Abstract
Under elevated loading conditions, the aggregation of fillers emerges as a pivotal factor driving the degradation of separation performance in mixed matrix membranes. The two-dimensional (2D) modification of fillers, aimed at enhancing interfacial contact with polymers, has been recognized as an effective strategy to improve interphase compatibility and increase filler loading capacity. However, it is worth noting that the BET surface area of 2D fillers is typically relatively low. In this study, a two-step approach was developed. First, a “diffusion-mediated” process was combined with a solvent optimization strategy based on first-principles (DFT) calculations, achieving a 20-fold suppression in ZIF-67 nucleation-crystallization rate. This enabled the successful synthesis of a 2D amorphous nanoflower structure. Subsequently, the processing parameters were fine-tuned to enhance the specific surface area of ZIF-67 to 403 m2/g while preserving its 2D structural integrity. Ultimately, the as-prepared 2D ZIF-67 was incorporated into a hydrogenated styrene-butadiene block copolymer (SEBS) matrix to fabricate a mixed matrix membrane. Remarkably, at a filler loading of 20 wt%, the CH4 permeability coefficient increased significantly from 11.7 barrer to 35.3 barrer, while the CH4/N2 selectivity was maintained at 3.21, indicating minimal interfacial defects and demonstrating the feasibility and effectiveness of the proposed methodology.
1. Introduction
Methane (CH4) stands as the second most copious greenhouse gas, possessing a Global Warming Potential (GWP) that is 84 times higher than that of CO2 within the 20-year time frame. Among the principal sources of methane emissions, the oil and gas industry discharged 72 million tonnes of CH4 in 2020, as per the database of the International Energy Agency [1]. In the process of oil and gas production, CH4 is frequently emitted in conjunction with N2. The separation of CH4 from N2 poses a significant challenge due to their comparable physical characteristics and minimal disparity in molecular diameters [2,3].
Membrane-based separation has emerged as an innovative and environmentally friendly technology, characterized by low energy consumption, high efficiency, and operational simplicity. In recent years, it has gained significant attention as an advanced separation strategy, appealing to both academic researchers and industrial practitioners alike [4,5,6,7]. However, during the commercialization process, the inherent denseness of polymer materials typically limits the gas processing capacity of separation membranes. The incorporation of metal–organic frameworks (MOFs) into polymer matrices to fabricate mixed matrix membranes (MMMs) has been identified as an effective approach to address this limitation [8]. Nevertheless, conventional MOF synthesis methods predominantly produce isotropic crystals with micrometer-scale dimensions. These crystals are prone to spontaneous aggregation within the polymer matrix, leading to interfacial defects that severely compromise the separation performance of the membranes.
To enhance interfacial compatibility, Gascon et al. [9] synthesized a 2D nanosheet CuBDC MOF (ns-CuBDC) with an aspect ratio exceeding 20, which was applied to CO2/CH4 separation. With the ns-CuBDC loading increased to 8.2 wt%, the CO2/CH4 selectivity improved from 59.8 for the pristine PI membrane to 78.7. However, a significant decline in the permeability of both CO2 and CH4 was observed, primarily attributed to the extremely low BET surface area of ns-CuBDC, which was only 53 m2/g. Zeolitic imidazolate frameworks (ZIFs), particularly exemplified by ZIF-67, have emerged as promising MOFs owing to their energy-efficient synthesis protocols, demonstrating exceptional separation capabilities for challenging gas pairs including He/CH4 [10], H2/CH4 [11], CO2/CH4, and CO2/N2 [12]. Kim et al. [13] synthesized 2D ZIF-L and incorporated it into PI matrices to fabricate MMMs for H2/CH4 separation. However, due to the relatively low surface area of ZIF-L (161 m2/g), even at a loading level of 20 wt%, the H2 permeability coefficient exhibited only a marginal increase from 219.7 barrer to 260.4 barrer. In recent years, extensive research has been devoted to 2D MOF-based MMMs to address these limitations and explore their potential in various separation applications [14,15,16,17]. Nonetheless, achieving filler architectures with adequate thinness and porosity remains a substantial and unresolved challenge.
Liu et al. [18] employed direct time-resolved in situ transmission electron microscopy (TEM) to investigate the nucleation and initial growth stages of ZIF-8, revealing the presence of a lamelliform amorphous cluster structure prior to crystallization. However, the amorphous clusters transformed into granular structures within 45 s, making it challenging to capture this transient state using conventional synthesis techniques. To address this challenge, the present study first employs solvent selection based on first-principles calculations, combined with a “diffusion-mediated” strategy, to significantly suppress the crystallization process of ZIF-67. Subsequently, process parameters are optimized to enhance the specific surface area of 2D ZIF-67 to 403 m2/g. Finally, SEBS MMMs with a 2D ZIF-67 loading of 20 wt% are fabricated, resulting in a remarkable increase in the CH4 permeability coefficient from 11.7 barrer to 35.3 barrer, while maintaining the CH4/N2 selectivity at 3.21.
2. Materials and Methods
2.1. Computational Details
The crystal structure of ZIF-67 was obtained from the Cambridge Crystallographic Data Centre (CCDC). DFT simulations were executed using Gaussian 16W [19]. For the geometry optimizations, all systems studied were optimized at the PBE0-D3(BJ) [20]/6-311G(d) [21] level, and the IEFPCM model [22] was employed to take into account the interactions between the solvent and the solute. All single-point energies were calculated using the DSD-PBEP86-D3(BJ) function [23] and def2-TZVPP basis set [24], and the SMD solvent model [25] was employed. Multiwfn3.7 [26] was used to analyze the electrostatic potential (ESP) and averaged local ionization energy (ALIE) information. All figures were plotted using VMD1.9.3 [27].
The entire process of a molecular reaction can be systematically categorized into two distinct stages. In the initial stage, the reactants gradually approach one another from a considerable distance. During this period, the electronic distribution within the molecules undergoes negligible change, and the long-range electrostatic interaction plays a crucial and decisive role. Subsequently, when the van der Waals surfaces of two molecules are on the verge of coming into contact, they continue to draw even closer. The chemical reaction is ultimately accomplished through the complex process of electronic structure rearrangement, with bond formation and bond cleavage being prominent features.
To accurately assess the two reaction processes of ZIF-67 molecules in various solvent environments, ESP and ALIE analysis are meticulously carried out, respectively. Here, ESP precisely describes the interaction energy between a unit positive charge located at a specific point r and the existing system. Regions with a relatively more positive (or more negative) electrostatic potential are generally regarded as more likely to attract the attack of nucleophilic (or electrophilic) reagents, thus effectively initiating a reaction. ALIE, on the other hand, refers to the average energy required for a molecule to lose an electron at a particular position. Notably, the lower this value is, the weaker the binding of electrons at this location becomes, and consequently, the more likely these electrons are to participate in electrophilic reactions. Both ESP and ALIE can be accurately expressed by Equations (1) and (2), respectively.
where ZA is the charge on nucleus A, located at RA, and ρ(r) is the electron density.
where ρ represents the total density, ρi represents the electron density corresponding to the i-th occupied molecular orbital and represents the energy of the i-th molecular orbital, reflects the ionization energy of electrons at a local position.
2.2. Materials
The chemical and polymer were utilized in the study, including Cobalt(II) nitrate hexahydrate [Co(NO3)2·6H2O, 99.99% purity], 2-Methylimidazole [2-MeIm, 98% purity], N,N-Dimethylformamide [DMF, 99.5% purity], SEBS [Kraton G1652, styrene/rubber: 30/70], Tetrabutylammonium bromide [TBAB, 99% purity], Toluene [99.5% purity], Ethanol [99.7% purity].
2.3. Synthesis of 2D Nanoflower ZIF-67 (ZIF-67nf)
The preparation procedure for the 2D nanoflower-like ZIF-67 was adapted from the synthesis of CuBDC nanosheets by Gascon et al. [6]. Initially, m1 g of Co(NO3)2·6H2O was completely dissolved in 15 mL of solvent to prepare Solution A. Subsequently, 1 g of 2-MeIm was thoroughly dissolved in 15 mL of solvent to obtain Solution B. In parallel, m2 g of TBAB was fully dissolved in V1 mL of solvent to prepare Solution C.
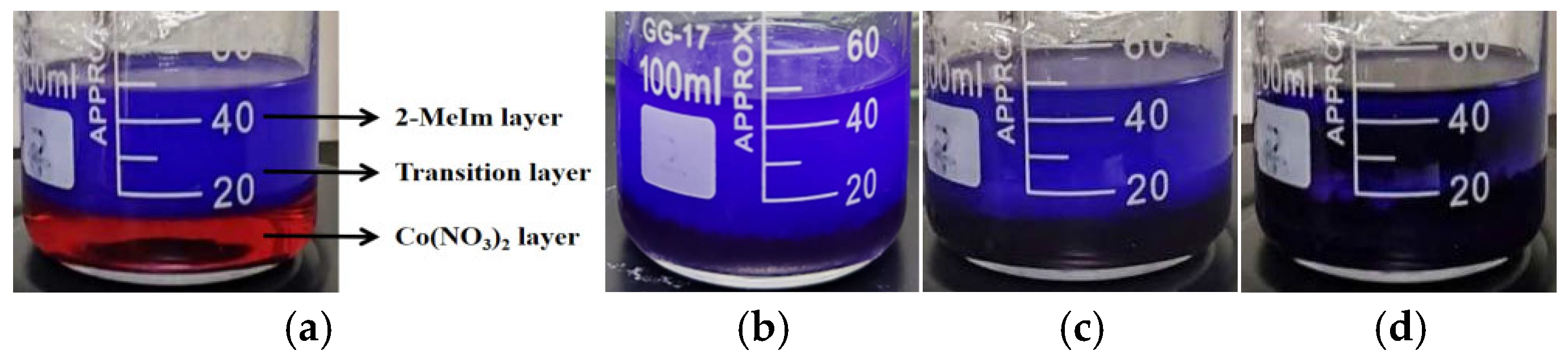
Following this, Solution A was transferred into a beaker. Solution C was then slowly added dropwise to Solution A, followed by the gradual dropwise addition of Solution B onto Solution C, thereby establishing a vertically layered solution system. The system was allowed to stand at room temperature (RT) for t1 hours, during which Co(NO3)2·6H2O and 2-MeIm slowly diffused into Solution B under the influence of a concentration gradient, resulting in the formation of ZIF-67. The resulting purple products were collected via centrifugation and washed with ethanol. The diffusion-mediated layered solution systems at different reaction durations are illustrated in Figure 1.

