Enhancement in Corrosion and Wear Resistance of FeCoNiCrAl High-Entropy Alloy Coating Through Dual Heat Treatment with 3:1 N2/H2 Atmosphere
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Materials and Coating Fabrication
2.2. Heat Treatment Method
2.3. Structural Characterization
2.4. Electrochemical Measurement
2.5. Tribological Property Assessments
3. Results
3.1. Structural Transformation
3.2. Electrochemical Corrosion Responses
3.2.1. EIS Responses
3.2.2. Analytical Standardization of EIS Data
3.2.3. Potentiodynamic Polarization Measurements
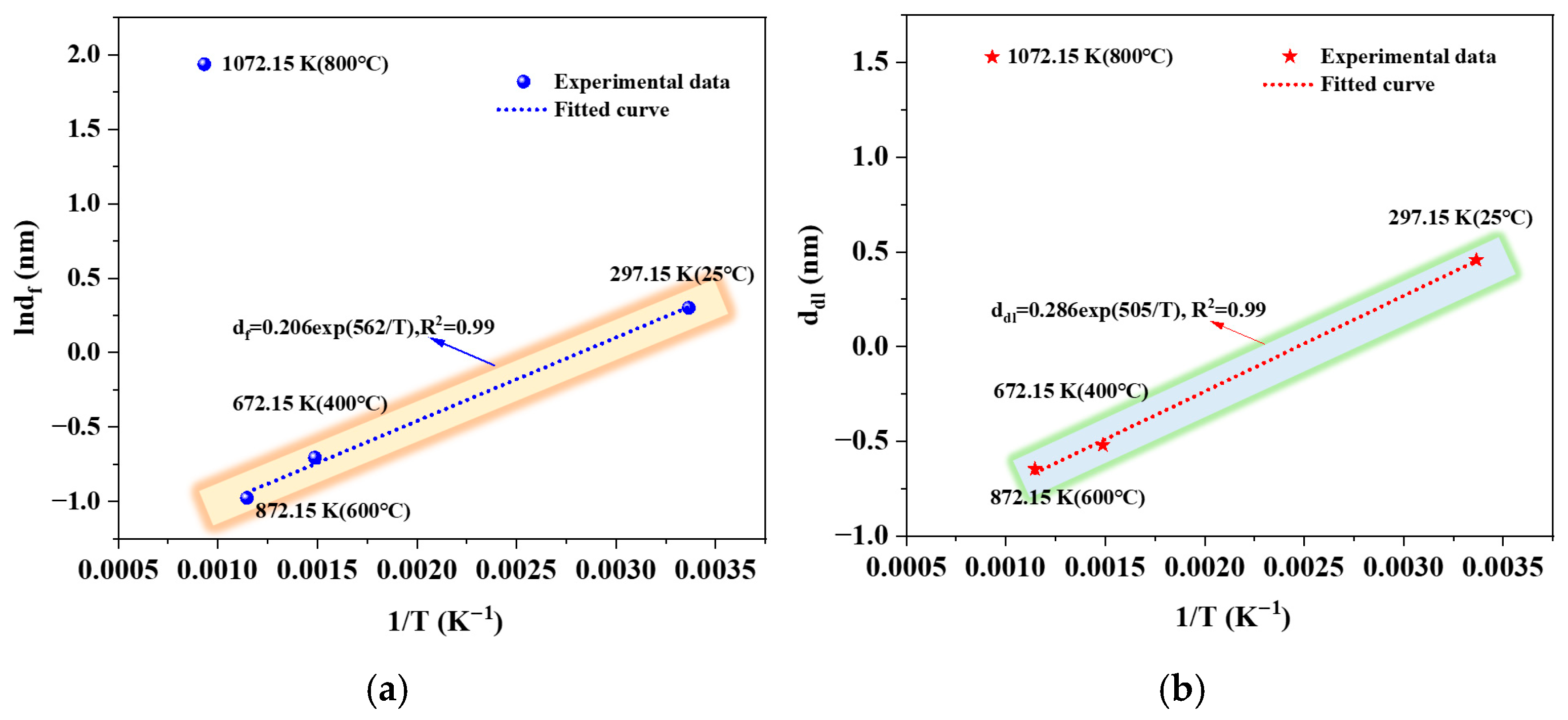
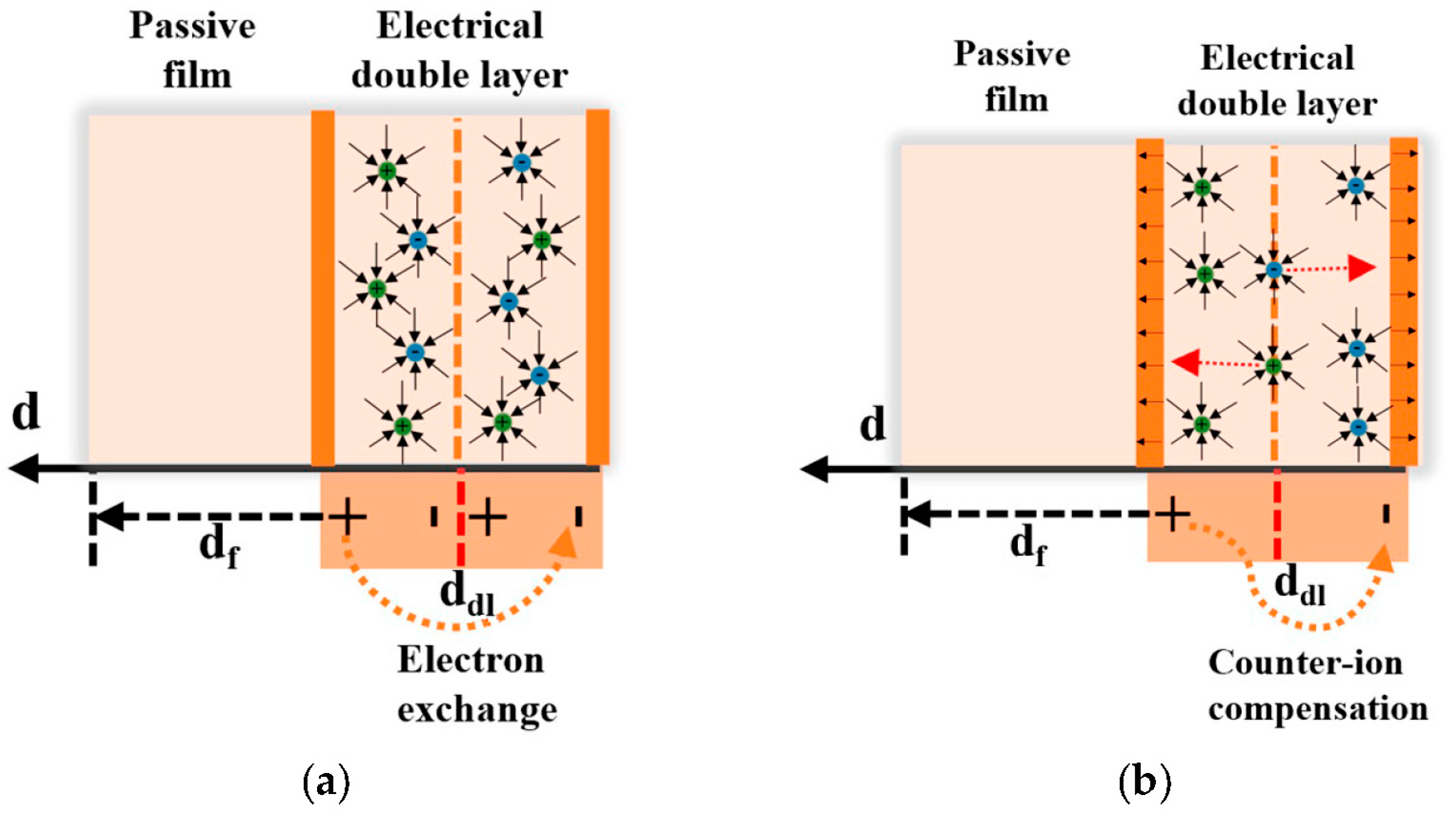
3.2.4. Effective Capacitance Model Analysis
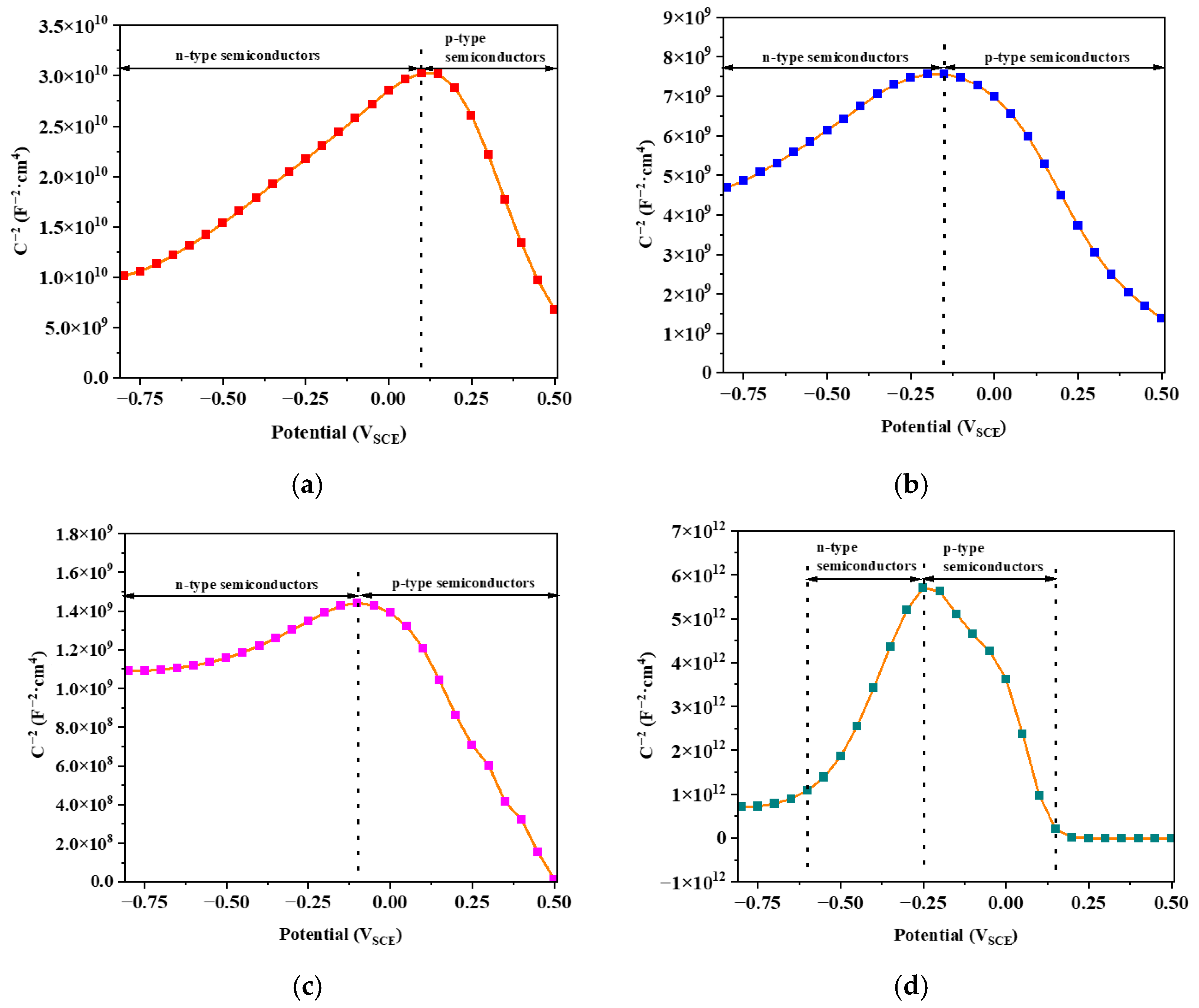
3.2.5. Semiconducting Performance
3.3. Tribological Properties
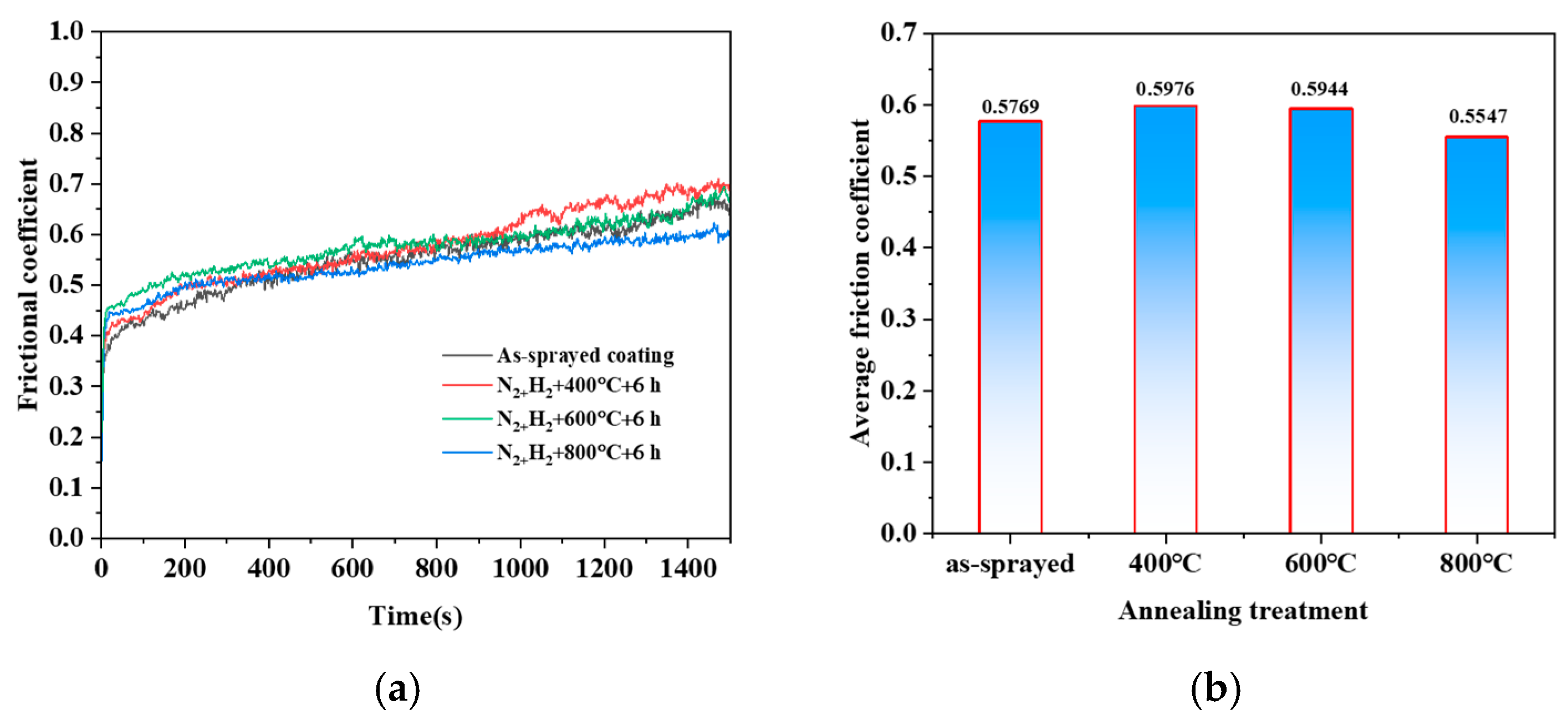
3.3.1. Frictional Coefficients
3.3.2. Worn Surfaces
4. Discussion
4.1. Molecular Orbital Theory-Based Analysis
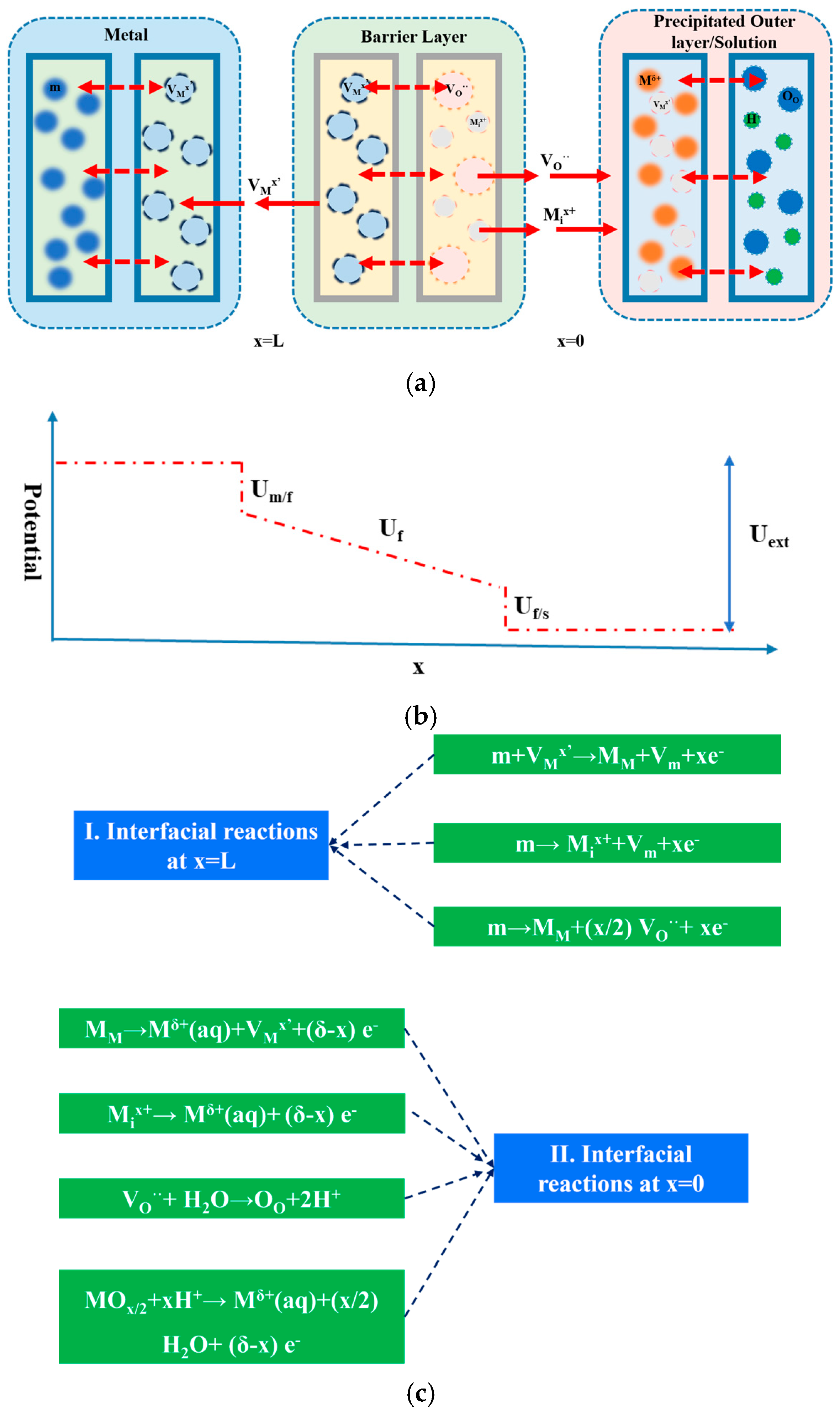
4.2. Point Defect Model (PDM) Elucidation
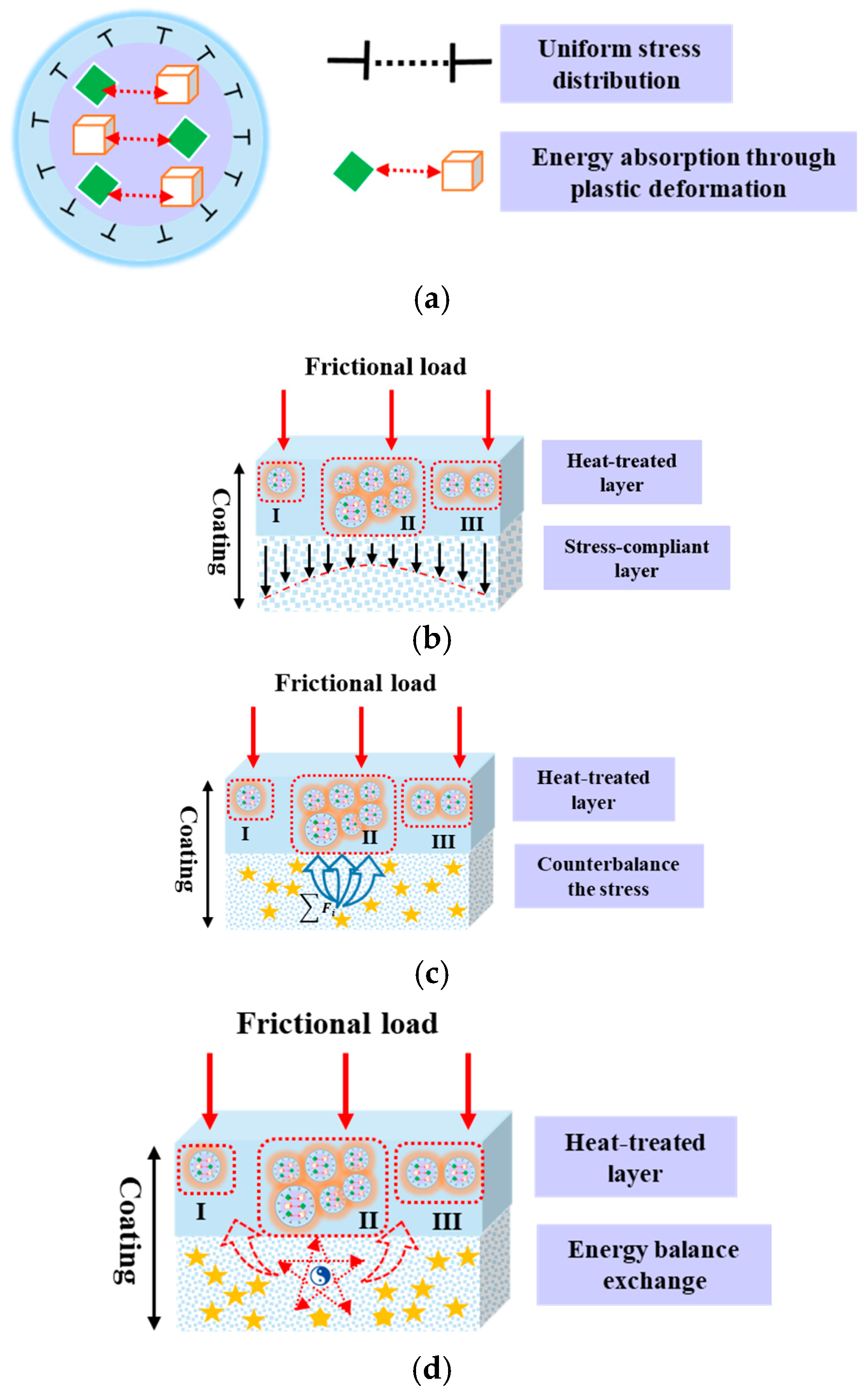
4.3. Wear Mechanisms
5. Conclusions
- (1)
- Annealing at 800 °C leads to the formation of a stable Fe0.64N0.36 nitride phase, which significantly enhances the properties of the FeCoNiCrAl coating.
- (2)
- Electrochemical assessments, guided by the point defect model (PDM) and effective capacitance model, reveal better corrosion resistance, characterized by a thicker passive film and enhanced thickness of the double-layer capacitance.
- (3)
- The formation of the nitride phase reduces internal stresses within the coating, contributing to improved wear performance, as evidenced by a lower friction coefficient and a smoother worn surface morphology.
- (4)
- Nitriding FeCoNiCrAl HEA coatings at 800 °C in a nitrogen–hydrogen atmosphere forms a heat-treated layer and stress-compliant layer that work synergistically to evenly distribute internal stress and efficiently dissipate frictional loads, thereby enhancing wear resistance and preventing premature failure.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kumar, S. Comprehensive review on high entropy alloy-based coating. Surf. Coat. Technol. 2024, 477, 130327. [Google Scholar] [CrossRef]
