Zinc Doped Hydroxyapatite Thin Films Prepared by Sol–Gel Spin Coating Procedure
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Thin Layer of Zinc Doped Hydroxyapatite
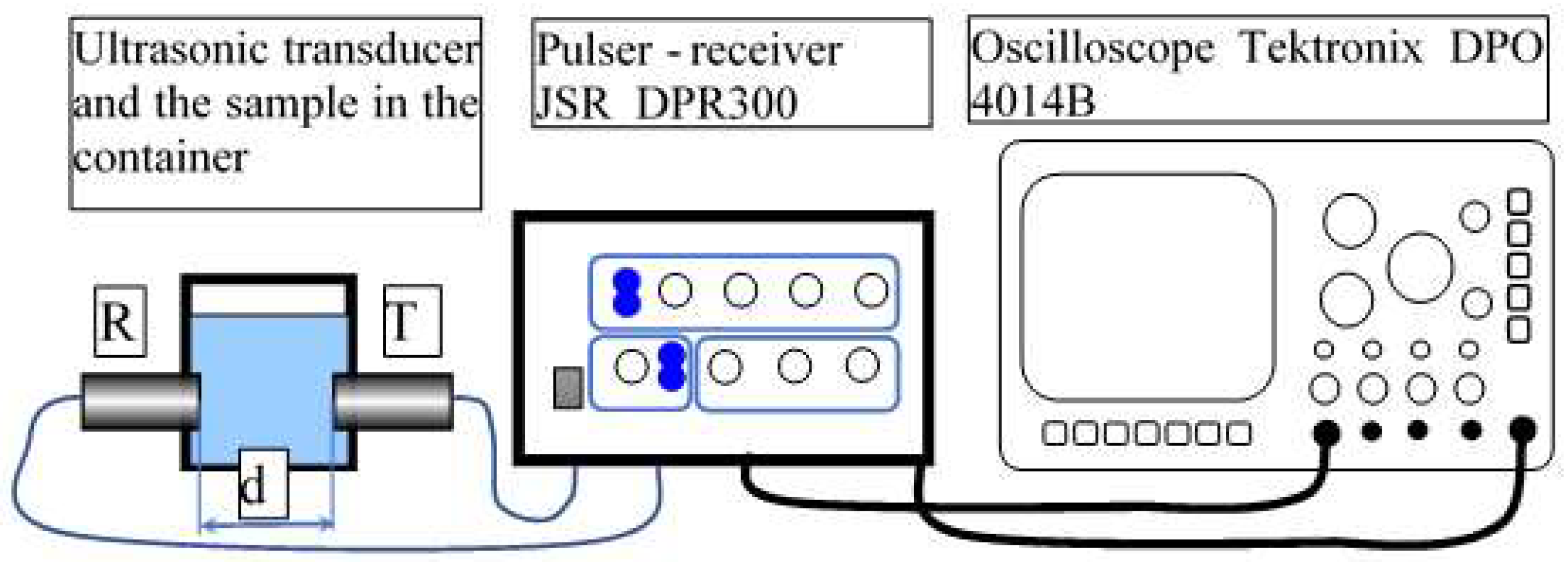
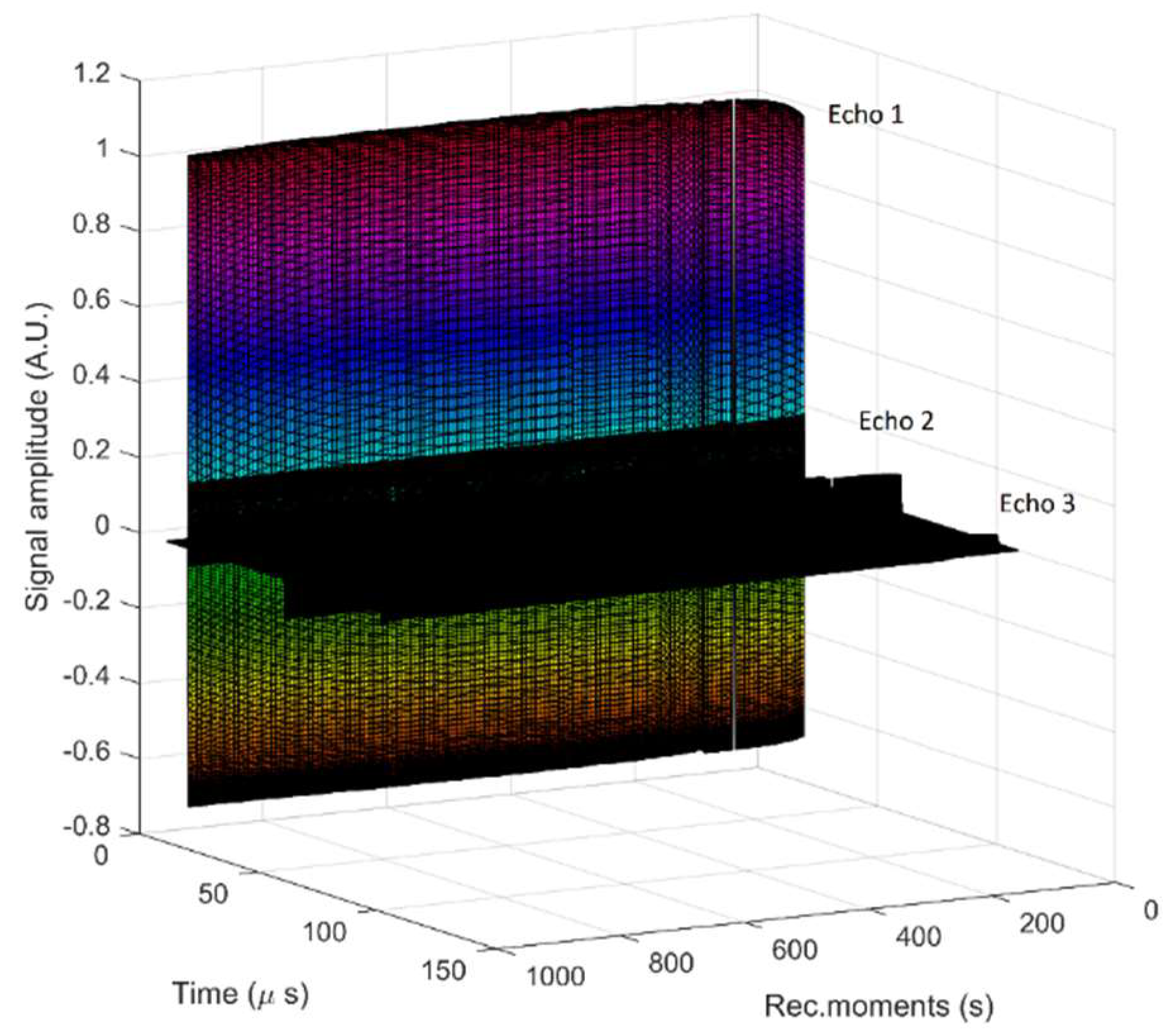
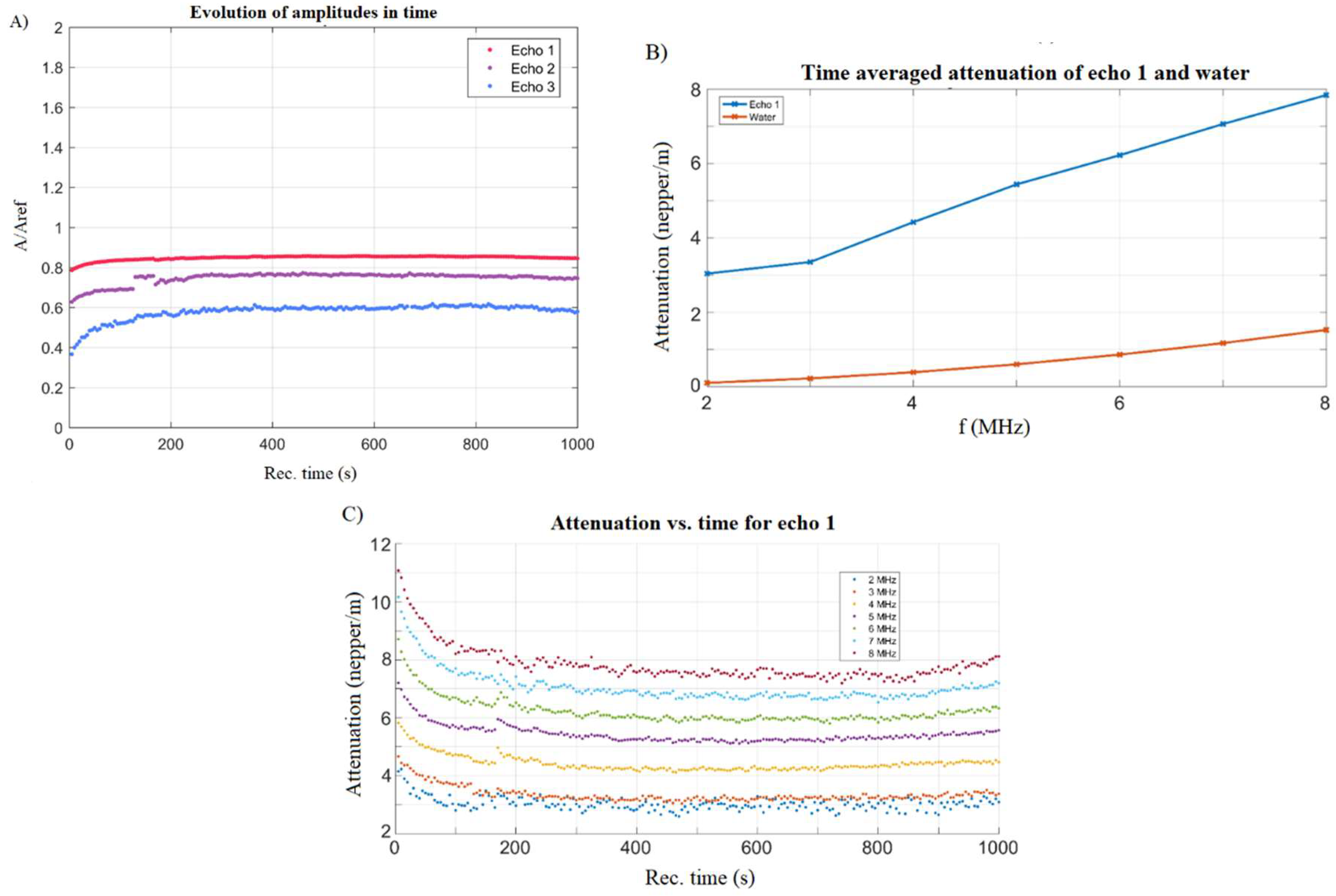
2.3. Characterization Methods
2.4. In Vitro Antifungal Activity
2.5. Hela Cell Viability Assays
2.6. Statistical Analysis
3. Results and Discussions
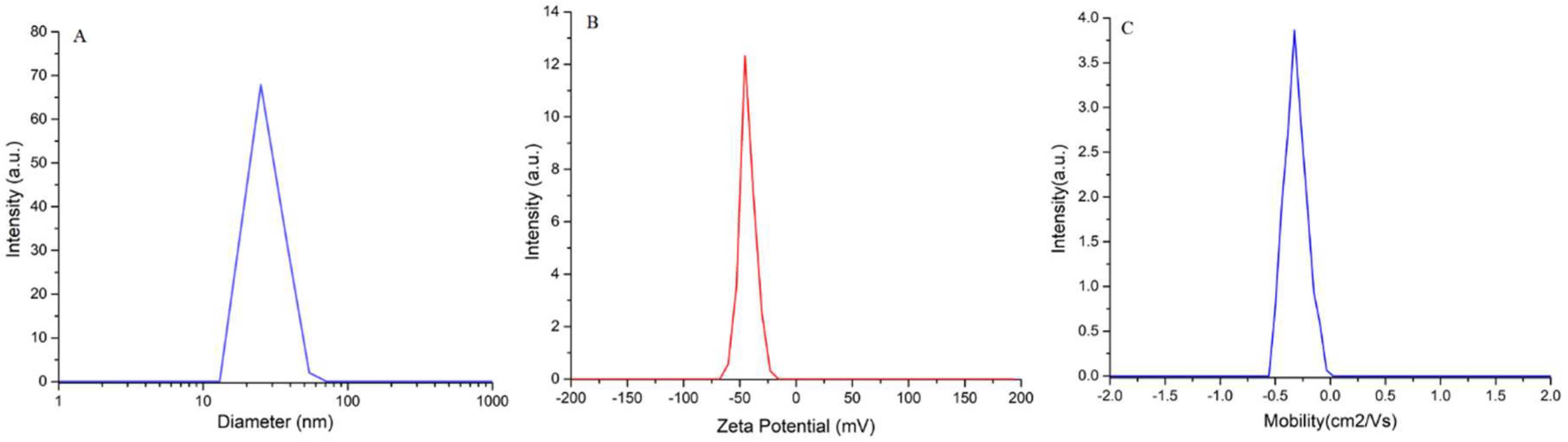
3.1. Characterization of Suspension
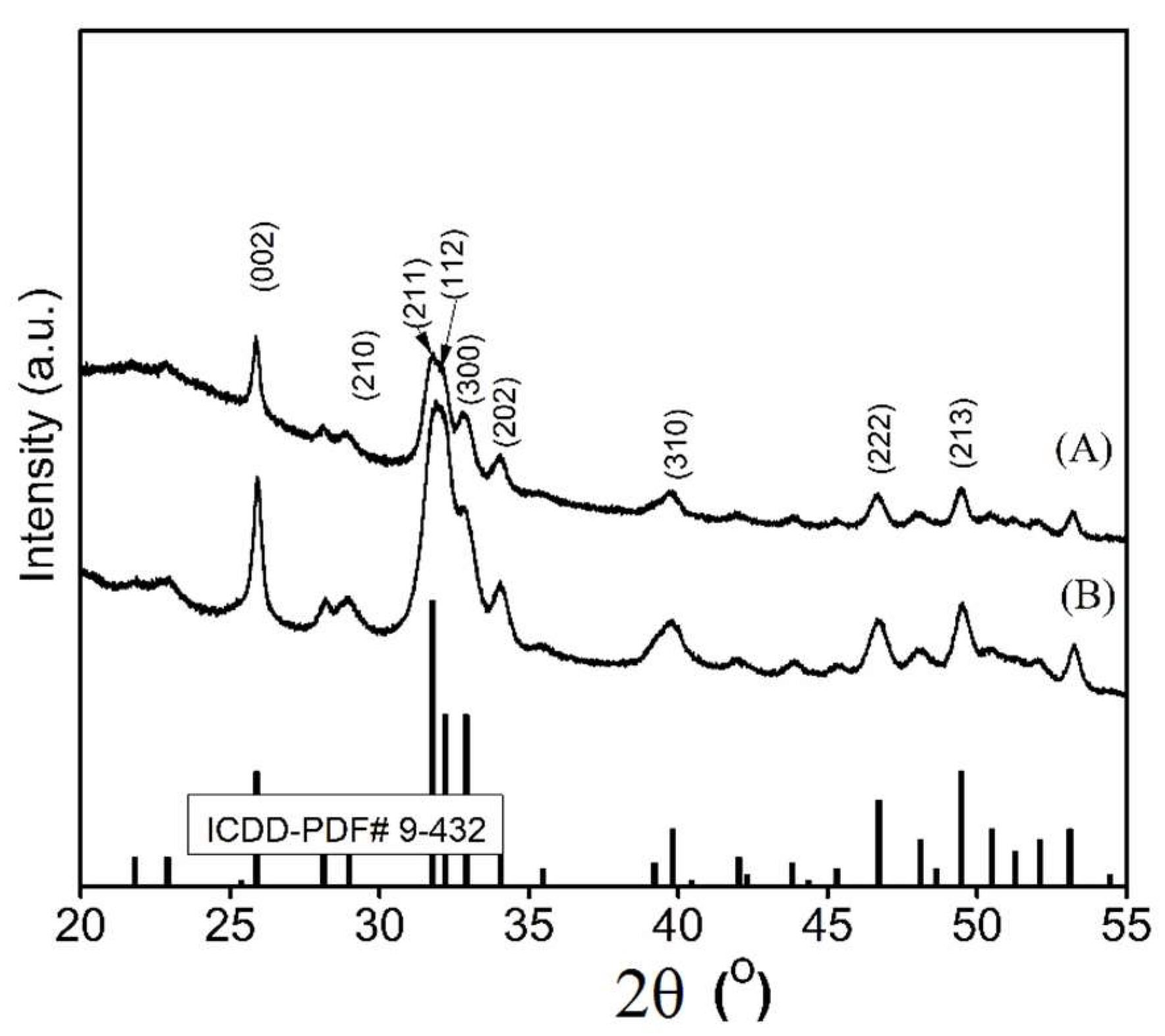
3.2. Structure and Morphology of the Layers
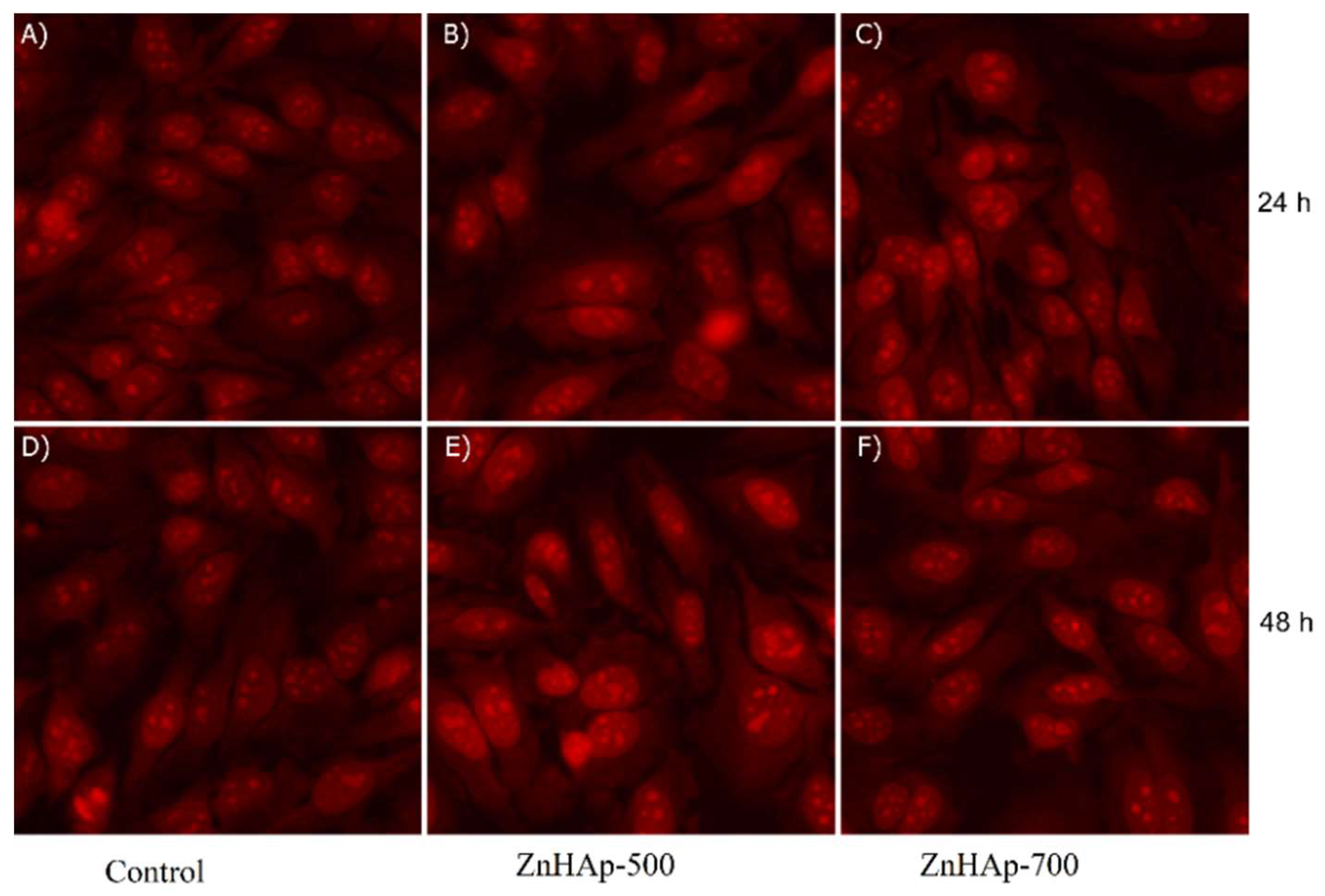
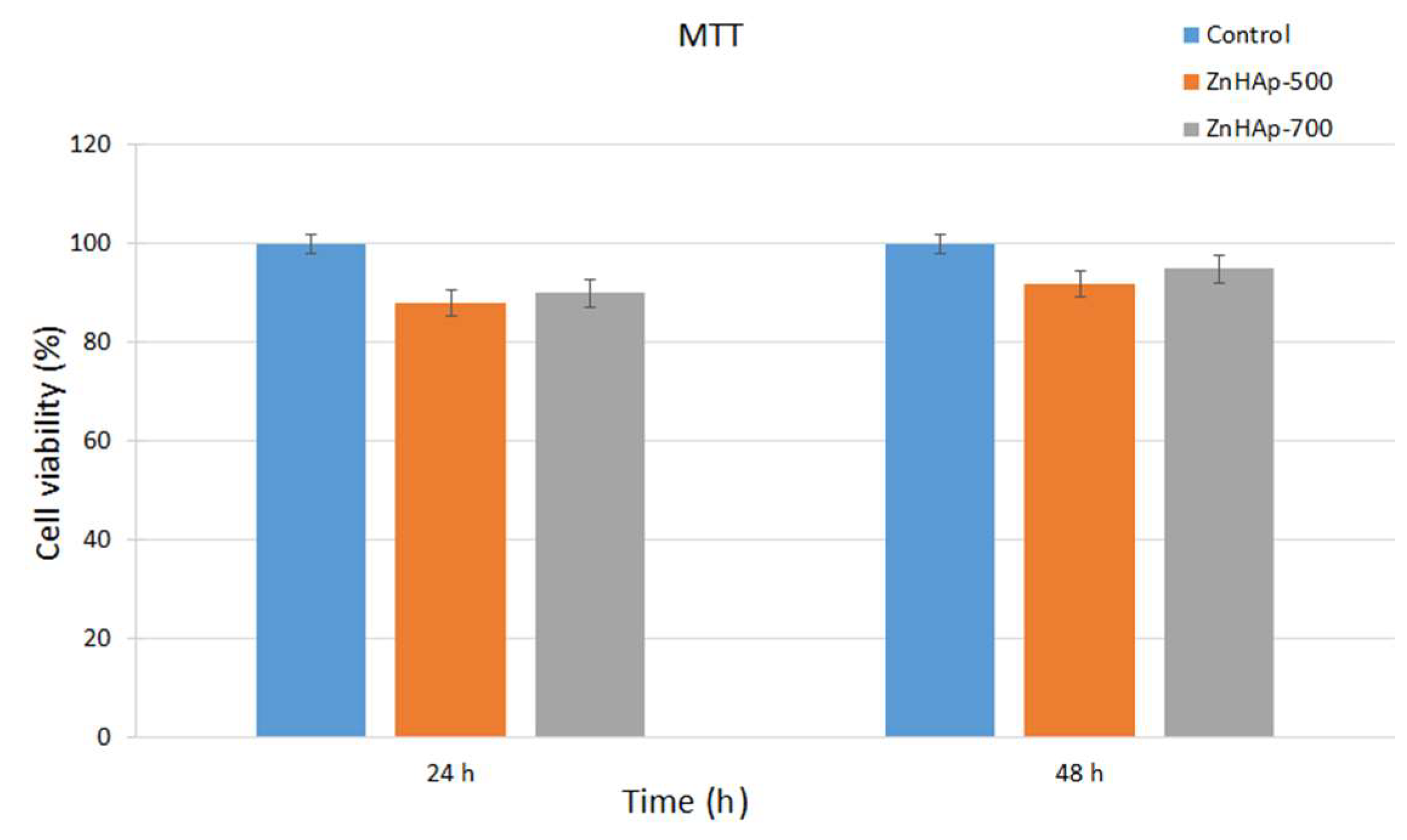
3.3. Cell Viability
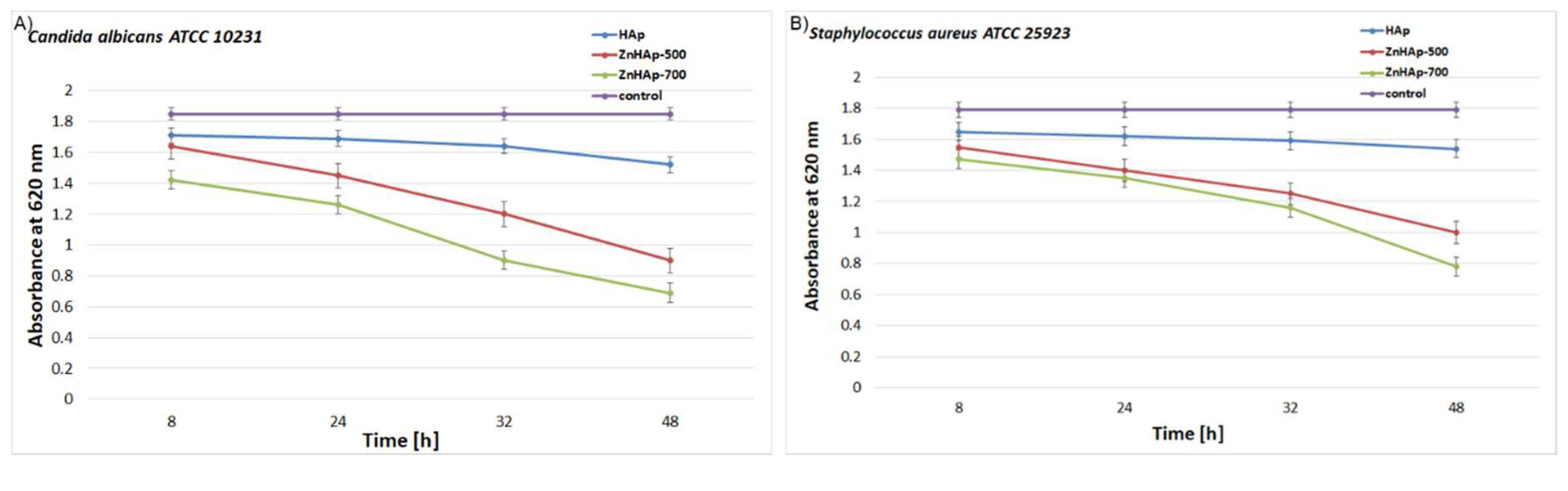
3.4. Antimicrobial Activity
3.5. Discussions
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Darouiche, R.O. Current concepts—Treatment of infections associated with surgical implants. N. Engl. J. Med. 2004, 350, 1422–1429. [Google Scholar] [CrossRef] [PubMed]
- Vallet-Regi, M.; Arcos, D. Biomimetic Nanoceramics in Clinical Use: From Materials to Applications, 1st ed.; Royal Society of Chemistry: Cambridge, UK, 2008. [Google Scholar]
- Prodan, A.M.; Beuran, M.; Turculet, C.S.; Popa, M.; Andronescu, E.; Bleotu, C.; Raita, S.M.; Soare, M.; Lupescu, O. In vitro evaluation of glycerol coated iron oxide nanoparticles in solution. Rom. Biotechnol. Lett. 2018, 23, 13901–13908. [Google Scholar]
- Kojic, E.M.; Darouiche, R.O. Candida infections of medical devices. Clin. Microbiol. Rev. 2004, 17, 255–267. [Google Scholar] [CrossRef] [PubMed]
- Chandra, J.; Guangyin, Z.; Ghannoum, M.A. Fungal biofilms and antimycotics. Curr. Drug Targets 2005, 6, 887–894. [Google Scholar] [CrossRef] [PubMed]
- Von Eiff, C.; Jansen, B.; Kohnen, W. Infections associated with medical devices: Pathogenesis, management and prophylaxis. Drugs 2005, 65, 179–214. [Google Scholar] [CrossRef] [PubMed]
