Melt-Spun, Cross-Section Modified Polycaprolactone Fibers for Use in Tendon and Ligament Tissue Engineering
Abstract
:1. Introduction
2. Materials and Methods
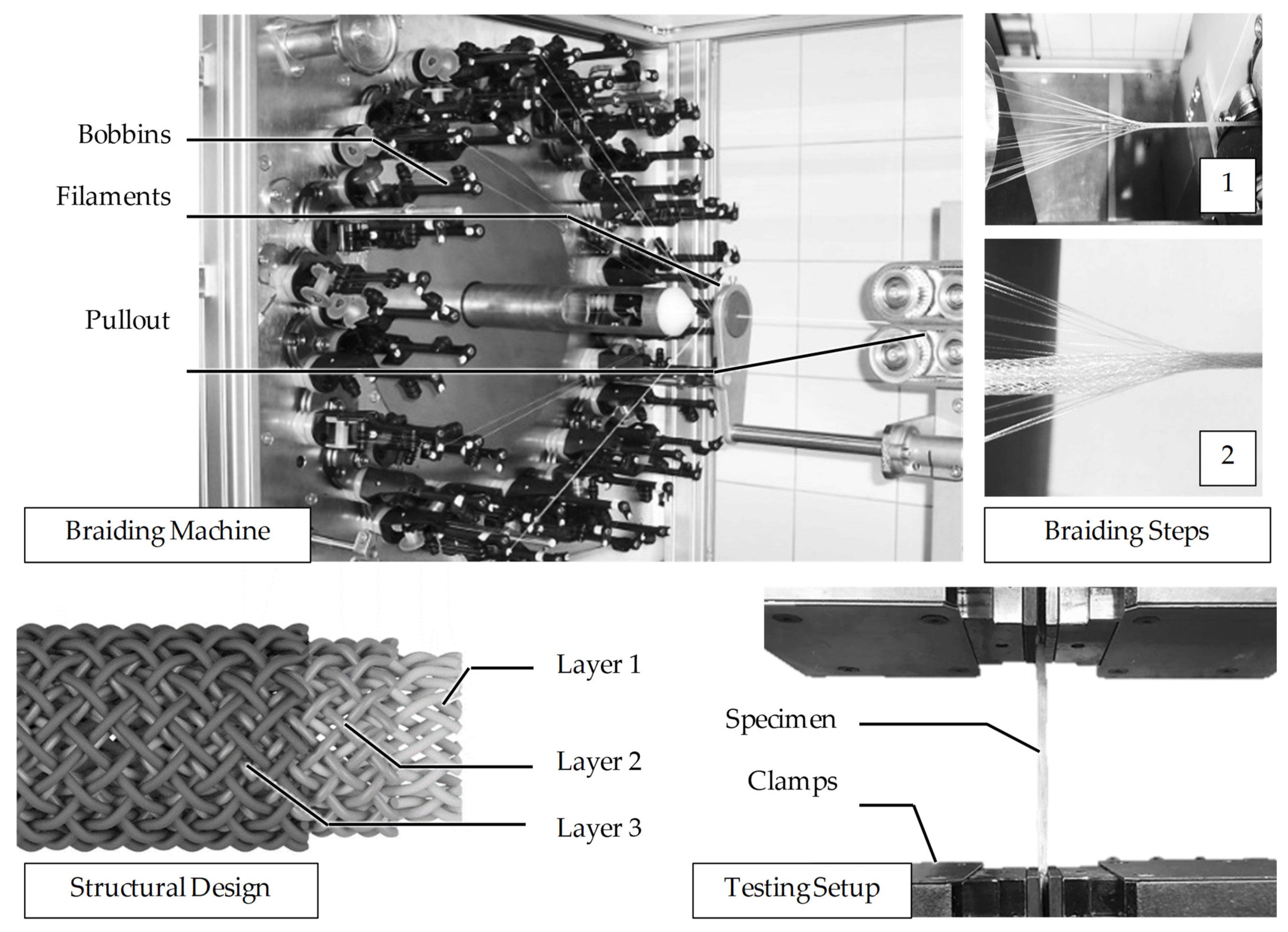
2.1. Fiber Fabrication
2.2. Braiding
2.3. Mechanical Characterization
2.4. Microscopic Analysis
2.5. Differential Scanning Calorimetry (DSC)
2.6. Wide-Angle X-ray Diffraction (WAXD)
2.7. Mechanical Fiber Characterization after Exposure to PBS at 37 °C
2.8. Statistical Analysis
3. Results
3.1. Fiber Fabrication
3.2. Mechanical Fiber Characterization after Exposure to PBS at 37 °C
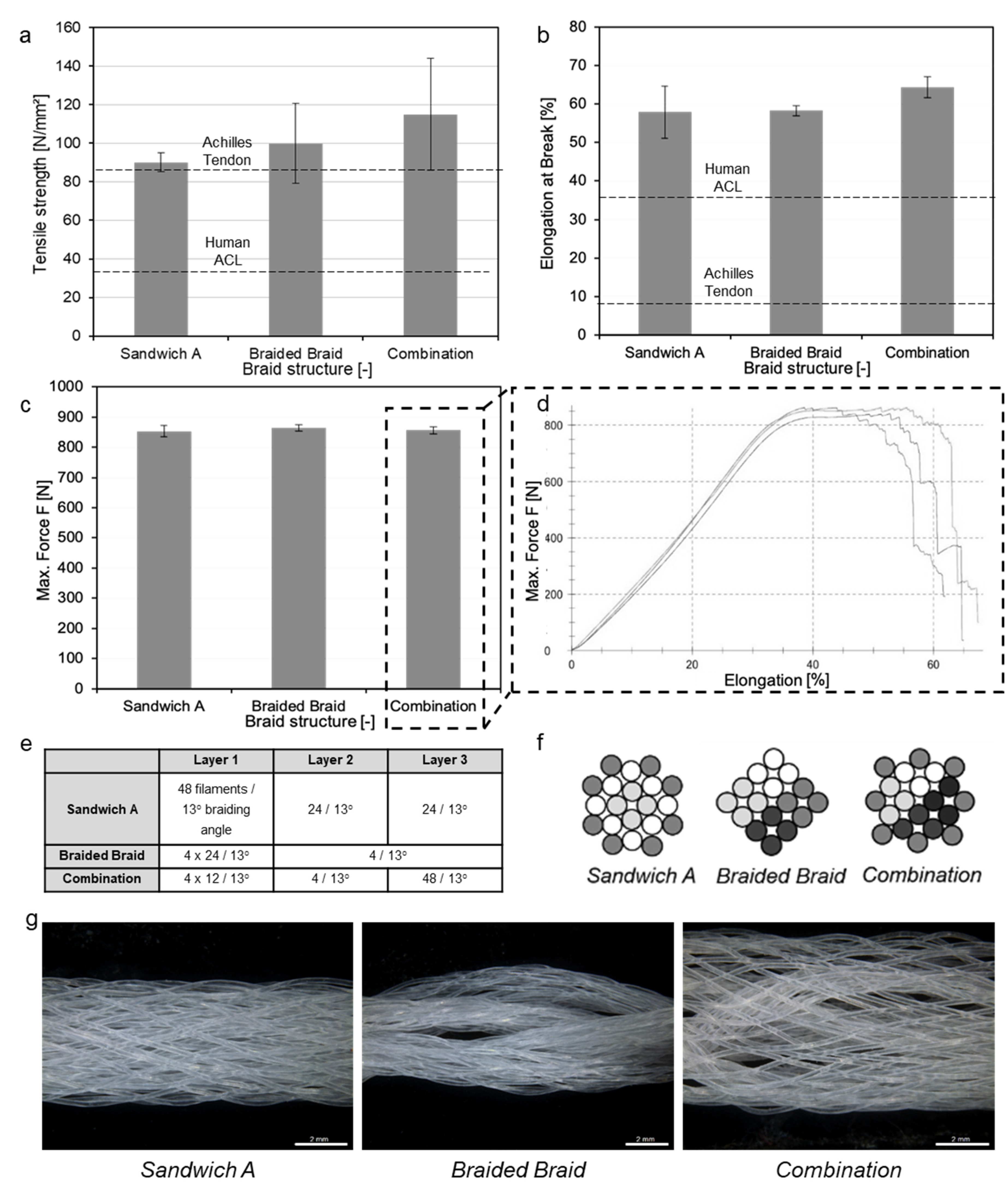
3.3. Scaffold Production
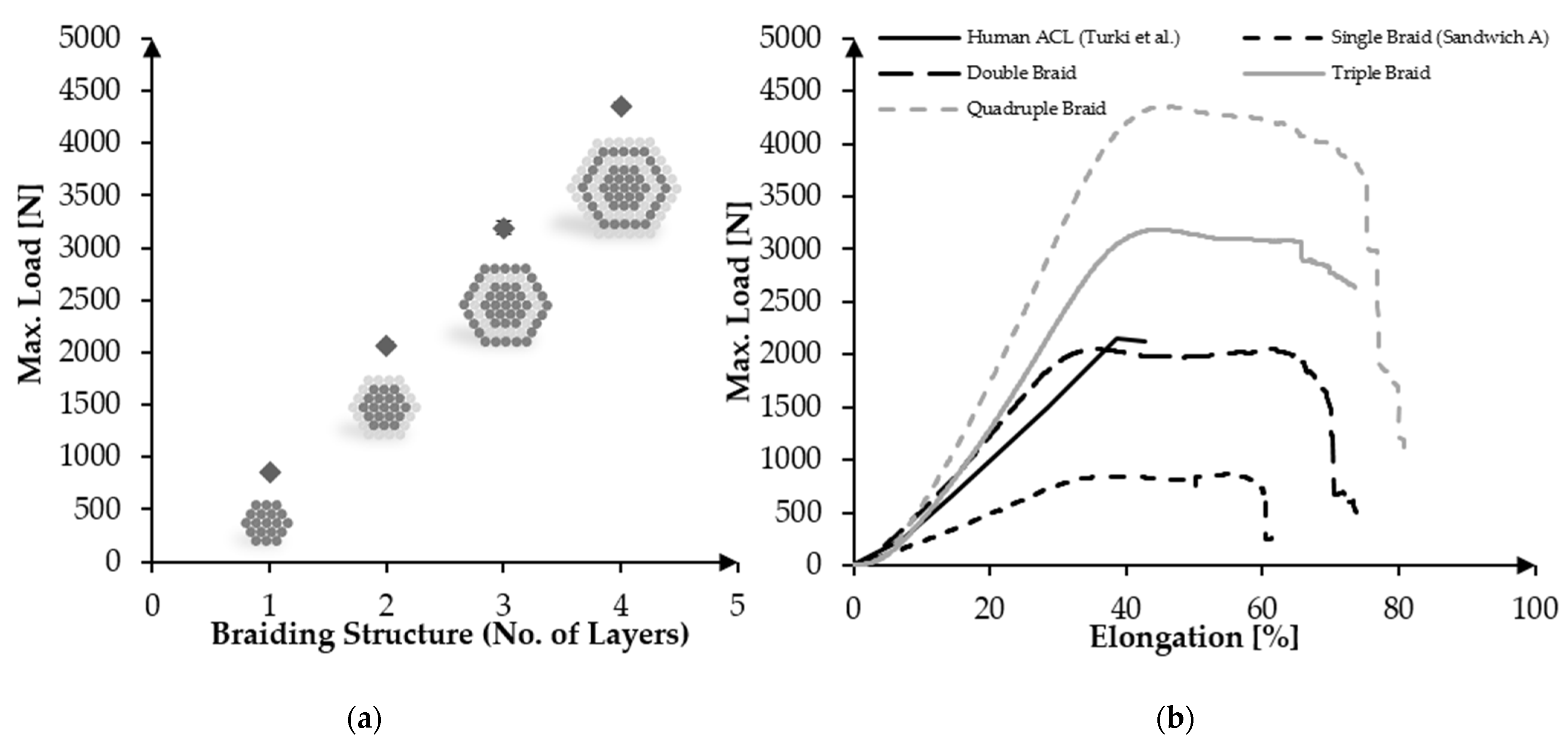
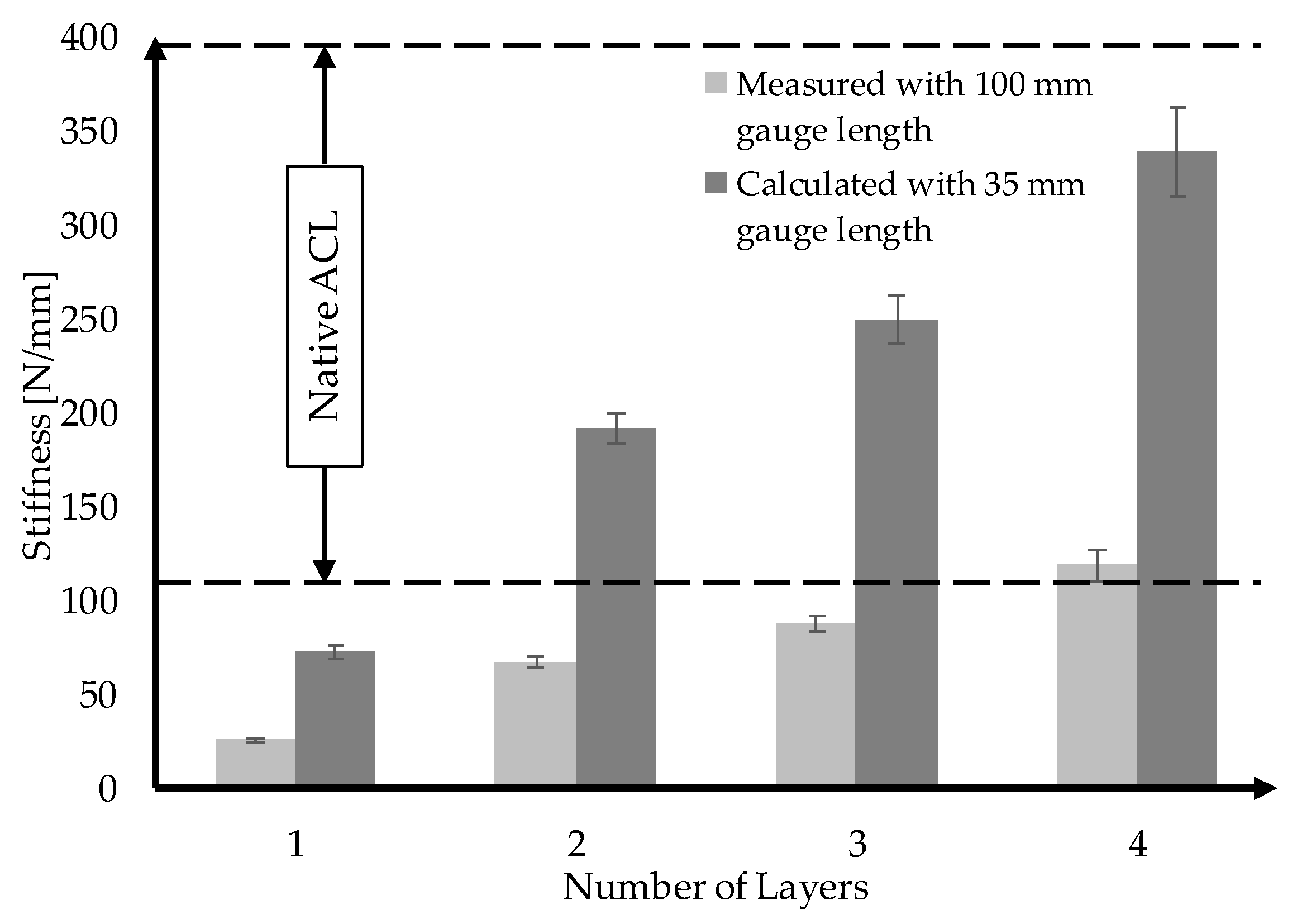
3.4. Upscaling towards Human ACL Scaffolds
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bogardus, R.L.; Martin, R.J.; Richman, A.R.; Kulas, A.S. Applying the Socio-Ecological Model to barriers to implementation of ACL injury prevention programs: A systematic review. J. Sport Health Sci. 2019, 8, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Nau, T.; Teuschl, A. Regeneration of the anterior cruciate ligament: Current strategies in tissue engineering. World J. Orthop. 2015, 6, 127–136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guidoin, M.-F.; Marois, Y.; Bejui, J.; Poddevin, N.; King, M.W.; Guidoin, R. Analysis of retrieved polymer fiber based replacements for the ACL. Biomaterials 2000, 21, 2461–2474. [Google Scholar] [CrossRef]
- Ge, Z.; Yang, F.; Goh, J.C.H.; Ramakrishna, S.; Lee, E.H. Biomaterials and scaffolds for ligament tissue engineering. J. Biomed. Mater. Res. A 2006, 77, 639–652. [Google Scholar] [CrossRef] [PubMed]
- Jedda, H.; Abessalem, S.B.; Sakli, F. The effect of cyclic loading on the mechanical properties of artificial ligaments. J. Textile Institute 2011, 102, 332–342. [Google Scholar] [CrossRef]
- Vieira, A.C.; Guedes, R.M.; Marques, A.T. Development of ligament tissue biodegradable devices: A review. J. Biomech. 2009, 42, 2421–2430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schlenker, H.-J. Tissue Engineering von Bandersatz: Einfluss Mechanischer Reize auf Humane Mesenchymale Progenitorzellen und Humane Kreuzbandzellen. Ph.D. Thesis, Universität Ulm, Ulm, Germany, 2006. [Google Scholar]
- Silva, M.; Ferreira, F.N.; Alves, N.M.; Paiva, M.C. Biodegradable polymer nanocomposites for ligament/tendon tissue engineering. J. Nanobiotechnol. 2020, 18, 23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Altman, G.H.; Horan, R.L.; Lu, H.H.; Moreau, J.; Martin, I.; Richmond, J.C.; Kaplan, D.L. Silk matrix for tissue engineered anterior cruciate ligaments. Biomaterials 2002, 23, 4131–4141. [Google Scholar] [CrossRef]
- Hutmacher, D.W. Scaffolds in tissue engineering bone and cartilage. Biomaterials 2000, 21, 2529–2543. [Google Scholar] [CrossRef]
