Characterization and Testing of a Novel Sprayable Crosslinked Edible Coating Based on Salmon Gelatin
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Salmon Gelatin Production
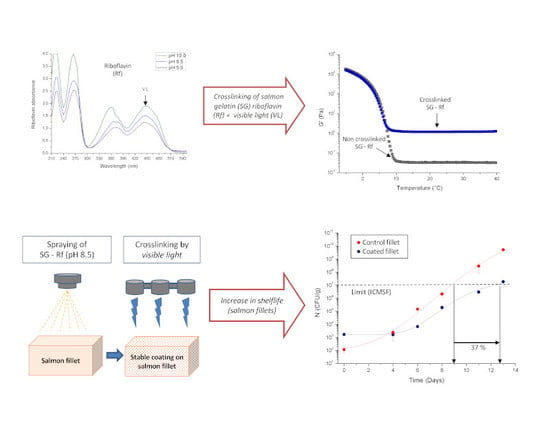
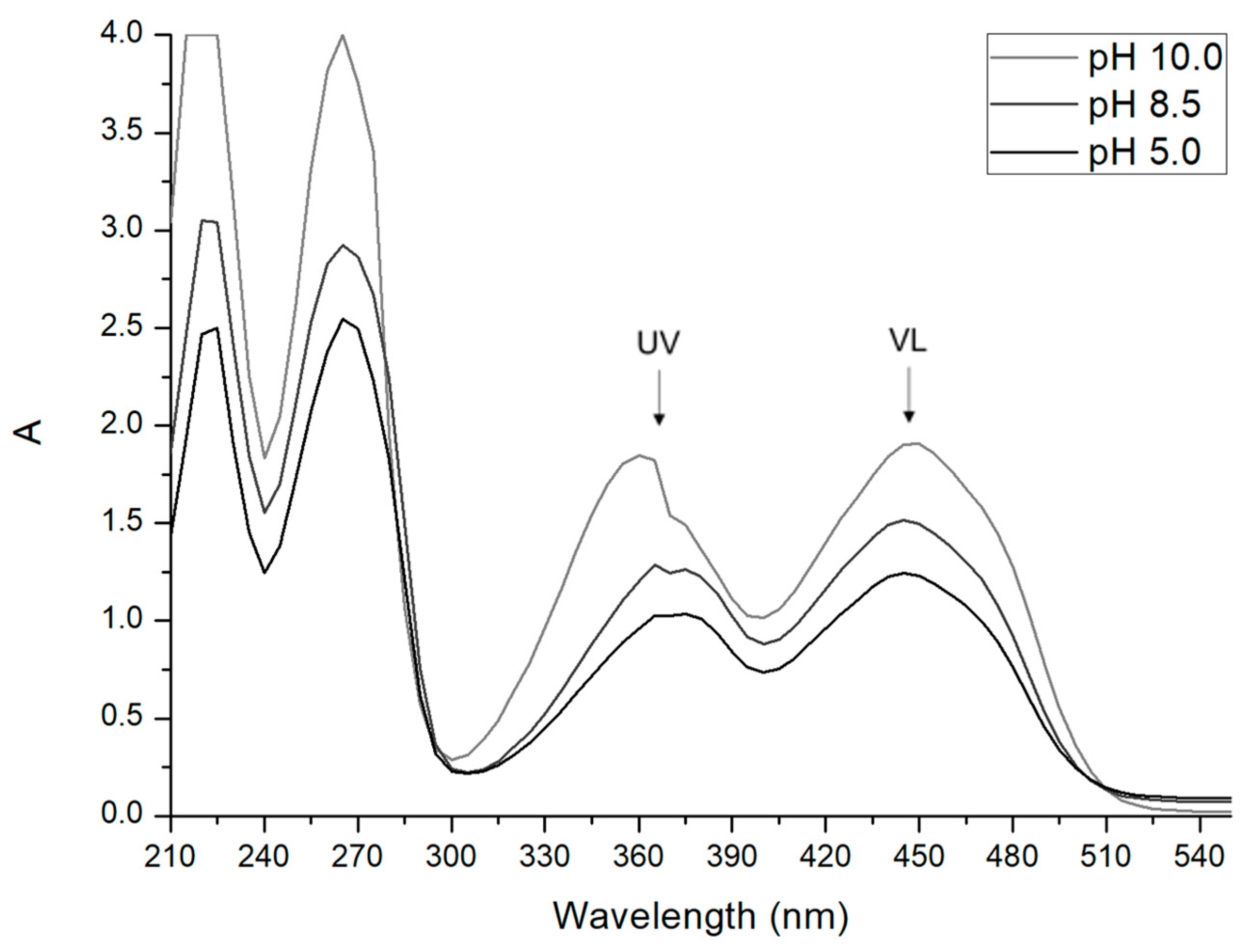
2.3. Effect of pH on Riboflavin Absorbance Spectrum
2.4. Preparation of Salmon Gelatin–Riboflavin (SG–Rf) Suspensions
2.5. Preparation of Salmon Gelatin–Riboflavin Hydrogels
2.6. Characterization of Salmon Gelatin–Riboflavin Hydrogels
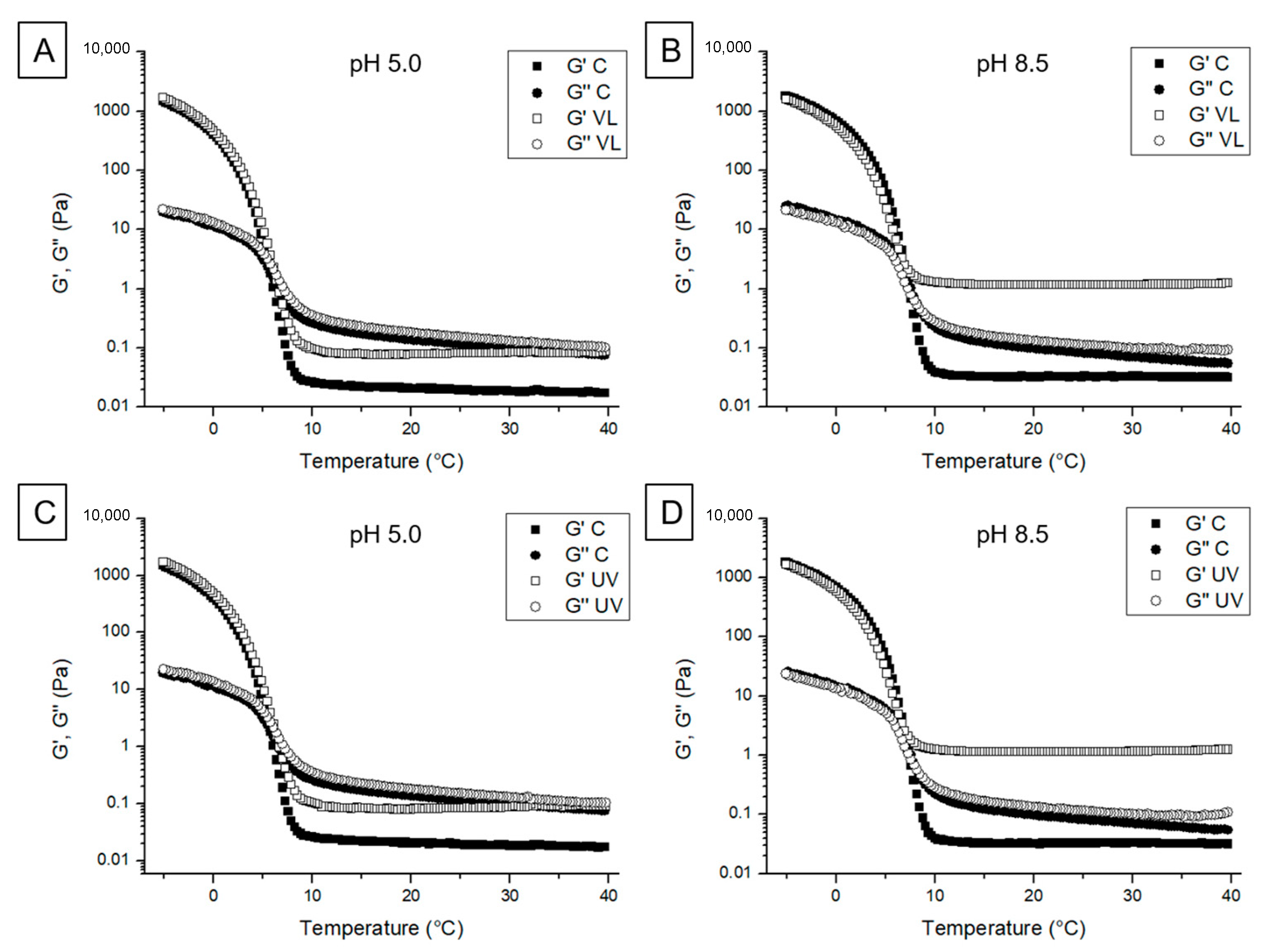
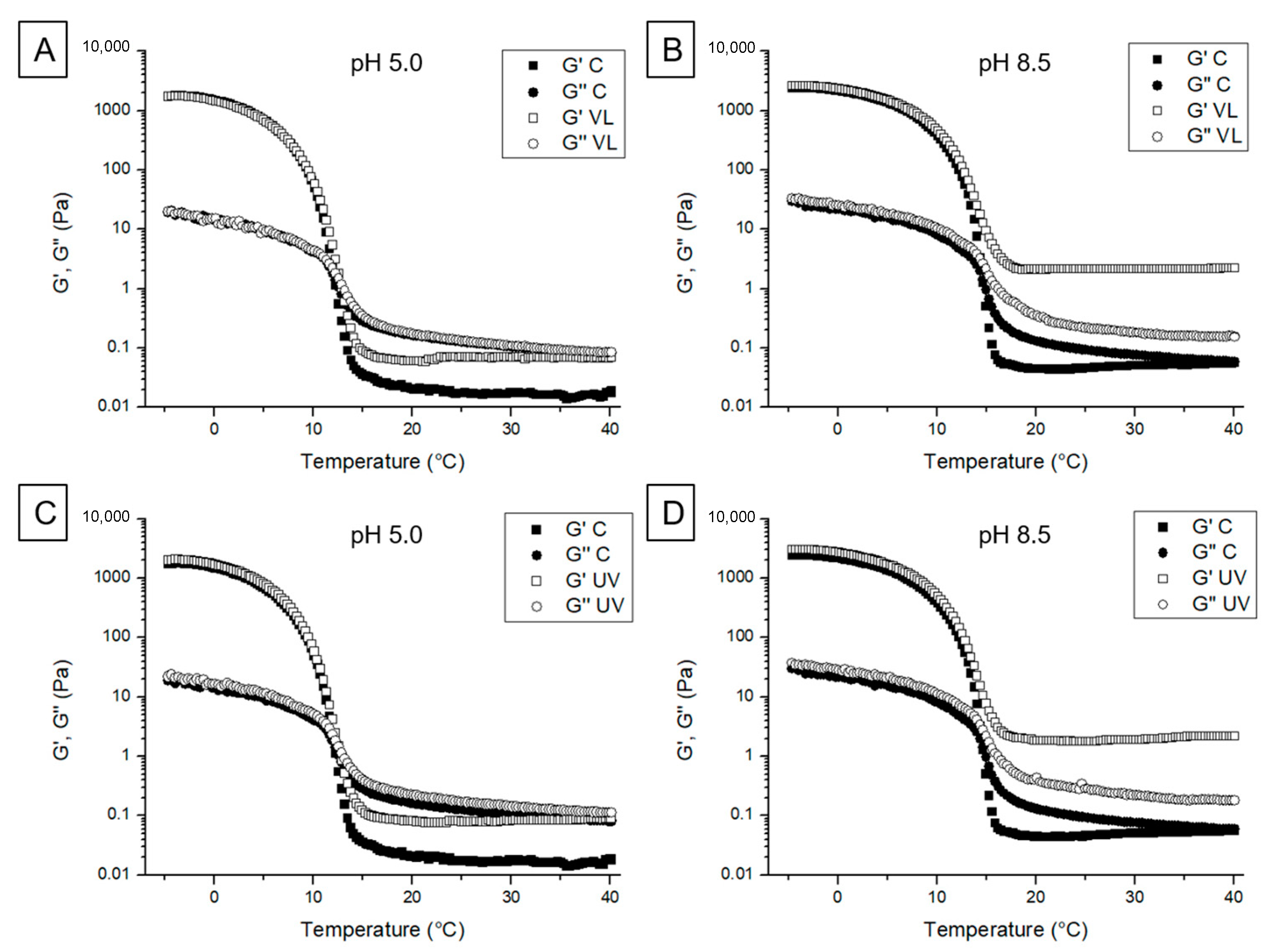
2.6.1. Viscoelastic Behavior
2.6.2. Apparent Viscosity
2.6.3. Thermal Properties
2.6.4. Light Transmittance
2.7. Evaluation of Salmon Gelatin–Riboflavin Hydrogels as a Sprayable Edible Coating
2.7.1. Coating of Salmon Fillets
2.7.2. Weight Loss of Salmon Fillets
2.7.3. pH Evolution of Salmon Fillets
2.7.4. Color Evolution of Salmon Fillets
2.7.5. Microbial Stability of Salmon Fillets
2.7.6. Statistical Analysis
3. Results and Discussion
3.1. Effect of pH on Riboflavin Absorbance Spectrum
3.2. Characterization of Salmon Gelatin Hydrogels
3.2.1. Viscoelastic Behavior
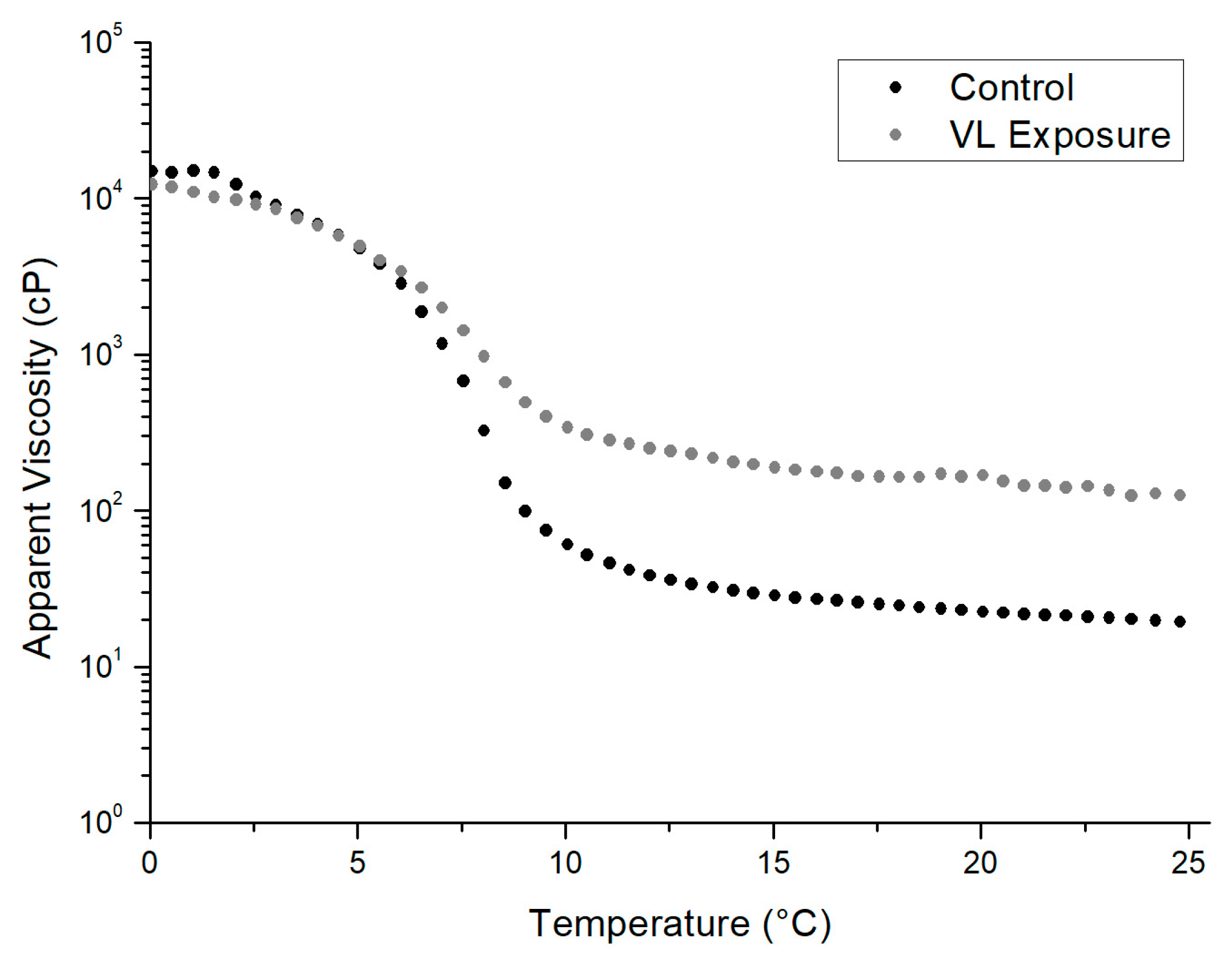
3.2.2. Apparent Viscosity
3.2.3. Thermal Properties
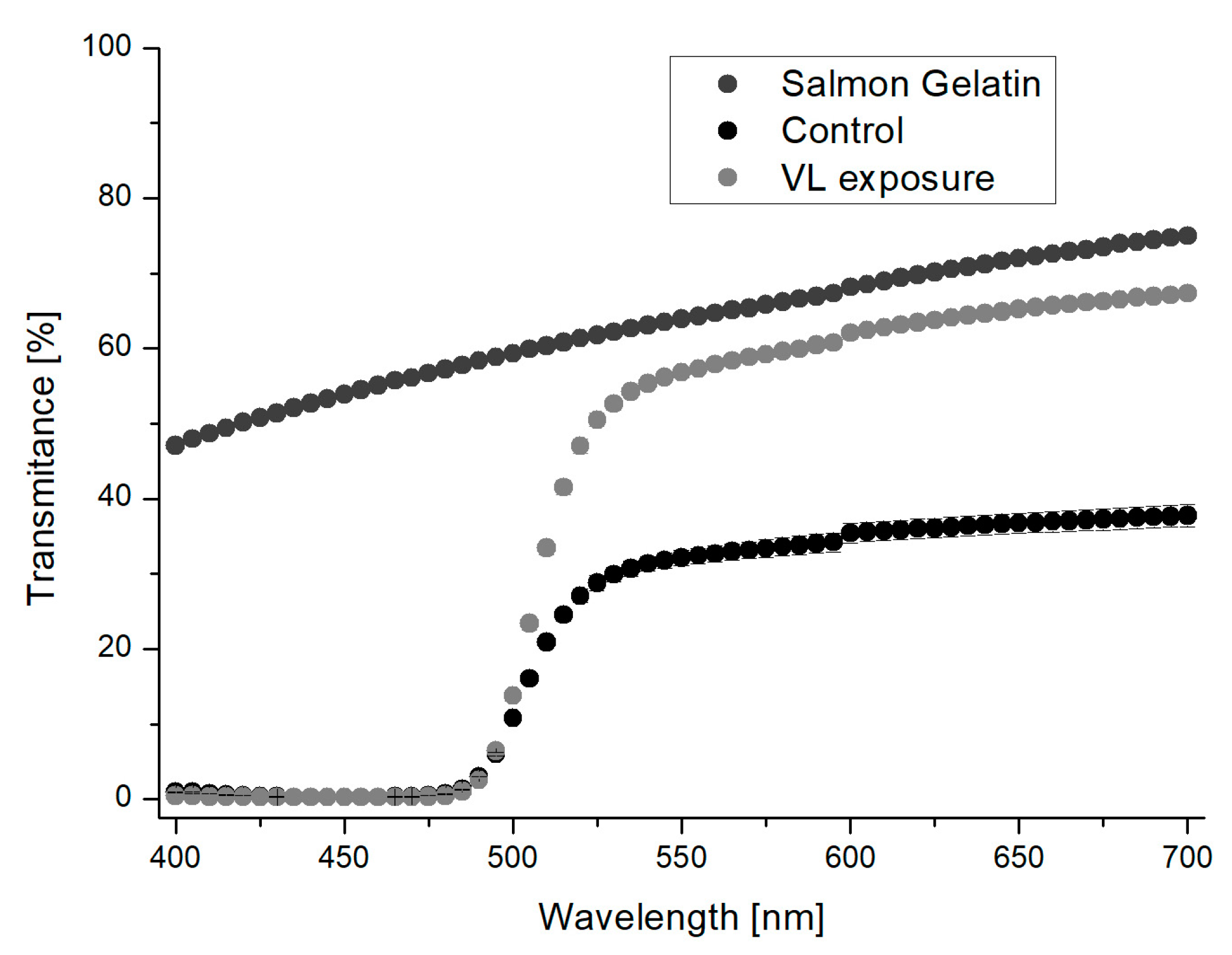
3.2.4. Light Transmittance
3.3. Evaluation of Salmon Gelatin–Riboflavin Hydrogels as a Sprayable Edible Coating
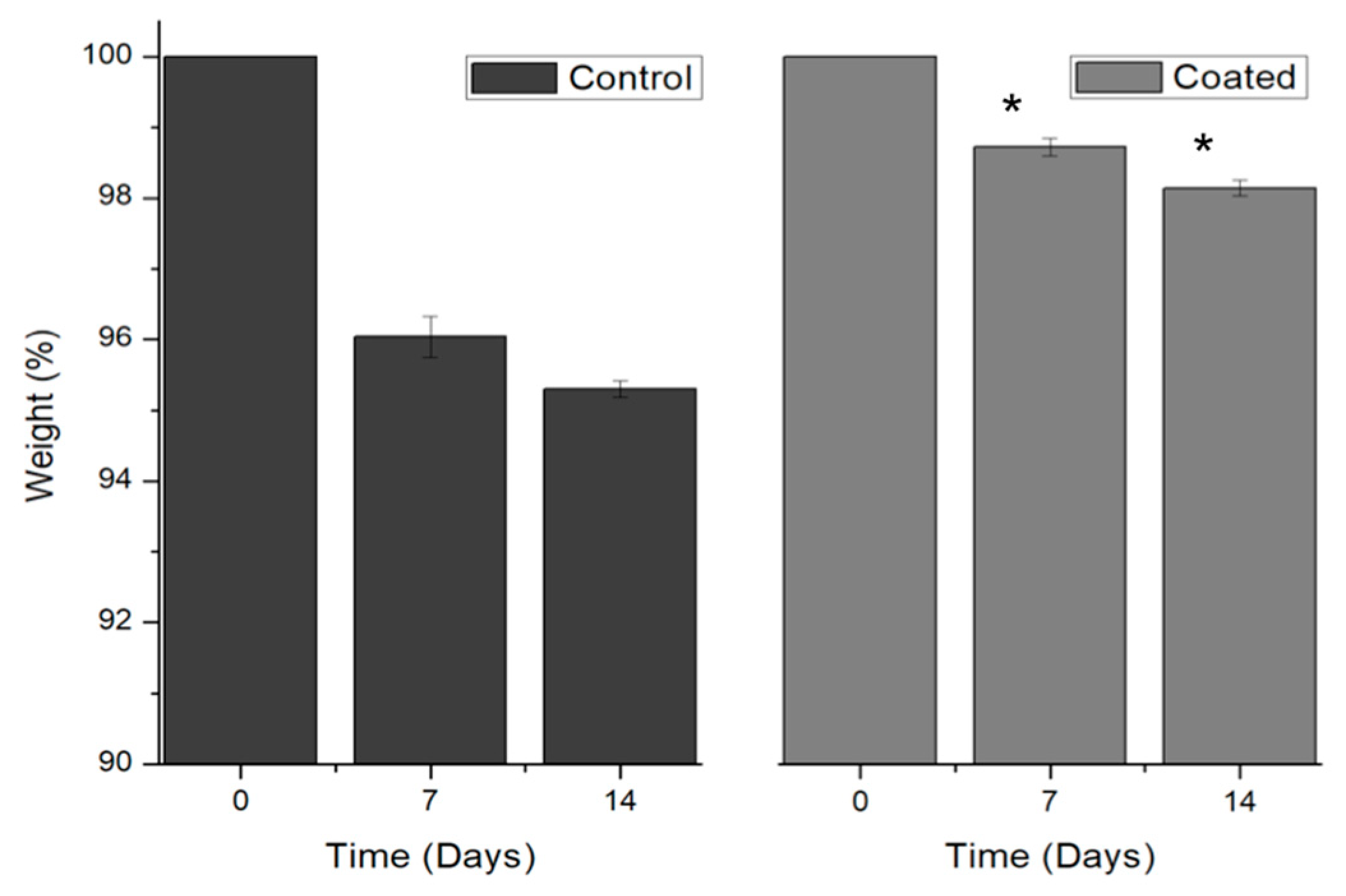
3.3.1. Moisture loss of coated salmon fillets
3.3.2. pH Evolution of Coated Salmon Fillets
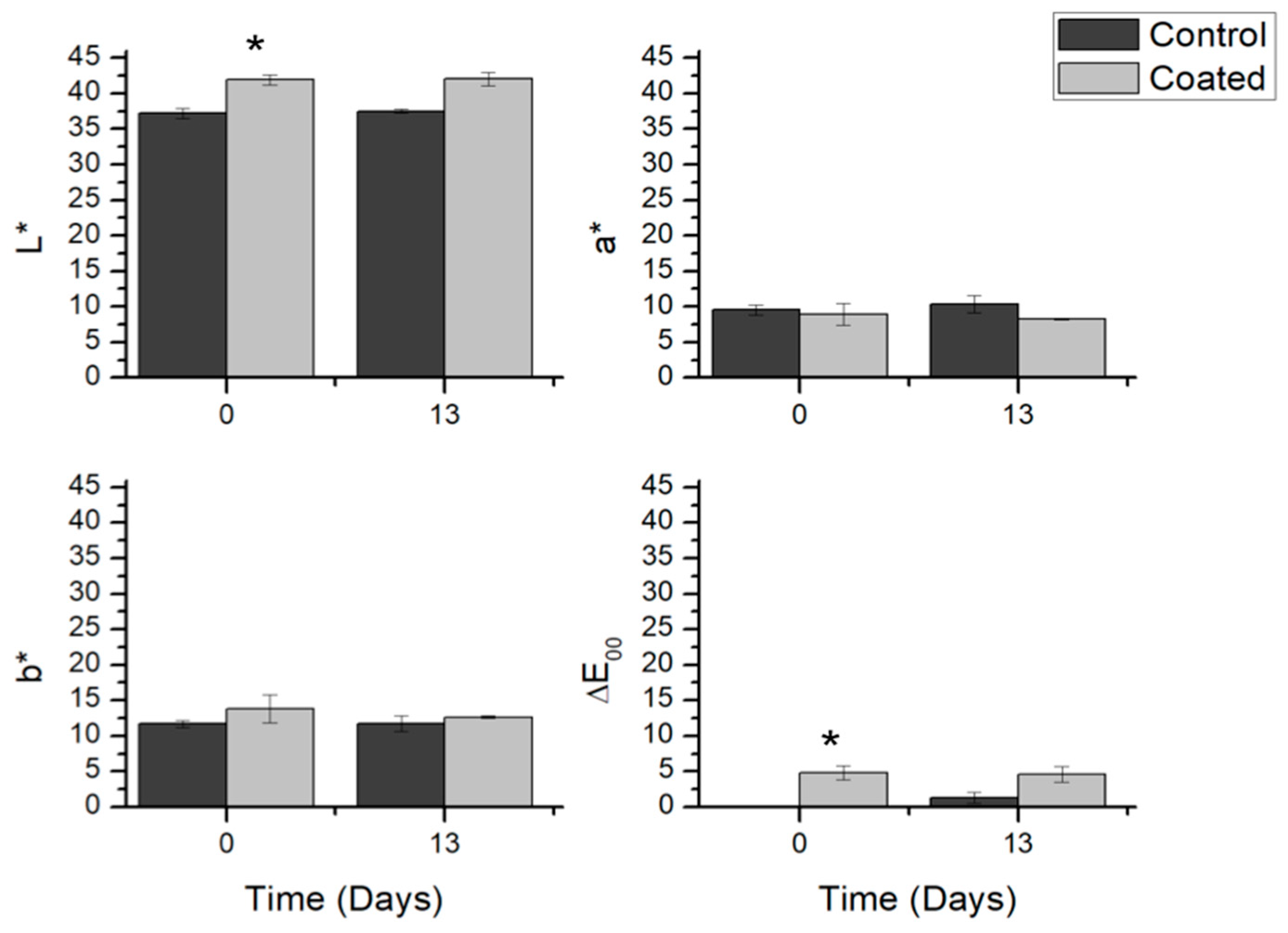
3.3.3. Color Evolution of Coated Salmon Fillets
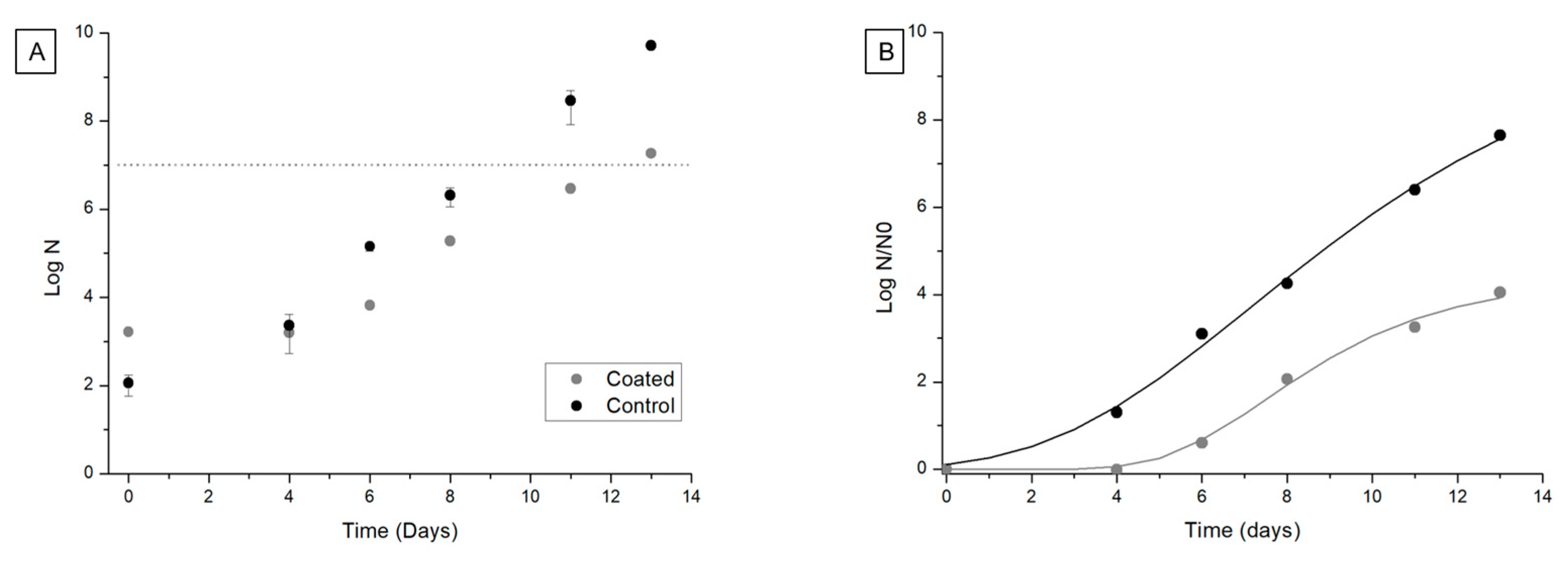
3.3.4. Microbiological Stability of Coated Salmon Fillets
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Karim, A.A.; Bhat, R. Fish Gelatin: Properties, Challenges, and Prospects as an Alternative to Mammalian Gelatins. Food Hydrocoll. 2009, 23, 563–576. [Google Scholar] [CrossRef]
- Djagny, K.B.; Wang, Z.; Xu, S.; Djagny, K.B.; Wang, Z.; Xu, S. Gelatin: A Valuable Protein for Food and Pharmaceutical Industries. Crit. Rev. Food Sci. Nutr. 2017, 41, 481–492. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, S.F.; Gómez-guillén, M.C. A State-of-the-Art Review on the Elaboration of Fish Gelatin as Bioactive Packaging: Special Emphasis on Nanotechnology-Based Approaches. Trends Food Sci. Technol. 2018, 79, 125–135. [Google Scholar] [CrossRef]