- Tao, X.W.; Zhang, Z.J.; Zhang, B.S.; Zhu, S.S.; Fan, Y.X.; Tian, H.L. Plasma sprayed CoNiCrMoNb(BSi) high-entropy amorphous alloy coating: The effect of spraying power on microstructure, mechanical and tribological properties. Mater. Chem. Phys. 2024, 314, 128887. [Google Scholar] [CrossRef]
- Boakye, G.B.; Straume, E.O.; Gunnarsson, B.G.; Kovalov, D.; Karlsdottir, S.N. Corrosion behaviour of HVOF developed Mo-based high entropy alloy coating and selected hard coatings for high temperature geothermal applications. Mater. Des. 2023, 235, 112431. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, S.D.; Sun, S.L.; Zhao, L.; Wu, L.P.; Ang, A.S.M. Study on the semiconductor properties of high entropy alloy FeCoNiCrAl coating prepared by air thermal spraying in 5% NaCl solution. Mater. Lett. 2024, 357, 135738. [Google Scholar] [CrossRef]
- Zhu, R.; Li, Y.P.; Sun, Y.M.; Feng, J.C.; Gong, W.B. Microstructure and properties of FeCoNiCrAl high-entropy alloy particle-reinforced Cu-matrix composites prepared via FSP. J. Alloys Compd. 2023, 940, 168906. [Google Scholar] [CrossRef]
- Rong, Z.Y.; Wang, C.H.; Wang, Y.; Dong, M.L.; You, Y.; Wang, J.N.; Liu, H.N.; Liu, J.Q.; Wang, Y.H.; Zhu, Z.Y. Microstructure and properties of FeCoNiCrX (X=Mn, Al) high-entropy alloy coatings. J. Alloys Compd. 2022, 921, 166061. [Google Scholar] [CrossRef]
- Gao, S.; Zhou, B.W.; Liu, Y.F.; Teng, D.; Xie, Y.X.; Zhang, X.G. Effect of heat treatment on mechanical properties, wear and corrosion resistance of HVAF sprayed FeCoNiCrMo high-entropy alloy coating. Mater. Charact. 2025, 226, 115199. [Google Scholar] [CrossRef]
- Ma, Y.L.; Wang, X.Y.; Li, Z.; Zhang, J.J.; Zhang, J. Effect of heat treatment on the interfacial element diffusion and hardness of FeCoNiCrAl high-entropy alloy coatings. Intermetallics 2025, 176, 108581. [Google Scholar] [CrossRef]
- Yu, B.X.; Ren, Y.S.; Zeng, Y.; Ma, W.H.; Morita, K.; Zhan, S.; Lei, Y.; Lv, G.Q.; Li, S.Y.; Wu, J.J. Recent progress in high-entropy alloys: A focused review of preparation processes and properties. J. Mater. Sci. Technol. 2024, 29, 2689–2719. [Google Scholar] [CrossRef]
- Wang, X.W.; Huang, B.S.; Li, T.N.; Wu, Y.Q.; Hong, X.L.; Zheng, J.N.; Zhu, Y.Y. Effect of heat treatment on microstructure and properties of CrMnFeCoNiMo high entropy alloy coating. J. Mater. Sci. Technol. 2024, 29, 311–322. [Google Scholar] [CrossRef]
