Anti-Icing Performance of a Coating Based on Nano/Microsilica Particle-Filled Amino-Terminated PDMS-Modified Epoxy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
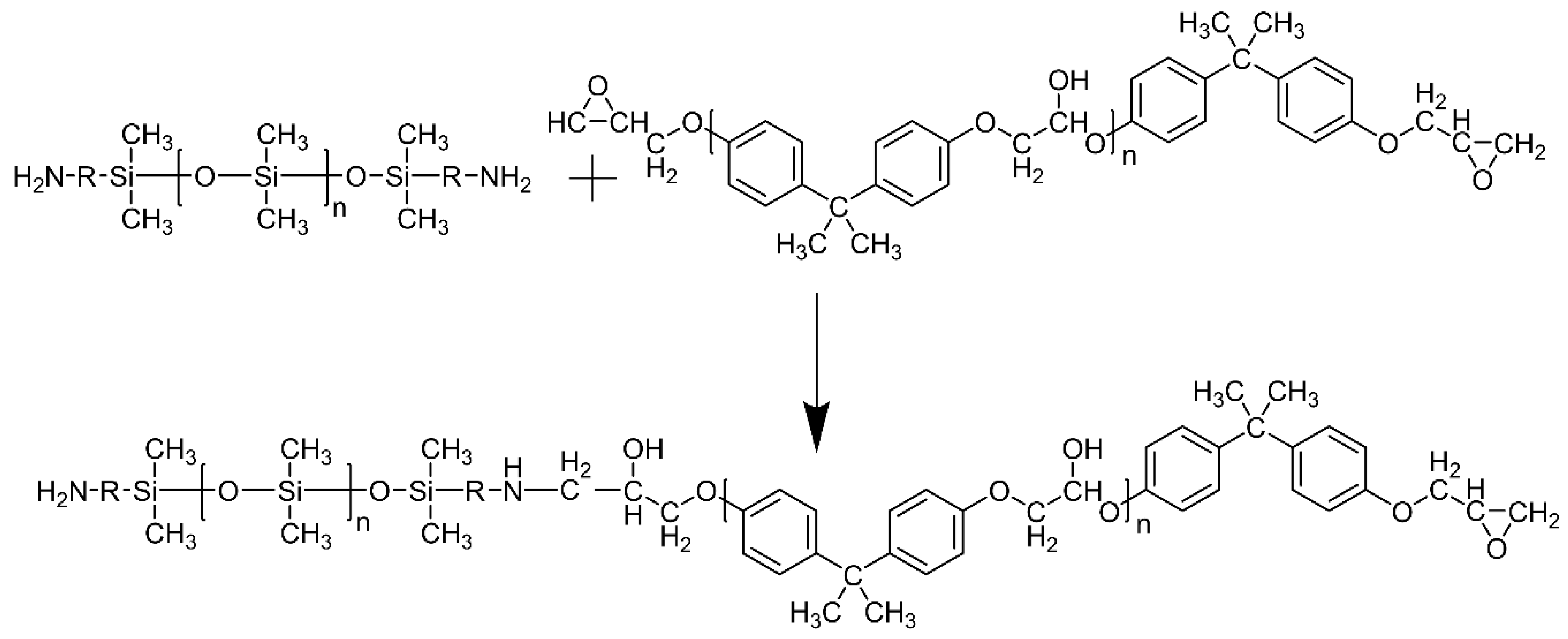
2.2. A-PDMS-Modified Epoxy Coating
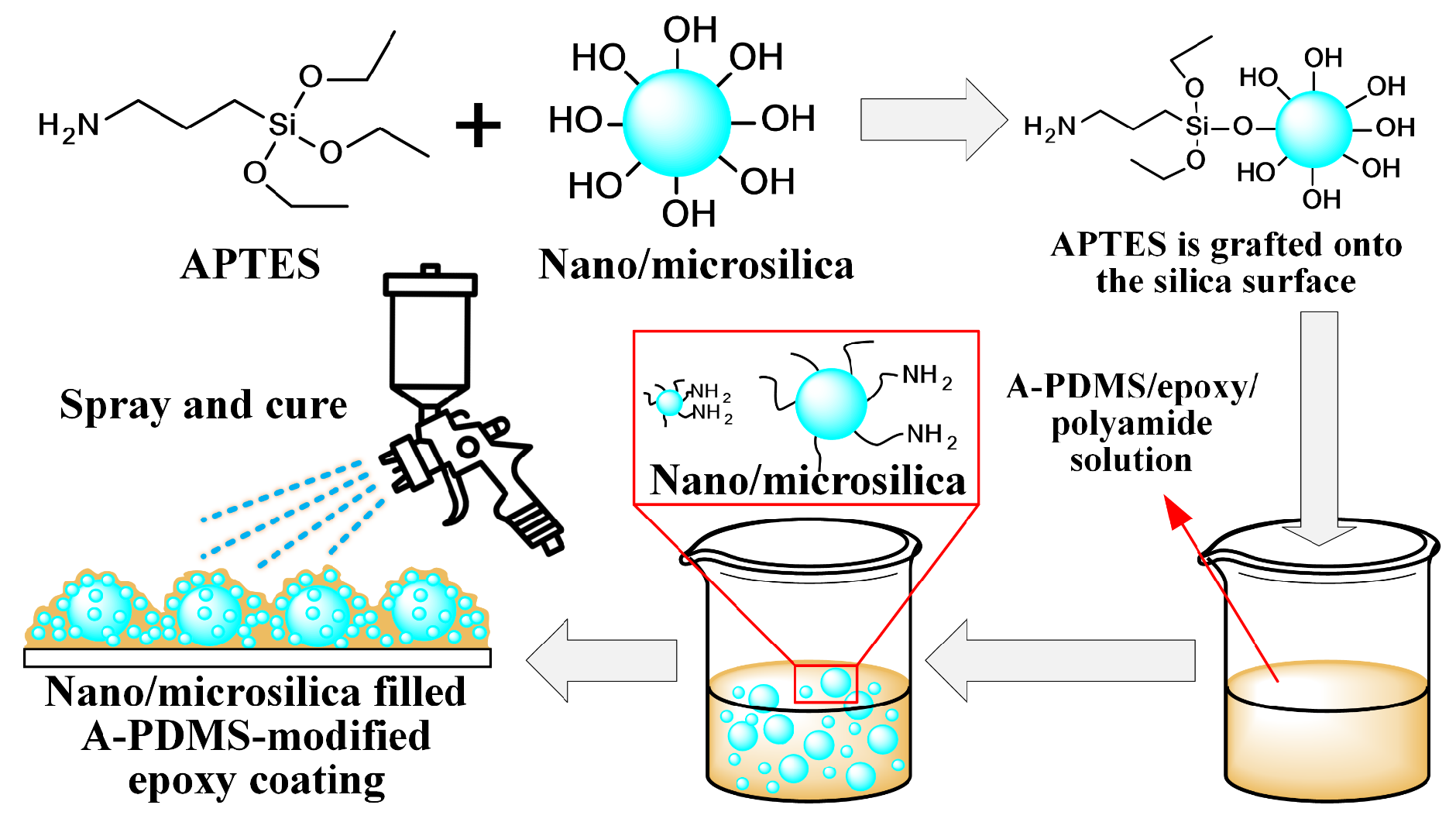
2.3. One-Step Spray Coating of Nano/Microsilica Filled A-PDMS-Modified Epoxy Coating
2.4. CA, CAH and Sliding Angle (SA) Measurements
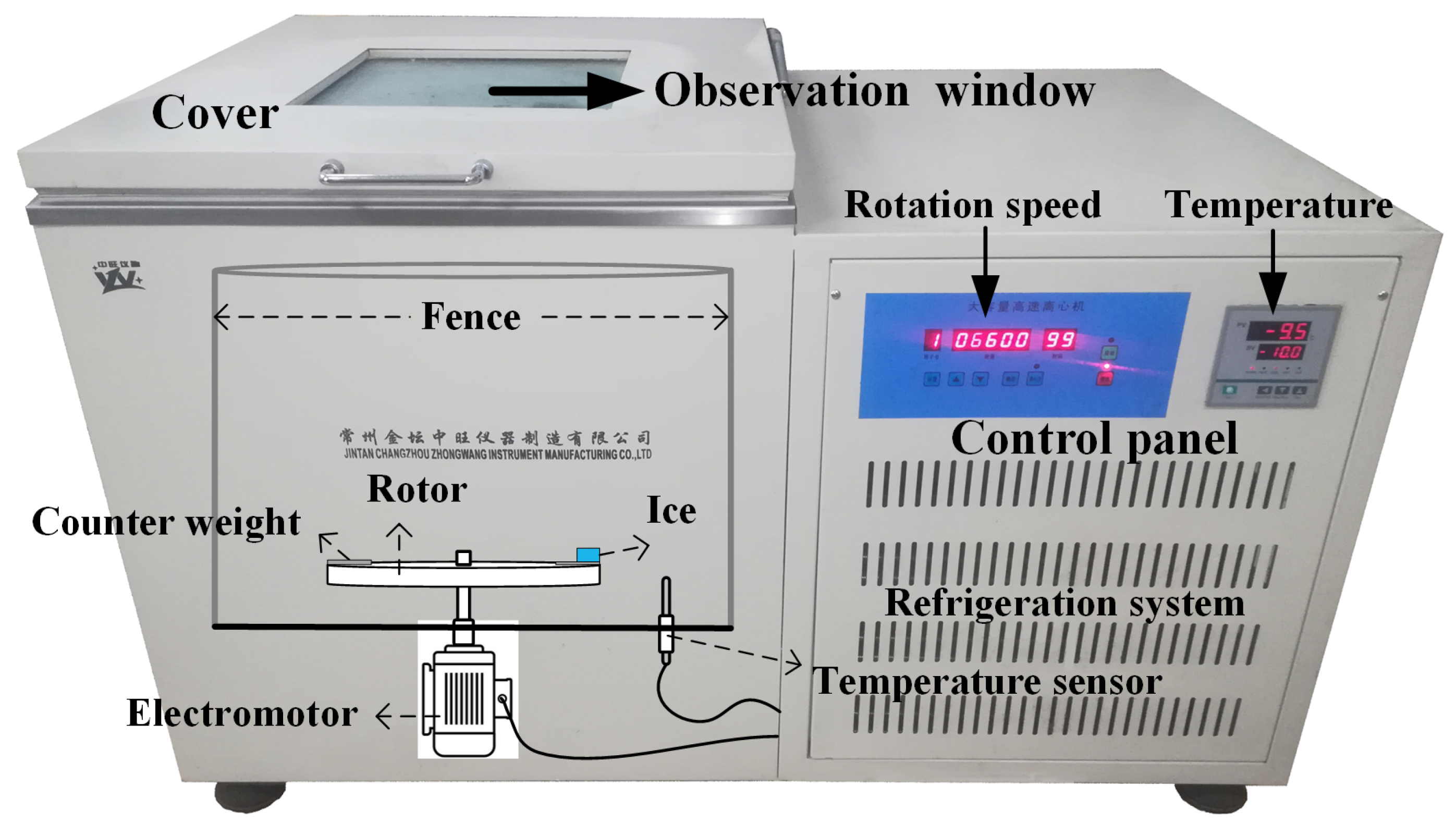
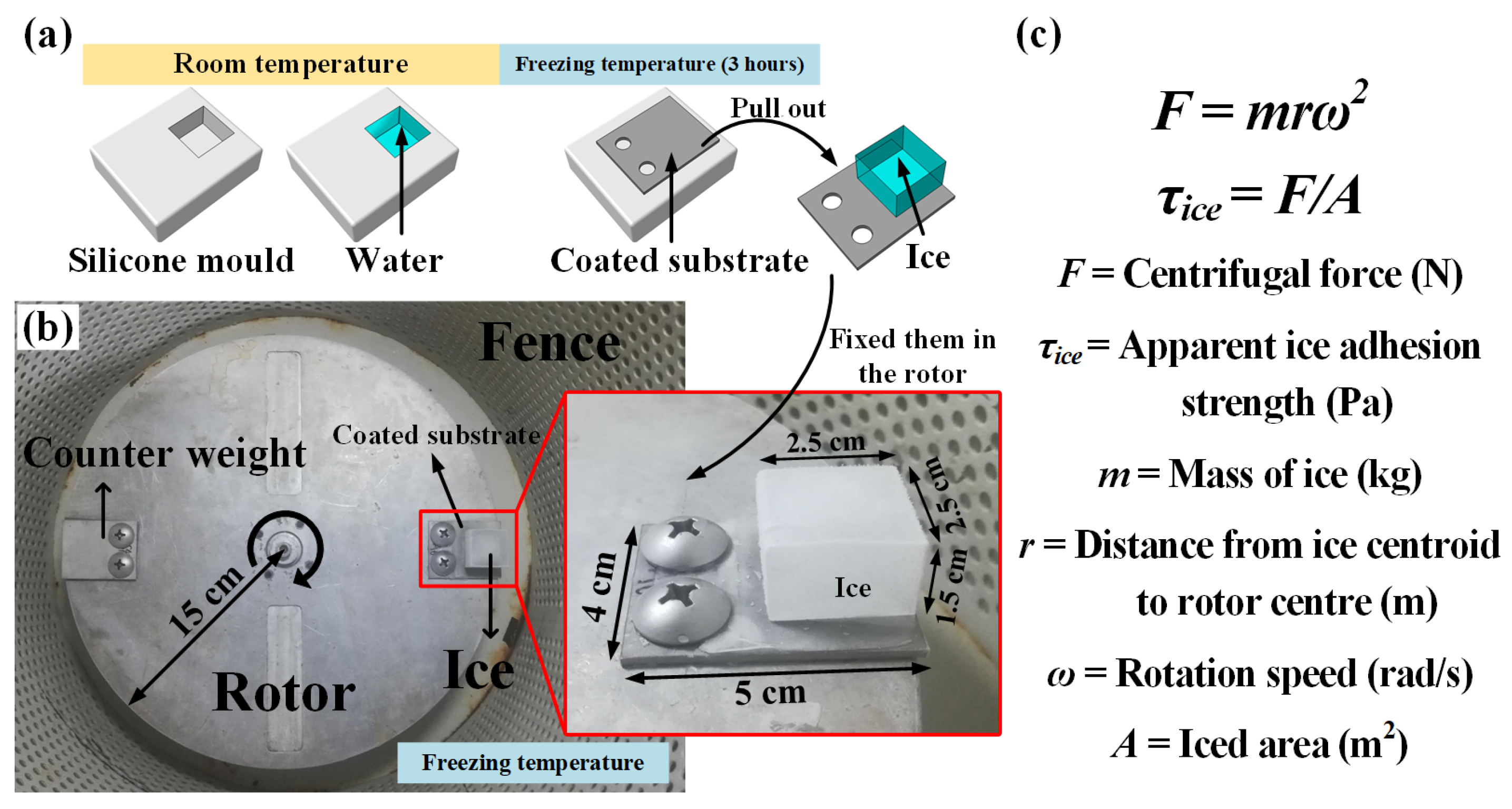
2.5. Ice Adhesion Strength Test
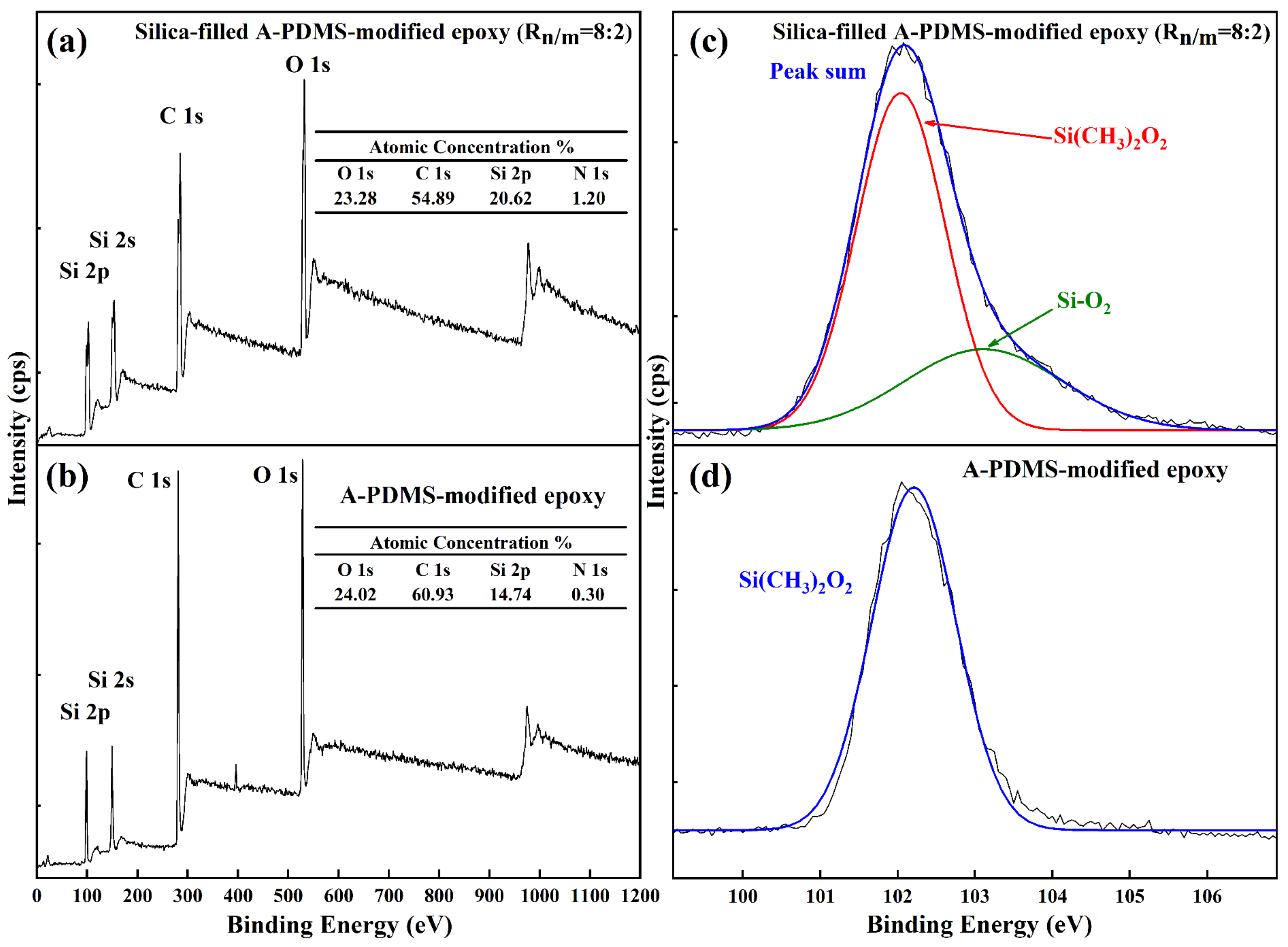
2.6. Scanning Electron Microscopy (SEM), X-ray Photoelectron Spectroscopy (XPS) and Surface Roughness
2.7. Formation of Rime Ice, Normal Ice and Glaze Ice
3. Results and Discussion
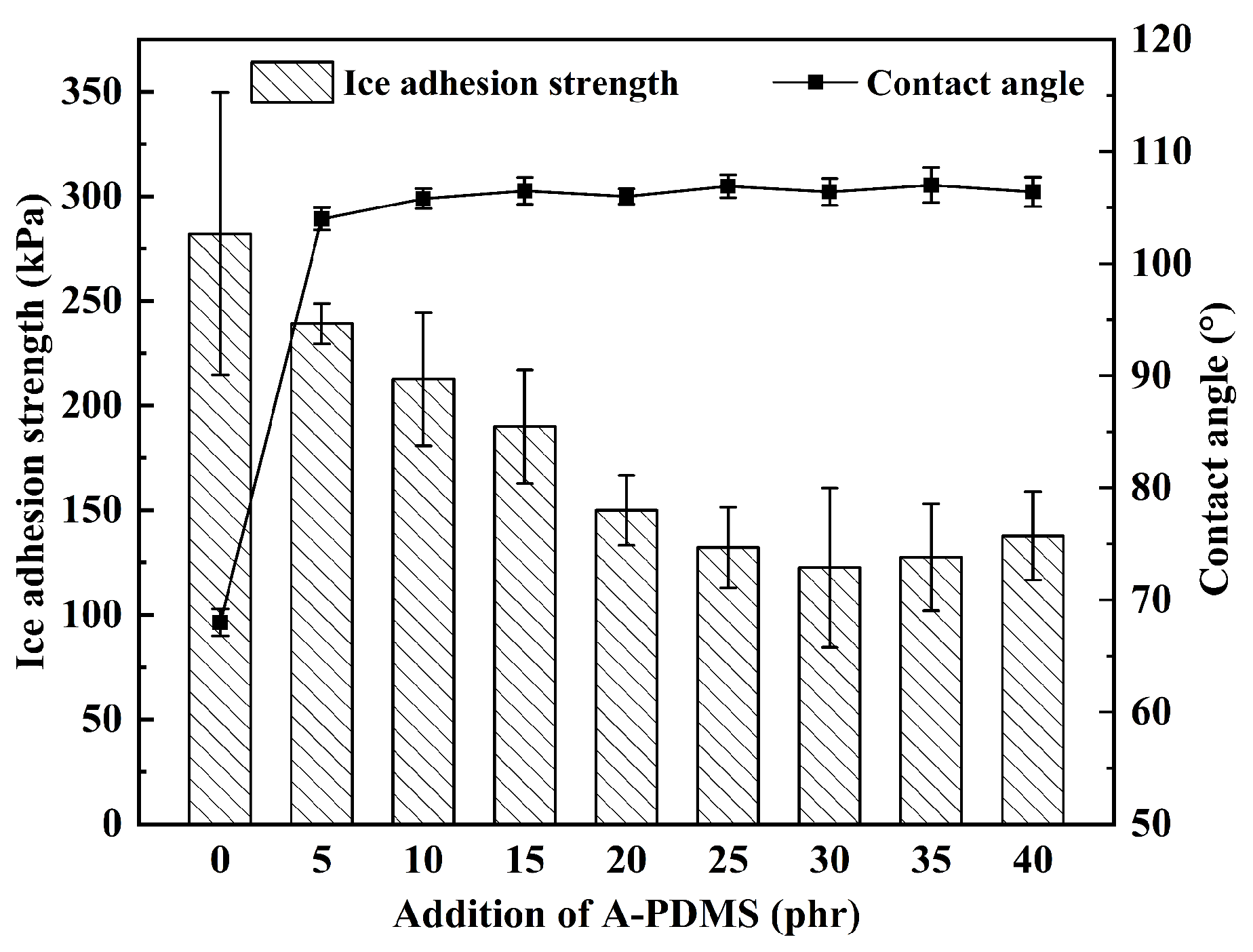
3.1. A-PDMS-Modified Epoxy Coating without Particles
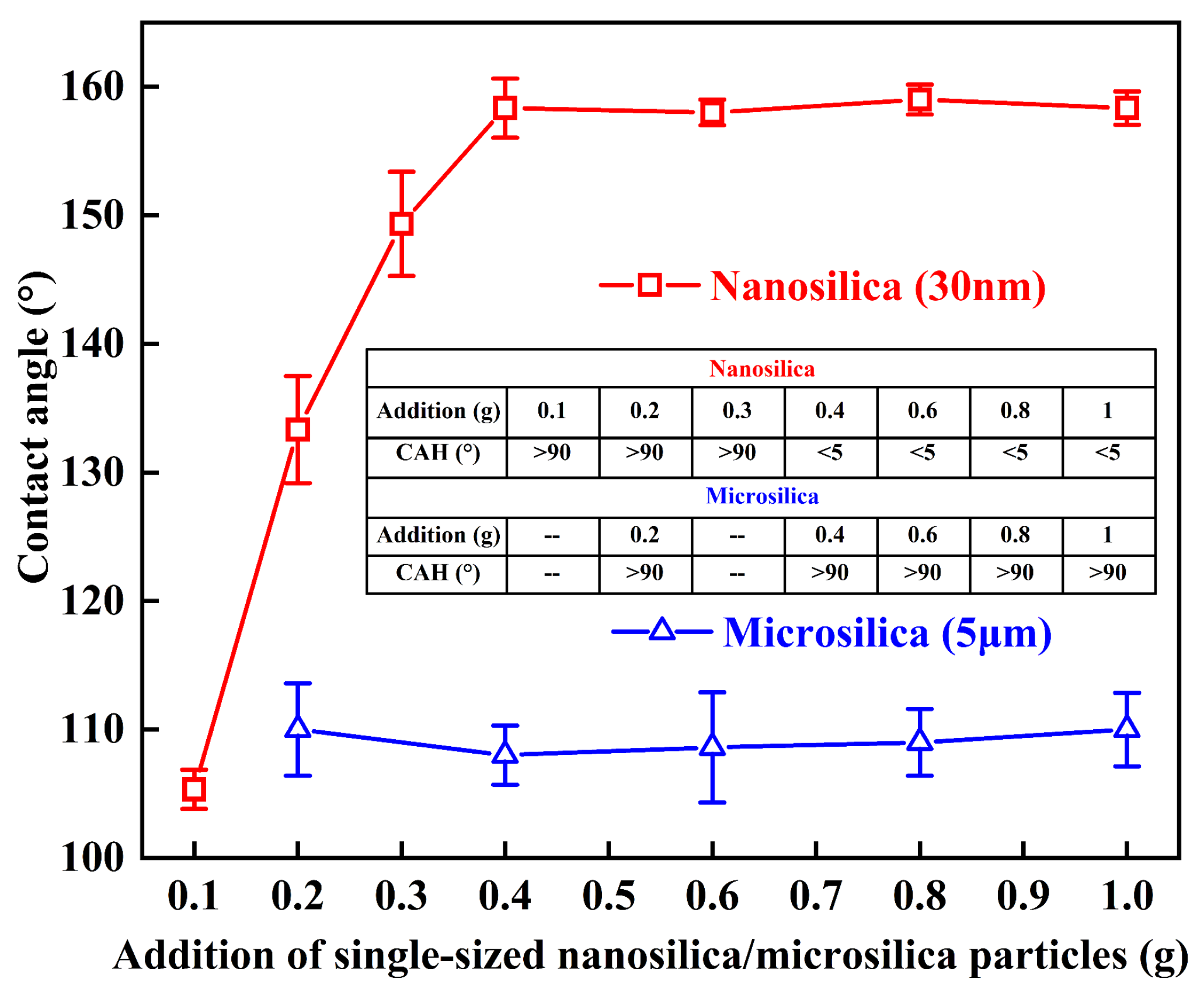