- Hanani, Z.A.N.; Roos, Y.H.; Kerry, J.P. Use and Application of Gelatin as Potential Biodegradable Packaging Materials for Food Products. Int. J. Biol. Macromol. 2014, 71, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Guillén, M.; Pérez-Mateos, M.; Gómez-Estaca, J.; López-Caballero, E.; Giménez, B.; Montero, P. Fish Gelatin: A Renewable Material for Developing Active Biodegradable Films. Trends Food Sci. Technol. 2009, 20, 3–16. [Google Scholar] [CrossRef]
- Wang, L.; Liu, L.; Holmes, J.; Kerry, J.F.; Kerry, J.P. Original Article Assessment of Film-Forming Potential and Properties of Protein and Polysaccharide-Based Biopolymer Films. Food Sci. Technol. 2007, 42, 1128–1138. [Google Scholar] [CrossRef]
- Antoniewski, M.N.; Barringer, C.L.; Knipe, C.L.; Zerby, H.N. Effect of a Gelatin Coating on the Shelf Life of Fresh Meat. Food Eng. Phys. Prop. 2007, 72, E382–E387. [Google Scholar] [CrossRef]
- Abdelhedi, O.; Jridi, M.; Nasri, R.; Mora, L.; Toldrá, F.; Nasri, M. Rheological and Structural Properties of Hemiramphus Far Skin Gelatin: Potential Use as an Active Fish Coating Agent. Food Hydrocoll. 2019, 87, 331–341. [Google Scholar] [CrossRef]
- Wang, Z.; Hu, S.; Gao, Y.; Ye, C.; Wang, H. Effect of Collagen-Lysozyme Coating on Fresh-Salmon Fillets Preservation. LWT-Food Sci. Technol. 2017, 75, 59–64. [Google Scholar] [CrossRef]
- Zhou, Q.; Li, P.; Fang, S.; Liu, W.; Mei, J.; Xie, J. Preservative Effects of Gelatin Active Coating Enriched with Eugenol Emulsion on Chinese Seabass (Lateolabrax Maculatus) during Superchilling (−0.9 °C) Storage. Coatings 2019, 9, 498. [Google Scholar] [CrossRef]
- Andreuccetti, C.; Carvalho, R.A.; Grosso, C.R.F. Gelatin-Based Films Containing Hydrophobic Plasticizers and Saponin from Yucca Schidigera as the Surfactant. Food Res. Int. 2010, 43, 1710–1718. [Google Scholar] [CrossRef]
- Díaz, P.; López, D.; Matiacevich, S.; Osorio, F.; Enrione, J. State Diagram of Salmon (Salmo Salar) Gelatin Films. J. Sci. Food Agric. 2011, 91, 2558–2565. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Miao, J.; Wu, J.; Chen, S.; Zhang, Q. Food Hydrocolloids Preparation and Characterization of Active Gelatin-Based Fi Lms Incorporated with Natural Antioxidants. Food Hydrocoll. 2014, 37, 166–173. [Google Scholar] [CrossRef]
- Ma, W.; Tang, C.; Yin, S.; Yang, X.; Wang, Q.; Liu, F.; Wei, Z. Characterization of Gelatin-Based Edible Films Incorporated with Olive Oil. FRIN-Food Res. Int. 2012, 49, 572–579. [Google Scholar] [CrossRef]
