Control over the Phase Formation in Metastable Transition Metal Nitride Thin Films by Tuning the Al+ Subplantation Depth
Abstract
1. Introduction
2. Experimental Methods
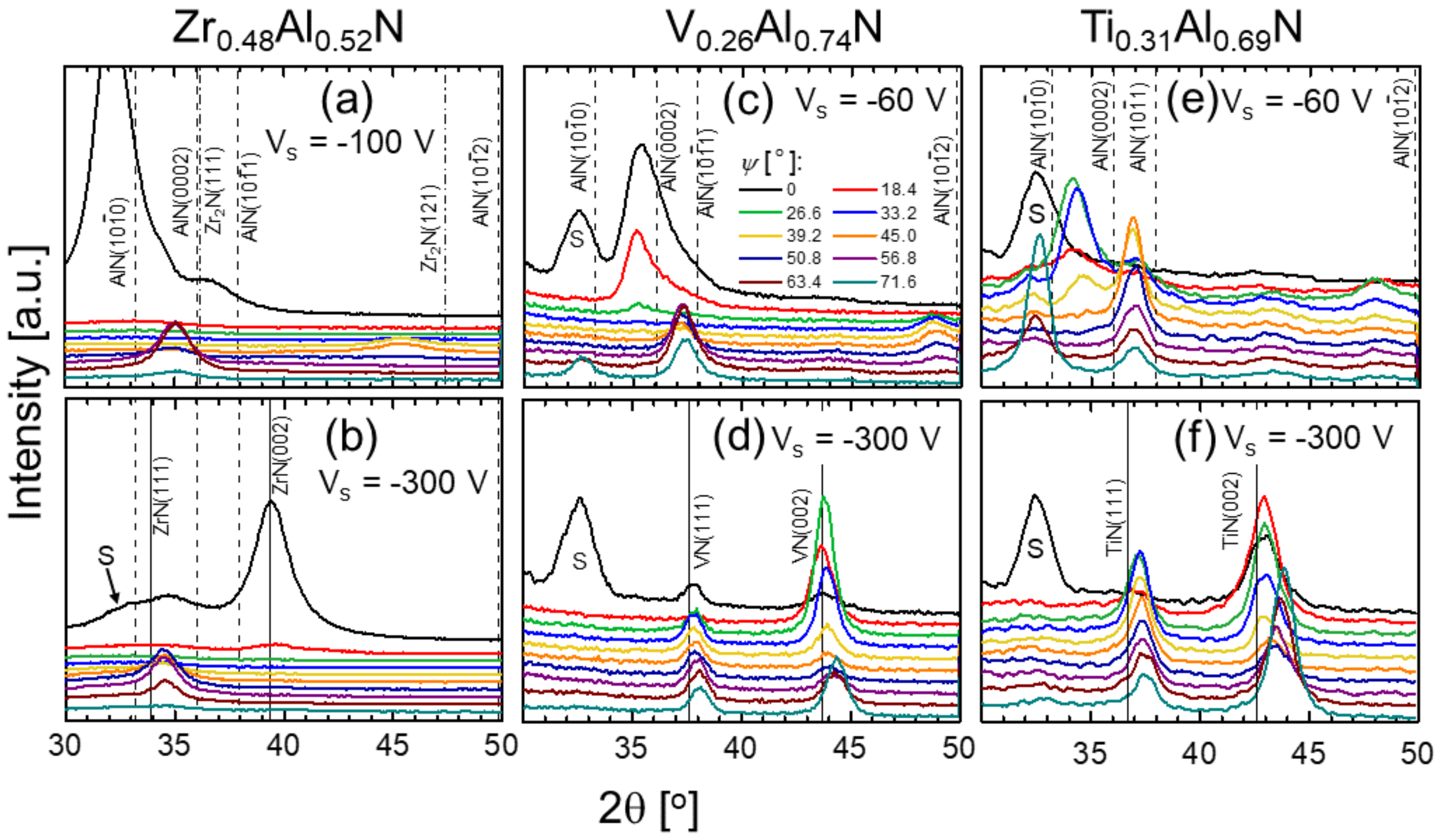
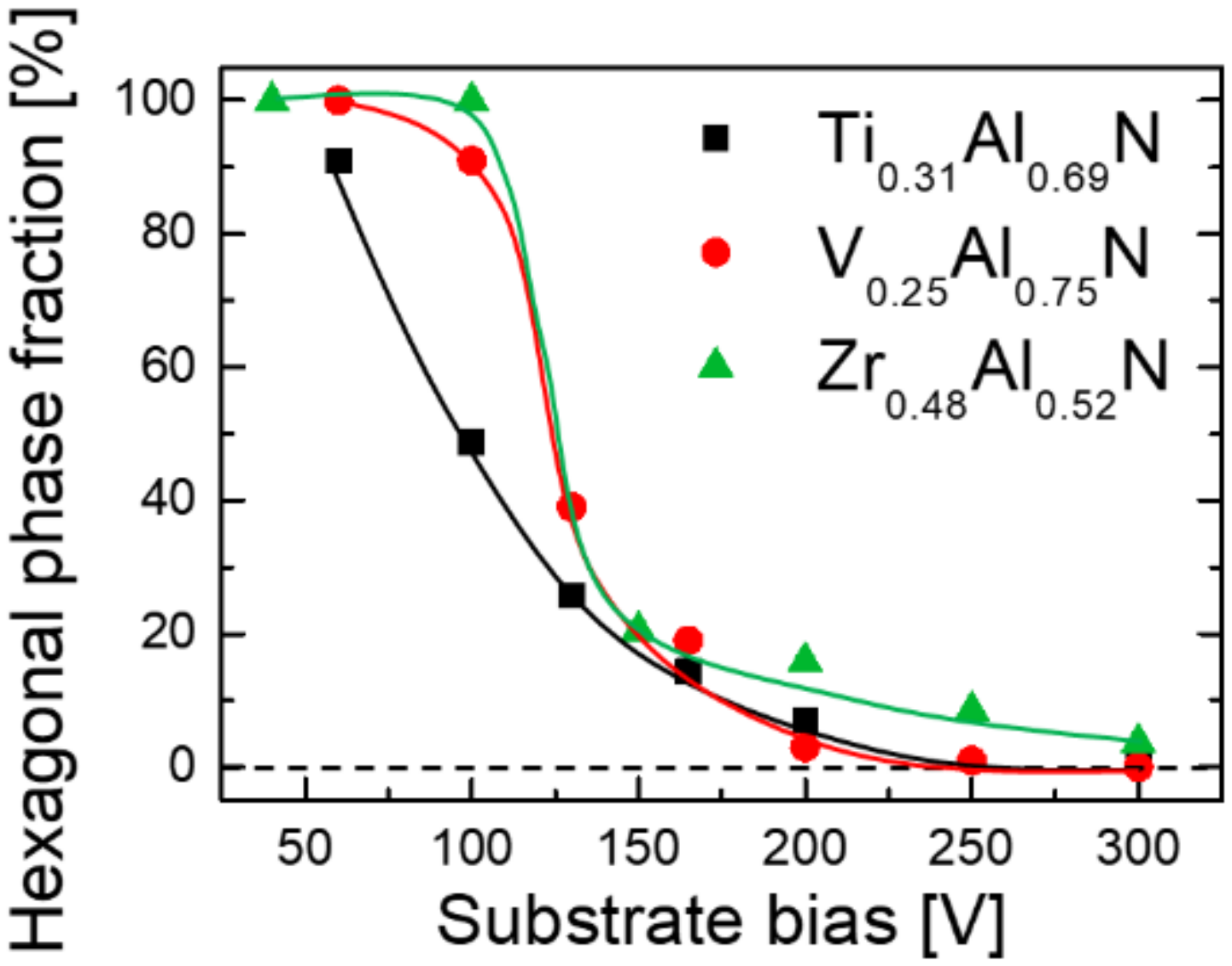
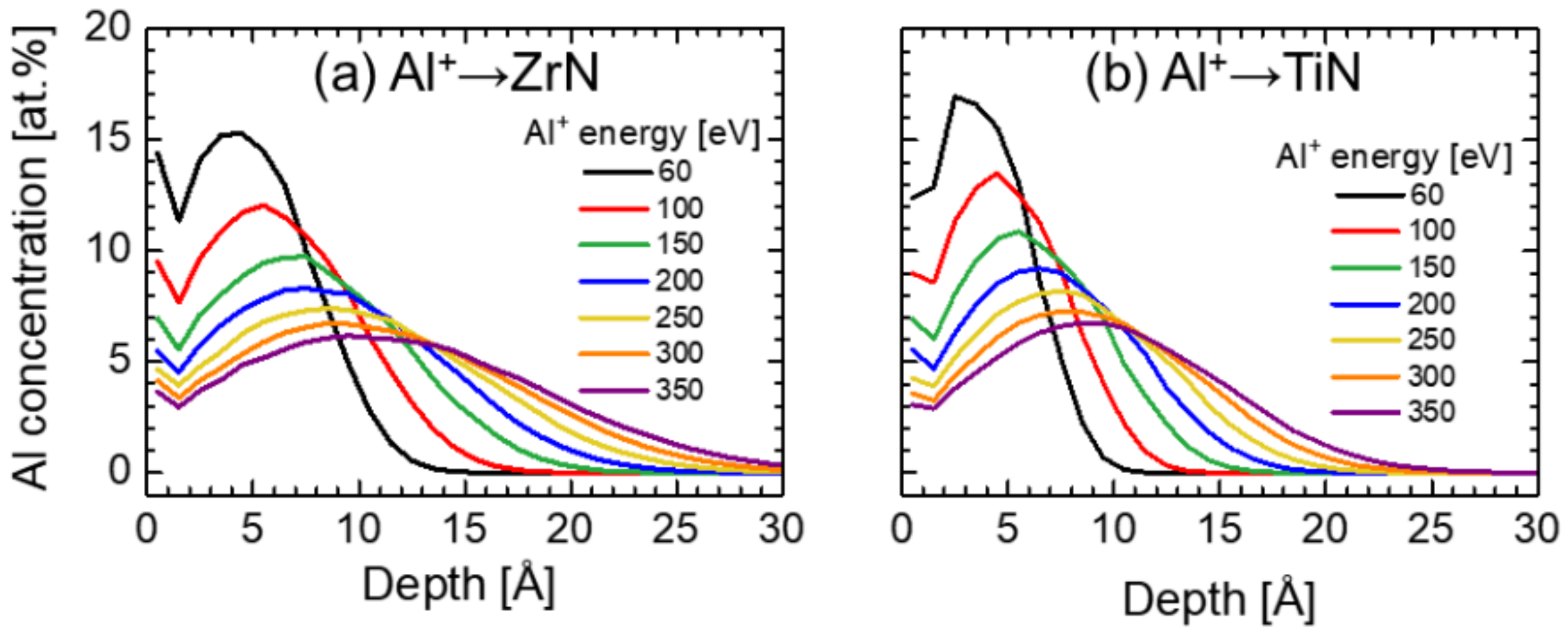
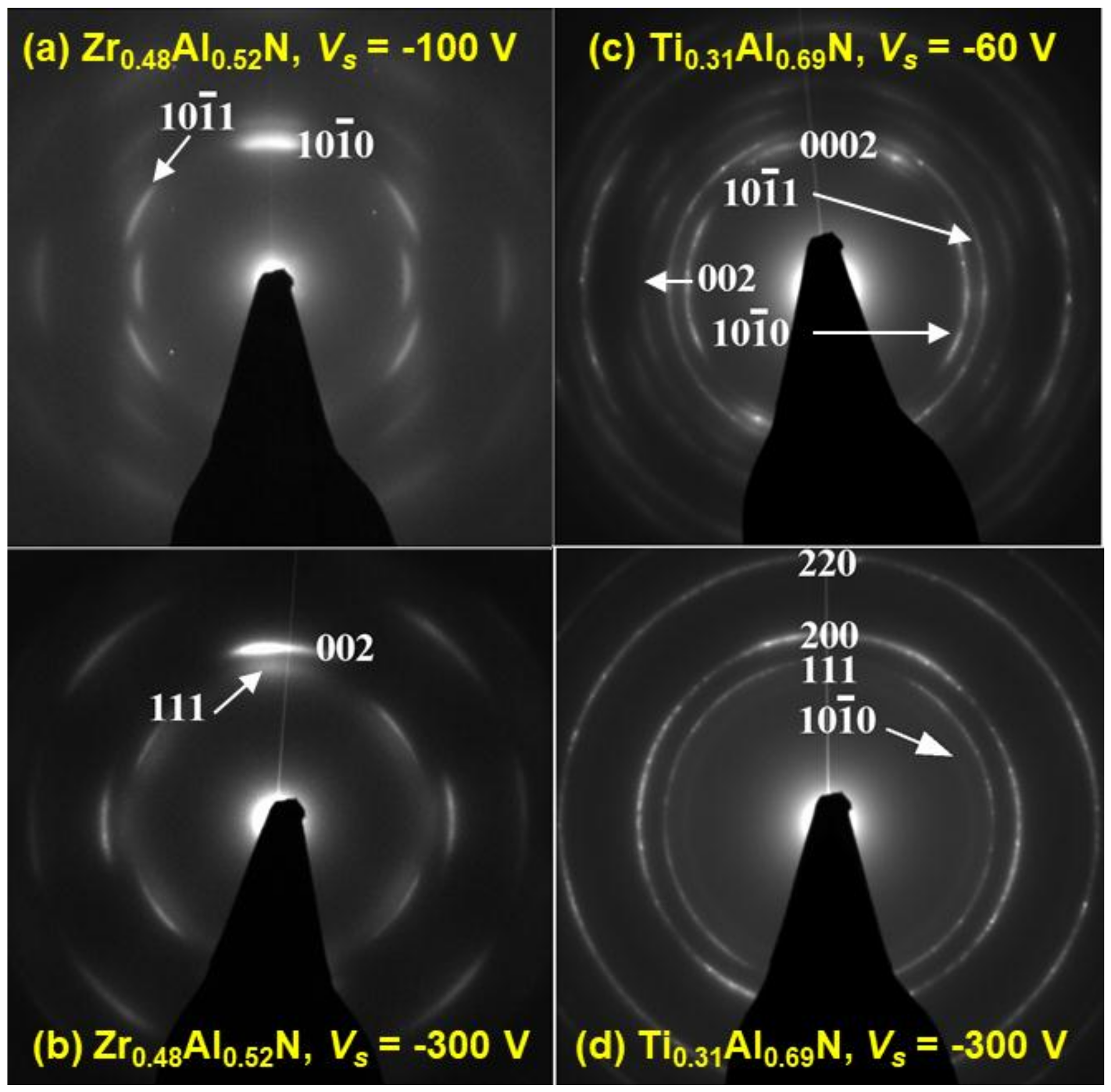
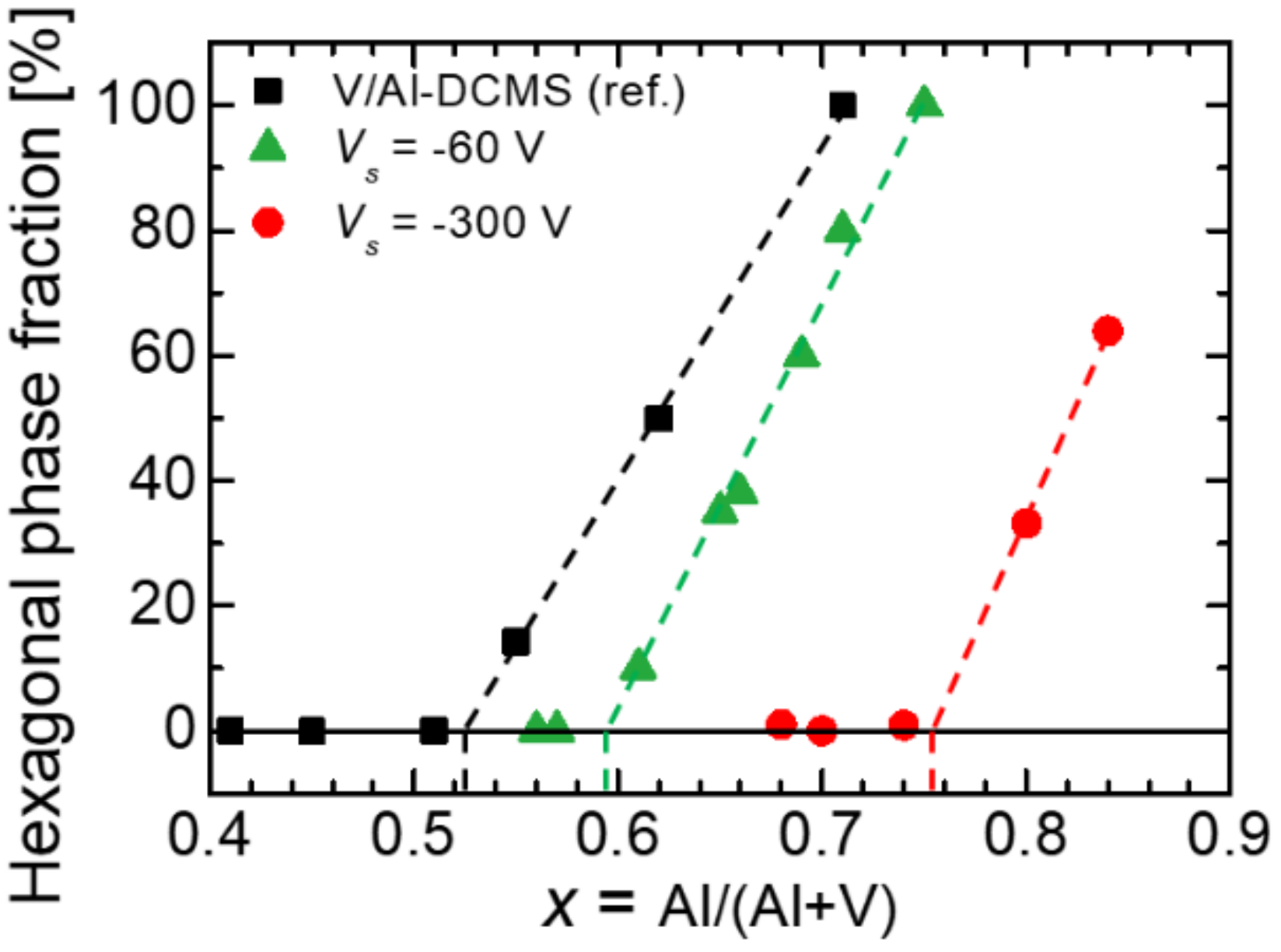
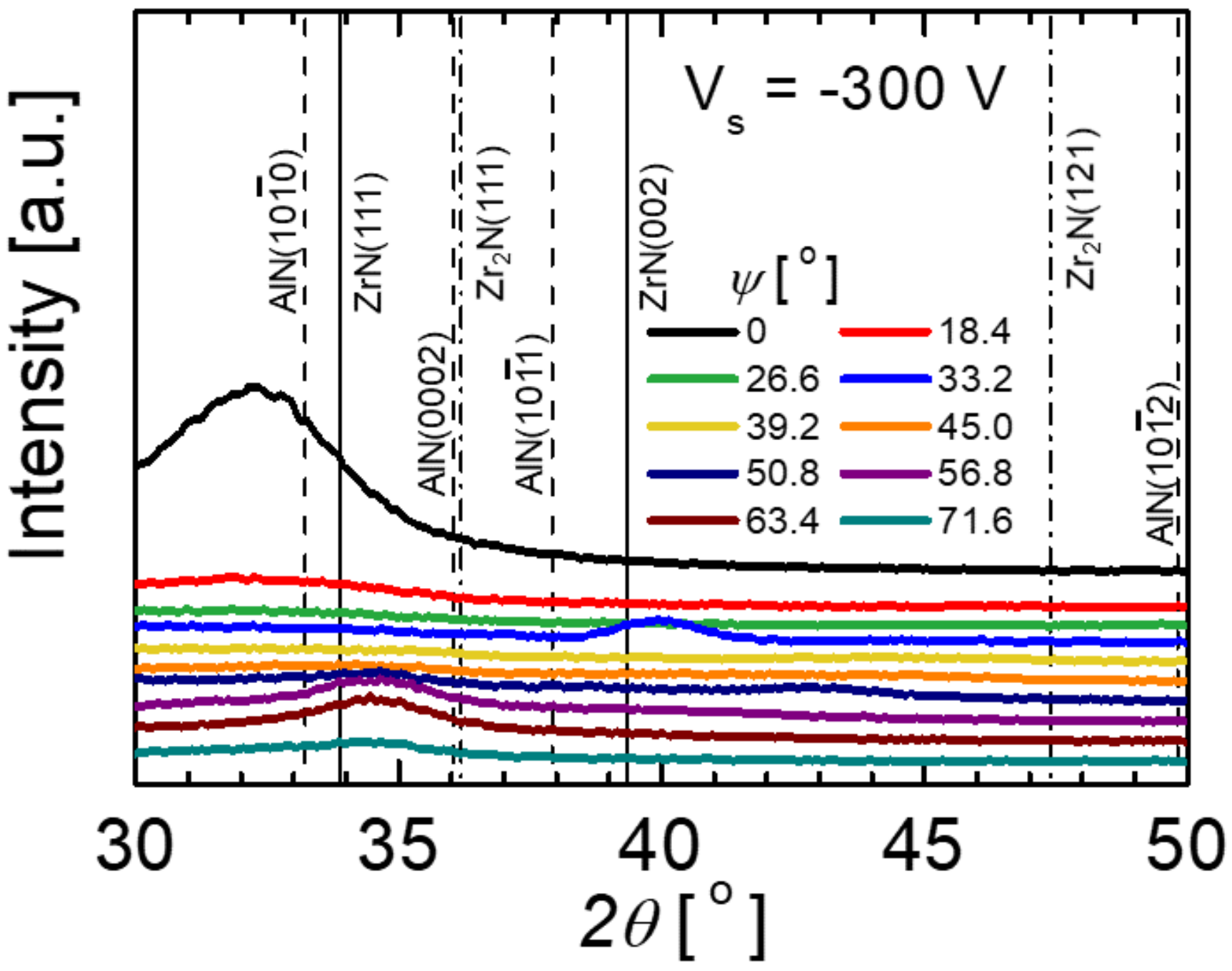
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References and Notes
- Knotek, O.; Münz, W.D.; Leyendecker, T. Industrial deposition of binary, ternary, and quaternary nitrides of titanium, zirconium, and aluminum. J. Vac. Sci. Technol. A 1987, 5, 2173–2179. [Google Scholar] [CrossRef]
- Mayrhofer, P.H.; Mitterer, C.; Hultman, L.; Clemens, H. Microstructural design of hard coatings. Prog. Mater. Sci. 2006, 51, 1032–1114. [Google Scholar] [CrossRef]
- Knotek, O.; Löffler, F.; Krämer, G. Multicomponent and multilayer physically vapour deposited coatings for cutting tools. Surf. Coat. Technol. 1992, 54–55, 241–248. [Google Scholar] [CrossRef]
- Munz, W.-D. Titanium aluminum nitride films: A new alternative to TiN coatings. J. Vac. Sci. Technol. A 1986, 4, 2717–2725. [Google Scholar] [CrossRef]
