Influence of Laser Colour Marking on the Corrosion Properties of Low Alloyed Ti
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Samples
2.2. Microstructural Examination
2.3. Electrochemical Measurements
2.4. Surface Analysis
3. Results and Discussion
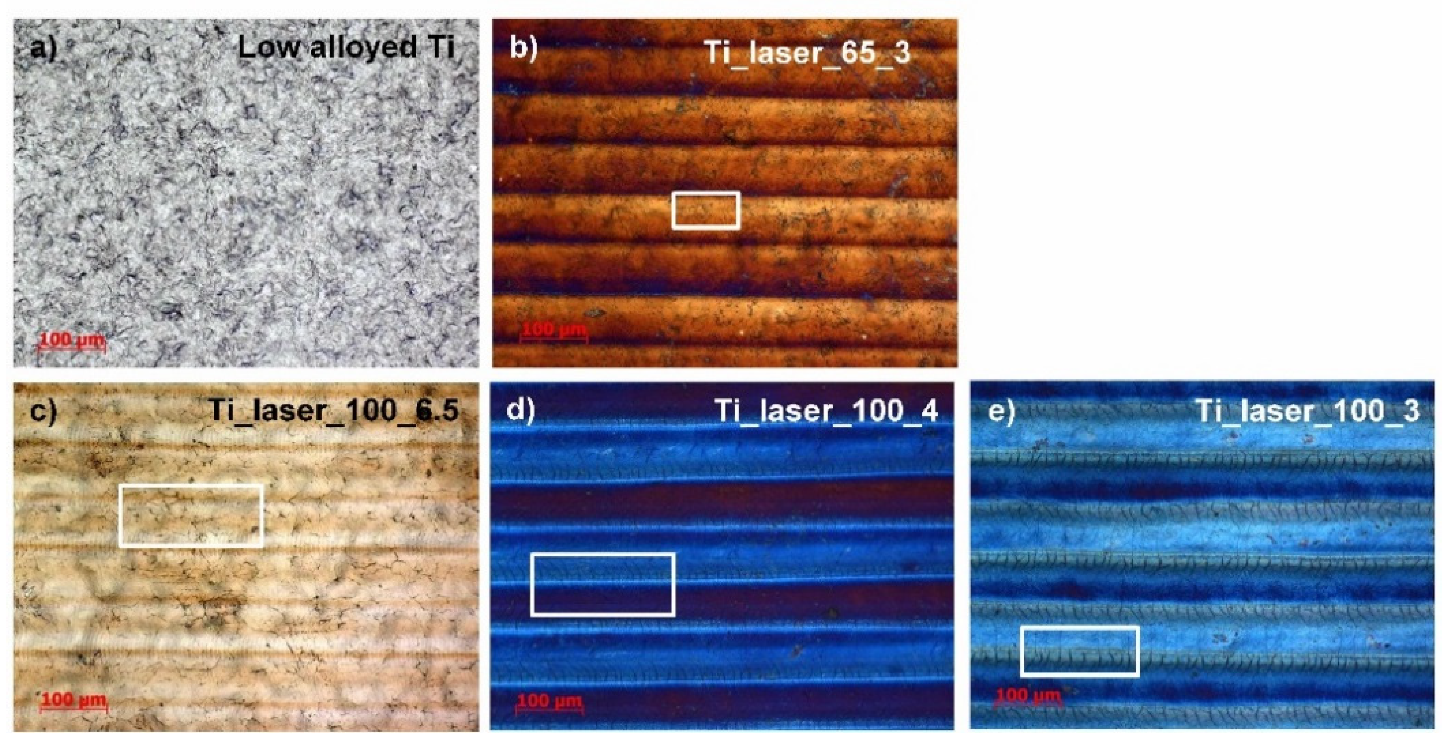
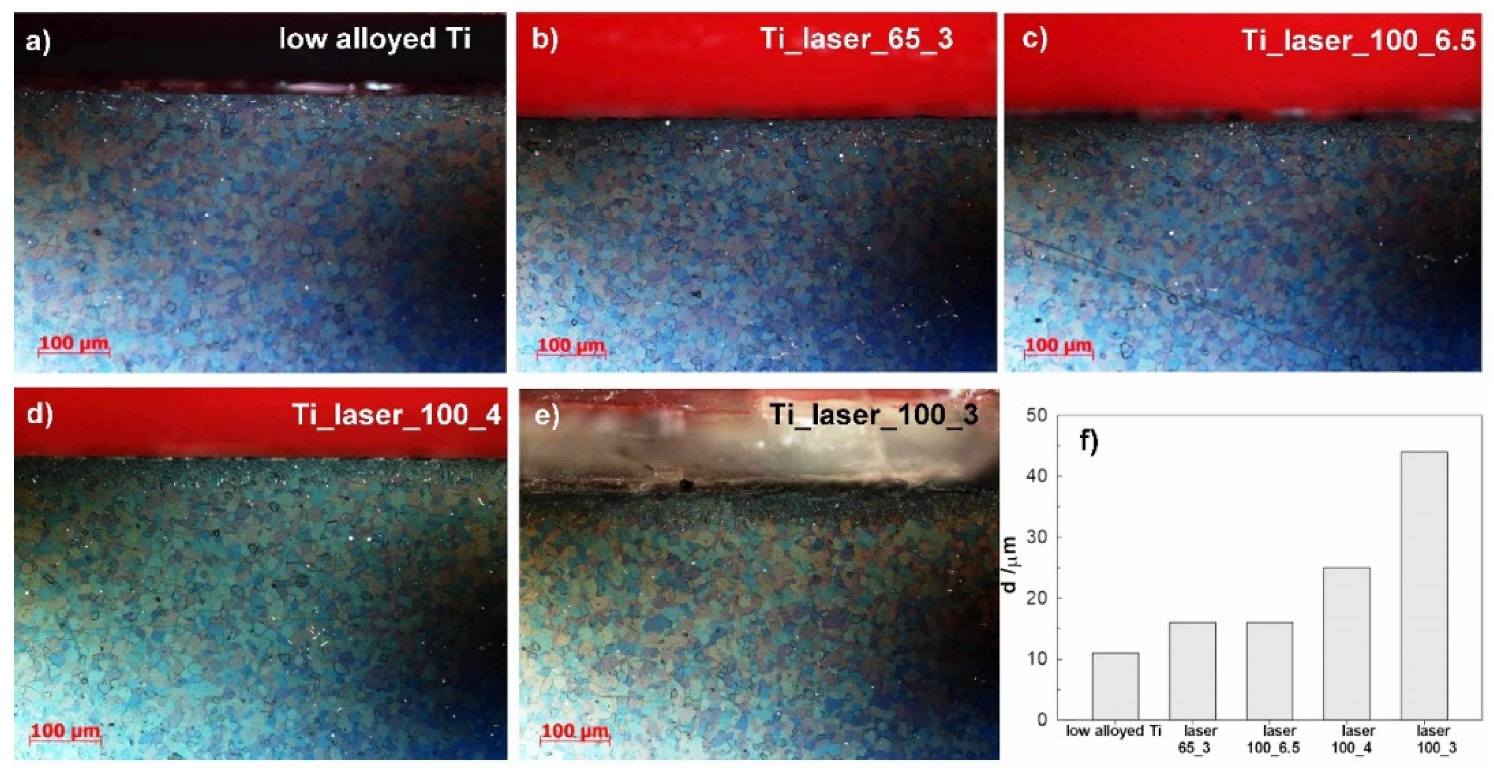
3.1. Light Mictroscopy and Microstructural Examination
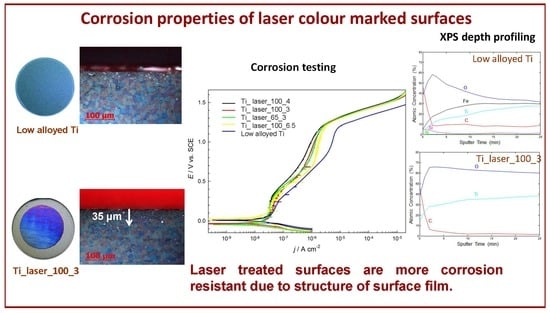
