Fabrication of Biomimetical TiO2@PVDF Composite Membrane with Omniphobicity via In-Situ Growth and Its Anti-Fouling Performance
Abstract
1. Introduction
2. Experimental
2.1. Materials and Chemicals
2.2. Fabrication of F-TiO2@PVDF Bio-Inspired Omniphobic Composite Membrane
- (1)
- PDA polymerization modification of the PVDF membrane surface
- (2)
- In situ growth of nanoparticles
- (3)
- Low-surface-energy grafting treatment
2.3. Membrane Characterization and Test
- (1)
- Microstructure morphology characterization of membranes
- (2)
- Composition analysis of membranes
- (3)
- Membrane wetting behavior
- (4)
- The membrane structural parameters
- (5)
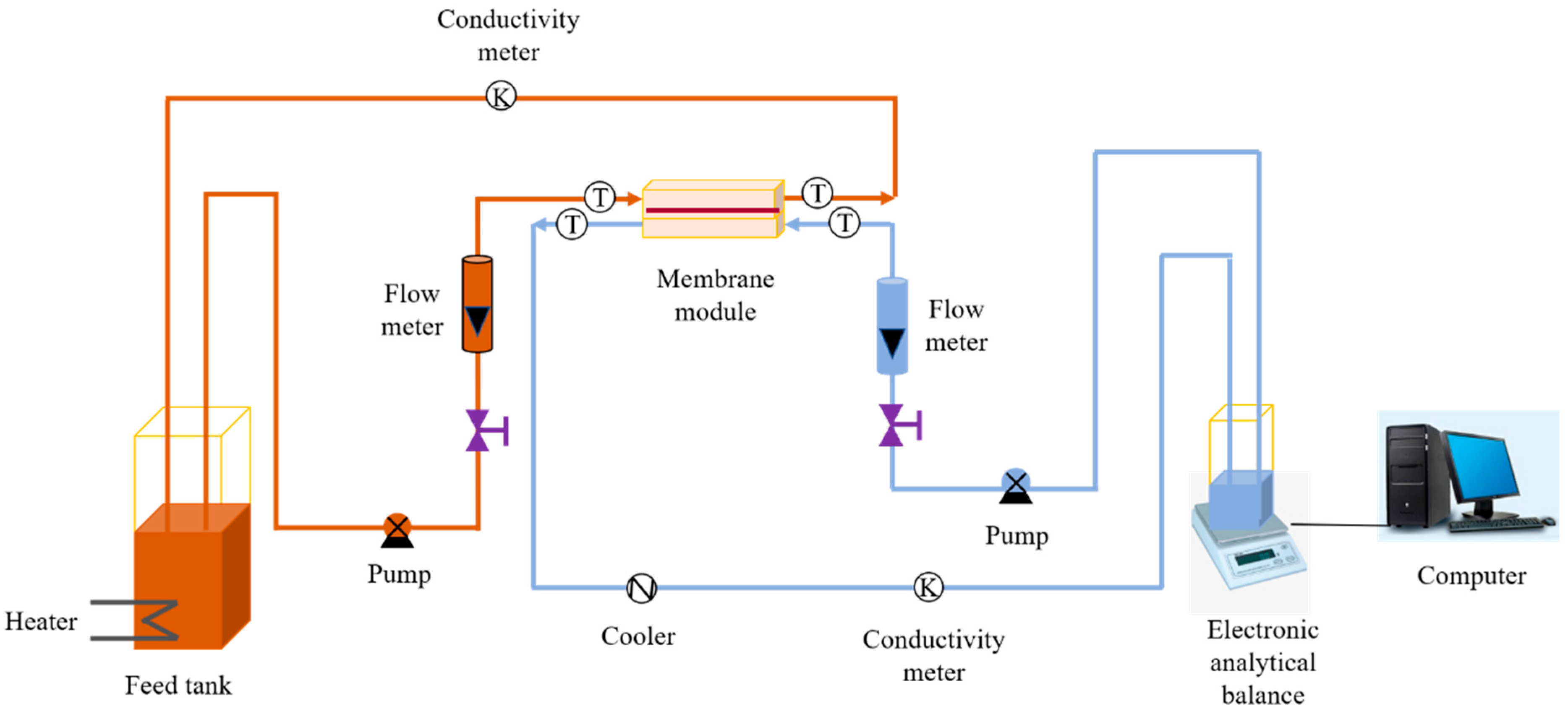
- Membrane anti-fouling performance testing
3. Results and Discussion
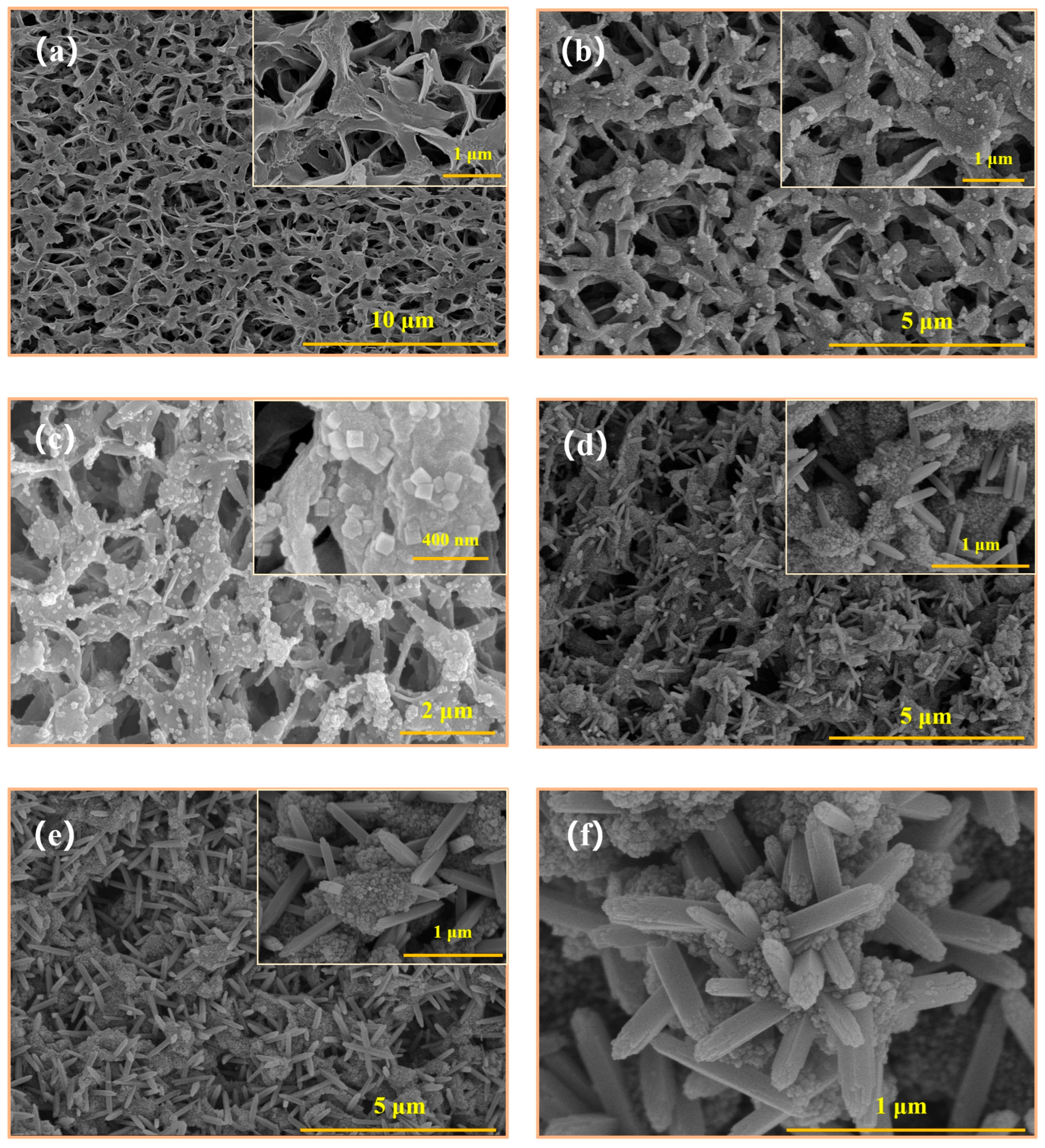
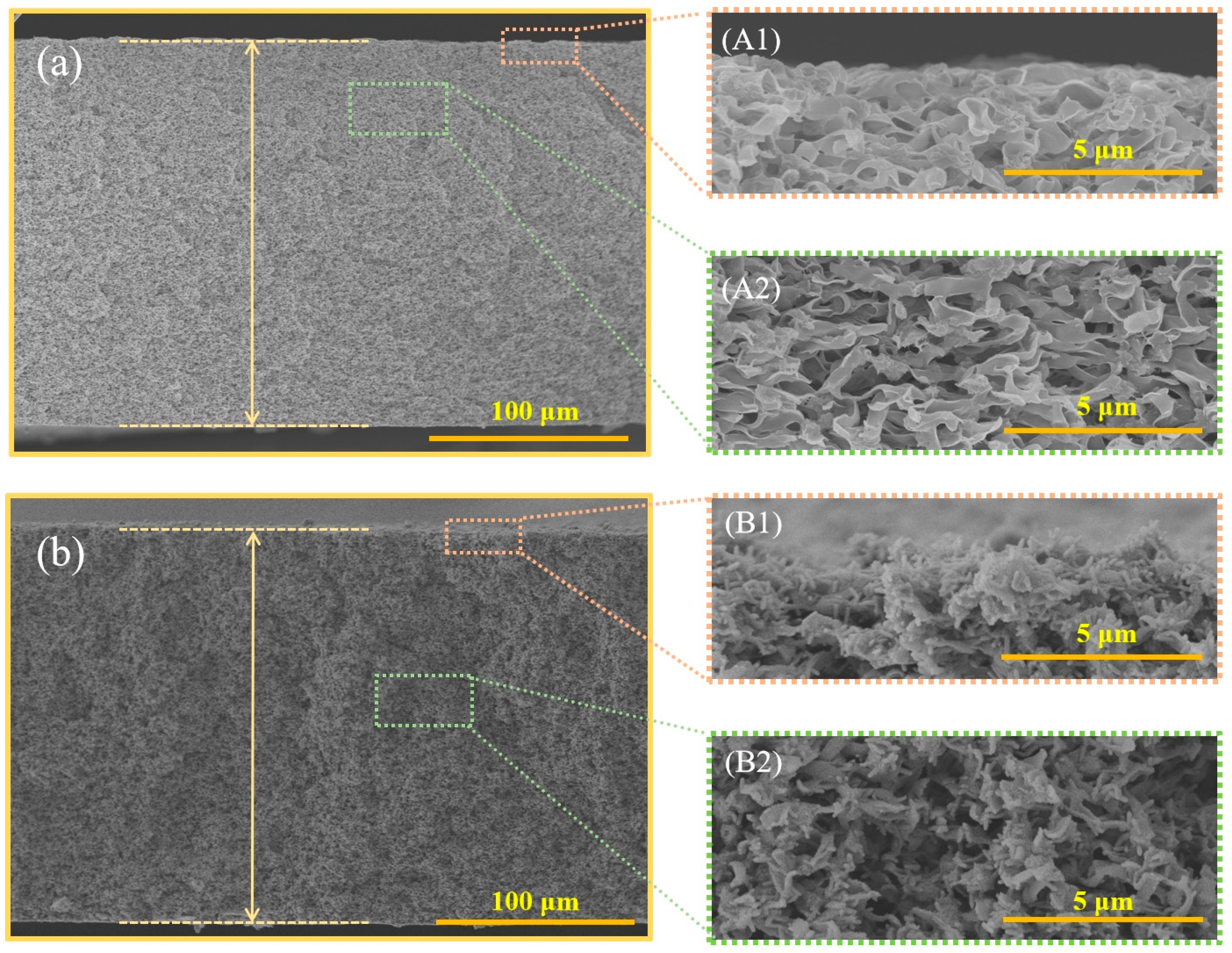
3.1. Membrane Morphology
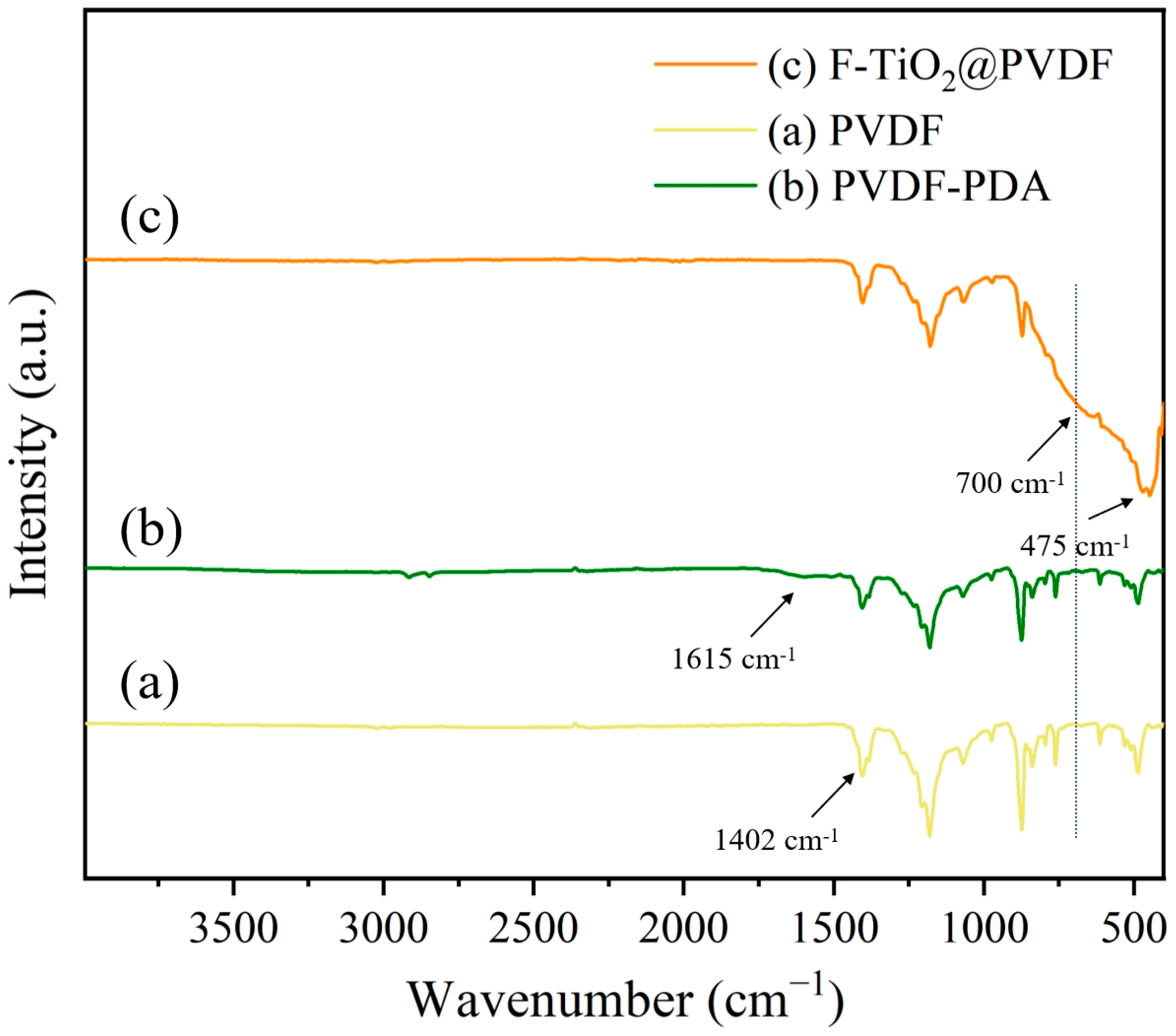
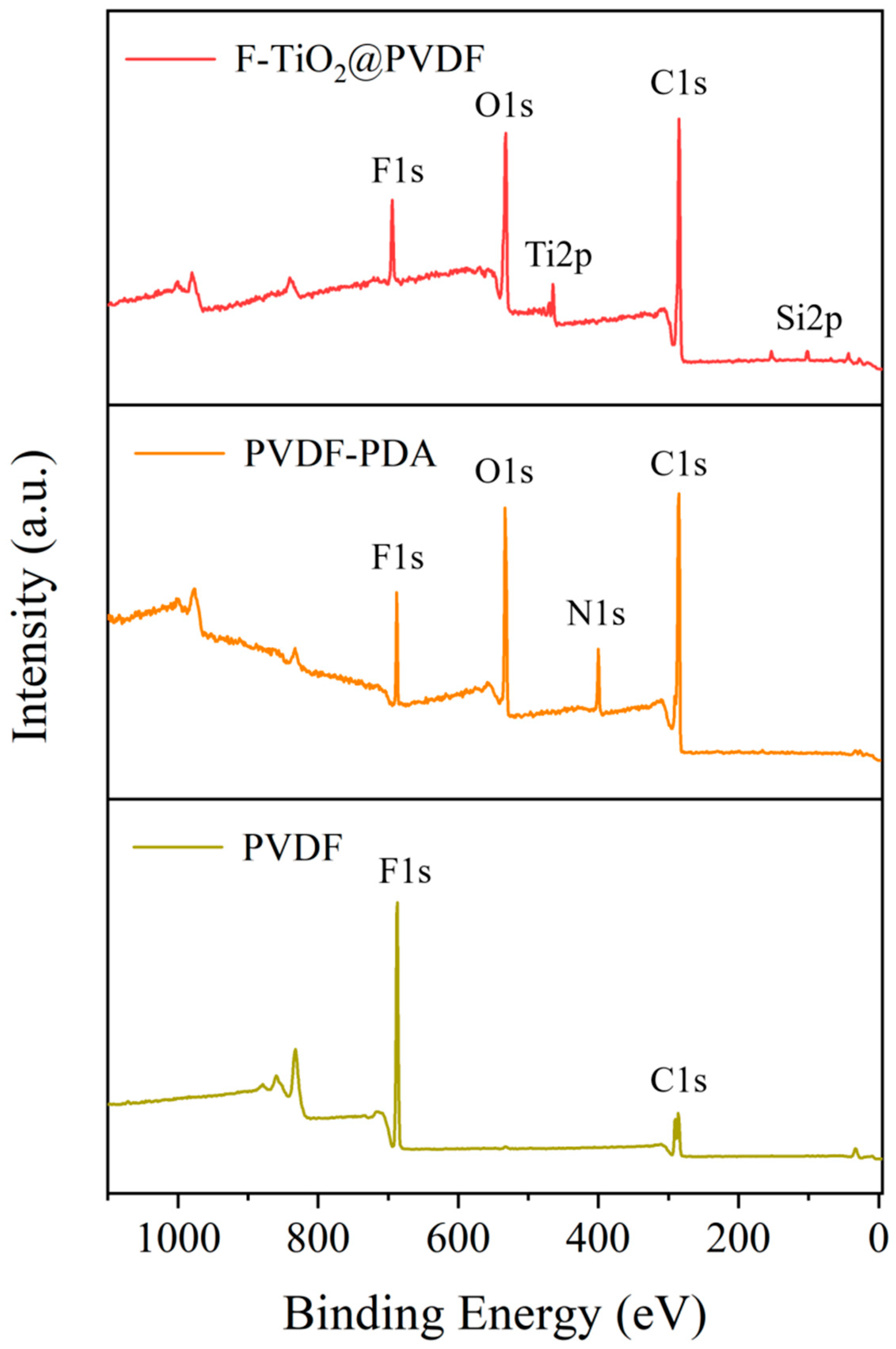
3.2. Membrane Chemical Composition
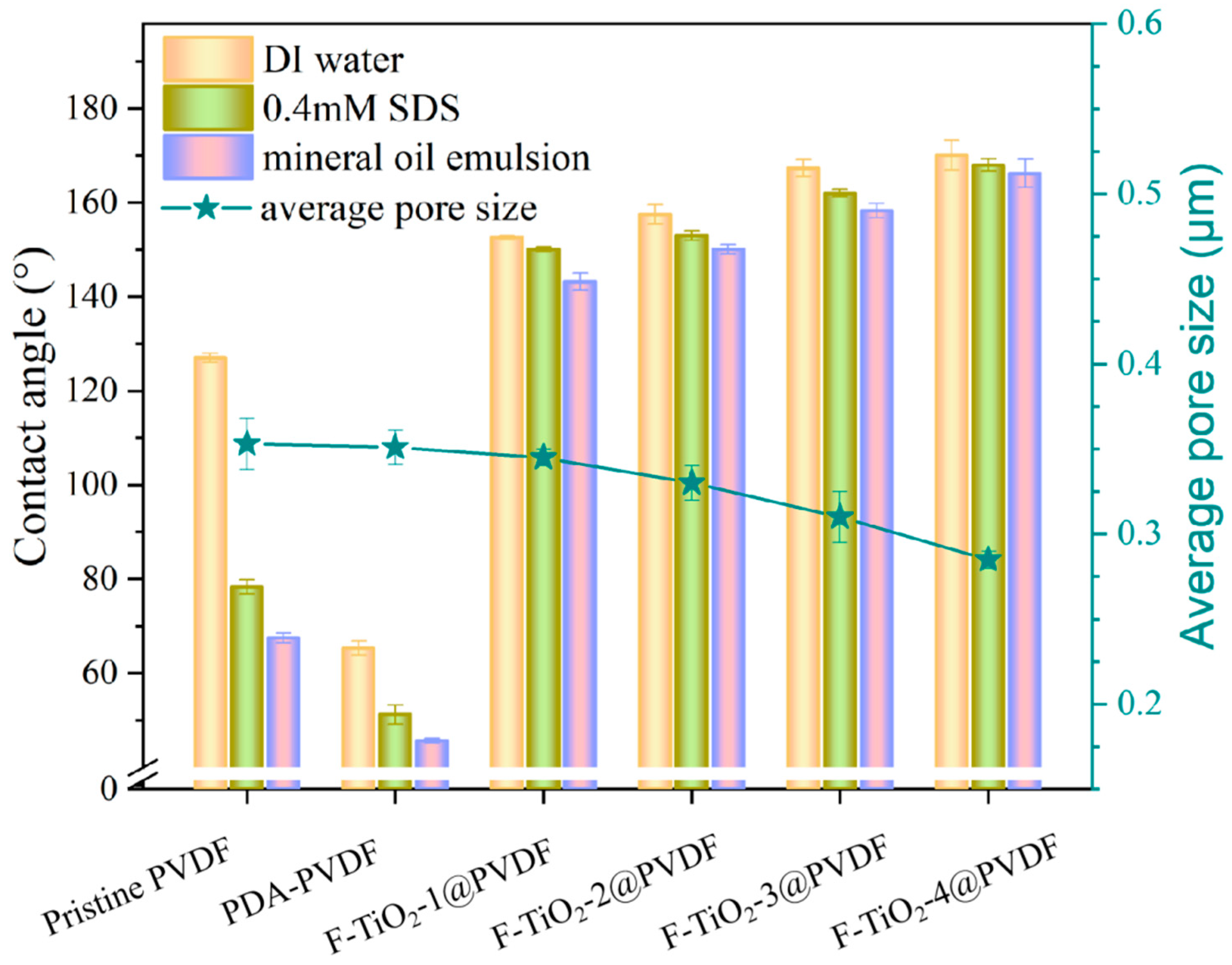
3.3. Membrane Wettability and Structural Parameter
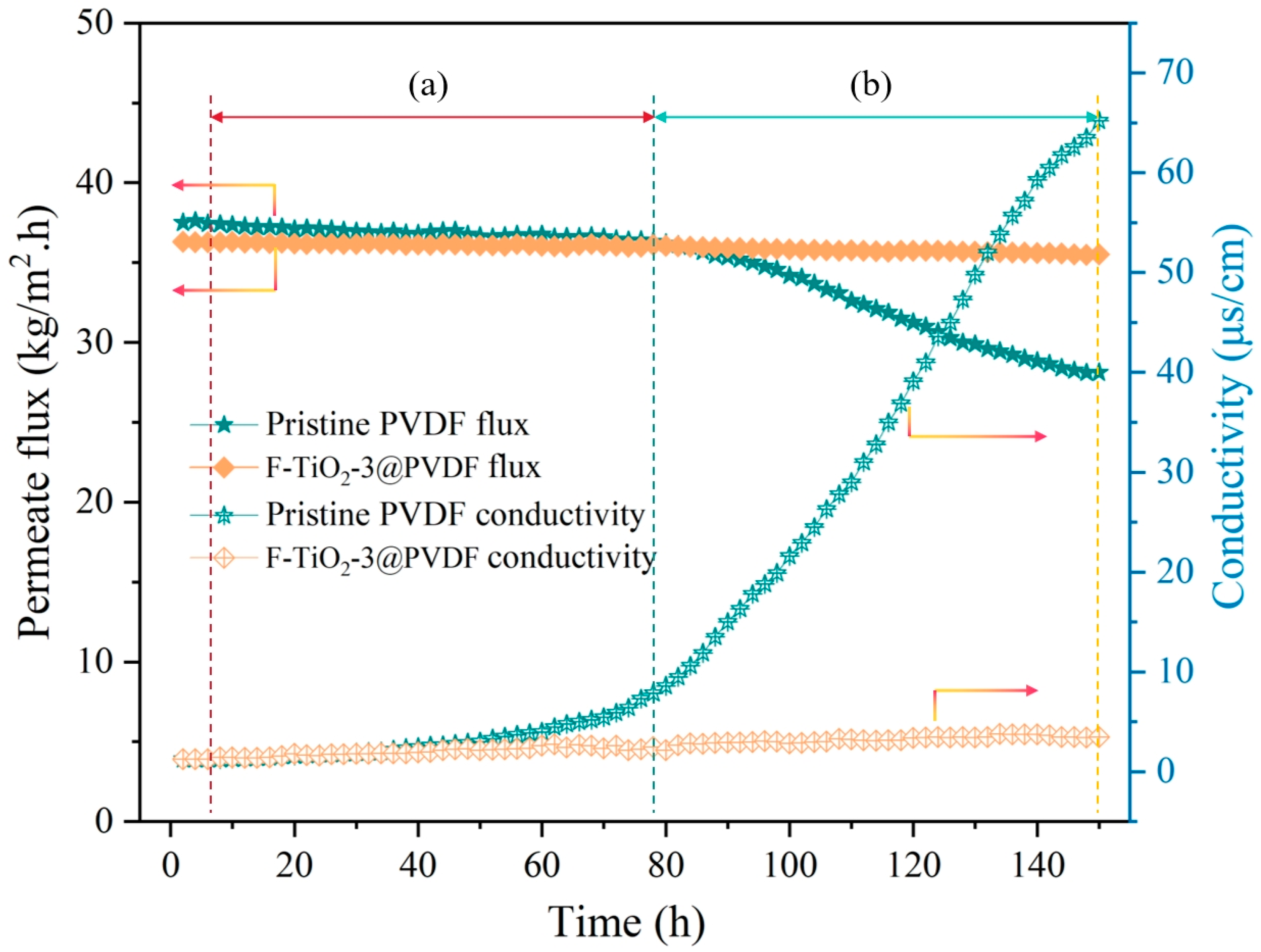
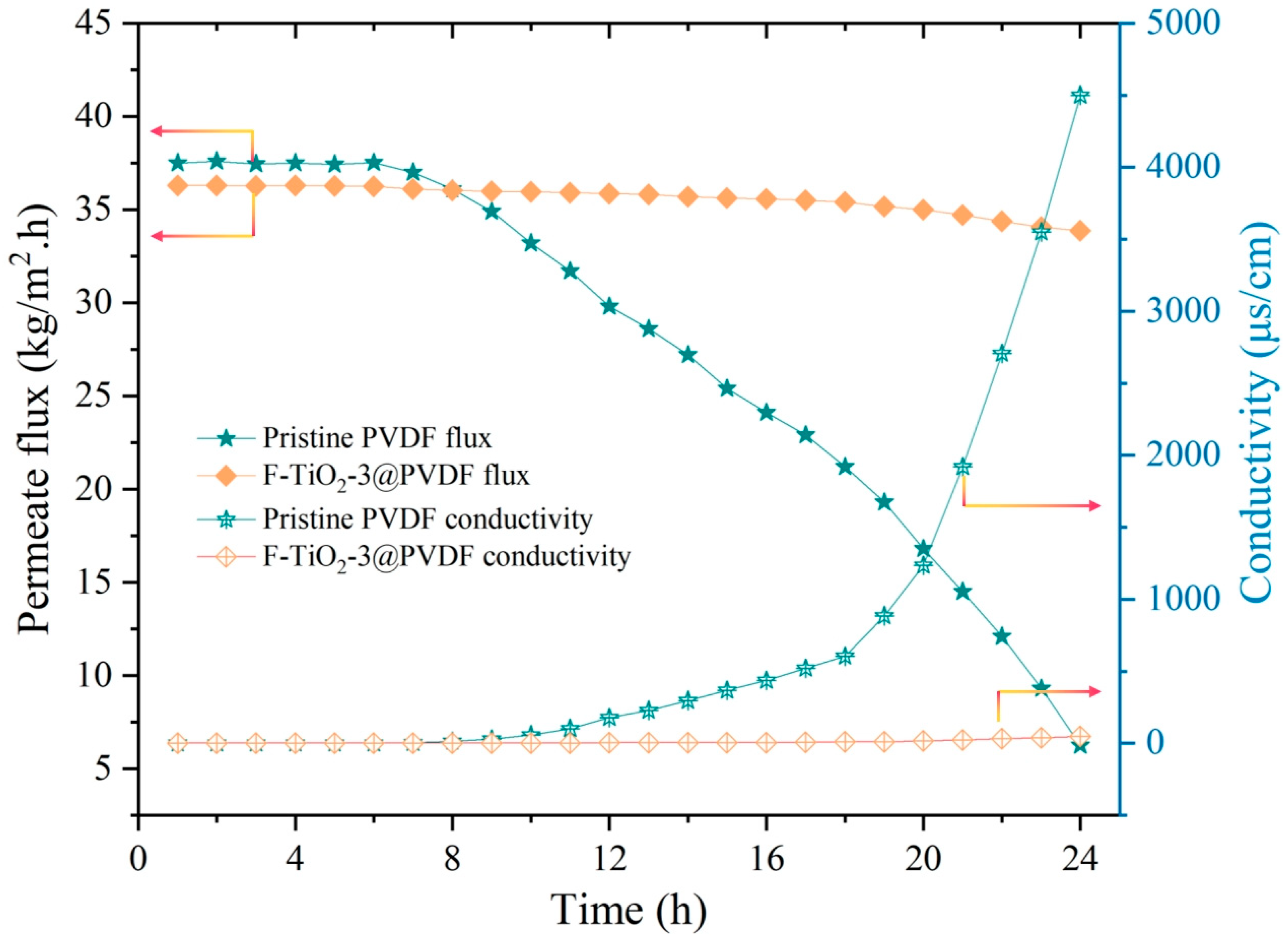
3.4. Membrane Distillation Fouling Test
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Shannon, M.A.; Bohn, P.W.; Elimelech, M.; Georgiadis, J.G.; Marinas, B.J.; Mayes, A.M. Science and technology for water purification in the coming decades. Nature 2018, 452, 301–310. [Google Scholar] [CrossRef]
- Gude, V.G.; Nirmalakhandan, N.; Deng, S.G. Renewable and sustainable approaches for desalination. Renew. Sustain. Energy Rev. 2010, 14, 2641–2654. [Google Scholar] [CrossRef]
