Cactus Mucilage for Food Packaging Applications
Abstract
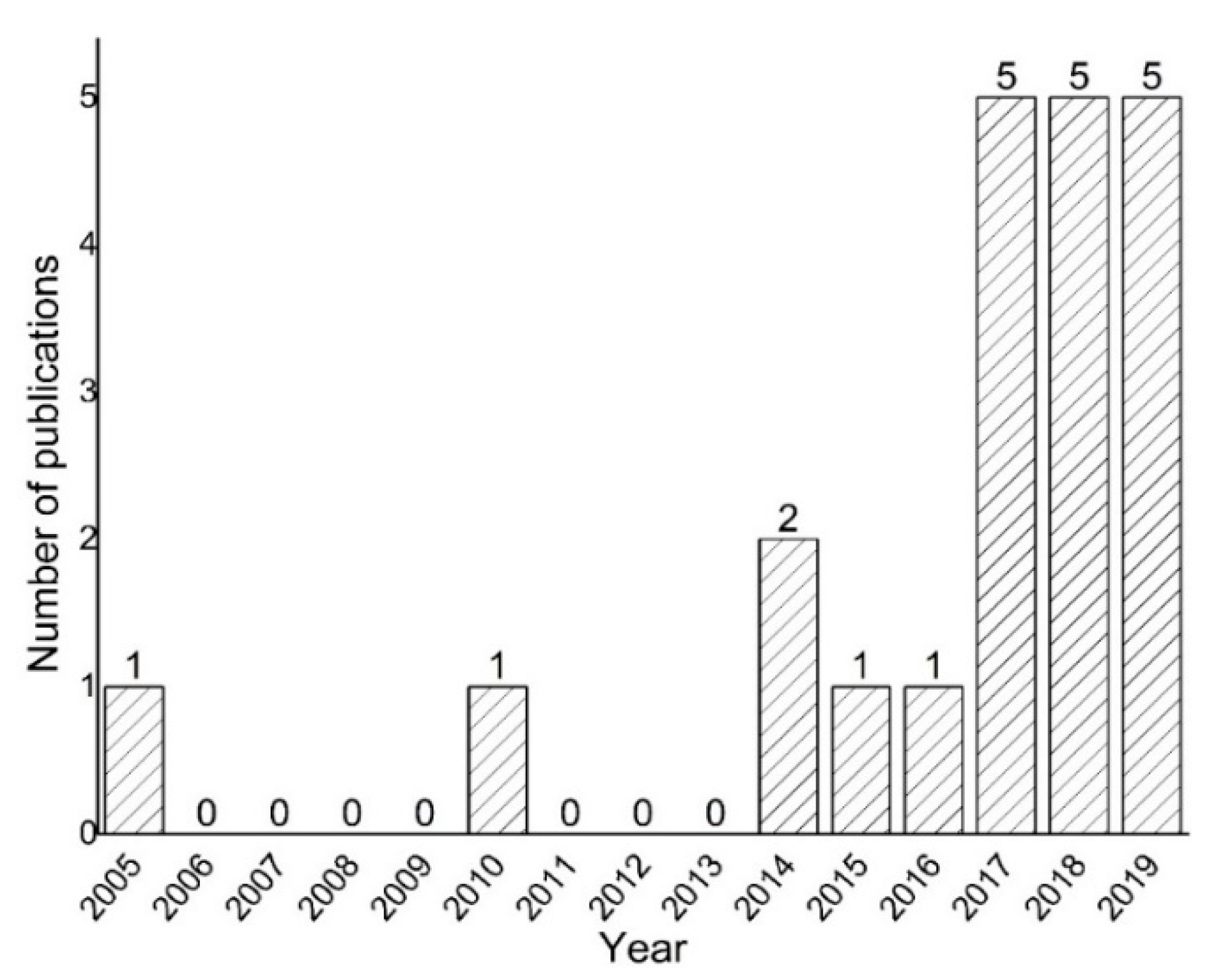
1. Introduction
2. Cactus Mucilage: Composition, Structure, Properties, and Applications
3. Cactus Mucilage for Developing Standalone Films
4. Cactus Mucilage as a Coating Material
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Morais, M.A.; Fonseca, K.S.; Viégas, E.K.D.; Almeida, S.L.; Maia, R.K.M.; Silva, V.N.S.; Simoes, A.N. Mucilage of spineless cactus in the composition of an edible coating for minimally processed yam (Dioscorea spp.). J. Food Meas. Charact. 2019, 13, 2000–2008. [Google Scholar] [CrossRef]
- Hassan, B.; Chatha, S.A.S.; Hussain, A.I.; Zia, K.M.; Akhtar, N. Recent advances on polysaccharides, lipids and protein based edible films and coatings: A review. Int. J. Biol. Macromol. 2018, 109, 1095–1107. [Google Scholar] [CrossRef] [PubMed]
- Aloui, H.; Khwaldia, K. Natural antimicrobial edible coatings for microbial safety and food quality enhancement. Compr. Rev. Food Sci. Food Saf. 2016, 15, 1080–1103. [Google Scholar] [CrossRef]
- Ozvural, E.B.; Huang, Q.; Chikindas, M.L. The comparison of quality and microbiological characteristic of hamburger patties enriched with green tea extract using three techniques: Direct addition, edible coating and encapsulation. LWT-Food Sci. Technol. 2016, 68, 385–390. [Google Scholar] [CrossRef]
- Aloui, H.; Jguirim, N.; Khwaldia, K. Effects of biopolymer-based active coatings on postharvest quality of okra pods in Tunisia. Fruits 2017, 72, 365–371. [Google Scholar] [CrossRef]
- Feng, Z.; Wu, G.; Liu, C.; Li, D.; Jiang, B.; Zhang, X. Edible coating based on whey protein isolate nanofibrils for antioxidation and inhibition of product browning. Food Hydrocoll. 2018, 79, 179–188. [Google Scholar] [CrossRef]
- Munhoz, D.R.; Moreira, F.K.V.; Bresolin, J.D.; Bernardo, M.P.; De Sousa, C.P.; Mattoso, L.H.C. Sustainable Production and in vitro biodegradability of edible films from yellow passion fruit co-products via continuous casting. ACS Sustain. Chem. Eng. 2018, 6, 9883–9892. [Google Scholar] [CrossRef]
- Ma, Q.; Liang, T.; Cao, L.; Wang, L. Intelligent poly (vinyl alcohol)-chitosan nanoparticles-mulberry extracts films capable of monitoring pH variations. Int. J. Biol. Macromol. 2018, 108, 576–584. [Google Scholar] [CrossRef]
- Ferreira, M.S.L.; Fai, A.E.C.; Andrade, C.T.; Picciani, P.H.; Azero, E.G.; Gonçalves, E.C. Edible films and coatings based on biodegradable residues applied to acerolas (Malpighiapunicifolia L.). J. Sci. Food Agric. 2016, 96, 1634–1642. [Google Scholar] [CrossRef]
- Valdés, A.; Garrigós, M.C. Carbohydrate-based advanced biomaterials for food sustainability: A review. Mater. Sci. Forum 2016, 842, 182–195. [Google Scholar] [CrossRef]
- Ochoa, M.J.; Barberab, G. History and economic and agro-ecological importance. In Crop Ecology, Cultivation and Uses of Cactus Pear; Mondragon, C., Nefzaoui, A., Sáenz, C., Eds.; The Food and Agriculture Organization of the United Nations and The International Center for Agricultural Research in the Dry Areas: Rome, Italy, 2017; pp. 1–11. [Google Scholar]
- Olatunji, O. Classification of Natural Polymers. In Natural Polymers; Olatunji, O., Ed.; Springer International Publishing: Cham, Switzerland, 2016; pp. 1–17. [Google Scholar]
- Kumar, A.; Madhusudana Rao, K.; Haider, A.; Han, S.S.; Son, T.W.; Kim, J.H.; Oh, T.H. Fabrication and characterization of multicomponent polysaccharide/nanohydroxyapatite composite scaffolds. Polym. Plast. Technol. Eng. 2017, 56, 983–991. [Google Scholar] [CrossRef]