- Laurent, C.P.; Durville, D.; Mainard, D.; Ganghoffer, J.-F.; Rahouadj, R. A multilayer braided scaffold for Anterior Cruciate Ligament: Mechanical modeling at the fiber scale. J. Mech. Behav. Biomed. Mater. 2012, 12, 184–196. [Google Scholar] [CrossRef]
- Vunjak-Novakovic, G.; Altman, G.; Horan, R.; Kaplan, D.L. Tissue engineering of ligaments. Annu. Rev. Biomed. Eng. 2004, 6, 131–156. [Google Scholar] [CrossRef] [PubMed]
- Legnani, C.; Ventura, A.; Terzaghi, C.; Borgo, E.; Albisetti, W. Anterior cruciate ligament reconstruction with synthetic grafts. A review of literature. Int. Orthop. 2010, 34, 465–471. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Wu, Y.; Pan, Z.; Sun, H.; Wang, J.; Yu, D.; Zhu, S.; Dai, J.; Chen, Y.; Tian, N.; et al. The effects of lactate and acid on articular chondrocytes function: Implications for polymeric cartilage scaffold design. Acta Biomater. 2016, 42, 329–340. [Google Scholar] [CrossRef] [PubMed]
- Gögele, C.; Hahn, J.; Elschner, C.; Breier, A.; Schröpfer, M.; Prade, I.; Meyer, M.; Schulze-Tanzil, G. Enhanced Growth of Lapine Anterior Cruciate Ligament-Derived Fibroblasts on Scaffolds Embroidered from Poly(l-lactide-co-ε-caprolactone) and Polylactic Acid Threads Functionalized by Fluorination and Hexamethylene Diisocyanate Cross-Linked Collagen Foams. Int. J. Mol. Sci. 2020, 21, 1132. [Google Scholar] [CrossRef] [Green Version]
- Fuoco, T.; Mathisen, T.; Finne-Wistrand, A. Minimizing the time gap between service lifetime and complete resorption of degradable melt-spun multifilament fibers. Polym. Degrad. Stab. 2019, 163, 43–51. [Google Scholar] [CrossRef]
- Sahoo, S.; Ouyang, H.; Goh, J.C.-H.; Tay, T.E.; Toh, S.L. Characterization of a Novel Polymeric Scaffold for Potential Application in Tendon/Ligament Tissue Engineering. Tissue Eng. 2006, 12, 91–99. [Google Scholar] [CrossRef] [Green Version]
- Mengsteab, P.Y.; Nair, L.S.; Laurencin, C.T. The past, present and future of ligament regenerative engineering. Regen. Med. 2016, 11, 871–881. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Ramanath, H.S.; Wang, D.-A. Tendon tissue engineering using scaffold enhancing strategies. Trends Biotechnol. 2008, 26, 201–209. [Google Scholar] [CrossRef]
- Liu, X.; Baldit, A.; de Brosses, E.; Velard, F.; Cauchois, G.; Chen, Y.; Wang, X.; de Isla, N.; Laurent, C. Characterization of Bone Marrow and Wharton’s Jelly Mesenchymal Stromal Cells Response on Multilayer Braided Silk and Silk/PLCL Scaffolds for Ligament Tissue Engineering. Polymers 2020, 12, 2163. [Google Scholar] [CrossRef]
- Zhang, P.; Han, F.; Chen, T.; Wu, Z.; Chen, S. “Swiss roll”-like bioactive hybrid scaffolds for promoting bone tissue ingrowth and tendon-bone healing after anterior cruciate ligament reconstruction. Biomater. Sci. 2020, 8, 871–883. [Google Scholar] [CrossRef] [PubMed]
- Woodruff, M.A.; Hutmacher, D.W. The return of a forgotten polymer—Polycaprolactone in the 21st century. Prog. Polym. Sci. 2010, 35, 1217–1256. [Google Scholar] [CrossRef] [Green Version]
