Shelf Life Assessment of Fresh Poultry Meat Packaged in Novel Bionanocomposite of Chitosan/Montmorillonite Incorporated with Ginger Essential Oil
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Films Production
2.3. In Vitro Active Characterization
2.3.1. Migration Assays
2.3.2. Antimicrobial Studies
2.4. In Situ Active Characterization
2.4.1. Physicochemical Characterization
2.4.2. Thiobarbituric Acid Reactive Substances (TBARS) Index
2.4.3. Microbiological Growth
2.5. Statistical Analysis
3. Results and Discussion
3.1. In Vitro Active Characterization
3.1.1. Migration Assay
3.1.2. Antimicrobial Studies
3.2. In Situ Active Characterization
3.2.1. Physicochemical Characterization
3.2.2. Thiobarbituric Acid Reactive Substances Index
3.2.3. Microbiological Growth
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- FAO. Poultry Development Review; FAO: Rome, Italy, 2013; ISBN 978-92-5-108067-2. [Google Scholar]
- OECD-FAO Agricultural Outlook 2016–2025; OECD Publishing: Paris, France, 2016; ISBN 9789264253223.
- Petrou, S.; Tsiraki, M.; Giatrakou, V.; Savvaidis, I.N. Chitosan dipping or oregano oil treatments, singly or combined on modified atmosphere packaged chicken breast meat. Int. J. Food Microbiol. 2012, 156, 264–271. [Google Scholar] [CrossRef] [PubMed]
- Latou, E.; Mexis, S.F.; Badeka, V.; Kontakos, S.; Kontominas, M.G. Combined effect of chitosan and modified atmosphere packaging for shelf life extension of chicken breast fillets. LWT Food Sci. Technol. 2014, 55, 263–268. [Google Scholar] [CrossRef]
- Arkoun, M.; Daigle, F.; Holley, R.A.; Heuzey, M.C.; Ajji, A. Chitosan-based nanofibers as bioactive meat packaging materials. Packag. Technol. Sci. 2018, 31, 185–195. [Google Scholar] [CrossRef]
- Souza, V.G.L.; Fernando, A.L. Nanoparticles in food packaging: Biodegradability and potential migration to food—A review. Food Packag. Shelf Life 2016, 8, 63–70. [Google Scholar] [CrossRef]
- Lee, K.T. Quality and safety aspects of meat products as affected by various physical manipulations of packaging materials. Meat Sci. 2010, 86, 138–150. [Google Scholar] [CrossRef] [PubMed]
- Prasad, P.; Kochhar, A. Active packaging in food industry: A Review. IOSR J. Environ. Sci. Toxicol. Food Technol. 2014, 8. [Google Scholar] [CrossRef]
- Dutta, P.K.; Tripathi, S.; Mehrotra, G.K.; Dutta, J. Perspectives for chitosan based antimicrobial films in food applications. Food Chem. 2009, 114, 1173–1182. [Google Scholar] [CrossRef]
- Otoni, C.G.; Espitia, P.J.P.; Avena-Bustillos, R.J.; McHugh, T.H. Trends in antimicrobial food packaging systems: Emitting sachets and absorbent pads. Food Res. Int. 2016, 83, 60–73. [Google Scholar] [CrossRef]
- Malhotra, B.; Keshwani, A.; Kharkwal, H. Antimicrobial food packaging: Potential and pitfalls. Front. Microbiol. 2015, 6, 611. [Google Scholar] [CrossRef] [PubMed]
- Yuan, G.; Chen, X.; Li, D. Chitosan films and coatings containing essential oils: The antioxidant and antimicrobial activity, and application in food systems. Food Res. Int. 2016, 89, 117–128. [Google Scholar] [CrossRef] [PubMed]
- Alboofetileh, M.; Rezaei, M.; Hosseini, H.; Abdollahi, M. Antimicrobial activity of alginate/clay nanocomposite films enriched with essential oils against three common foodborne pathogens. Food Control 2014, 36, 1–7. [Google Scholar] [CrossRef]
- De Melo, A.A.; Geraldine, R.M.; Silveira, M.F.; Torres, M.C.; e Rezende, C.S.; Silva, C.; Fernandes, T.H.; de Oliveira, A.N. Microbiological quality and other characteristics of refrigerated chicken meat in contact with cellulose acetate-based film incorporated with rosemary essential oil. Braz. J. Microbiol. 2012, 43, 1419–1427. [Google Scholar] [CrossRef] [PubMed]
- Zivanovic, S.; Chi, S.; Draughon, A. Antimicrobial activity of chitosan. Science 2005, 70, 45–51. [Google Scholar] [CrossRef]
- Darder, M.; Colilla, M.; Ruiz-Hitzky, E. Biopolymer-clay nanocomposites based on chitosan intercalated in montmorillonite. Chem. Mater. 2003, 15, 3774–3780. [Google Scholar] [CrossRef]
- Ferreira, A.R.V.; Torres, C.A.V.; Freitas, F.; Sevrin, C.; Grandfils, C.; Reis, M.A.M.; Alves, V.D.; Coelhoso, I.M. Development and characterization of bilayer films of FucoPol and chitosan. Carbohydr. Polym. 2016, 147, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Srinivasa, P.C.; Ramesh, M.N.; Tharanathan, R.N. Effect of plasticizers and fatty acids on mechanical and permeability characteristics of chitosan films. Food Hydrocoll. 2007, 21, 1113–1122. [Google Scholar] [CrossRef]
- Azeredo, H.M.C.; Mattoso, L.H.C.; Avena-Bustillos, R.J.; Filho, G.C.; Munford, M.L.; Wood, D.; McHugh, T.H. Nanocellulose reinforced chitosan composite films as affected by nanofiller loading and plasticizer content. J. Food Sci. 2010, 75, N1–N7. [Google Scholar] [CrossRef] [PubMed]
