Synthesis and Characterization of Keratin-Based Scaffold for Potential Tissue Engineering Applications
Abstract
Highlights
- Physicochemical characterization, surface morphology analysis, and tensile strength testing of the fabricated Keratin-Gelatin (KG) and Keratin-Gelatin-Hydroxyapatite (HAp) (KGH) scaffolds demonstrated promising results for tissue engineering applications.
- Specifically, the KGH scaffold exhibited higher cell viability compared to the KG scaffold, highlighting the potential of the HAp-enriched scaffold as a promising candidate for bone regeneration.
- The potential of human nail-derived keratin and HAp as promising biomaterials for tissue engineering scaffolds.
- The use of human nail waste as a keratin source and marine shell waste for HAp supports the principles of a circular economy, promoting sustainable and value-added reuse of biological waste materials.
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Keratin Extraction
2.3. Hydroxyapatite Synthesis
2.4. Scaffold Fabrication
2.4.1. Fabrication of Keratin–Gelatin Scaffold
2.4.2. Fabrication of Keratin–Gelatin–Hydroxyapatite Scaffold
2.5. Characterization Techniques
2.5.1. Functional Group Analysis
2.5.2. Crystallinity Analysis
2.5.3. Surface Morphology
2.5.4. Water Uptake Capability and Water Retention Capability
2.5.5. Porosity
2.5.6. Tensile Strength Testing
2.6. Biocompatibility Study
2.6.1. Experimental Setup
2.6.2. Scaffold Preparation
2.6.3. Cell Culture and Seeding
2.6.4. Cell Proliferation Assay
2.6.5. Trypan Blue Assay
2.7. Statistical Analysis
3. Results and Discussions
3.1. Confirmatory Test for Keratin Protein
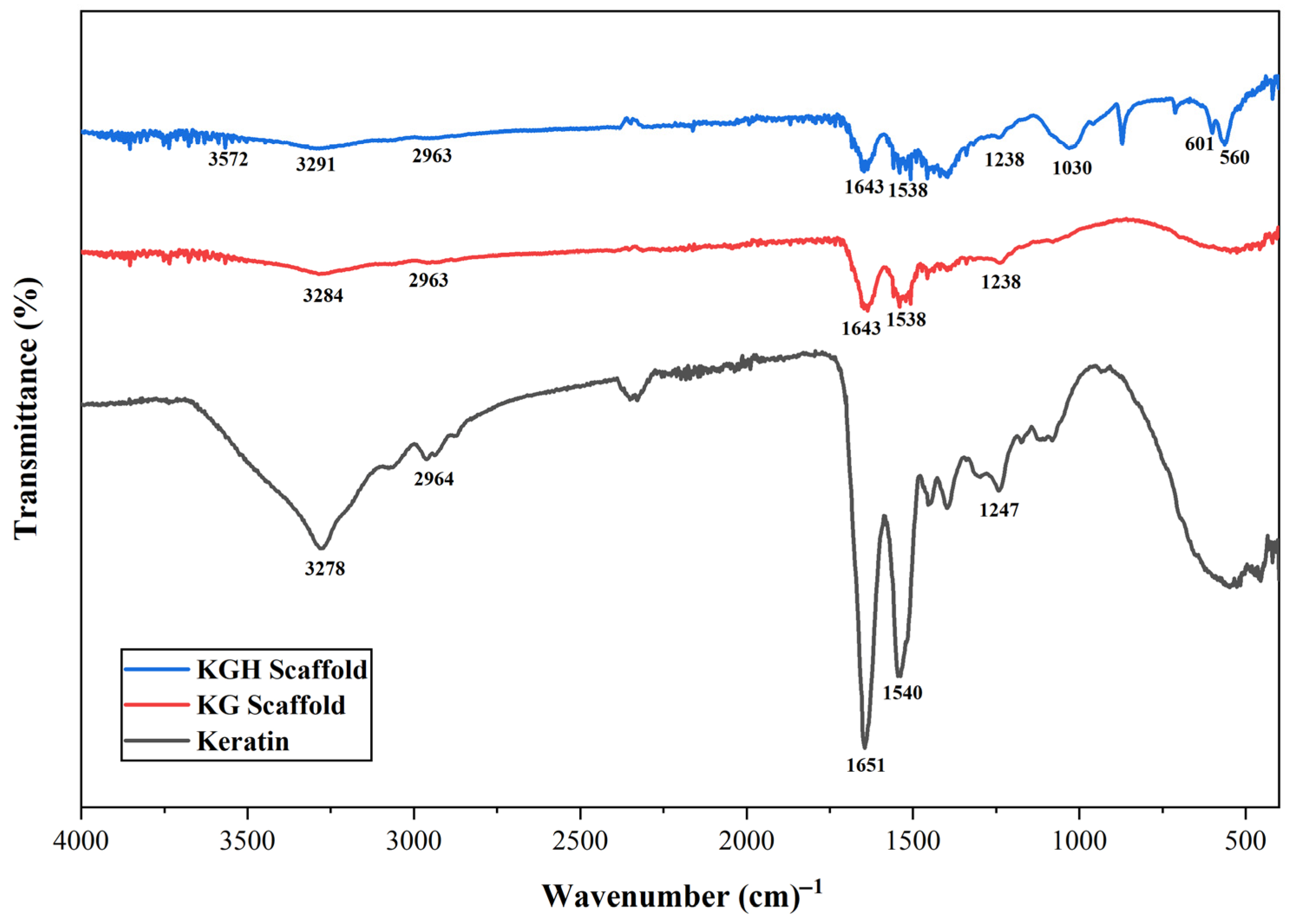
3.2. FTIR Analysis
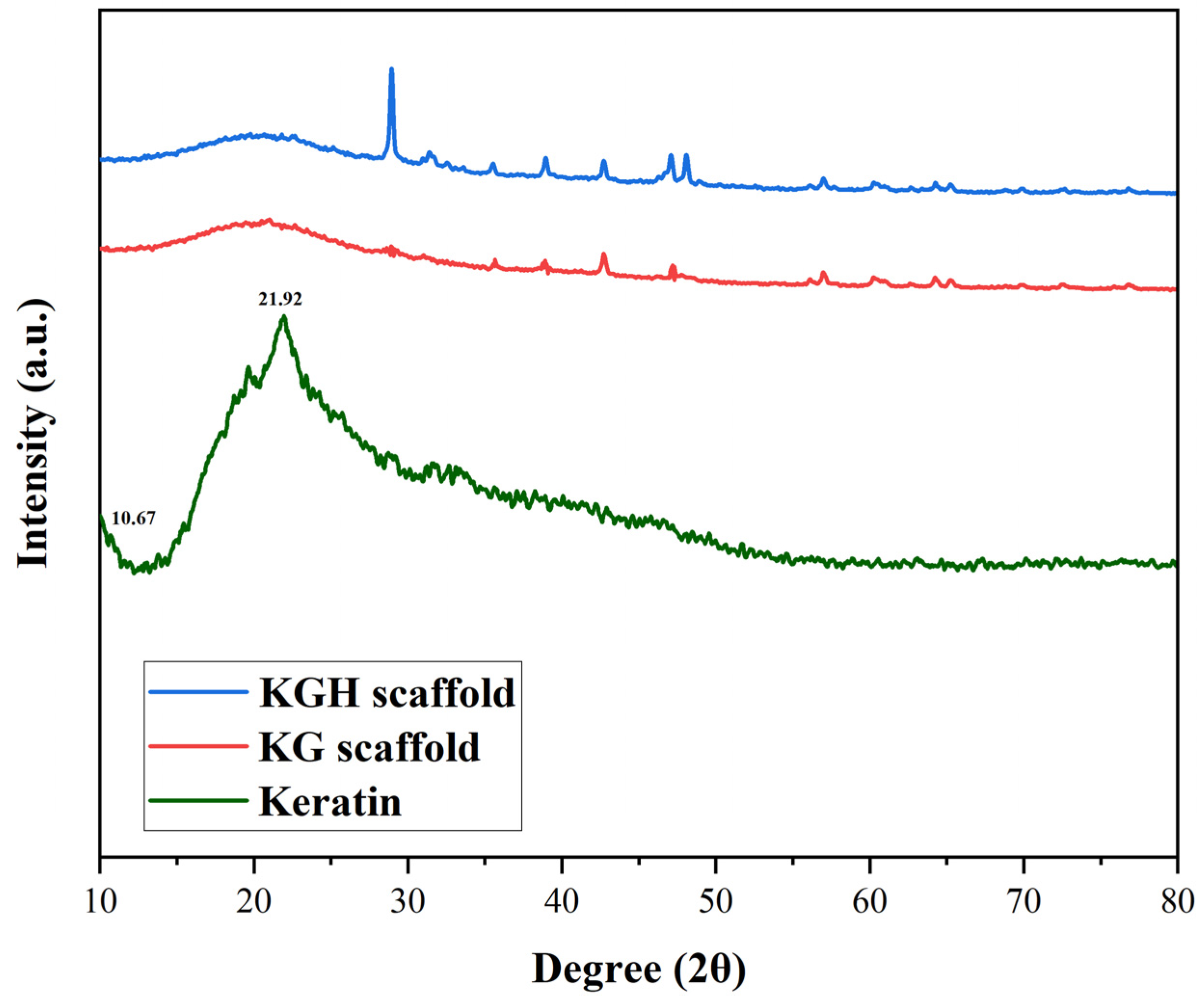
3.3. XRD Analysis
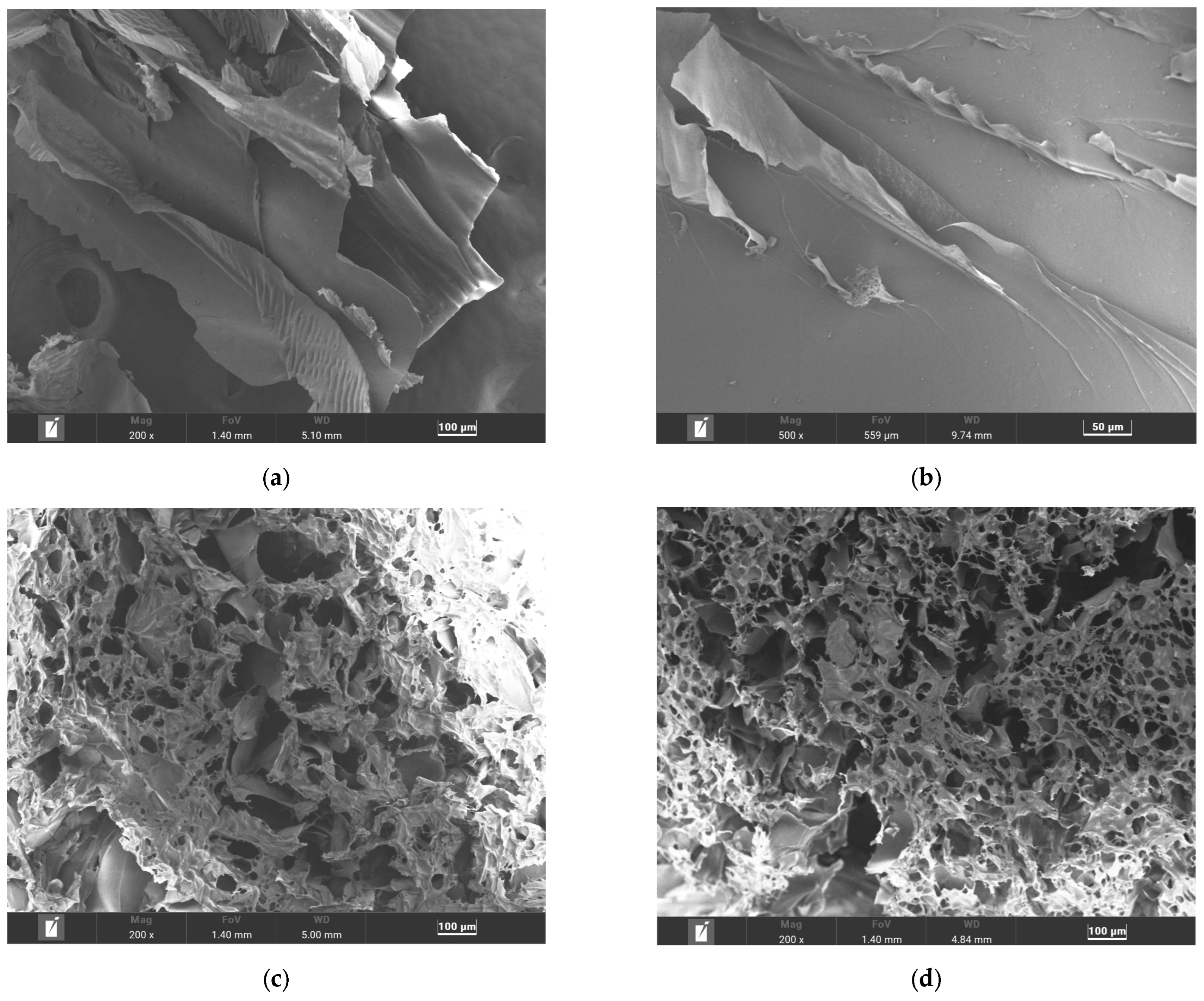
3.4. Microstructure and Pore Size Determination
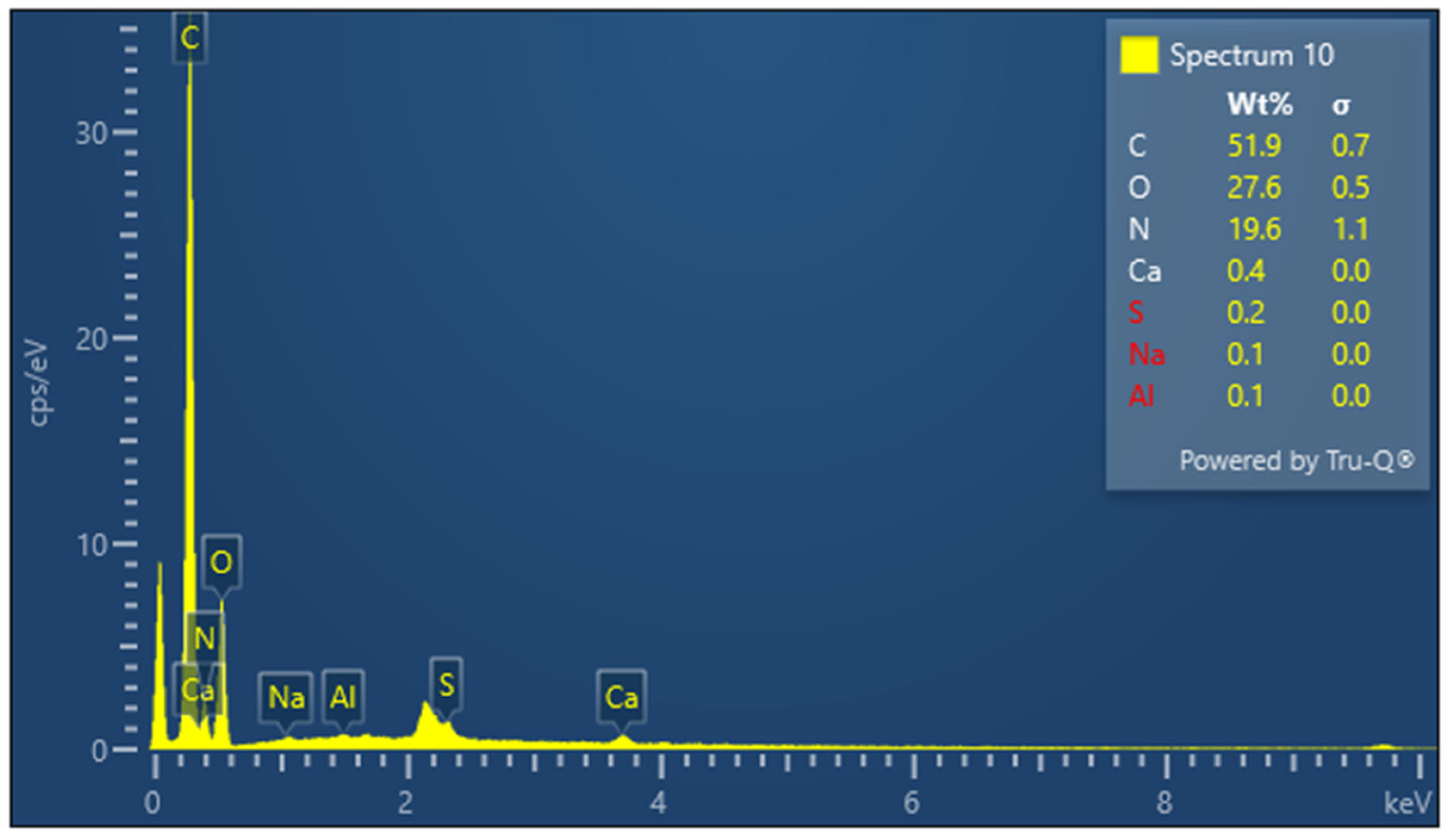
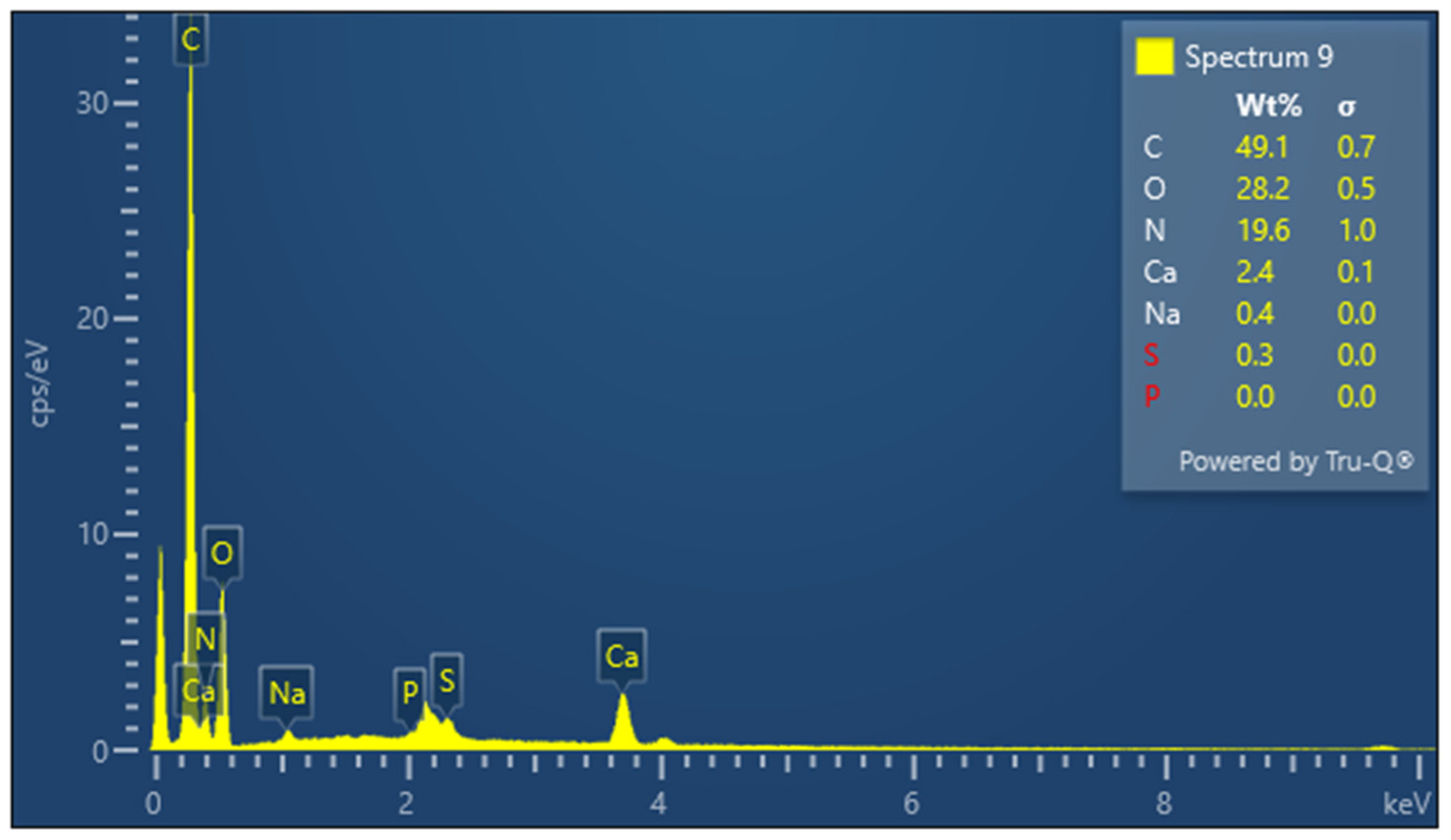
3.5. Elemental Composition Analysis
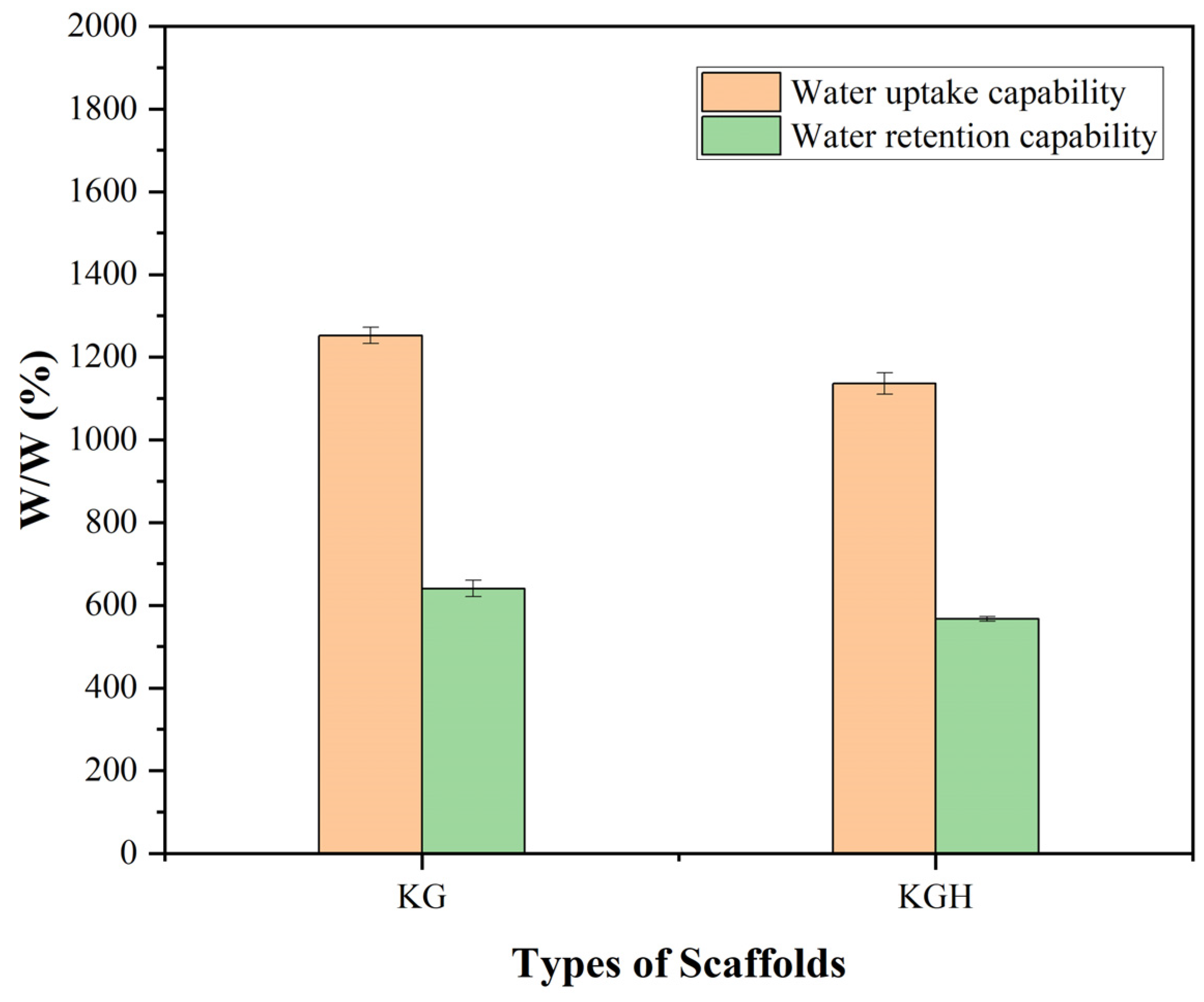
3.6. Water Uptake and Retention Capability
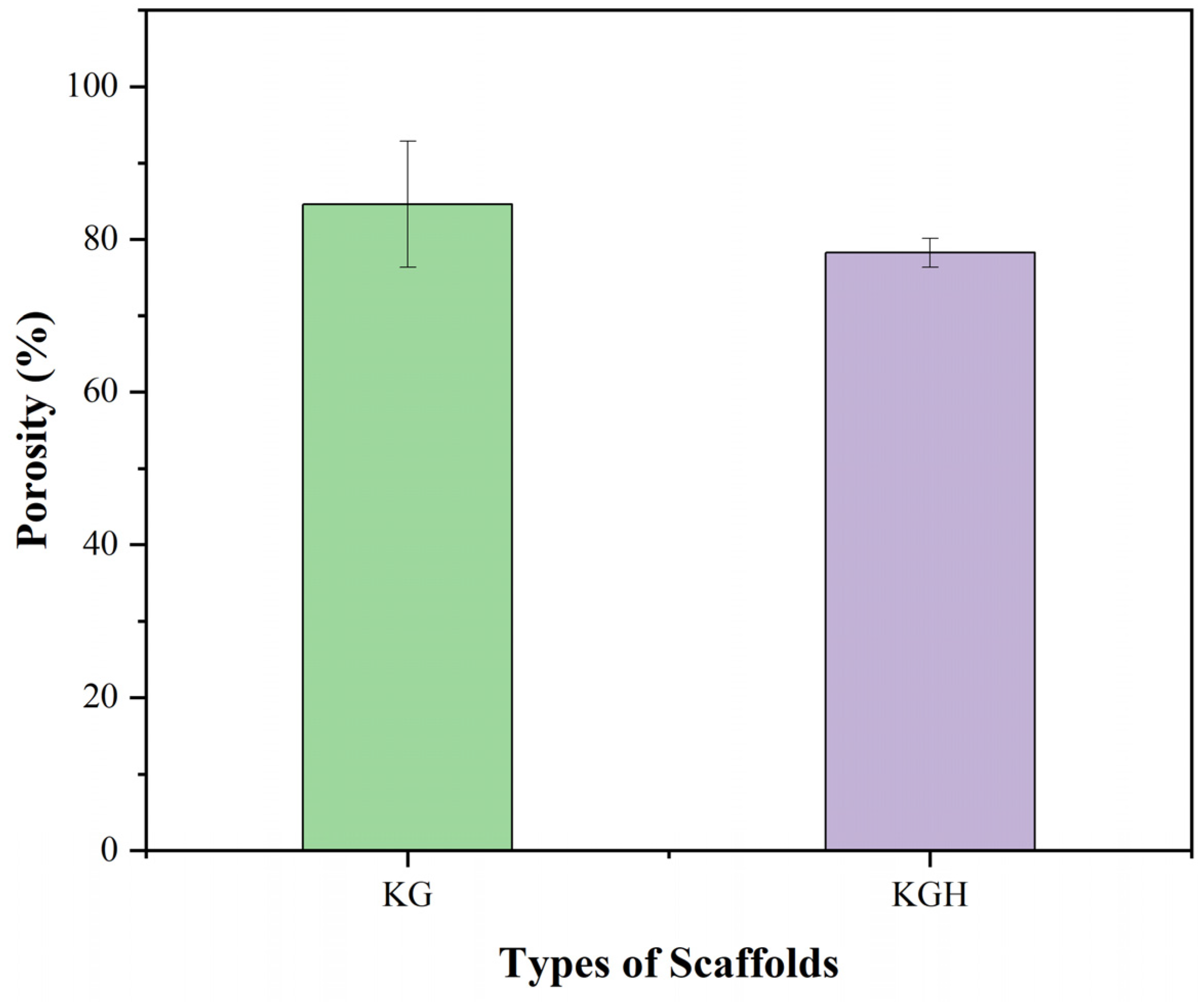
3.7. Porosity
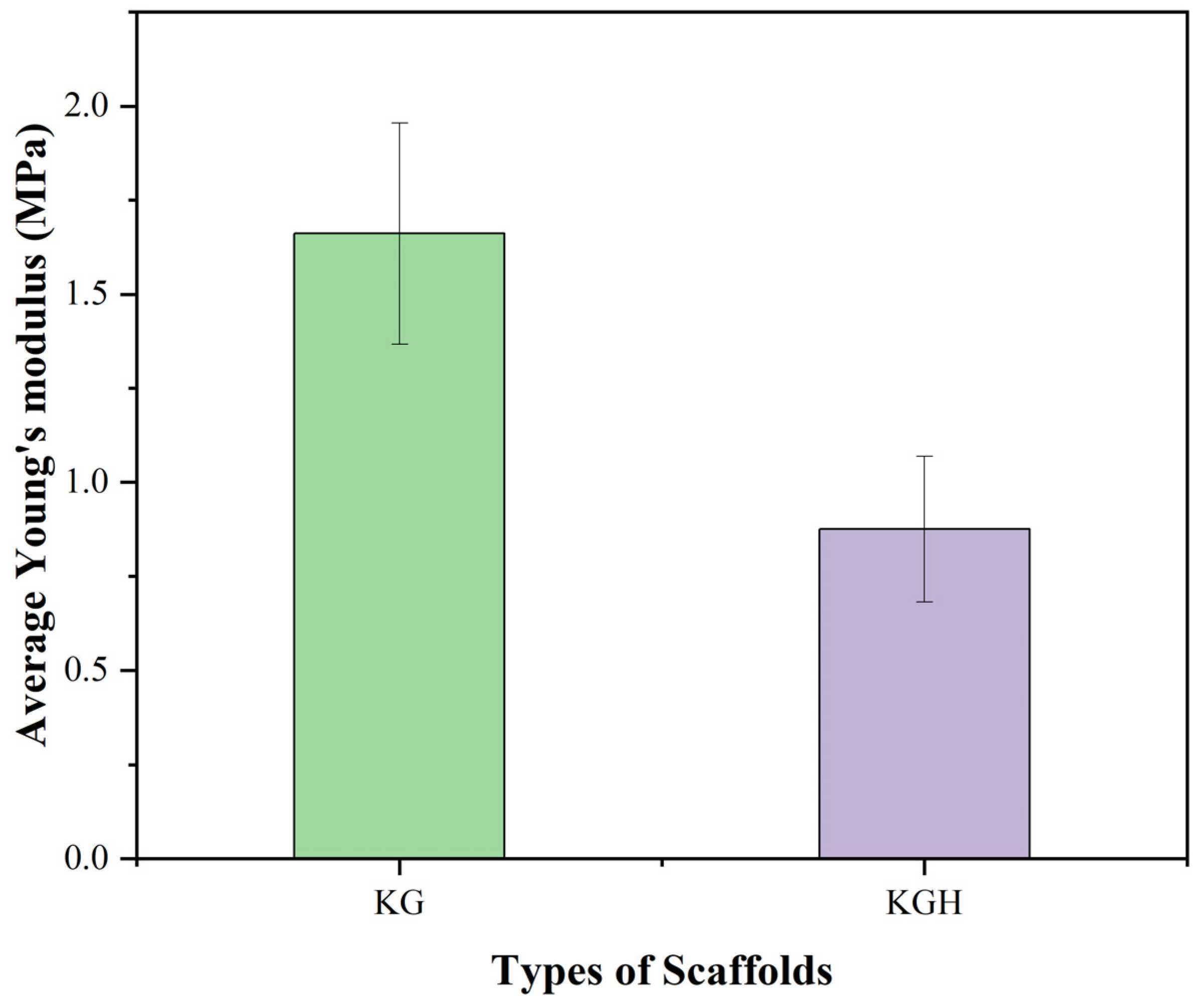
3.8. Tensile Strength
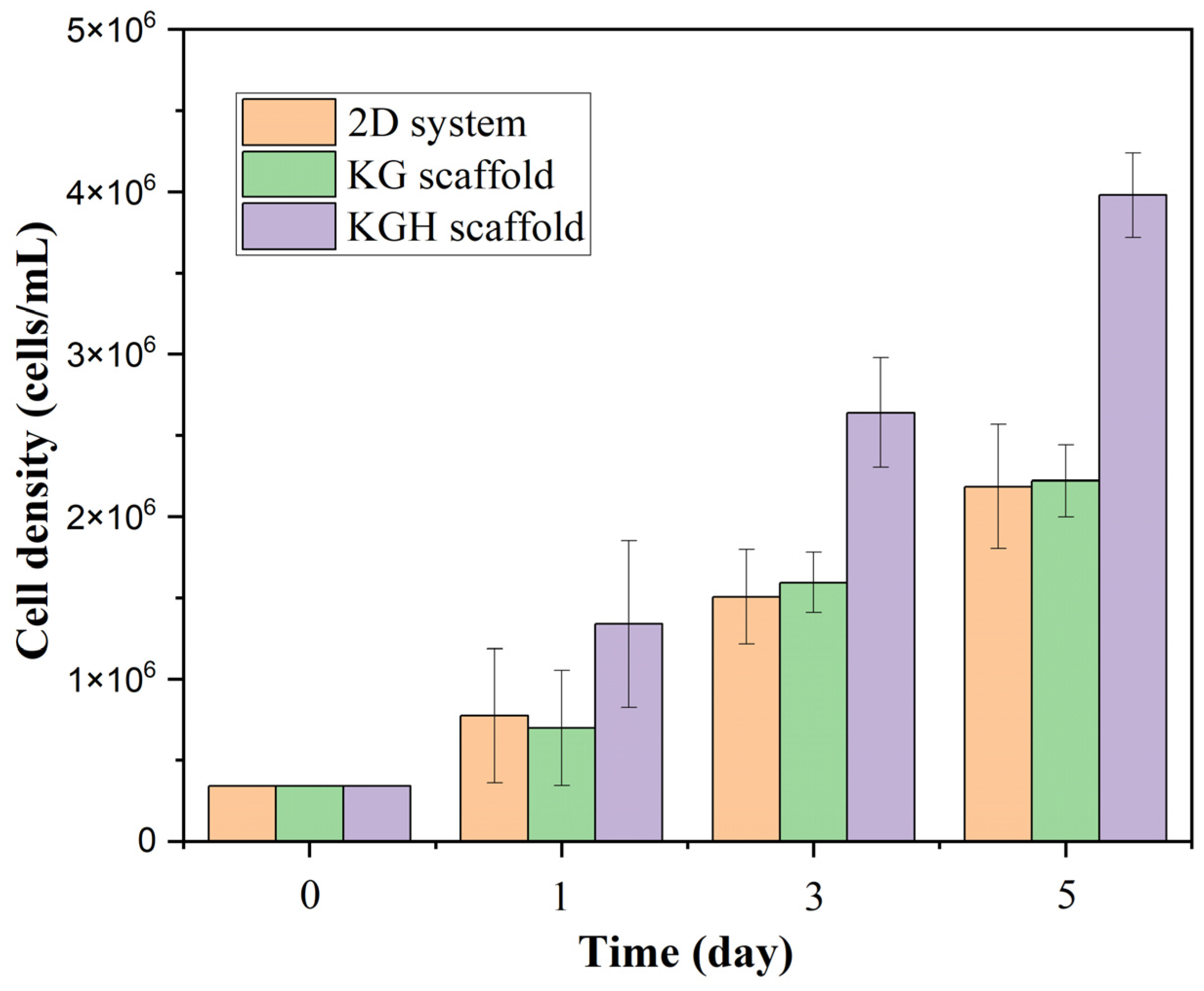
3.9. Biocompatability Analysis
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Baneshi, N.; Moghadas, B.K.; Adetunla, A.; Yusof, M.Y.P.M.; Dehghani, M.; Khandan, A.; Saber-Samandari, S.; Toghraie, D. Investigation the mechanical properties of a novel multicomponent scaffold coated with a new bio-nanocomposite for bone tissue engineering: Fabrication, simulation and characterization. J. Mater. Res. Technol. 2021, 15, 5526–5539. [Google Scholar] [CrossRef]
- Li, X.; Wang, Y.; Wang, Z.; Qi, Y.; Li, L.; Zhang, P.; Chen, X.; Huang, Y. Composite PLA/PEG/nHA/Dexamethasone Scaffold Prepared by 3D Printing for Bone Regeneration. Macromol. Biosci. 2018, 18, e1800068. [Google Scholar] [CrossRef] [PubMed]
- Krishani, M.; Shin, W.Y.; Suhaimi, H.; Sambudi, N.S. Development of Scaffolds from Bio-Based Natural Materials for Tissue Regeneration Applications: A Review. Gels 2023, 9, 100. [Google Scholar] [CrossRef] [PubMed]
