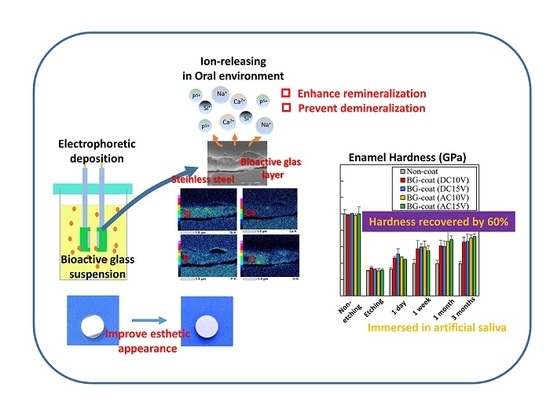
Electrophoretic Deposition as a New Bioactive Glass Coating Process for Orthodontic Stainless Steel
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. EPD Process
2.3. Color Measurements
2.4. Characterization of the Coatings
2.5. Mechanical Properties of the Coatings
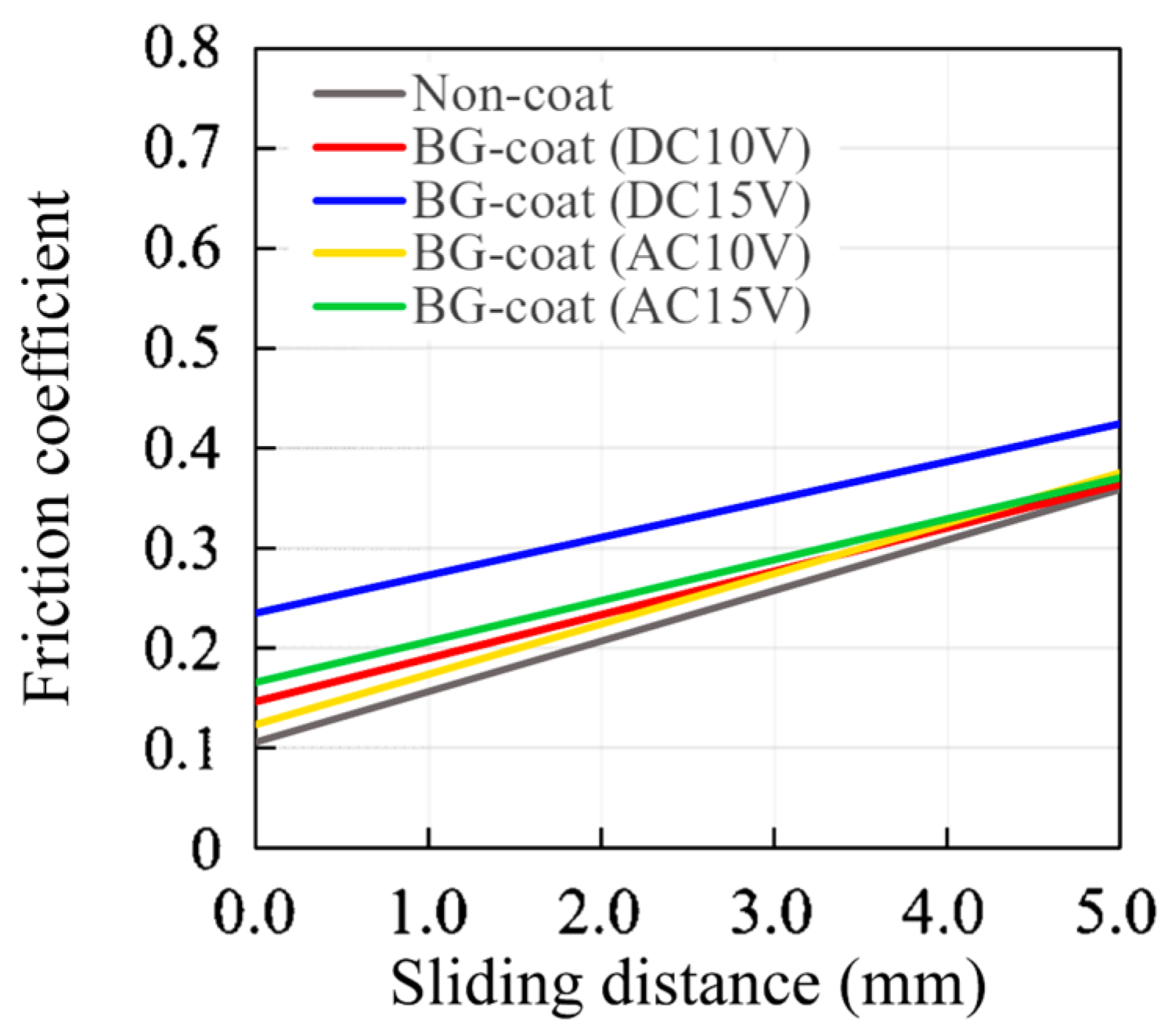
2.6. Frictional Properties Measured by the Progressive Load Scratch Test
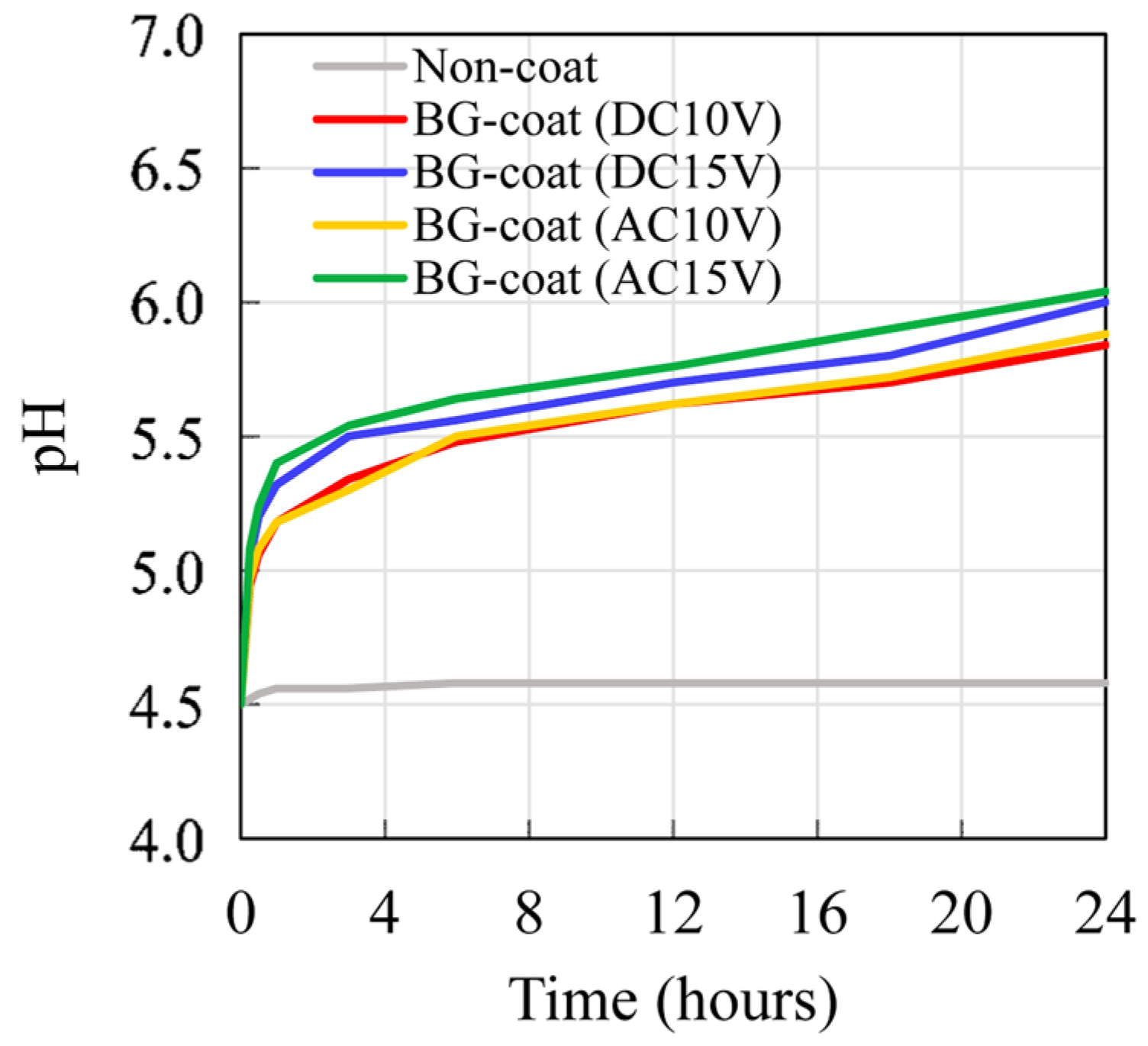
2.7. Acid-Neutralizing Ability
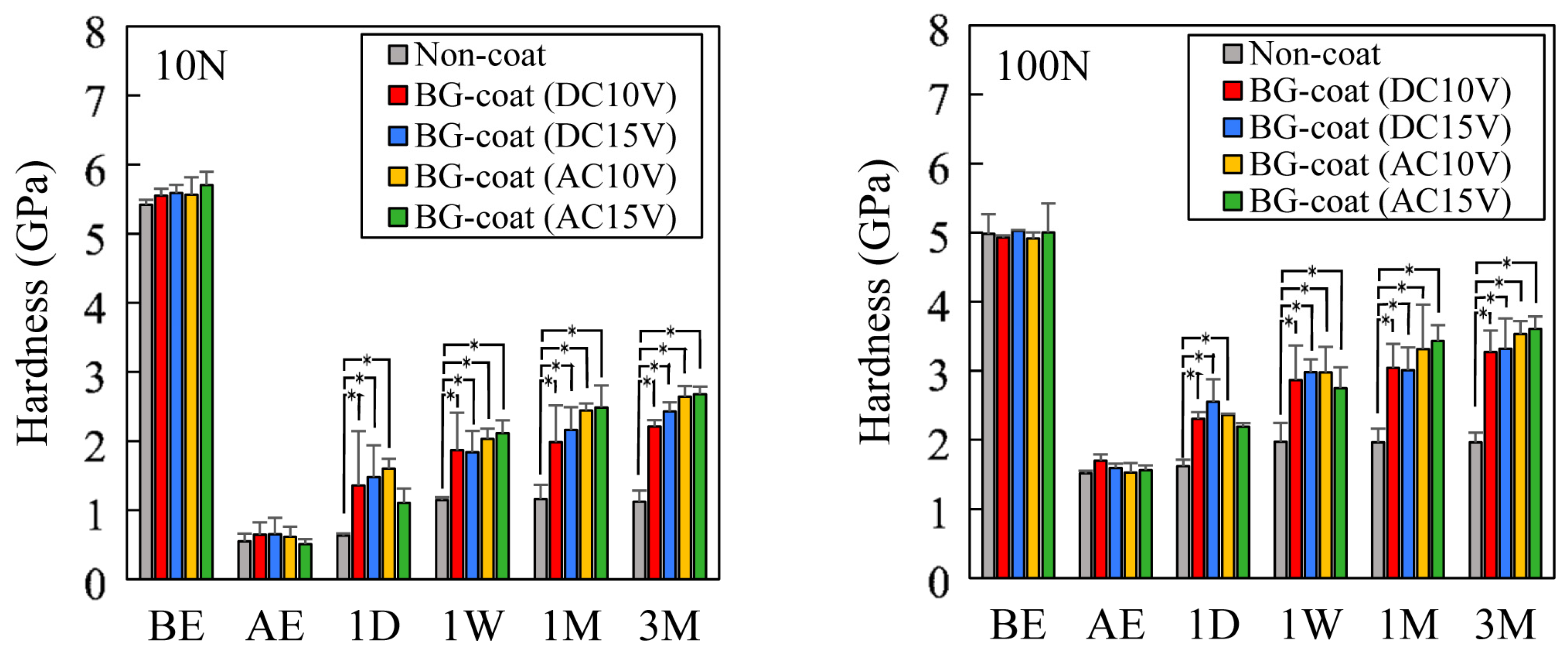
2.8. Enamel Remineralization Ability and Changes in the Mechanical Properties
2.9. Cytocompatibility
2.10. Statistical Analyses
3. Results
3.1. Color Measurements
3.2. Crystal Structures, Morphological Features, and Compositions of the Coating Layers
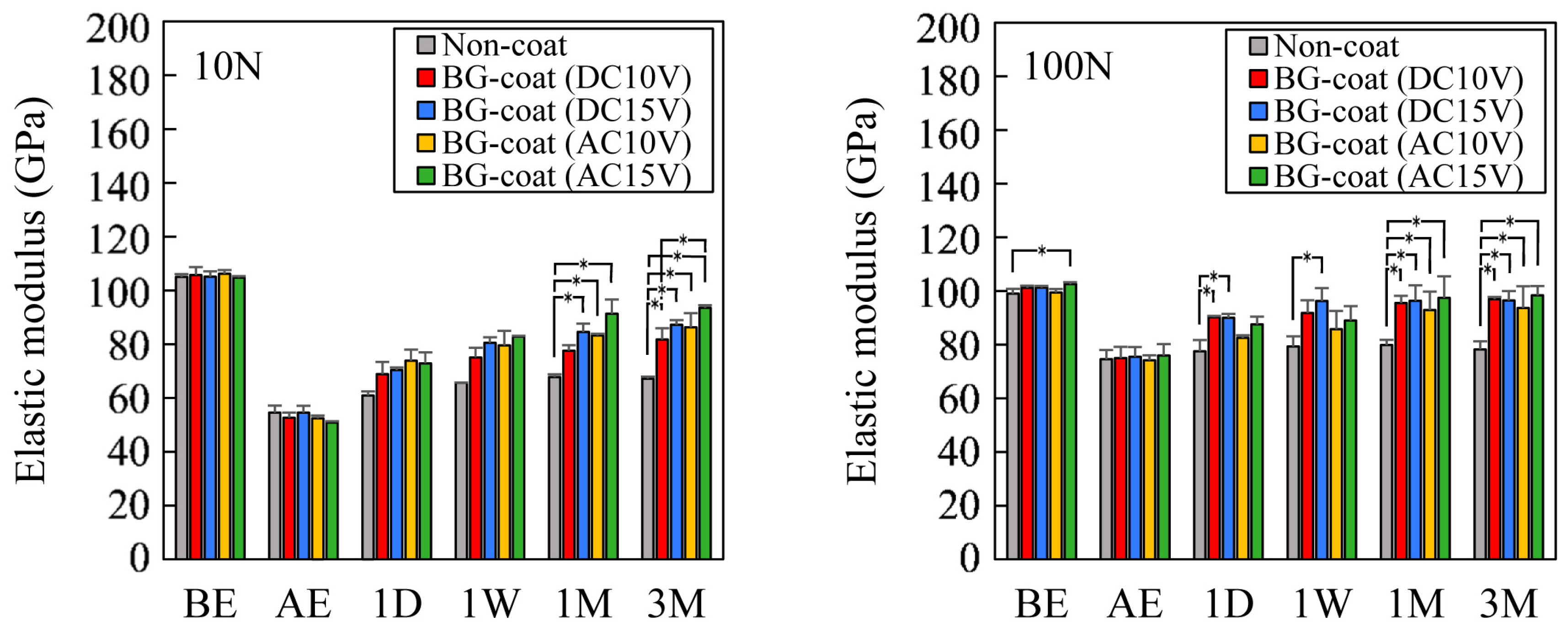
3.3. Evaluation of the Mechanical Properties of the Coating Layers by Nanoindentation
3.4. Frictional Properties Measured by the Progressive Load Scratch Test
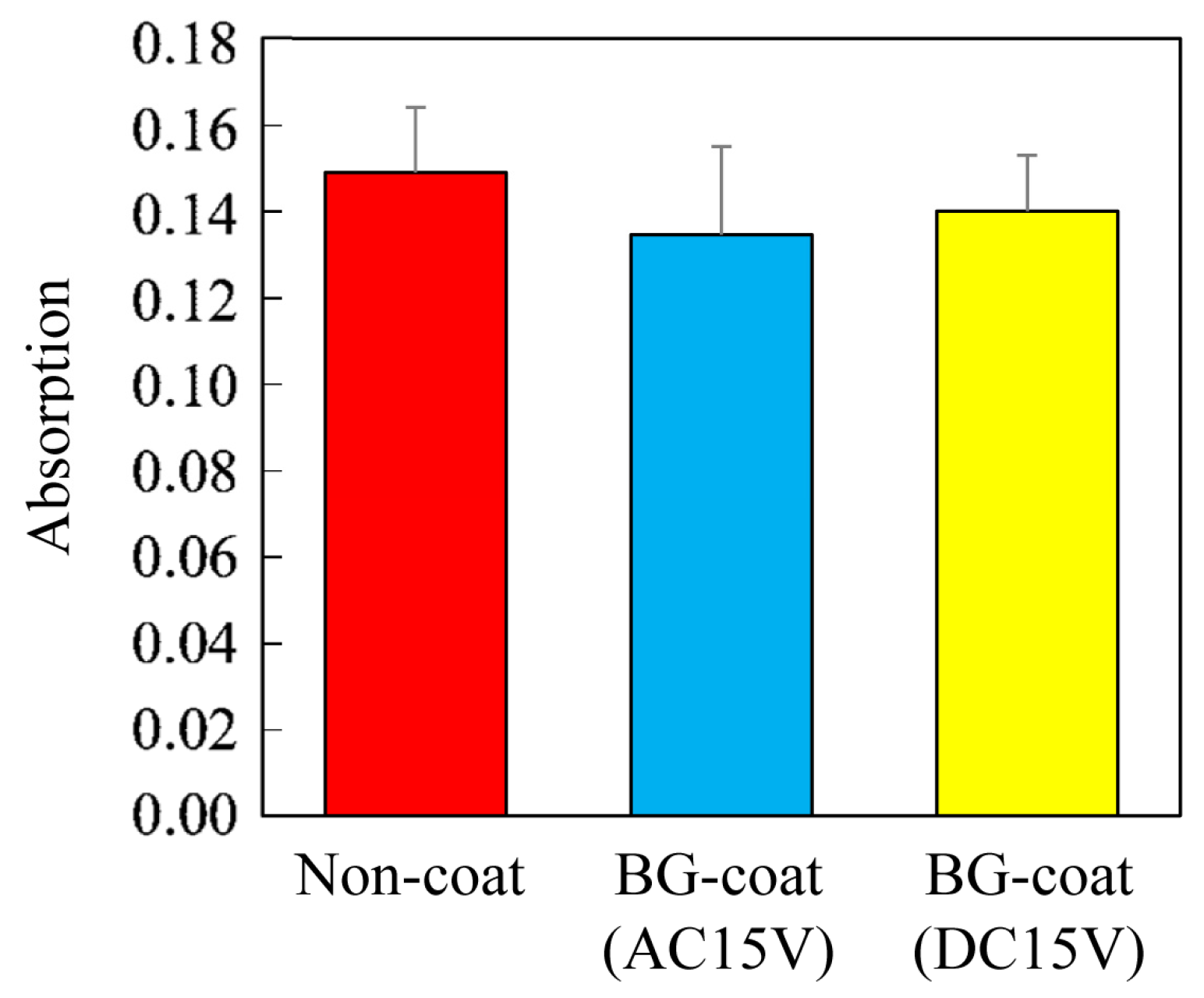
3.5. Analysis of Acid-Neutralizing Ability
3.6. Enamel Remineralization Ability in Artificial Saliva and Mechanical Property Changes Determined by Nanoindentation