- Mihindukulasuriya, S.D.F.; Lim, L.-T. Nanotechnology development in food packaging: A review. Trends Food Sci. Technol. 2014, 40, 149–167. [Google Scholar] [CrossRef]
- Souza, V.G.L.; Pires, J.R.A.; Rodrigues, P.F.; Lopes, A.A.S.; Fernandes, F.M.B.; Duarte, M.P.; Coelhoso, I.M.; Fernando, A.L. Bionanocomposites of chitosan/montmorillonite incorporated with Rosmarinus officinalis essential oil: Development and physical characterization. Food Packag. Shelf Life 2018, 16, 148–156. [Google Scholar] [CrossRef]
- Coelho, A.C.V.; Santos, P.D.S.; Santos, H.D.S. Argilas especiais: Argilas quimicamente modificadas-uma revisão. Quim. Nova 2007, 30, 1282–1294. [Google Scholar] [CrossRef]
- El-Ghorab, A.H.; Nauman, M.; Anjum, F.M.; Hussain, S.; Nadeem, M. A comparative study on chemical composition and antioxidant activity of ginger (Zingiber officinale) and cumin (Cuminum cyminum). J. Agric. Food Chem. 2010, 58, 8231–8237. [Google Scholar] [CrossRef] [PubMed]
- López, E.I.C.; Balcázar, M.F.H.; Mendoza, J.M.R.; Ortiz, A.D.R.; Melo, M.T.O.; Parrales, R.S.; Delgado, T.H. Antimicrobial activity of essential oil of Zingiber officinale Roscoe (Zingiberaceae). Am. J. Plant Sci. 2017, 8, 1511–1524. [Google Scholar] [CrossRef]
- Noshirvani, N.; Ghanbarzadeh, B.; Gardrat, C.; Rezaei, M.R.; Hashemi, M.; Le Coz, C.; Coma, V. Cinnamon and ginger essential oils to improve antifungal, physical and mechanical properties of chitosan-carboxymethyl cellulose films. Food Hydrocoll. 2017, 70, 36–45. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, A.; Wang, W.; Ye, R.; Liu, Y.; Xiao, J.; Wang, K. Characterisation of microemulsion nanofilms based on Tilapia fish skin gelatine and ZnO nanoparticles incorporated with ginger essential oil: Meat packaging application. Int. J. Food Sci. Technol. 2017, 52, 1670–1679. [Google Scholar] [CrossRef]
- Noori, S.; Zeynali, F.; Almasi, H. Antimicrobial and antioxidant efficiency of nanoemulsion-based edible coating containing ginger (Zingiber officinale) essential oil and its effect on safety and quality attributes of chicken breast fillets. Food Control 2018, 84, 312–320. [Google Scholar] [CrossRef]
- Klangmuang, P.; Sothornvit, R. Active hydroxypropyl methylcellulose-based composite coating powder to maintain the quality of fresh mango. LWT Food Sci. Technol. 2018, 91, 541–548. [Google Scholar] [CrossRef]
- BYK. Cloisite®Na+ Technical Data Sheet. Available online: http://www.byk.com/en/additives/additives-by-name/cloisite-na.php (accessed on 5 February 2018).
- Souza, V.G.L.; Fernando, A.L.; Pires, J.R.A.; Rodrigues, P.F.; Lopes, A.A.S.S.; Fernandes, F.M.B. Physical properties of chitosan films incorporated with natural antioxidants. Ind. Crops Prod. 2017, 107, 565–572. [Google Scholar] [CrossRef]
- Dias, M.V.; Machado Azevedo, V.; Borges, S.V.; Soares, N.D.F.F.; de Barros Fernandes, R.V.; Marques, J.J.; Medeiros, É.A.A. Development of chitosan/montmorillonite nanocomposites with encapsulated α-tocopherol. Food Chem. 2014, 165, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Souza, V.G.L.; Rodrigues, P.F.; Duarte, M.P.; Fernando, A.L. Antioxidant migration studies in chitosan films incorporated with plant extracts. J. Renew. Mater. 2018. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-ciocalteu. Methods Enzymol. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Chungy, D.; Papadakis, S.E.; Yamy, K.L. Simple models for assessing migration from food-packaging films. Food Addit. Contam. 2002, 19, 611–617. [Google Scholar] [CrossRef] [PubMed]
- Nouri, A.; Yaraki, M.T.; Ghorbanpour, M.; Agarwal, S.; Gupta, V.K. Enhanced antibacterial effect of chitosan film using montmorillonite/CuO nanocomposite. Int. J. Biol. Macromol. 2017, 109, 1219–1231. [Google Scholar] [CrossRef] [PubMed]
- ASTM E2149 Standard Test Methods for Determining the Antimicrobial Activity of Immobilized Antimicrobial Agents Under Dynamic Contact Conditions; ASTM International: West Conshohocken, PA, USA, 2001.
- AOAC. Official Methods of Analysis of the Association of Official Analytical Chemists, 20th ed.; AOAC International: Gaithersburg, MD, USA, 2016. [Google Scholar]
- Rosmini, M.R.; Perlo, F.; Pérez-Alvarez, J.A.; Pagán-Moreno, M.J.; Gago-Gago, A.; López-Santoveña, F.; Aranda-Catalá, V. TBA test by an extractive method applied to “paté”. Meat Sci. 1996, 42, 103–110. [Google Scholar] [CrossRef]
- ISO 4833-1:2013 Microbiology of the Food Chain—Horizontal Method for the Enumeration of Microorganisms—Part 1: Colony Count at 30 Degrees C by the Pour Plate Technique; International Organization for Standardization: Geneva, Switzerland, 2013.
