Diammonium Hydrogenphosphate Treatment on Dolostone: The Role of Mg in the Crystallization Process
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
3. Results
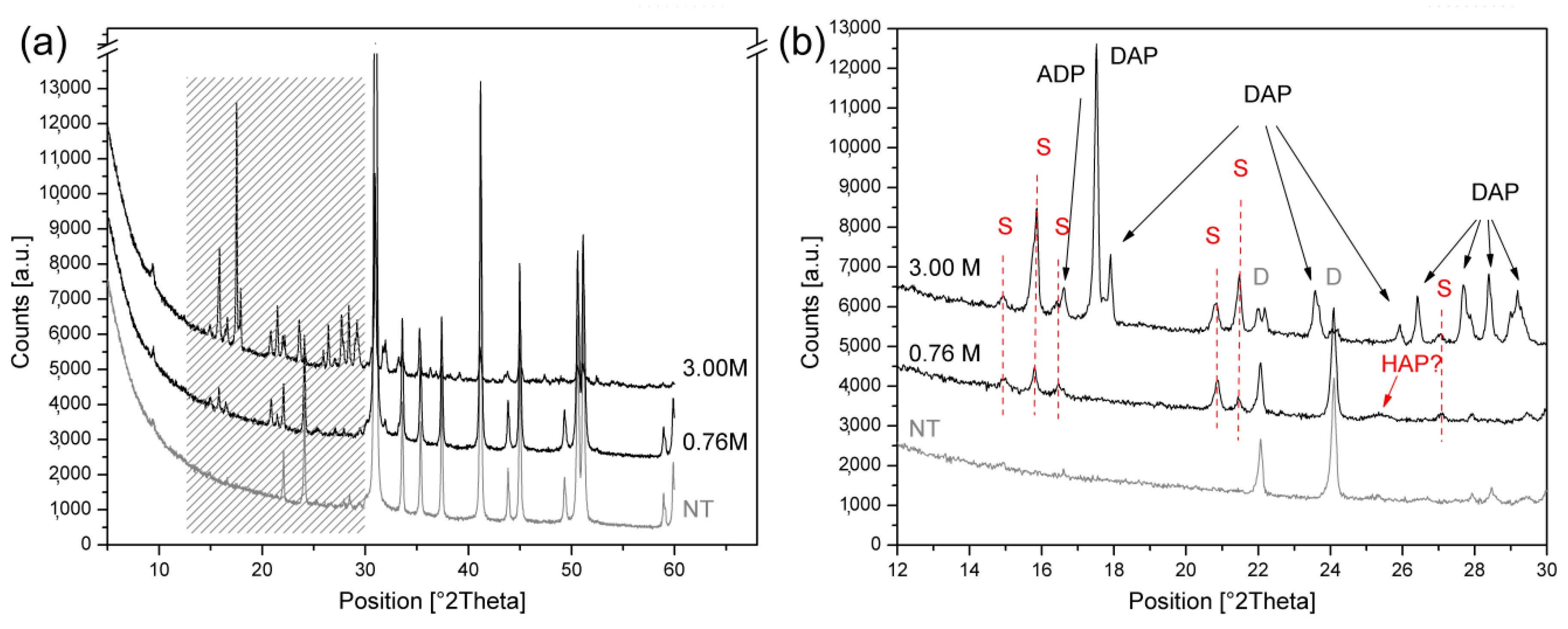
3.1. Analysis of the Newly-Formed Phases on Angera Stone
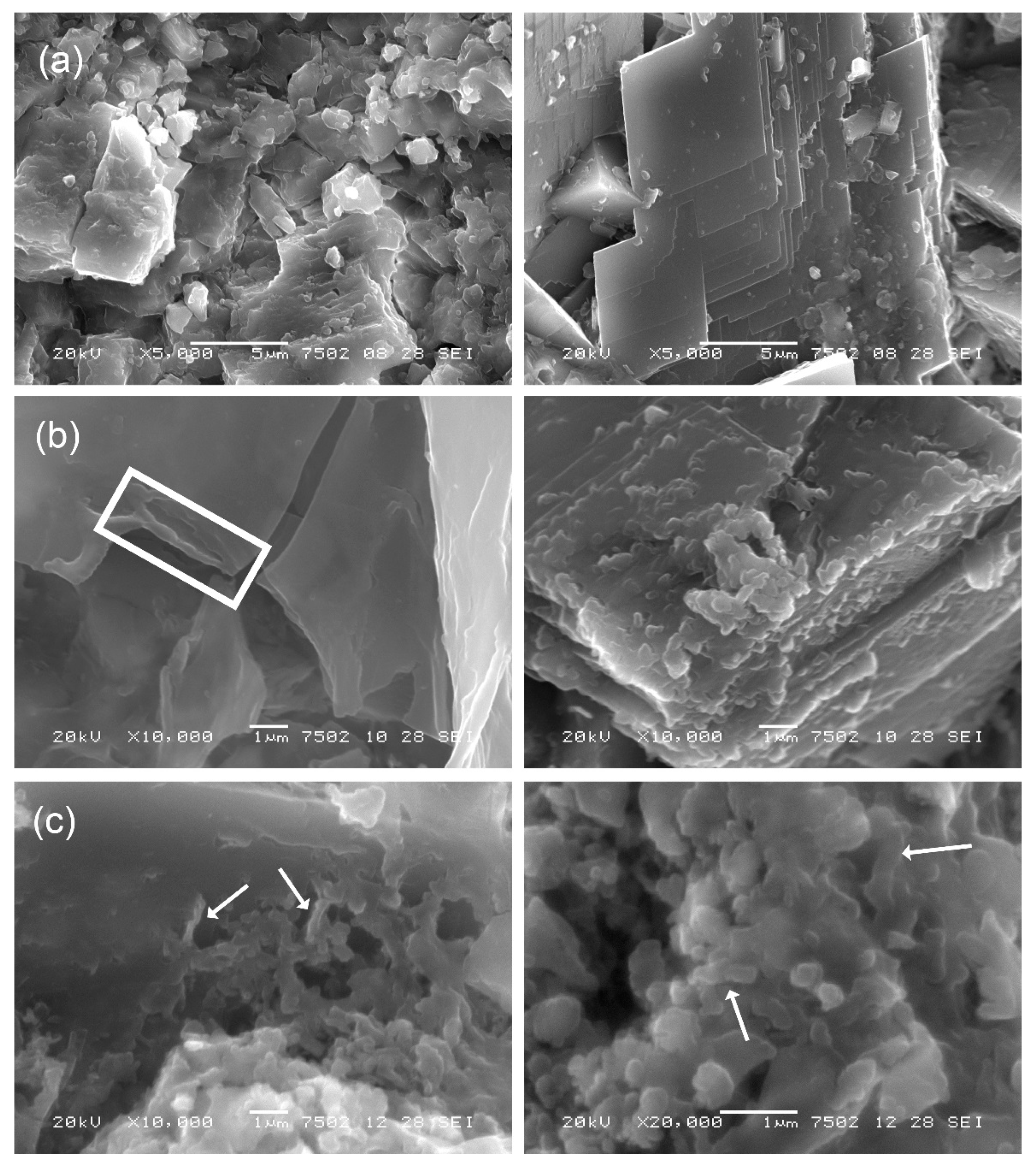
3.2. Micro-Morphological Investigations of the Stone Surface
3.3. Evidence of HAP Formation by Thermal Treatments
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Matteini, M.; Rescic, S.; Fratini, F.; Botticelli, G. Ammonium Phosphates as Consolidating Agents for Carbonatic Stone Materials Used in Architecture and Cultural Heritage: Preliminary Research. Int. J. Archit. Herit. Conserv. Anal. Restor. 2011, 5, 717–736. [Google Scholar] [CrossRef]
- Sassoni, E.; Naidu, S.; Scherer, G.W. The use of hydroxyapatite as a new inorganic consolidant for damaged carbonate stones. J. Cult. Herit. 2011, 12, 346–355. [Google Scholar] [CrossRef]
- Yang, F.; Zhang, B.; Liu, Y.; Wei, G.; Zhang, H.; Chen, W.; Xu, Z. Biomimic conservation of weathered calcareous stones by apatite. New J. Chem. 2011, 35, 887. [Google Scholar] [CrossRef]
- Matteini, M.; Colombo, C.; Botticelli, G.; Casati, M.; Conti, C.; Negrotti, R.; Realini, M.; Possenti, E. Ammonium phosphates to consolidate carbonatic stone materials: An inorganic-mineral treatment greatly promising. In Built Heritage 2013 Monitoring Conservation Management; Springer International Publishing: Cham, Switzerland, 2013; pp. 1278–1286. [Google Scholar]
- Ni, M.; Ratner, B.D. Nacre surface transformation to hydroxyapatite in a phosphate buffer solution. Biomaterials 2003, 24, 4323–4331. [Google Scholar] [CrossRef]
- Kasioptas, A.; Perdikouri, C.; Putnis, C.V.; Putnis, A. Pseudomorphic replacement of single calcium carbonate crystals by polycrystalline apatite. Mineral. Mag. 2008, 72, 77–80. [Google Scholar] [CrossRef]
- Sassoni, E. Hydroxyapatite and Other Calcium Phosphates for the Conservation of Cultural Heritage: A Review. Materials 2018, 11, 557. [Google Scholar] [CrossRef] [PubMed]