- Leong, N.L.; Petrigliano, F.A.; McAllister, D.R. Current tissue engineering strategies in anterior cruciate ligament reconstruction. J. Biomed. Mater. Res. A 2014, 102, 1614–1624. [Google Scholar] [CrossRef] [PubMed]
- Laurent, C.P.; Ganghoffer, J.-F.; Babin, J.; Six, J.-L.; Wang, X.; Rahouadj, R. Morphological characterization of a novel scaffold for anterior cruciate ligament tissue engineering. J. Biomech. Eng. 2011, 133, 65001. [Google Scholar] [CrossRef] [PubMed]
- Lim, W.L.; Liau, L.L.; Ng, M.H.; Chowdhury, S.R.; Law, J.X. Current Progress in Tendon and Ligament Tissue Engineering. Tissue Eng. Regen. Med. 2019, 16, 549–571. [Google Scholar] [CrossRef]
- Rinoldi, C.; Kijeńska-Gawrońska, E.; Khademhosseini, A.; Tamayol, A.; Swieszkowski, W. Fibrous Systems as Potential Solutions for Tendon and Ligament Repair, Healing, and Regeneration. Adv. Healthc. Mater. 2021, 10, e2001305. [Google Scholar] [CrossRef]
- Wu, Y.; Han, Y.; Wong, Y.S.; Fuh, J.Y.H. Fibre-based scaffolding techniques for tendon tissue engineering. J. Tissue Eng. Regen. Med. 2018, 12, 1798–1821. [Google Scholar] [CrossRef]
- Li, X.; Chen, Z.; Zhang, H.; Zhuang, Y.; Shen, H.; Chen, Y.; Zhao, Y.; Chen, B.; Xiao, Z.; Dai, J. Aligned Scaffolds with Biomolecular Gradients for Regenerative Medicine. Polymers 2019, 11, 341. [Google Scholar] [CrossRef] [Green Version]
- Rothrauff, B.B.; Lauro, B.B.; Yang, G.; Debski, R.E.; Musahl, V.; Tuan, R.S. Braided and Stacked Electrospun Nanofibrous Scaffolds for Tendon and Ligament Tissue Engineering. Tissue Eng. Part A 2017, 23, 378–389. [Google Scholar] [CrossRef]
- Orr, S.B.; Chainani, A.; Hippensteel, K.J.; Kishan, A.; Gilchrist, C.; Garrigues, N.W.; Ruch, D.S.; Guilak, F.; Little, D. Aligned multilayered electrospun scaffolds for rotator cuff tendon tissue engineering. Acta Biomater. 2015, 24, 117–126. [Google Scholar] [CrossRef] [Green Version]
- Beason, D.P.; Connizzo, B.K.; Dourte, L.M.; Mauck, R.L.; Soslowsky, L.J.; Steinberg, D.R.; Bernstein, J. Fiber-aligned polymer scaffolds for rotator cuff repair in a rat model. J. Shoulder Elbow Surg. 2012, 21, 245–250. [Google Scholar] [CrossRef]
- Willbold, E.; Wellmann, M.; Welke, B.; Angrisani, N.; Gniesmer, S.; Kampmann, A.; Hoffmann, A.; de Cassan, D.; Menzel, H.; Hoheisel, A.L.; et al. Possibilities and limitations of electrospun chitosan-coated polycaprolactone grafts for rotator cuff tear repair. J. Tissue Eng. Regen. Med. 2020, 14, 186–197. [Google Scholar] [CrossRef] [PubMed]
- Thayer, P.S.; Goldstein, A.S. Bio-Instructive Scaffolds for Tendon/Ligament Regeneration. Bio-Instructive Scaffolds for Musculoskeletal Tissue Engineering and Regenerative Medicine; Elsevier: Amsterdam, The Netherlands, 2017; pp. 87–112. ISBN 9780128033944. [Google Scholar]