- ISO 4831:2006(en) Microbiology of Food and Animal Feeding Stuffs—Horizontal Method for the Detection and Enumeration of Coliforms—Most Probable Number Technique; International Organization for Standardization: Geneva, Switzerland, 2006.
- Lopez de Dicastillo, C.; Nerin, C.; Alfaro, P.; Catala, R.; Gavara, R.; Hernandez-Munoz, P. Development of new antioxidant active packaging films based on ethylene vinyl alcohol copolymer (EVOH) and green tea extract. J. Agric. Food Chem. 2011, 59, 7832–7840. [Google Scholar] [CrossRef] [PubMed]
- Calatayud, M.; López-de-Dicastillo, C.; López-Carballo, G.; Vélez, D.; Hernández Muñoz, P.; Gavara, R. Active films based on cocoa extract with antioxidant, antimicrobial and biological applications. Food Chem. 2013, 139, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Bolumar, T.; Andersen, M.L.; Orlien, V. Antioxidant active packaging for chicken meat processed by high pressure treatment. Food Chem. 2011, 129, 1406–1412. [Google Scholar] [CrossRef]
- Souza, V.G.L.; Pires, J.R.A.; Vieira, E.T.; Coelhoso, I.M.; Duarte, M.P.; Fernando, A.L. Activity of chitosan-montmorillonite bionanocomposites incorporated with rosemary essential oil: From in vitro assays to aplication in fresh poultry meat (unpublished, manuscript in preparation).
- Abdollahi, M.; Rezaei, M.; Farzi, G. A novel active bionanocomposite film incorporating rosemary essential oil and nanoclay into chitosan. J. Food Eng. 2012, 111, 343–350. [Google Scholar] [CrossRef]
- Ribeiro-Santos, R.; Andrade, M.; de Melo, N.R.; Sanches-Silva, A. Use of essential oils in active food packaging: Recent advances and future trends. Trends Food Sci. Technol. 2017, 61, 132–140. [Google Scholar] [CrossRef]
- Regnier, T.; Combrinck, S.; Du Plooy, W. Essential oils and other plant extracts as food preservatives. In Progress in Food Preservation; Bhat, R., Alias, A.K., Paliyath, G., Eds.; Wiley-Blackwell: Oxford, UK, 2012; pp. 539–579. [Google Scholar]
- Teixeira, B.; Marques, A.; Ramos, C.; Neng, N.R.; Nogueira, J.M.F.; Saraiva, J.A.; Nunes, M.L. Chemical composition and antibacterial and antioxidant properties of commercial essential oils. Ind. Crops Prod. 2013, 43, 587–595. [Google Scholar] [CrossRef]
- Singh, P.; Singh, S.; Kapoor, I.P.S.; Singh, G.; Isidorov, V.; Szczepaniak, L. Chemical composition and antioxidant activities of essential oil and oleoresins from Curcuma zedoaria rhizomes, part-74. Food Biosci. 2013, 3, 42–48. [Google Scholar] [CrossRef]
- López, P.; Sánchez, C.; Batlle, R.; Nerín, C. Solid- and vapor-phase antimicrobial activities of six essential oils: Susceptibility of selected foodborne bacterial and fungal strains. J. Agric. Food Chem. 2005, 53, 6939–6946. [Google Scholar] [CrossRef] [PubMed]
