Preparation and Characterization of Polydopamine-Modified Ni/Carbon Nanotubes Friction Composite Coating
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Modification of CNTs@pDA
2.3. Preparation of Ni/CNTs@pDA Composite Coating
2.4. Characterization of CNTs@pDA
2.5. Friction-Wear and Corrosion Tests of Ni/CNTs@pDA Composite Coating
3. Results and Discussions
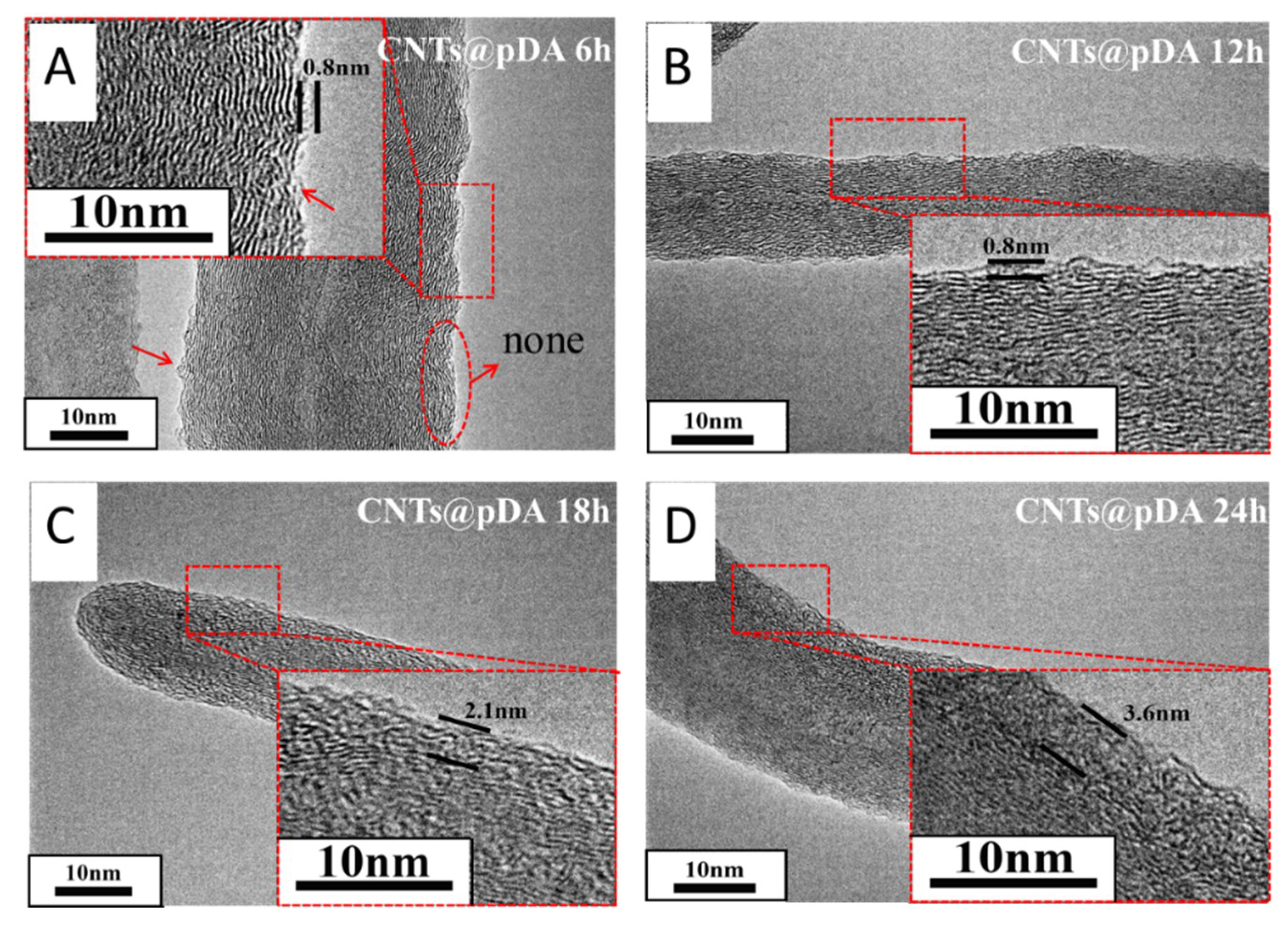
3.1. Surface Morphology of CNTs@pDA
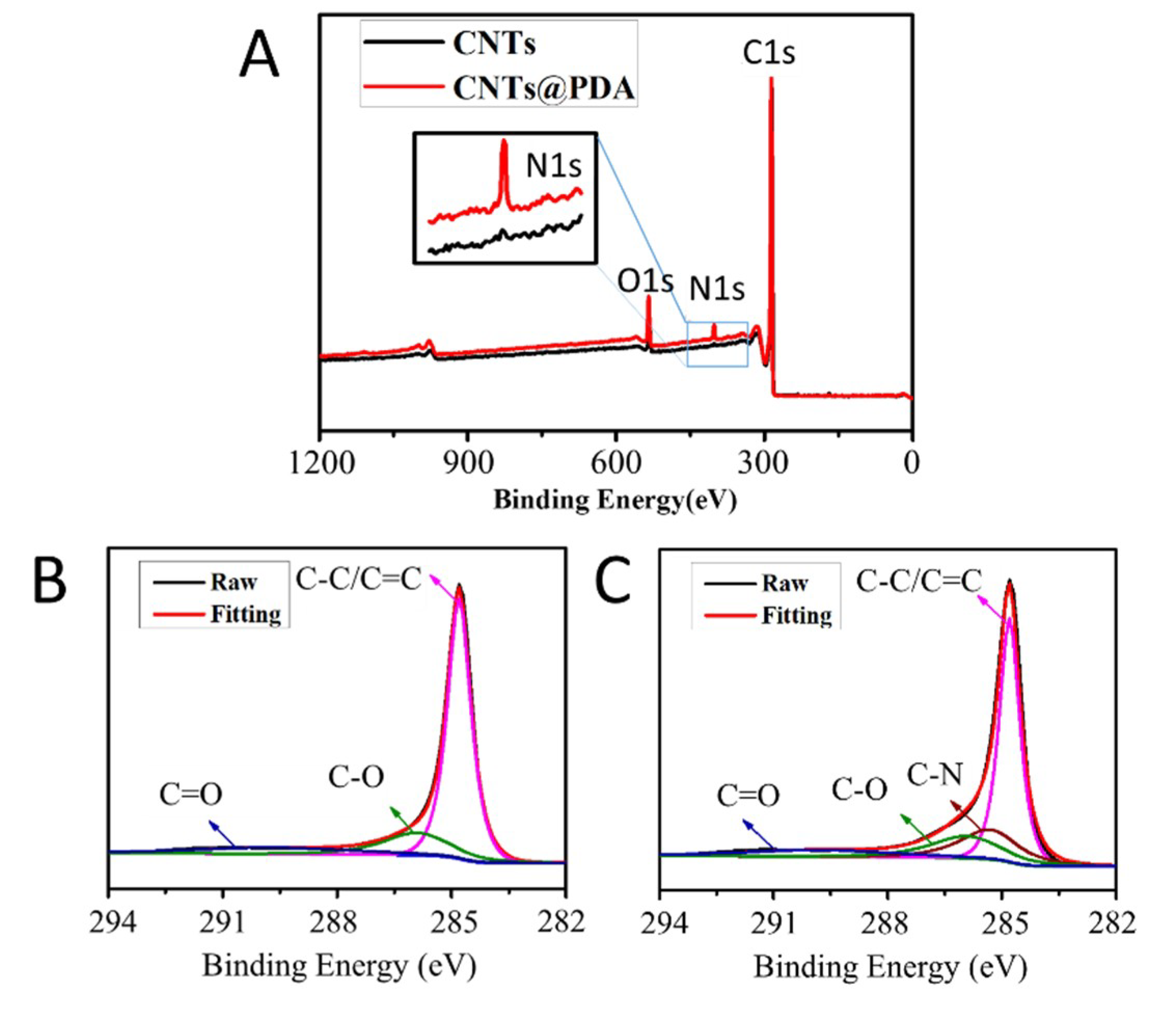
3.2. XPS Detection of CNTs@pDA
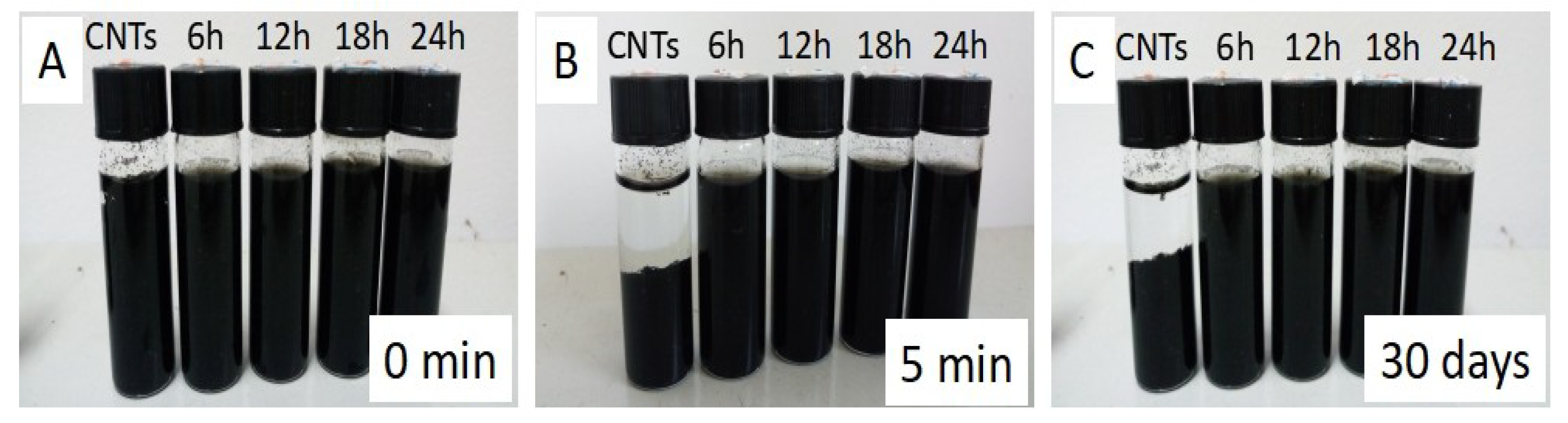
3.3. Dispersion Observation of CNTs
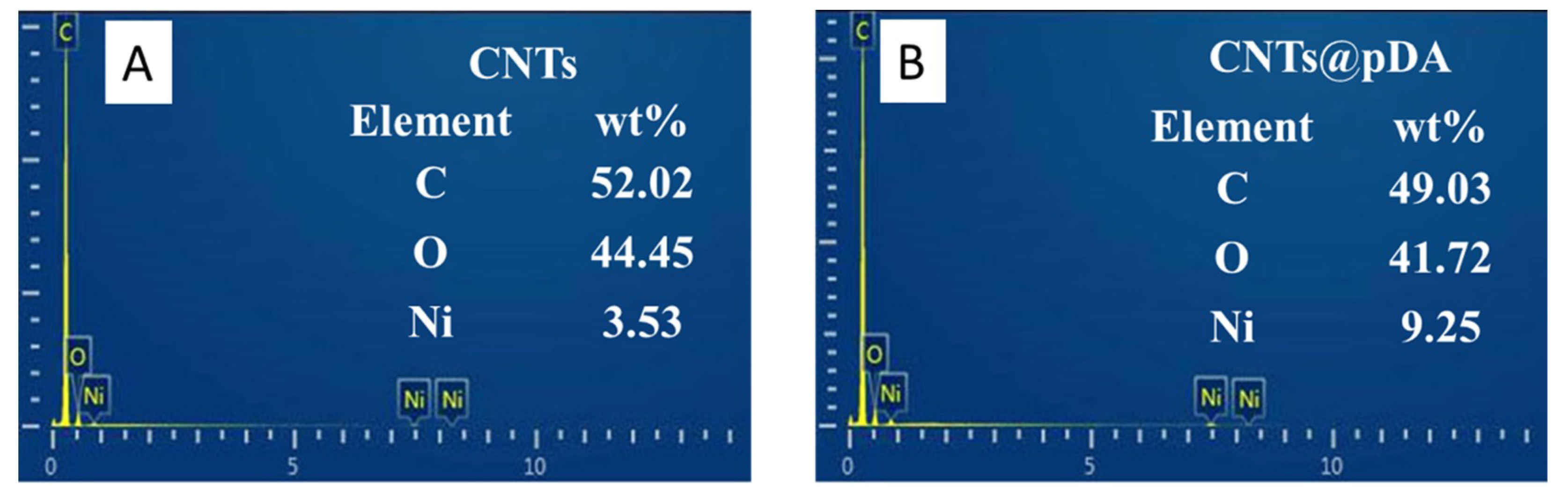
3.4. CNTs@pDA Deposition
3.5. Surface Analysis
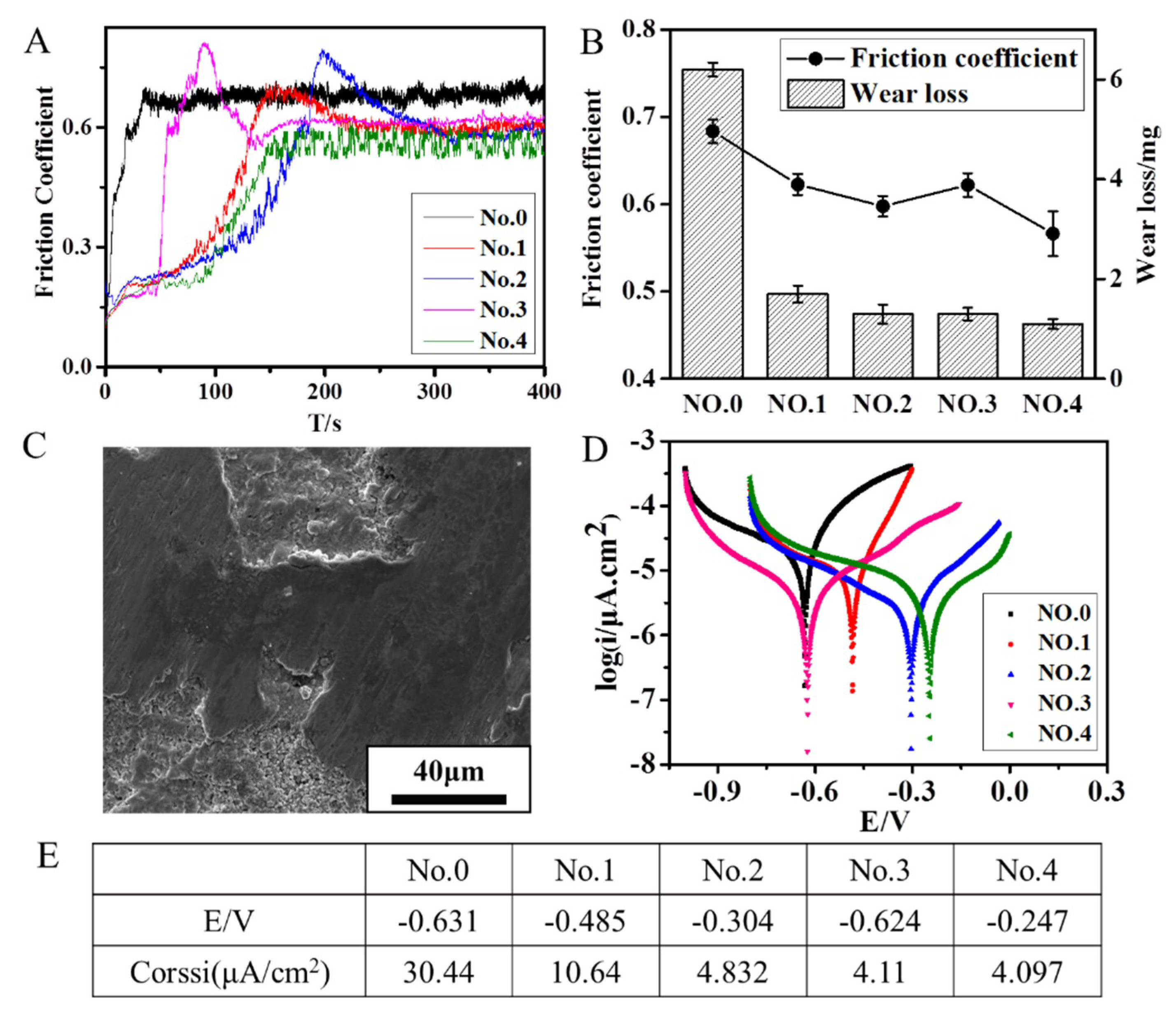
3.6. Friction and Wear Behavior
3.7. Corrosion Resistance
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Iijima, S. Helical microtubules of graphitic carbon. Nature 1991, 354, 56–58. [Google Scholar] [CrossRef]
- Lee, C.; Wei, X.; Kysar, J.W.; Hone, J. Measurement of the elastic properties and intrinsic strength of monolayer graphene. Science 2008, 321, 385–388. [Google Scholar] [CrossRef] [PubMed]
- Alandin, A.A. Thermal properties of graphene and nanostructured carbon materials. Nat. Mater. 2011, 10, 569–581. [Google Scholar] [CrossRef] [PubMed]