Figure 1.
Diffusion-mediated layered solution system: (a) 0 min; (b) 15 min; (c) 30 min; (d) 60 min.
2.4. Preparation of MMMs
ZIF-67 was ultrasonically dispersed in 5 g of toluene to form Solution D. Meanwhile, SEBS was completely dissolved in 6.33 g of toluene under vigorous stirring to generate Solution E. Subsequently, Solutions D and E were combined and stirred continuously for 0.5 h, resulting in Solution F. After ultrasonic dispersion, Solution F was placed in a vacuum oven to eliminate air bubbles. The MMMs were then cast onto a glass plate and covered for 48 h to minimize solvent evaporation. Finally, the MMMs were dried under vacuum to remove residual solvent. In this process, the total mass of ZIF-67 and SEBS was fixed at 2 g, with ZIF-67 loadings set at 5 wt%, 10 wt%, 15 wt%, and 20 wt%, respectively. For comparative purposes, MMMs incorporating a three-dimensional (3D) filler morphology with identical loading levels were also prepared.
2.5. Characterization
A Panalytical X’Pert PRO MPD Diffractometer (Cu Kα, 40 kV, 40 mA) was used to obtain X-ray diffraction (XRD) patterns, ranging from 5° to 45° with a step size of 0.016° [28]. The ZIF-67 nanoflower morphologies were examined using a Hitachi S4800 scanning electron microscope (SEM). To investigate the surface area and pore size distribution, Brunauer–Emmett–Teller (BET) and Barrett-Joyner-Halenda (BJH) were tested on Micromeritics ASAP 2460. In order to evaluate the separation performance of the prepared MMMs in our work, the gas separation capabilities were assessed using pure CH4 and N2 permeation experiments. Measurements were conducted at 25 °C under an applied pressure difference of 100 kPa, employing a differential pressure gas permeameter (Labthink G2/110) with a standardized membrane area of 1 cm2. Real-time analysis of permeate flux was performed via online gas chromatography, with data acquisition initiated upon establishment of steady-state permeation conditions.
3. Results and Discussion
3.1. Aggregation and Reactivity
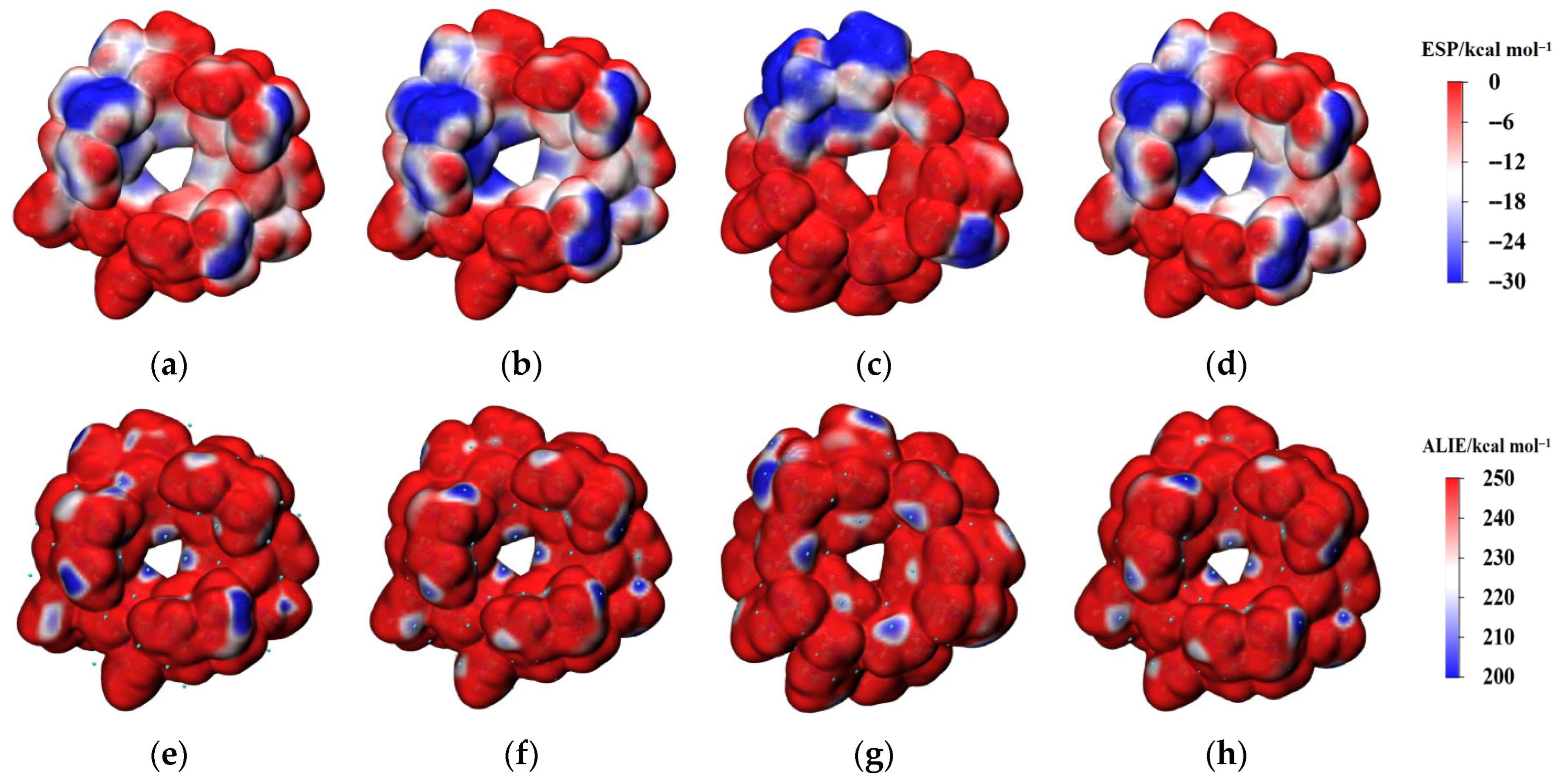
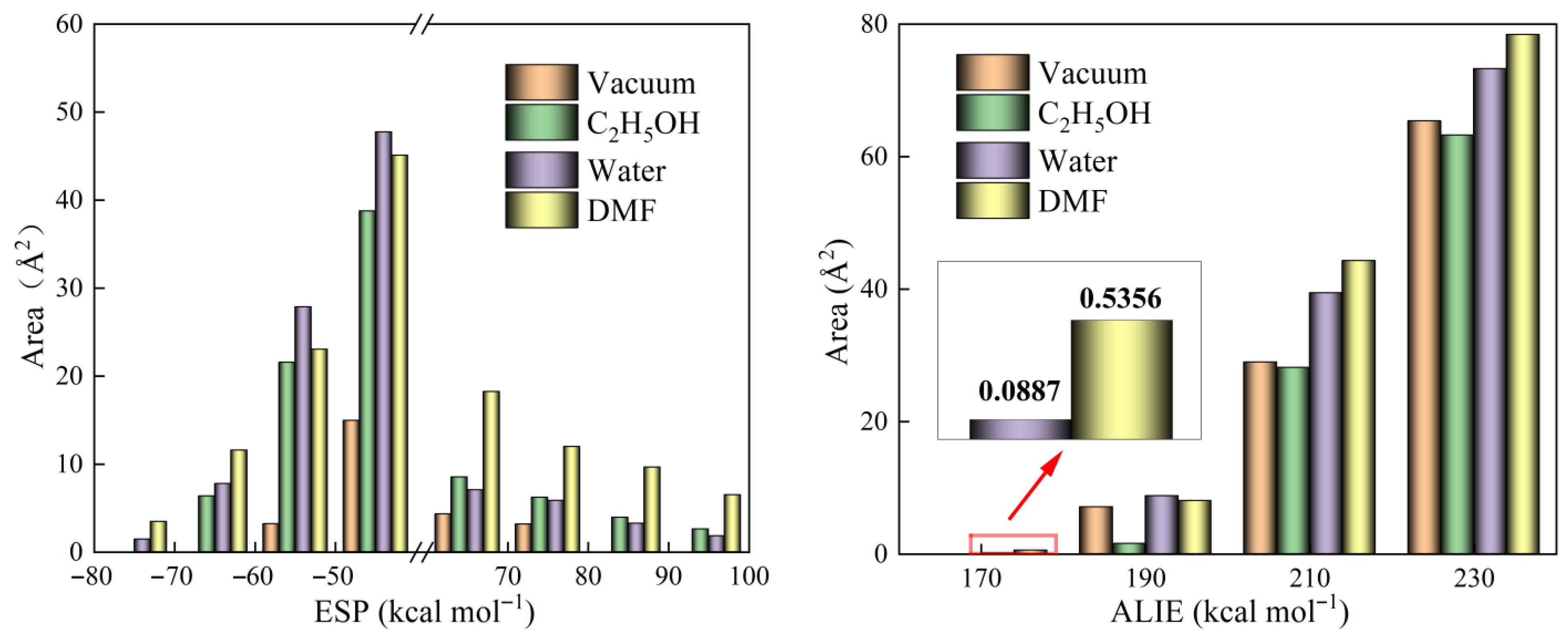
ESP and ALIE analyses were conducted to evaluate the aggregation and reactivity characteristics of the ZIF-67 unit cell. Figure 2 depicts the distributions of ESP and ALIE on the surface of ZIF-67 molecules under vacuum and in various solvent environments. Figure 3 provides a quantitative analysis of the surface areas of ZIF-67 molecules corresponding to different ESP and ALIE distribution intervals. As shown in Figure 3, in the ethanol environment, both the negative ESP and the positive ALIE occupy the smallest molecular surface areas, indicating that ZIF-67 is least prone to aggregation and reaction in this environment. Therefore, ethanol is an optimal reaction solvent for ZIF-67, as it minimizes the crystallization rate and facilitates the formation of 2D aggregate structures.