- Kumar, A.; Rao, K.M.; Han, S.S. Application of xanthan gum as polysaccharide in tissue engineering: A review. Carbohydr. Polym. 2018, 180, 128–144. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Negi, Y.S.; Bhardwaj, N.K.; Choudhary, V. Synthesis and characterization of methylcellulose/PVA based porous composite. Carbohydr. Polym. 2012, 88, 1364–1372. [Google Scholar] [CrossRef]
- Stintzing, F.C.; Carle, R. Cactus stems (Opuntia spp.): A review on their chemistry, technology, and uses. Mol. Nutr. Food Res. 2005, 49, 175–194. [Google Scholar] [CrossRef] [PubMed]
- Salehi, E.; Emam-Djomeh, Z.; Fathi, M.; Askari, G. Opuntiaficus-indica Mucilage. In Emerging Natural Hydrocolloids; Razavi, S.M.A., Ed.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2019; pp. 425–449. [Google Scholar]
- Ribeiro, E.M.O.; Silva, N.H.; Lima Filho, J.L.; Brito, J.Z.; da Silva, M.P.C. Study of carbohydrates present in the cladodes of Opuntiaficus-indica (fodder palm), according to age and season. Ciênc. Tecnol. Aliment. 2010, 30, 933–939. [Google Scholar] [CrossRef]
- Sepúlveda, E.; Sáenz, C.; Aliaga, E.; Aceituno, C. Extraction and characterization of mucilage in Opuntia spp. J. Arid Environ. 2007, 68, 534–545. [Google Scholar] [CrossRef]
- Sáenz, C.; Sepúlveda, E.; Matsuhiro, B. Opuntia sppmucilage’s: A functional component with industrial perspectives. J. Arid Environ. 2004, 57, 275–290. [Google Scholar] [CrossRef]
- Petera, B.; Delattre, C.; Pierre, G.; Wadouachi, A.; Elboutachfaiti, R.; Engel, E.; Poughon, L.; Michaud, P.; Fenoradosoa, T.A. Characterization of arabinogalactan-rich mucilage from Cereus triangularis cladodes. Carbohydr. Polym. 2015, 127, 372–380. [Google Scholar] [CrossRef]
- Habibi, Y.; Mahrouz, M.; Marais, M.F.; Vignon, M.R. An arabinogalactan from the skin of Opuntiaficus-indica prickly pear fruits. Carbohydr. Res. 2004, 339, 1201–1205. [Google Scholar] [CrossRef]
- Matsuhiro, B.; Lillo, L.E.; Sáenz, C.; Urzúa, C.C.; Zárate, O. Chemical characterization of the mucilage from fruits of Opuntiaficusindica. Carbohydr. Polym. 2006, 63, 263–267. [Google Scholar] [CrossRef]
- Ammar, I.; Ennouri, M.; Attia, H. Phenolic content and antioxidant activity of cactus (Opuntiaficus-indica L.) flowers are modified according to the extraction method. Ind. Crop. Prod. 2015, 64, 97–104. [Google Scholar] [CrossRef]
- Amin, E.S.; Awad, O.M.; El-Sayed, M.M. The mucilage of Opuntiaficus-indica Mill. Carbohydr. Res. 1970, 15, 159–161. [Google Scholar] [CrossRef]
- Mc Garvie, D.; Parolis, H. The mucilage of Opuntiaficus-indica. Carbohydr. Res. 1979, 69, 171–179. [Google Scholar] [CrossRef]
- Trachtenberg, S.; Mayer, A.M. Composition and properties of Opuntiaficus-indica mucilage. Phytochemistry 1981, 20, 2665–2668. [Google Scholar] [CrossRef]
- Fox, D.I.; Pichler, T.; Yeh, D.H.; Alcantar, N.A. Removing heavy metals in water: The interaction of cactus mucilage and arsenate (As (V)). Environ. Sci. Technol. 2012, 46, 4553–4559. [Google Scholar] [CrossRef] [PubMed]
- Souza, M.T.F.; Almeida, C.A.; Ambrosio, E.; Santos, L.B.; Freitas, T.K.F.S.; Manholer, D.D.; Carvalho, G.M.; Garcia, J.C. Extraction and use of Cereus peruvianus cactus mucilage in the treatment of textile effluents. J. Taiwan Inst. Chem. Eng. 2016, 67, 174–183. [Google Scholar] [CrossRef]