- Sassoni, E.; D’Amen, E.; Roveri, N.; Scherer, G.W.; Franzoni, E. Durable self-cleaning coatings for architectural surfaces by incorporation of TiO2 nano-particles into hydroxyapatite films. Materials 2018, 11, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Possenti, E.; Colombo, C.; Conti, C.; Gigli, L.; Merlini, M.; Plaisier, J.R.; Realini, M.; Sali, D.; Gatta, G.D. Diammonium hydrogenphosphate for the consolidation of building materials. Investigation of newly-formed calcium phosphates. Constr. Build. Mater. 2019, 195, 557–563. [Google Scholar] [CrossRef]
- Possenti, E.; Colombo, C.; Bersani, D.; Bertasa, M.; Botteon, A.; Conti, C.; Lottici, P.P.; Realini, M. New insight on the interaction of diammonium hydrogenphosphate conservation treatment with carbonatic substrates: A multi-analytical approach. Microchem. J. 2016, 127, 79–86. [Google Scholar] [CrossRef]
- Molina, E.; Rueda-Quero, L.; Benavente, D.; Burgos-Cara, A.; Ruiz-Agudo, E.; Cultrone, G. Gypsum crust as a source of calcium for the consolidation of carbonate stones using a calcium phosphate-based consolidant. Constr. Build. Mater. 2017, 143, 298–311. [Google Scholar] [CrossRef]
- Sassoni, E.; Graziani, G.; Franzoni, E. Repair of sugaring marble by ammonium phosphate: Comparison with ethyl silicate and ammonium oxalate and pilot application to historic artifact. Mater. Des. 2015, 88, 1145–1157. [Google Scholar] [CrossRef]
- Wang, L.; Nancollas, G.H. Calcium Orthophosphates: Crystallization and Dissolution. Chem. Rev. 2008, 108, 4628–4669. [Google Scholar] [CrossRef] [PubMed]
- Dorozhkin, S.V. Calcium orthophosphates. J. Mater. Sci. 2007, 42, 1061–1095. [Google Scholar] [CrossRef]
- Calore, N.; Botteon, A.; Colombo, C.; Comunian, A.; Possenti, E.; Realini, M.; Sali, D.; Conti, C. High Resolution ATR μ-FTIR to map the diffusion of conservation treatments applied to painted plasters. Vib. Spectrosc. 2018, 98, 105–110. [Google Scholar] [CrossRef]
- Possenti, E. Inorganic Products Used in the Conservation of Cultural Heritage: Interaction with Carbonatic Substrates and Newly-Formed Crystalline Phases. Ph.D. Thesis, University of Milan, Milan, Italy, 7 February 2019. [Google Scholar]
- Possenti, E.; Colombo, C.; Conti, C.; Gigli, L.; Merlini, M.; Plaisier, J.R.; Realini, M.; Gatta, G.D. Grazing incidence synchrotron X-ray diffraction of marbles consolidated with diammonium hydrogen phosphate treatments: Non-destructive probing of buried minerals. Appl. Phys. A 2018, 124, 383. [Google Scholar] [CrossRef]