- Ramos, M.; Valdes, A.; Beltran, A.; Garrigos, M.C. Gelatin-Based Films and Coatings for Food Packaging Applications. Coatings 2016, 6, 41. [Google Scholar] [CrossRef]
- Wihodo, M.; Moraru, C.I. Physical and Chemical Methods Used to Enhance the Structure and Mechanical Properties of Protein Films: A Review. J. Food Eng. 2013, 114, 292–302. [Google Scholar] [CrossRef]
- Fakhouri, F.M.; Martelli, S.M.; Caon, T.; Velasco, J.I.; Mei, L.H.I. Edible Films and Coatings Based on Starch/Gelatin: Film Properties and Effect of Coatings on Quality of Refrigerated Red Crimson Grapes. Postharvest Biol. Technol. 2015, 109, 57–64. [Google Scholar] [CrossRef]
- Nata, I.F.; Irawan, C.; Ramadhan, L.; Ramadhani, M.R. Influence of Soy Protein Isolate on Gelatin-Based Edible Film Properties. MATEC Web Conf. 2018, 156, 01014. [Google Scholar] [CrossRef][Green Version]
- Rivero, S.; García, M.A.; Pinotti, A. Composite and Bi-Layer Films Based on Gelatin and Chitosan. J. Food Eng. 2009, 90, 531–539. [Google Scholar] [CrossRef]
- Quero, F.; Coveney, A.; Lewandowska, A.E.; Richardson, R.M.; Lee, K.; Eichhorn, S.J.; Alam, M.A.; Enrione, J. Stress Transfer Quantification in Gelatin-Matrix Natural Composites with Tunable Optical Properties. Biomacromolecules 2015, 16, 1784–1793. [Google Scholar] [CrossRef]
- Carvalho, R.A.; De Grosso, C.R.F. Characterization of Gelatin Based Films Modified with Transglutaminase, Glyoxal and Formaldehyde. Food Hydrocoll. 2004, 18, 717–726. [Google Scholar] [CrossRef]
- Mu, C.; Guo, J.; Li, X.; Lin, W.; Li, D. Food Hydrocolloids Preparation and Properties of Dialdehyde Carboxymethyl Cellulose Crosslinked Gelatin Edible Fi Lms. Food Hydrocoll. 2012, 27, 22–29. [Google Scholar] [CrossRef]
- Arnesen, J.A.; Gildberg, A. Extraction and Characterisation of Gelatine from Atlantic Salmon (Salmo Salar) Skin. Bioresour. Technol. 2007, 98, 53–57. [Google Scholar] [CrossRef]
- Silvipriya, K.S.; Kumar, K.K.; Bhat, A.R.; Kumar, B.D.; John, A.; Lakshmanan, P. Collagen: Animal Sources and Biomedical Application. J. Appl. Pharm. Sci. Vol. 2015, 5, 123–127. [Google Scholar] [CrossRef]
- Haug, I.J.; Draget, K.I.; Smidsrød, O. Physical and Rheological Properties of Fish Gelatin Compared to Mammalian Gelatin. Food Hydrocoll. 2004, 18, 203–213. [Google Scholar] [CrossRef]