- Sivasothy, Y.; Chong, W.K.; Hamid, A.; Eldeen, I.M.; Sulaiman, S.F.; Awang, K. Essential oils of Zingiber officinale var. rubrum Theilade and their antibacterial activities. Food Chem. 2011, 124, 514–517. [Google Scholar] [CrossRef]
- Shariatinia, Z.; Fazli, M. Mechanical properties and antibacterial activities of novel nanobiocomposite films of chitosan and starch. Food Hydrocoll. 2015, 46, 112–124. [Google Scholar] [CrossRef]
- Singh, G.; Kapoor, I.P.S.; Singh, P.; de Heluani, C.S.; de Lampasona, M.P.; Catalan, C.A.N. Chemistry, antioxidant and antimicrobial investigations on essential oil and oleoresins of Zingiber officinale. Food Chem. Toxicol. 2008, 46, 3295–3302. [Google Scholar] [CrossRef] [PubMed]
- Trajano, V.N.; Lima, O.; Leite, E.; Travassos, R. Propriedade antibacteriana de óleos essenciais de especiarias sobre bactérias contaminantes de alimentos. 2009, 29, 542–545. [Google Scholar] [CrossRef]
- Hafsa, J.; Smach, M.; Ben Khedher, M.R.; Charfeddine, B.; Limem, K.; Majdoub, H.; Rouatbi, S. Physical, antioxidant and antimicrobial properties of chitosan films containing Eucalyptus globulus essential oil. LWT Food Sci. Technol. 2016, 68, 356–364. [Google Scholar] [CrossRef]
- Siripatrawan, U.; Vitchayakitti, W. Improving functional properties of chitosan films as active food packaging by incorporating with propolis. Food Hydrocoll. 2016, 61, 695–702. [Google Scholar] [CrossRef]
- Abdollahi, M.; Rezaei, M.; Farzi, G. Original article Improvement of active chitosan film properties with rosemary essential oil for food packaging. Food Sci. Technol. 2012, 47, 847–853. [Google Scholar] [CrossRef]
- Verlee, A.; Mincke, S.; Stevens, C.V. Recent developments in antibacterial and antifungal chitosan and its derivatives. Carbohydr. Polym. 2017, 164, 268–283. [Google Scholar] [CrossRef] [PubMed]
- Siripatrawan, U.; Harte, B.R. Physical properties and antioxidant activity of an active film from chitosan incorporated with green tea extract. Food Hydrocoll. 2010, 24, 770–775. [Google Scholar] [CrossRef]
- Lavorgna, M.; Piscitelli, F.; Mangiacapra, P.; Buonocore, G.G. Study of the combined effect of both clay and glycerol plasticizer on the properties of chitosan films. Carbohydr. Polym. 2010, 82, 291–298. [Google Scholar] [CrossRef]
- MINOLTA. Precise Color Communication: Color Control from Perception to Instrumentation; Minolta Co., Ltd.: Osaka, Japan, 2007. [Google Scholar]
- Rojas, M.C.; Brewer, M.S. Effect of natural antioxidants on oxidative stability of frozen, vacuum-packaged beef and pork. J. Food Qual. 2008, 31, 173–188. [Google Scholar] [CrossRef]
- Contini, C.; Álvarez, R.; O’Sullivan, M.; Dowling, D.P.; Gargan, S.Ó.; Monahan, F.J. Effect of an active packaging with citrus extract on lipid oxidation and sensory quality of cooked turkey meat. Meat Sci. 2014, 96, 1171–1176. [Google Scholar] [CrossRef] [PubMed]
