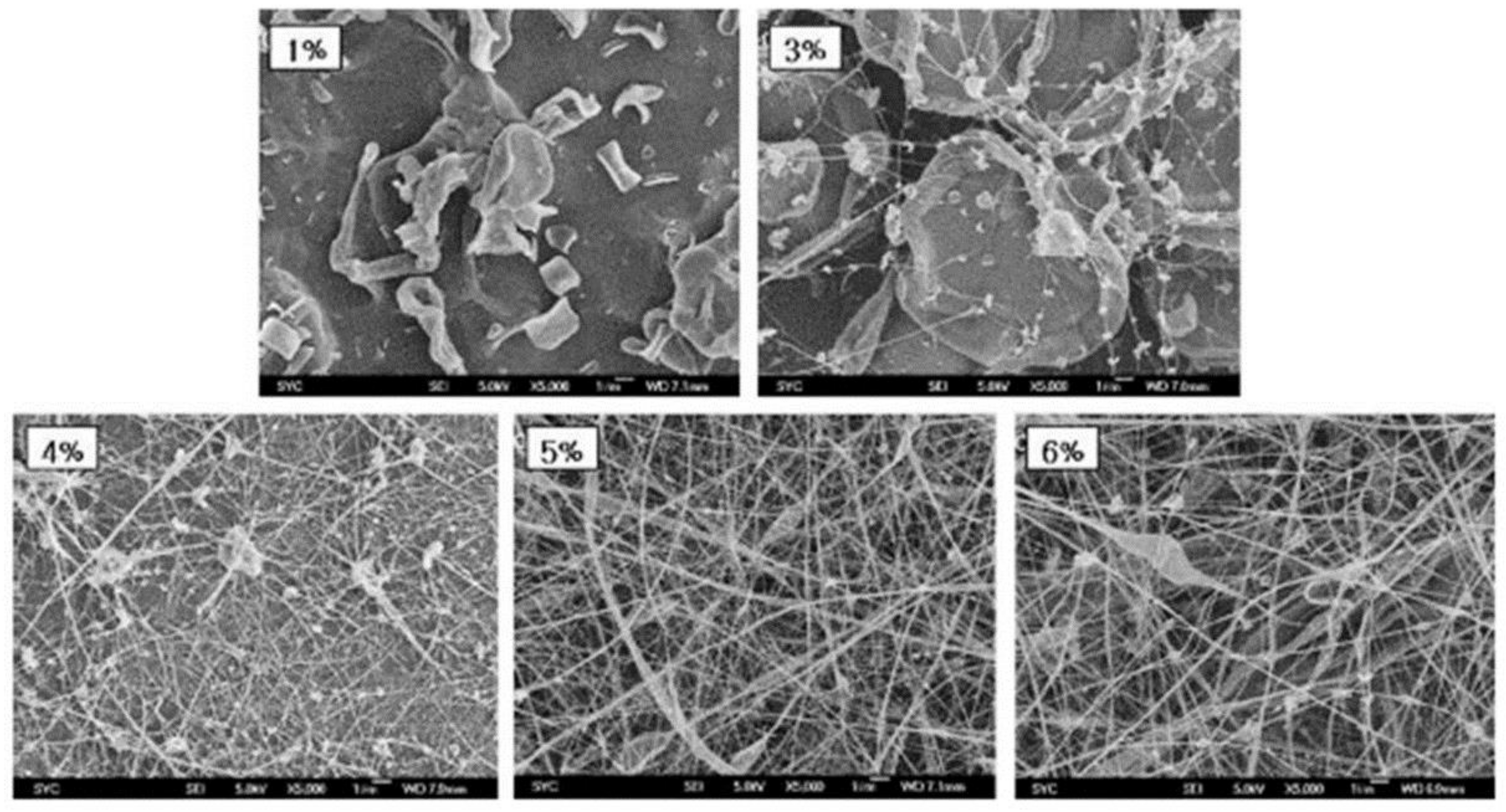
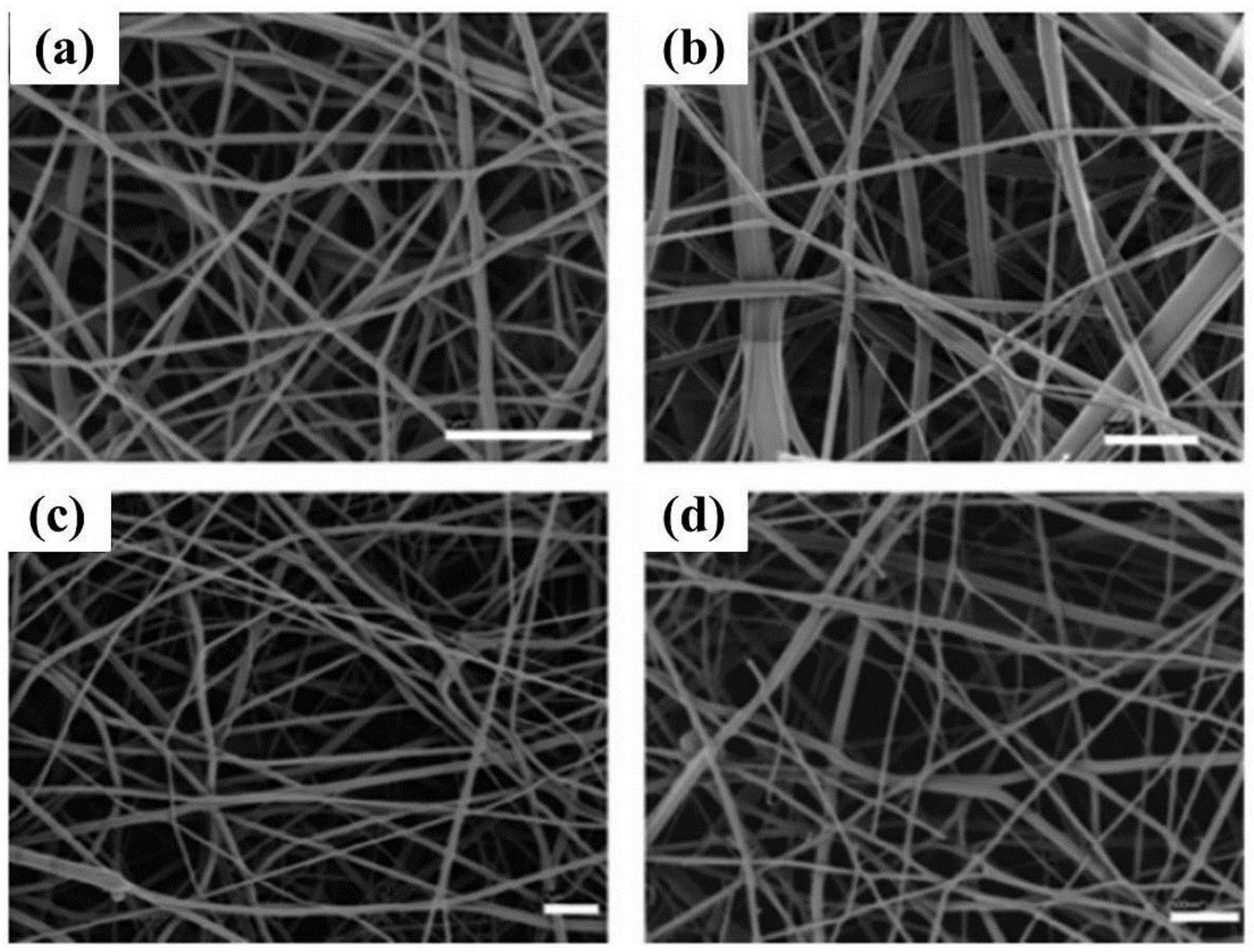
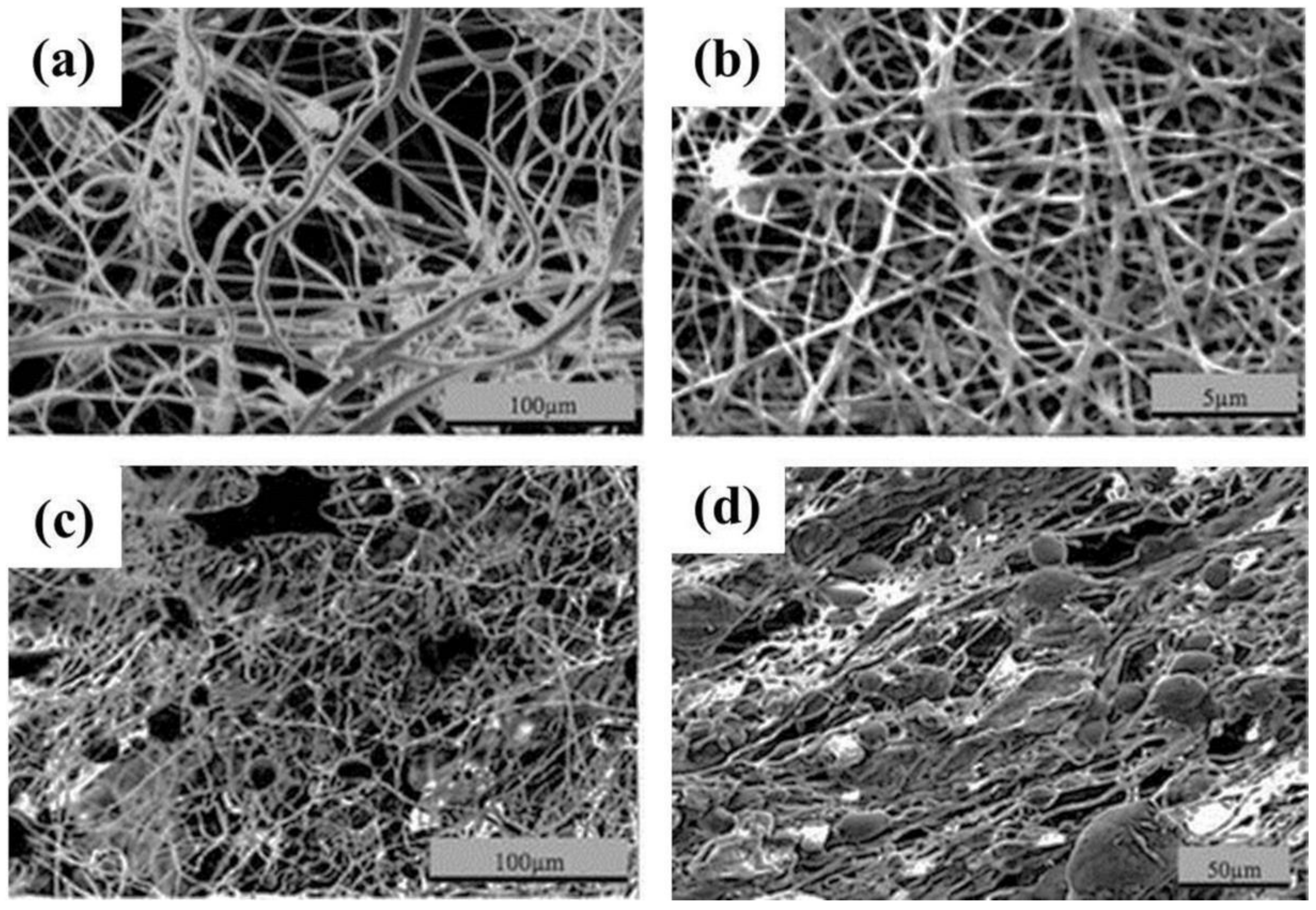
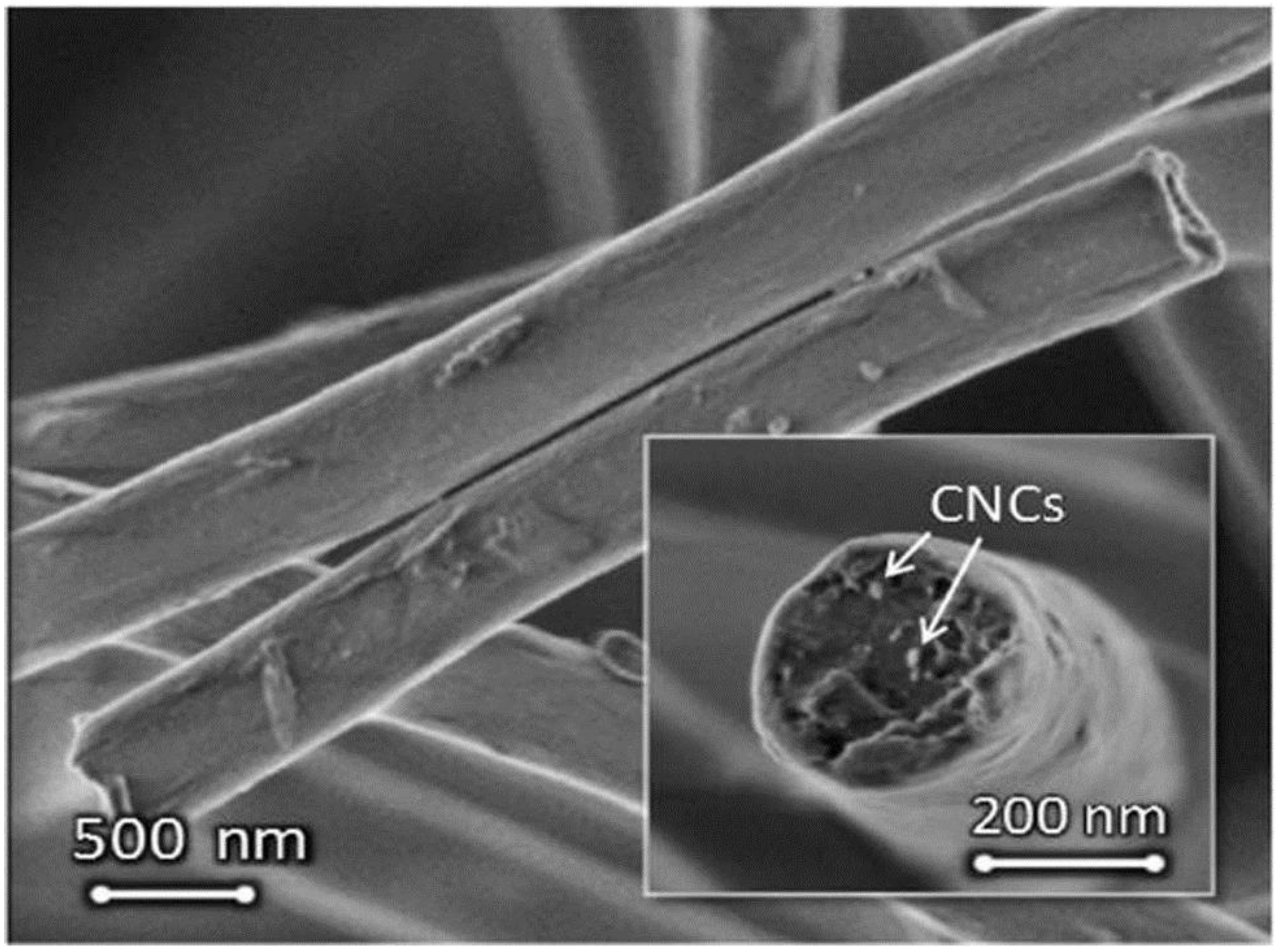
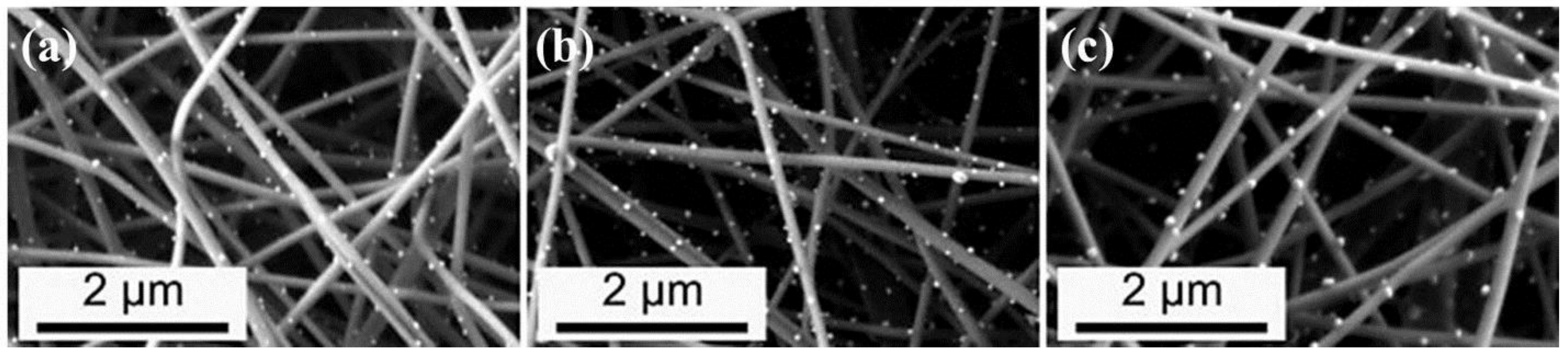
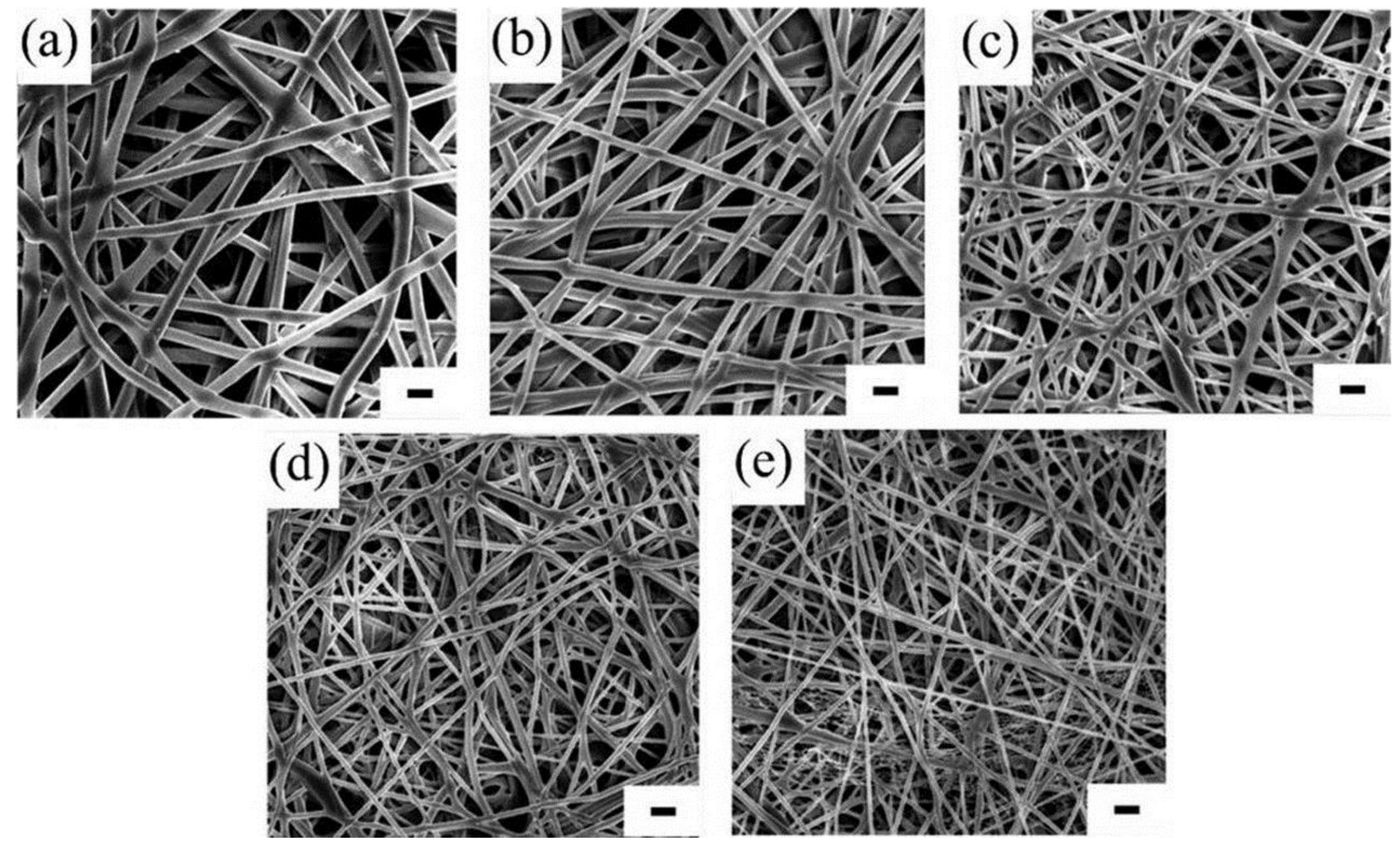
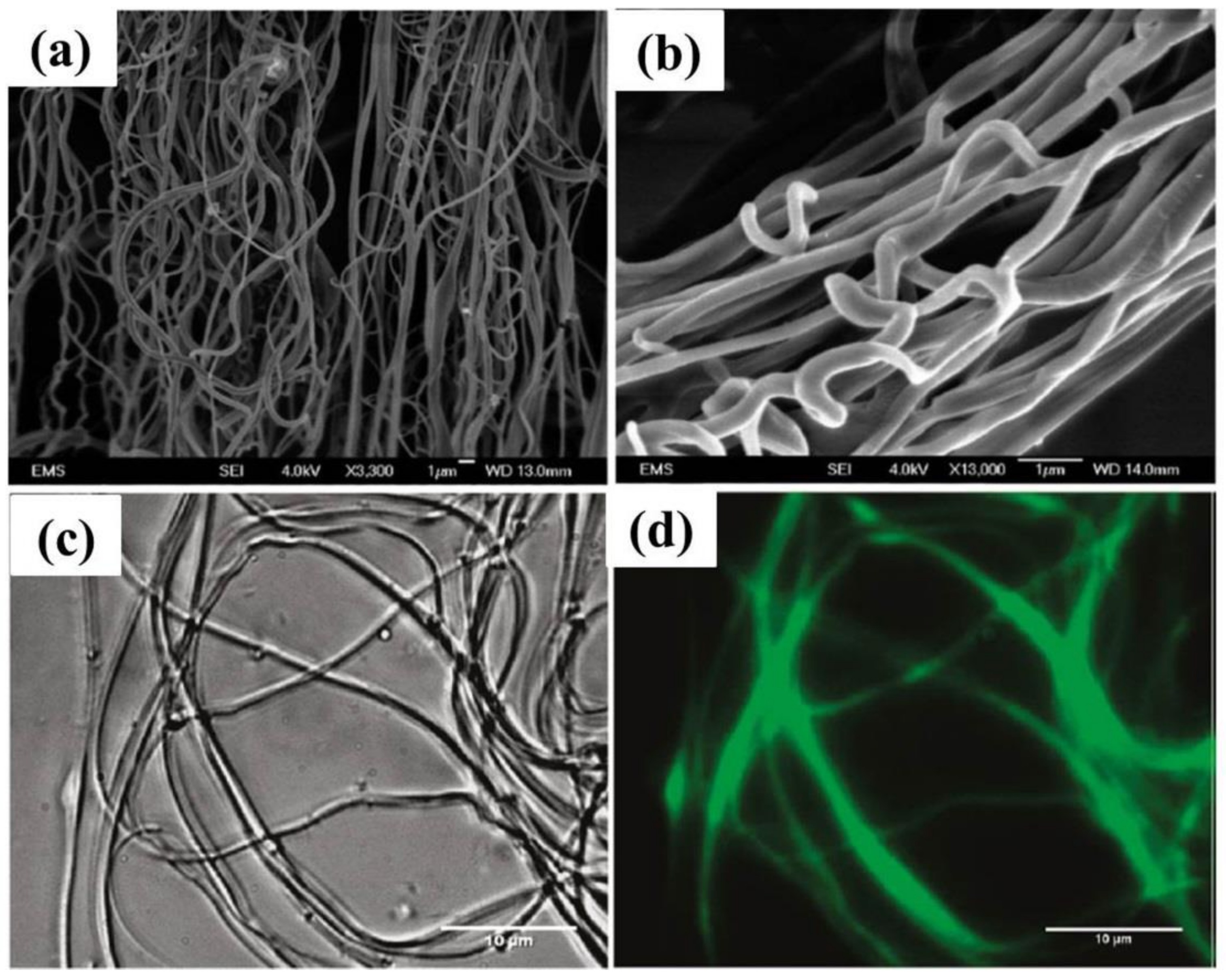

| Polymer | Solvent Used | Voltage (kV) | Collecting Distance (cm) | Flow-Rate (mL/h) | Diameter (nm) | References |
|---|---|---|---|---|---|---|
| Cellulose | NMMO/water | 9–10 | 10–15 | Not specified | 200–400 | [93] |
| HNO3/H3PO4 | 12 | 8 | 10 | 3940 | [96] | |
| Cellulose Acetate | H2O/Chloroform | 5 | 4 | Not specified | 16–2000 | [120] |