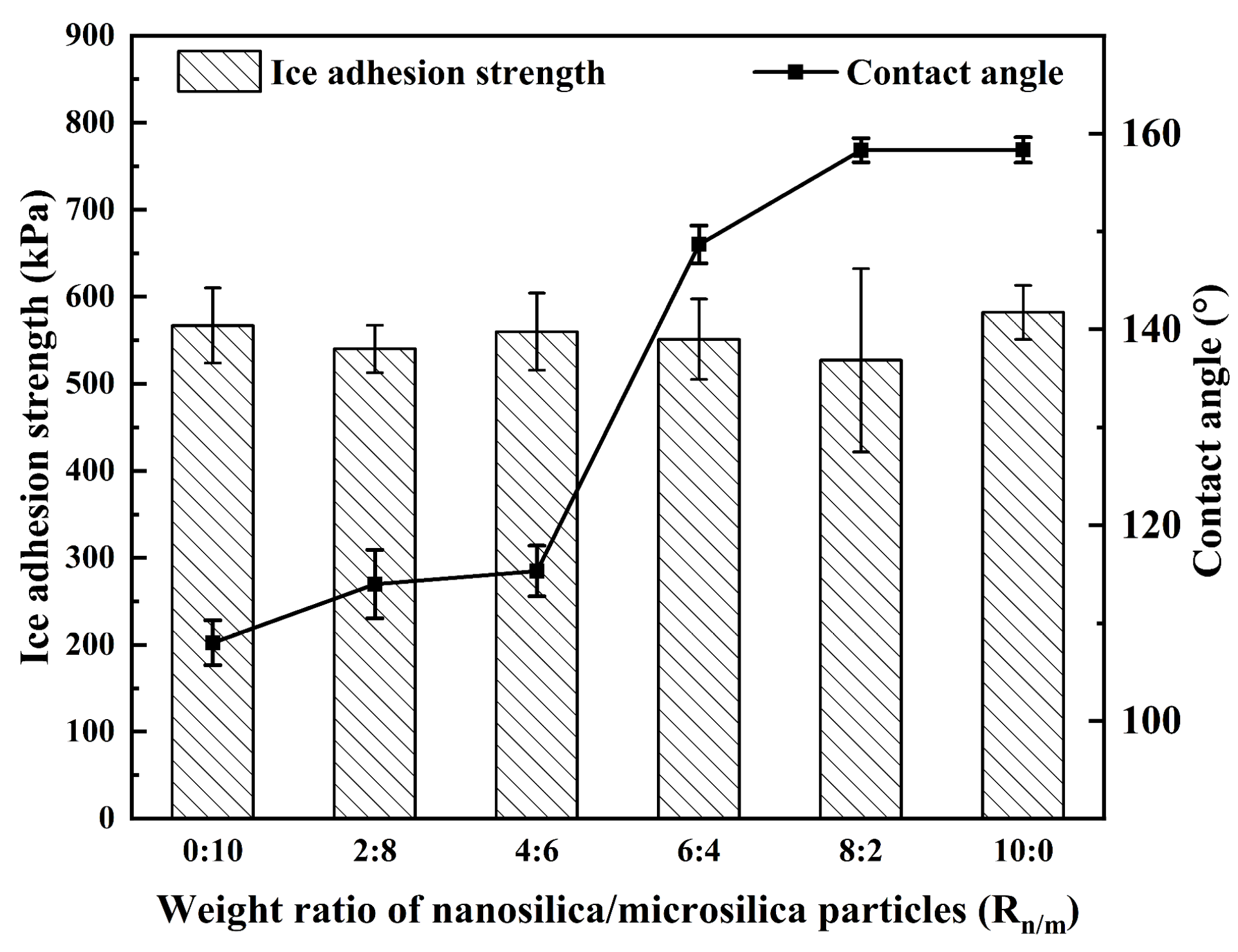
3.2. Nano/Microsilica-Filled A-PDMS-Modified Epoxy Coating
3.3. Icing Delay Test
3.4. The Influences of Temperature and Ice Type on Ice Adhesion Strength
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lv, J.; Song, Y.; Jiang, L.; Wang, J. Bio-Inspired strategies for anti-icing. ACS Nano 2014, 8, 3152–3169. [Google Scholar] [CrossRef] [PubMed]
- Bormashenko, E. Physics of pre-wetted, lubricated and impregnated surfaces: A review. Philos. Trans. A Math. Phys. Eng. Sci. 2018, 377, 20180264. [Google Scholar] [CrossRef] [PubMed]
- Rykaczewski, K.; Anand, S.; Subramanyam, S.B.; Varanasi, K.K. Mechanism of frost formation on lubricant-impregnated surfaces. Langmuir 2013, 29, 5230–5238. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Xue, J.; Wang, Y.; Chen, Q.; Ding, J.; Wang, Q. Ice-Phobic coatings based on silicon-oil-infused polydimethylsiloxane. ACS Appl. Mater. Interfaces 2013, 5, 4053–4062. [Google Scholar] [CrossRef] [PubMed]