- Di Lorenzo, F.; Silipo, A.; Molinaro, A.; Parrilli, M.; Schiraldi, C.; D’Agostino, A.; Izzo, E.; Rizza, L.; Bonina, A.; Bonina, F.; et al. The polysaccharide and low molecular weight components of Opuntiaficus-indica cladodes: Structure and skin repairing properties. Carbohydr. Polym. 2017, 157, 128–136. [Google Scholar] [CrossRef]
- Malviya, R.; Srivastava, P.; Kulkarni, G.T. Applications ofmucilages in drug delivery—Areview. Adv. Biol. Res. 2011, 5, 1–7. [Google Scholar]
- Del-Valle, V.; Hernández-Muñoz, P.; Guarda, A.; Galotto, M.J. Development of a cactus-mucilage edible coating (Opuntiaficusindica) and its application to extend strawberry (Fragariaananassa) shelf-life. Food Chem. 2005, 91, 751–756. [Google Scholar] [CrossRef]
- Gheribi, R.; Puchot, L.; Verge, P.; Jaoued-Grayaa, N.; Mezni, M.; Habibi, Y.; Khwaldia, K. Development of plasticized edible films from Opuntiaficus-indica mucilage: A comparative study of various polyol plasticizers. Carbohydr. Polym. 2018, 190, 204–211. [Google Scholar] [CrossRef]
- Gheribi, R.; Gharbi, M.A.; El Ouni, M.; Khwaldia, K. Enhancement of the physical, mechanical and thermal properties of cactus mucilage films by blending with polyvinyl alcohol. Food Packag. Shelf Life 2019, 22, 100386. [Google Scholar] [CrossRef]
- Lopez-Garcia, F.; Jimenez-Martinez, C.; Guzman-Lucero, D.; Maciel-Cerda, A.; Delgado-Macuil, R.; Cabrero-Palomino, D.; Terres-Rojas, E.; Arzate-Vazquez, I. Physical and chemical characterization of a biopolymer film Made with corn starch and nopalxoconostle (Opuntiajoconsotle) Mucilage. Rev. Mex. Ing. Quim. 2017, 16, 147–158. [Google Scholar]
- Guadarrama-Lezama, Y.; Castano, J.; Velazquez, G.; Carrillo-Navas, H.; Alvarez-Ramirez, J. Effect of nopal mucilage addition on physical, barrier and mechanical properties of citric pectin-based films. J. Food Sci. Technol. 2018, 55, 3739–3748. [Google Scholar] [CrossRef] [PubMed]
- Felkai-Haddache, L.; Remini, H.; Dulong, V.; Mamou-Belhabib, K.; Picton, L.; Madani, K.; Rihouey, C. Conventional and Microwave-Assisted Extraction of Mucilage from Opuntiaficus-indica Cladodes: Physico-Chemical and Rheological Properties. Food Bioprocess Technol. 2016, 9, 481–492. [Google Scholar] [CrossRef]
- Medina-Torres, L.; De La Fuente, E.B.R.; Torrestiana-Sanchez, B.; Katthain, R. Rheological properties of the mucilage gum (Opuntiaficusindica). Food Hydrocoll. 2000, 14, 417–424. [Google Scholar] [CrossRef]
- Kalegowda, P.; Chauhan, A.S.; Urs, S.M.N. Opuntiadillenii (Ker-Gawl) Haw cladode mucilage: Physico-chemical, rheological and functional behavior. Carbohydr. Polym. 2017, 157, 1057–1064. [Google Scholar] [CrossRef] [PubMed]
- Salehi, E.; Emam-Djomeh, Z.; Askari, G.; Fathi, M. Opuntiaficusindica fruit gum: Extraction, characterization, antioxidant activity and functional properties. Carbohydr. Polym. 2019, 206, 565–572. [Google Scholar] [CrossRef]
- Gheribi, R.; Habibi, Y.; Khwaldia, K. Prickly pear peels as a valuable resource of added-value polysaccharide: Study of structural, functional and film forming properties. Int. J. Biol. Macromol. 2019, 126, 238–245. [Google Scholar] [CrossRef]
- Han, Y.L.; Gao, J.; Yin, Y.Y.; Jin, Z.Y.; Xu, X.M.; Chen, H.Q. Extraction optimization by response surface methodology of mucilage polysaccharide from the peel of Opuntiadillenii haw. fruits and their physicochemical properties. Carbohydr. Polym. 2016, 151, 381–391. [Google Scholar] [CrossRef]
- Jouki, M.; Yazidi, F.T.; Mortazavi, S.A.; Koocheki, A. Quince seed mucilage films incorporated with oregano essential oil: Physical, thermal, barrier, antioxidant and antibacterial properties. Food Hydrocoll. 2014, 36, 9–19. [Google Scholar] [CrossRef]