- Ciubotariu, A.C.; Benea, L.; Lakatos Varsanyi, M.; Dragan, V. Electrochemical impedance spectroscopy and corrosion behaviour of Al2O3-Ni nano composite coatings. Electrochim. Acta 2008, 53, 4557–4563. [Google Scholar] [CrossRef]
- Alirezaei, S.; Monirvaghefi, S.M.; Salehi, M.; Saatchi, A. Effect of alumina content on surface morphology and hardness of Ni-P-Al2O3(α) electroless composite coatings. Surf. Coat. Tech. 2004, 184, 170–175. [Google Scholar] [CrossRef]
- Dehgahi, S.; Amini, R.; Alizadeh, M. Corrosion, passivation and wear behaviors of electrodeposited Ni–Al2O3–SiC nano-composite coatings. Surf. Coat. Tech. 2016, 304, 502–511. [Google Scholar] [CrossRef]
- Vaezi, M.R.; Sadrnezhaad, S.K.; Nikzad, L. Electrodeposition of Ni–SiC nano-composite coatings and evaluation of wear and corrosion resistance and electroplating characteristics. Colloids Surf. A Physicochem. Eng. Asp. 2008, 315, 176–182. [Google Scholar] [CrossRef]
- Parida, G.; Chaira, D.; Chopkar, M.; Basu, A. Synthesis and characterization of Ni-TiO2 composite coatings by electro-co-deposition. Surf. Coat. Tech. 2011, 205, 4871–4879. [Google Scholar] [CrossRef]