3.7. Cytocompatibility Assays
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Iijima, M.; Zinelis, S.; Papageorgiou, S.N.; Brantley, W.; Eliades, T. Orthodontic brackets. In Orthodontic Application of Biomaterials; Eliades, T., Brantley, W., Eds.; Woodhead Publishing: Sawston, UK, 2017; pp. 75–96. [Google Scholar]
- Iijima, M.; Muguruma, T.; Brantley, W.; Choe, H.C.; Nakagaki, S.; Alapati, S.B.; Mizoguchi, I. Effect of coating on properties of esthetic orthodontic nickel-titanium wires. Angle Orthod. 2012, 82, 319–325. [Google Scholar] [CrossRef] [PubMed]
- Lopes Filho, H.; Maia, L.E.; Araújo, M.V.; Ruellas, A.C. Influence of optical properties of esthetic brackets (color, translucence, and fluorescence) on visual perception. Am. J. Orthod. Dent. Orthop. 2012, 141, 460–467. [Google Scholar] [CrossRef] [PubMed]
- Karamouzos, A.; Athanasiou, A.E.; Papadopoulos, M.A. Clinical characteristics and properties of ceramic brackets: A comprehensive review. Am. J. Orthod. Dent. Orthop. 1997, 112, 34–40. [Google Scholar] [CrossRef]