- Mistry, A.S.; Mikos, A.G. Tissue engineering strategies for bone regeneration. In Regenerative Medicine II. Advances in Biochemical Engineering; Yannas, I.V., Ed.; Springer: Berlin/Heidelberg, Germany, 2005; Volume 94. [Google Scholar] [CrossRef]
- Alizadeh-Osgouei, M.; Li, Y.; Wen, C. A comprehensive review of biodegradable synthetic polymer-ceramic composites and their manufacture for biomedical applications. Bioact. Mater. 2019, 4, 22–36. [Google Scholar] [CrossRef] [PubMed]
- Asadian, M.; Chan, K.V.; Norouzi, M.; Grande, S.; Cools, P.; Morent, R.; De Geyter, N. Fabrication and plasma modification of nanofibrous tissue engineering scaffolds. Nanomaterials 2020, 10, 119. [Google Scholar] [CrossRef] [PubMed]
- Sabir, M.I.; Xu, X.; Li, L. A review on biodegradable polymeric materials for bone tissue engineering applications. J. Mater. Sci. 2009, 44, 5713–5724. [Google Scholar] [CrossRef]
- Mariani, E.; Lisignoli, G.; Borzì, R.M.; Pulsatelli, L. Biomaterials: Foreign bodies or tuners for the immune response? Int. J. Mol. Sci. 2019, 20, 636. [Google Scholar] [CrossRef] [PubMed]
- Ranjit, E.; Hamlet, S.; George, R.; Sharma, A.; Love, R.M. Biofunctional approaches of wool-based keratin for tissue engineering. J. Sci. Adv. Mater. Devices 2022, 7, 100398. [Google Scholar] [CrossRef]
- McLellan, J.; Thornhill, S.G.; Shelton, S.; Kumar, M. Keratin-based biofilms, hydrogels, and biofibers. In Keratin as a Protein Biopolymer: Extraction from Waste Biomass and Applications; Sharma, S., Kumar, A., Eds.; Springer: Cham, Switzerland, 2019; pp. 187–200. [Google Scholar]
- Cardamone, J.M.; Nuñez, A.; Garcia, R.A.; Aldema-Ramos, M.; Van Vliet, K.J. Characterizing wool keratin. Adv. Mater. Sci. Eng. 2009, 2009, 1–6. [Google Scholar] [CrossRef]
- Fraser, R.D.B.; MacRae, T.P.; Rogers, G.E. Keratins: Their Composition, Structure, and Biosynthesis; Thomas: Springfield, IL, USA, 1972. [Google Scholar]
- Schweizer, J.; Bowden, P.E.; Coulombe, P.A.; Langbein, L.; Lane, E.B.; Magin, T.M.; Maltais, L.; Omary, M.B.; Parry, D.A.; Rogers, M.A.; et al. New consensus nomenclature for mammalian keratins. J. Cell Biol. 2006, 174, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, Y.; Ye, J.; Yuan, J.; Xiao, Y. Fabrication of poly (ε-caprolactone)/keratin nanofibrous mats as a potential scaffold for vascular tissue engineering. Mater. Sci. Eng. C 2016, 68, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Navarro, J.; Swayambunathan, J.; Lerman, M.; Santoro, M.; Fisher, J.P. Development of keratin-based membranes for potential use in skin repair. Acta Biomater. 2019, 83, 177–188. [Google Scholar] [CrossRef] [PubMed]
- Potter, N.A.; Van Dyke, M. Effects of differing purification methods on properties of keratose biomaterials. ACS Biomater. Sci. Eng. 2018, 4, 1316–1323. [Google Scholar] [CrossRef] [PubMed]
- Spearman, R.I.C. On the nature of the horny scales of the pangolin. Zool. J. Linn. Soc. 1967, 46, 267–273. [Google Scholar] [CrossRef]