- Benea, L.; Başa, S.; Dănăilă, E.; Caron, N.; Raquet, O.; Ponthiaux, P.; Celis, J. Fretting and wear behaviors of Ni/nano-WC composite coatings in dry and wet conditions. Mater. Des. 2015, 65, 550–558. [Google Scholar] [CrossRef]
- Zarebidaki, A.; Allahkaram, S. Effect of surfactant on the fabrication and characterization of Ni-P-CNT composite coatings. J. Alloy. Compd. 2011, 509, 1836–1840. [Google Scholar] [CrossRef]
- Suzuki, T.; Konno, T. Improvement in tool life of electroplated diamond tools by Ni-based carbon nanotube composite coatings. Precis. Eng. 2014, 38, 659–665. [Google Scholar] [CrossRef]
- Chen, X.H.; Chen, C.S.; Xiao, H.N.; Liu, H.B.; Zhou, L.P.; Li, S.L.; Zhang, G. Dry friction and wear characteristics of nickel/carbon nanotube electroless composite deposits. Tribol. Int. 2006, 39, 22–28. [Google Scholar] [CrossRef]
- Karousis, N.; Tagmatarchis, N.; Tasis, D. Current progress on the chemical modification of carbon nanotubes. Chem. Rev. 2010, 110, 5366–5397. [Google Scholar] [CrossRef] [PubMed]
- Le, V.T.; Ngo, C.L.; Le, Q.T.; Ngo, T.T.; Nguyen, D.N.; Vu, M.T. Surface modification and functionalization of carbon nanotube with some organic compounds. Adv. Nat. Sci. Nanosci. Nanotechnol. 2013, 4, 035017. [Google Scholar] [CrossRef]