- Predoi, D.; Iconaru, S.L.; Buton, N.; Badea, M.L.; Marutescu, L. Antimicrobial activity of new materials based on lavender and basil essential oils and hydroxyapatite. Nanomaterials 2018, 8, 291. [Google Scholar] [CrossRef] [PubMed]
- Furko, M.; Jiang, Y.; Wilkins, T.; Balázsi, C. Development and characterization of silver and zinc doped bioceramic layer on metallic implant materials for orthopedic application. Ceram. Int. 2016, 42, 4924–4931. [Google Scholar] [CrossRef]
- Bosetti, M.; Massé, A.; Tobin, E.; Cannas, M. Silver coated materials for external fixation devices: In vitro biocompatibility and genotoxicity. Biomaterials 2002, 23, 887–892. [Google Scholar] [CrossRef]
- Jung, W.K.; Koo, H.C.; Kim, K.W.; Shin, S.; Kim, S.H.; Park, Y.H. Antibacterial activity and mechanism of action of the silver ion in Staphylococcus aureus and Escherichia coli. Appl. Environ. Microbiol. 2008, 74, 2171–2178. [Google Scholar] [CrossRef] [PubMed]
- Schierholz, J.M.; Lucasj, L.J.; Rump, A. Efficacy of silver-coated medical devices. J. Hosp. Infect. 1998, 40, 257–262. [Google Scholar] [CrossRef]
- Huang, Y.; Zhang, X.; Qiao, H.; Hao, M.; Zhang, H.; Xu, Z.; Zhang, X.; Pang, X.; Lin, H. Corrosion resistance and cytocompatibility studies of zinc-doped fluorohydroxyapatite nanocomposite coatings on titanium implant. Ceram. Int. 2016, 42, 1903–1915. [Google Scholar] [CrossRef]
- Kolmas, J.; Groszyk, E.; Kwiatkowska-Róhycka, D. Substituted hydroxyapatites with antibacterial properties. BioMed Res. Int. 2014, 2014, 178123. [Google Scholar] [CrossRef] [PubMed]
- Kurtjak, M.; Aničić, N.; Vukomanovicć, M. Inorganic nanoparticles: Innovative tools for antimicrobial agents. In Antibacterial Agents; Kumavath, R.N., Ed.; IntechOpen: London, UK, 2017. [Google Scholar]