| Acetone/acetic acid/(DMAc) | 5–18 | 6–13 | Not specified | 100–1000 | [43] | |
| Acetone/water | 17 | 10 | 3 | 600–1910 | [112] | |
| Acetone/DMF/trifluoroethylene | 25 | 15 | 4 | 200–1000 | [121] | |
| Acetone/(DMAc) | 17 | 10 | 3 | 100–1000 | [119] | |
| Acetone/(DMAc) | 15 | 15 | 1 | 100–1000 | [122] | |
| Cellulose Acetate/PVP | Acetone/Acetic acid | 7–20 | 6 | 1.8–6.0 | 1000 | [97] |
| Cellulose/LiCl | LiCl/N,N-DMAc | 1–4 | 7–13 | 0.6 | 150–500 | [101] |
| LiCl/N,N- (DMAc)/N- (NMMO)/water | 1–4 | 10–20 | 3 | 270–750 | [107] | |
| Cellulose/PLA | Chloroform/acetone | 10 | 12 | 2.5 | 500–3000 | [123] |
| Ethyl Cellulose | THF and DMAc | 20 | 10 | Not specified | 100–2200 | [124] |
| Ethyl-Cyanoethyl Cellulose | (THF) | 20–50 | 5–20 | Not specified | 200 | [125] |
| Hydroxypropyl Cellulose | Anhydrous ethanol and 2-propanol | 10–30 | 10–15 | Not specified | <100 | [126] |
| Polymer | Solvent | Voltage Applied (kV) | Collecting Distance (cm) | Flowrate (mL/h) | Diameter (nm) | References |
|---|---|---|---|---|---|---|
| Lignin/PAN | N.N-DMF | 15 | 10 | 1.2 | 300 | [178] |
| DMF | 20 | 20 | 1 | 200–500 | [179] | |
| DMF | 26 | 25 | 1 | ~200 | [180] | |
| DMF | 16 | Not Specified | 1.6 | 400–100 | [181] | |
| Lignin/PEO | Ethanol/DMF/Water | 5.5–13.5 | 22.5 | 0.1 | ~400 | [182] |
| DMF | 6.5–7.0 | 10 | 1 | ~1000 | [183] | |