- Hörling, A.; Hultman, L.; Odén, M.; Sjölén, J.; Karlsson, L. Mechanical properties and machining performance of Ti1−xAlxN-coated cutting tools. Surf. Coat. Technol. 2005, 191, 384–392. [Google Scholar] [CrossRef]
- Kimura, A.; Hasegawa, H.; Yamada, K.; Suzuki, T. Effects of Al content on hardness, lattice parameter and microstructure of Ti1−xAlxN films. Surf. Coat. Technol. 1999, 120–121, 438–441. [Google Scholar] [CrossRef]
- Hörling, A.; Hultman, L.; Odén, M.; Sjölén, J.; Karlsson, L. Thermal stability of arc evaporated high aluminum-content Ti1−xAlxN thin films. J. Vac. Sci. Technol. A 2002, 20, 1815–1823. [Google Scholar] [CrossRef]
- Mayrhofer, P.H.; Hörling, A.; Karlsson, L.; Mitterer, C.; Hultman, L. Self-organized nanostructures in the Ti–Al–N system. Appl. Phys. Lett. 2003, 83, 2049–2051. [Google Scholar] [CrossRef]
- Knutsson, A.; Johansson, M.P.; Persson, P.O.Å.; Odén, M. Thermal decomposition products in arc evaporated TiAlN/TiN multilayers. Appl. Phys. Lett. 2008, 93, 143110. [Google Scholar] [CrossRef]
- Holec, D.; Rachbauer, R.; Chen, L.; Wang, L.; Luef, D.; Mayrhofer, P.H. Phase stability and alloy-related trends in Ti–Al–N, Zr–Al–N and Hf–Al–N systems from first principles. Surf. Coat. Technol. 2011, 206, 1698–1704. [Google Scholar] [CrossRef]