- Ghaderi-Ghahfarokhi, M.; Barzegar, M.; Sahari, M.A.; Ahmadi Gavlighi, H.; Gardini, F. Chitosan-cinnamon essential oil nano-formulation: Application as a novel additive for controlled release and shelf life extension of beef patties. Int. J. Biol. Macromol. 2017, 102, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Abdollahi, M.; Rezaei, M.; Farzi, G. Influence of chitosan/clay functional bionanocomposite activated with rosemary essential oil on the shelf life of fresh silver carp. Int. J. Food Sci. Technol. 2014, 49, 811–818. [Google Scholar] [CrossRef]
- Remya, S.; Mohan, C.O.; Bindu, J.; Sivaraman, G.K.; Venkateshwarlu, G.; Ravishankar, C.N. Effect of chitosan based active packaging film on the keeping quality of chilled stored barracuda fish. J. Food Sci. Technol. 2016, 53, 685–693. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Hu, S.; Lu, Y.; Yang, H.; Zhao, Y.; Li, L. Effects of coatings of polyethyleneimine and thyme essential oil combined with chitosan on sliced fresh channa argus during refrigerated storage. J. Food Process Eng. 2015, 38, 225–233. [Google Scholar] [CrossRef]
- Atarés, L.; Pérez-Masiá, R.; Chiralt, A. The role of some antioxidants in the HPMC film properties and lipid protection in coated toasted almonds. J. Food Eng. 2011, 104, 649–656. [Google Scholar] [CrossRef]
- European Commission. Commmission Regulation (EC) No 2073/2005 of 15 November 2005 on microbiological criteria for foodstuffs. Off. J. Eur. Union 2005, L338, 1–19. Available online: https://www.fsai.ie/uploadedFiles/Reg2073_2005%281%29.pdf (accessed on 5 February 2018).
- Economou, T.; Pournis, N.; Ntzimani, A.; Savvaidis, I.N. Nisin-EDTA treatments and modified atmosphere packaging to increase fresh chicken meat shelf-life. Food Chem. 2009, 114, 1470–1476. [Google Scholar] [CrossRef]
- Khanjari, A.; Karabagias, I.K.; Kontominas, M.G. Combined effect of N,O-carboxymethyl chitosan and oregano essential oil to extend shelf life and control Listeria monocytogenes in raw chicken meat fillets. LWT Food Sci. Technol. 2013, 53, 94–99. [Google Scholar] [CrossRef]
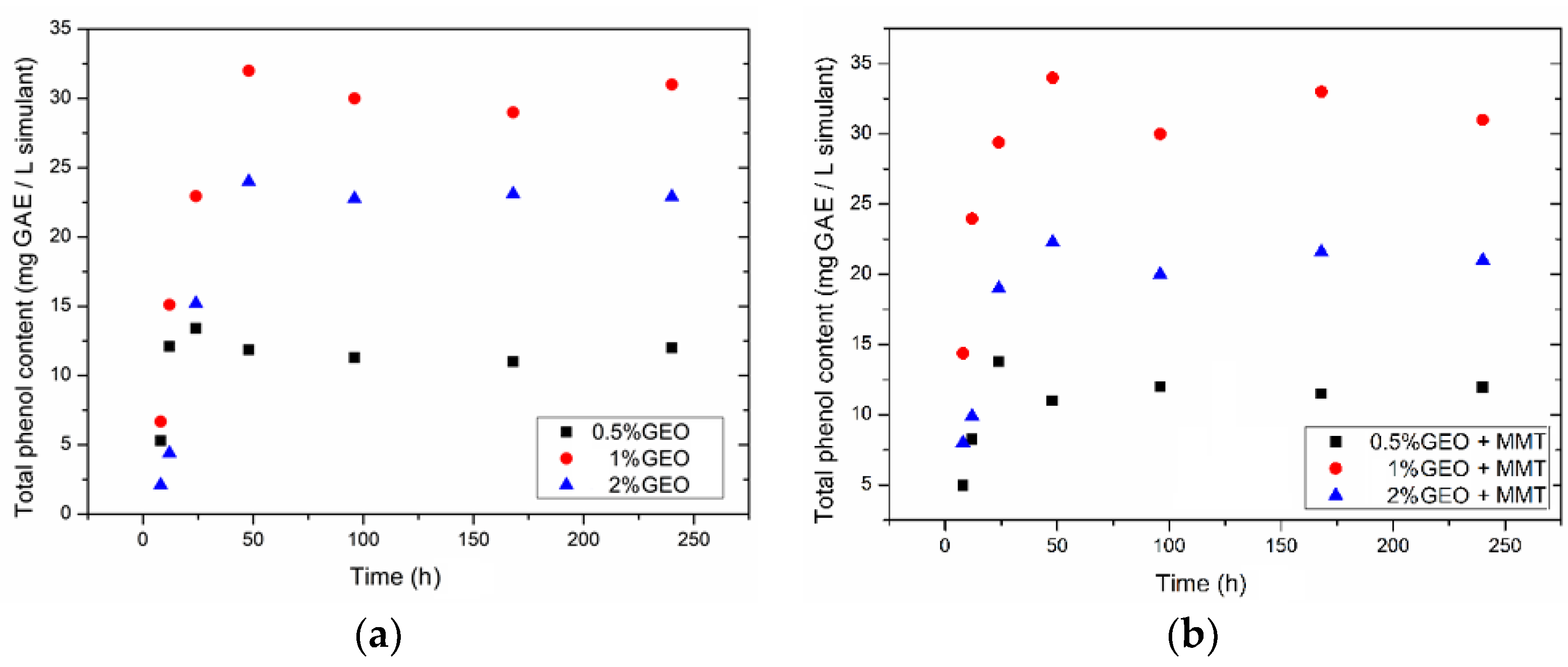
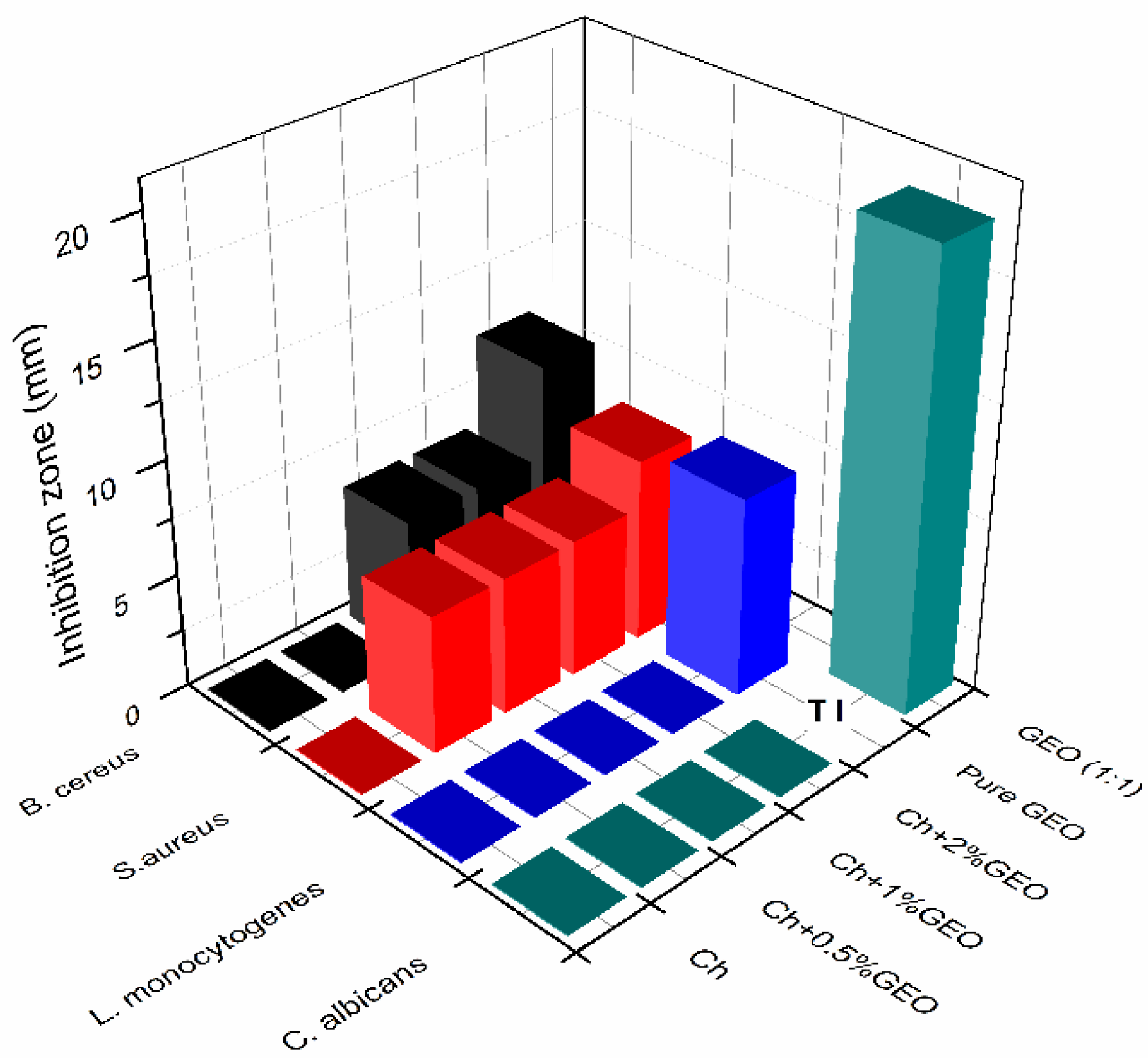
| Film | Antibacterial Activity (Log Reduction) | Diffusion Coefficient of TPC (10−11 m2/s) | Maximum GEO Diffused/Total Incorporated into Films | Maximum % Radical Scavenging (DPPH) | |
|---|---|---|---|---|---|
| B. cereus | S. enterica | ||||
| Ch | 7.3 ± 0.1 A | 5.3 ± 0.6 A | No migration | – | – |
| Ch + MMT | 6.7 ± 1 A | 3.5 ± 0.7 B | No migration | – | – |
| Ch + 0.5% GEO | 3.4 ± 0.2 B | 3.8 ±0.1 B | 2.06 ± 0.2 A,B | 0.71 | 6.3 |
| Ch + MMT + 0.5% GEO | 2.3 ± 0.1 B | 3.2 ±1.0 B | 3.77 ± 0.48 A,B | 0.82 | 40.6 |