- Sathish, M.; Radhika, N.; Saleh, B. Microstructure and dry sliding wear evaluation of functionally graded coating deposited via atmospheric plasma spray. Sci. Rep. 2024, 14, 22272. [Google Scholar] [CrossRef]
- Chen, W.; Hilhorst, A.; Bokas, G.; Gorsse, S.; Jacques, P.J.; Hautier, G. A map of single-phase high-entropy alloys. Nat. Commun. 2023, 14, 2856. [Google Scholar] [CrossRef]
- Baruffi, B.; Curtin, C.A. Theory of spontaneous grain boundary roughening in high entropy alloys. Acta Mater. 2022, 234, 118011. [Google Scholar] [CrossRef]
- Yang, Y.F.; Hu, F.; Xia, T.; Li, R.H.; Bai, J.Y.; Zhu, J.Q.; Xu, J.Y.; Zhang, G.F. High entropy alloys: A review of preparation techniques, properties and industry applications. J. Alloys Compd. 2025, 1010, 177691. [Google Scholar] [CrossRef]
- Zhang, Z.Z.; Wang, Y.S.; Chen, L.X.; Liang, X.L. Tribological behaviors of FeCoNiCrAlx sliding against Si3N4 ceramics under high temperature condition. Ceram. Int. 2023, 49, 20480–20494. [Google Scholar] [CrossRef]
- Basem, A.; Hassan, M.A.; Elkady, O.A.; El-Shekeil, Y.A.; Bendoukha, S.; Barhoumi, N.; Refaey, H.A.; Elsheikh, A. Characterization of FeCoNiCr high-entropy alloys manufactured by powder metallurgy technique. J. Mater. Sci. Technol. 2024, 30, 88–100. [Google Scholar] [CrossRef]
- Hsu, W.L.; Tsai, C.T.; Yeh, A.C.; Yeh, J.W. Clarifying the four core effects of high-entropy materials. Nat. Rev. Chem. 2024, 8, 471–485. [Google Scholar] [CrossRef]
- Verma, V.; Belcher, C.H.; Apelian, D.; Lavernia, E.J. Diffusion in High Entropy Alloy Systems-A Review. Prog. Mater. Sci. 2024, 142, 101245. [Google Scholar] [CrossRef]
- Wang, H.; Yang, P.Y.; Zhao, W.J.; Ma, S.H.; Hou, J.H.; He, Q.F.; Wu, C.L.; Chen, H.A.; Wang, Q.; Cheng, Q.; et al. Lattice distortion enabling enhanced strength and plasticity in high entropy intermetallic alloy. Nat. Commun. 2024, 15, 6782. [Google Scholar] [CrossRef] [PubMed]
- Feng, H.; Han, Y.; Li, H.B.; Tian, Y.Z.; Zhu, H.C.; Jiang, Z.H.; He, T.; Zhou, G. Enhancement in impact toughness of CoCrFeMnNi high-entropy alloy via nitrogen addition. J. Alloys Compd. 2023, 932, 167615. [Google Scholar] [CrossRef]