| (DMF)/methanol/methylene chloride | 5.5–13.5 | 22.5 | 0.1 | 85–875 | [169] | |
| DMF | 15 | 20 | Not Specified | 400–3261 | [184] | |
| Water | 20 | 22 | 0.2 | 300–12,000 | [185] | |
| DMF | 70 × 103 V/m | 0.2 m | 4.2 × 10−10 m3 · s−1 | 26–809 | [186] | |
| DMF | 20 | 10 | 0.5 | 300–1100 | [187] | |
| Ethanol | 9–14 | 14–20 | 1.8 | 234–1363 | [188] | |
| Lignin/PVA | Water | 26 | 25 | 1.2 | ~300 | [189] |
| 2-propanol | 26 | 25 | 1.2 | ~120 | [190] | |
| Water | 22 | 15 | 1 | 70–290 | [191] | |
| Lignin/Alumina | Ethanol | 14 | 15–20 | 0.5–4.0 | 500–4000 | [192] |
| Lignin/Cellulose | Water | 19 | 22 | 1.6 | 200–400 | [193] |
| Lignin/Cellulose CNCs | Water | 19 | 22 | 1.6 | 30,000–80,000 | [194] |
| Water | 19 | 22 | 1.6 | 4–200 | [195] | |
| Lignin/Cellulose/PET | TFA | 25 | 8 | 0.3 | 150–430 | [196] |
| Lignin/Chitosan/PVA | DMF | 14 | 22.5 | 0.1 | 77–1920 | [197] |
| Lignin/H3PO4 | Ethanol | 24 | Not Specified | 0.3 | >1000 | [174] |
| Lignin/Phosphorous/Platinum | Ethanol | 14 | 25 | 3 | 600–1000/600–3000 | [198] |
| Lignin/platinum-acetyl-acetonate | Ethanol | 12 | 20–25 | 0.06–0.8 | 400–1000 | [173] |
| Lignocellulosic | 1-ethyl-3methylimidazolium acetate | 35 | 15 | Not Specified | 100–1800 | [175] |
| Polymer | Solvent Used | Voltage Applied (kV) | Collecting Distance (cm) | Flow Rate (mL/h) | Diameter (nm) | References |