- Sheng, S.H.; Zhang, R.F.; Veprek, S. Phase stabilities and thermal decomposition in the Zr1−xAlxN system studied by ab initio calculation and thermodynamic modeling. Acta Mater. 2008, 56, 968–976. [Google Scholar] [CrossRef]
- Alling, B.; Ruban, A.V.; Karimi, A.; Peil, O.E.; Simak, S.I.; Hultman, L.; Abrikosov, I.A. Mixing and decomposition thermodynamics of c−Ti1−xAlxN from first-principles calculations. Phys. Rev. B 2007, 75, 045123. [Google Scholar] [CrossRef]
- Hans, M.; Music, D.; Chen, Y.-T.; Patterer, L.; Eriksson, A.O.; Kurapov, D.; Ramm, J.; Arndt, M.; Rudigier, H.; Schneider, J.M. Crystallite size-dependent metastable phase formation of TiAlN coatings. Sci. Rep. 2017, 7, 16096. [Google Scholar] [CrossRef] [PubMed]
- Wahlström, U.; Hultman, L.; Sundgren, J.-E.; Adibi, F.; Petrov, I.; Greene, J.E. Crystal growth and microstructure of polycrystalline Ti1−xAlxN alloy films deposited by ultra-high-vacuum dual-target magnetron sputtering. Thin Solid Films 1993, 235, 62–70. [Google Scholar] [CrossRef]
- Adibi, F.; Petrov, I.; Greene, J.E.; Wahlstrom, U.; Sundgren, J.-E. Design and characterization of a compact two-target ultrahigh vacuum magnetron sputter deposition system: Application to the growth of epitaxial Ti1−xAlxN alloys and TiN/Ti1−xAlxN superlattices. J. Vac. Sci. Technol. A 1993, 11, 136–142. [Google Scholar] [CrossRef]