- Liedtke, J.; Vahjen, W. In vitro antibacterial activity of zinc oxide on a broad range of reference strains of intestinal origin. Vet. Microbiol. 2012, 160, 251–255. [Google Scholar] [CrossRef] [PubMed]
- Iconaru, S.L.; Prodan, A.M.; Turculet, C.S.; Beuran, M.; Ghita, R.V.; Costescu, A.; Groza, A.; Chifiriuc, M.C.; Chapon, P.; Gaiaschi, S.; et al. Enamel based composite layers deposited on titanium substrate with antifungal activity. J. Spectrosc. 2016, 2016, 4361051. [Google Scholar] [CrossRef]
- Groza, A.; Ciobanu, C.S.; Popa, C.L.; Iconaru, S.L.; Chapon, P.; Luculescu, C.; Ganciu, M.; Predoi, D. Structural properties and antifungal activity against Candida albicans biofilm of different composite layers based on Ag/Zn doped hydroxyapatite-polydimethylsiloxanes. Polymers 2016, 8, 131. [Google Scholar] [CrossRef]
- Ciobanu, C.S.; Massuyeau, F.; Constantin, L.V.; Predoi, D. Structural and physical properties of antibacterial Ag-doped nano-hydroxyapatite synthesized at 100 °C. Nanoscale Res. Lett. 2011, 6, 613. [Google Scholar] [CrossRef] [PubMed]
- Predoi, D.; Iconaru, S.L.; Deniaud, A.; Chevallet, M.; Michaud-Soret, I.; Buton, N.; Prodan, A.M. Textural, structural and biological evaluation of hydroxyapatite doped with zinc at low concentrations. Materials 2017, 10, 229. [Google Scholar] [CrossRef] [PubMed]
- Popa, C.L.; Deniaud, A.; Michaud-Soret, I.; Guégan, R.; Motelica-Heino, M.; Predoi, D. Structural and biological assessment of zinc doped hydroxyapatite nanoparticles. J. Nanomater. 2016, 2016, 1062878. [Google Scholar] [CrossRef]
- Ciobanu, C.S.; Popa, C.L.; Predoi, D. Sm:HAp Nanopowders present antibacterial activity against Enterococcus faecalis. J. Nanomater. 2014, 2014, 780686. [Google Scholar] [CrossRef]
- Zebarjad, S.M.; Sajjadi, S.A.; Ebrahimi Sdrabadi, T.; Sajjadi, S.A.; Yaghmaei, A.; Naderi, B. A Study on mechanical properties of PMMA/hydroxyapatite nanocomposite. Engineering 2011, 3, 795–801. [Google Scholar] [CrossRef]