- Dick, M.; Costa, T.M.H.; Gomaa, A.; Subirade, M.; Rios, A.O.; Flôres, S.H. Edible film production from chia seed mucilage: Effect of glycerol concentration on its physicochemical and mechanical properties. Carbohydr. Polym. 2015, 130, 198–205. [Google Scholar] [CrossRef] [PubMed]
- Karami, N.; Kamkar, A.; Shahbazi, Y.; Misaghi, A. Edible films based on chitosan-flaxseed mucilage: In vitro antimicrobial and antioxidant properties and their application on survival of food-borne pathogenic bacteria in raw minced trout fillets. Pharm. Biomed. Res. 2019, 5, 10–16. [Google Scholar]
- Sadeghi-Varkani, A.; Emam-Djomeh, Z.; Askari, G. Physicochemical and microstructural properties of a novel edible film synthesized from Balangu seed mucilage. Int. J. Biol. Macromol. 2018, 108, 1110–1119. [Google Scholar] [CrossRef] [PubMed]
- Beigomi, M.; Mohsenzadeh, M.; Salari, A. Characterization of a novel biodegradable edible film obtained from Dracocephalummoldavica seed mucilage. Int. J. Biol. Macromol. 2018, 108, 874–883. [Google Scholar] [CrossRef] [PubMed]
- Cotrim, M.; Mottin, A.; Ayres, E. Preparation and characterization of okra mucilage (Abelmoschusesculentus) edible films. Macromol. Symp. 2016, 367, 90–100. [Google Scholar] [CrossRef]
- Espino-Díaz, M.; De Jesús Ornelas-Paz, J.; Martínez-Téllez, M.A.; Santillán, C.; Barbosa-Cánovas, G.V.; Zamudio-Flores, P.B.; Olivas, G.I. Development and characterization of edible films based on mucilage of Opuntiaficus-Indica (L.). J. Food Sci. 2010, 75, E347–E352. [Google Scholar] [CrossRef] [PubMed]
- Lira-Vargas, A.A.; Corrales-Garcia, J.J.E.; Valle-Guadarrama, S.; Peña-Valdivia, C.B.; Trejo-Marquez, M.A. Biopolymeric films based on cactus (Opuntiaficus-indica) mucilage incorporated with gelatin and bees wax. J. Prof. Assoc. Cactus Dev. 2014, 16, 51–70. [Google Scholar]
- Dominguez-Martinez, B.M.; Martinez-Flores, H.E.; Berrios, J.J.; Otoni, C.G.; Wood, D.F.; Velazquez, G. Physical characterization of biodegradable films based on chitosan, polyvinyl alcohol and Opuntia Mucilage. J. Polym. Environ. 2017, 25, 683–691. [Google Scholar] [CrossRef]
- Damas, M.S.P.; Pereira Junior, V.A.; Nishihora, R.K.; Quadri, M.G.N. Edible films from mucilage of Cereus hildmannianus fruits: Development and characterization. J. Appl. Polym. Sci. 2017, 134, 45223. [Google Scholar] [CrossRef]
- Oliveira, N.L.; Rodrigues, A.A.; Neves, I.C.O.; Lago, A.M.T.; Borges, S.V.; De Resende, J.V. Development and characterization of biodegradable films based on Pereskiaaculeata Miller mucilage. Ind. Crop. Prod. 2019, 130, 499–510. [Google Scholar] [CrossRef]
- Trevino-Garza, M.Z.; García, S.; Heredia, N.; Alanís-Guzmán, M.G.; Arévalo-Nino, K. Layer-by-layer edible coatings based on mucilages, pullulan and chitosan and its effect on quality and preservation of fresh-cut pineapple (Ananascomosus). Postharvest Biol. Technol. 2017, 128, 163–175. [Google Scholar] [CrossRef]
- Oluwaseun, A.C.; Samuel, O.F.; Sunday, A.E. Effects of Opuntia cactus mucilage extract and storage under evaporative coolant system on the shelf life of Carica papaya fruits. J. Agrobiotechnol. 2014, 5, 49–66. [Google Scholar]
- Allegra, A.; Sortino, G.; Inglese, P.; Settanni, L.; Todaro, A.; Gallotta, A. The effectiveness of Opuntiaficus-indica mucilage edible coating on post-harvest maintenance of ‘Dottato’fig (Ficuscarica L.) fruit. Food Packag. Shelf Life 2017, 12, 135–141. [Google Scholar] [CrossRef]