- Alrejaye, N.; Pober, R.; Giordano, I.R. Torsional strength of computer-aided design/computer-aided manufacturing-fabricated esthetic orthodontic brackets. Angle Orthod. 2017, 87, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Imai, T.; Yamagata, S.; Watari, F.; Kobayashi, M.; Nagayama, K.; Toyoizumi, H.; Uga, M.; Nakamura, S. Temperature-dependence of the mechanical properties of FRP orthodontic wire. Dent. Mater. J. 1999, 18, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Zufall, S.W.; Kusy, R.P. Sliding mechanics of coated composite wires and the development of an engineering model for binding. Angle Orthodont. 2000, 70, 34–47. [Google Scholar] [PubMed]
- Burstone, C.J.; Liebler, S.A.H.; Goldberg, A.J. Polyphenylene polymers as esthetic orthodontic archwires. Am. J. Orthod. Dent. Orthop. 2011, 139, e391–e398. [Google Scholar] [CrossRef] [PubMed]
- Tanimoto, Y.; Inami, T.; Yamaguchi, M.; Nishiyama, N.; Kasai, K. Preparation, mechanical, and in vitro properties of glass fiber-reinforced polycarbonate composites for orthodontic application. J. Biomed. Mater. Res. B 2015, 103, 743–750. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, D.L.; Mattos, C.T.; Simão, R.A.; De Oliveira Ruellas, A.C. Coating stability and surface characteristics of esthetic orthodontic coated archwires. Angle Orthod. 