- Prasadh, S.; Wong, R.C.W. Unraveling the mechanical strength of biomaterials used as a bone scaffold in oral and maxillofacial defects. Oral Sci. Int. 2018, 15, 48–55. [Google Scholar] [CrossRef]
- Predoi, D.; Iconaru, S.L.; Predoi, M.V. Bioceramic layers with antifungal properties. Coatings 2018, 8, 276. [Google Scholar] [CrossRef]
- Turculet, C.S.; Prodan, A.M.; Negoi, I.; Teleanu, G.; Popa, M.; Andronescu, E.; Beuran, M.; Stanciu, G.A.; Hristu, R.; Badea, M.L.; et al. Preliminary evaluation of the antifungal activity of samarium doped hydroxyapatite thin films. Rom. Biotechnol. Lett. 2018, 23, 13928–13932. [Google Scholar]
- Andronescu, E.; Iordache, F.; Ciobanu, C.S.; Badea, M.L.; Costescu, A.; Prodan, A. Optical properties of bioactive europium doped hydroxyapatite (HAp:Eu3+). Optoelectron. Adv. Mat. 2015, 9, 1155–1159. [Google Scholar]
- Negrila, C.C.; Predoi, M.V.; Iconaru, S.L.; Predoi, D. Development of Zinc-doped hydroxyapatite by sol–gel method for medical applications. Molecules 2018, 23, 2986. [Google Scholar] [CrossRef] [PubMed]
- Johal, K.K. In vivo response of strontium and zinc-based ionomeric cement implants in bone. J. Mater. Sci. Mater. Med. 2002, 13, 375–379. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, M. Role of zinc in bone formation and bone resorption. J. Trace Elem. Exp. Med. 1998, 11, 119–135. [Google Scholar] [CrossRef]
- Ohtsu, N.; Kakuchi, Y.; Ohtsuki, T. Antibacterial effect of zinc oxide/hydroxyapatite coatings prepared by chemical solution deposition. Appl. Surf. Sci. 2018, 445, 596–600. [Google Scholar] [CrossRef]
- Iconaru, S.L.; Prodan, A.M.; Buton, N.; Predoi, D. Structural characterization and antifungal studies of zinc-doped hydroxyapatite coatings. Molecules 2017, 22, 604. [Google Scholar] [CrossRef] [PubMed]