- Xing, Q.; Huang, T.H.; Kong, D.H.; Wang, T.; Zhai, R.X.; He, X.; Yi, J.H.; Song, P. Sintering densification behavior of nano-ITO powder with high oxygen vacancy induced via plasma stimulation. Ceram. Int. 2024, 50, 22922–22935. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, Q.; Zhen, Y.Z.; Xu, F.H.; Ma, Z.; Gao, L.H.; Liu, Y.B.; Liu, L.; Tian, X.C. Oxygen vacancy induced superhydrophobicity of air plasma spraying deposited Y2O3 coatings. J. Eur. Ceram. Soc. 2025, 45, 116871. [Google Scholar] [CrossRef]
- Wu, Y.; Xiao, S.; Chen, Y.N.; Dong, W.L.; Liu, J.C.; Huang, Y.; Shi, K.J.; Fan, S.Y.; Ye, Z.S.; Tang, G.L.; et al. Oxygen plasma-assisted magnetron sputtering deposition of non-stoichiometric Y2O3 films: Influence of oxygen vacancies on etching resistance. Surf. Coat. Technol. 2024, 494, 131448. [Google Scholar] [CrossRef]
- Myhr, O.R.; Marioara, C.D.; Engler, O. Modeling the Effect of Excess Vacancies on Precipitation and Mechanical Properties of Al-Mg-Si Alloys. Metall. Mater. Trans. A 2023, 55, 291–302. [Google Scholar] [CrossRef]
- Xia, D.H.; Deng, C.M.; Macdonald, D.; Jamali, S.; Mills, D.; Luo, J.L.; Strebl, M.G.; Amiri, M.; Jin, W.X.; Song, S.Z.; et al. Electrochemical measurements used for assessment of corrosion and protection of metallic materials in the field: A critical review. J. Mater. Sci. Technol. 2022, 112, 151–183. [Google Scholar] [CrossRef]
- Zhao, C.K.; Ding, Z.Y.; Zhang, K.Y.; Du, Z.T.; Fang, H.Q.; Chen, L.; Jiang, H.; Wang, M.; Wu, M.B. Comprehensive Chlorine Suppression: Advances in Materials and System Technologies for Direct Seawater Electrolysis. Nano-Micro Lett. 2025, 17, 113. [Google Scholar] [CrossRef]
- Wang, H.J.; Feng, H.; Li, H.B.; Zhang, S.C.; Zhu, H.J.; Jiao, W.C.; Jiang, Z.H. Thermal stability and softening mechanism of a new type of high nitrogen hot-work die steel 3Cr5Mo2SiVN. Mater. Charact. 2024, 210, 113789. [Google Scholar] [CrossRef]
- Bairagi, D.; Duley, P.; Paliwal, M.; Mandal, S. Influence of second phase precipitates on mechanical and in-vitro corrosion behaviour of Mg-4Zn-0.5Ca-0.8Mn alloy in optimum homogenized conditions. J. Magnes. Alloys 2023, 11, 1343–1366. [Google Scholar] [CrossRef]