- Joly-Duhamel, C.; Hellio, D.; Djabourov, M. All Gelatin Networks: 1. Biodiversity and Physical Chemistry. Langmuir 2002, 18, 7208–7217. [Google Scholar] [CrossRef]
- Ninan, G.; Joseph, J. A Comparative Study on the Physical, Chemical and Functional Properties of Carp Skin and Mammalian Gelatins. J. Food Sci. Technol. 2014, 51, 2085–2091. [Google Scholar] [CrossRef]
- Nurul, A.G.; Sarbon, N.M. Effects of PH on Functional, Rheological and Structural Properties of Eel (Monopterus Sp.) Skin Gelatin Compared to Bovine Gelatin. Int. Food Res. J. 2015, 22, 572–583. [Google Scholar]
- Díaz-Calderón, P.; Flores, E.; González-Muñoz, A.; Pepczynska, M.; Quero, F.; Enrione, J. Influence of Extraction Variables on the Structure and Physical Properties of Salmon Gelatin. Food Hydrocoll. 2017, 71, 118–128. [Google Scholar] [CrossRef]
- Cho, S.M.; Gu, Y.S.; Kim, S.B. Extracting Optimization and Physical Properties of Yellowfin Tuna (Thunnus Albacares) Skin Gelatin Compared to Mammalian Gelatins. Food Hydrocoll. 2005, 19, 221–229. [Google Scholar] [CrossRef]
- Elharfaoui, N.; Djabourov, M.; Babel, W. Molecular Weight Influence on Gelatin Gels: Structure, Enthalpy and Rheology. Macromol. Symp. 2007, 256, 149–157. [Google Scholar] [CrossRef]
- Eysturskarð, J.; Haug, I.J. Mechanical Properties of Mammalian and Fish Gelatins as a Function of the Contents of α-Chain, β-Chain, and Low and High Molecular Weight Fractions. Food Biophys. 2010, 5, 9–16. [Google Scholar] [CrossRef]
- Gómez-Estaca, J.; Montero, P.; Fernández-Martín, F.; Gómez-Guillén, M. Physico-Chemical and Film-Forming Properties of Bovine-Hide and Tuna-Skin Gelatin: A Comparative Study. J. Food Eng. 2009, 90, 480–486. [Google Scholar] [CrossRef]
- Bigi, A.; Cojazzi, G.; Panzavolta, S.; Rubini, K.; Roveri, N. Mechanical and Thermal Properties of Gelatin Films at Different Degrees of Glutaraldehyde Crosslinking. Biomaterials 2001, 22, 763–768. [Google Scholar] [CrossRef]
- Chiou, B.S.; Avena-Bustillos, R.J.; Shey, J.; Yee, E.; Bechtel, P.J.; Imam, S.H.; Glenn, G.M.; Orts, W.J. Rheological and Mechanical Properties of Cross-Linked Fish Gelatins. Polymer (Guildford) 2006, 47, 6379–6386. [Google Scholar] [CrossRef]
- Ofner, C.M.; Zhang, Y.E.; Jobeck, V.C.; Bowman, B.J. Crosslinking Studies in Gelatin Capsules Treated with Formaldehyde and in Capsules Exposed to Elevated Temperature and Humidity. J. Pharm. Sci. 2001, 90, 79–88. [Google Scholar] [CrossRef]