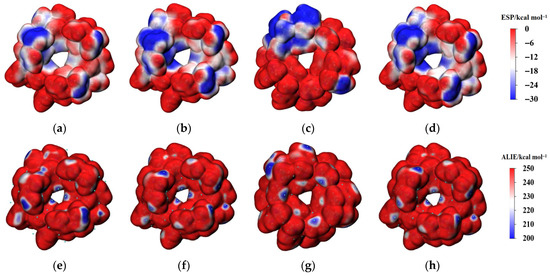
Figure 2.
Distribution of ESP (isovalue = 0.001 a.u.) and ALIE (isovalue = 0.0005 a.u.) on the surface of ZIF-67 molecules under different surroundings. (a,e): vacuum; (b,f): ethanol; (c,g): DMF; (d,h): water.
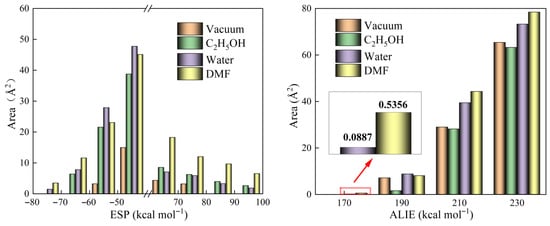
Figure 3.
The areas occupied by different ESP and ALIE intervals on the surface of ZIF-67 molecules.
3.2. SEM, XRD and BET
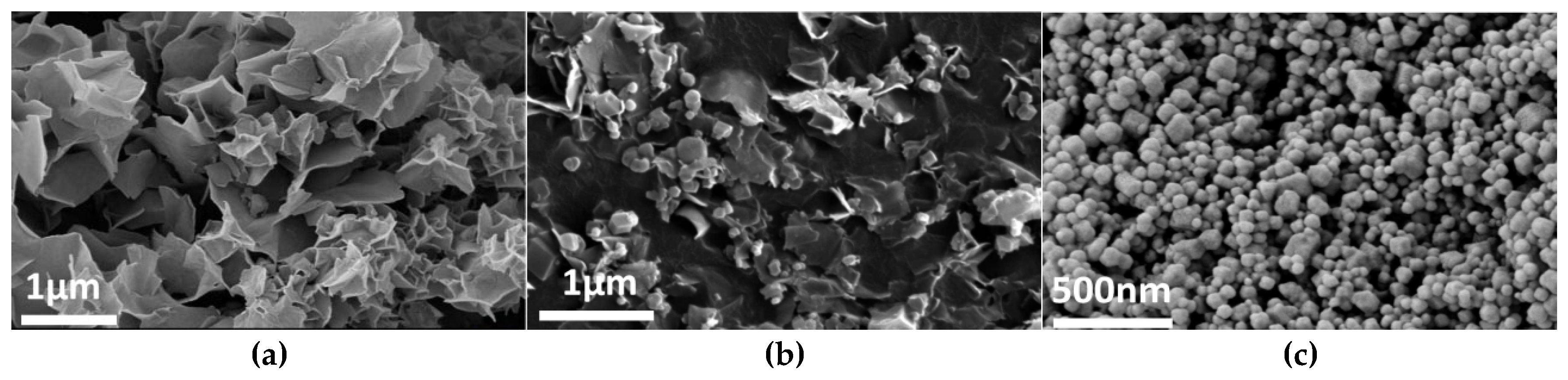
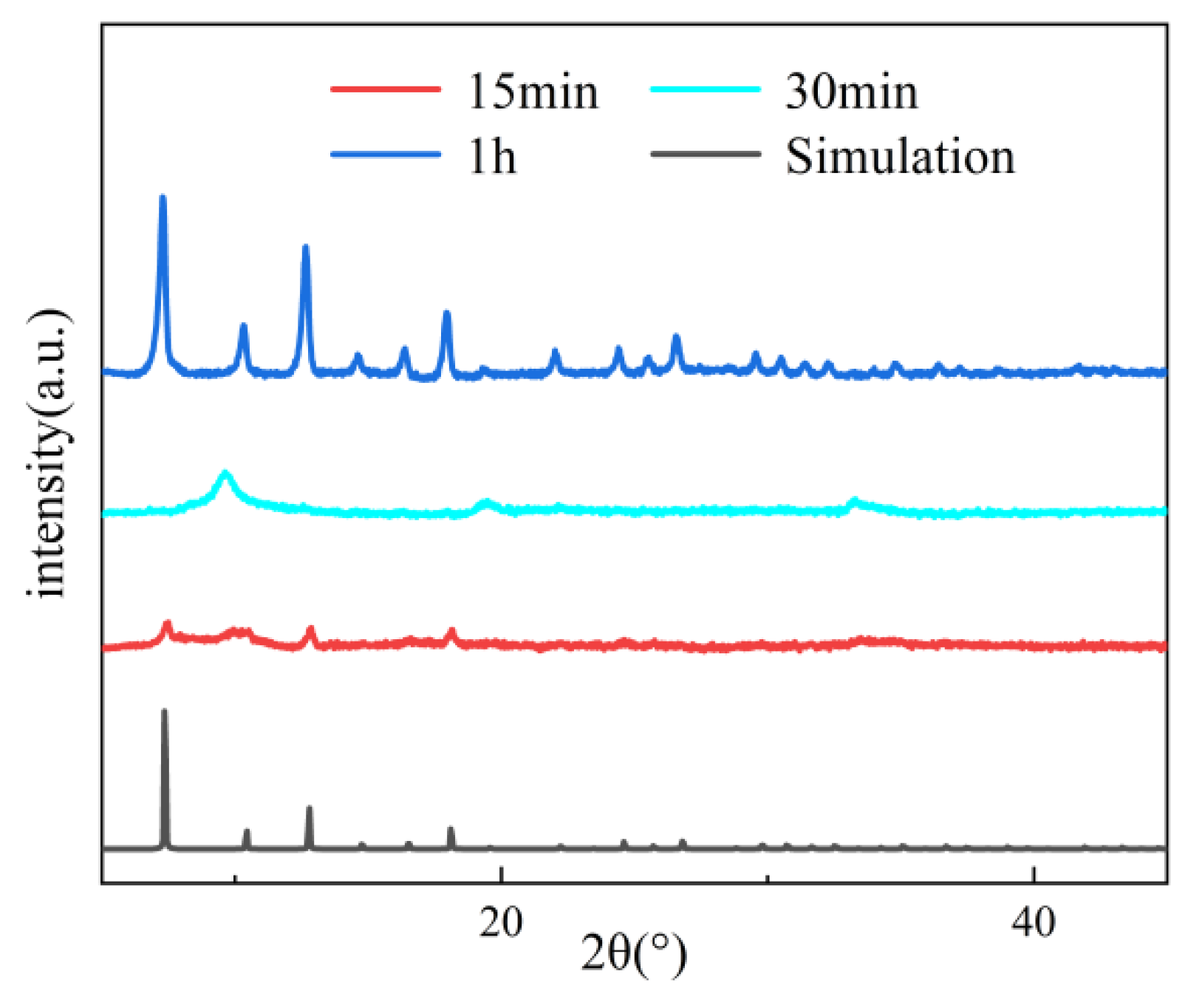
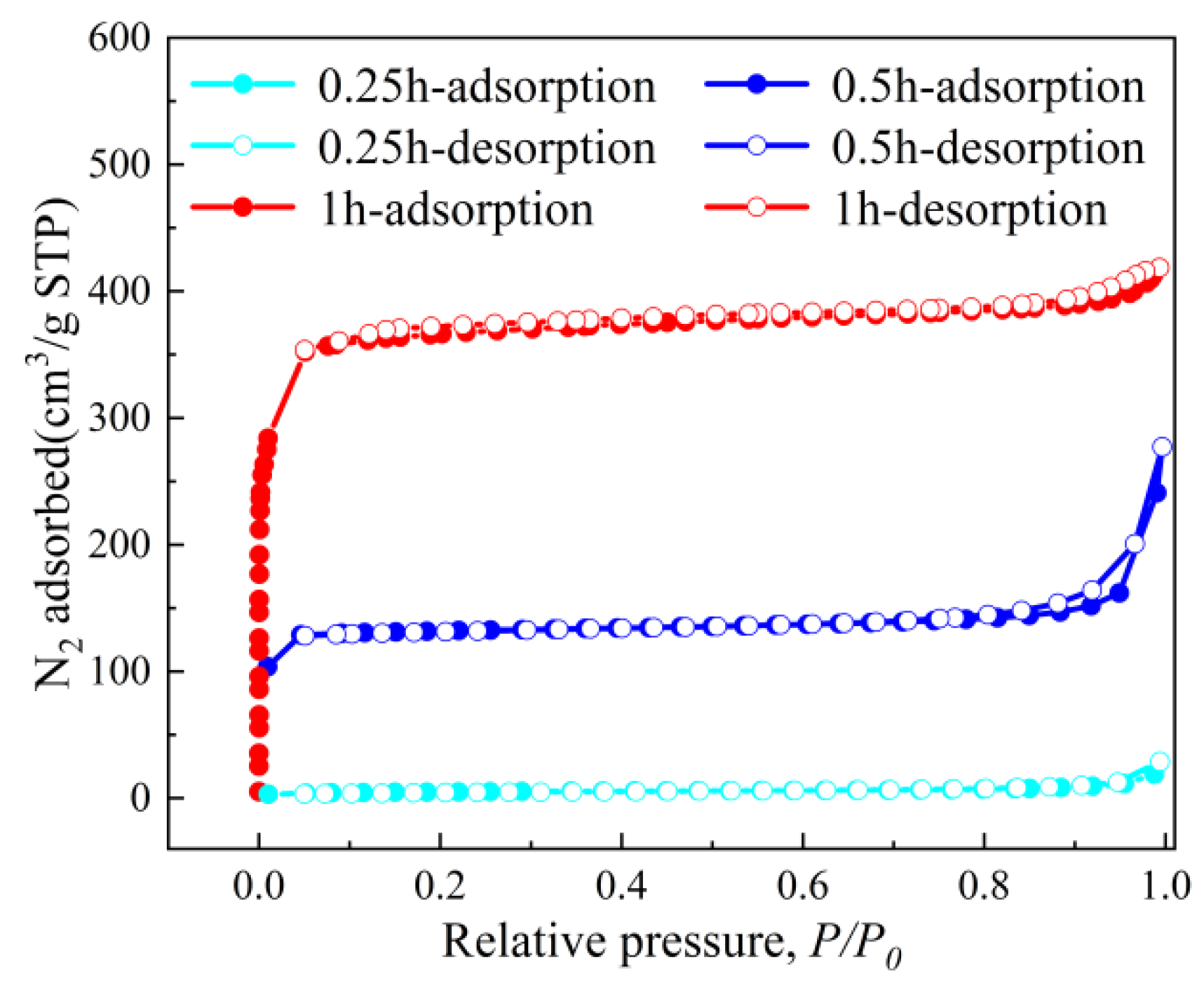
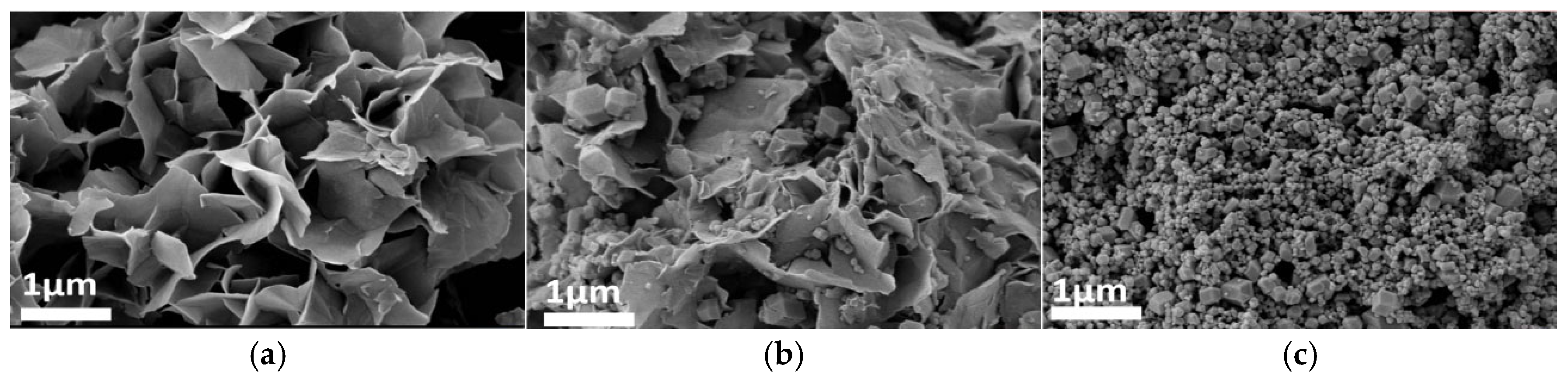
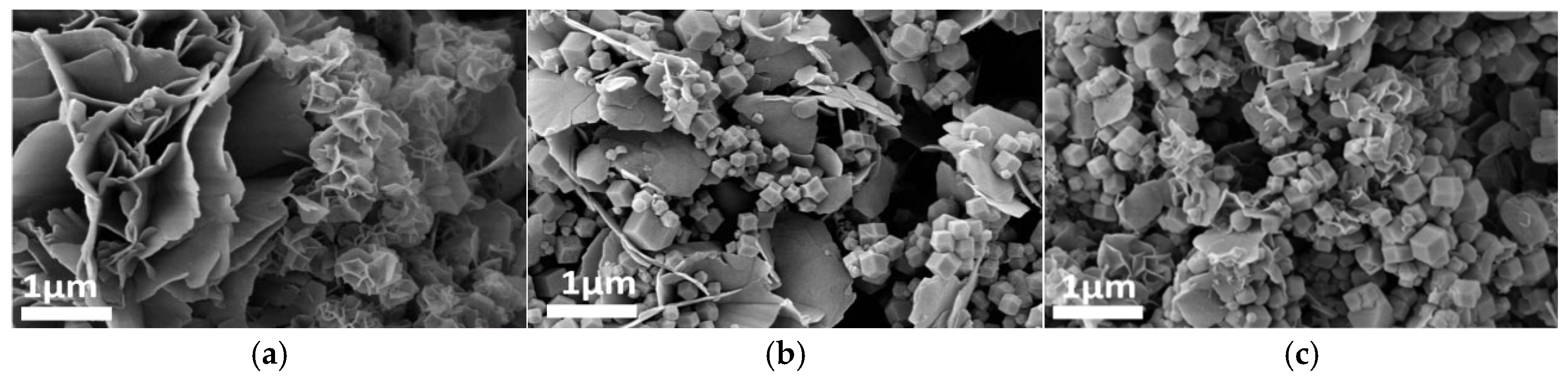
Figure 4, Figure 5 and Figure 6 systematically illustrate the morphological evolution, crystallinity, and gas adsorption characteristics of ZIF-67 synthesized under varying reaction durations. As depicted in Figure 4a, ZIF-67 synthesized with a reaction duration of 0.25 h (denoted as ZIF-670.25h) exhibits a well-defined 2D nanoflower morphology with ultrathin petal-like structures. However, prolonging the reaction duration to 0.5 h and 1.0 h triggers