- Wang, M.Q.; Zhou, Z.H.; Wang, Q.J.; Liu, Y.Y.; Wang, Z.H.; Zhang, X. Box-Behnken design to enhance the corrosion resistance of plasma sprayed Fe-based amorphous coating. Results Phys. 2019, 15, 102708. [Google Scholar] [CrossRef]
- Ravishankar, S.; Bisquert, J.; Kirchartz, T. Interpretation of Mott–Schottky plots of photoanodes for water splitting. Chem. Sci. 2022, 13, 4828–4837. [Google Scholar] [CrossRef]
- Wu, L.T.; Zhou, Z.H.; Zhang, K.C.; Zhang, X.; Wang, G.Y. Electrochemical and passive film evaluation on the corrosion resistance variation of Fe-based amorphous coating affected by high temperature. J. Non-Cryst. Solids 2022, 597, 121892. [Google Scholar] [CrossRef]
- Wang, X.X.; Yu, X.H.; Jin, M.; Zhou, Y.C.; Liu, J.P. Cations effect on the passive film for carbon steels in concrete simulated pore solutions. J. Build. Eng. 2024, 94, 109945. [Google Scholar] [CrossRef]
- Wu, Y.Y.; Jiang, J.Y.; Sun, Q.; An, Y.Y.; Zhao, R.; Zheng, H.L.; Li, H. Efficient removal of both positively and negatively charged colloidal contaminants using amphoteric starch-based flocculants synthesized by low-pressure UV initiation. Sep. Purif. Technol. 2022, 282, 120120. [Google Scholar] [CrossRef]
- Veluchamy, A.; Sherwood, D.; Emmanuel, B.; Cole, I.S. Critical review on the passive film formation and breakdown on iron electrode and the models for the mechanisms underlying passivity. J. Electroanal. Chem. 2017, 785, 186–215. [Google Scholar] [CrossRef]
- Xu, J.; Peng, S.; Li, Z.Y.; Jiang, S.Y.; Xie, Z.H.; Munroe, P. The influence of semiconducting properties of passive films on the cavitation erosion resistance of a NbN nanoceramic coating. Ultrason. Sonochem. 2021, 71, 105406. [Google Scholar] [CrossRef] [PubMed]
- Bösing, I. Modeling electrochemical oxide film growth-passive and transpassive behavior of iron electrodes in halide-free solution. NPJ Mater. Degrad. 2023, 7, 53. [Google Scholar] [CrossRef]
- Supekar, R.; Nair, R.B.; McDonald, A.; Stoyanov, P. Sliding wear behavior of high entropy alloy coatings deposited through cold spraying and flame spraying: A comparative assessment. Wear 2023, 516–517, 204596. [Google Scholar] [CrossRef]
- Zhou, Y.; Chen, Z.X.; Jiang, Y.H.; Wang, Y.; Wang, Z.Y.; You, S.H.; Song, Z.H.; Wu, C.; Ren, X.; Meng, C. Study on the microstructure, wear and corrosion resistance of CoCrFeNiMn high-entropy alloy coating via ultrasonic impact treatment. Surf. Coat. Technol. 2025, 512, 132329. [Google Scholar] [CrossRef]