- Fraser, R.D.B.; Parry, D.A.D. The structural basis of the filament-matrix texture in the avian/reptilian group of hard β-keratins. J. Struct. Biol. 2011, 173, 391–405. [Google Scholar] [CrossRef] [PubMed]
- Squire, J.; Vibert, P.J.; Elliott, A. Fibrous Protein Structure: A Volume Dedicated to Dr Arthur Elliott; Academic Press: London, UK; San Diego, CA, USA, 1987. [Google Scholar]
- Park, M.; Kim, B.-S.; Shin, H.K.; Park, S.-J.; Kim, H.-Y. Preparation and characterization of keratin-based biocomposite hydrogels prepared by electron beam irradiation. Mater. Sci. Eng. C 2013, 33, 5051–5057. [Google Scholar] [CrossRef] [PubMed]
- Poole, A.J.; Lyons, R.E.; Church, J.S. Dissolving feather keratin using sodium sulfide for bio-polymer applications. J. Polym. Environ. 2011, 19, 995–1004. [Google Scholar] [CrossRef]
- Korniłłowicz-Kowalska, T.; Bohacz, J. Biodegradation of keratin waste: Theory and practical aspects. Waste Manag. 2011, 31, 1689–1701. [Google Scholar] [CrossRef] [PubMed]
- Tonin, C.; Aluigi, A.; Varesano, A.; Vineis, C. Keratin-based nanofibers. In Nanofibers; Kumar, A., Ed.; InTech: Croatia, Yugoslavia, 2010; pp. 139–158. [Google Scholar]
- Sarrami, P.; Karbasi, S.; Farahbakhsh, Z.; Bigham, A.; Rafienia, M. Fabrication and characterization of novel polyhydroxybutyrate-keratin/nanohydroxyapatite electrospun fibers for bone tissue engineering applications. Int. J. Biol. Macromol. 2022, 220, 1368–1389. [Google Scholar] [CrossRef] [PubMed]
- Siyum, S. Human Hair Keratin Protein, Hair Fibers and Hydroxyapatite Composite Scaffold for Bone Tissue Regeneration. Master’s Thesis, Cleveland State University, Cleveland, OH, USA, 2014. [Google Scholar]
- Kitahara, T.; Ogawa, H. The extraction and characterization of human nail keratin. J. Dermatol. Sci. 1991, 2, 402–406. [Google Scholar] [CrossRef] [PubMed]
- Bean, W.B. Nail growth. Thirty-five years of observation. Arch. Intern. Med. 1980, 140, 73–76. [Google Scholar] [CrossRef] [PubMed]
- Chilakamarry, C.R.; Mahmood, S.; Saffe, S.N.B.M.; Bin Arifin, M.A.; Gupta, A.; Sikkandar, M.Y.; Begum, S.S.; Narasaiah, B. Extraction and application of keratin from natural resources: A review. 3 Biotech 2021, 11, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Baswan, S.; Kasting, G.B.; Li, S.K.; Wickett, R.; Adams, B.; Eurich, S.; Schamper, R. Understanding the formidable nail barrier: A review of the nail microstructure, composition and diseases. Mycoses 2017, 60, 284–295. [Google Scholar] [CrossRef] [PubMed]
- Feroz, S.; Muhammad, N.; Ratnayake, J.; Dias, G. Keratin—Based materials for biomedical applications. Bioact. Mater. 2020, 5, 496–509. [Google Scholar] [CrossRef] [PubMed]
- Skopinska-Wisniewska, J.; Tuszynska, M.; Olewnik-Kruszkowska, E. Comparative study of gelatin hydrogels modified by various cross-linking agents. Materials 2021, 14, 396. [Google Scholar] [CrossRef] [PubMed]