- Kuijpers, A.J.; Engbers, G.H.M.; Krijgsveld, J.; Zaat, S.A.J.; Feijen, J. Cross-Linking and Characterisation of Gelatin Matrices for Biomedical Applications. J. Biomater. Sci. Polym. Ed. 2000, 11, 225–243. [Google Scholar] [CrossRef] [PubMed]
- Klotz, B.J.; Gawlitta, D.; Rosenberg, A.J.W.P.; Malda, J.; Melchels, F.P.W. Gelatin-Methacryloyl Hydrogels: Towards Biofabrication-Based Tissue Repair. Trends Biotechnol. 2016, 34, 394–407. [Google Scholar] [CrossRef]
- Yue, K.; Trujillo-de Santiago, G.; Alvarez, M.M.; Tamayol, A.; Annabi, N.; Khademhosseini, A. Biomaterials Synthesis, Properties, and Biomedical Applications of Gelatin Methacryloyl (GelMA) Hydrogels. Biomaterials 2015, 73, 254–271. [Google Scholar] [CrossRef]
- Yang, G.; Xiao, Z.; Ren, X.; Long, H.; Qian, H.; Ma, K.; Guo, Y. Enzymatically Crosslinked Gelatin Hydrogel Promotes the Proliferation of Adipose Tissue-Derived Stromal Cells. PeerJ 2016, 4, e2497. [Google Scholar] [CrossRef]
- Heo, J.; Koh, R.H.; Shim, W.; Kim, H.D.; Yim, H.; Hwang, N.S. Riboflavin-Induced Photo-Crosslinking of Collagen Hydrogel and Its Application in Meniscus Tissue Engineering. Drug Deliv. Transl. Res. 2015, 6, 148–158. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.; Kim, T.G.; Kim, B.S.; Kim, S.-W.; Kwon, S.-M.; Cho, D.-W. Tailoring Mechanical Properties of Decellularized Extracellular Matrix Bioink by Vitamin B2-Induced Photo-Crosslinking. Acta Biomater. 2016, 33, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Tirella, A.; Liberto, T.; Ahluwalia, A. Riboflavin and Collagen: New Crosslinking Methods to Tailor the Stiffness of Hydrogels. Mater. Lett. 2012, 74, 58–61. [Google Scholar] [CrossRef]
- Wang, K.; Wang, W.; Wu, X.; Xiao, J.; Liu, Y. Effect of Photochemical UV/ Riboflavin-Mediated Cross-Links on Different Properties of Fish Gelatin Films. J. Food Process Eng. 2017, 40, e12536. [Google Scholar] [CrossRef]
- Zhang, Y.; Conrad, A.H.; Conrad, G.W. Effects of Ultraviolet-A and Riboflavin on the Interaction of Collagen and Proteoglycans during Corneal Cross-Linking. J. Biol. Chem. 2011, 286, 13011–13022. [Google Scholar] [CrossRef]
- Ibusuki, S.; Halbesma, G.J.; Randolph, M.A.; Redmond, R.W.; Kochevar, I.E.; Gill, T.J. Photochemically Cross-Linked Collagen Gels as Three-Dimensional Scaffolds for Tissue Engineering. Tissue Eng. 2007, 13, 1995–2001. [Google Scholar] [CrossRef]