- Gu, J.; Sun, Y.N.; Cheng, W.J.; Chong, Z.Z.; Ma, X.F.; Huang, L.F.; Zhang, S.L.; Chen, Y.F. Microstructure and wear resistance of multi-layer graphene doped AlCoCrFeNi2.1 high-entropy alloy self-lubricating coating prepared by laser cladding. Intermetallics 2025, 176, 108578. [Google Scholar] [CrossRef]
- Chang, C.C.; Hsiao, Y.T.; Chen, Y.L.; Tsai, C.Y.; Lee, Y.J.; Ko, P.H.; Chang, S.Y. Lattice distortion or cocktail effect dominates the performance of Tantalum-based high-entropy nitride coatings. Appl. Surf. Sci. 2022, 577, 151894. [Google Scholar] [CrossRef]
- Sun, X.; Xu, H.X.; Cao, Y.J.; Deng, J.S.; Kang, Y.T.; Wang, J.Z.; Cui, J.H. Progress in the design of molecular structure of amino acid corrosion inhibitors based on molecular orbital theory and their mechanisms: A review. J. Mol. Liq. 2025, 427, 127374. [Google Scholar] [CrossRef]
- Riccardi, P.; Dukes, C.A. Excitation of the triplet 2p4(3P)3s2 autoionizing state of Neon by molecular orbital electron promotion at solid surfaces. Chem. Phys. Lett. 2022, 798, 139610. [Google Scholar] [CrossRef]
- Li, Y.H.; Gao, S.; Zhang, L.; Chen, H.C.; Wang, C.C.; Ji, H.D. Insights on selective Pb adsorption via O 2p orbit in UiO-66 containing rich-zirconium vacancies. Chin. Chem. Lett. 2024, 35, 109894. [Google Scholar] [CrossRef]
- Chen, E.X.; He, L.; Qiu, M.; Zhang, Y.F.; Sun, Y.Y.; Li, W.H.; Xiao, J.Z.; Chen, J.; Xu, G.; Lin, Q.P. Regulating electron transfer and orbital interaction within metalloporphyrin-MOFs for highly sensitive NO2 sensing. Chem. Sci. 2024, 15, 6833–6841. [Google Scholar] [CrossRef]
- Kim, J.U.; Lim, E.S.; Park, J.Y.; Jung, D.; Lee, S. Radical approaches for C(sp3)–N bond cleavage in deaminative transformations. Chem. Commun. 2025, 61, 6997–7008. [Google Scholar] [CrossRef]
- Wen, S.F.; Gong, B.S.; Gong, K.; Mao, F.X. Study on growth kinetics of passivation film of Ti-6Al-3Nb-2Zr-1Mo alloy Based on point defect model. J. Electroanal. Chem. 2024, 973, 118660. [Google Scholar] [CrossRef]
- Zhao, T.Y.; Chen, S.; Qiu, J.; Sun, L.; Macdonald, D.D. Study on the passivation properties of austenitic stainless steel 316LN based on the point defect model. Corros. Sci. 2024, 237, 112293. [Google Scholar] [CrossRef]