- Thian, E.S.; Konishi, T.; Kawanobe, Y.; Lim, P.N.; Choong, C.; Ho, B.; Aizawa, M. Zinc-substituted hydroxyapatite: A biomaterial with enhanced bioactivity and antibacterial properties. J. Mater. Sci. Mater. Med. 2013, 24, 437–445. [Google Scholar] [CrossRef] [PubMed]
- Radovanović, Ž.; Veljović, D.; Jokić, B.; Dimitrijević, S.; Bogdanović, G.; Kojić, V.; Petrović, R.; Janaćković, D. Biocompatibility and antimicrobial activity of zinc(II)-doped hydroxyapatite, synthesized by a hydrothermal method. J. Serb. Chem. Soc. 2012, 77, 1787–1798. [Google Scholar] [CrossRef]
- Adamiak, B.; Wiatrowski, A.; Domaradzki, J.; Kaczmarek, D.; Wojcieszak, D.; Mazur, M. Preparation of multicomponent thin films by magnetron co-sputtering method: The Cu–Ti case study. Vacuum 2019, 161, 419–428. [Google Scholar] [CrossRef]
- Rajendra Kumar, R.T.; Karunagaran, B.; Senthil Kumar, V.; Jeyachandran, Y.L.; Mangalaraj, D.; Narayandass, S.K. Structural properties of V2O5 thin films prepared by vacuum evaporation. Mater. Sci. Semicond. Process. 2003, 6, 543. [Google Scholar] [CrossRef]
- Wang, X.; Shi, F.; Gao, X.; Fan, C.; Huang, W.; Feng, X. A sol–gel dip/spin coating method to prepare titanium oxide films. Thin Solid Films 2013, 548, 34–39. [Google Scholar] [CrossRef]
- Calnan, S.; Upadhyaya, M.H.; Dann, E.S.; Thwaites, J.M.; Tiwari, N.A. Effects of target bias voltage on indium tin oxide films deposited by high target utilisation sputtering. Thin Solid Films 2007, 515, 8500–8504. [Google Scholar] [CrossRef]
- Yuen, C.; Yu, S.F.; Leong, E.S.P.; Lau, S.P.; Pita, K.; Yang, H.Y.; Chen, T.P. Room temperature deposition of p-type arsenic doped ZnO polycrystalline films by laser-assist filtered cathodic vacuum arc technique. J. Appl. Phys. 2007, 101, 094905. [Google Scholar] [CrossRef]