|---|---|---|---|---|---|---|
| Whey protein | Water | 0–30 | 7 | 10 | Not specified | [225] |
| Acetic acid/methanol | 60 | 18 | Not specified | 680–860 | [226] | |
| DI | 7.5–25 | 10 | 1–3 | 100–400 | [227] | |
| Soy-Protein | Acetic acid/DI | 28 | 15 | Not specified | 200–1200 | [221] |
| TFE/HFIP | 20–30 | 26 | 2.4 | 200–260 | [228] | |
| DMF | 20 | 13 | 2 | 100–205 | [212] | |
| Chloroform/DMF | 20–30 | 26 | 2.4 | 200–2000 | [229] | |
| HFIP | 25 | 25 | 90 | 200–300 | [230] | |
| Poly (DL-lactide)/BSA | THF | 20 | 15 | 1.6 | 47–634 | [231] |
| Poly (DL-lactide)/lysozyme | THF | Not specified | Not specified | Not specified | 110–620 | [206] |
| Poly (lactidecocaprolactone)/BSA | Chloroform | 15 | 15 | 1.0 | 20–910 | [208] |
| Dextran/PLGA/BSA | Chloroform/DMF/TFE | 13-15 | 15 | 0.6 | 214–548 | [232] |
| Globular proteins/Gelatin | Ethanol | 25 | 7.5–15 | 0.03–1.2 | 100–100,000 | [233] |
| Polycaprolactone/poly (ethylene-oxide)/lysozyme | Chloroform | 15 | 12 | 1.2 | 1000–1430 | [234] |
| Poly (lactic acid) | Dimethyl carbonate | Not specified | Not specified | Not specified | 220–970 | [235] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kakoria, A.; Sinha-Ray, S. A Review on Biopolymer-Based Fibers via Electrospinning and Solution Blowing and Their Applications. Fibers 2018, 6, 45. https://doi.org/10.3390/fib6030045
Kakoria A, Sinha-Ray S. A Review on Biopolymer-Based Fibers via Electrospinning and Solution Blowing and Their Applications. Fibers. 2018; 6(3):45. https://doi.org/10.3390/fib6030045
Chicago/Turabian StyleKakoria, Ashish, and Sumit Sinha-Ray. 2018. "A Review on Biopolymer-Based Fibers via Electrospinning and Solution Blowing and Their Applications" Fibers 6, no. 3: 45. https://doi.org/10.3390/fib6030045
APA StyleKakoria, A., & Sinha-Ray, S. (2018). A Review on Biopolymer-Based Fibers via Electrospinning and Solution Blowing and Their Applications. Fibers, 6(3), 45. https://doi.org/10.3390/fib6030045