- Makino, Y.; Moria, M.; Miyakea, S.; Saitob, K.; Asami, K. Characterization of Zr–Al–N films synthesized by a magnetron sputtering method. Surf. Coat. Technol. 2005, 193, 219–222. [Google Scholar] [CrossRef]
- Araiza, J.J.; Sanchez, O.; Albella, J.M. Influence of the aluminum incorporation on the structure of sputtered ZrNx films deposited at low temperatures. Vacuum 2009, 83, 1236–1239. [Google Scholar] [CrossRef]
- Lamni, R.; Sanjinés, R.; Parlinska-Wojtan, M.; Karimi, A.; Lévy, F. Microstructure and nanohardness properties of Zr–Al–N and Zr–Cr–N thin films. J. Vac. Sci. Technol. A 2005, 23, 593–598. [Google Scholar] [CrossRef]
- Rovere, F.; Music, D.; Ershov, S.; Baben, M.t.; Fuss, H.-G.; Mayrhofer, P.H.; Schneider, J.M. Experimental and computational study on the phase stability of Al-containing cubic transition metal nitrides. J. Phys. D Appl. Phys. 2010, 43, 035302. [Google Scholar] [CrossRef]
- Greczynski, G.; Mráz, S.; Ruess, H.; Hans, M.; Lu, J.; Hultman, L.; Schneider, J.M. Extended metastable Al solubility in cubic VAlN by metal-ion bombardment during pulsed magnetron sputtering: Film stress vs subplantation. J. Appl. Phys. 2017, 122, 025304. [Google Scholar] [CrossRef]