- Natu, M.V.; Sardinha, J.P.; Correia, I.J.; Gil, M.H. Controlled release gelatin hydrogels and lyophilisates with potential application as ocular inserts. Biomed. Mater. 2007, 2, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Echave, M.C.; Hernáez-Moya, R.; Iturriaga, L.; Pedraz, J.L.; Lakshminarayanan, R.; Dolatshahi-Pirouz, A.; Taebnia, N.; Orive, G. Recent advances in gelatin-based therapeutics. Expert Opin. Biol. Ther. 2019, 19, 773–779. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Fu, X. Naturally derived materials-based cell and drug delivery systems in skin regeneration. J. Control. Release 2010, 142, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Jaipan, P.; Nguyen, A.; Narayan, R.J. Gelatin-based hydrogels for biomedical applications. MRS Commun. 2017, 7, 416–426. [Google Scholar] [CrossRef]
- Young, S.; Wong, M.; Tabata, Y.; Mikos, A.G. Gelatin as a delivery vehicle for the controlled release of bioactive molecules. J. Control. Release 2005, 109, 256–274. [Google Scholar] [CrossRef] [PubMed]
- Bello, A.B.; Kim, D.; Kim, D.; Park, H.; Lee, S.-H. Engineering and Functionalization of Gelatin Biomaterials: From Cell Culture to Medical Applications. Tissue Eng. Part B Rev. 2020, 26, 164–180. [Google Scholar] [CrossRef] [PubMed]
- Bigi, A.; Cojazzi, G.; Panzavolta, S.; Roveri, N.; Rubini, K. Stabilization of gelatin films by crosslinking with genipin. Biomaterials 2002, 23, 4827–4832. [Google Scholar] [CrossRef] [PubMed]
- Tonda-Turo, C.; Gentile, P.; Saracino, S.; Chiono, V.; Nandagiri, V.K.; Muzio, G.; Canuto, R.A.; Ciardelli, G. Comparative analysis of gelatin scaffolds crosslinked by genipin and silane coupling agent. Int. J. Biol. Macromol. 2011, 49, 700–706. [Google Scholar] [CrossRef] [PubMed]
- Ranganathan, S.; Balagangadharan, K.; Selvamurugan, N. Chitosan and gelatin-based electrospun fibers for bone tissue engineering. Int. J. Biol. Macromol. 2019, 133, 354–364. [Google Scholar] [CrossRef] [PubMed]
- Hossan, M.J.; Gafur, M.A.; Kadir, M.R.; Karim, M.M. Preparation and Characterization of Gelatin-Hydroxyapatite Composite for Bone Tissue Engineering. Int. J. Eng. Technol. Sci. 2014, 14, 24. [Google Scholar]
- Rad, M.M.; Saber-Samandari, S.; Sadighi, M.; Tayebi, L.; Aghdam, M.M.; Khandan, A. Macro-and micromechanical modelling of HA-Elastin scaffold fabricated using freeze drying technique Nanocomposite Scaffold Mechanical property Wollastonite-HA ceramic Micromechanical model. J. Nanoanal. 2021, 8, 15. [Google Scholar]
- Krishani, M.; Suhaimi, H.; Sambudi, N.S. A Review of Hydroxyapatite: Sustainable Product Development in Terms of Waste Valorization; Nova Science Publishers: New York, NY, USA, 2023. [Google Scholar]
- Tu, H.; Yu, W.; Duan, L. Structural studies and macro-performances of hydroxyapatite-reinforced keratin thin films for biological applications. J. Mater. Sci. 2016, 51, 9573–9588. [Google Scholar] [CrossRef]
- Murugiah, K.; Zakaria, M.I.; Suhaimi, H.; Caesarendra, W.; Sambudi, N.S. Synthesis and Characterisation of Hydroxyapatite (HAp) from Asiatic Hard Clam (Meretrix meretrix) and Blood Cockle Clam (Anadara granosa) Using Wet Precipitation Process. In Proceedings of the 2021 IEEE National Biomedical Engineering Conference (NBEC), Kuala Lumpur, Malaysia, 9–10 November 2021; pp. 1–6. [Google Scholar] [CrossRef]