- Ogunniyi, E.O.; Richards, B.S. Renewable energy powered membrane technology: Electro-hydraulic control system design for managing pump shutdowns in a photovoltaic-membrane water desalination system. Desalination 2025, 608, 118784–118800. [Google Scholar] [CrossRef]
- Jones, E.; Qadir, M.; Vliet, M.T.; Smakhtin, V.; Kang, S. The state of desalination and brine production: A global outlook. Sci. Total Environ. 2019, 657, 1343–1356. [Google Scholar] [CrossRef]
- Ali, A.; Tufa, R.A.; Macedonio, F.; Curcio, E.; Drioli, E. Membrane technology in renewable-energy-driven desalination. Renew. Sustain. Energy Rev. 2018, 81, 1–21. [Google Scholar] [CrossRef]
- Grant, S.B.; Saphores, J.D.; Feldman, D.L.; Hamilton, A.J.; Fletcher, T.D.; Cook, P.M.; Stewardson, M.; Sanders, B.F.; Levin, L.A.; Ambrose, R.F.; et al. Taking the “waste” out of “wastewater” for human water security and ecosystem sustainability. Science 2012, 337, 681–686. [Google Scholar] [CrossRef]
- Gittins, J.R.; Hemingway, J.R.; Dajka, J.C. How a water-resources crisis highlights social-ecological disconnects. Water Res. 2021, 194, 116937–116941. [Google Scholar] [CrossRef]
- Duckett, D.; Troldborg, M.; Hendry, S.; Cousin, H. Making waves: Promoting municipal water reuse without a prevailing scarcity driver. Water Res. 2024, 249, 120965–120970. [Google Scholar] [CrossRef]
- Deshmukh, A.; Boo, C.; Karanikola, V.; Lin, S.H.; Straub, A.P.; Tong, T.Z.; Warsinger, D.M.; Elimelech, M. Membrane distillation at the water-energy nexus: Limits, opportunities, and challenges. Energy Environ. Sci. 2018, 11, 1177–1196. [Google Scholar] [CrossRef]
- Gude, V.G. Desalination and sustainability—An appraisal and current perspective. Water Res. 2016, 89, 87–106. [Google Scholar] [CrossRef]