- Possenti, E.; Colombo, C.; Conti, C.; Gigli, L.; Merlini, M.; Plaisier, J.R.; Realini, M.; Gatta, G.D. What’s underneath? A non-destructive depth profile of painted stratigraphies by synchrotron grazing incidence X-ray diffraction. Analyst 2018, 143, 4290–4297. [Google Scholar] [CrossRef] [PubMed]
- Gulotta, D.; Bertoldi, M.; Bortolotto, S.; Fermo, P.; Piazzalunga, A.; Toniolo, L. The Angera stone. A challenging conservation issue in the polluted environment of Milan (Italy). Environ. Earth Sci. 2013, 69, 1085–1094. [Google Scholar] [CrossRef]
- Schultheiss, S.; Sethmann, I.; Schlosser, M.; Kleebe, H.-J. Pseudomorphic transformation of Ca/Mg carbonates into phosphates with focus on dolomite conversion. Mineral. Mag. 2013, 77, 2725–2737. [Google Scholar] [CrossRef]
- Pesonen, J.; Myllymäki, P.; Vervecken, G.; Hu, T.; Prokkola, H.; Tuomikoski, S. Use of calcined dolomite as chemical coagulant in the simultaneous removal of nitrogen and phosphorus. In Proceedings of the 6th International Conference on Sustainable Solid Waste Management, Naxos Island, Greece, 13–16 June 2018; pp. 1–8. [Google Scholar]
- Beeson, K.C. Chemical Reactions in Fertilizer Mixtures Reactions of Diammonium Phosphate with Limestone and with Dolomite. Ind. Eng. Chem. 1937, 29, 705–708. [Google Scholar] [CrossRef]
- Keenen, F.G.; Morgan, W.A. Rate of Dolomite Reactions in Mixed Fertilizers. Ind. Eng. Chem. 1937, 29, 197–201. [Google Scholar] [CrossRef]
- Cavallo, A.; Bigioggero, B.; Colombo, A.; Tunesi, A. The Verbano Cusio Ossola province: A land of quarries in northern Italy (Piedmont). Period. Mineral. 2004, 73, 197–210. [Google Scholar]
- Alessandrini, G.; Bugini, R.; Peruzzi, R. I materiali lapidei impiegati nei monumenti lombardi e i loro problemi di conservazione. In Materiali Lapidei, Bollettino d’Arte—Ministero per i beni e le attività culturali; Istituto Poligrafico e Zecca dello Stato: Rome, Italy, 1987; pp. 145–156. [Google Scholar]
- Alessandrini, G. Le pietre del monumento. In La Ca’ Granda di Milano. L’intervento Conservativo sul Cortile Richiniano; SNAM-Amilcare Pizzi: Milano, Italy, 1993; pp. 173–203. ISBN 88-366-0435-8. [Google Scholar]