- Sharma, G.; Wu, W.; Dalal, E.N. The CIEDE2000 Color-Difference Formula: Implementation Notes, Supplementary Test Data, and Mathematical Observations. Color Res. Appl. 2005, 30, 21–30. [Google Scholar] [CrossRef]
- International Organization for Standardization. International Standard ISO/IEC Information Technology—Security Techniques—Information Security Management Systems—Requirements; International Organization for Standardization: Geneva, Switzerland, 2013. [Google Scholar]
- Zwietering, M.H.; Jongenburger, I.; Rombouts, F.M.; van’t Riet, K. Modeling of the Bacterial Growth Curve. Appl. Environ. Microbiol. 1990, 56, 1875–1881. [Google Scholar]
- Pina-Pérez, M.C.; González, A.; Moreno, Y.; Ferrús, M.A. Helicobacter Pylori Growth Pattern in Reference Media and Extracts from Selected Minimally Processed Vegetables. Food Control 2018, 86, 389–396. [Google Scholar] [CrossRef]
- Zanetti-Polzi, L.; Aschi, M.; Daidone, I.; Amadei, A. Theoretical Modeling of the Absorption Spectrum of Aqueous Riboflavin. Chem. Phys. Lett. 2017, 669, 119–124. [Google Scholar] [CrossRef]
- Kasimova, G.K.; Astanov, S.K.; Kurtaliev, E.N.; Nizomov, N.N. Structure of Self-Assembled Riboflavin Molecules in Solutions. J. Mol. Struct. 2019, 1185, 107–111. [Google Scholar] [CrossRef]
- Huang, R.; Hyun, J.K.; Min, D.B. Photosensitizing Effect of Riboflavin, Lumiflavin, and Lumichrome on the Generation of Volatiles in Soy Milk. J. Agric. Food Chem. 2006, 54, 2359–2364. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; Yan, L.; Mu, C.; Li, W.; Zhang, M.; Zhu, Q. Effect of PH on Gelatin Self-Association Investigated by Laser Light Scattering and Atomic Force Microscopy. Polym. Int. 2002, 51, 233–238. [Google Scholar] [CrossRef]
- Zaupa, A.; Byres, N.; Dal Zovo, C.; Acevedo, C.A.; Angelopoulos, I.; Terraza, C.; Nestle, N.; Abarzúa-Illanes, P.N.; Quero, F.; Díaz-Calderón, P.; et al. Cold-Adaptation of a Methacrylamide Gelatin towards the Expansion of the Biomaterial Toolbox for Specialized Functionalities in Tissue Engineering. Mater. Sci. Eng. C 2019, 102, 373–390. [Google Scholar] [CrossRef] [PubMed]
- Chiou, B.-S.; Avena-Bustillos, R.J.; Bechtel, P.J.; Jafri, H.; Narayan, R.; Imam, S.H.; Glenn, G.M.; Orts, W.J. Cold Water Fish Gelatin Films: Effects of Cross-Linking on Thermal, Mechanical, Barrier, and Biodegradation Properties. Eur. Polym. J. 2008, 44, 3748–3753. [Google Scholar] [CrossRef]