3.2. Electrochemical Measurements in 0.1 M NaCl Solution
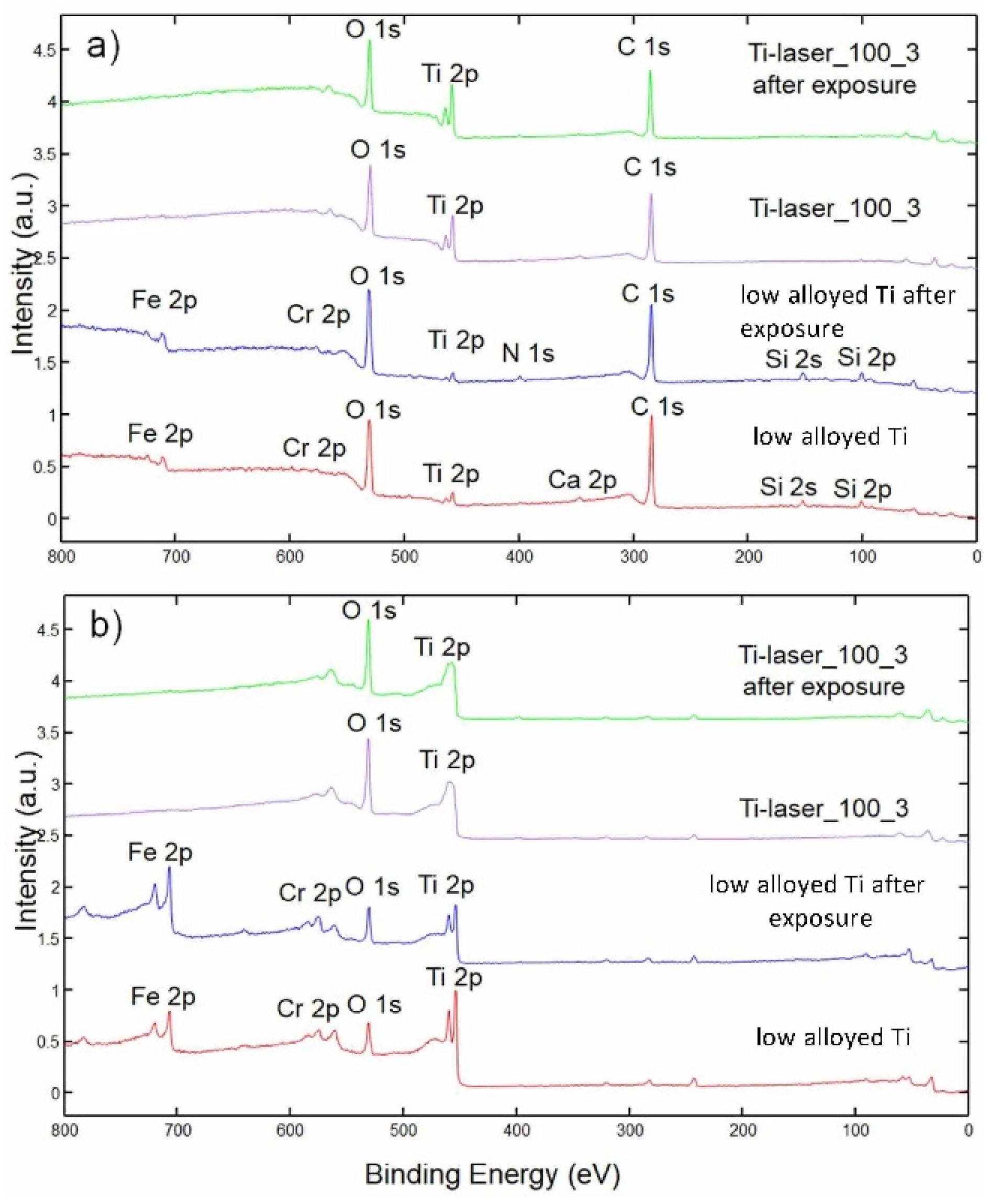
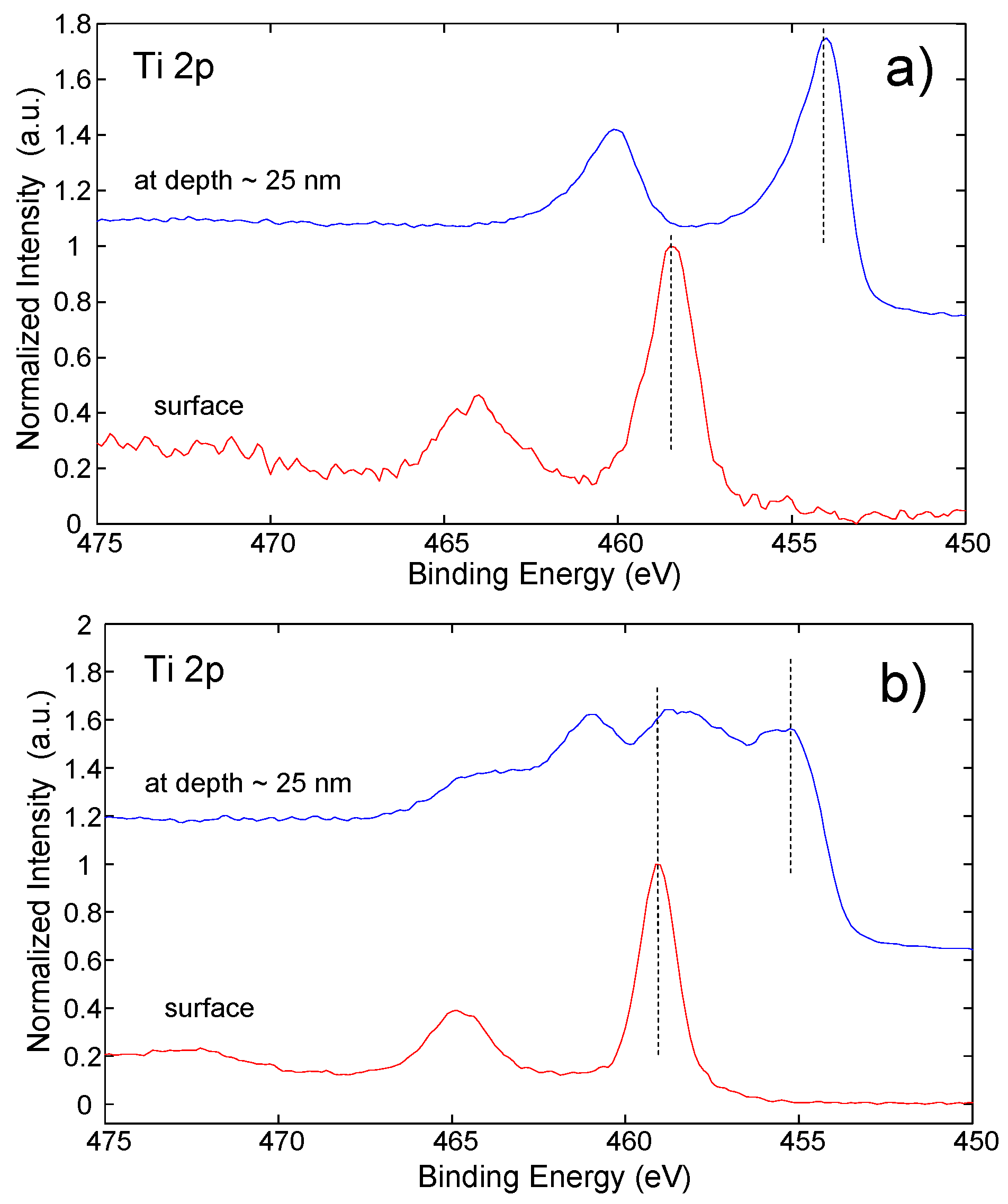
3.3. XPS Analysis
4. Conclusions
- The depth of microstructural changes in the cross-sections of laser treated surfaces was related to the laser power and production speed of the laser. It was the highest for 100% power and the lowest production speed of the laser.