- Zhu, P.; Ge, F.; Li, S.; Xue, Q.; Huang, F. Microstructure, chemical states, and mechanical properties of magnetron co-sputtered V1−xAlxN coatings. Surf. Coat. Technol. 2013, 232, 311–318. [Google Scholar] [CrossRef]
- Greczynski, G.; Mráz, S.; Hans, M.; Primetzhofer, D.; Lu, J.; Hultman, L.; Schneider, J.M. Unprecedented Al supersaturation in single-phase rock salt structure VAlN films by Al+ subplantation. J. Appl. Phys. 2017, 121, 171907. [Google Scholar] [CrossRef]
- Kouznetsov, V.; Macak, K.; Schneider, J.M.; Helmersson, U.; Petrov, I. A novel pulsed magnetron sputter technique utilizing very high target power densities. Surf. Coat. Technol. 1999, 122, 290–293. [Google Scholar] [CrossRef]
- Greczynski, G.; Lu, J.; Johansson, M.; Jensen, J.; Petrov, I.; Greene, J.E.; Hultman, L. Role of Tin+ and Aln+ ion irradiation (n = 1, 2) during Ti1-xAlxN alloy film growth in a hybrid HIPIMS/magnetron mode. Surf. Coat. Technol. 2012, 206, 4202–4211. [Google Scholar] [CrossRef]
- Greczynski, G.; Patscheider, J.; Lu, J.; Alling, B.; Ektarawong, A.; Jensen, J.; Petrov, I.; Greene, J.E.; Hultman, L. Control of Ti1−xSixN nanostructure via tunable metal-ion momentum transfer during HIPIMS/DCMSco-deposition. Surf. Coat. Technol. 2015, 280, 174–184. [Google Scholar] [CrossRef]