- Zegbe, J.A.; Mena-Covarrubias, J.; Dominguez-Canales, V.S.I. Cactus mucilage as a coating film to enhance shelf life of unprocessed guavas (Psidiumguajava L.). Acta Hortic. 2015, 1067, 423–427. [Google Scholar]
- Bernardino-Nicanor, A.; Montañez-Soto, J.L.; Conde-Barajas, E.; Negrete-Rodríguez, M.L.X.; Teniente-Martínez, G.; Vargas-León, E.A.; Juárez-Goiz, J.M.S.; Acosta-García, G.; González-Cruz, L. Spectroscopic andstructuralanalysesof Opuntia Robusta Mucilage and itspotential as anediblecoating. Coatings 2018, 8, 466. [Google Scholar] [CrossRef]
- Zambrano, J.; Valera, A.M.; Materano, W.; Maffei, M.; Quintero, I.; Ruiz, Y.; Marcano-Belmonte, D. Effect of edible coatings based on cactus mucilage (Opuntiaelatior Mill.) in the physicochemical and sensory properties of guava (Psidiumguajava L.) under controlled storage. Rev. Fac. Agron. 2018, 35, 476–495. [Google Scholar]
- GirmaAbera, N.; Kebede, W.; Wassu, M. Effect of Aloe gel and cactus mucilage coating on chemical quality and sensory attributes of mango (Mangiferaindica L.). J. Postharvest Technol. 2019, 7, 31–43. [Google Scholar]
- Allegra, A.; Gallotta, A.; Carimi, F.; Mercati, F.; Inglese, P.; Martinelli, F. Metabolic profiling and post-harvest behavior of “Dottato” Fig (Ficuscarica L.) fruit covered with an edible coating from O. ficus-indica. Front. Plant Sci. 2018, 9, 1321. [Google Scholar] [CrossRef]
- Allegra, A.; Inglese, P.; Sortino, G.; Settanni, L.; Todaro, A.; Liguori, G. The influence of Opuntiaficus-indica mucilage edible coating on the quality of ‘Hayward’ kiwifruit slices. Postharvest Biol. Technol. 2016, 120, 45–51. [Google Scholar] [CrossRef]
| Raw Material | Extraction | Yield of Extraction (% Dry Weight) | Composition and Structure | Properties and Applications | Reference |
|---|---|---|---|---|---|
| Cladodes Opuntiaficusindica | Homogenization in water with blender Filtration Centrifugation Lyophilization Resuspension in TCA solution Dialyze against water Addition of ethanol Centrifugation lyophilization | 1.124 mg/mL of tissue | Arabinose 67% Galactose 6% Xylose 20% Rhamnose 5% | MW 4.3 × 106 g/mol Water holding capacity | [27] |
| Cladodes Opuntia ficus indica | Mechanical press | - | Two polysaccharidic entities: Linear β-(1-4)-galactose polymer and highly branched xyloarabinan | Moisturize and heal cutis favoring cutaneous reparative processes Wound healing properties | [30] |
| Cladodes Opuntia ficus indica | Mechanical press of cladodes inner part Precipitation with ethanol | 14% | Galactose 40% Arabinose 30% Xylose, rhamnose, glucose: Minor sugars NMR specific signals of arabinogalactan polysaccharide | Film-forming properties | [33] |
| Cladodes Opuntia ficus indica | Maceration in water, assisted with a microwave Precipitation with ethanol | 8% | Arabinose Galactose Rhamnose Xylose Acide galacturonique | Viscoelastic behavior MW 16.7–17.5 × 106 g/mol. | [37] |
| Cladodes Opuntia ficus indica | Maceration Centrifugation Decantation Precipitation with acetone Washing with isopropyl alcohol | - | Arabinose 44% Galactose 20% Xylose 22% Rhamnose 7% Galacturonic acid 6% | MW 2.3 × 104 g/mol Non-Newtonian shear-thinning behavior High elastic properties similar to synthetic polymers like polyisobutylene At low concentrations (<3%): Typical behavior of dilute solution At high concentrations: Weak gel behavior | [38] |
| Cladodes Opuntia dillenii haw | Maceration in water, Precipitation with ethanol | 6% | Arabinose 39% Galactose 33% Rhamnose 16% Xylose 5% Glucose 5% | Pseudo plastic behavior Good swelling index High water-holding capacity Anti-obesity property through lipase inhibition | [39] |