- Gurav, K.V.; Patil, U.M.; Shin, S.W.; Pawar, S.M.; Kim, J.H.; Lokhande, C.D. Morphology evolution of ZnO thin films from aqueous solutions and their application to liquefied petroleum gas (LPG) sensor. J. Alloy. Compd. 2012, 525, 1–7. [Google Scholar] [CrossRef]
- Jamesh, M.; Kumar, S.; Sankara Narayanan, T.S.N. Electrodeposition of hydroxyapatite coating on magnesium for biomedical applications. J. Coat. Technol. Res. 2012, 9, 495–502. [Google Scholar] [CrossRef]
- Hornberger, H.; Virtanen, S.; Boccaccini, A.R. Biomedical coatings on magnesium alloys—A review. Acta Biomater. 2012, 8, 2442–2455. [Google Scholar] [CrossRef] [PubMed]
- Wen, C.E.; Xu, W.; Hu, W.Y.; Hodgson, P.D. Hydroxyapatite/titania sol–gel coatings on titanium–zirconium alloy for biomedical applications. Acta Biomater. 2007, 3, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Kakimoto, M.-A.; Suzuki, M.-A.; Konishi, T.; Imai, Y.; Iwamoto, M.; Hino, T. Preparation of mono- and multilayer films of aromatic polyimides using Langmuir-Blodgett technique. Chem. Lett. 1986, 15, 823–826. [Google Scholar] [CrossRef]
- Tsay, C.Y.; Fan, K.S.; Wang, Y.W.; Chang, C.J.; Tseng, Y.K.; Lin, C.K. Transparent semiconductor zinc oxide thin films deposited on glass substrates by sol-gel process. Cermic. Int. 2010, 36, 1791–1795. [Google Scholar] [CrossRef]
- Emslie, A.G.; Bonner, F.T.; Peck, L.G. Flow of a viscous liquid on a rotating disk. J. Appl. Phys. 1958, 29, 858–862. [Google Scholar] [CrossRef]
- Motelica-Heino, M.; Donard, O.F. Comparison of UV and IR laser ablation ICP-MS on silicate reference materials and implementation of normalisation factors for quantitative measurements. Geostand. Geoanal. Res. 2001, 25, 345–359. [Google Scholar] [CrossRef]
- ASTM E2149–13a Standard Test Method for Determining the Antimicrobial Activity of Antimicrobial Agents under Dynamic Contact Conditions; ASTM International: West Conshohocken, PA, USA, 2013.