2013, 83, 994–1001. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, D.L.; Mattos, C.T.; Sant’Anna, E.F.; Ruellas, A.C.; Elias, C.N. Cross-section dimensions and mechanical properties of esthetic orthodontic coated archwires. Am. J. Orthod. Dent. Orthop. 2013, 143, S85–S91. [Google Scholar] [CrossRef] [PubMed]
- Bradley, T.G.; Berzins, D.W.; Valeri, N.; Pruszynski, J.; Eliades, T.; Katsaros, C. An investigation into the mechanical and aesthetic properties of new generation coated nickel-titanium wires in the as-received state and after clinical use. Eur. J. Orthod. 2014, 36, 290–296. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.H.; Park, H.S.; Kim, Y.K.; Kim, K.H.; Kwon, T.Y. Comparative short-term in vitro analysis of mutans streptococci adhesion on esthetic, nickel-titanium, and stainless-steel arch wires. Angle Orthod. 2014, 84, 680–686. [Google Scholar] [CrossRef] [PubMed]
- Rudge, P.; Sherriff, M.; Bister, D. A comparison of roughness parameters and friction coefficients of aesthetic archwires. Eur. J. Orthod. 2015, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Muguruma, T.; Iijima, M.; Yuasa, T.; Kawaguchi, K.; Mizoguchi, I. Characterization of the coatings covering esthetic orthodontic archwires and their influence on the bending and frictional properties. Angle Orthod. 2017, 87, 610–617. [Google Scholar] [CrossRef] [PubMed]
- Newman, G.V. Epoxy adhesives for orthodontic attachments: progress report. Am. J. Orthod. Dent. Orthop. 1965, 51, 901–912. [Google Scholar] [CrossRef]
- Eliades, T. Orthodontic materials research and applications: Part 1. Current status and projected future developments in bonding and adhesives. Am. J. Orthod. Dent. Orthop. 2006, 130, 445–451. [Google Scholar] [CrossRef] [PubMed]
- Iijima, M.; Muguruma, T.; Brantley, W.A.; Ito, S.; Yuasa, T.; Saito, T.; Mizoguchi, I. Effect of bracket bonding on nanomechanical properties of enamel. Am. J. Orthod. Dent. Orthop. 2010, 138, 735–740. [Google Scholar] [CrossRef] [PubMed]
- Feathestone, J.D. Remineralization, the natural caries repair process—The need for new approaches. Adv. Dent. Res. 2009, 21, 4–7. [Google Scholar] [CrossRef] [PubMed]
- Tschoppe, P.; Zandim, D.L.; Martus, P.; Kielbassa, A.M. Enamel and dentin remineralization by nano-hydroxyapatite toothpaste. J. Dent. 2011, 39, 430–437. [Google Scholar] [CrossRef] [PubMed]