- Tamayol, A.; Akbari, M.; Annabi, N.; Paul, A.; Khademhosseini, A.; Juncker, D. Fiber-based tissue engineering: Progress, challenges, and opportunities. Biotechnol. Adv. 2013, 31, 669–687. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, S.; Wang, Y.; Streubel, P.N.; Duan, B. Living nanofiber yarn-based woven biotextiles for tendon tissue engineering using cell tri-culture and mechanical stimulation. Acta Biomater. 2017, 62, 102–115. [Google Scholar] [CrossRef] [PubMed]
- Li, X. A Hybrid Graft of Silk-Based Artificial Ligament and TCP/PEEK Anchorage for ACL Reconstruction; ETH Zurich: Zurich, Switzerland, 2013. [Google Scholar]
- Freeman, J.W.; Woods, M.D.; Laurencin, C.T. Tissue engineering of the anterior cruciate ligament using a braid-twist scaffold design. J. Biomech. 2007, 40, 2029–2036. [Google Scholar] [CrossRef] [Green Version]
- Ramakrishna, H.; Li, T.; He, T.; Temple, J.; King, M.W.; Spagnoli, A. Tissue engineering a tendon-bone junction with biodegradable braided scaffolds. Biomater. Res. 2019, 23, 11. [Google Scholar] [CrossRef]
- Cooper, J.A.; Sahota, J.S.; Gorum, W.J.; Carter, J.; Doty, S.B.; Laurencin, C.T. Biomimetic tissue-engineered anterior cruciate ligament replacement. Proc. Natl. Acad. Sci. USA 2007, 104, 3049–3054. [Google Scholar] [CrossRef] [Green Version]
- Cooper, J.A.; Lu, H.H.; Ko, F.K.; Freeman, J.W.; Laurencin, C.T. Fiber-based tissue-engineered scaffold for ligament replacement: Design considerations and in vitro evaluation. Biomaterials 2005, 26, 1523–1532. [Google Scholar] [CrossRef]
- Lu, H.H.; Cooper, J.A.; Manuel, S.; Freeman, J.W.; Attawia, M.A.; Ko, F.K.; Laurencin, C.T. Anterior cruciate ligament regeneration using braided biodegradable scaffolds: In vitro optimization studies. Biomaterials 2005, 26, 4805–4816. [Google Scholar] [CrossRef]
- Freeman, J.W.; Woods, M.D.; Cromer, D.A.; Ekwueme, E.C.; Andric, T.; Atiemo, E.A.; Bijoux, C.H.; Laurencin, C.T. Evaluation of a hydrogel-fiber composite for ACL tissue engineering. J. Biomech. 2011, 44, 694–699. [Google Scholar] [CrossRef]
- Mengsteab, P.Y.; Conroy, P.; Badon, M.; Otsuka, T.; Kan, H.-M.; Vella, A.T.; Nair, L.S.; Laurencin, C.T. Evaluation of a bioengineered ACL matrix’s osteointegration with BMP-2 supplementation. PLoS ONE 2020, 15, e0227181. [Google Scholar] [CrossRef] [Green Version]
- Madhavarapu, S.; Rao, R.; Libring, S.; Fleisher, E.; Yankannah, Y.; Freeman, J.W. Design and characterization of three-dimensional twist-braid scaffolds for anterior cruciate ligament regeneration. Technology 2017, 5, 98–106. [Google Scholar] [CrossRef]
- Zheng, Z.; Ran, J.; Chen, W.; Hu, Y.; Zhu, T.; Chen, X.; Yin, Z.; Heng, B.C.; Feng, G.; Le, H.; et al. Alignment of collagen fiber in knitted silk scaffold for functional massive rotator cuff repair. Acta Biomater. 2017, 51, 317–329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ouyang, H.W.; Goh, J.C.H.; Thambyah, A.; Teoh, S.H.; Lee, E.H. Knitted poly-lactide-co-glycolide scaffold loaded with bone marrow stromal cells in repair and regeneration of rabbit Achilles tendon. Tissue Eng. 2003, 9, 431–439. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.L.; Yin, Z.; Shen, W.L.; Chen, X.; Heng, B.C.; Zou, X.H.; Ouyang, H.W. Efficacy of hESC-MSCs in knitted silk-collagen scaffold for tendon tissue engineering and their roles. Biomaterials 2010, 31, 9438–9451. [Google Scholar] [CrossRef] [PubMed]