- Criscuoli, A.; Macedonio, F.; Brunetti, A.; Tocci, E.; Drioli, E. Impact of Membrane Engineering on the Process Engineering Progresses: Towards a Sustainable Development. Chem. Eng. Process. Process Intensif. 2023, 189, 109385. [Google Scholar] [CrossRef]
- Buggenhout, S.V.; Verbeke, R.; Davenport, D.M.; Vankelecom, I.F. Drying polymer membranes for preservation: A review. J. Membr. Sci. 2025, 731, 124190–124207. [Google Scholar] [CrossRef]
- Alkhudhiri, A.; Darwish, N.; Hilal, N. Membrane distillation: A comprehensive review. Desalination 2012, 287, 2–18. [Google Scholar] [CrossRef]
- Lawson, K.W.; Lloyd, D.R. Membrane distillation. J. Membr. Sci. 1997, 124, 1–25. [Google Scholar] [CrossRef]
- Khayet, M. Membranes and theoretical modeling of membrane distillation: A review. Adv. Colloid Interface Sci. 2011, 164, 56–88. [Google Scholar] [CrossRef]
- Drioli, E.; Ali, A.; Macedonio, F. Membrane distillation: Recent developments and Perspectives. Desalination 2015, 356, 56–84. [Google Scholar] [CrossRef]
- El-Bourawi, M.S.; Ding, Z.; Ma, R.; Khayet, M. A framework for better understanding membrane distillation separation process. J. Membr. Sci. 2006, 285, 4–29. [Google Scholar] [CrossRef]
- Tong, T.; Elimelech, M. The global rise of zero liquid discharge for wastewater management: Drivers, technologies, and future directions. Environ. Sci. Technol. 2016, 50, 6846–6855. [Google Scholar] [CrossRef]
- Frédette, C.; Comeau, Y.; Brisson, J. Design of a zero liquid discharge leachate treatment system using an evapotranspiration willow bed. Water Res. 2022, 209, 117950–117959. [Google Scholar] [CrossRef]
- Wang, P.; Deng, H.; Yao, J.; Cheng, W.; Wu, C.; Zhang, T.; Ma, J.; Wang, W. Unraveling the intricate fouling behaviors of landfill leachate components during membrane distillation concentration toward zero liquid discharge. J. Membr. Sci. 2024, 703, 122851–122861. [Google Scholar] [CrossRef]