- Greczynski, G.; Lu, J.; Petrov, I.; Greene, J.E.; Bolz, S.; Kölker, W.; Schiffers, C.; Lemmer, O.; Hultman, L. Novel strategy for low-temperature, high-rate growth of dense, hard, and stress-free refractory ceramic thin films. J. Vac. Sci. Technol. A 2014, 32, 041515. [Google Scholar] [CrossRef]
- Greczynski, G.; Petrov, I.; Greene, J.E.; Hultman, L. Strategy for tuning the average charge state of metal ions incident at the growing film during HIPIMS deposition. Vacuum 2015, 116, 36–41. [Google Scholar] [CrossRef]
- Greczynski, G.; Mráz, S.; Hultman, L.; Schneider, J.M. Selectable phase formation in VAlN thin films by controlling Al+ subplantation depth. Sci. Rep. 2017, 7, 17544. [Google Scholar] [CrossRef]
- Available online: https://www.cemecon.de/en/coating-plants/cc-800-hipims (accessed on 27 December 2018).
- Greczynski, G.; Mráz, S.; Hultman, L.; Schneider, J.M. Venting temperature determines surface chemistry of magnetron sputtered TiN films. Appl. Phys. Lett. 2016, 108, 041603. [Google Scholar] [CrossRef]
- Ström, P.; Petersson, P.; Rubel, M.; Possnert, G. A combined segmented anode gas ionization chamber and time-of-flight detector for heavy ion elastic recoil detection analysis. Rev. Sci. Instrum. 2016, 87, 103303. [Google Scholar] [CrossRef]