- Corrosion properties were studied by potentiodynamic measurements and electrochemical impedance spectroscopy. It was found that laser treated surfaces exhibited lower current densities in the passive region, while the stability of such surfaces is enhanced in long-term exposure in an aggressive environment.
- XPS analysis showed that the low alloyed Ti surface was different from the laser marked surface.
- A mixture of Ti and Fe/Cr oxides of thickness of 10 nm was found on low alloyed Ti surfaces, however mainly pure TiO2 oxide layer was detected on laser marked surfaces. TiO2 oxide was thicker (25 nm) than those on laser non-treated surface (about 10 nm).
- Different constitution and thickness of oxide layer of low alloyed Ti surface (Cr and Fe presence in TiO2 surface film) and laser marked surfaces (thick TiO2) affected long-term corrosion susceptibility. Low alloyed Ti surfaces were less stable than laser marked surfaces.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ng, T.; Yeo, S. Aesthetic laser marking assessment using luminance ratios. Opt. Lasers Eng. 2001, 35, 177–186. [Google Scholar] [CrossRef]
- Zheng, H.; Lim, G.C. Laser-effected darkening in TPEs with TiO2 additives. Opt. Lasers Eng. 2004, 41, 791–800. [Google Scholar] [CrossRef]
- Liu, C.; Lu, Y.; Xiong, Y.; Zhang, Q.; Cao, Z. Recognition of laser-marked quick response codes on polypropylene surfaces. Polym. Degrad. Stab. 2018, 147, 115–122. [Google Scholar] [CrossRef]
- Zelenska, K.; Zelensky, S.E.; Poperenko, L.V.; Kanev, K.; Mizeikis, V.; Gnatyuk, V.A. Thermal mechanisms of laser marking in transparent polymers with light-absorbing microparticles. Opt. Laser Technol. 2016, 76, 96–100. [Google Scholar] [CrossRef]
- Fujimoto, S.; Tsujino, K.; Shibata, T. Growth and properties of Cr-rich thick and porous oxide films on Type 304 stainless steel formed by square wave potential pulse polarisation. Electrochim. Acta 2001, 47, 543–551. [Google Scholar] [CrossRef]
- Antonczak, A.J.; Stepak, B.; Koziol, P.E.; Abramski, K.M. The influence of process parameters on the laser-induced coloring of titanium. Appl. Phys. A 2014, 115, 1003–1013. [Google Scholar] [CrossRef]
- Del Pino, A.P.; Serra, P.; Morenza, J. Oxidation of titanium through Nd: YAG laser irradiation. Appl. Surf. Sci. 2002, 197, 887–890. [Google Scholar] [CrossRef]
- Diamanti, M.V.; Del Curto, B.; Pedeferri, M. Interference colors of thin oxide layers on titanium. Color Res. Appl. 2018, 33, 221–228. [Google Scholar] [CrossRef]
- Veiko, V.; Odintsova, G.; Gorbunova, E.; Ageev, E.; Shimko, A.; Karlagina, Y.; Andreeva, Y. Development of complete color palette based on spectrophotometric measurements of steel oxidation results for enhancement of color laser marking technology. Mater. Des. 2016, 89, 684–688. [Google Scholar] [CrossRef]