- Hench, L.L.; Hench, L.L.; Splinter, R.J.; Allen, W.C.; Greenlee, T.K. Bonding mechanisms at the interface of ceramic prosthetic materials. J. Biomed. Mater. Res. 1971, 5, 117–141. [Google Scholar] [CrossRef]
- Kokubo, T. Apatite formation on surface of ceramics, metals and polymers in body environment. Acta Mater. 1998, 46, 2519–2527. [Google Scholar] [CrossRef]
- Hoppe, A.; Güldal, N.S.; Boccaccini, A.R. A review of the biological response to ionic dissolution products from bioactive glasses and glass-ceramics. Biomaterials 2011, 32, 2757–2774. [Google Scholar] [CrossRef] [PubMed]
- Fredholm, Y.C.; Karpukhina, N.; Brauer, D.S.; Jones, J.R.; Law, R.V.; Hill, R.G. Influence of strontium for calcium substitution in bioactive glasses on degradation, ion release and apatite formation. J. R. Soc. Interface 2012, 9, 880–889. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Rawlinson, S.C.; Hill, R.G.; Fortune, F. Fluoride incorporation in high phosphate containing bioactive glasses and in vitro osteogenic, angiogenic and antibacterial effects. Dent. Mater. 2016, 32, 412–422. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, J.S.; Gentile, P.; Pires, R.A.; Reis, R.L.; Hatton, P.V. Multifunctional bioactive glass and glass-ceramic biomaterials with antibacterial properties for repair and regeneration of bone tissue. Acta Biomater. 2017, 59, 2–11. [Google Scholar] [CrossRef] [PubMed]
- Besra, L.; Liu, M. A review on fundamentals and applications of electrophoretic deposition (EPD). Prog. Mater. Sci. 2007, 52, 1–61. [Google Scholar] [CrossRef]
- Chen, Q.; Garcia, R.P.; Munoz, J.; Pérez de Larraya, U.; Garmendia, N.; Yao, Q.; Boccaccini, A.R. Cellulose nanocrystals—Bioactive glass hybrid coating as bone substitutes by electrophoretic co-deposition: In situ control of mineralization of bioactive glass and enhancement of osteoblastic performance. ACS Appl. Mater. Interfaces 2015, 7, 24715–24725. [Google Scholar] [CrossRef] [PubMed]
- Commission International de L’Eclairage. Colormetry—Technical Report CIE Pub. No. 15, 3rd ed.; Bureau Central de la CIE: Vienna, Austria, 2004. [Google Scholar]
- ISO 14577-1 Metallic Materials—Instrumented Indentation Test for Hardness and Materials Parameters—Part 1: Test Method; International Organization for Standardization: Geneva, Switzerland, 2002.