| Cladodes Cereus triangularis | Maceration in water Precipitation with ethanol | 24% | Galactan backbone composed of (1→4) linked β-D-Galp residues substituted by L-arabinofuranosyl residues | MW 8.4 × 106 g/mol Antioxidant activity | [21] |
| Pulp Cereus peruvianus | The plant was manually pealed, and the pulp was recovered Solvent extraction in saline solution composed of NaCl, KCl, and NaNO3 Filtration | - | Characteristic FTIR pics of complex polysaccharides | Partially crystalline structure Treatment of textile effluents by coagulation/flocculation | [29] |
| Fruit pulp Opuntiaficusindica | Blending with screw press Filtration, centrifugation Dialysis against water Precipitation with ethanol | 3.8% | Uronic acid 23% Arabinose, rhamnose, xylose, galactose: 1.0:1.7:2.5:4.1 (ratio) complex mixture of polysaccharides | - | [23] |
| Fruit pulp Opuntiaficusindica | Mixing in water Microwave-assisted extraction Filtration Freeze drying | - | Glucose 78% Arabinose 13% Xylose 5% Galactose 2% Mannose 2% Arabinoglucan structure | MW 3.67 × 106 g/mol Shear-thinning behavior Thickening, stabilizing, and antioxidant properties Anti-DPPH radical scavenging activity comparable to that of BHT | [40] |
| Fruit peels Opuntiaficusindica | Maceration in water Precipitation with ethanol | 4% | Arabinose 33% Galactose 23% Galacturonic acid 14% Arabinogalactan structure | - | [22] |
| Fruit peels Opuntia ficus indica | Mechanical press Precipitation with ethanol | 3% | Galactose 54% Arabinose 34% Xylose 10% Galacturonic acid 9% Backbone chain made of (1➔4) linked β-D-Galp residues | Film-forming properties Emulsifying and foaming properties Good water-holding capacity | [41] |
| Fruit peels Opuntiadillenii haw | Microwave assisted extraction Precipitation with ethanol | 16% | - | Gelling properties Good thermal stability | [42] |
| Composition | Mucilage Extraction | Film-Forming Conditions | Main Properties | References |
|---|---|---|---|---|
| Mucilage (cladodes of Opuntia ficus indica + PVA | Pressing of cladodes inner part, filtration, precipitation with ethanol, drying (50 °C, 24 h) | Mucilage/PVA (90:10, 80:20, 70:30 and 60:40) PEG 200 30% Casting onto plastic petri dishes Drying at 50 °C for 24 h Storage at 53% RH and 25 °C | Thickness 0.16–0.19 mm WVP 35–474 g mm/m2 d kPa TS 2–6 MPa EB50–60% WCA90°–115° Tg 39–60 °C Tm 198–213 °C | [34] |
| Mucilage (Opuntia ficus indica fruit peels) | Pressing of cladodes inner part, filtration, precipitation with ethanol, drying (50 °C, 24 h) | Mucilage 4% wt/wt Glycerol 40% Casting onto plastic petri dishes Drying at 40 °C for 48 h Storage at 53% RH and 25 °C | Thickness 0.17 mm WVP 53 g mm/m2 d kPa TS ~1 MPa EB ~66% WCA ~91° Tg 41 °C | [41] |
| Mucilage (Pereskia aculeata Miller leaves) | Homogenization with water in a blender, filtration, centrifugation, precipitation, freeze drying | Mucilage 1.5–2% Glycerol 20–25% | TS 1.2–5.2 MPa EB 22%–46% YB 5.4–69 MPa | [53] |
| Citric pectin + Mucilage (Opuntia ficus indica cladodes) | Immersion in CaCl2 solution for 24 h, filtration, storage at 4 °C | Citric pectin 2 g/100 mL water Cactus mucilage 5, 10, 12, 14 16, 18, and 20 g/100 g water Glycerol 5 mL Casting onto acrylic plates Drying at 50 °C, overnight Storage 52% RH at 25 °C | WVP 1.5–1.7 × 10−9 g/m d Pa TS 0.5–0.8 MPa YM 0.9–1.7 MPa EB 25%–41% Tm 209–310 °C | [36] |