- Yao, M.W.; Tijing, L.D.; Naidu, G.; Kim, S.H.; Matsuyama, H.; Fane, A.G.; Shon, H.K. A review of membrane wettability for the treatment of saline water deploying membrane distillation. Desalination 2020, 479, 114312–114333. [Google Scholar] [CrossRef]
- Naidu, G.; Tijing, L.; Johir, M.; Shon, H.; Vigneswaran, S. Hybrid membrane distillation: Resource, nutrient and energy recovery. J. Membr. Sci. 2020, 599, 117832–117852. [Google Scholar] [CrossRef]
- Gontarek-Castro, E.; Castro-Muñoz, R. How to make membrane distillation greener: A review of environmentally friendly and sustainable aspects. Green Chem. 2024, 26, 164–185. [Google Scholar] [CrossRef]
- González, D.; Amigo, J.; Suárez, F. Membrane distillation: Perspectives for sustainable and improved desalination. Renew. Sustain. Energy Rev. 2017, 80, 238–259. [Google Scholar] [CrossRef]
- Yu, S.; Zhao, Q.; Zhu, J.; Gong, G.; Hu, Y. Incorporating TiO2 nanocages into electrospun nanofibrous membrane for efficient and anti-fouling membrane distillation. J. Membr. Sci. 2024, 698, 122614–122623. [Google Scholar] [CrossRef]
- Chang, H.; Zhu, Y.; Huang, L.; Yan, Z.; Qu, F.; Liang, H. Mineral scaling induced membrane wetting in membrane distillation for water treatment: Fundamental mechanism and mitigation strategies. Water Res. 2023, 247, 120807–120828. [Google Scholar] [CrossRef]
- Siyal, M.I.; Lee, C.K.; Park, C.; Khan, A.A.; Kim, J.O. A review of membrane development in membrane distillation for emulsified industrial or shale gas wastewater treatments with feed containing hybrid impurities. J. Environ. Manag. 2019, 243, 45–66. [Google Scholar] [CrossRef]