- Oliver, W.C.; Pharr, G.M. An improved technique for determining hardness and elastic modulus using load and displacement sensing indentation experiments. J. Mater. Res. 1992, 7, 1564–1583. [Google Scholar] [CrossRef]
- Rho, J.Y.; Pharr, G.M. Nanoindentation testing of bone. In Mechanical Testing of Bone and the Bone-Implant Interface; An, Y.H., Draughn, R.A., Eds.; CRC Press: Boca Raton, FL, USA, 1999; pp. 257–269. [Google Scholar]
- Iijima, M.; Muguruma, T.; Brantley, W.A.; Mizoguchi, I. Comparisons of nanoindentation, 3-point bending, and tension tests for orthodontic wires. Am. J. Orthod. Dent. Orthop. 2011, 140, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.K. Colour and translucency of tooth-colored orthodontic brackets. Eur. J. Orthod. 2008, 30, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Esan, T.A.; Olusile, A.O.; Akeredolu, P.A. Factors influencing tooth shade selection for completely edentulous patients. J. Contemp. Dent. Pract. 2006, 7, 80–87. [Google Scholar] [PubMed]
- Seuss, S.; Lehmann, M.; Boccaccini, A.R. Alternating current electrophoretic deposition of antibacterial bioactive glass-chitosan composite coatings. Int. J. Mol. Sci. 2014, 15, 12231–12242. [Google Scholar] [CrossRef] [PubMed]
- Kusy, R.P.; Tobin, E.J.; Whitley, J.Q.; Sioshansi, P. Frictional coefficients of ion-implanted alumina against ion-implanted beta-titanium in the low load, low velocity, single pass regime. Dent. Mater. 1992, 8, 167–172. [Google Scholar] [CrossRef]
- Burrow, S.J. Friction and resistance to sliding in orthodontics: A critical review. Am. J. Orthod. Dent. Orthop. 2009, 135, 442–447. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Featherstone, J.D. Prevention of enamel demineralization: An in-vitro study using light-cured filled sealant. Am. J. Orthod. Dent. Orthop. 2005, 128, 592–600. [Google Scholar] [CrossRef] [PubMed]
- Gorton, J.; Featherstone, J.D. In vivo inhibition of demineralization around orthodontic brackets. Am. J. Orthod. Dentofacial Orthop. 2003, 123, 10–14. [Google Scholar] [CrossRef] [PubMed]
- De Almeida, P.D.V.; Grégio, A.M.; Machado, M.A.; de Lima, A.A.; Azevedo, L.R. Saliva composition and functions: A comprehensive review. J. Contemp. Dent. Pract. 2008, 9, 72–80. [Google Scholar]




| Coordinate | Non-Coat | BG-Coat (DC 10 V) | BG-Coat (DC 15 V) | BG-Coat (AC 10 V) | BG-Coat (AC 15 V) | p Value | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| mean | S.D. | mean | S.D. | mean | S.D. | mean | S.D. | mean | S.D. | ||
| L* | 78.02 a | 0.14 | 54.88 a | 0.34 | 63.79 c | 0.14 | 61.83 d | 0.36 | 70.42 e | 0.71 | 0.000 |
| a* | −0.12 a | 0.03 | −0.50 b | 0.05 | −0.48 b | 0.10 | −0.48 b | 0.02 | −0.41 b | 0.11 | 0.000 |
| b* | 2.31 a | 0.10 | 7.89 b | 0.30 | 6.12 c | 0.65 | 8.12 b | 0.10 | 6.70 c | 0.26 | 0.000 |
| Mechanical Properties | Non-Coat | BG-Coat (DC 10 V) | BG-Coat (DC 15 V) | BG-Coat (AC 10 V) | BG-Coat (AC 15 V) | p Value | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| mean | S.D. | mean | S.D. | mean | S.D. | mean | S.D. | mean | S.D. | ||
| Hardness (GPa) | 6.11 a | 0.22 | 0.49 a | 0.10 | 1.99 c | 0.52 | 0.85 b | 0.48 | 1.98 c | 0.73 | 0.000 |
| Elastic modulus (GPa) | 192.46 a | 4.96 | 70.47 b | 23.22 | 109.12 c | 23.39 | 84.32 bc | 15.28 | 128.5 d | 27.65 | 0.000 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kawaguchi, K.; Iijima, M.; Endo, K.; Mizoguchi, I. Electrophoretic Deposition as a New Bioactive Glass Coating Process for Orthodontic Stainless Steel. Coatings 2017, 7, 199. https://doi.org/10.3390/coatings7110199
Kawaguchi K, Iijima M, Endo K, Mizoguchi I. Electrophoretic Deposition as a New Bioactive Glass Coating Process for Orthodontic Stainless Steel. Coatings. 2017; 7(11):199. https://doi.org/10.3390/coatings7110199
Chicago/Turabian StyleKawaguchi, Kyotaro, Masahiro Iijima, Kazuhiko Endo, and Itaru Mizoguchi. 2017. "Electrophoretic Deposition as a New Bioactive Glass Coating Process for Orthodontic Stainless Steel" Coatings 7, no. 11: 199. https://doi.org/10.3390/coatings7110199
APA StyleKawaguchi, K., Iijima, M., Endo, K., & Mizoguchi, I. (2017). Electrophoretic Deposition as a New Bioactive Glass Coating Process for Orthodontic Stainless Steel. Coatings, 7(11), 199. https://doi.org/10.3390/coatings7110199