- Chen, D.H.; Dong, C.F.; Engelhardt, G.R.; Qiu, J.; Macdonald, D.D. Determination of kinetic parameters in the point defect model (PDM) for iron using electrochemical impedance spectroscopy and first-principles calculations. Corros. Sci. 2025, 248, 112779. [Google Scholar] [CrossRef]
- Kolotinskii, D.A.; Nikolaev, V.S.; Stegailov, V.V.; Timofeev, A.V. Point Defect Model for the kinetics of oxide film growth on the surface of T91 steel in contact with lead-bismuth eutectic. Corros. Sci. 2023, 211, 110829. [Google Scholar] [CrossRef]
- Li, Y.H.; Macdonald, D.D.; Yang, J.; Qiu, J.; Wang, S.Z. Point defect model for the corrosion of steels in supercritical water: Part I, film growth kinetics. Corros. Sci. 2020, 163, 108280. [Google Scholar] [CrossRef]
- Zhang, H.F.; Zhang, X.; Zhang, J.; Pan, M.M. Vacuum plasma sprayed AlCoCrFeNi high-entropy alloy bond Coating: Vacuum heat-treatment, microstructure evolution and oxidation behavior analysis. Intermetallics 2025, 184, 108836. [Google Scholar] [CrossRef]
- Tunalioğlu, M.Ş.; Tuç, B. Theoretical and experimental investigation of wear in internal gears. Wear 2014, 309, 208–215. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, W.F.; Peng, J.; Wang, A.; Fan, W.J.; Li, J. Unveiling wear property and multiscale tribological mechanism of laser cladding-nitriding synergistically enhanced high-entropy alloy coatings. Appl. Surf. Sci. 2025, 710, 163878. [Google Scholar] [CrossRef]
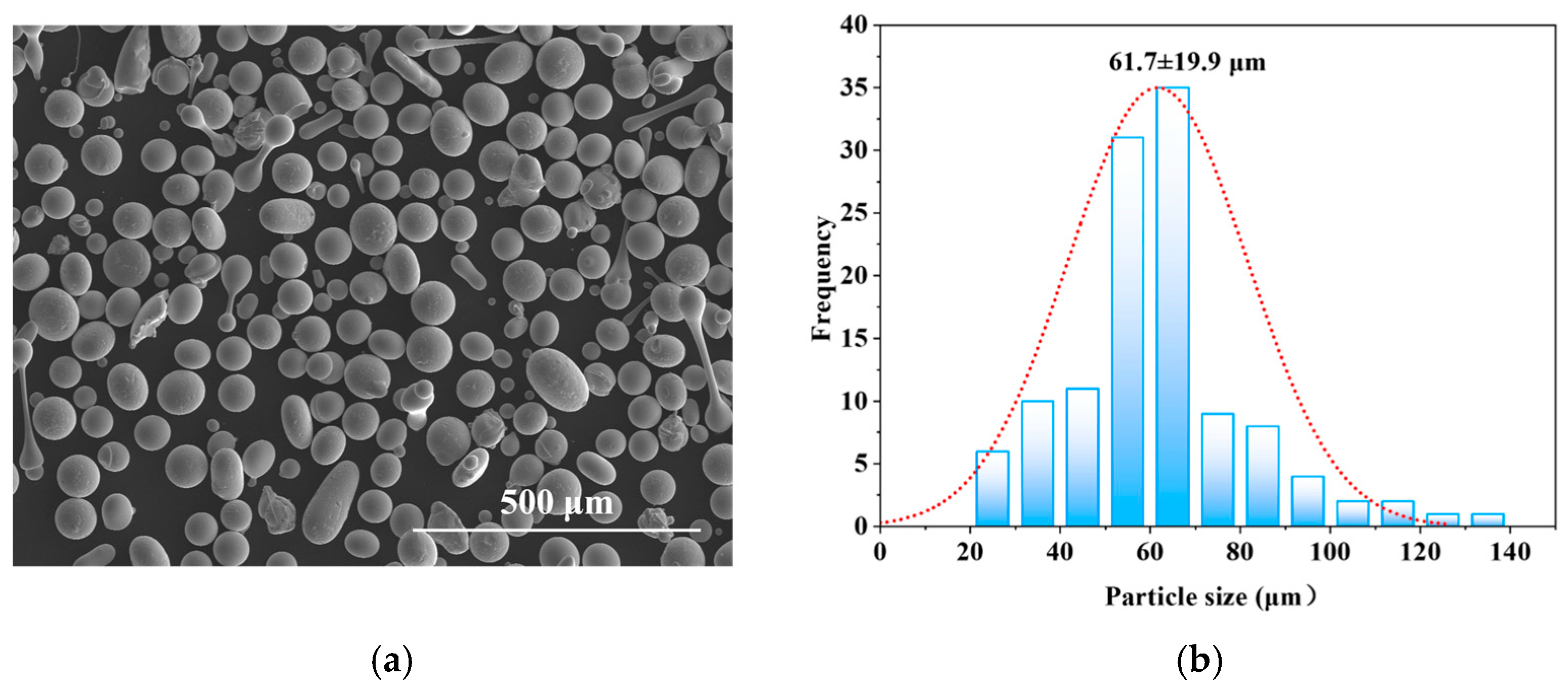
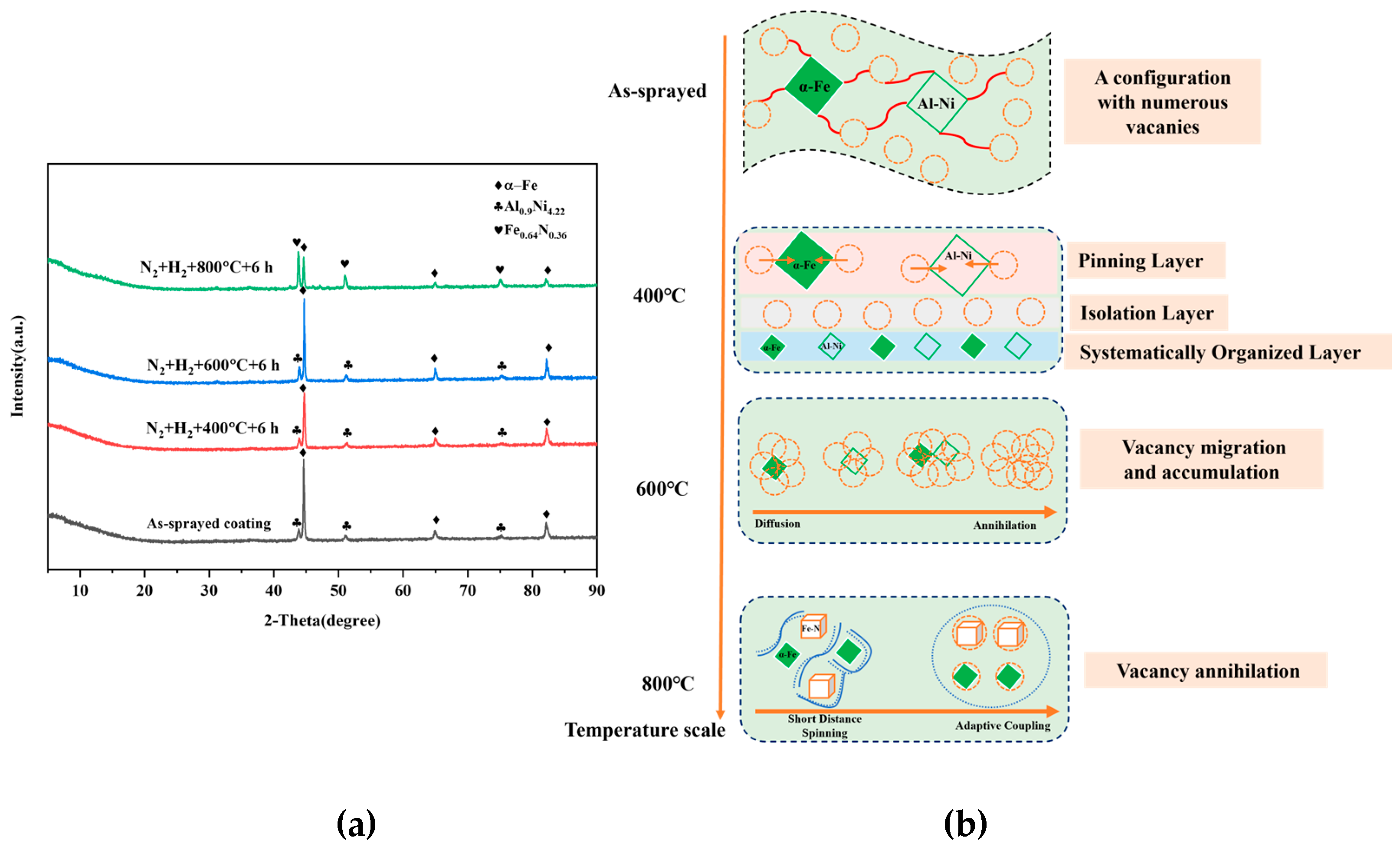
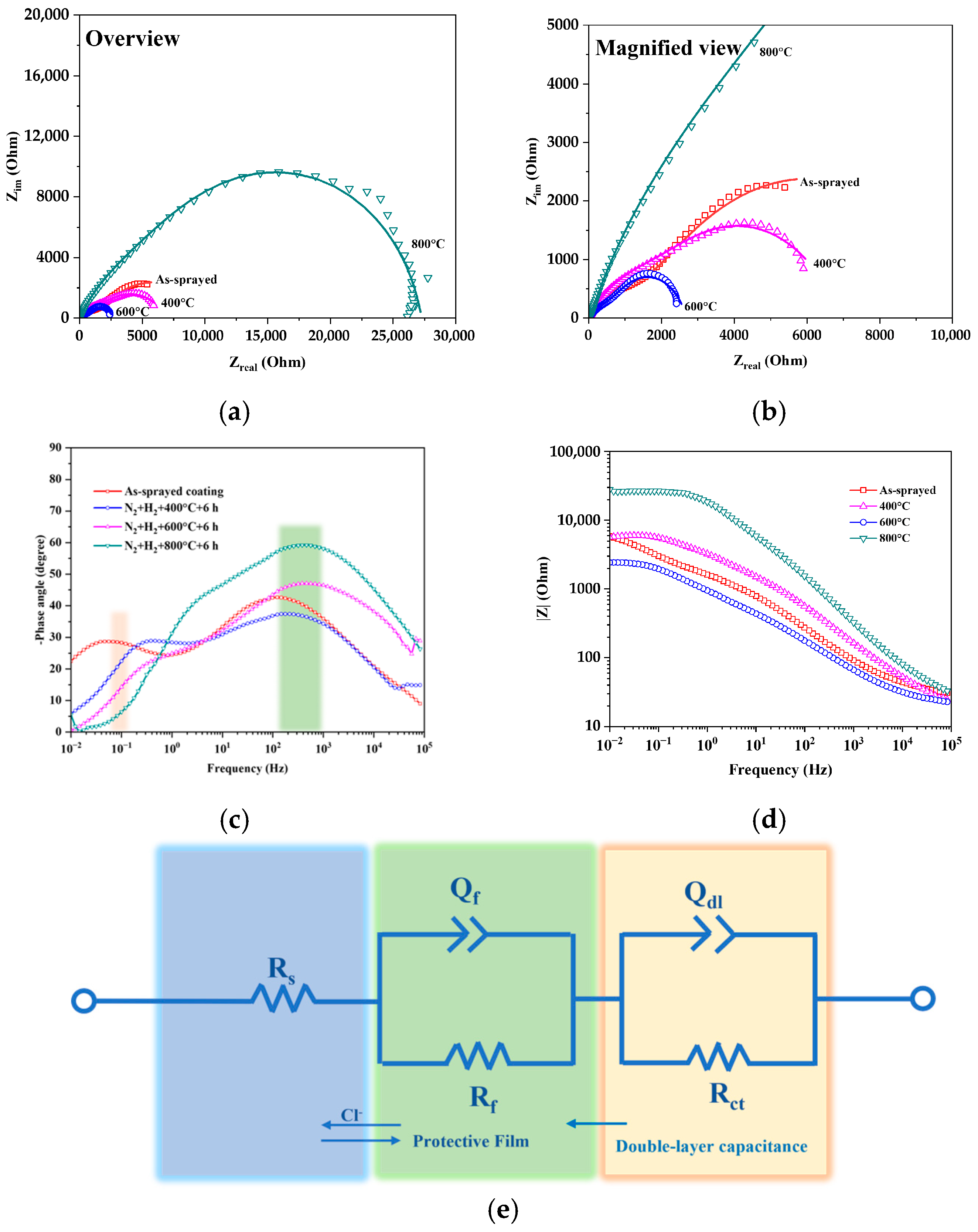

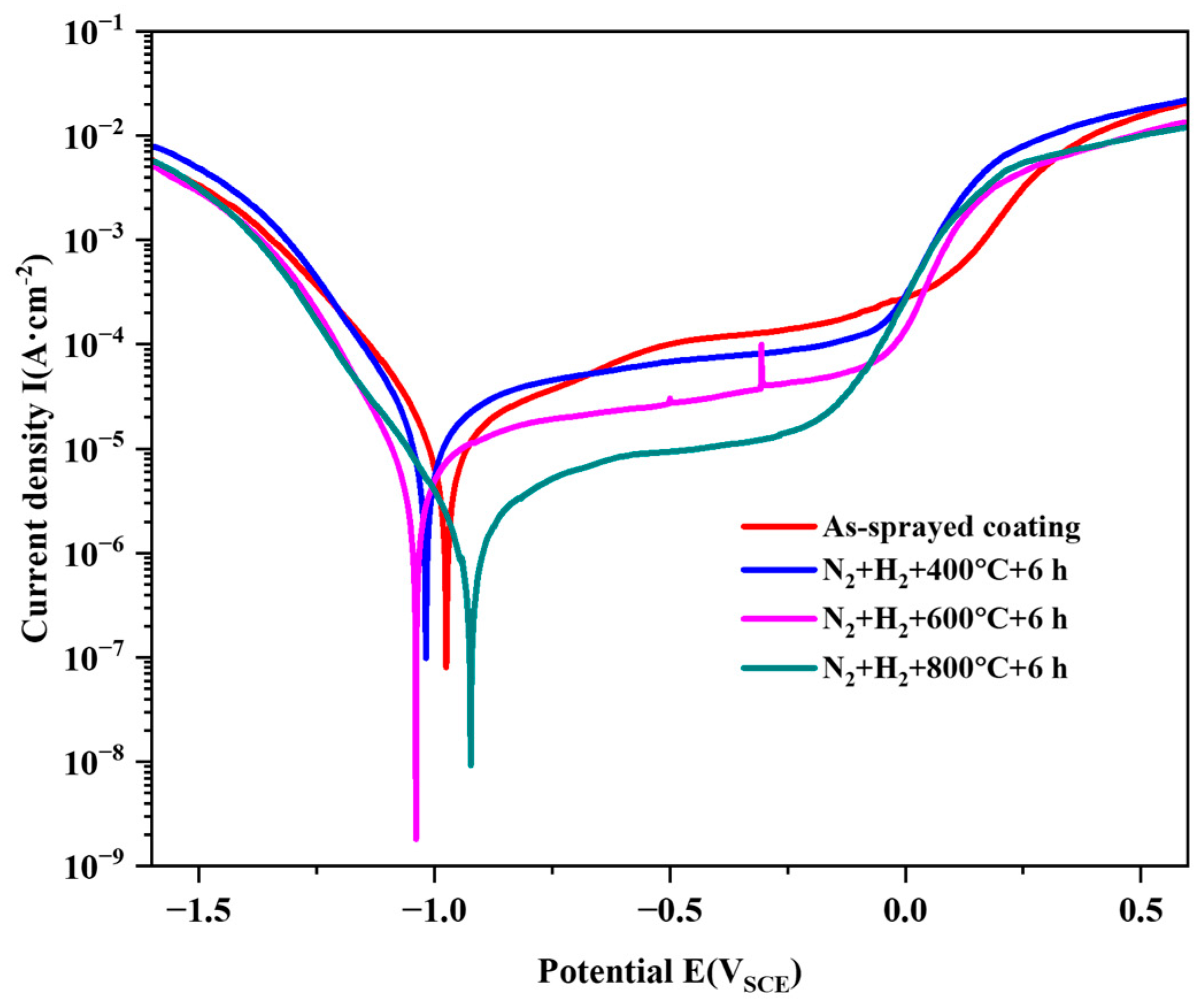
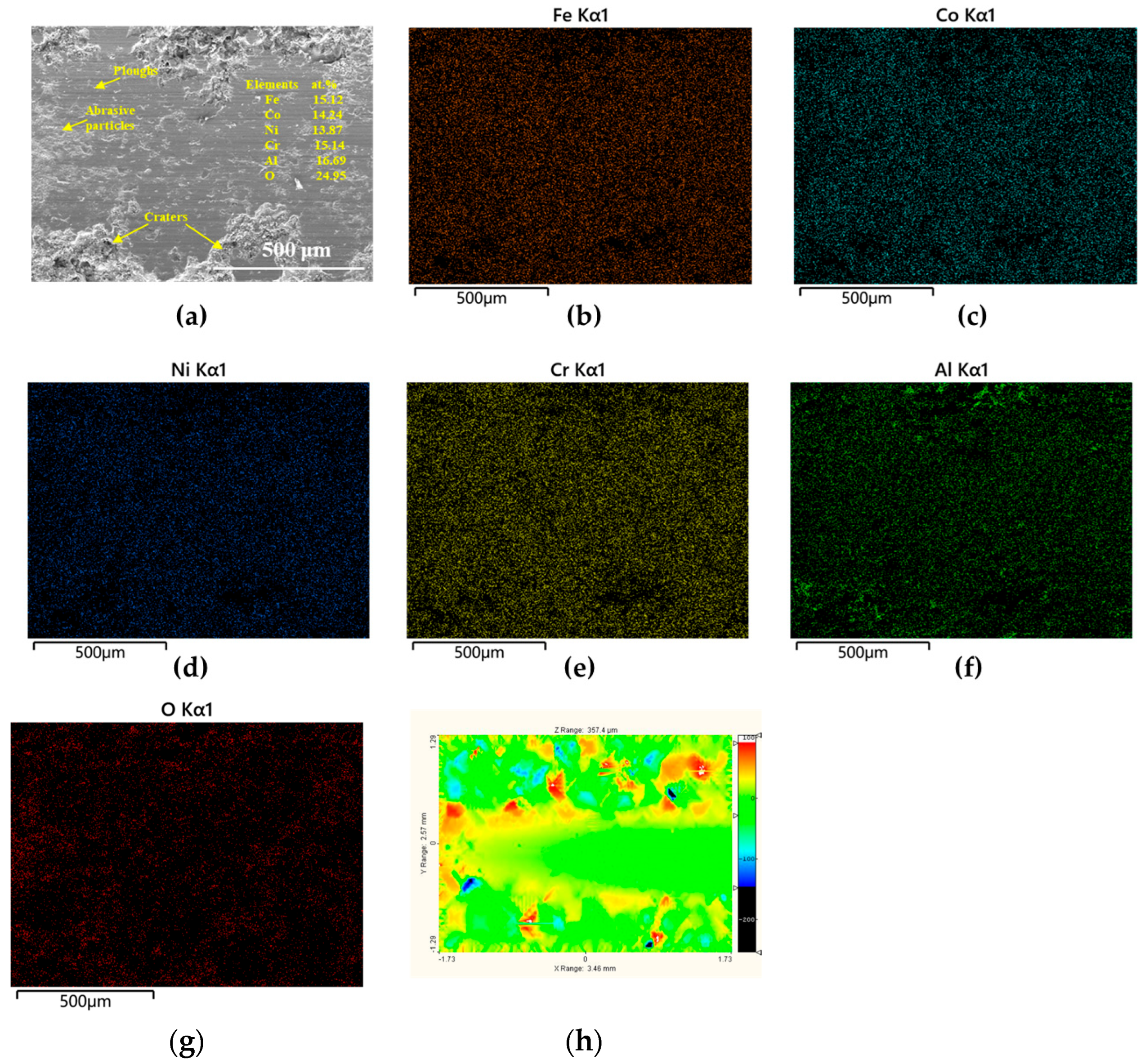
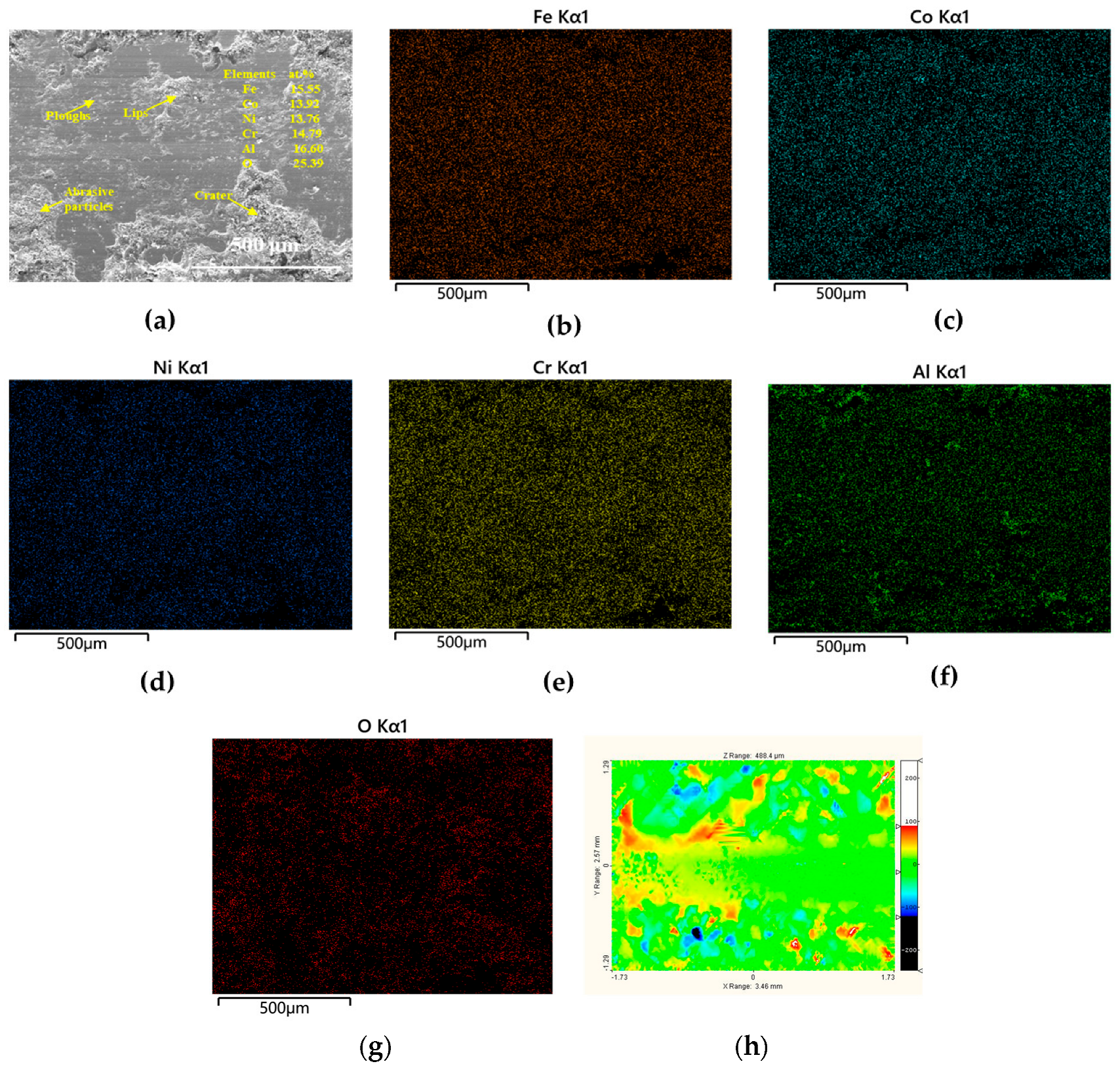
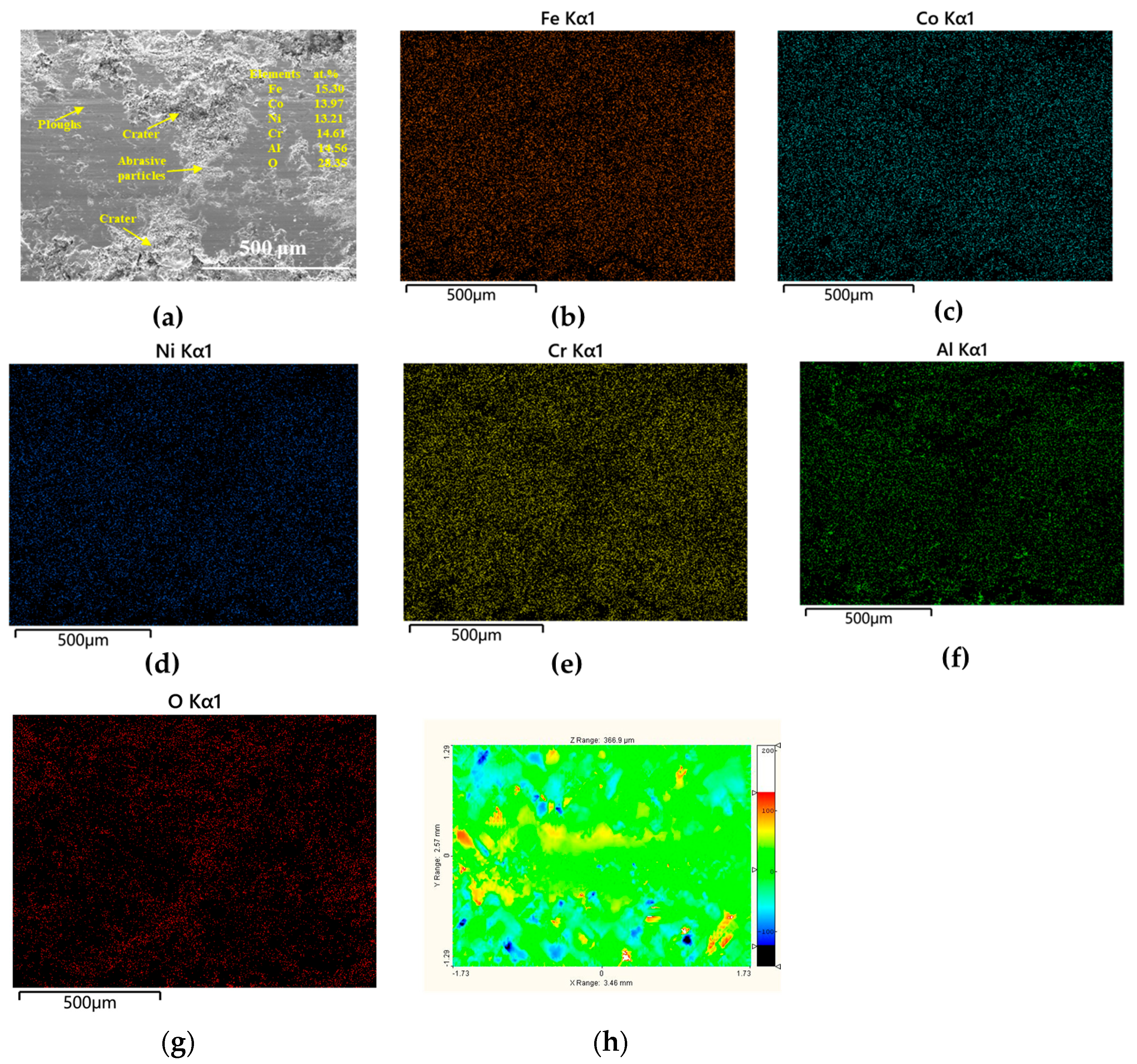
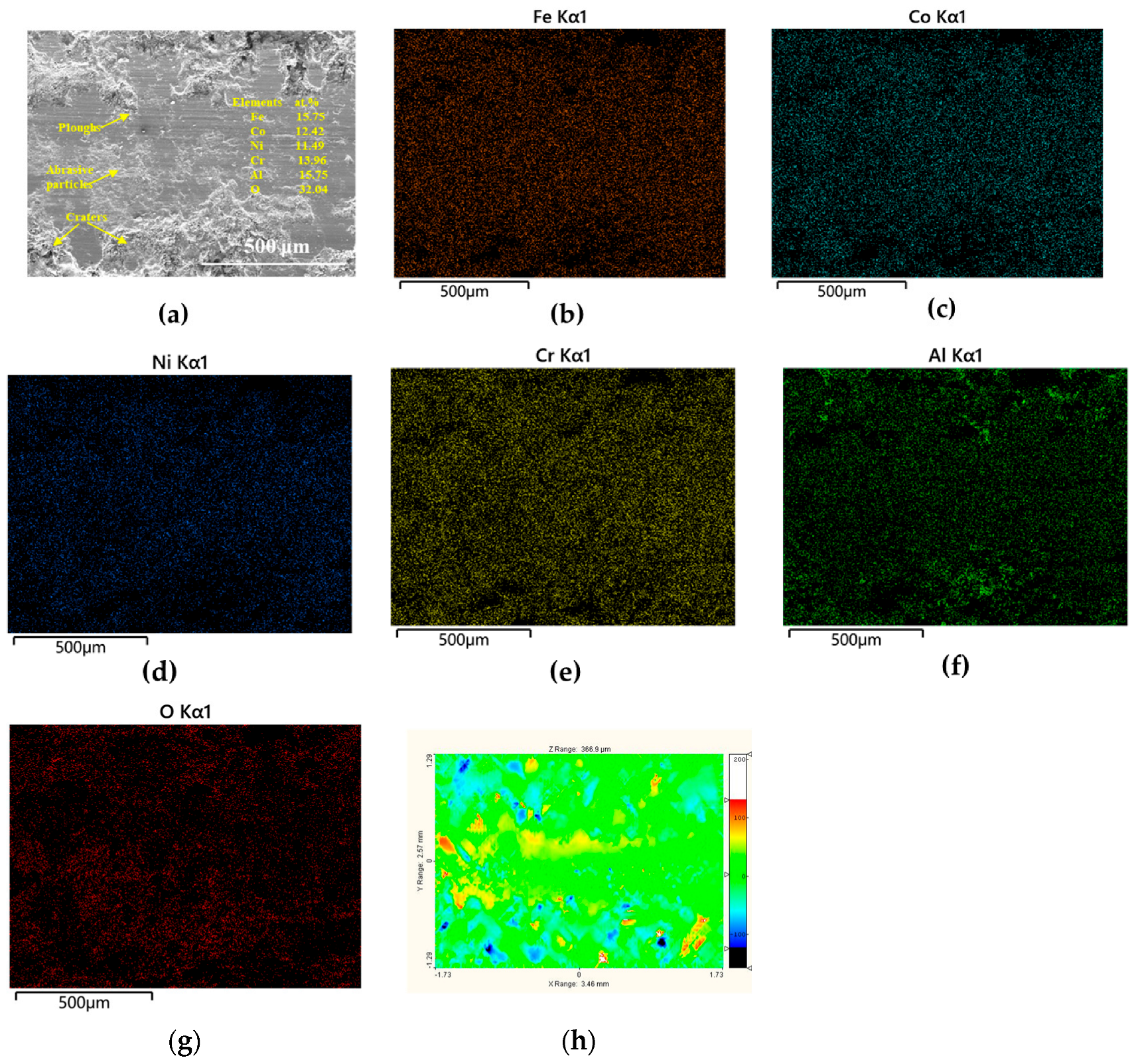

| Tailored Parameters | Input Values |
|---|---|
| Supplied power (kW) | 46.1 |
| Primary gas supply rate (L/min) | 45.4 |
| Secondary gas supply rate (L/min) | 24.0 |
| Carrier gas supply rate (L/min) | 7.5 |
| Cross-traversal velocity (mm/s) | 140 |
| Coating passes | 3 |
| Temperature (°C) | Phase Composition (wt.%) | ||
|---|---|---|---|
| α-Fe | Al0.9Ni4.22 | Fe0.64N0.36 | |
| 25 | 90.91 | 9.09 | / |
| 400 | 91.20 | 8.80 | / |