- Demirbay, B.; Akaoğlu, C.; Ulusaraç, İ.; Gülay Acar, F. Thermal and UV Radiation Effects on Dynamic Viscosity of Gelatin-Based Riboflavin Solutions. J. Mol. Liq. 2017, 225, 147–150. [Google Scholar] [CrossRef]
- Goudoulas, T.B.; Germann, N. Phase Transition Kinetics and Rheology of Gelatin-Alginate Mixtures. Food Hydrocoll. 2017, 66, 49–60. [Google Scholar] [CrossRef]
- Demirbay, B.; Ata Ayhan, A.; Cereyan, N.; Akaoğlu, C.; Ulusaraç, İ.; Koyuncu, N.; Gülay Acar, F. Rheological Properties of Dextrin-Riboflavin Solutions under Thermal and UV Radiation Effects. J. Mol. Liq. 2017, 240, 597–603. [Google Scholar] [CrossRef]
- Feng, X.; Bansal, N.; Yang, H. Fish Gelatin Combined with Chitosan Coating Inhibits Myofibril Degradation of Golden Pomfret (Trachinotus Blochii) Fillet during Cold Storage. Food Chem. 2016, 200, 283–292. [Google Scholar] [CrossRef]
- Alparslan, Y.; Yapıcı, H.H.; Metin, C.; Baygar, T.; Günlü, A.; Baygar, T. Quality Assessment of Shrimps Preserved with Orange Leaf Essential Oil Incorporated Gelatin. LWT-Food Sci. Technol. 2016, 72, 457–466. [Google Scholar] [CrossRef]
- Yagiz, Y.; Kristinsson, H.G.; Balaban, M.O.; Welt, B.A.; Raghavan, S.; Marshall, M.R. Correlation between Astaxanthin Amount and A* Value in Fresh Atlantic Salmon (Salmo Salar) Muscle during Different Irradiation Doses. Food Chem. 2010, 120, 121–127. [Google Scholar] [CrossRef]
- Møretrø, T.; Moen, B.; Heir, E.; Hansen, A.Å.; Langsrud, S. Contamination of Salmon Fillets and Processing Plants with Spoilage Bacteria. Int. J. Food Microbiol. 2016, 237, 98–108. [Google Scholar] [CrossRef] [PubMed]
- ICMSF. Microorganisms in Foods 2: Sampling for Microbiological Analysis: Principles and Specific Applications; Blackwell Scientific Publications: Oxford, UK, 1986. [Google Scholar]
- Sarmast, E.; Fallah, A.A.; Habibian Dehkordi, S.; Rafieian-Kopaei, M. Impact of Glazing Based on Chitosan-Gelatin Incorporated with Persian Lime (Citrus Latifolia) Peel Essential Oil on Quality of Rainbow Trout Fillets Stored at Superchilled Condition. Int. J. Biol. Macromol. 2019, 136, 316–323. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, R.; Maheswarappa, N.B. Superchilling of Muscle Foods: Potential Alternative for Chilling and Freezing. Crit. Rev. Food Sci. Nutr. 2019, 59, 1256–1263. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, S.F.; Rezaei, M.; Zandi, M.; Ghavi, F.F. Effect of Fish Gelatin Coating Enriched with Oregano Essential Oil on the Quality of Refrigerated Rainbow Trout Fillet. J. Aquat. Food Prod. Technol. 2016, 25, 835–842. [Google Scholar] [CrossRef]