- Mahmoodian, H.; Moradi, O.; Shariatzadeh, B. Grafting chitosan and polyHEMA on carbon nanotubes surfaces: “Grafting to” and “Grafting from” methods. Int J. Biol. Macromol. 2014, 63, 92–97. [Google Scholar] [CrossRef] [PubMed]
- Poochai, C.; Pongprayoon, T. Enhancing dispersion of carbon nanotube in polyacrylonitrile matrix using admicellar polymerization. Colloids Surf. A Physicochem. Eng. Asp. 2014, 456, 67–74. [Google Scholar] [CrossRef]
- Pierard, N.; Fonseca, A.; Colomer, J.; Bossuot, C.; Benoit, J.M.; Tendeloo, G.V.; Pirard, J.; Nagy, J.B. Ball milling effect on the structure of single-wall carbon nanotubes. Carbon 2004, 42, 1691–1697. [Google Scholar] [CrossRef]
- Huang, W.; Lin, Y.; Taylor, S.; Gaillard, J.; Rao, A.A.M.; Sun, Y. Sonication-assisted functionalization and solubilization of carbon nanotubes. Nano. Lett. 2002, 2, 231–234. [Google Scholar] [CrossRef]
- Wepasnick, K.A.; Smith, B.A.; Schrote, K.E.; Wilson, H.K.; Diegelmann, S.R.; Fairbrother, D.H. Surface and structural characterization of multi-walled carbon nanotubes following different oxidative treatments. Carbon 2011, 49, 24–36. [Google Scholar] [CrossRef]
- Chen, C.S.; Chen, X.H.; Xu, L.S.; Yang, Z.; Li, W.H. Modification of multi-walled carbon nanotubes with fatty acid and their tribological properties as lubricant additive. Carbon 2005, 43, 1660–1666. [Google Scholar] [CrossRef]