- Penide, J.; Quintero, F.; Riveiroa, A.; Fernández, A.; del Val, J.; Comesaña, R.; Lusquiños, F.; Pou, J. High contrast laser marking of alumina. Appl. Surf. Sci. 2015, 336, 118–128. [Google Scholar] [CrossRef]
- Durdu, S.; Usta, M.; Berkem, A.S. Bioactive coatings on Ti6Al4V alloy formed by plasma electrolytic oxidation. Surf. Coat. Technol. 2016, 301, 85–93. [Google Scholar] [CrossRef]
- O’Hana, S.; Pinkerton, A.J.; Shoba, K.; Gale, A.W.; Li, L. Laser surface colouring of titanium for contemporary jewellery. Surf. Eng. 2008, 24, 147–153. [Google Scholar] [CrossRef]
- Akman, E.; Cerkezoglu, E. Compositional and micro-scratch analyses of laser induced colored surface of titanium. Opt. Lasers Eng. 2016, 84, 37–43. [Google Scholar] [CrossRef]
- Amara, E.; Haid, F.; Noukaz, A. Experimental investigations on fiber laser color marking of steels. Appl. Surf. Sci. 2015, 351, 1–12. [Google Scholar] [CrossRef]
- Antończak, A.J.; Kocoń, D.; Nowak, M.; Kozioł, P.; Abramski, K.M. Laser-induced colour marking—Sensitivity scaling for a stainless steel. Appl. Surf. Sci. 2013, 264, 229–236. [Google Scholar] [CrossRef]
- Antończak, A.J.; Skowroński, Ł.; Trzcinski, M.; Kinzhybalo, V.V.; Łazarek, Ł.K.; Abramski, K.M. Laser-induced oxidation of titanium substrate: Analysis of the physicochemical structure of the surface and sub-surface layers. Appl. Surf. Sci. 2015, 325, 217–226. [Google Scholar] [CrossRef]
- Astarita, A.; Genna, S.; Leone, C.; Minutolo, F.M.C.; Squillace, A.; Velotti, C. Study of the laser marking process of cold sprayed titanium coatings on aluminium substrates. Opt. Laser Technol. 2016, 83, 168–176. [Google Scholar] [CrossRef]
- Qi, J.; Wang, K.; Zhu, Y. A study on the laser marking process of stainless steel. J. Mat. Process. Technol. 2003, 139, 273–276. [Google Scholar] [CrossRef]
- Salou, L.; Hoornaert, A.; Louarn, G.; Layrolle, P. Enhanced osseointegration of titanium implants with nanostructured surfaces: An experimental study in rabbits. Acta Biomater. 2015, 11, 494–502. [Google Scholar] [CrossRef]
- Veiko, V.; Karlagina, Y.; Moskvin, M.; Mikhailovskii, V.; Odintsova, G.; Olshin, P.; Pankin, D.; Romanov, V.; Yatsuk, R. Metal surface coloration by oxide periodic structures formed with nanosecond laser pulses. Opt. Lasers Eng. 2017, 96, 63–67. [Google Scholar] [CrossRef]
- Teng, K.; Delplancke, J.-L.; Zhang, J.; O’Keefe, T.J. Electrochemical characterization of copper deposited on plasma and thermally modified titanium surfaces. Metall. Mater. Trans. B 1998, 29, 749–754. [Google Scholar] [CrossRef]
- Wang, H.-Y.; Zhu, R.-F.; Lu, Y.-P.; Xiao, G.-Y.; Ma, X.-N.; Li, Y. Structures and properties of layered bioceramic coatings on pure titanium using a hybrid technique of sandblasting and micro-arc oxidation. Appl. Surf. Sci. 2013, 282, 271–280. [Google Scholar] [CrossRef]