| Mucilage (cladodes of Opuntia ficus indica) + plasticizers (glycerol, sorbitol, PEG 200 and PEG 400) | Pressing of cladodes inner part, filtration, precipitation with ethanol, drying (50 °C, 24 h) | Mucilage 4%, plasticizer 40% Casting onto plastic petri dishes Drying 40 °C, 48 h Storage 53% RH, 25 °C, 48 h | Thickness ~0.2 mm WVP 22–64 g mm/m2 d kPa TS 1–2.5 MPa EB 50%–65% WCA 85° Tg 30–50 °C | [33] |
| Starch + PVA + mucilage (Opuntia joconsotle) + chitosan+ glycerol | Direct mucilage: Grinding, filtration, centrifugation Extracted mucilage: Precipitation with ethanol, pH adjusted to 3.5 with HCl | Mucilage 2.5–27% PVA 11–14% Chitosan 11–16% Starch 27–36% Glycerol 22–30% Casting onto glass petri dishes Drying in 35 °C for 48 h Storage in polyethylene bags in a desiccator at 22 °C | YM ~0.2 GPa H 19–22 MPa | [35] |
| PVA + chitosan + mucilage (Opuntia tomentosa) | Mixing in blender, centrifugation, precipitation with ethanol | Mucilage 10% PVA 8%, 23%, 38%, 53%, and 68% Chitosan 8%, 23%, 38%, 53%, and 68% Glycerol 14% Casting onto glass plates Drying with a convective dehydrator at 40 °C for 4 h | Thickness 0.05–0.07 mm WVP 3066–852 mL/mm2 d Pa TS 30–50 MPa EB 10–70% | [51] |
| Mucilage (Cereus hildmannianus fruits) | Water extraction at 60 °C, Filtration, centrifugation, Precipitation with ethanol, washing with acetone, drying (40 °C, 24 h) | Mucilage 1% Glycerol 1%–4% Casting onto Teflon plates Drying at 23 °C for 48 h Storage at 55% RH and 23 °C | Thickness 0.1–0.17 mm WVP 0.32–1.1 g mm/m2 h kPa TS 3–28 MPa EB 0.4–19% YM40-2359 MPa WCA 75°–108° | [52] |
| Mucilage (Opuntia ficus indica cladodes) + gelatin + beeswax | Mixing with water at 90 °C, decantation, centrifugation, precipitation with ethanol, dialyze, freeze drying | Mucilage 0.5% (30 °C) Gelatin 0.25–0.5% (60 °C) Beeswax 0.25–0.5% (60 °C) Glycerol 0.6%, Tween 80 0.4% Casting and drying at 24 °C, 50% RH for 1–3 days | Thickness 0.02–0.04 mm WVP 13–116 × 10–12 mol m/s m2 Pa O2P 3–14 × 10−12 mol m/s m2 Pa CO2P 3–9 × 10−12 mol m/s m2 Pa TS 0.5–2.7 MPa | [50] |
| Mucilage (Opuntiaficus indica cladodes) + glycerol + CaCl2 | Crushing, homogenization in water at 85°, filtration, centrifugation, precipitation with ethanol, washing with ethanol, freeze drying | Mucilage 4%, glycerol 50%, CaCl2 30% pH (3, 4, 5.6, 7, 8) RH 30%, 25 °C. Casting onto glass petri dishes coated with Teflon Drying at room temperature for 24h Storage at 50% RH and 25 °C | Thickness 0.109–0.131 mm WVP 98–147 g mm/m2 d KPa TS 0.3–0.95 MPa EB 15–24% | [49] |
| Composition | Mucilage Extraction | Coated Product | Coating Method and Conditions | Main Effects | References |
|---|---|---|---|---|---|
| Mucilage (spineless cactus cladodes) + cassava starch + glycerol | Immersion in a solution containing 5 mg/L citric acid | Minimally processed yam (Dioscorea spp.) | Immersion Storage in Nylon packages for 10 days at 5 °C. | Fresh mass loss was reduced Visual and sensory quality were maintained Increase in phenolic compounds | [1] |
| Mucilage (cladodes of O. ficusindica +Aloe debrana) | Cladodes were pressed and sieved | Mango (Mangiferaindica L.) | Dipping | Quality deterioration was slowed Good appearance was maintained Total soluble solids content was maintained after 16 days of storage Organoleptic properties of mucilage-coated fruits were better than control and aloe gel-coated fruits | [60] |
| Mucilage (cladodes of O. elatior Mill.) | - | Guava (Psidiumguajava L.) | Immersion Storage at 10 °C for 4–16 days | Reduction of weight and firmness loss | [59] |