- Lee, H.; Dellatore, S.M.; Miller, W.M.; Messersmith, P.B. Mussel-inspired surface chemistry for multifunctional coatings. Science 2007, 318, 426–430. [Google Scholar] [CrossRef] [PubMed]
- Fei, B.; Qian, B.; Yang, Z.; Wang, R.; Liu, W.C.; Mak, C.L.; Xin, J.H. Coating carbon nanotubes by spontaneous oxidative polymerization of dopamine. Carbon 2008, 46, 1795–1797. [Google Scholar] [CrossRef]
- Liao, Y.; Cao, B.; Wang, W.; Zhang, L.; Wu, D.; Jin, R. A facile method for preparing highly conductive and reflective surface-silvered polyimide films. Appl Surf. Sci. 2009, 255, 8207–8212. [Google Scholar] [CrossRef]
- Xi, Z.; Xu, Y.; Zhu, L.; Wang, Y.; Zhu, B. A facile method of surface modification for hydrophobic polymer membranes based on the adhesive behavior of poly(DOPA) and poly(dopamine). J. Membr. Sci. 2009, 327, 244–253. [Google Scholar] [CrossRef]
- Jiang, Y.; Lu, Y.; Zhang, L.; Liu, L.; Dai, Y.; Wang, W. Preparation and characterization of silver nanoparticles immobilized on multi-walled carbon nanotubes by poly(dopamine) functionalization. J. Nanopart Res. 2012, 14. [Google Scholar] [CrossRef]
- Sureshkumar, M.; Siswanto, D.Y.; Chen, Y.; Lee, C.; Wang, M. Antibacterial and biocompatible surfaces based on dopamine autooxidized silver nanoparticles. J. Polym. Sci. Part. B 2013, 51, 303–310. [Google Scholar] [CrossRef]