- Guo, P.; Zheng, Y.; Wen, M.; Song, C.; Lin, Y.; Jiang, L. Icephobic/Anti-Icing Properties of Micro/Nanostructured Surfaces. Adv. Mater. 2012, 24, 2642–2648. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, W.; Lu, J.; Zhu, C.; Lin, W.; Feng, J. Aqueous epoxy-based superhydrophobic coatings: Fabrication and stability in water. Prog. Org. Coat. 2018, 121, 201–208. [Google Scholar] [CrossRef]
- Wu, Y.; Zhao, M.; Guo, Z. Robust, Heat-Resistant and multifunctional superhydrophobic coating of carbon microflowers with molybdenum trioxide nanoparticles. J. Colloid Interface Sci. 2017, 506, 649–658. [Google Scholar] [CrossRef] [PubMed]
- Li, J.-H.; Weng, R.; Di, X.-Q.; Yao, Z.-W. Gradient and weather resistant hybrid super-hydrophobic coating based on fluorinated epoxy resin. J. Appl. Polym. Sci. 2014, 131, 40955. [Google Scholar] [CrossRef]
- Zhang, F.; Qian, H.; Wang, L.; Wang, Z.; Du, C.; Li, X.; Zhang, D. Superhydrophobic carbon nanotubes/epoxy nanocomposite coating by facile one-step spraying. Surf. Coat. Technol. 2018, 341, 15–23. [Google Scholar] [CrossRef]
- Peng, W.; Gou, X.; Qin, H.; Zhao, M.; Zhao, X.; Guo, Z. Creation of a multifunctional superhydrophobic coating for composite insulators. Chem. Eng. J. 2018, 352, 774–781. [Google Scholar] [CrossRef]
- Sarkar, D.K.; Farzaneh, M. Superhydrophobic coatings with reduced ice adhesion. J. Adhes. Sci. Technol. 2009, 23, 1215–1237. [Google Scholar] [CrossRef]
- Wu, X.; Zheng, S.; Daniel, A.B.-A.; Vadim, V.S.; Chen, Z. Transparent icephobic coatings using bio-based epoxy resin. Mater. Des. 2018, 140, 516–523. [Google Scholar] [CrossRef]
- Cheng, T.; He, R.; Zhang, Q.; Zhan, X.; Chen, F. Magnetic particle-based super-hydrophobic coatings with excellent anti-icing and thermoresponsive deicing performance. J. Mater. Chem. A 2015, 43, 21637–21646. [Google Scholar] [CrossRef]
- Kulinich, S.A.; Farzaneh, M. Ice adhesion on super-hydrophobic surfaces. Appl. Sur. Sci. 2009, 255, 8153–8157. [Google Scholar] [CrossRef]
- Zheng, S.; Daniel, A.B.-A.; Wu, X.; Zhan, X.; Huang, Y.; Zeng, X.; Zhang, Q.; Chen, Z. Durable waterborne hydrophobic bio-epoxy coating with improved anti-icing and self-cleaning performance. ACS Sustain. Chem. Eng. 2018, 7, 641–649. [Google Scholar] [CrossRef]
- Guerin, F.; Laforte, C.; Farinas, M.-I.; Perron, J. Analytical model based on experimental data of centrifuge ice adhesion tests with different substrates. Cold Reg. Sci. Technol. 2016, 121, 93–99. [Google Scholar] [CrossRef]
- ISO 25178-2: 2012 Geometrical Product Specifications (GPS)-Surface Texture: Areal-Part 2: Terms, Definitionsand Surface Texture Parameters; ISO: Geneva, Switzerland, 2012.