- Abid, M.B. Advancements in Omniphobic membranes: Properties, fabrication techniques, and applications in membrane distillation. Desalination Water Treat. 2025, 322, 101214–101225. [Google Scholar] [CrossRef]
- Li, J.; Ren, L.F.; Huang, M.; Yang, J.; Shao, J.; He, Y. Facile preparation of omniphobic PDTS-ZnO-PVDF membrane with excellent anti-wetting property in direct contact membrane distillation (DCMD). J. Membr. Sci. 2022, 650, 120404–120415. [Google Scholar] [CrossRef]
- Feng, H.; Li, H.; Li, M.; Zhang, X. Construction of omniphobic PVDF membranes for membrane distillation: Investigating the role of dimension, morphology, and coating technology of silica nanoparticles. Desalination 2022, 525, 115498. [Google Scholar] [CrossRef]
- Zhu, Z.; Tan, G.; Lei, D.; Yang, Q.; Tan, X.; Liang, N.; Ma, D. Omniphobic membrane with process optimization for advancing flux and durability toward concentrating reverse-osmosis concentrated seawater with membrane distillation. J. Membr. Sci. 2021, 639, 119763–119772. [Google Scholar] [CrossRef]
- Liao, X.; Goh, K.; Liao, Y.; Wang, R.; Razaqpur, A.G. Bio-inspired super liquid-repellent membranes for membrane distillation: Mechanisms, fabrications and applications. Adv. Colloid. Interface Sci. 2021, 297, 102547–102573. [Google Scholar] [CrossRef]
- Tolan, D.; El-Sawaf, A.; Ahmed, A.S.A.; Nassar, A.; Mohamed, N.M.; Alhindawy, I.G.; Elshehy, E.A.; Utgikar, V. Enhanced photocatalytic activity of (In–Sr–P) tridoped TiO2/Bi2O3 composite loaded on mesoporous carbon: A facile sol-hydrothermal synthesis approach. Mater. Chem. Phys. 2024, 322, 129570–129581. [Google Scholar] [CrossRef]