| 600 | 82.27 | 17.73 | / |
| 800 | 44.54 | / | 55.46 |
| Temperature (°C) | Rs (Ω·cm2) | Qf (Ω−1·cm−2·sn1) | Rf (Ω·cm2) | n1 | df (nm) | Qdl (Ω−1·cm−2·sn2) | Rct (Ω·cm2) | n2 | ddl (nm) |
|---|---|---|---|---|---|---|---|---|---|
| 25 | 25.46 | 0.0001960 | 3415 | 0.78 | 1.35 | 0.0001676 | 2790 | 0.78 | 1.58 |
| 400 | 18.88 | 0.0006180 | 1878 | 0.77 | 0.49 | 0.0007926 | 1741 | 0.74 | 0.59 |
| 600 | 16.48 | 0.001082 | 779.3 | 0.75 | 0.38 | 0.001041 | 796.2 | 0.73 | 0.52 |
| 800 | 23.51 | 0.0000943 | 8073 | 0.88 | 6.93 | 0.00001079 | 19,190 | 0.90 | 4.61 |
| Temperature (°C) | Ecorr (mV) | Icorr (μA·cm−2) | Epit (mV) | Ipass (μA·cm−2) | Rp (Ω·cm2) |
|---|---|---|---|---|---|
| 25 | −975 | 9.71 | 81.9 | 380 | 3876 |
| 400 | −1018 | 12.6 | −53.5 | 257 | 3008 |
| 600 | −1039 | 5.39 | −45.0 | 93.3 | 6782 |
| 800 | −923 | 1.64 | −183.4 | 33.3 | 24,206 |
| Temperature (°C) | Donor Density (cm−3) | Acceptor Density (cm−3) |
|---|---|---|
| 25 | 3.41 × 1020 | 2.13 × 1020 |
| 400 | 1.08 × 1021 | 1.37 × 1021 |
| 600 | 6.39 × 1021 | 6.56 × 1021 |
| 800 | 9.86 × 1017 | 3.15 × 1018 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, M.; Li, B.; He, C.; Sun, J.; Li, L.; Liu, A.; Shi, F. Enhancement in Corrosion and Wear Resistance of FeCoNiCrAl High-Entropy Alloy Coating Through Dual Heat Treatment with 3:1 N2/H2 Atmosphere. Coatings 2025, 15, 986. https://doi.org/10.3390/coatings15090986
Wang M, Li B, He C, Sun J, Li L, Liu A, Shi F. Enhancement in Corrosion and Wear Resistance of FeCoNiCrAl High-Entropy Alloy Coating Through Dual Heat Treatment with 3:1 N2/H2 Atmosphere. Coatings. 2025; 15(9):986. https://doi.org/10.3390/coatings15090986
Chicago/Turabian StyleWang, Miqi, Buxiang Li, Chi He, Jing Sun, Liyuan Li, Aihui Liu, and Fang Shi. 2025. "Enhancement in Corrosion and Wear Resistance of FeCoNiCrAl High-Entropy Alloy Coating Through Dual Heat Treatment with 3:1 N2/H2 Atmosphere" Coatings 15, no. 9: 986. https://doi.org/10.3390/coatings15090986
APA StyleWang, M., Li, B., He, C., Sun, J., Li, L., Liu, A., & Shi, F. (2025). Enhancement in Corrosion and Wear Resistance of FeCoNiCrAl High-Entropy Alloy Coating Through Dual Heat Treatment with 3:1 N2/H2 Atmosphere. Coatings, 15(9), 986. https://doi.org/10.3390/coatings15090986