- Chen, J.; Liu, J.; He, M.; Li, K.; Cui, D.; Zhang, Q.; Zeng, X.; Zhang, Y.; Wang, J.; Song, Y. Superhydrophobic surfaces cannot reduce ice adhesion. Appl. Phys. Lett. 2012, 101, 111603. [Google Scholar] [CrossRef]
- Vali, G. Repeatability and randomness in heterogeneous freezing nucleation. Atmos. Chem. Phys. 2008, 8, 5017–5031. [Google Scholar] [CrossRef]
- Boinovich, L.B.; Emelyanenko, A.M.; Emelyanenko, K.A.; Modin, E.B. Modus operandi of protective and anti-icing mechanisms underlying the design of longstanding outdoor icephobic coatings. ACS Nano 2019, 13, 4335–4346. [Google Scholar] [CrossRef] [PubMed]
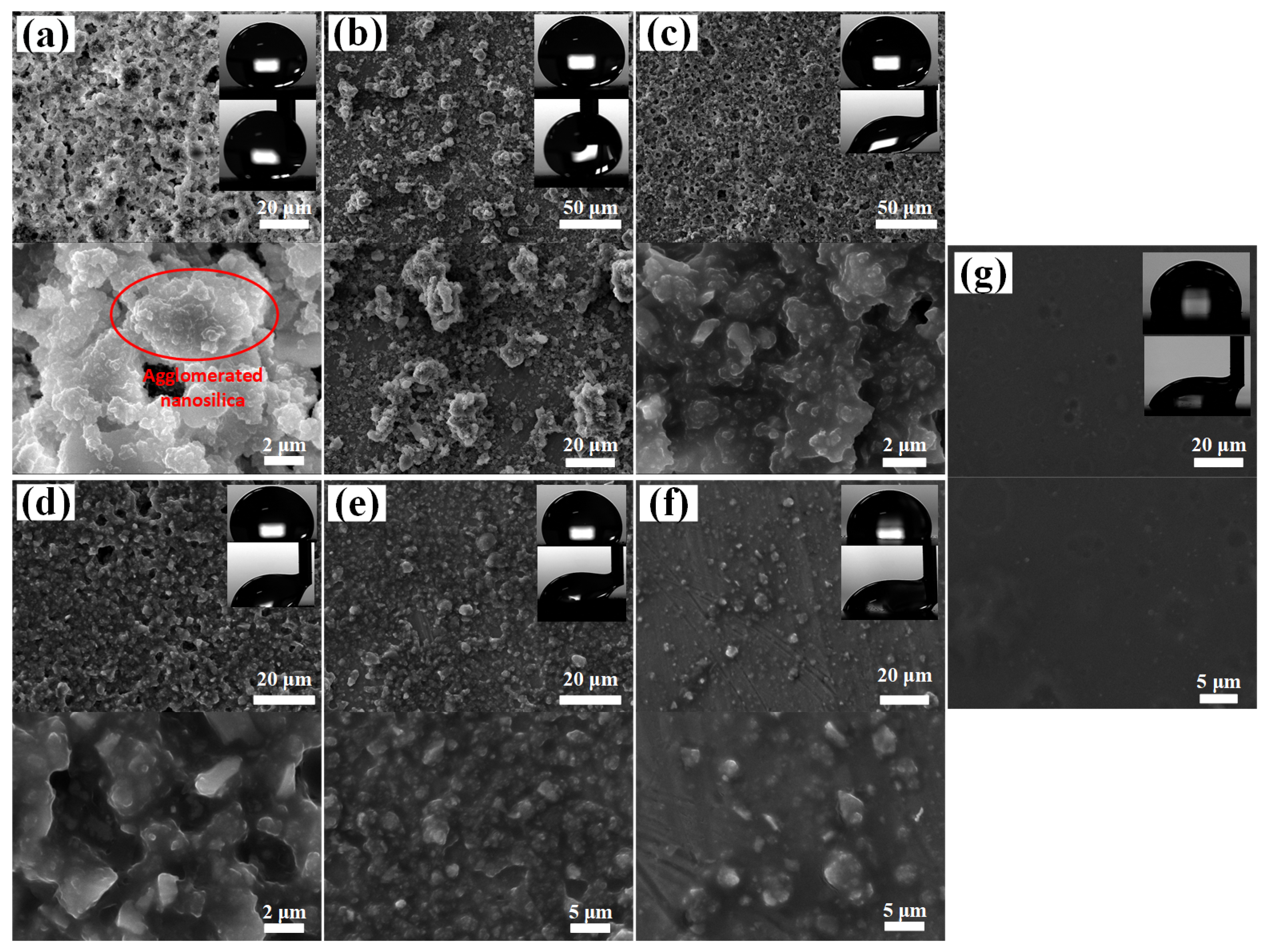

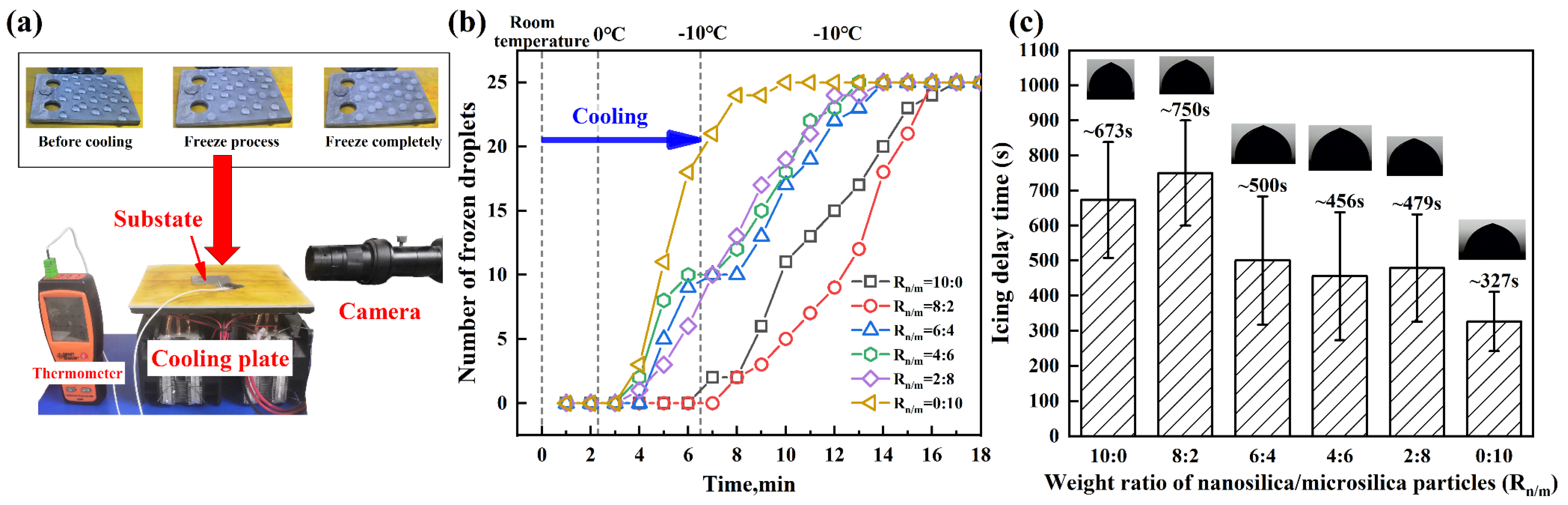
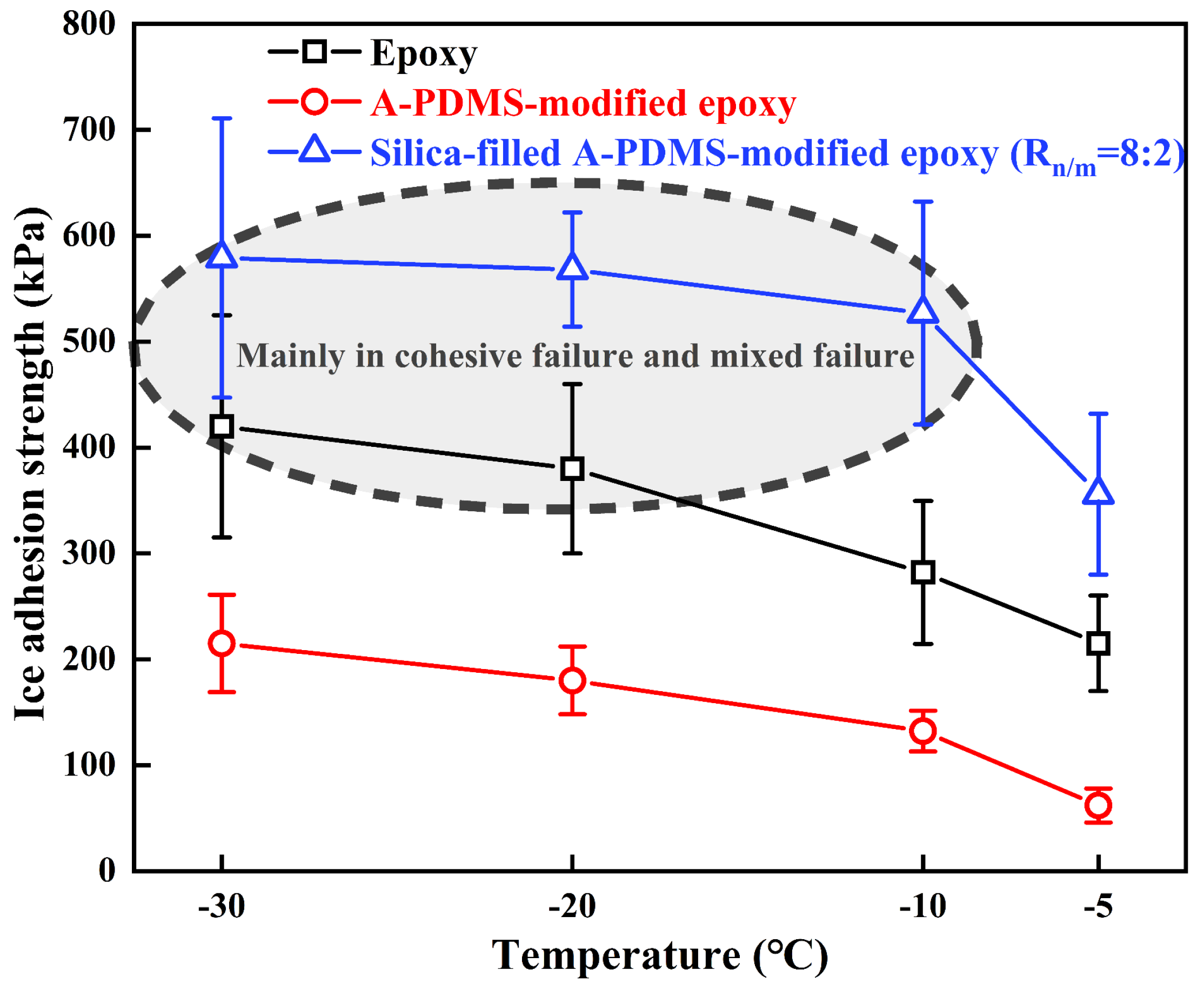
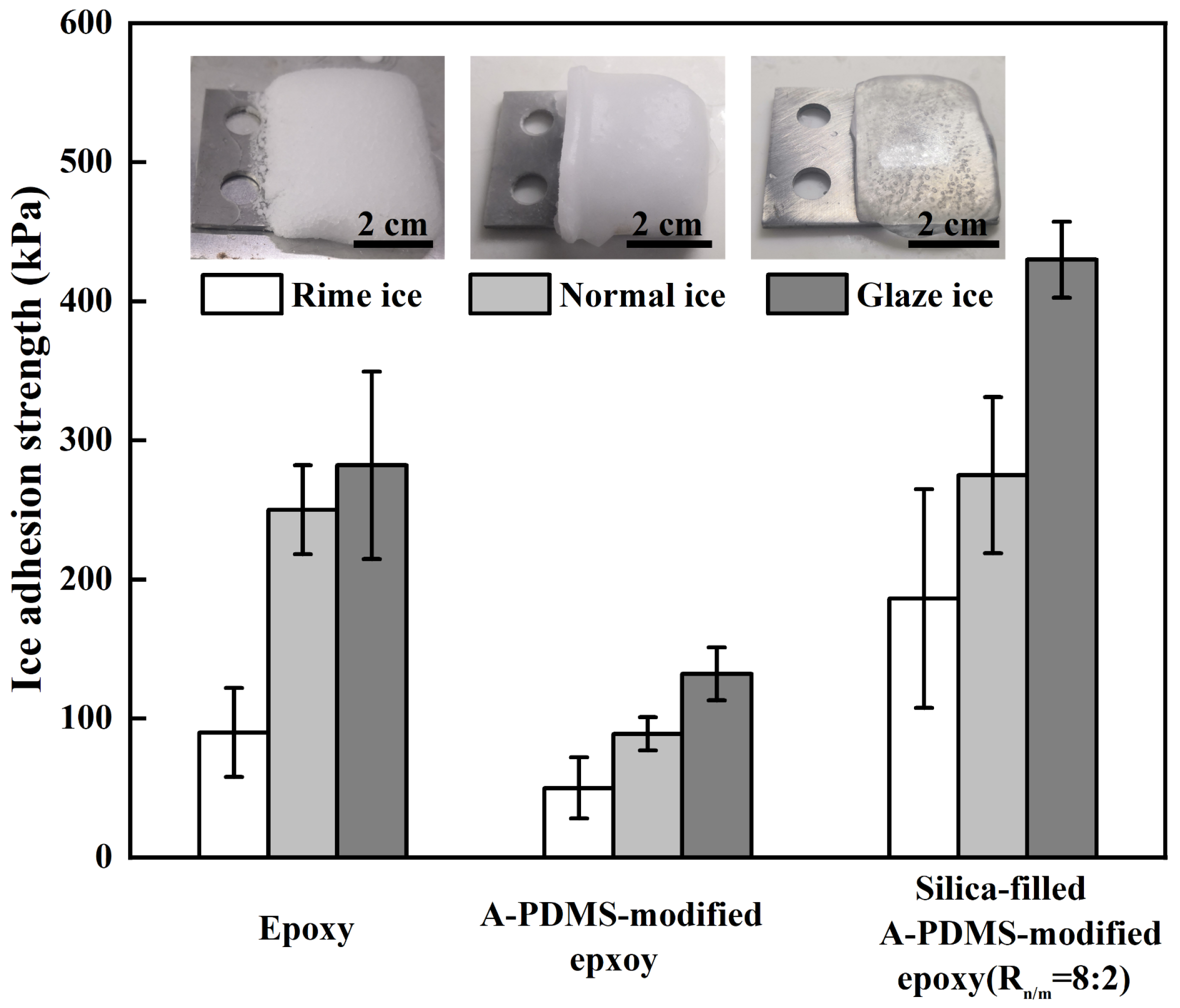
| Coatings | CA (°) | CAH (°) | Frequency of Failure Modes | ||
|---|---|---|---|---|---|
| Adhesive Failure | Cohesive Failure | Mixed Failure | |||
| Epoxy | 68.0 ± 1.2 | >30 | 3 | - | 2 |
| Epoxy + 5 phr of A-PDMS | 105.0 ± 1.1 | >30 | 5 | - | - |