- Learn, G.D.; McClellan, P.E.; Knapik, D.M.; Cumsky, J.L.; Webster-Wood, V.; Anderson, J.M.; Gillespie, R.J.; Akkus, O. Woven collagen biotextiles enable mechanically functional rotator cuff tendon regeneration during repair of segmental tendon defects in vivo. J. Biomed. Mater. Res. Part B Appl. Biomater. 2019, 107, 1864–1876. [Google Scholar] [CrossRef]
- Laranjeira, M.; Domingues, R.M.A.; Costa-Almeida, R.; Reis, R.L.; Gomes, M.E. 3D Mimicry of Native-Tissue-Fiber Architecture Guides Tendon-Derived Cells and Adipose Stem Cells into Artificial Tendon Constructs. Small 2017, 13. [Google Scholar] [CrossRef]
- Dong, Q.; Cai, J.; Wang, H.; Chen, S.; Liu, Y.; Yao, J.; Shao, Z.; Chen, X. Artificial ligament made from silk protein/Laponite hybrid fibers. Acta Biomater. 2020, 106, 102–113. [Google Scholar] [CrossRef]
- Hahn, J.; Breier, A.; Brünig, H.; Heinrich, G. Long-term hydrolytic degradation study on polymer-based embroidered scaffolds for ligament tissue engineering. J. Ind. Text. 2018, 47, 1305–1320. [Google Scholar] [CrossRef]
- Hahn, J. Herstellung und Charakterisierung Gestickter Trägerstrukturen auf Basis Abbaubarer, Polymerer Fadenmaterialien für das Tissue Engineering des Vorderen Kreuzbandes. Ph.D. Thesis, Technische Universität Dresden, Dresden, Germany, 2021. [Google Scholar]
- Pal, J.; Kankariya, N.; Sanwaria, S.; Nandan, B.; Srivastava, R.K. Control on molecular weight reduction of poly(ε-caprolactone) during melt spinning-a way to produce high strength biodegradable fibers. Mater. Sci. Eng. C Mater. Biol. Appl. 2013, 33, 4213–4220. [Google Scholar] [CrossRef]
- Lam, C.X.F.; Hutmacher, D.W.; Schantz, J.-T.; Woodruff, M.A.; Teoh, S.H. Evaluation of polycaprolactone scaffold degradation for 6 months in vitro and in vivo. J. Biomed. Mater. Res. A 2009, 90, 906–919. [Google Scholar] [CrossRef]
- Leroux, A.; Ngoc Nguyen, T.; Rangel, A.; Cacciapuoti, I.; Duprez, D.; Castner, D.G.; Migonney, V. Long-term hydrolytic degradation study of polycaprolactone films and fibers grafted with poly(sodium styrene sulfonate): Mechanism study and cell response. Biointerphases 2020, 15, 61006. [Google Scholar] [CrossRef] [PubMed]
- Gurarslan, A.; Caydamli, Y.; Shen, J.; Tse, S.; Yetukuri, M.; Tonelli, A.E. Coalesced poly(ε-caprolactone) fibers are stronger. Biomacromolecules 2015, 16, 890–893. [Google Scholar] [CrossRef] [PubMed]
- Nair, L.S.; Laurencin, C.T. Biodegradable polymers as biomaterials. Prog. Polym. Sci. 2007, 32, 762–798. [Google Scholar] [CrossRef]
- Rohindra, D.R.; Khurma, J.R. Miscibility, melting and crystallization of poly(ε-caprolactone) and poly (vinyl formal) blend. S. Pac. J. Nat. Appl. Sci. 2007, 25, 53. [Google Scholar] [CrossRef] [Green Version]