- Alessandrini, G. Lo stato di conservazione dei materiali lapidei: Morfologia e cause di degrado. In La Ca’ Granda di Milano. L’intervento Conservativo sul Cortile Richiniano; SNAM-Amilcare Pizzi: Milano, Italy, 1993; pp. 219–239. ISBN 88-366-0435-8. [Google Scholar]
- Riganti, V.; Rosetti, R.; Soggetti, F.; Veniale, F.; Zezza, U. Alterazione e protezione delle pietre dei monumenti storici dell’Università di Pavia; Società Italiana di Scienze Naturali Corso Venezia: Milano, Italy, 1978. [Google Scholar]
- Pittaluga, D.; Fratini, F.; Nielsen, A.; Rescic, S. Industrial archaeological sites and architectonic remains: The problem of consolidation in humid areas. In Proceedings of the Scienza e Beni Culturali XXVIII, Bressanone, Italy, 10–13 July 2012; pp. 303–312. [Google Scholar]
- Sassoni, E. Phosphate-based treatments for conservation of stone. RILEM Tech. Lett. 2017, 2, 14. [Google Scholar] [CrossRef]
- Karampas, I.A.; Kontoyannis, C.G. Characterization of calcium phosphates mixtures. Vib. Spectrosc. 2013, 64, 126–133. [Google Scholar] [CrossRef]
- Bhuiyan, M.I.H.; Mavinic, D.S.; Koch, F.A. Thermal decomposition of struvite and its phase transition. Chemosphere 2008, 70, 1347–1356. [Google Scholar] [CrossRef] [PubMed]
- Gunasekaran, S.; Anbalagan, G. Thermal decomposition of natural dolomite. Bull. Mater. Sci. 2007, 30, 339–344. [Google Scholar] [CrossRef]
- Olszak-Humienik, M.; Jablonski, M. Thermal behavior of natural dolomite. J. Therm. Anal. Calorim. 2015, 119, 2239–2248. [Google Scholar] [CrossRef]
- Ramlogan, M.V.; Rouff, A.A. An investigation of the thermal behavior of magnesium ammonium phosphate hexahydrate. J. Therm. Anal. Calorim. 2016, 123, 145–152. [Google Scholar] [CrossRef]
- Tansel, B.; Lunn, G.; Monje, O. Struvite formation and decomposition characteristics for ammonia and phosphorus recovery: A review of magnesium-ammonia-phosphate interactions. Chemosphere 2018, 194, 504–514. [Google Scholar] [CrossRef] [PubMed]
- Koutsopoulos, S. Synthesis and characterization of hydroxyapatite crystals: A review study on the analytical methods. J. Biomed. Mater. Res. 2002, 62, 600–612. [Google Scholar] [CrossRef] [PubMed]
- Dorozhkin, S.V. Amorphous calcium (ortho)phosphates. Acta Biomater. 2010, 6, 4457–4475. [Google Scholar] [CrossRef] [PubMed]
- Dorozhkin, S.V. Calcium Orthophosphates in Nature, Biology and Medicine. Materials 2009, 2, 399–498. [Google Scholar] [CrossRef]