a morphological transition to 3D granular aggregates (Figure 4b,c), consistent with the nucleation-crystallization mechanism proposed for ZIF-8 by Liu et al. [12]. This structural evolution is corroborated by XRD analysis (Figure 5), where ZIF-670.25h displays no discernible diffraction peaks, confirming its amorphous nature. In contrast, ZIF-671.0h exhibits sharp Bragg reflections matching the simulated ZIF-67 pattern, indicative of high crystallinity.

Figure 4.
SEM images: m1 = 0.89, m2 = 0, V1 = 15, (a) t1 = 0.25; (b) t1 = 0.5; (c) t1 = 1; Here, m1 denotes the mass of Co(NO3)2·6H2O, g; V1 represents the mass of the solvent employed for the transient layer, ml; m2 refers to the mass of TBAB, g; t1 denotes the duration of the reaction, h. The same definitions apply to the parameters presented in Figure 7, Figure 8, Figure 9 and Figure 10.
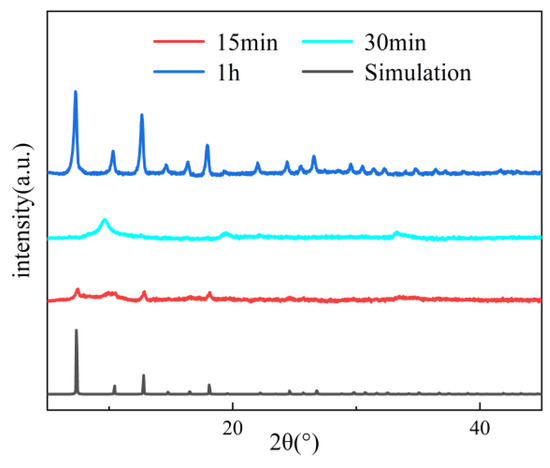
Figure 5.
Comparison of PXRD patterns of ZIF-67 with different reaction duration.
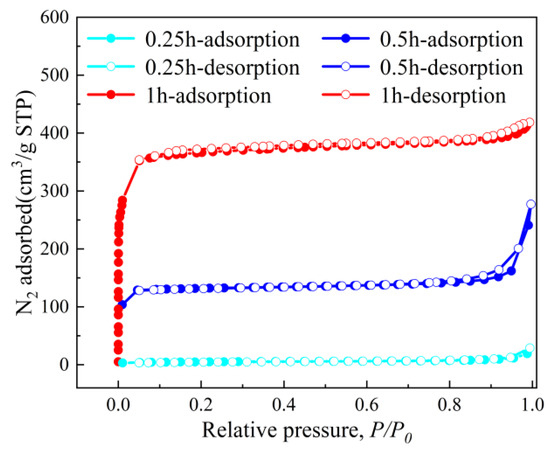
Figure 6.
N2 adsorption–desorption isotherms at 77 K.