| Mucilage (from parenchymatous and chlorenchymatous tissues of O. Robusta) | Extraction with water or ethanol from parenchyma or chlorenchyma (high speed blending), filtration, drying. | Tomatoes (Lycopersicumsculentum) | Brushing (3 times) Storage at 20 °C | Enhanced firmness Reduced weight loss Fruit ripening during storage was delayed | [58] |
| Mucilage (cladodes of O. ficusindica) + glycerol | Crushing of cladodes, homogenization in water, filtration, precipitation with ethanol, drying | ‘Dottato’ fig (Ficuscarica L.) fruit | Dipping Storage in refrigerator at 4 °C and 85% RH for 14 days | Weight loss was decreased Fig shelf life was extended Brightness, visual appearance, and firmness were maintained Lower microbial cell densities Reduced Enterobacteriaceae counts Coating attenuated the decrease in amino acids’ content and increased the amount of carbohydrates and other key metabolites | [57,61] |
| Mucilage (cladodes of O. ficusindica) + glycerol +chitosan | Blending of cladodes, homogenization in water, centrifugation, precipitation with ethanol, drying | Fresh-cut pineapple (Ananascomosus) | Dipping using layer-by-layer process Storage in plastic containers at 4 °C for 18 days. | Weight loss and softening of fruits were reduced Color, odor, flavor, and texture were preserved Sensory acceptance was extended by 6 days in comparison with the control | [54] |
| Mucilage (cladodes of O. ficusindica) + glycerol/tween 20 | Crushing of cladodes, homogenization in water, filtration, precipitation with ethanol, drying | Fresh kiwifruit (Actinidiadeliciosa) slices | Dipping Storage in sealed polyethylene terephthalate packages at 5 °C and 90% RH for 12 days | Firmness as well as ascorbic acid and pectin contents were maintained Visual quality and flavor were preserved | [62] |
| Mucilage (cladodes of O. ficusindica) + glycerol/PEG | Homogenization in water, filtration, precipitation with ethanol, drying | Unprocessed Guavas (PsidiumGuajava L.) | Fruits were coated with processed films Storage for 6–8 days at 27 °C and 20% RH | Extended shelf life Quality attributes were maintained High firmness Total soluble solids and dry matter concentrations were maintained | [57] |
| Mucilage (cladodes of O. ficusindica) | Homogenization in water, centrifugation | Carica papaya Fruit | Dipping, drying Storage for 6 weeks at 27 °C and 55%–60% RH | Higher firmness Extended shelf life Lower microbial load (total aerobic psychrotrophic) | [37] |
| Mucilage (cladodes of O. ficusindica) + glycerol | Homogenization in water, centrifugation | Strawberry (Fragariaananassa) | Dipping, drying Storage for 10 days at 5 °C and 75% RH | Extended shelf life Greater firmness Color was not affected by coating Sensorial analysis revealed consumer’s preference for coated fruits over uncoated ones | [32] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gheribi, R.; Khwaldia, K. Cactus Mucilage for Food Packaging Applications. Coatings 2019, 9, 655. https://doi.org/10.3390/coatings9100655
Gheribi R, Khwaldia K. Cactus Mucilage for Food Packaging Applications. Coatings. 2019; 9(10):655. https://doi.org/10.3390/coatings9100655
Chicago/Turabian StyleGheribi, Rim, and Khaoula Khwaldia. 2019. "Cactus Mucilage for Food Packaging Applications" Coatings 9, no. 10: 655. https://doi.org/10.3390/coatings9100655
APA StyleGheribi, R., & Khwaldia, K. (2019). Cactus Mucilage for Food Packaging Applications. Coatings, 9(10), 655. https://doi.org/10.3390/coatings9100655