- Fereshteh, Z. Freeze-drying technologies for 3D scaffold engineering. In Functional 3D Tissue Engineering Scaffolds: Materials, Technologies, and Applications; Elsevier: Amsterdam, The Netherlands, 2018; pp. 151–174. [Google Scholar] [CrossRef]
- Paramasivam, S.; Perumal, S.S. Methanolic extract of O.umbellata L. exhibits anti-osteoporotic effect via promoting osteoblast proliferation in MG-63 cells and inhibiting osteoclastogenesis in RANKL-stimulated RAW 264.7 cells. J. Ethnopharmacol. 2023, 315, 116641. [Google Scholar] [CrossRef] [PubMed]
- Aliyu, Z.; Mahmud, A.D.; Oluwatoyin, A.B.; Aliyu, A.; Yahaya, A.S.; Saleh, A.M. Extraction and Characterization of Keratin Protein from Chicken Feathers using Alkaline Hydrolysis Method: Effects of Sodium Sulphide Concentration and Shelf-life Evaluation. Malays. J. Catal. 2023, 7, 37–41. [Google Scholar] [CrossRef]
- Sun, Q.; Yu, L.; Zhang, Z.; Qian, C.; Fang, H.; Wang, J.; Wu, P.; Zhu, X.; Zhang, J.; Zhong, L.; et al. A novel gelatin/carboxymethyl chitosan/nano-hydroxyapatite/β-tricalcium phosphate biomimetic nanocomposite scaffold for bone tissue engineering applications. Front. Chem. 2022, 10, 958420. [Google Scholar] [CrossRef] [PubMed]
- Thein-Han, W.; Misra, R. Biomimetic chitosan–nanohydroxyapatite composite scaffolds for bone tissue engineering. Acta Biomater. 2009, 5, 1182–1197. [Google Scholar] [CrossRef] [PubMed]
- Bazgir, M.; Saeinasab, M.; Zhang, W.; Zhang, X.; Tsui, K.M.; Sarvestani, A.M.; Nawaz, S.; Coates, P.; Youseffi, M.; Elies, J.; et al. Investigation of Cell Adhesion and Cell Viability of the Endothelial and Fibroblast Cells on Electrospun PCL, PLGA and Coaxial Scaffolds for Production of Tissue Engineered Blood Vessel. J. Funct. Biomater. 2022, 13, 282. [Google Scholar] [CrossRef] [PubMed]
- Suhaimi, H.; Das, D.B. Glucose diffusivity in cell-seeded tissue engineering scaffolds. Biotechnol. Lett. 2015, 38, 183–190. [Google Scholar] [CrossRef] [PubMed]
- El-Bahrawy, N.R.; Elgharbawy, H.; Elmekawy, A.; Salem, M.; Morsy, R. Development of porous hydroxyapatite/PVA/gelatin/alginate hybrid flexible scaffolds with improved mechanical properties for bone tissue engineering. Mater. Chem. Phys. 2024, 319. [Google Scholar] [CrossRef]
- Hernandez, A.M.; Santos, C.V.; De Icaza, M.; Castano, V. Microstructural characterisation of keratin fibres from chicken feathers. Int. J. Environ. Pollut. 2005, 23, 162–178. [Google Scholar] [CrossRef]
- Senoz, E.; Wool, R.P. Microporous carbon-nitrogen fibers from keratin fibers by pyrolysis. J. Appl. Polym. Sci. 2010, 118, 1752–1765. [Google Scholar] [CrossRef]
- Sharma, S.; Gupta, A.; Kumar, A.; Kee, C.G.; Kamyab, H.; Saufi, S.M. An efficient conversion of waste feather keratin into ecofriendly bioplastic film. Clean Technol. Environ. Policy 2018, 20, 2157–2167. [Google Scholar] [CrossRef]
- Vasconcelos, A.; Freddi, G.; Cavaco-Paulo, A. Biodegradable materials based on silk fibroin and keratin. Biomacromolecules 2008, 9, 1299–1305. [Google Scholar] [CrossRef] [PubMed]