- Elliott, J.C. Hydroxyapatite and Nonstoichiometric Apatites. In Structure and Chemistry of the Apatites and Other Calcium Orthophosphates; Elsevier: Amsterdam, The Netherlands, 1994; pp. 111–189. [Google Scholar]
- Drouet, C. Apatite formation: Why it may not work as planned, and how to conclusively identify apatite compounds. Biomed. Res. Int. 2013, 2013, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Ariyanto, E.; Ha Ming, A.; Tushar, S. Effect of initial solution pH on solubility and morphology of struvice crystals. In Proceedings of the CHEMECA Conference, Sydney, Australia, 18–21 September 2011; pp. 1–10. [Google Scholar]
- Ren, F.; Leng, Y.; Xin, R.; Ge, X. Synthesis, characterization and ab initio simulation of magnesium-substituted hydroxyapatite. Acta Biomater. 2010, 6, 2787–2796. [Google Scholar] [CrossRef] [PubMed]
- Naidu, S.; Scherer, G.W. Nucleation, growth and evolution of calcium phosphate films on calcite. J. Colloid Interface Sci. 2014, 435, 128–137. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Harris, W. Carbonate and magnesium interactive effect on calcium phosphate precipitation. Environ. Sci. Technol. 2008, 42, 436–442. [Google Scholar] [CrossRef] [PubMed]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Possenti, E.; Conti, C.; Gatta, G.D.; Realini, M.; Colombo, C. Diammonium Hydrogenphosphate Treatment on Dolostone: The Role of Mg in the Crystallization Process. Coatings 2019, 9, 169. https://doi.org/10.3390/coatings9030169
Possenti E, Conti C, Gatta GD, Realini M, Colombo C. Diammonium Hydrogenphosphate Treatment on Dolostone: The Role of Mg in the Crystallization Process. Coatings. 2019; 9(3):169. https://doi.org/10.3390/coatings9030169
Chicago/Turabian StylePossenti, Elena, Claudia Conti, G. Diego Gatta, Marco Realini, and Chiara Colombo. 2019. "Diammonium Hydrogenphosphate Treatment on Dolostone: The Role of Mg in the Crystallization Process" Coatings 9, no. 3: 169. https://doi.org/10.3390/coatings9030169
APA StylePossenti, E., Conti, C., Gatta, G. D., Realini, M., & Colombo, C. (2019). Diammonium Hydrogenphosphate Treatment on Dolostone: The Role of Mg in the Crystallization Process. Coatings, 9(3), 169. https://doi.org/10.3390/coatings9030169