| Epoxy + 10 phr of A-PDMS | 105.8 ± 0.9 | >30 | 5 | - | - |
| Epoxy + 15 phr of A-PDMS | 106.5 ± 1.2 | >30 | 5 | - | - |
| Epoxy + 20 phr of A-PDMS | 106.0 ± 0.7 | >30 | 4 | - | 1 |
| Epoxy + 25 phr of A-PDMS | 106.9 ± 1.0 | >30 | 5 | - | - |
| Epoxy + 30 phr of A-PDMS | 106.4 ± 1.2 | >30 | 5 | - | - |
| Epoxy + 35 phr of A-PDMS | 107.0 ± 1.5 | >30 | 5 | - | - |
| Epoxy + 40 phr of A-PDMS | 106.4 ± 1.3 | >30 | 5 | - | - |
| Epoxy + 25 phr of A-PDMS + silica (Rn/m = 0:10) | 108.1 ± 2.3 | >90 | - | 2 | 3 |
| Epoxy + 25 phr of A-PDMS + silica (Rn/m = 2:8) | 114.0 ± 3.5 | >90 | - | 1 | 4 |
| Epoxy + 25 phr of A-PDMS + silica (Rn/m = 4:6) | 115.3 ± 2.6 | >90 | - | 3 | 2 |
| Epoxy + 25 phr of A-PDMS + silica (Rn/m = 6:4) | 148.7 ± 1.9 | >90 | 1 | 2 | 2 |
| Epoxy + 25 phr of A-PDMS + silica (Rn/m = 8:2) | 158.3 ± 1.2 | <5 | 1 | 1 | 3 |
| Epoxy + 25 phr of A-PDMS + silica (Rn/m = 10:0) | 158.3 ± 1.3 | <5 | - | 3 | 2 |
| Temperature (°C) | 22 | 10 | 5 | 1 |