- Zhao, B.; Zhao, B.H.; Zhang, W.; Wang, D.N.; Feng, W.; Liu, Y.; Lin, Z.; Du, K.Q.; Deng, C.F. Effect of Zn content on cytoactivity and bacteriostasis of micro-arc oxidation coatings on pure titanium. Surf. Coat. Technol. 2013, 228, S428–S432. [Google Scholar] [CrossRef]
- Diamanti, M.V.; Del Curto, B.; Masconale, V.; Pedeferri, M. Production and anodic colouring of newly-designed titanium jewels. Colour Des. Creat. 2010, 16, 1–9. [Google Scholar]
- Lavisse, L.; Grevey, D.; Langlade, C.; Vannes, B. The early stage of the laser-induced oxidation of titanium substrates. Appl. Surf. Sci. 2002, 186, 150–155. [Google Scholar] [CrossRef]
- Banerjee, D.; Williams, J. Perspectives on titanium science and technology. Acta Mater. 2013, 61, 844–879. [Google Scholar] [CrossRef]
- Li, Z.; Zheng, H.Y.; Teh, K.M.; Liu, Y.C.; Lim, G.C.; Seng, H.L.; Yakovlev, N.L. Analysis of oxide formation induced by UV laser coloration of stainless steel. Appl. Surf. Sci. 2009, 256, 1582–1588. [Google Scholar] [CrossRef]
- Kasman, S. Impact of parameters on the process response: A Taguchi orthogonal analysis for laser engraving. Measurement 2013, 46, 2577–2584. [Google Scholar] [CrossRef]
- Adams, D.; Murphy, R.D.; Saiz, D.J.; Hirschfeld, D.A.; Rodriguez, M.A.; Kotula, P.G.; Jared, B.H. Nanosecond pulsed laser irradiation of titanium: Oxide growth and effects on underlying metal. Surf. Coat. Technol. 2014, 248, 38–45. [Google Scholar] [CrossRef]
- Kung, K.-C.; Lee, T.-M.; Lui, T.-S. Bioactivity and corrosion properties of novel coatings containing strontium by micro-arc oxidation. J. Alloys Compd. 2010, 508, 384–390. [Google Scholar] [CrossRef]
- Dong, H.; Li, X.Y. Oxygen boost diffusion for the deep-case hardening of titanium alloys. Mat. Sci. Eng. A 2000, 280, 303–310. [Google Scholar] [CrossRef]
- Valete, S.; Steyer, P.; Richard, L.; Forest, B.; Donnet, C.; Audoarrd, E. Influence of femtosecond laser marking on the corrosion resistance of stainless steels. Appl. Surf. Sci. 2006, 25, 4696–4701. [Google Scholar] [CrossRef]
- Calvert, K.; Kosaka, Y. Evaluation of titanium alloys after High temperature air exposure. In Proceedings of the 13th World Conference on Titanium, San Diego, CA, USA, 16–20 August 2015; The Minerals, Metals & Materials Society: Pittsburgh, PA, USA, 2016. [Google Scholar]
- Tait, W.S. An Introduction to Electrochemical Corrosion Testing for Practicing Engineers and Scientists; Ohio State University: Columbus, OH, USA, 1994. [Google Scholar]
- Pan, J.; Thierry, D.; Leygraf, C. Electrochemical impedance spectroscopy study of passive oxide film on titanium for implant application. Electrochim. Acta 1996, 41, 1143–1153. [Google Scholar] [CrossRef]
- Raistrick, I.D.; MacDonald, J.R.; Francschetti, D.R. Impedance Spectroscopy Emphasizing Solid Materials and Systems; MacDonald, J.R., Ed.; John Wiley & Sons: New York, NY, USA, 1987. [Google Scholar]