- Mochizuki, M.; Nakayama, K.; Qian, R.; Jiang, B.-Z.; Hirami, M.; Hayashi, T.; Masuda, T.; Nakajima, A. Studies on the biodegradable poly (hexano-6-lactone) fibers 1. Structure and properties of drawn poly (hexano-6-lactone) fibers (Technical Report). Pure Appl. Chem. 1997, 69, 2567–2576. [Google Scholar] [CrossRef]
- Mochizuki, M.; Hirano, M.; Kanmuri, Y.; Kudo, K.; Tokiwa, Y. Hydrolysis of polycaprolactone fibers by lipase: Effects of draw ratio on enzymatic degradation. J. Appl. Polym. Sci. 1995, 55, 289–296. [Google Scholar] [CrossRef]
- Leroux, A.; Maurice, E.; Viateau, V.; Migonney, V. Feasibility Study of the Elaboration of a Biodegradable and Bioactive Ligament Made of Poly(ε-caprolactone)-pNaSS Grafted Fibers for the Reconstruction of Anterior Cruciate Ligament: In Vivo Experiment. IRBM 2019, 40, 38–44. [Google Scholar] [CrossRef]
- Noyes, F.; Grood, E. The strength of the anterior cruciate ligament in humans and Rhesus. J. Bone Joint Surg. Am. 1976, 58, 1074–1082. [Google Scholar] [CrossRef]
- Chandrashekar, N.; Slauterbeck, J.; Hashemi, J. Sex-based differences in the anthropometric characteristics of the anterior cruciate ligament and its relation to intercondylar notch geometry: A cadaveric study. Am. J. Sports Med. 2005, 33, 1492–1498. [Google Scholar] [CrossRef]
- Kamiya, R.; Cheeseman, B.A.; Popper, P.; Chou, T.-W. Some recent advances in the fabrication and design of three-dimensional textile preforms: A review. Compos. Sci. Technol. 2000, 60, 33–47. [Google Scholar] [CrossRef]
- Steinmann, W.; Walter, S.; Gries, T.; Seide, G.; Roth, G. Modification of the mechanical properties of polyamide 6 multifilaments in high-speed melt spinning with nano silicates. Text. Res. J. 2012, 82, 1846–1858. [Google Scholar] [CrossRef]
- Vad, T.; Wulfhorst, J.; Pan, T.-T.; Steinmann, W.; Dabringhaus, S.; Beckers, M.; Seide, G.; Gries, T.; Sager, W.F.C.; Heidelmann, M.; et al. Orientation of Well-Dispersed Multiwalled Carbon Nanotubes in Melt-Spun Polymer Fibers and Its Impact on the Formation of the Semicrystalline Polymer Structure: A Combined Wide-Angle X-ray Scattering and Electron Tomography Study. Macromolecules 2013, 46, 5604–5613. [Google Scholar] [CrossRef]
- Wren, T.A.L.; Yerby, S.A.; Beaupré, G.S.; Carter, D.R. Mechanical properties of the human achilles tendon. Clin. Biomech. 2001, 16, 245–251. [Google Scholar] [CrossRef]
- Turki, S.; Marzougui, S.; Ben Abdessalem, S. Impact of polyurethane yarns on the mechanical properties of braided artificial ligaments. J. Text. Inst. 2015, 106, 912–918. [Google Scholar] [CrossRef]
- Karmani, S.; Ember, T. The anterior cruciate ligament—1. Curr. Orthop. 2003, 17, 369–377. [Google Scholar] [CrossRef]
- Negahi Shirazi, A.; Chrzanowski, W.; Khademhosseini, A.; Dehghani, F. Anterior Cruciate Ligament: Structure, Injuries and Regenerative Treatments. Adv. Exp. Med. Biol. 2015, 881, 161–186. [Google Scholar] [CrossRef]