- Fuchs, A.V.; Ritz, S.; Pütz, S.; Mailänder, V.; Landfester, K.; Ziener, U. Bioinspired phosphorylcholine containing polymer films with silver nanoparticles combining antifouling and antibacterial properties. Biomater. Sci. 2013, 1, 470–477. [Google Scholar] [CrossRef]
- Ciobanu, C.S.; Iconaru, S.L.; Gyorgy, E.; Radu, M.; Costache, M.; Dinischiotu, A.; Le Coustumer, P.; Lafdi, K.; Predoi, D. Biomedical properties and preparation of iron oxide-dextran nanostructures by MAPLE technique. Chem. Cent. J. 2012, 6, 17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Egelhaaf, S.U.; Wehrli, E.; Muller, M.; Adrian, M.; Schurtenberger, P. Determination of the size distribution of lecithin liposomes: A comparative study using freeze fracture, cryoelectron microscopy and dynamic light scattering. J. Microsc. 1996, 184, 214–228. [Google Scholar] [CrossRef]
- Xu, G.; Aksay, I.A.; Groves, J.T. Continuous crystalline carbonate apatite thin films. A Biomimetic approach. J. Am. Chem. Soc. 2001, 123, 2196–2203. [Google Scholar] [CrossRef] [PubMed]
- Jokanovic, V.; Uskokovic, D. Calcium hydroxyapatite thin films on titanium substrates prepared by ultrasonic spray pyrolysis. Mater. Trans. 2005, 46, 228–235. [Google Scholar] [CrossRef]
- Anwar, A.; Akbar, S.; Sadiqa, A.; Kazmi, M. Novel continuous flow synthesis, characterization and antibacterial studies of nanoscale zinc substituted hydroxyapatite bioceramics. Inorg. Chim. Acta 2016, 453, 16–22. [Google Scholar] [CrossRef]
- Chen, X.; Tang, Q.L.; Zhu, Y.J.; Zhu, C.L.; Feng, X.P. Synthesis and antibacterial property of zinc loaded hydroxyapatite nanorods. Mater. Lett. 2012, 89, 233–235. [Google Scholar] [CrossRef]
- Ren, F.; Xin, R.; Ge, X.; Leng, Y. Characterization and structural analysis of zinc-substituted hydroxyapatites. Acta Biomater. 2009, 5, 3141–3149. [Google Scholar] [CrossRef] [PubMed]
- Ben-Nissan, B.; Choi, A.H. Sol–gel production of bioactive nano-coatings for medical applications. Part 1: An introduction. Nanomedicine 2006, 1, 311–319. [Google Scholar] [CrossRef] [PubMed]