- Baben, M.; Hans, M.; Primetzhofer, D.; Evertz, S.; Ruess, H.; Schneider, J.M. Unprecedented thermal stability of inherently metastable titanium aluminum nitride by point defect engineering. Mater. Res. Lett. 2017, 5, 158–169. [Google Scholar] [CrossRef]
- The JCPDS Database, Data Set Number: 25-1133; International Center for Diffraction Data: Newtown Square, PA, USA, 1998.
- The JCPDS Database, Data Set Number: 35-0753; International Center for Diffraction Data: Newtown Square, PA, USA, 1998.
- Kohn, J.A.; Cotter, P.G.; Potter, R.A. Synthesis of aluminum nitride monocrystals. Am. Miner. 1956, 41, 355–359. [Google Scholar]
- Birkholz, M.; Genzel, C. Residual stress analysis. In Thin Film Analysis by X-Ray Scattering; Birkholz, M., Ed.; Wiley-VCH: Weinheim, Germany, 2006; pp. 239–295. [Google Scholar]
- The JCPDS Database, Data Set Number: 35-0768; International Center for Diffraction Data: Newtown Square, PA, USA, 1998.
- The JCPDS Database, Data Set Number: 38-1420; International Center for Diffraction Data: Newtown Square, PA, USA, 1998.
- The first ~1 Å from the surface was not considered in the fraction calculation of the implanted Al+.