- Kek-Merl, D.; Lappalainen, J.; Tuller, H.L. Electrical properties of nanocrystalline CeO2 thin films deposited by in situ pulsed laser deposition. J. Electrochem. Soc. 2006, 153, J15–J20. [Google Scholar] [CrossRef]
- Hashimonto, S.; Tanaka, A. Alteration of Ti 2p XPS spectrum for titanium oxide by low-energy Ar ion bombardment. Surf. Interface Anal. 2002, 34, 262–265. [Google Scholar] [CrossRef]




| Element | Si | Al | Cr | Fe | Ti |
|---|---|---|---|---|---|
| Content (wt %) | 0.48 | 0.4 | 1.27 | 2.81 | balance |
| Sample | Speed [%] | Speed [mm/s] | Average Power [%] | Average Power [W] | PRR [kHz] | Observed Colour |
|---|---|---|---|---|---|---|
| (a) Low alloyed Ti | – | – | – | – | – | Natural grey |
| (b) Ti_laser_65-3 | 3 | 60 | 65 | 19.5 | 35 | Brown |
| (c) Ti_laser_100-6.5 | 6.5 | 130 | 100 | 30 | Yellow | |
| (d) Ti_laser_100-4 | 4 | 80 | 100 | 30 | Light blue | |
| (e) Ti_laser_100-3 | 3 | 60 | 100 | 30 | Blue |
| Sample | Ecorr/V | jcorr */nA·cm−2 | Eb/V | ΔE/V |
|---|---|---|---|---|
| Low alloyed Ti | 0.011 | 27.0 ± 2.2 | 1.17 | 1.16 |
| Ti Laser 100_6.5 | 0.030 | 47.9 ± 1.0 | 1.22 | 1.19 |
| Ti Laser 100_4 | 0.009 | 16.1 ± 0.6 | 1.22 | 1.21 |
| Ti Laser 100_3 | −0.033 | 28.5 ± 0.5 | 1.22 | 1.25 |
| Ti Laser 65_3 | −0.044 | 29 ± 9 | 1.22 | 1.27 |
| Sample | Re | Q1 | C1 | n1 | R1 | Q2 | C2 | n2 | R2 | Chi2 |
|---|---|---|---|---|---|---|---|---|---|---|
| low alloyed Ti | 87 | 1.86 × 10−5 | 13.6 × 10−6 | 0.941 | 310 | 6.887 × 10−6 | 31.7 × 10−6 | 0.749 | 1.38 × 107 | 0.0007 |
| Laser 65_3 | 29 | 2.38 × 10−5 | 12.6 × 10−6 | 0.904 | 1043 | 3.028 × 10−6 | 4.76 × 10−6 | 0.892 | 1.38 × 107 | 0.003 |
| Laser 100_6.5 | 13 | 1.31 × 10−5 | 13.1 × 10−6 | 1 | 541 | 1.108 × 10−5 | 39.4 × 10−6 | 0.754 | 4.4 × 106 | 0.0008 |
| Laser 100_4 | 30 | 2.09 × 10−5 | 15.2 × 10−6 | 0.929 | 701 | 1.97 × 10−6 | 65.0 × 10−6 | 0.5 | 1.67 × 107 | 0.002 |
| laser 100_3 | 15 | 1.02 × 10−6 | 10.2 × 10−6 | 1 | 552 | 1.24 × 10−5 | 63.9 × 10−6 | 0.779 | 1.31 × 107 | 0.00057 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kosec, T.; Legat, A.; Kovač, J.; Klobčar, D. Influence of Laser Colour Marking on the Corrosion Properties of Low Alloyed Ti. Coatings 2019, 9, 375. https://doi.org/10.3390/coatings9060375
Kosec T, Legat A, Kovač J, Klobčar D. Influence of Laser Colour Marking on the Corrosion Properties of Low Alloyed Ti. Coatings. 2019; 9(6):375. https://doi.org/10.3390/coatings9060375
Chicago/Turabian StyleKosec, Tadeja, Andraž Legat, Janez Kovač, and Damjan Klobčar. 2019. "Influence of Laser Colour Marking on the Corrosion Properties of Low Alloyed Ti" Coatings 9, no. 6: 375. https://doi.org/10.3390/coatings9060375
APA StyleKosec, T., Legat, A., Kovač, J., & Klobčar, D. (2019). Influence of Laser Colour Marking on the Corrosion Properties of Low Alloyed Ti. Coatings, 9(6), 375. https://doi.org/10.3390/coatings9060375