- Silva, O.A.; Rossin, A.R.S.; Lima, A.M.d.O.; Valente, A.D.; Garcia, F.P.; Nakamura, C.V.; Follmann, H.D.M.; Silva, R.; Martins, A.F. Synthesis of Keratin Nanoparticles Extracted from Human Hair through Hydrolysis with Concentrated Sulfuric Acid: Characterization and Cytotoxicity. Materials 2024, 17, 3759. [Google Scholar] [CrossRef] [PubMed]
- Das, M.P.; Suguna, P.R.; Prasad, K.; Vijaylakshmi, J.V.; Renuka, M. Extraction and Characterization of Gelatin: A Functional Biopolymer. Int. J. Pharm. Pharm. Sci. 2017, 9, 239. [Google Scholar] [CrossRef]
- Padmanabhan, V.P.; Kulandaivelu, R.; Panneer, D.S.; Vivekananthan, S.; Sagadevan, S.; Lett, J.A. Microwave synthesis of hydroxyapatite encumbered with ascorbic acid intended for drug leaching studies. Mater. Res. Innov. 2019, 24, 171–178. [Google Scholar] [CrossRef]
- Feughelman, M.; Lyman, D.; Willis, B. The parallel helices of the intermediate filaments of α-keratin. Int. J. Biol. Macromol. 2002, 30, 95–96. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Ke, G.; Wu, J.; Wang, X. Modification of wool fiber using steam explosion. Eur. Polym. J. 2006, 42, 2168–2173. [Google Scholar] [CrossRef]
- Khosa, M.A.; Ullah, A. In-situ modification, regeneration, and application of keratin biopolymer for arsenic removal. J. Hazard. Mater. 2014, 278, 360–371. [Google Scholar] [CrossRef] [PubMed]
- Poole, A.J.; Church, J.S. The effects of physical and chemical treatments on Na2S produced feather keratin films. Int. J. Biol. Macromol. 2015, 73, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Mukasheva, F.; Adilova, L.; Dyussenbinov, A.; Yernaimanova, B.; Abilev, M.; Akilbekova, D. Optimizing scaffold pore size for tissue engineering: Insights across various tissue types. Front. Bioeng. Biotechnol. 2024, 12, 1444986. [Google Scholar] [CrossRef] [PubMed]
- Balaji, S.; Kumar, R.; Sripriya, R.; Kakkar, P.; Ramesh, D.V.; Reddy, P.N.K.; Sehgal, P. Preparation and comparative characterization of keratin–chitosan and keratin–gelatin composite scaffolds for tissue engineering applications. Mater. Sci. Eng. C 2012, 32, 975–982. [Google Scholar] [CrossRef]
- Hübner, C.; Vadalà, M.; Voges, K.; Lupascu, D.C. Poly(vinyl alcohol) freeze casts with nano-additives as potential thermal insulators. Sci. Rep. 2023, 13, 1020. [Google Scholar] [CrossRef] [PubMed]
- Ko, Y.G. Formation of oriented fishbone-like pores in biodegradable polymer scaffolds using directional phase-separation processing. Sci. Rep. 2020, 10, 14472. [Google Scholar] [CrossRef] [PubMed]
- Diaz, F.; Forsyth, N.; Boccaccini, A.R. Aligned Ice Templated Biomaterial Strategies for the Musculoskeletal System. Adv. Healthc. Mater. 2023, 12, e2203205. [Google Scholar] [CrossRef] [PubMed]
- Kakkar, P.; Verma, S.; Manjubala, I.; Madhan, B. Development of keratin–chitosan–gelatin composite scaffold for soft tissue engineering. Mater. Sci. Eng. C 2014, 45, 343–347. [Google Scholar] [CrossRef] [PubMed]
- Angulo, D.E.L.; Sobral, P.J.D.A. The effect of processing parameters and solid concentration on the microstructure and pore architecture of gelatin-chitosan scaffolds produced by freeze-drying. Mater. Res. 2016, 19, 839–845. [Google Scholar] [CrossRef]