The CO2 adsorption–desorption isotherms (Figure 6) further quantify the impact of structural evolution on porosity. ZIF-670.25h demonstrates negligible gas uptake due to its non-porous 2D architecture, yielding a BET surface area of only 18.2 m2/g. Conversely, ZIF-670.5h and ZIF-671.0h exhibit Type I isotherms characteristic of microporous materials, with BET surface areas increasing dramatically to 434.1 m2/g and 1011.5 m2/g, respectively. This enhancement correlates with the development of 3D granular structures, which introduce hierarchical porosity and accessible adsorption sites. Notably, the steep adsorption at low relative pressures (P/P0 < 0.1) for ZIF-671.0h underscores its dominant microporosity, while the hysteresis loop at higher pressures (0.4 < P/P0 < 0.9) suggests the coexistence of mesopores formed during particle aggregation.
Collectively, these findings highlight the critical role of reaction duration in governing ZIF-67’s structural and textural properties. The amorphous 2D nanoflower morphology (ZIF-670.25h) offers minimal porosity but serves as a precursor for subsequent crystallite growth. By contrast, extended reaction durations promote crystallization and 3D granular formation, significantly enhancing surface area and pore volume. This structural-property relationship underscores the necessity of precise kinetic control in tailoring ZIF-67 for specific applications, such as gas separation membranes, where balanced crystallinity and porosity are essential for optimizing performance.
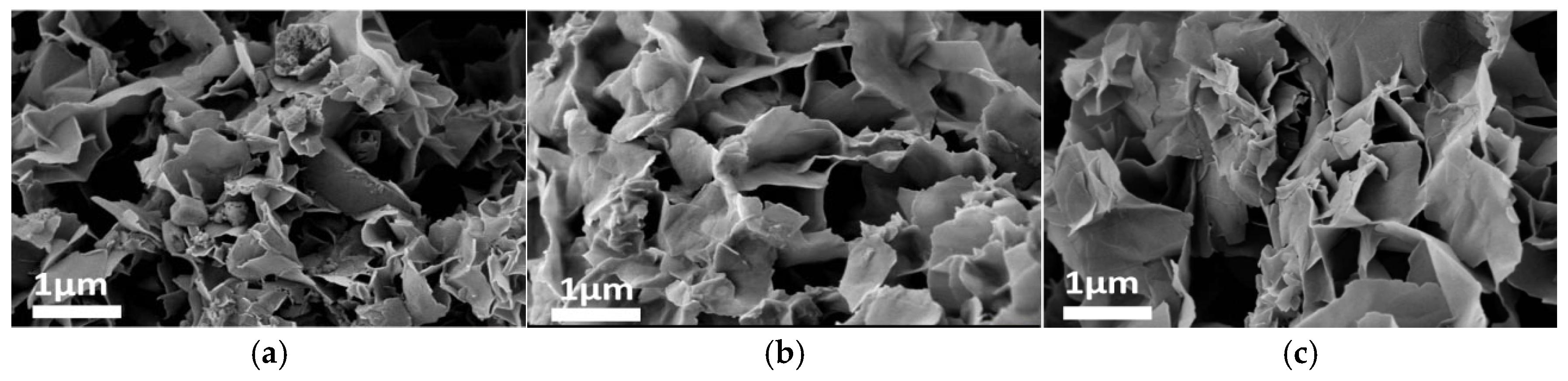
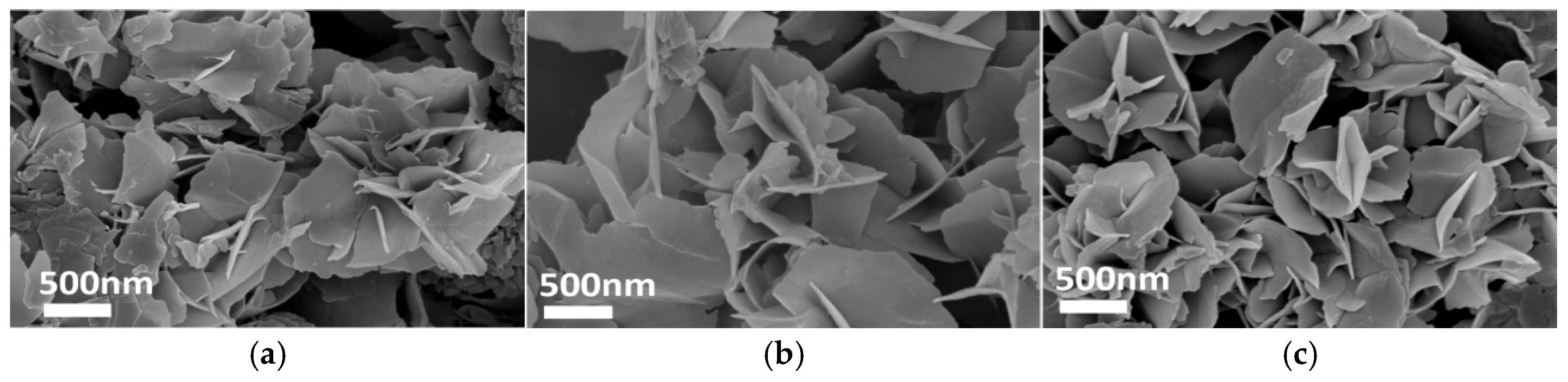
Figure 7, Figure 8, Figure 9 and Figure 10 present SEM images of ZIF-67 synthesized under varying parameters, including molar ratios of precursors (m1), transition layer volumes (V1), ethanol/water solvent ratios, and template agent (TBAB) loadings. As shown in Figure 7a, ZIF-67 synthesized with m1 = 0.66 exhibited a well-defined 2D nanoflower morphology. However, reducing the precursor ratio (m1 = 0.44 and 0.22) led to