- Gupta, T.; Samriti; Cho, J.; Prakash, J. Hydrothermal synthesis of TiO2 nanorods: Formation chemistry, growth mechanism, and tailoring of surface properties for photocatalytic activities. Mater. Today Chem. 2021, 20, 100428–100467. [Google Scholar] [CrossRef]
- Zhang, W.; Li, Y.; Liu, J.; Li, B.; Wang, S. Fabrication of hierarchical poly (vinylidene fluoride) micro/nano-composite membrane with anti-fouling property for membrane distillation. J. Membr. Sci. 2017, 535, 258–267. [Google Scholar] [CrossRef]
- Zhang, W.; Lu, Y.; Liu, J.; Li, X.; Li, B.; Wang, S. Preparation of re-entrant and anti-fouling PVDF composite membrane with omniphobicity for membrane distillation. J. Membr. Sci. 2020, 595, 117563–117576. [Google Scholar] [CrossRef]
- Zhang, W.; Hu, B.; Wang, Z.; Li, B. Fabrication of omniphobic PVDF composite membrane with dual-scale hierarchical structure via chemical bonding for robust membrane distillation. J. Membr. Sci. 2021, 622, 119038–119052. [Google Scholar] [CrossRef]
- Tong, Y.; Wu, Y.; Xu, Z.; Luo, L.; Jia, R.; Han, R. Hydrolysis co-deposition of bio-inspired hybrid hydrophilic network antifouling loose nanofiltration membrane for effective dye/salt separation. J. Membr. Sci. 2024, 694, 122444–122458. [Google Scholar] [CrossRef]
- Mameda, N.; Park, H.; Kim, J.; Shah, S.S.; Rahman, S.; Sherugar, P.; Lee, H.; Choo, K.H. Self-assembled electrocatalytic TiO2 nanowire membrane for multifunctional water purification. J. Membr. Sci. 2025, 734, 124422–124434. [Google Scholar] [CrossRef]
- Cao, N.; Gu, M.; Gao, M.; Li, C.; Liu, K.; Zhao, X.; Feng, J.; Ren, Y.; Wei, T. A three-layer photocatalyst carbon fibers/TiO2 seed/TiO2 nanorods with high photocatalytic degradation under visible light. Appl. Surf. Sci. 2020, 530, 147289–147295. [Google Scholar] [CrossRef]