| Ch + 1% GEO | 1.5 ± 0.3 B | 3.8 ± 0.4 B | 3.15 ± 0.29 A,B | 0.68 | 10.5 |
| Ch + MMT + 1% GEO | 3.6 ± 0.8 B | 3.2 ± 0.3 B | 11.4 ± 0.63 A | 1.0 | 8.32 |
| Ch + 2% GEO | 3.6 ± 0.3 B | 3.6 ± 0.3 B | 1.24 ± 0.43 B | 0.37 | 18.9 |
| Ch + MMT + 2% GEO | 5.3 ± 0.0 A | 2.3 ± 0.2 B | 0.13 ± 0.01 B | 0.31 | 6.05 |
| Parameter | Day | Unwrapped | PVC | Ch | Ch + 0.5% GEO | Ch + 1% GEO | Ch + 2% GEO | Ch + MMT | Ch + MMT + 0.5% GEO | Ch + MMT + 1% GEO | Ch + MMT + 2% GEO |
|---|---|---|---|---|---|---|---|---|---|---|---|
| TBARS (mg MDA/kg meat) | 0 | 0.11 ± 0.05 a,C | 0.11 ± 0.05 a,B | 0.11 ± 0.05 a,B | 0.11 ± 0.05 a,A | 0.11 ± 0.05 a,A | 0.11 ± 0.05 a,A,B | 0.11 ± 0.05 a,B | 0.11 ± 0.05 a,C | 0.11 ± 0.05 a,C | 0.11 ± 0.05 a,B |
| 3 | 0.13 ± 0.06 b,C | 0.11 ± 0.01 b,B | 0.19 ± 0.06 b,B | 0.16 ± 0.02 b,A | 0.07 ± 0.0 b,B | 0.10 ± 0.06 b,A,B | 0.34 ± 0.02 a,B | 0.15 ± 0.01 b,C | 0.15 ± 0.03 b,C | 0.18 ± 0.09 b,B | |
| 7 | 0.88 ± 0.20 a,B | 0.07 ± 0.01 c,B | 0.12 ± 0.02 c,B | 0.19 ± 0.10 c,A | 0.05 ± 0.02 c,B | 0.06 ± 0.02 c,A,B | 0.49 ± 0.03 b,A,B | 0.17 ± 0.04 c,C | 0.16 ± 0.02 c,C | 0.13 ± 0.02 c,B | |
| 10 | 1.48 ± 0.33 a,A | 0.65 ± 0.25 b,c,A | 0.23 ± 0.06 d,e,A,B | 0.20 ± 0.02 d,e,A | 0.04 ± 0.01 e,B | 0.02 ± 0.01 e,B | 0.58 ± 0.04 b,c,d,A,B | 0.78 ± 0.00 b,B | 0.57 ± 0.00 b,c,d,B | 0.28 ± 0.03 c,d,e,B | |
| 15 | 1.97 ± 0.28 a,A | 0.65 ± 0.02 b,c,d,A | 0.42 ± 0.12 c,d,A | 0.25 ± 0.0 c,d,A | 0.20 ± 0.04 d,A | 0.15 ± 0.06 d,A | 1.11 ± 0.54 b,A | 1.09 ± 0.20 b,A | 0.83 ± 0.12 b,c,A | 0.51 ± 0.12 b,c,d,A | |
| Hue (°) | 0 | 57 ± 1 a,E | 57 ± 1 a,C | 57 ± 1 a,A | 57 ± 1 a,B | 57 ± 1 a,A | 57 ± 1 a,A | 57 ± 1 a,A | 57 ± 1 a,A | 57 ± 1 a,B | 57 ± 1 a,B,C |
| 3 | 62 ± 0 a,b,D | 61 ± 1 a,b,c,B | 59 ± 2 b,c,d,A | 56 ± 1 d,e,B | 52 ± 1 e,A | 53 ± 1 e,B | 62 ± 0 a,b,A | 60 ± 2 a,b,c,A | 58 ± 1 c,d,B | 62 ± 0 a,A | |
| 7 | 64 ± 0 a,b,C | 67 ± 0 a,A | 59 ± 1 b,c,A | 56 ± 1 c,d,B | 57 ± 4 c,d,A | 57 ± 2 c,d,A | 62 ± 3 b,c,A | 59 ± 1 b,c,A | 64 ± 0 a,b,A | 53 ± 2 d,C | |
| 10 | 67 ± 0 a,B | 66 ± 1 a,A | 59 ± 1 c,A | 64 ± 1 a,b,A | 58 ± 2 c,A | 60 ± 1 b,c,A | 61 ± 2 b,c,A | 59 ± 1 c,A | 60 ± 0 b,c,B | 61 ± 2 b,c,A,B | |
| 15 | 70 ± 1 a,A | 67 ± 1 a,b,A | 60 ± 2 c,d,A | 55 ± 0 e,B | 55 ± 2 e,A | 57 ± 1 d,e,A | 62 ± 0 b,c,A | 60 ± 0 c,d,A | 57 ± 4 c,d,e,B | 60 ± 3 c,d,e,A,B | |
| pH | 0 | 5.87 ± 0.11 a,C | 5.96 ± 0.15 a,C | 5.87 ± 0.11 a,D | 5.72 ± 0.02 a,A,B | 5.71 ± 0.21 a,A | 5.71 ± 0.21 a,B,C | 5.96 ± 0.15 a,B | 5.96 ± 0.15 a,A | 5.96 ± 0.15 a,B | 5.96 ± 0.15 a,B |
| 3 | 6.09 ± 0.35 a,C | 6.19 ± 0.08 a,C | 6.01 ± 0.20 a,C,D | 5.57 ± 0.07 b,B | 5.38 ± 0.02 b,B | 5.45 ± 0.05 b,C | 6.01 ± 0.00 a,B | 6.12 ± 0.05 a,A | 6.03 ± 0.02 a,B | 5.96 ± 0.09 a,b,B | |