- Fahami, A.; Beall, G.W.; Betancourt, T. Synthesis, bioactivity and zeta potential investigations of chlorine and fluorine substituted hydroxyapatite. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 59, 78–85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J. Biocompatibility and anti-bacterial activity of Zn-containing HA/TiO2 hybrid coatings on Ti substrate. J. Hard. Tissue Biol. 2013, 22, 311–318. [Google Scholar] [CrossRef]
- Tank, K.P.; Chudasama, K.S.; Thaker, V.S.; Joshi, M.J. Pure and zinc doped nano-hydroxyapatite: Synthesis, characterization, antimicrobial and hemolytic studies. J. Cryst. Growth 2014, 401, 474–479. [Google Scholar] [CrossRef]

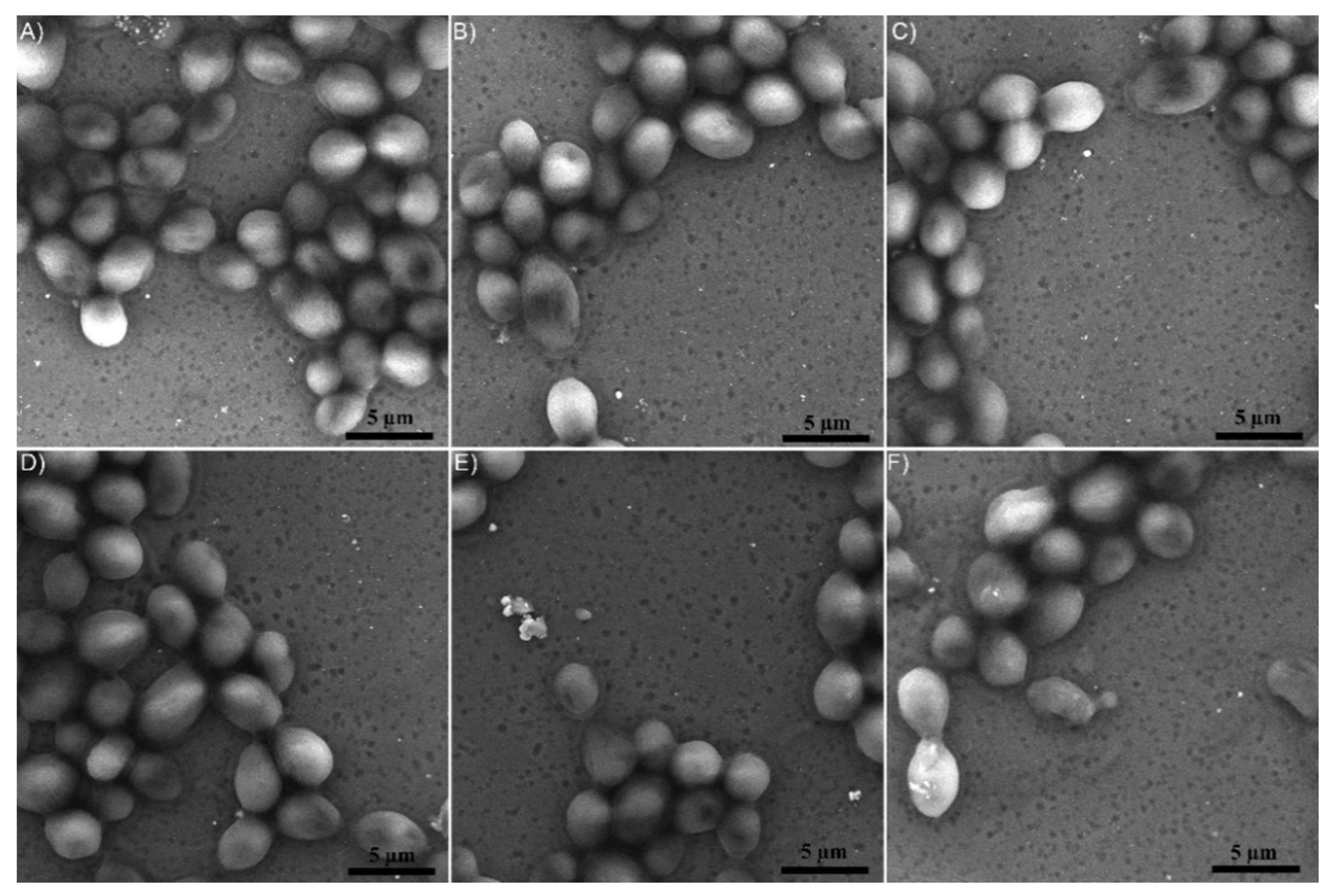
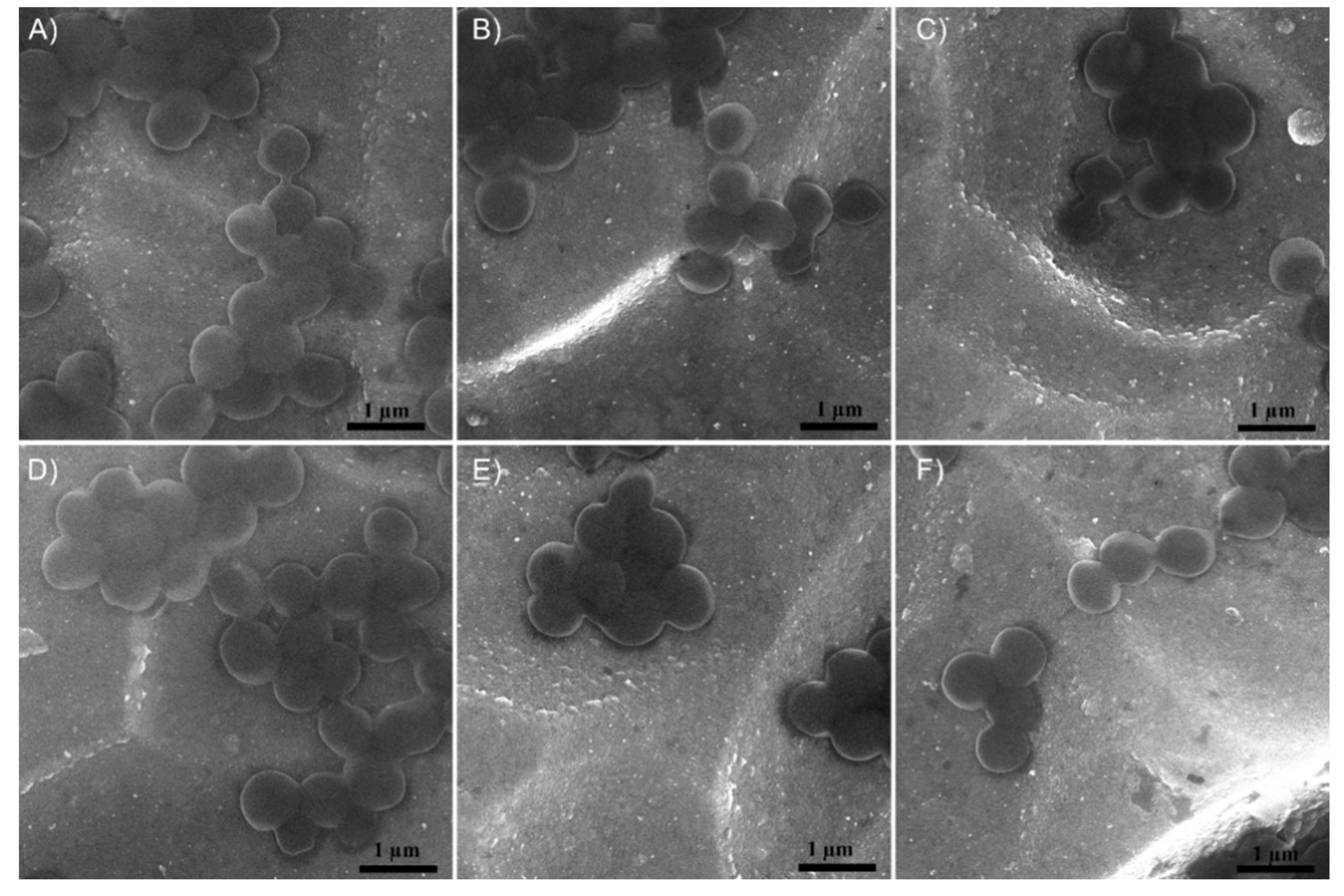
| Sample | Candida albicans ATCC 10231 | Staphylococcus aureus ATCC 25923 | ||||||
|---|---|---|---|---|---|---|---|---|
| 8 h | 24 h | 32 h | 48 h | 8 h | 24 h | 32 h | 48 h | |
| HAp | ± | ± | ± | + | ± | + | + | + |
| ZnHAp-500 | + | ++ | +++ | +++ | + | ++ | +++ | +++ |
| ZnHAp-700 | ++ | +++ | +++ | ++++ | ++ | +++ | ++++ | ++++ |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Predoi, D.; Iconaru, S.L.; Predoi, M.V.; Buton, N.; Motelica-Heino, M. Zinc Doped Hydroxyapatite Thin Films Prepared by Sol–Gel Spin Coating Procedure. Coatings 2019, 9, 156. https://doi.org/10.3390/coatings9030156
Predoi D, Iconaru SL, Predoi MV, Buton N, Motelica-Heino M. Zinc Doped Hydroxyapatite Thin Films Prepared by Sol–Gel Spin Coating Procedure. Coatings. 2019; 9(3):156. https://doi.org/10.3390/coatings9030156
Chicago/Turabian StylePredoi, Daniela, Simona Liliana Iconaru, Mihai Valentin Predoi, Nicolas Buton, and Mikael Motelica-Heino. 2019. "Zinc Doped Hydroxyapatite Thin Films Prepared by Sol–Gel Spin Coating Procedure" Coatings 9, no. 3: 156. https://doi.org/10.3390/coatings9030156
APA StylePredoi, D., Iconaru, S. L., Predoi, M. V., Buton, N., & Motelica-Heino, M. (2019). Zinc Doped Hydroxyapatite Thin Films Prepared by Sol–Gel Spin Coating Procedure. Coatings, 9(3), 156. https://doi.org/10.3390/coatings9030156