irregular granular structures (Figure 7b,c), highlighting the critical role of stoichiometric control in morphology development. Similarly, Figure 8, Figure 9 and Figure 10 demonstrate that optimizing the transition layer volume (V1 = 15), solvent composition (VC2H5OH:VH2O = 50:50), and TBAB loading (m2 = 0.5 g) significantly enhanced structural uniformity and porosity.

Figure 7.
SEM images: m2 = 0, V1 = 15, t1 = 0.25, (a) m1 = 0.66; (b) m1 = 0.44; (c) m1 = 0.22.

Figure 8.
SEM images: m1 = 0.66, m2 = 0, t1 = 0.25, (a) V1 = 0; (b) V1 = 5; (c) V1 = 10.

Figure 9.
SEM images: m1 = 0.66, m2 = 0, t1 = 0.25, V1 = 15, (a) V1(C2H5OH):V1(H2O) = 85:15; (b) V1(C2H5OH):V1(H2O) = 70:30; (c) V1(C2H5OH):V1(H2O) = 50:50.

Figure 10.
SEM images: m1 = 0.66, t1 = 0.25, V1 = 15, (a) m2 = 0.1; (b) m2 = 0.3; (c) m2 = 0.5.
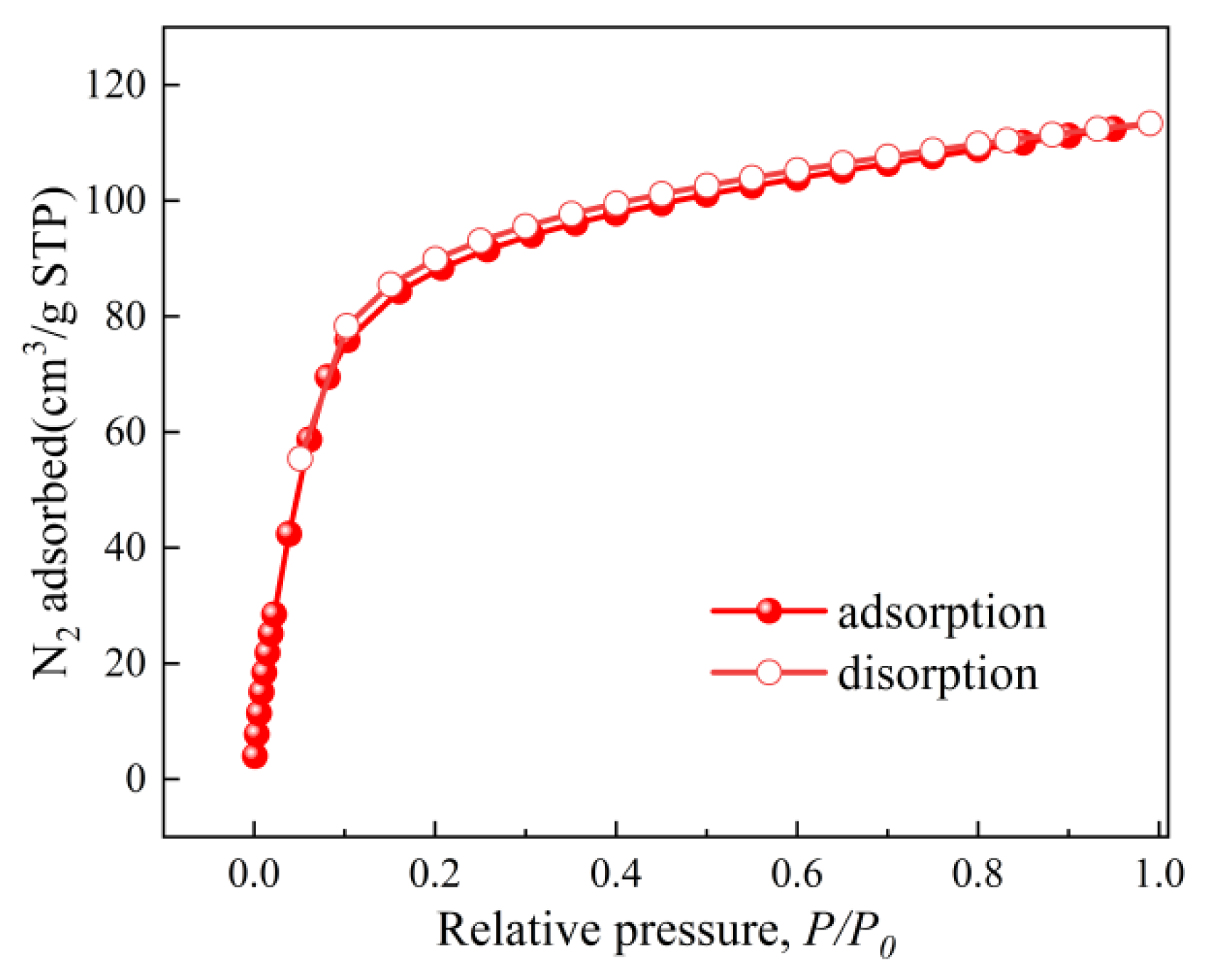
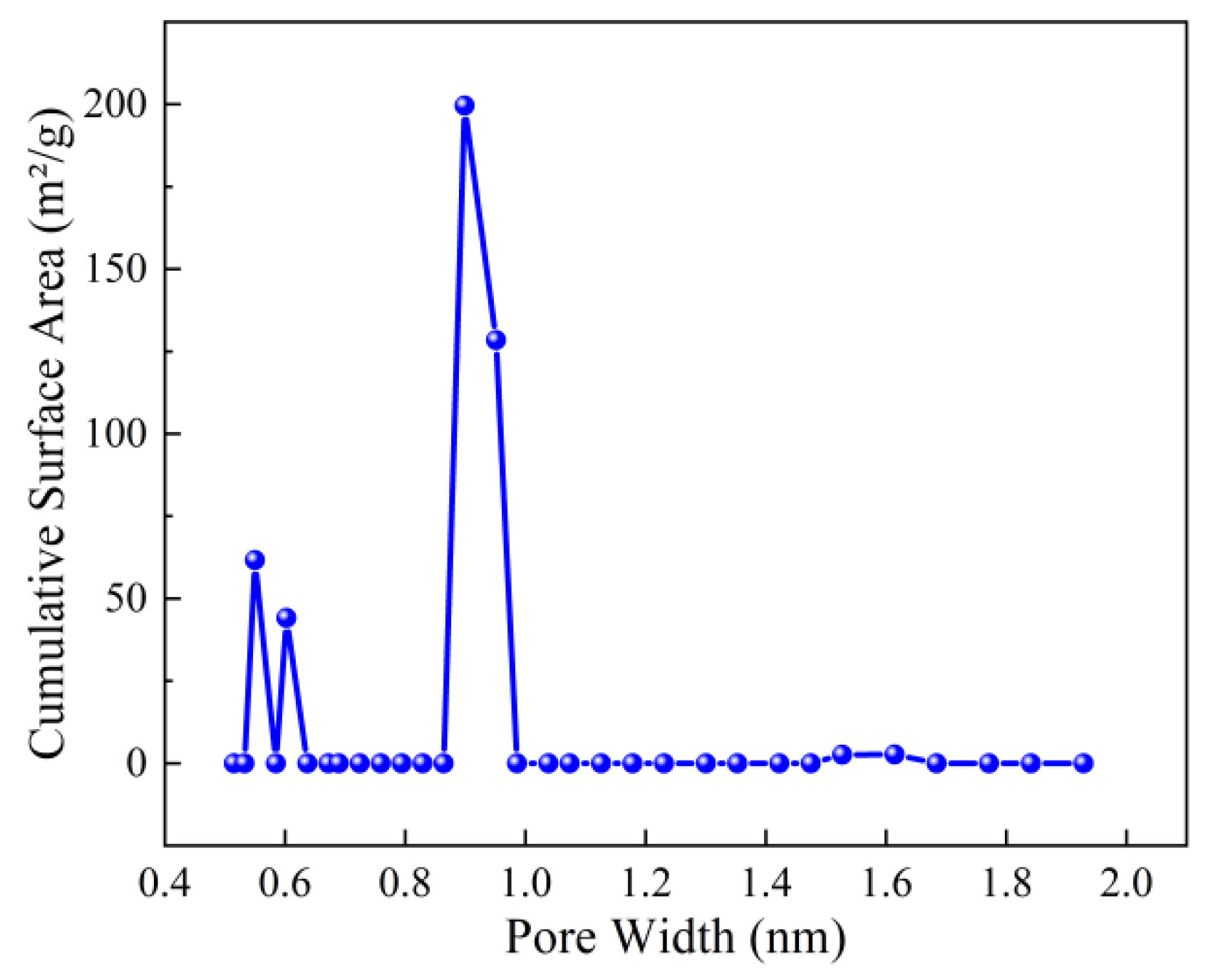
The BET surface area and pore structure of the optimized ZIF-67nf were evaluated through CO2 adsorption–desorption isotherms (Figure 11) and pore size distribution analysis (Figure 12). The isotherm of ZIF-67nf displays a Type IV hysteresis loop, indicative of mesoporous materials, with a steep adsorption slope at low relative pressures (P/P0 < 0.1), confirming the presence of micropores. The BET surface area of ZIF-67nf reached 403 m2/g, a substantial improvement compared to the non-porous 2D ZIF-670.25h (18.2 m2/g, Figure 6). The pore volume increased to 0.197 cm3/g, with a narrow pore size distribution centered at ~0.9 nm (Figure 12), suggesting a homogeneous mesoporous structure. These structural enhancements are attributed to the synergistic effects of solvent optimization, templating agents, and diffusion-mediated synthesis, which suppressed rapid crystallization and promoted controlled growth of the 2D nanoflower architecture.
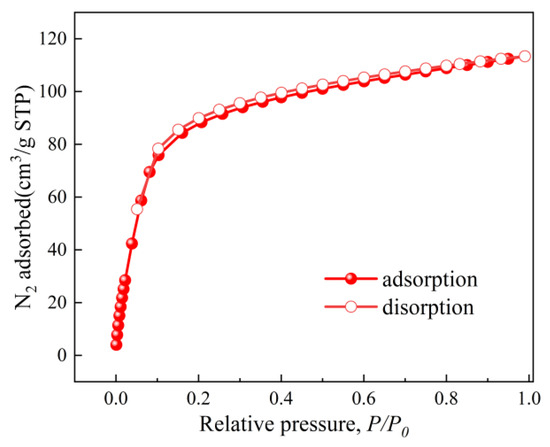
Figure 11.
N2 adsorption–desorption isotherms of ZIF-67ns at 77 K.
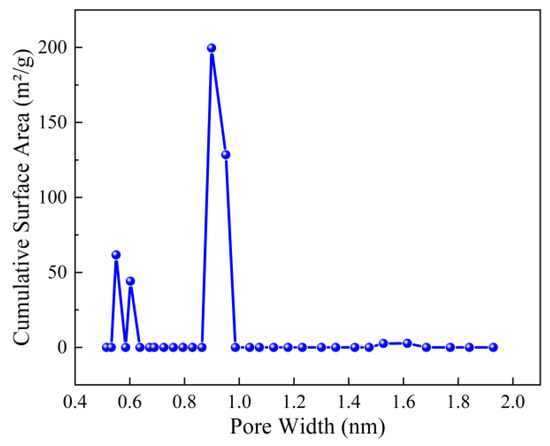
Figure 12.
Pore size distribution of ZIF-67nf.