| 7 | 7.16 ± 0.30 a,B | 6.50 ± 0.12 b,B | 6.33 ± 0.02 b,c,B,C | 5.62 ± 0.01 f,A,B | 5.74 ± 0.04 e,f,A | 5.76 ± 0.10 e,f,B | 5.84 ± 0.02 d,f,B | 6.02 ± 0.04 c,d,e,A | 6.10 ± 0.04 c,d,B | 5.94 ± 0.08 d,e,f,B | |
| 10 | 7.55 ± 0.02 a,B | 6.79 ± 0.04 b,B | 6.51 ± 0.21 b,c,B | 5.58 ± 0.02 e,B | 5.60 ± 0.03 e,A,B | 5.52 ± 0.04 e,B,C | 6.01 ± 0.04 d,B | 6.14 ± 0.20 d,A | 6.29 ± 0.07 c,d,B | 6.00 ± 0.20 d,B | |
| 15 | 8.17 ± 0.14 a,A | 7.26 ± 0.13 b,c,A | 7.14 ± 0.23 b,c,A | 5.85 ± 0.2 d,e,A | 5.71 ± 0.09 e,A | 6.22 ± 0.01 d,e,A | 7.27 ± 0.36 b,c,A | 6.50 ± 0.55 c,d,e,A | 7.33 ± 0.36 b,A | 6.61 ± 0.24 b,c,d,A | |
| Acidity (% oleic acid equivalent) | 0 | 1.80 ± 0.39 a,A | 1.68 ± 0.22 a,A | 1.80 ± 0.39 a,A | 2.12 ± 0.11 a,A | 2.12 ± 0.11 a,A | 2.12 ± 0.11 a,A | 1.68 ± 0.22 a,A | 1.68 ± 0.22 a,A | 1.68 ± 0.22 a,A | 1.68 ± 0.22 a,A |
| 3 | 1.24 ± 0.37 b,A,B | 1.32 ± 0.08 b,B | 1.13 ± 0.01 b,B | 1.70 ± 0.02 a,B | 1.75 ± 0.08 a,B | 1.75 ± 0.03 a,B | 1.19 ± 0.17 b,B | 1.04 ± 0.19 b,B | 1.04 ± 0.09 b,C,D | 1.26 ± 0.05 b,A,B | |
| 7 | 0.42 ± 0.14 f,C | 1.27 ± 0.07 c,d,B | 0.85 ± 0.05 e,B | 1.47 ± 0.01 a,b,B,C | 1.55 ± 0.04 a,C | 1.41 ± 0.03 a,b,c,C | 1.49 ± 0.00 a,b,A,B | 1.22 ± 0.00 c,d,A,B | 1.33 ± 0.13 b,c,d,B,C | 1.16 ± 0.02 d,B | |
| 10 | 0.73 ± 0.15 c,B,C | 1.15 ± 0.06 b,B,C | 1.13 ± 0.18 b,c,B | 1.30 ± 0.20 a,b,C | 1.46 ± 0.09 a,b,C | 1.36 ± 0.04 a,b,C | 1.46 ± 0.10 a,b,A,B | 1.58 ± 0.23 a,A,B | 1.52 ± 0.06 a,b,A,B | 1.65 ± 0.15 a,A | |
| 15 | 0.66 ± 0.14 b,B,C | 0.87 ± 0.02 a,b,C | 1.22 ± 0.30 a,A,B | 0.73 ± 0.11 a,b,D | 1.22 ± 0.03 a,D | 1.11 ± 0.08 a,b,D | 0.78 ± 0.15 a,b,C | 1.24 ± 0.34 a,A,B | 0.76 ± 0.09 a,b,D | 1.13 ± 0.29 a,b,B | |
| Water content (%) | 0 | 75.5 ± 0.2 a,B | 75.5 ± 0.2 a,B | 75.5 ± 0.2 a,A | 75.5 ± 0.2 a,A | 75.5 ± 0.2 a,A | 75.5 ± 0.2 a,A | 75.5 ± 0.2 a,A | 75.5 ± 0.2 a,A | 75.5 ± 0.2 a,A | 75.5 ± 0.2 a,A |
| 3 | 75.4 ± 0.2 a,b,B | 75.8 ± 0.4 a,B | 73.4 ± 0.1 c,B,C | 74.7 ± 0.4 b,A | 73.3 ± 0.0 c,A,B | 73.6 ± 0.2 c,B | 73.8 ± 0.2 c,B | 73.1 ± 0.4 c,B | 73.4 ± 0.2 c,B | 73.0 ± 0.2 c,C | |
| 7 | 76.0 ± 0.2 a,A,B | 75.1 ± 0.4 a,B | 72.5 ± 0.1 b,C | 73.2 ± 0.3 b,B | 72.6 ± 0.6 b,B | 72.8 ± 0.5 b,B,C | 73.2 ± 0.3 b,B | 73.1 ± 0.3 b,B | 72.8 ± 0.0 b,B | 72.9 ± 0.7 b,C | |
| 10 | 76.2 ± 0.4 a,A,B | 75.6 ± 0.3 a,b,B | 74.5 ± 0.3 b,c,A,B | 74.4 ± 0.5 c,A,B | 72.0 ± 0.1 d,B | 70.7 ± 0.7 e,C,D | 73.6 ± 0.2 c,B | 74.1 ± 0.2 c,B | 73.4 ± 0.6 c,B | 74.1 ± 0.2 c,B | |
| 15 | 77.3 ± 1.0 a,A | 76.7 ± 0.1 a,b,A | 73.4 ± 0.8 c,d,e,B,C | 71.6 ± 0.8 e,C | 72.0 ± 2.0 d,e,B | 71.3 ± 0.4 e,D | 75.9 ± 0.6 a,b,c,A | 74.2 ± 0.9 b,c,d,B | 74.8 ± 0.2 a,b,c,A | 73.6 ± 0.2 c,d,e,B,C |
| Parameter | Day | Unwrapped | PVC | Ch | Ch + 0.5% GEO | Ch + 1% GEO | Ch + 2% GEO | Ch + MMT | Ch + MMT + 0.5% GEO | Ch + MMT + 1% GEO | Ch + MMT + 2% GEO |
|---|---|---|---|---|---|---|---|---|---|---|---|