- Chu, Z.; Qiu, L.; Chen, Y.; Zhuang, Z.; Du, P.; Xiong, J. TiO2-loaded carbon fiber: Microwave hydrothermal synthesis and photocatalytic activity under UV light irradiation. J. Phys. Chem. C 2020, 136, 109138–109144. [Google Scholar] [CrossRef]
- Jiang, X.; Duan, Y.; Tian, Y.; Chen, M.; Li, M.; Liu, H.; Yang, W.; Tian, M. Facile one-pot hydrothermal method to prepare Sn(II) and N co-doped TiO2 photocatalyst for water splitting under visible light irradiation. Rare Met. 2022, 41, 406–414. [Google Scholar] [CrossRef]
- Maaref, S.; Kantzas, A.; Bryant, S.L. The effect of silanization assisted nanoparticle hydrophobicity on emulsion stability through droplet size diatribution analysis. Chem. Eng. Sci. 2019, 201, 175–190. [Google Scholar] [CrossRef]
- Chen, F.; Zhao, L.; Yu, W.; Wang, Y.; Zhang, H.; Guo, L. Dynamic monitoring and regulation of pentachlorophenol photodegradation process by chemiluminescence and TiO2/PDA. J. Hazard. Mater. 2020, 399, 123073–123081. [Google Scholar] [CrossRef]
- Boussu, K.; De Baerdemaeker, J.; Dauwe, C.; Weber, M.; Lynn, K.G.; Depla, D.; Aldea, S.; Vankelecom, I.F.; Vandecasteele, C.; Van der Bruggen, B. Physico-chemical characterization of nanofiltration membranes. Chem. Phys. Chem. 2007, 8, 370–379. [Google Scholar] [CrossRef] [PubMed]
- Erbil, H.Y.; Cansoy, C.E. Range of applicability of the Wenzel and Cassie-Baxter equations for superhydrophobic surfaces. Langmuir 2009, 25, 14135–14145. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Li, J.; Qiu, J.; Sun, Y. Biomimetic nano/microfabrication techniques in multi-bioinspired superhydrophobic wood: New insight on theory, design and applications. Surf. Interfaces 2024, 48, 104217–104235. [Google Scholar] [CrossRef]