- Yu, F.; Chen, S.; Chen, Y.; Li, H.; Yang, L.; Chen, Y.; Yin, Y. Experimental and theoretical analysis of polymerization reaction process on the polydopamine membranes and its corrosion protection properties for 304 Stainless Steel. J. Mol. Struct. 2010, 982, 152–161. [Google Scholar] [CrossRef]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Chen, J. Preparation and Characterization of Polydopamine-Modified Ni/Carbon Nanotubes Friction Composite Coating. Coatings 2019, 9, 596. https://doi.org/10.3390/coatings9100596
Wang Y, Chen J. Preparation and Characterization of Polydopamine-Modified Ni/Carbon Nanotubes Friction Composite Coating. Coatings. 2019; 9(10):596. https://doi.org/10.3390/coatings9100596
Chicago/Turabian StyleWang, Ye, and Jianfeng Chen. 2019. "Preparation and Characterization of Polydopamine-Modified Ni/Carbon Nanotubes Friction Composite Coating" Coatings 9, no. 10: 596. https://doi.org/10.3390/coatings9100596
APA StyleWang, Y., & Chen, J. (2019). Preparation and Characterization of Polydopamine-Modified Ni/Carbon Nanotubes Friction Composite Coating. Coatings, 9(10), 596. https://doi.org/10.3390/coatings9100596