- Gómez-Guillén, M.C.; López-Caballero, M.E.; Alemán, A.; Lopez de Lacey, A.; Giménez, B.; Montero, P. Antioxidant and Antimicrobial Peptide Fractions from Squid and Tuna Skin Gelatin. In Sea By-Products as Real Material: New Ways of Application; Bihan, E.L., Ed.; Transworld Research Network: Trivandrum, India, 2010; pp. 89–115. [Google Scholar]
- Giménez, B.; Alemán, A.; Montero, P.; Gómez-Guillén, M.C. Antioxidant and Functional Properties of Gelatin Hydrolysates Obtained from Skin of Sole and Squid. Food Chem. 2009, 114, 976–983. [Google Scholar] [CrossRef]
| Treatment | Tgel (°C) | ΔHgel (Jg−1) |
|---|---|---|
| Control | 9.9 ± 0.1 a | 8.0 ± 0.8 a |
| VL exposure | 9.9 ± 0.1 a | 8.2 ± 0.5 a |
| Sample | Time (days) | ||||
|---|---|---|---|---|---|
| 0 | 4 | 6 | 8 | 13 | |
| Control | 6.21 ± 0.05 a | 6.10 ± 0.03 a | 6.12 ± 0.03 a | 6.02 ± 0.03 b | 6.46 ± 0.08 b |
| Coated | 6.20 ± 0.02 a | 6.10 ± 0.08 a | 6.13 ± 0.03 a | 6.11 ± 0.03 a | 6.14 ± 0.02 a |
| Treatment | λ (d) | μm (d−1) | A | Growth Cycles | R2 | R2aj | RMSE |
|---|---|---|---|---|---|---|---|
| Control | 2.4 | 1.8 | 23.3 | 10.1 | 99.7 | 99.5 | 0.21 |
| Coating | 5.1 | 1.5 | 10.0 | 4.3 | 99.5 | 99.1 | 0.16 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Char, C.; Padilla, C.; Campos, V.; Pepczynska, M.; Díaz-Calderón, P.; Enrione, J. Characterization and Testing of a Novel Sprayable Crosslinked Edible Coating Based on Salmon Gelatin. Coatings 2019, 9, 595. https://doi.org/10.3390/coatings9100595
Char C, Padilla C, Campos V, Pepczynska M, Díaz-Calderón P, Enrione J. Characterization and Testing of a Novel Sprayable Crosslinked Edible Coating Based on Salmon Gelatin. Coatings. 2019; 9(10):595. https://doi.org/10.3390/coatings9100595
Chicago/Turabian StyleChar, Cielo, Cristina Padilla, Vanessa Campos, Marzena Pepczynska, Paulo Díaz-Calderón, and Javier Enrione. 2019. "Characterization and Testing of a Novel Sprayable Crosslinked Edible Coating Based on Salmon Gelatin" Coatings 9, no. 10: 595. https://doi.org/10.3390/coatings9100595
APA StyleChar, C., Padilla, C., Campos, V., Pepczynska, M., Díaz-Calderón, P., & Enrione, J. (2019). Characterization and Testing of a Novel Sprayable Crosslinked Edible Coating Based on Salmon Gelatin. Coatings, 9(10), 595. https://doi.org/10.3390/coatings9100595