| SA (°) | 5 ± 2 | 10 ± 3 | 23 ± 7 | 37 ± 6 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, Q.; Hao, T.; Zhang, J.; Wang, C.; Zhang, R.; Qi, H. Anti-Icing Performance of a Coating Based on Nano/Microsilica Particle-Filled Amino-Terminated PDMS-Modified Epoxy. Coatings 2019, 9, 771. https://doi.org/10.3390/coatings9120771
Xie Q, Hao T, Zhang J, Wang C, Zhang R, Qi H. Anti-Icing Performance of a Coating Based on Nano/Microsilica Particle-Filled Amino-Terminated PDMS-Modified Epoxy. Coatings. 2019; 9(12):771. https://doi.org/10.3390/coatings9120771
Chicago/Turabian StyleXie, Qiang, Tianhui Hao, Jifeng Zhang, Chao Wang, Rongkui Zhang, and Hui Qi. 2019. "Anti-Icing Performance of a Coating Based on Nano/Microsilica Particle-Filled Amino-Terminated PDMS-Modified Epoxy" Coatings 9, no. 12: 771. https://doi.org/10.3390/coatings9120771
APA StyleXie, Q., Hao, T., Zhang, J., Wang, C., Zhang, R., & Qi, H. (2019). Anti-Icing Performance of a Coating Based on Nano/Microsilica Particle-Filled Amino-Terminated PDMS-Modified Epoxy. Coatings, 9(12), 771. https://doi.org/10.3390/coatings9120771