| TMAB (log CFU/g meat) | 0 | 5.1 ± 0.1 a,C | 5.1 ± 0.1 a,C | 5.1 ± 0.1 a,C | 5.1 ± 0.1 a,C | 5.1 ± 0.1 a,B | 5.1 ± 0.1 a,B | 5.1 ± 0.1 a,C | 5.1 ± 0.1 a,C | 5.1 ± 0.1 a,B | 5.1 ± 0.1 a,B |
| 3 | 8.8 ± 0.1 a,B | 8.7 ± 0.0 a,B | 7.1 ± 0.3 b,B | 7.1 ± 0.4 b,B | 6.7 ± 0.2 b,A | 6.8 ± 0.2 b,A | 6.7 ± 0.0 b,B | 7.1 ± 0.3 b,B | 5.3 ± 0.2 c,B | 5.9 ± 0.0 c,B | |
| 7 | 10.2 ± 0.1 a,A | 9.5 ± 0.3 a,b,A | 8.5 ± 0.2 c,d,A | 7.4 ± 0.0 e,f,B | 7.8 ± 0.1 d,f,A | 8.1 ± 0.1 c,d,e,A | 7.2 ± 0.7 f,A,B | 8.7 ± 0.1 b,c,A | 8.7 ± 0.1 c,A | 8.3 ± 0.2 c,d,A | |
| 10 | 10.1 ± 0.0 a,A | 9.6 ± 0.1 a,b,A | 8.7 ± 0.5 c,A | 8.3 ± 0.2 c,d,A | 7.5 ± 0.1 e,A | 7.7 ± 0.5 d,e,A | 8.5 ± 0.0 c,A | 8.8 ± 0.0 c,A | 8.9 ± 0.1 b,c,A | 8.8 ± 0.0 c,A | |
| Total coliforms (log MPN/g meat) | 0 | 0.8 ± 0.4 a,C | 0.8 ± 0.4 a,C | 0.8 ± 0.4 a,C | 0.8 ± 0.4 a,B | 0.8 ± 0.4 a,C | 0.8 ± 0.4 a,B | 0.8 ± 0.4 a,C | 0.8 ± 0.4 a,C | 0.8 ± 0.4 a,D | 0.8 ± 0.4 a,C |
| 3 | 3.0 ± 0.4 b,c,B,C | 3.4 ± 0.0 a,b,B | 2.8 ± 0.5 b,c,B | 3.2 ± 0.2 a,b,c,A | 4.0 ± 0.0 a,A | 2.8 ± 0.4 b,c,A | 2.9 ± 0.5 b,c,B | 2.9 ± 0.5 b,c,B | 2.2 ± 0.2 c,C | 2.2 ± 0.2 c,B | |
| 7 | 4.5 ± 1.7 a,A,B | 3.9 ± 0.5 a,b,c,B | 4.0 ± 0.9 a,b,A,B | 3.4 ± 0.3 a,b,c,A | 2.3 ± 0.00 b,c,B | 2.1 ± 0.1 c,A | 3.0 ± 0.3 a,b,c,B | 3.6 ± 0.4 a,b,c,B | 3.7 ± 0.4 a,b,c,B | 2.4 ± 0.1 b,c,B | |
| 10 | 6.7 ± 0.6 a,A | 6.4 ± 0.0 a,A | 5.3 ± 1.0 a,A | 3.2 ± 0.0 b,A | 2.6 ± 0.6 b,B | 2.2 ± 0.8 b,A | 5.9 ± 0.2 a,A | 6.2 ± 0.2 a,A | 5.9 ± 0.5 a,A | 5.9 ± 0.5 a,A |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Souza, V.G.L.; Pires, J.R.A.; Vieira, É.T.; Coelhoso, I.M.; Duarte, M.P.; Fernando, A.L. Shelf Life Assessment of Fresh Poultry Meat Packaged in Novel Bionanocomposite of Chitosan/Montmorillonite Incorporated with Ginger Essential Oil. Coatings 2018, 8, 177. https://doi.org/10.3390/coatings8050177
Souza VGL, Pires JRA, Vieira ÉT, Coelhoso IM, Duarte MP, Fernando AL. Shelf Life Assessment of Fresh Poultry Meat Packaged in Novel Bionanocomposite of Chitosan/Montmorillonite Incorporated with Ginger Essential Oil. Coatings. 2018; 8(5):177. https://doi.org/10.3390/coatings8050177
Chicago/Turabian StyleSouza, Victor G. L., João R. A. Pires, Érica T. Vieira, Isabel M. Coelhoso, Maria P. Duarte, and Ana L. Fernando. 2018. "Shelf Life Assessment of Fresh Poultry Meat Packaged in Novel Bionanocomposite of Chitosan/Montmorillonite Incorporated with Ginger Essential Oil" Coatings 8, no. 5: 177. https://doi.org/10.3390/coatings8050177
APA StyleSouza, V. G. L., Pires, J. R. A., Vieira, É. T., Coelhoso, I. M., Duarte, M. P., & Fernando, A. L. (2018). Shelf Life Assessment of Fresh Poultry Meat Packaged in Novel Bionanocomposite of Chitosan/Montmorillonite Incorporated with Ginger Essential Oil. Coatings, 8(5), 177. https://doi.org/10.3390/coatings8050177