- Bormashenko, E. Why does the Cassie–Baxter equation apply? Colloids Surf. A 2008, 324, 47–50. [Google Scholar] [CrossRef]


| Feed | Inlet Temperature of the Feed (°C) | Inlet Temperature of the Permeate (°C) | Feed Velocity (m/s) | Permeate Velocity (m/s) |
|---|---|---|---|---|
| 35 g/L NaCl [28,36] | ~65 | ~15 | ~0.38 | ~0.38 |
| 35 g/L NaCl + 1.26 g/L CaCl2 + 1.61 g/L MgSO4 + 10 mg/L HA [28,36] | ||||
| 35 g/L NaCl + 1.26 g/L CaCl2 + 1.61 g/L MgSO4 + 10 mg/L HA + 0.4 mM SDS [37] | ||||
| 35 g/L NaCl + 1.26 g/L CaCl2 + 1.61 g/L MgSO4 + 10 mg/L HA + 0.20% mineral oil emulsion [37] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, W.; Zhu, X.; Li, B.; Hu, B.; Shen, L.; Meng, Y.; Gao, H. Fabrication of Biomimetical TiO2@PVDF Composite Membrane with Omniphobicity via In-Situ Growth and Its Anti-Fouling Performance. Coatings 2025, 15, 965. https://doi.org/10.3390/coatings15080965
Zhang W, Zhu X, Li B, Hu B, Shen L, Meng Y, Gao H. Fabrication of Biomimetical TiO2@PVDF Composite Membrane with Omniphobicity via In-Situ Growth and Its Anti-Fouling Performance. Coatings. 2025; 15(8):965. https://doi.org/10.3390/coatings15080965
Chicago/Turabian StyleZhang, Wei, Xuran Zhu, Baoan Li, Boyang Hu, Leyu Shen, Yanzong Meng, and Haifeng Gao. 2025. "Fabrication of Biomimetical TiO2@PVDF Composite Membrane with Omniphobicity via In-Situ Growth and Its Anti-Fouling Performance" Coatings 15, no. 8: 965. https://doi.org/10.3390/coatings15080965
APA StyleZhang, W., Zhu, X., Li, B., Hu, B., Shen, L., Meng, Y., & Gao, H. (2025). Fabrication of Biomimetical TiO2@PVDF Composite Membrane with Omniphobicity via In-Situ Growth and Its Anti-Fouling Performance. Coatings, 15(8), 965. https://doi.org/10.3390/coatings15080965