- Abbasi, N.; Hamlet, S.; Love, R.M.; Nguyen, N.-T. Porous scaffolds for bone regeneration. J. Sci. Adv. Mater. Devices 2020, 5, 1–9. [Google Scholar] [CrossRef]
- Di Luca, A.; Ostrowska, B.; Lorenzo-Moldero, I.; Lepedda, A.; Swieszkowski, W.; Van Blitterswijk, C.; Moroni, L. Gradients in pore size enhance the osteogenic differentiation of human mesenchymal stromal cells in three-dimensional scaffolds. Sci. Rep. 2016, 6, 22898. [Google Scholar] [CrossRef] [PubMed]
- Rasoulianboroujeni, M.; Kiaie, N.; Tabatabaei, F.S.; Yadegari, A.; Fahimipour, F.; Khoshroo, K.; Tayebi, L. Dual Porosity Protein-based Scaffolds with Enhanced Cell Infiltration and Proliferation. Sci. Rep. 2018, 8, 14889. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Wang, W.; Liu, D.; Zhang, H.; Gao, P.; Geng, L.; Yuan, Y.; Lu, J.; Wang, Z. The promotion of angiogenesis induced by three-dimensional porous beta-tricalcium phosphate scaffold with different interconnection sizes via activation of PI3K/Akt pathways. Sci. Rep. 2015, 5, 9409. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, J.M.M.; Alqahtani, A.; Al Fatease, A.; Alqahtani, T.; Khan, B.A.; Ashmitha, B.; Vijaya, R. Human hair keratin composite scaffold: Characterisation and biocompatibility study on nih 3t3 fibroblast cells. Pharmaceuticals 2021, 14, 781. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Hwangbo, H.; Koo, Y.; Kim, G. Fabrication of mechanically reinforced gelatin/hydroxyapatite bio-composite scaffolds by core/shell nozzle printing for bone tissue engineering. Int. J. Mol. Sci. 2020, 21, 3401. [Google Scholar] [CrossRef] [PubMed]
- Nayak, K.K.; Gupta, P. In vitro biocompatibility study of keratin/agar scaffold for tissue engineering. Int. J. Biol. Macromol. 2015, 81, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Razali, K.; Nasir, N.M.; Cheng, E.; Mamat, N.; Mazalan, M.; Wahab, Y.; Roslan, M.M. The effect of gelatin and hydroxyapatite ratios on the scaffolds′ porosity and mechanical properties. In Proceedings of the IECBES 2014, Conference Proceedings—2014 IEEE Conference on Biomedical Engineering and Sciences: “Miri, Where Engineering in Medicine and Biology and Humanity Meet”, Sarawak, Malaysia, 8–10 December 2014; pp. 256–259. [Google Scholar] [CrossRef]
- Lei, C.; Zhu, H.; Li, J.; Li, J.; Feng, X.; Chen, J. Preparation and characterization of polyhydroxybutyrate-co-hydroxyvalerate/silk fibroin nanofibrous scaffolds for skin tissue engineering. Polym. Eng. Sci. 2014, 55, 907–916. [Google Scholar] [CrossRef]
- Jarząbek, D.M. The impact of weak interfacial bonding strength on mechanical properties of metal matrix – Ceramic reinforced composites. Compos. Struct. 2018, 201, 352–362. [Google Scholar] [CrossRef]
- Lutzweiler, G.; Halili, A.N.; Vrana, N.E. The overview of porous, bioactive scaffolds as instructive biomaterials for tissue regeneration and their clinical translation. Pharmaceutics 2020, 12, 602. [Google Scholar] [CrossRef] [PubMed]