- Möller, W.; Eckstein, W.; Biersack, J.P. TRIDYN—Binary collision simulation of atoms collisions and dynamic composition changes in solids. Comput. Phys. Commun. 1988, 51, 355–368. [Google Scholar] [CrossRef]
- Mayrhofer, P.H.; Sonnleitner, D.; Bartosik, M.; Holec, D. Structural and mechanical evolution of reactively and non-reactively sputtered Zr–Al–N thin films during annealing. Surf. Coat. Technol. 2014, 244, 52–56. [Google Scholar] [CrossRef] [PubMed]
- Ghafoor, N.; Johnson, L.; Klenov, D.O.; Demeulemeester, J.; Desjardins, P.; Petrov, I.; Hultman, L.; Odén, M. Nanolabyrinthine ZrAlN thin films by self-organization of interwoven single-crystal cubic and hexagonal phases. Appl. Phys. Lett. Mater. 2013, 1, 022105. [Google Scholar] [CrossRef]
- Hultman, L.; Sundgren, J.-E.; Greene, J.E. Formation of polyhedral N2 bubbles during reactive sputter deposition of epitaxial TiN (100) films. J. Appl. Phys. 1989, 66, 536–544. [Google Scholar] [CrossRef]
- Davis, C.A. A simple model for the formation of compressive stress in thin films by ion bombardment. Thin Solid Films 1993, 226, 30–34. [Google Scholar] [CrossRef]
- Ulrich, S.; Theel, T.; Schwan, J.; Ehrhardt, H. Magnetron-sputtered superhard materials. Surf. Coat. Technol. 1997, 97, 45–59. [Google Scholar] [CrossRef]
- Teixeira, V. Mechanical integrity in PVD coatings due to the presence of residual stresses. Thin Solid Films 2001, 392, 276–281. [Google Scholar] [CrossRef]
- Oettel, H.; Wiedemann, R. Residual stresses in PVD hard coatings. Surf. Coat. Technol. 1995, 76, 265–273. [Google Scholar] [CrossRef]
- Lide, D.R. CRC Handbook of Chemistry and Physics, 84th ed.; CRC Press: Boca Raton, FL, USA, 2003. [Google Scholar]
- Petrov, I.; Hultman, L.; Helmersson, U.; Sundgren, J.-E.; Greene, J.E. Microstructure modification of TiN by ion bombardment during reactive sputter deposition. Thin Solid Films 1989, 169, 299–314. [Google Scholar] [CrossRef]
- Petrov, I.; Barna, P.B.; Hultman, L.; Greene, J.E. Microstructural evolution during film growth. J. Vac. Sci. Technol. A 2003, 21, S117–S128. [Google Scholar] [CrossRef]
- Music, D.; Banko, L.; Ruess, H.; Engels, M.; Hecimovic, A.; Grochla, D.; Rogalla, D.; Brögelmann, T.; Ludwig, A.; von Keudell, A.; Bobzin, K. Correlative plasma-surface model for metastable Cr–Al–N: Frenkel pair formation and influence of the stress state on the elastic properties. J. Appl. Phys. 2017, 121, 215108. [Google Scholar] [CrossRef]
- Greczynski, G.; Hultman, L. Peak amplitude of target current determines deposition rate loss during high power pulsed magnetron sputtering. Vacuum 2016, 124, 1–4. [Google Scholar] [CrossRef]
- Greczynski, G.; Zhirkov, I.; Petrov, I.; Greene, J.E.; Rosen, J. Gas rarefaction effects during high power pulsed magnetron sputtering of groups IVb and VIb transition metals in Ar. J. Vac. Sci. Technol. A 2017, 35, 060601. [Google Scholar] [CrossRef]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Greczynski, G.; Mráz, S.; Hans, M.; Lu, J.; Hultman, L.; Schneider, J.M. Control over the Phase Formation in Metastable Transition Metal Nitride Thin Films by Tuning the Al+ Subplantation Depth. Coatings 2019, 9, 17. https://doi.org/10.3390/coatings9010017
Greczynski G, Mráz S, Hans M, Lu J, Hultman L, Schneider JM. Control over the Phase Formation in Metastable Transition Metal Nitride Thin Films by Tuning the Al+ Subplantation Depth. Coatings. 2019; 9(1):17. https://doi.org/10.3390/coatings9010017
Chicago/Turabian StyleGreczynski, Grzegorz, Stanislav Mráz, Marcus Hans, Jun Lu, Lars Hultman, and Jochen M. Schneider. 2019. "Control over the Phase Formation in Metastable Transition Metal Nitride Thin Films by Tuning the Al+ Subplantation Depth" Coatings 9, no. 1: 17. https://doi.org/10.3390/coatings9010017
APA StyleGreczynski, G., Mráz, S., Hans, M., Lu, J., Hultman, L., & Schneider, J. M. (2019). Control over the Phase Formation in Metastable Transition Metal Nitride Thin Films by Tuning the Al+ Subplantation Depth. Coatings, 9(1), 17. https://doi.org/10.3390/coatings9010017