- Eickhoff, R.M.; Bolle, T.; Kossel, K.; Heise, D.; Kroh, A.; Lambertz, A.; Blaeser, A.; Gries, T.; Jockenhoevel, S.; Neumann, U.P.; et al. Improved biocompatibility of profiled sutures through lower macrophages adhesion. J. Biomed. Mater. Res. Part B Appl. Biomater. 2019, 107, 1772–1778. [Google Scholar] [CrossRef]
- Burkersroda, F.v.; Schedl, L.; Göpferich, A. Why degradable polymers undergo surface erosion or bulk erosion. Biomaterials 2002, 23, 4221–4231. [Google Scholar] [CrossRef]
- Chen, D.R.; Bei, J.Z.; Wang, S.G. Polycaprolactone microparticles and their biodegradation. Polym. Degrad. Stab. 2000, 67, 455–459. [Google Scholar] [CrossRef]
- Park, S.J.; Lee, B.-K.; Na, M.H.; Kim, D.S. Melt-spun shaped fibers with enhanced surface effects: Fiber fabrication, characterization and application to woven scaffolds. Acta Biomater. 2013, 9, 7719–7726. [Google Scholar] [CrossRef]
- Babaarslan, O.; Hacioğullari, S.Ö. Effect of fibre cross-sectional shape on the properties of POY continuous filaments yarns. Fibers Polym 2013, 14, 146–151. [Google Scholar] [CrossRef]
- Tsuji, H.; Mizuno, A.; Ikada, Y. Properties and morphology of poly(L-lactide). III. Effects of initial crystallinity on long-termin vitro hydrolysis of high molecular weight poly(L-lactide) film in phosphate-buffered solution. J. Appl. Polym. Sci. 2000, 77, 1452–1464. [Google Scholar] [CrossRef]
- Gleadall, A.; Pan, J.; Atkinson, H. A simplified theory of crystallisation induced by polymer chain scissions for biodegradable polyesters. Polym. Degrad. Stab. 2012, 97, 1616–1620. [Google Scholar] [CrossRef] [Green Version]
- Beslikas, T.; Gigis, I.; Goulios, V.; Christoforides, J.; Papageorgiou, G.Z.; Bikiaris, D.N. Crystallization study and comparative in vitro-in vivo hydrolysis of PLA reinforcement ligament. Int. J. Mol. Sci. 2011, 12, 6597–6618. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Laurent, C.; Du, Q.; Targa, L.; Cauchois, G.; Chen, Y.; Wang, X.; de Isla, N. Mesenchymal stem cell interacted with PLCL braided scaffold coated with poly-l-lysine/hyaluronic acid for ligament tissue engineering. J. Biomed. Mater. Res. A 2018, 106, 3042–3052. [Google Scholar] [CrossRef] [PubMed]
- Mengsteab, P.Y.; Freeman, J.; Barajaa, M.A.; Nair, L.S.; Laurencin, C.T. Ligament Regenerative Engineering: Braiding Scalable and Tunable Bioengineered Ligaments Using a Bench-Top Braiding Machine. Regen. Eng. Transl. Med. 2021, 7, 524–532. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Mengsteab, P.Y.; Narayanan, G.; Nair, L.S.; Laurencin, C.T. Enhancing the Surface Properties of a Bioengineered Anterior Cruciate Ligament Matrix for Use with Point-of-Care Stem Cell Therapy. Engineering 2021, 7, 153–161. [Google Scholar] [CrossRef]
- Kohn, D.; Rose, C. Primary stability of interference screw fixation. Influence of screw diameter and insertion torque. Am. J. Sports Med. 1994, 22, 334–338. [Google Scholar] [CrossRef]
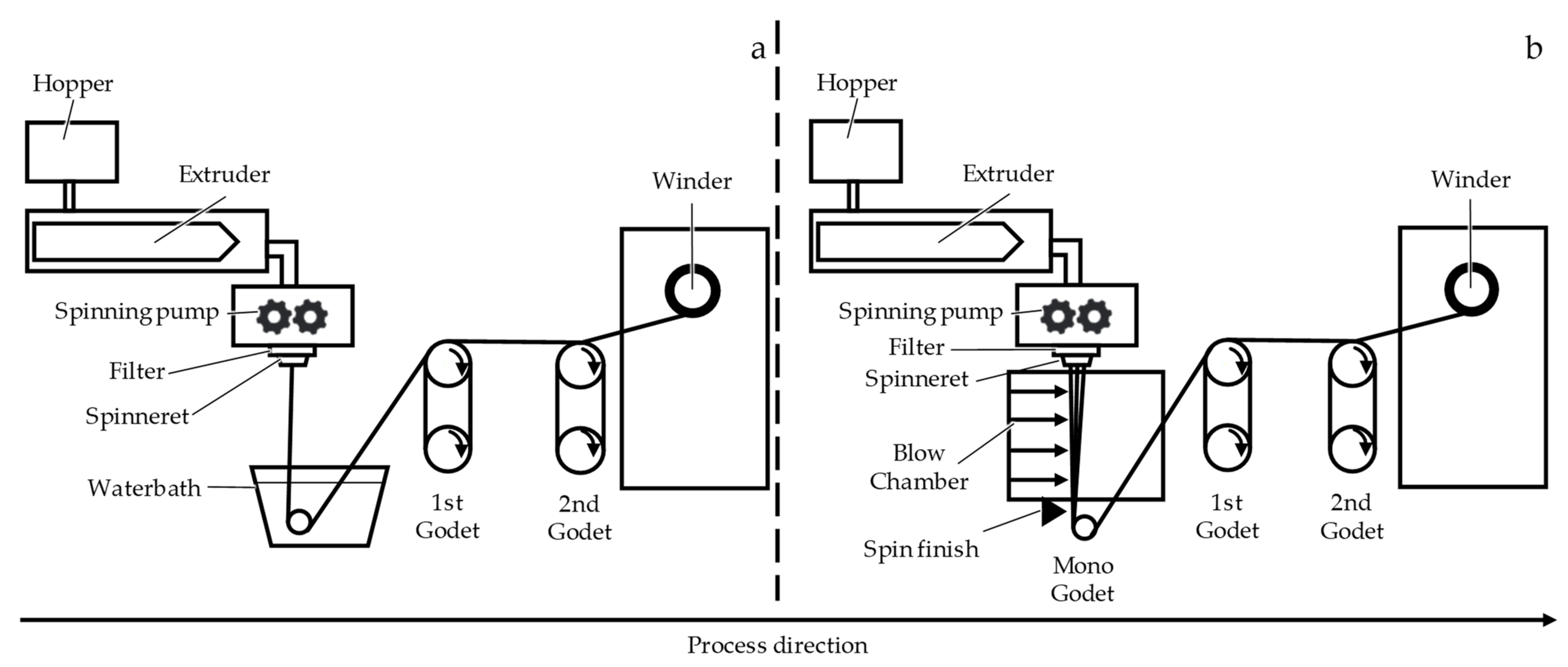
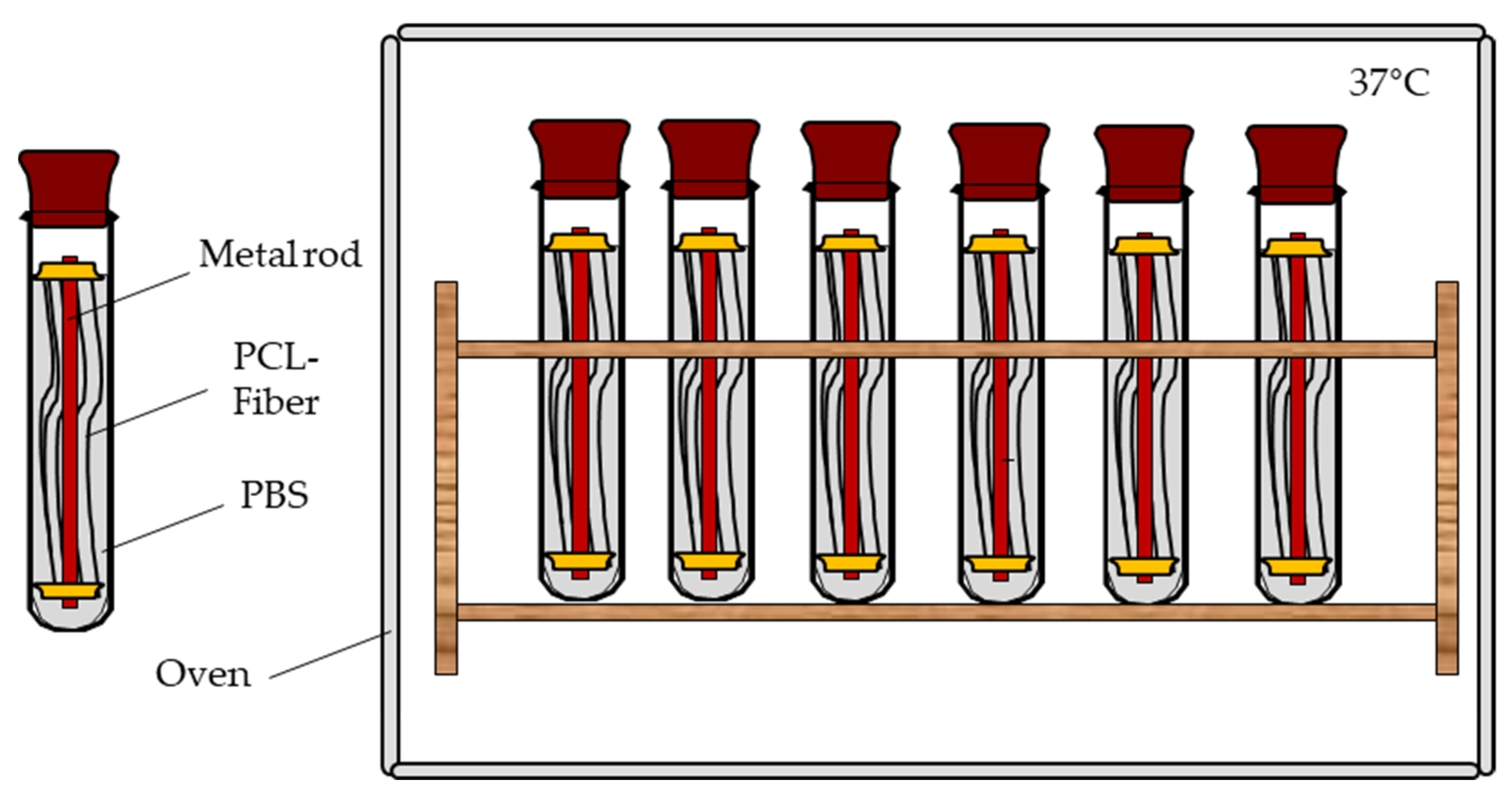
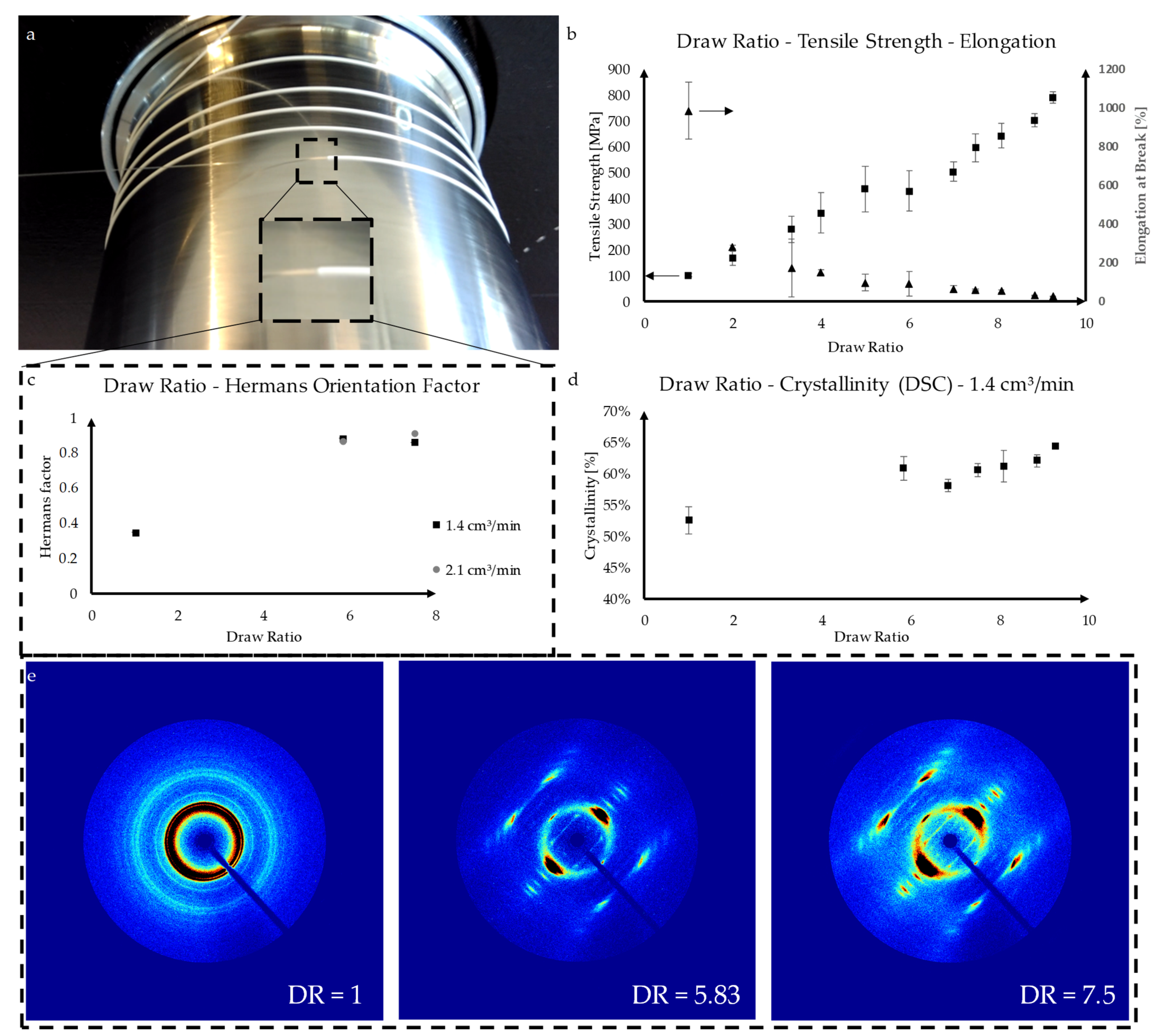