3.3. MMMs Performance
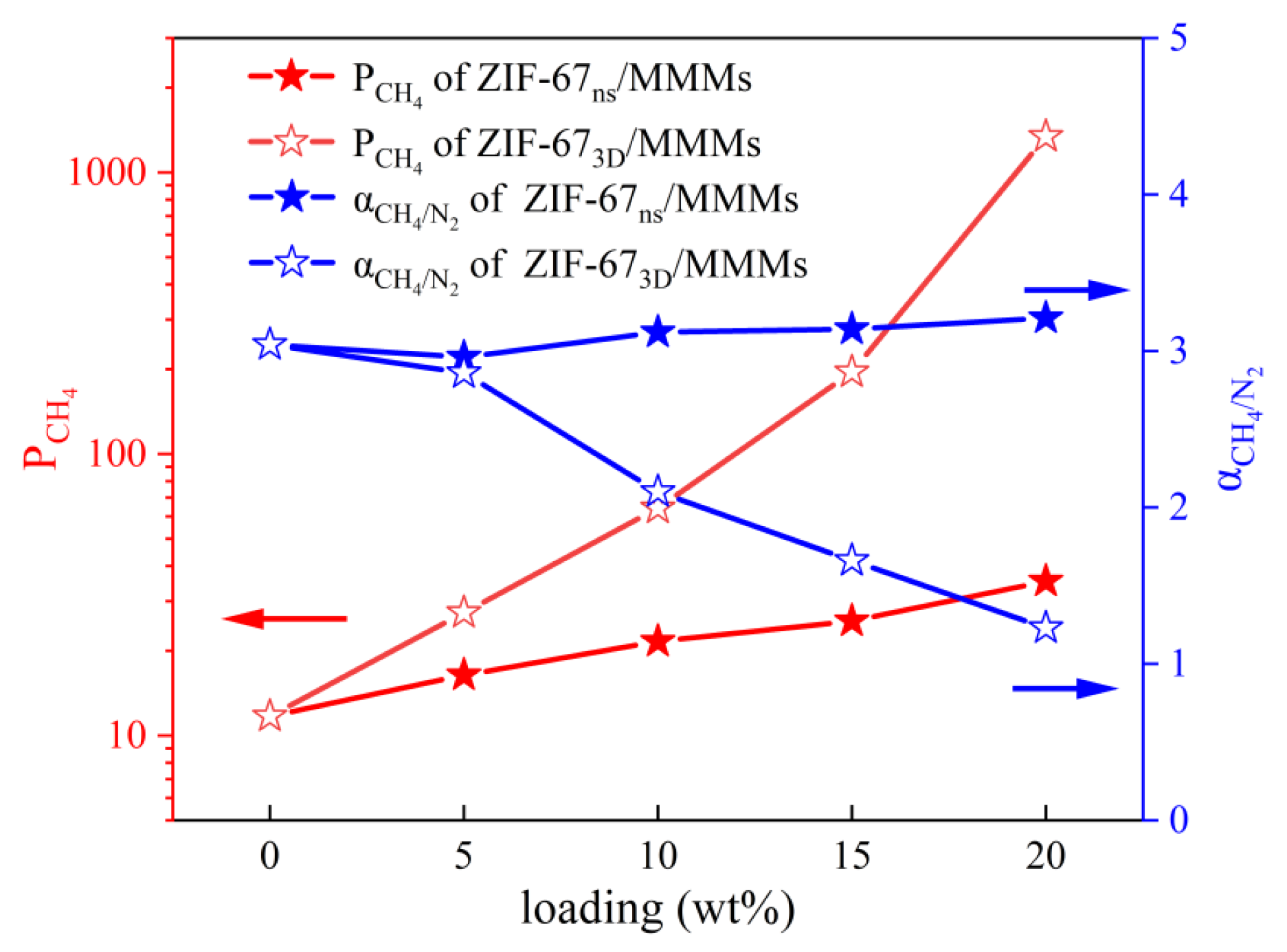
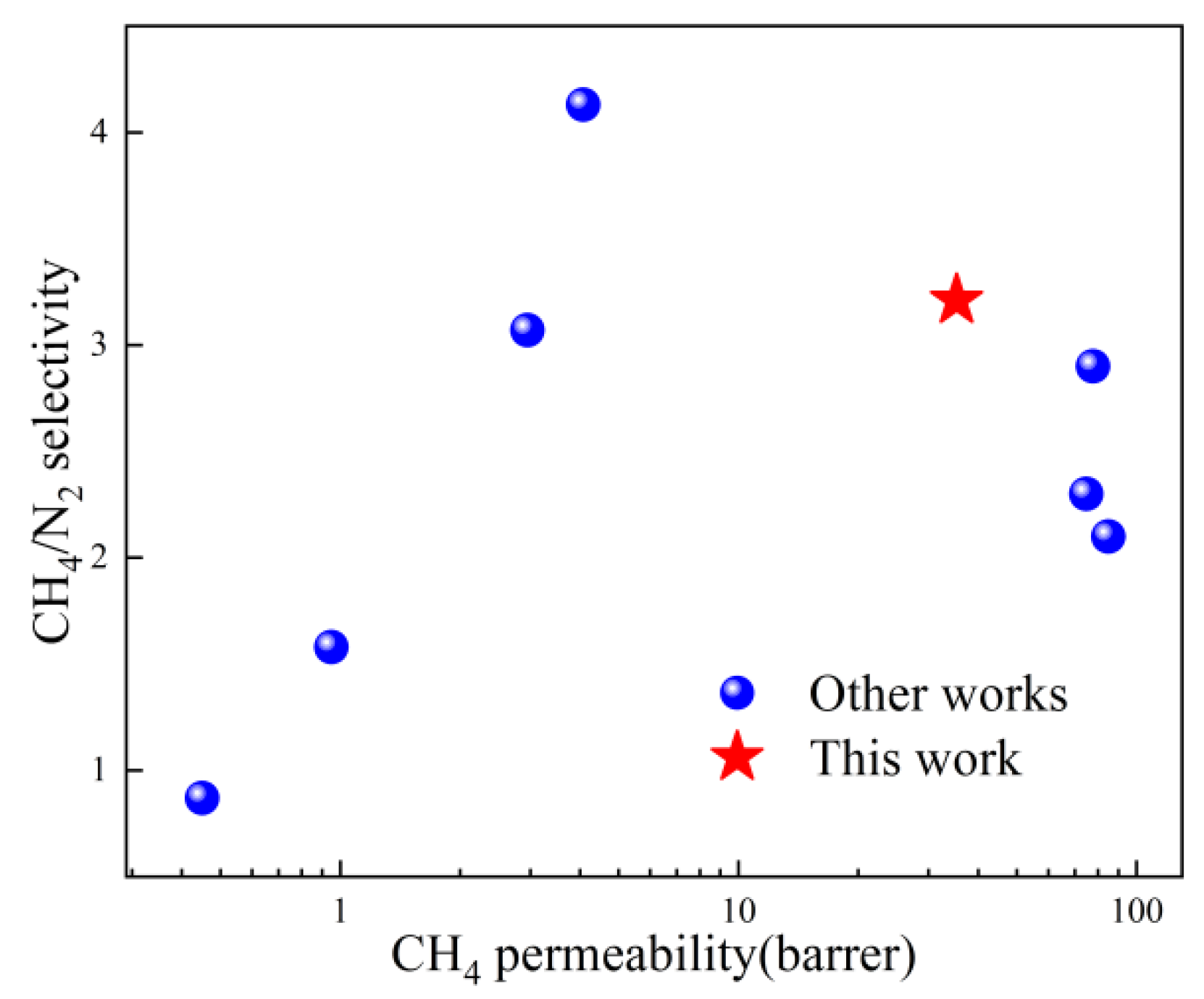
To evaluate the enhancement effect of ZIF-67nf on the membrane separation performance, MMMs with different loadings were prepared. Meanwhile, for the purpose of comparison, MMMs with the same loadings using 3D ZIF-67 as the filler were also prepared. The CH4/N2 separation performance is shown in Figure 13. When the ZIF-67nf loading in the MMMs reaches 20 wt%, the CH4 permeability coefficient increases steadily from 11.7 barrer to 35.3 barrer, while maintaining a CH4/N2 selectivity above 3. In contrast, MMMs incorporating 3D ZIF-67 particles show a more pronounced increase in CH4 permeability; however, this is accompanied by a sharp decline in CH4/N2 selectivity, highlighting the presence of significant interfacial defects in the membrane structure. This indicates that 2D-structured fillers possess significant potential advantages in enhancing interfacial compatibility within MMMs. Table 1 and Figure 14 compare the CH4/N2 separation performance of the polymeric membranes fabricated in this work with those reported in other studies, demonstrating promising potential when considering both permeability and selectivity.
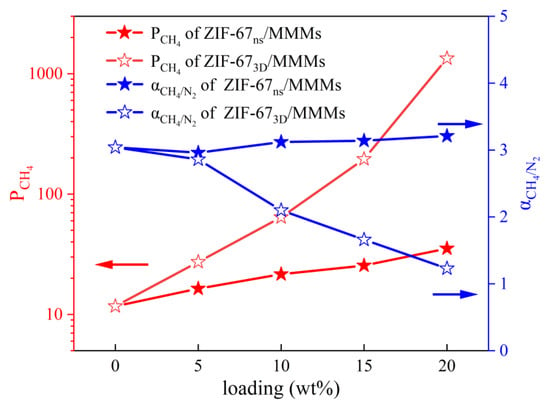
Figure 13.
The CH4/N2 separation performance of MMMs fabricated at varying ZIF-67 loadings in this work.

Table 1.
Comparative of CH4/N2 separation performance of MMMs.
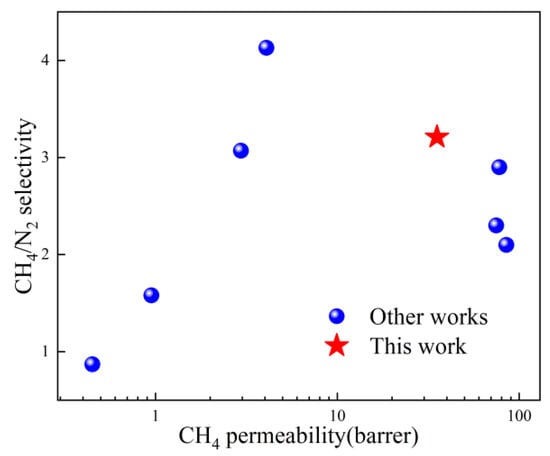
Figure 14.
Comparative analysis of CH4/N2 separation performance between MMMs developed in this work and other reported works.
4. Conclusions
In summary, to obtain ZIF-67 with a high aspect ratio, we successfully reduced the crystallization rate of ZIF-67 by more than one order of magnitude using a solvent screening strategy based on first-principles calculations combined with a diffusion-mediated process, resulting in the formation of a 2D amorphous nanoflower structure. Furthermore, by introducing a templating agent into the reaction system, the formation of pore structures was promoted to some extent, increasing the BET surface area from 18.2 m2/g to 403 m2/g while preserving the 2D nanoflower morphology. Finally, MMMs with a 20 wt% loading of this 2D nanoflower ZIF-67 were fabricated. Due to the synergistic effect of high filler loading and the relatively high specific surface area, the CH4 permeability coefficient increased from 11.7 barrer to 35.3 barrer, representing a 201% improvement, while maintaining a CH4/N2 selectivity above 3, demonstrating excellent interfacial compatibility. Although existing 2D MOF-based MMMs achieve high filler loadings, their gas separation performance remains critically limited by the ultra low specific surface area of the fillers. Future research must prioritize developing 2D MOFs with engineered pore architectures to enable breakthrough enhancements in membrane permeability/selectivity combinations beyond current Robeson upper bounds.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The author Dongze Li was employed by the company SINOPEC Research Institute of Safety Engineering Co., Ltd.
References
- IEA. Methane from Oil & Gas-Methane Tracker 2021-Analysis; IEA: Paris, France, 2021; Available online: https://www.iea.org/fuels-and-technologies/methane-abatement (accessed on 7 December 2021).