- Raucci, M.G.; D’AMora, U.; Ronca, A.; Demitri, C.; Ambrosio, L. Bioactivation routes of gelatin-based scaffolds to enhance at nanoscale level bone tissue regeneration. Front. Bioeng. Biotechnol. 2019, 7, 27. [Google Scholar] [CrossRef] [PubMed]
- Mahboudi, S.; Pezeshki-Modaress, M.; Noghabi, K.A. The study of fibroblast cell growth on the porous scaffold of gelatin-starch blend using the salt-leaching and lyophilization method. Int. J. Polym. Mater. Polym. Biomater. 2015, 64, 653–659. [Google Scholar] [CrossRef]
- Rezwan, K.; Chen, Q.Z.; Blaker, J.J.; Boccaccini, A.R. Biodegradable and bioactive porous polymer/inorganic composite scaffolds for bone tissue engineering. Biomaterials 2006, 27, 3413–3431. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.-J.; Kim, D.-H.; Ryoo, J.-H.; Park, S.-M.; Kwon, H.-C.; Keum, D.-H.; Shin, D.-M.; Han, S.-G. Influence of Gelatin on Adhesion, Proliferation, and Adipogenic Differentiation of Adipose Tissue-Derived Stem Cells Cultured on Soy Protein–Agarose Scaffolds. Foods 2024, 13, 2247. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Cheong, N.; He, Z.; Zhang, T. Application of Hydroxyapatite Composites in Bone Tissue Engineering: A Review. J. Funct. Biomater. 2025, 16, 127. [Google Scholar] [CrossRef] [PubMed]
- Aminatun; Sujak MK, A.; Izak R, D.; Hadi, S.; Sari, Y.W.; Gunawarman; Cahyati, N.; Yusuf, Y.; Abdullah, C.A.C. Fabrication and biocompatibility evaluation of hydroxyapatite-polycaprolactone-gelatin composite nanofibers as a bone scaffold. RSC Adv. 2024, 14, 24815–24827. [Google Scholar] [CrossRef] [PubMed]

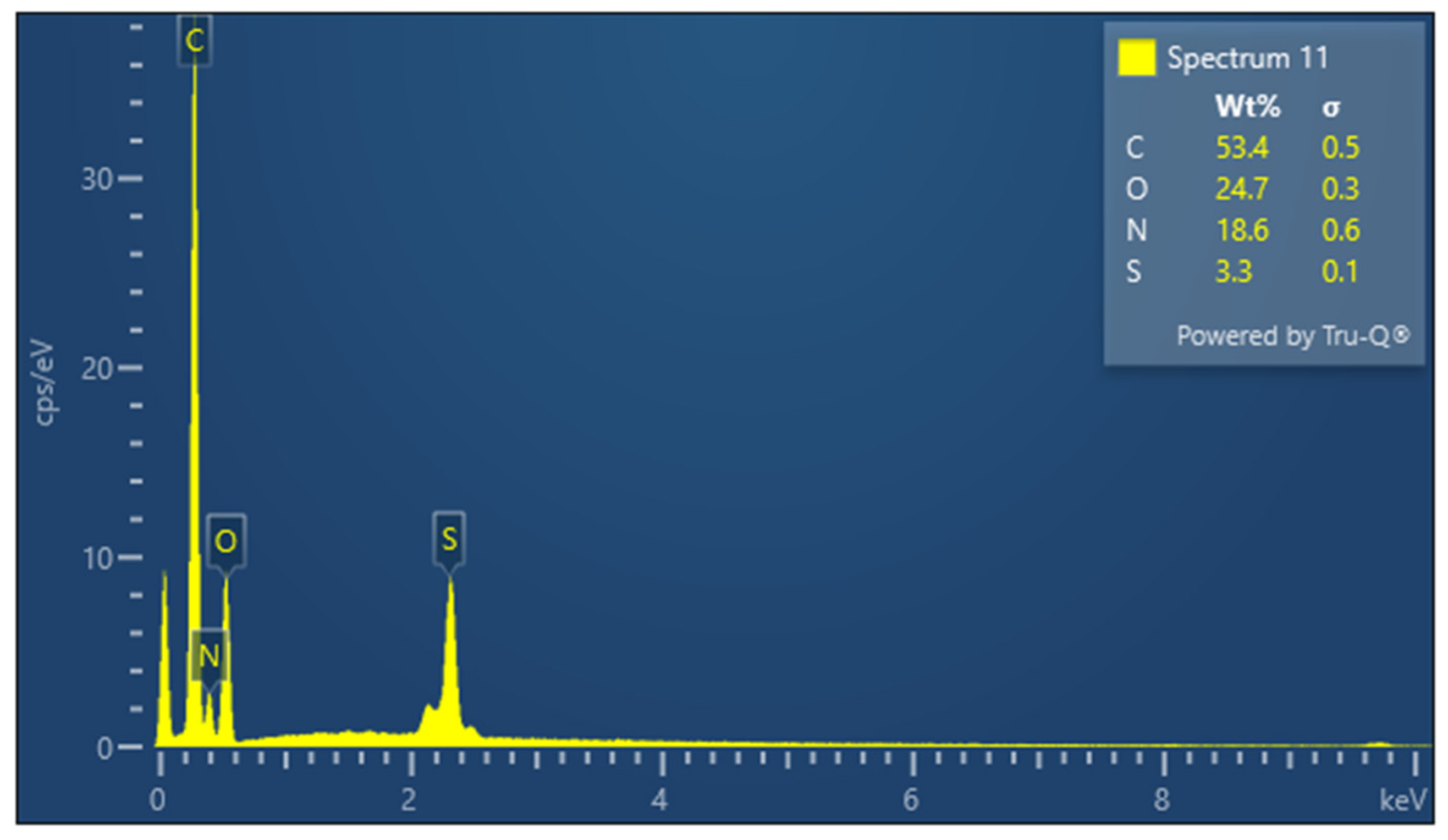
| Sample | Element | Weight Percentage (%) | Standard Deviation (σ) |
|---|---|---|---|
| Keratin | C | 53.4 | 0.5 |
| O | 24.7 | 0.3 | |
| N | 18.6 | 0.6 | |
| S | 3.3 | 0.1 | |
| KG scaffold | C | 51.9 | 0.7 |
| O | 27.6 | 0.5 | |
| N | 19.6 | 1.1 | |
| S | 0.2 | 0.0 | |
| Ca | 0.4 | 0.0 | |
| Na | 0.1 | 0.0 | |
| Al | 0.1 | 0.0 | |
| KGH scaffold | C | 49.1 | 0.7 |
| O | 28.2 | 0.5 | |
| N | 19.6 | 1.0 | |
| Ca | 2.4 | 0.1 | |
| Na | 0.4 | 0.0 | |
| S | 0.3 | 0.0 | |
| P | 0.0 | 0.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krishani, M.; Chong, J.N.; Lim, W.R.; Jusoh, N.; Sambudi, N.S.; Suhaimi, H. Synthesis and Characterization of Keratin-Based Scaffold for Potential Tissue Engineering Applications. Fibers 2025, 13, 97. https://doi.org/10.3390/fib13070097
Krishani M, Chong JN, Lim WR, Jusoh N, Sambudi NS, Suhaimi H. Synthesis and Characterization of Keratin-Based Scaffold for Potential Tissue Engineering Applications. Fibers. 2025; 13(7):97. https://doi.org/10.3390/fib13070097
Chicago/Turabian StyleKrishani, Murugiah, Jia Ning Chong, Wan Rong Lim, Norwahyu Jusoh, Nonni Soraya Sambudi, and Hazwani Suhaimi. 2025. "Synthesis and Characterization of Keratin-Based Scaffold for Potential Tissue Engineering Applications" Fibers 13, no. 7: 97. https://doi.org/10.3390/fib13070097
APA StyleKrishani, M., Chong, J. N., Lim, W. R., Jusoh, N., Sambudi, N. S., & Suhaimi, H. (2025). Synthesis and Characterization of Keratin-Based Scaffold for Potential Tissue Engineering Applications. Fibers, 13(7), 97. https://doi.org/10.3390/fib13070097