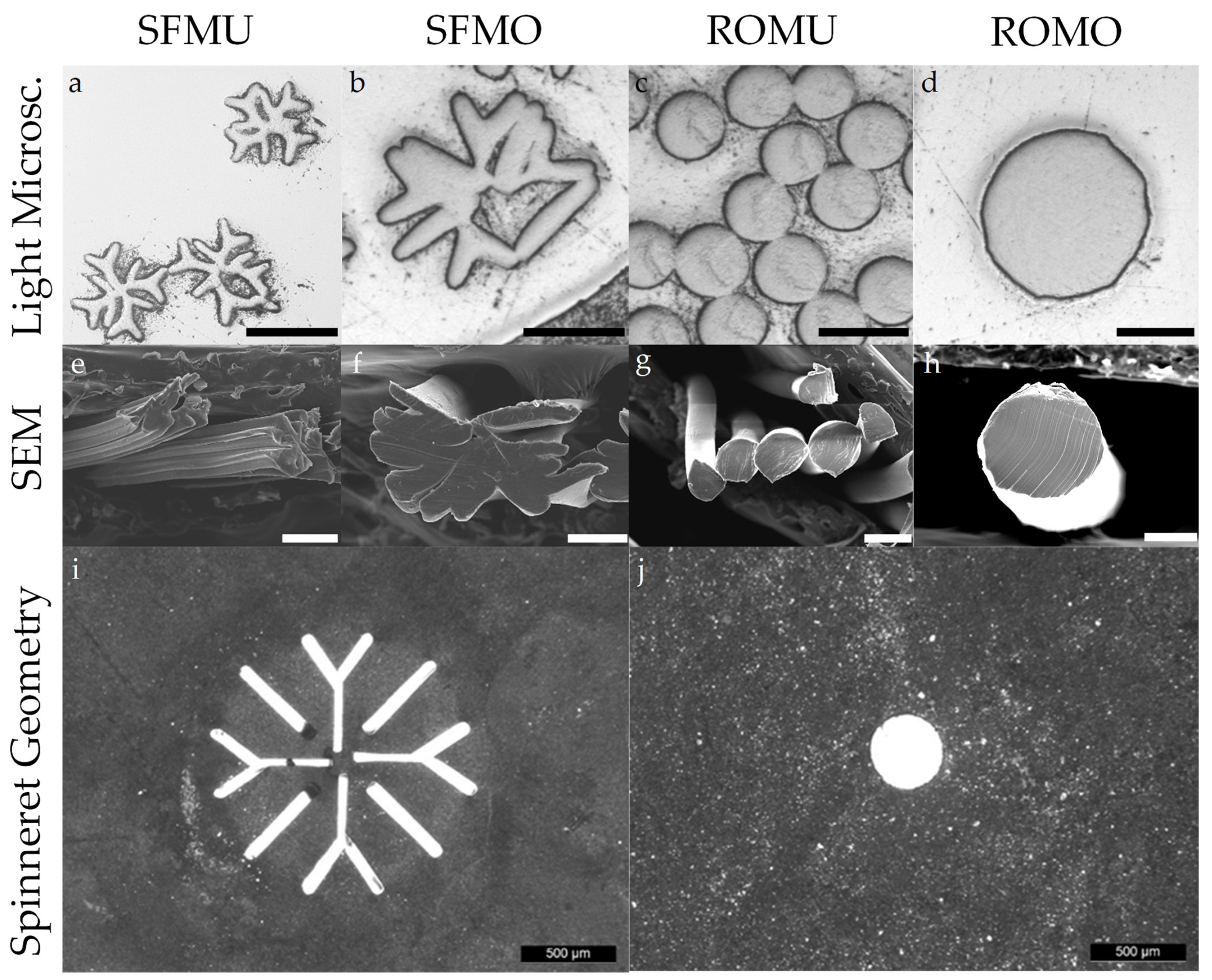
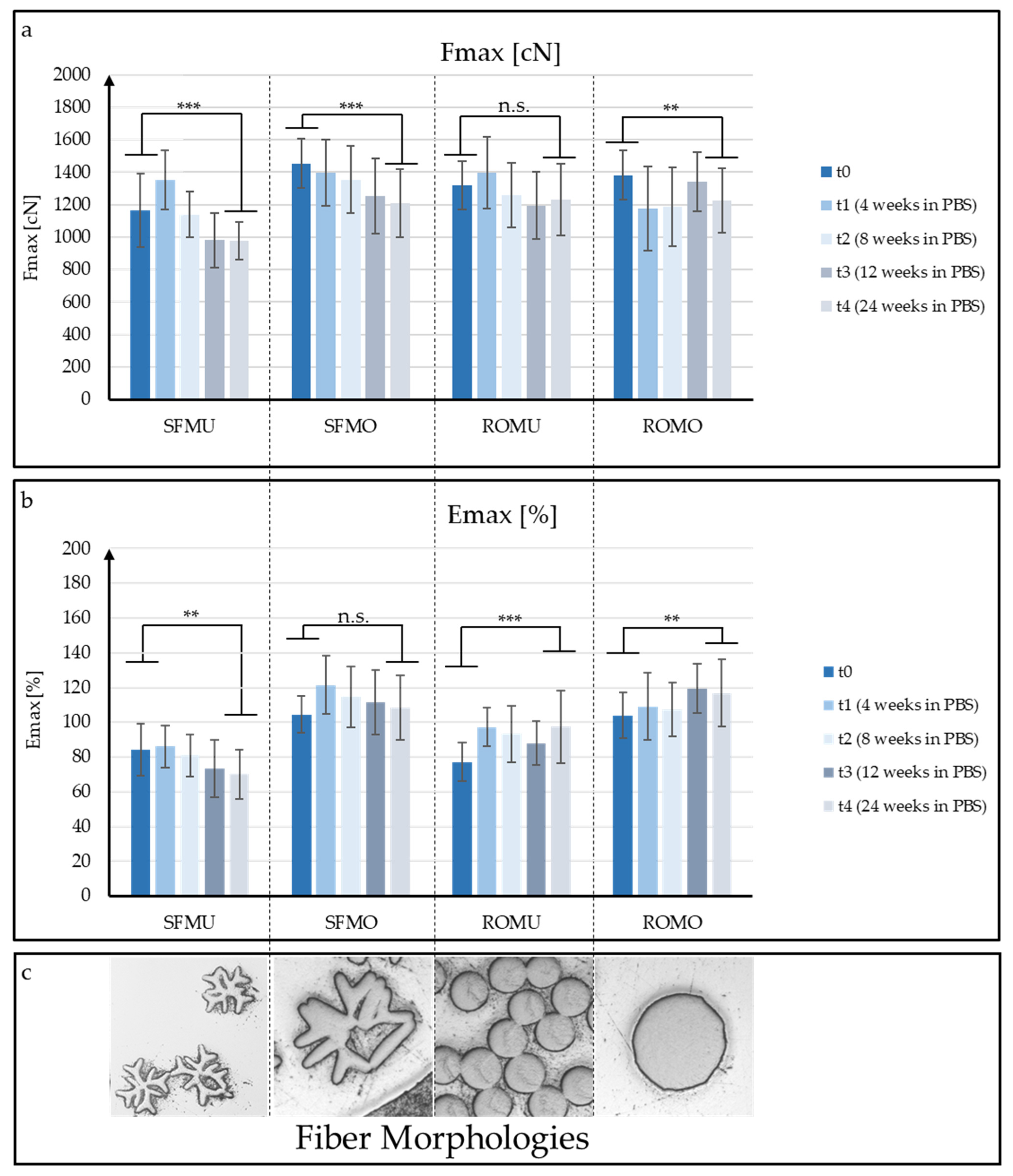
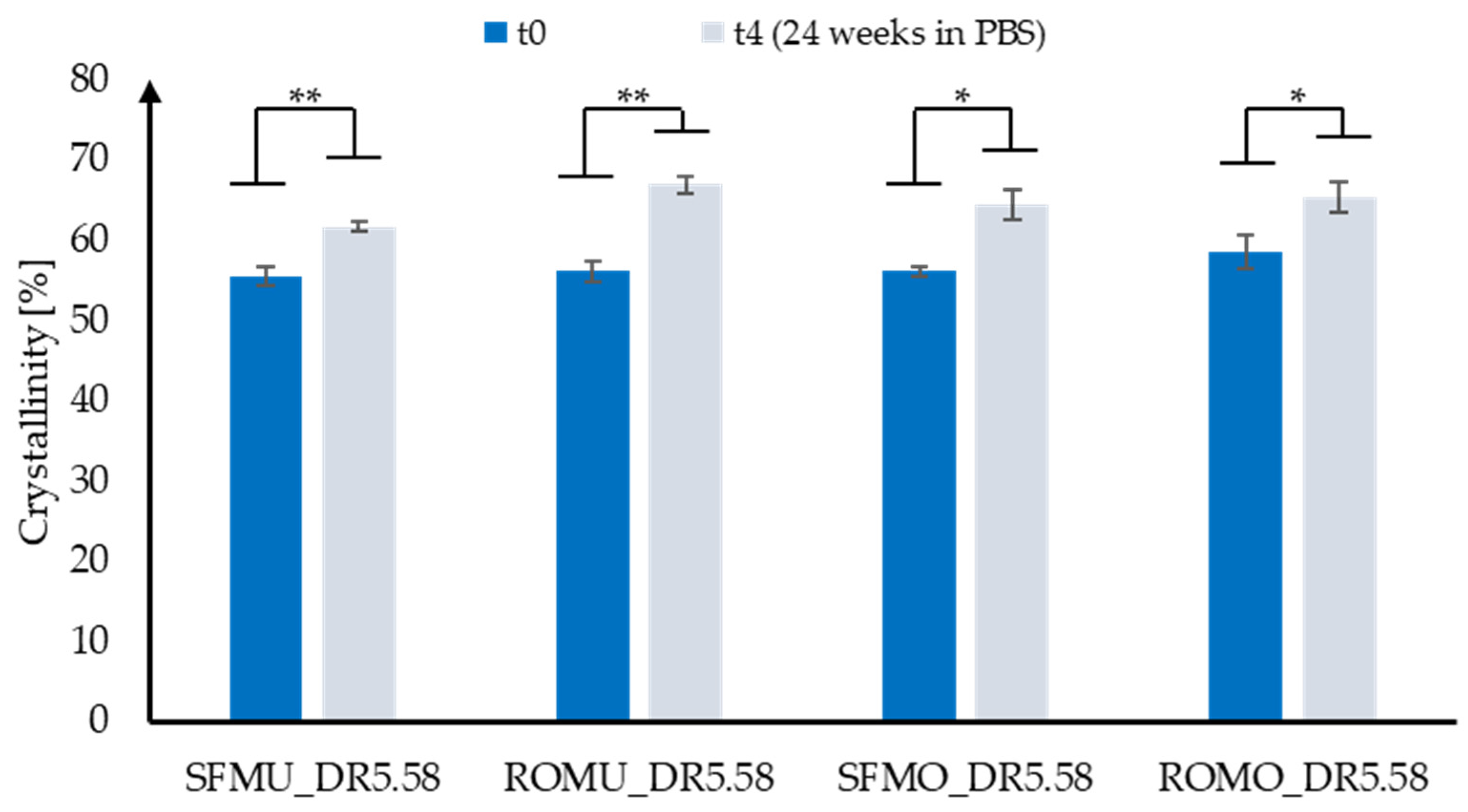
| Sample | Shape | Yarn Structure | DR | Yarn Count [dtex] | Ref. Diameter [µm] | Max. Load [cN] | Elongation at Max. Load [%] |
|---|---|---|---|---|---|---|---|
| ROMO_5.58 | Round | Monofilament | 5.58 | 428.97 | ~222 | 1530.56 | 94.55 |
| ROMU_5.58 | Round | Multifilament | 5.58 | 469.14 | ~76 | 1559.17 | 100.24 |
| SFMO_5.58 | Snowflake | Monofilament | 5.58 | 452.33 | ~236 | 1624.93 | 88.33 |
| SFMU_5.58 | Snowflake | Multifilament | 5.58 | 480.70 | ~87 | 1588.70 | 95.10 |
| Full Name | Abbreviation |
|---|---|
| Round monofilament | (ROMO) |
| Snowflake monofilament | (SFMO) |
| Round multifilament | (ROMU) |
| Snowflake multifilament | (SFMU) |
| Layer Design (n = 3) | Load at Failure [N] | Diameter D1 [mm] | Diameter D2 [mm] | Cross-Section A [mm2] | UTS [MPa] |
|---|---|---|---|---|---|
| Sandwich A | 852.8 ± 18.79 | 4.50 ± 0.55 | 2.70 ± 0.26 | 9.47 ± 0.30 | 90.03 ± 4.80 |
| Braided Braid | 864.23 ± 11.09 | 5.29 ± 0.75 | 2.07 ± 0.40 | 8.57 ± 1.66 | 99.88 ± 20.77 |
| Combination | 855.77 ± 12.49 | 4.12 ± 0.86 | 2.33 ± 0.25 | 7.53 ± 1.81 | 114.84 ± 29.04 |
| Sample (n = 3) | No. of Layers | Load at Failure [N] | Diameter D1 [mm] | Diameter D2 [mm] | Cross-Section A [mm2] | UTS [MPa] |
|---|---|---|---|---|---|---|
| A-96f | 1 | 852.8 ± 18.79 | 4.50 ± 0.55 | 2.70 ± 0.26 | 9.47 ± 0.30 | 90.03 ± 4.80 |
| A-192f | 2 | 2067.38 ± 32.00 | 5.91 ± 0.41 | 4.04 ± 0.10 | 18.77 ± 1.59 | 110.14 ± 11.02 |
| A-288f | 3 | 3191.03 ± 60.00 | 9.00 ± 0.93 | 4.62 ± 0.95 | 32.30 ± 5.46 | 98.78 ± 18.57 |
| A-384f | 4 | 4353.88 ± 37.30 | 12.90 ± 1.87 | 5.35 ± 1.93 | 53.32 ± 16.80 | 81.64 ± 26.42 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bauer, B.; Emonts, C.; Bonten, L.; Annan, R.; Merkord, F.; Vad, T.; Idrissi, A.; Gries, T.; Blaeser, A. Melt-Spun, Cross-Section Modified Polycaprolactone Fibers for Use in Tendon and Ligament Tissue Engineering. Fibers 2022, 10, 23. https://doi.org/10.3390/fib10030023
Bauer B, Emonts C, Bonten L, Annan R, Merkord F, Vad T, Idrissi A, Gries T, Blaeser A. Melt-Spun, Cross-Section Modified Polycaprolactone Fibers for Use in Tendon and Ligament Tissue Engineering. Fibers. 2022; 10(3):23. https://doi.org/10.3390/fib10030023
Chicago/Turabian StyleBauer, Benedict, Caroline Emonts, Louisa Bonten, Rokaya Annan, Felix Merkord, Thomas Vad, Akram Idrissi, Thomas Gries, and Andreas Blaeser. 2022. "Melt-Spun, Cross-Section Modified Polycaprolactone Fibers for Use in Tendon and Ligament Tissue Engineering" Fibers 10, no. 3: 23. https://doi.org/10.3390/fib10030023
APA StyleBauer, B., Emonts, C., Bonten, L., Annan, R., Merkord, F., Vad, T., Idrissi, A., Gries, T., & Blaeser, A. (2022). Melt-Spun, Cross-Section Modified Polycaprolactone Fibers for Use in Tendon and Ligament Tissue Engineering. Fibers, 10(3), 23. https://doi.org/10.3390/fib10030023