- Wang, Q.; Yu, Y.; Li, Y.; Min, X.; Zhang, J.; Sun, T. Methane separation and capture from nitrogen rich gases by selective adsorption in microporous Materials: A review. Sep. Purif. Technol. 2021, 283, 120206. [Google Scholar] [CrossRef]
- Zhang, C.; Chen, Y.; Wu, H.; Li, H.; Li, X.; Tu, S.; Qiao, Z.; An, D.; Xia, Q. Mechanochemical synthesis of a robust cobalt-based metal–organic framework for ads;orption separation methane from nitrogen. Chem. Eng. J. 2021, 435, 133876. [Google Scholar] [CrossRef]
- Winarta, J.; Meshram, A.; Zhu, F.; Li, R.; Jafar, H.; Parmar, K.; Liu, J.; Mu, B. Metal-organic framework-based mixed-matrix membranes for gas separation: An overview. J. Polym. Sci. 2020, 58, 2518–2546. [Google Scholar] [CrossRef]
- Foo, K.; Liang, Y.Y.; Goh, P.S.; Fletcher, D.F. Computational fluid dynamics simulations of membrane gas separation: Overview, challenges and future perspectives. Process Saf. Environ. Prot. 2023, 191, 127–140. [Google Scholar] [CrossRef]
- Elahi, F.; Wang, Y.; Hazazi, K.; Kumar, V.; Balcik, M.; Wehbe, N.; Xu, F.; Pinnau, I. Long-term pure- and mixed-gas performance of carbon molecular sieve membranes derived from a tetraphenylethylene-based ladder polymer of intrinsic microporosity (TPE-PIM) for propylene/propane separation. J. Membr. Sci. 2024, 713, 123255. [Google Scholar] [CrossRef]
- Li, B.; Qi, B.; Han, J.; Qian, X.; Yang, C.; Cai, S. Separation of oil–water emulsion by biomimetic polycaprolactone tannic acid hydrophilic modified membranes. Fuel 2025, 386, 134242. [Google Scholar] [CrossRef]
- He, S.; Zhu, B.; Li, S.; Zhang, Y.; Jiang, X.; Lau, C.H.; Shao, L. Recent progress in PIM-1 based membranes for sustainable CO2 separations: Polymer structure manipulation and mixed matrix membrane design. Sep. Purif. Technol. 2022, 284, 120277. [Google Scholar] [CrossRef]
- Rodenas, T.; Luz, I.; Prieto, G.; Seoane, B.; Miro, H.; Corma, A.; Kapteijn, F.; Llabrés, I.; Xamena, F.X.; Gascon, J. Metal-organic framework nanosheets in polymer composite materials for gas separation. Nat. Mater. 2014, 14, 48–55. [Google Scholar] [CrossRef]
- Zhang, J.; Dong, H.; Guo, F.; Yi, H.; Jiang, X.; He, G.; Xiao, W. Construction of Transport Channels by HNTs@ZIF-67 Composites in a Mixed-Matrix Membrane for He/CH4 Separation. Membranes 2025, 15, 197. [Google Scholar] [CrossRef]
- Jonnalagedda, A.; Choudhari, S.A.; Nayak, B.; Pani, A.K.; Kuncharam, B.V.R. Asymmetric mixed matrix membranes with zeolite imidazolate frameworks (ZIF-8, ZIF-67, bimetallic ZIF-8/67) and polyethersulfone for high flux and high selective hydrogen separation. Polym. Eng. Sci. 2025, 65, 3209–3225. [Google Scholar] [CrossRef]
- Missaoui, N.; Chrouda, A.; Kahri, H.; Gross, A.J.; Ardani, M.R.; Pang, A.L.; Ahmadipour, M. PEG-templated synthesis of ultramicroporous n-ZIF-67 nanoparticles with high selectivity for the adsorption and uptake of CO2 over CH4 and N2. Sep. Purif. Technol. 2023, 316, 123755. [Google Scholar] [CrossRef]
- Kim, S.; Shamsaei, E.; Lin, X.; Hu, Y.; Simon, G.P.; Seong, J.G.; Kim, J.S.; Lee, W.H.; Lee, Y.M.; Wang, H. The enhanced hydrogen separation performance of mixed matrix membranes by incorporation of two-dimensional ZIF-L into polyimide containing hydroxyl group. J. Membr. Sci. 2017, 549, 260–266. [Google Scholar] [CrossRef]
- Zhu, W.; Li, X.; Sun, Y.; Guo, R.; Ding, S. Introducing hydrophilic ultra-thin ZIF-L into mixed matrix membranes for CO2/CH4 separation. RSC Adv. 2019, 9, 23390–23399. [Google Scholar] [CrossRef]
- Datta, S.J.; Mayoral, A.; Bettahalli, N.M.S.; Bhatt, P.M.; Karunakaran, M.; Carja, I.D.; Fan, D.; Mileo, P.G.M.; Semino, R.; Maurin, G.; et al. Rational design of mixed-matrix metal-organic framework membranes for molecular separations. Science 2022, 376, 1080–1087. [Google Scholar] [CrossRef]
- Zhao, Q.; Lian, S.; Li, R.; Yang, Y.; Zang, G.; Song, C. Fabricating Leaf-like hierarchical ZIF-67 as Intra-Mixed matrix membrane microarchitecture for efficient intensification of CO2 separation. Sep. Purif. Technol. 2022, 305, 122460. [Google Scholar] [CrossRef]
- Zhu, C.; Peng, Y.; Li, K.; Liu, L.; Yang, W. Regulation of MOF-74 Nanosheet Channels for Precise H2 Purification. J. Membr. Sci. 2024, 709, 123140. [Google Scholar] [CrossRef]
- Liu, X.; Chee, S.W.; Raj, S.; Sawczyk, M.; Král, P.; Mirsaidov, U. Three-step nucleation of metal-organic framework nanocrystals. Proc. Natl. Acad. Sci. USA 2021, 118, e2008880118. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian, 1.6. Revision, A.0.3; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Adamo, C.; Barone, V. Toward reliable density functional methods without adjustable parameters: The PBE0 model. J. Chem. Phys. 1999, 110, 6158–6170. [Google Scholar] [CrossRef]
- Krishnan, R.; Binkley, J.S.; Seeger, R.; Pople, J.A. Self-consistent molecular orbital methods. XX. A basis set for correlated wave functions. J. Chem. Phys. 1980, 72, 650–654. [Google Scholar] [CrossRef]
- Mennucci, B.; Tomasi, J. Continuum solvation models: A new approach to the problem of solute’s charge distribution and cavity boundaries. J. Chem. Phys. 1997, 106, 5151–5158. [Google Scholar] [CrossRef]
- Kozuch, S.; Martin, J.M.L. DSD-PBEP86. In search of the best double-hybrid DFT with spin-component scaled MP2 and dispersion corrections. Phys. Chem. Chem. Phys. 2011, 13, 20104. [Google Scholar] [CrossRef]
- Weigend, F.; Ahlrichs, R. Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: Design and assessment of accuracy. Phys. Chem. Chem. Phys. 2005, 7, 3297. [Google Scholar] [CrossRef]
- Marenich, A.V.; Cramer, C.J.; Truhlar, D.G. Universal Solvation Model Based on Solute Electron Density and on a Continuum Model of the Solvent Defined by the Bulk Dielectric Constant and Atomic Surface Tensions. J. Phys. Chem. B 2009, 113, 6378–6396. [Google Scholar] [CrossRef]
- Lu, T.; Chen, F. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 2011, 33, 580–592. [Google Scholar] [CrossRef]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual molecular dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Guo, Z.; Zheng, L.; Du, M.; Han, J.; Yang, C. Effect of modified EVA-GMX bionic nanocomposite pour point depressants on the rheological properties of waxy crude oil. Fuel 2025, 403, 136025. [Google Scholar] [CrossRef]
- Perez, E.V.; Balkus, K.J.; Ferraris, J.P.; Musselman, I.H. Mixed-matrix membranes containing MOF-5 for gas separations. J. Membr. Sci. 2009, 328, 165–173. [Google Scholar] [CrossRef]
- Perez, E.V.; Balkus, K.J.; Ferraris, J.P.; Musselman, I.H. Metal-organic polyhedra 18 mixed-matrix membranes for gas separation. J. Membr. Sci. 2014, 463, 82–93. [Google Scholar] [CrossRef]
- Wang, S.; Guo, Q.; Liang, S.; Li, P.; Li, X.; Luo, J. [Ni3(HCOO)6]/Poly(styrene-b-butadiene-b-styrene) Mixed-Matrix Membranes for CH4/N2 Gas Separation. Chem. Eng. Technol. 2017, 41, 353–366. [Google Scholar] [CrossRef]
- Wang, S.; Guo, Q.; Liang, S.; Li, P.; Luo, J. Preparation of Ni-MOF-74/SBS mixed matrix membranes and its application of CH4/N2 separation. Sep. Purif. Technol. 2018, 199, 206–213. [Google Scholar] [CrossRef]
- Chi, W.S.; Hwang, S.; Lee, S.-J.; Park, S.; Bae, Y.-S.; Ryu, D.Y.; Kim, J.H.; Kim, J. Mixed matrix membranes consisting of SEBS block copolymers and size-controlled ZIF-8 nanoparticles for CO2 capture. J. Membr. Sci. 2015, 495, 479–488. [Google Scholar] [CrossRef]
- Miri, S.; Omidkhah, M.; Ebadi Amooghin, A.; Matsuura, T. Membrane-based gas separation accelerated by quaternary mixed matrix membranes. J. Nat. Gas Sci. Eng. 2020, 84, 103655. [Google Scholar] [CrossRef]
- Estahbanati, E.G.; Omidkhah, M.; Amooghin, A.E. Interfacial Design of Ternary Mixed Matrix Membranes Containing Pebax 1657/Silver-Nanopowder/[BMIM][BF4] for Improved CO2 Separation Performance. ACS Appl. Mater. Interfaces 2017, 9, 10094–10105. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).