Preparation of High Self-Healing Diels–Alder (DA) Synthetic Resin and Its Influence on the Surface Coating Properties of Poplar Wood and Glass
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Self-Healing Coating Preparation Method
2.3. Preparation of DA Polymers and Semi-IPN Structured Polymers and Coating Production
2.4. Characterization
3. Results and Discussion
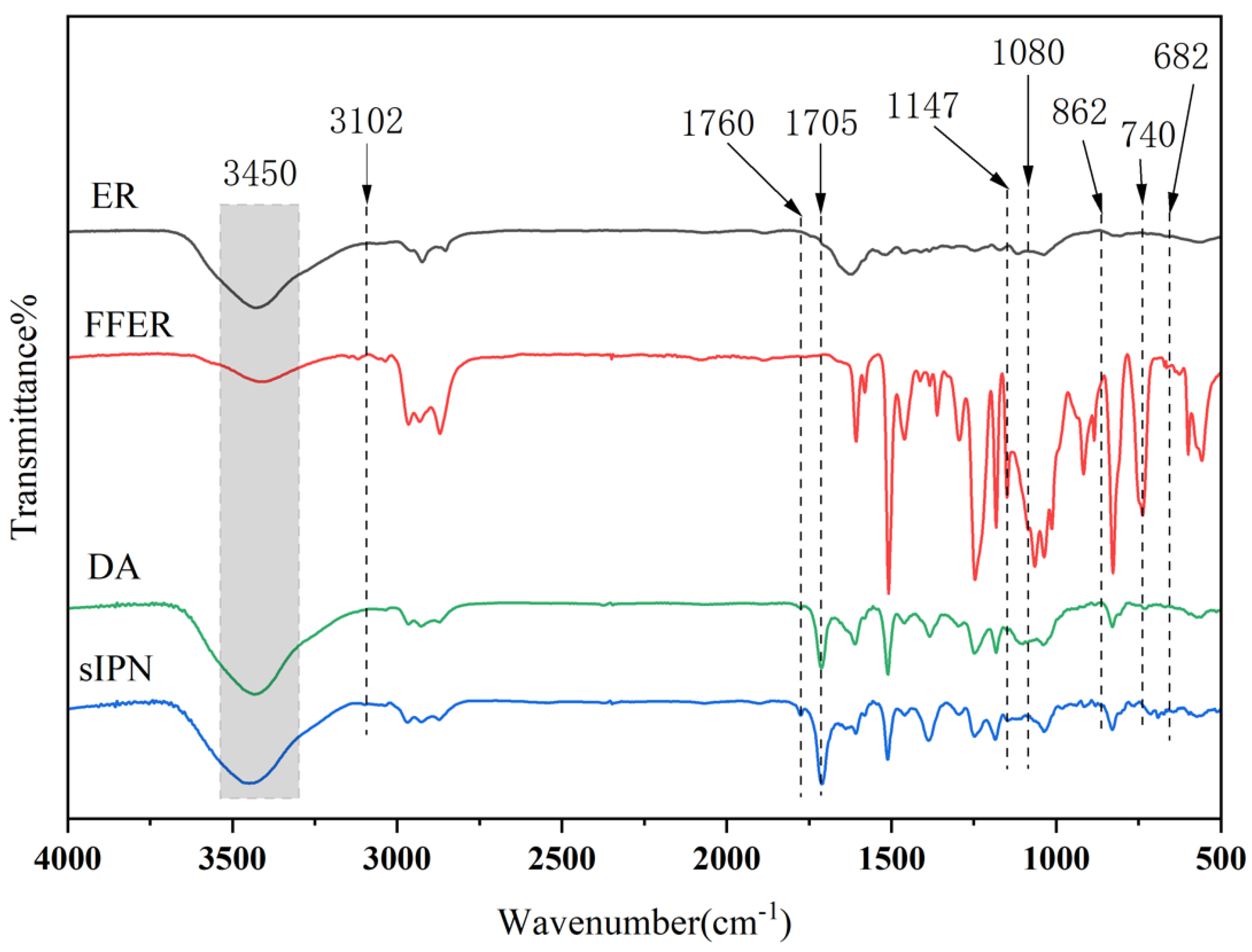
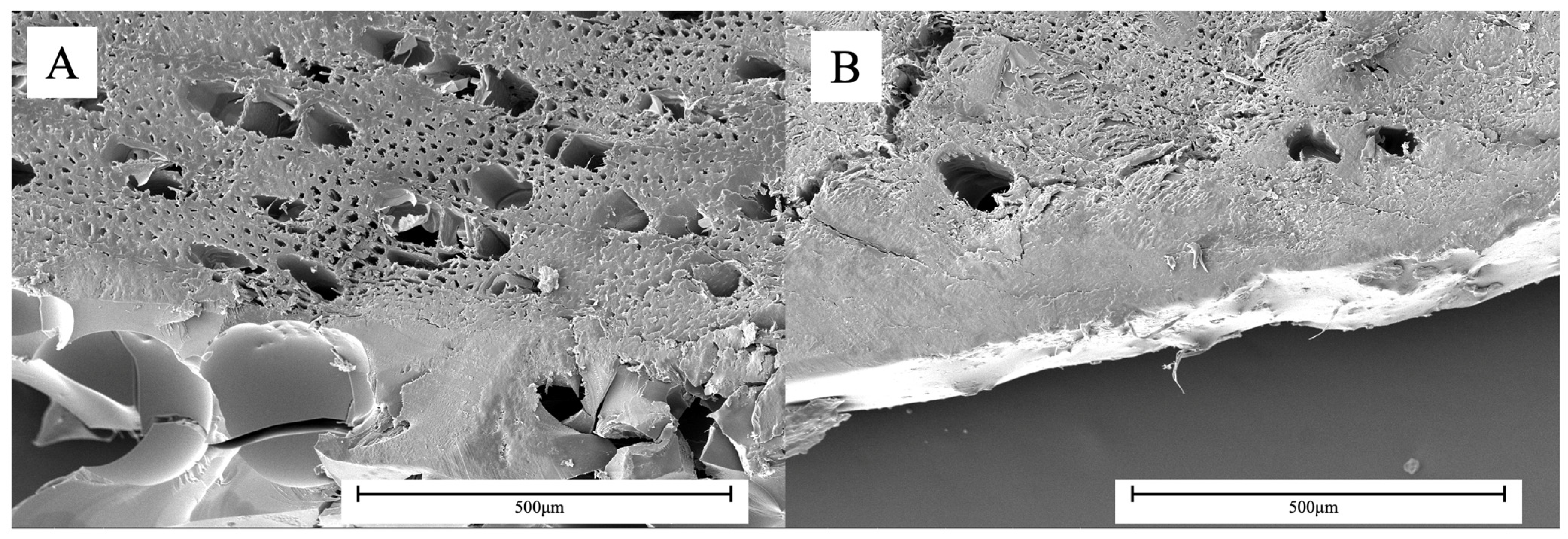
3.1. Chemical Analysis of Resins
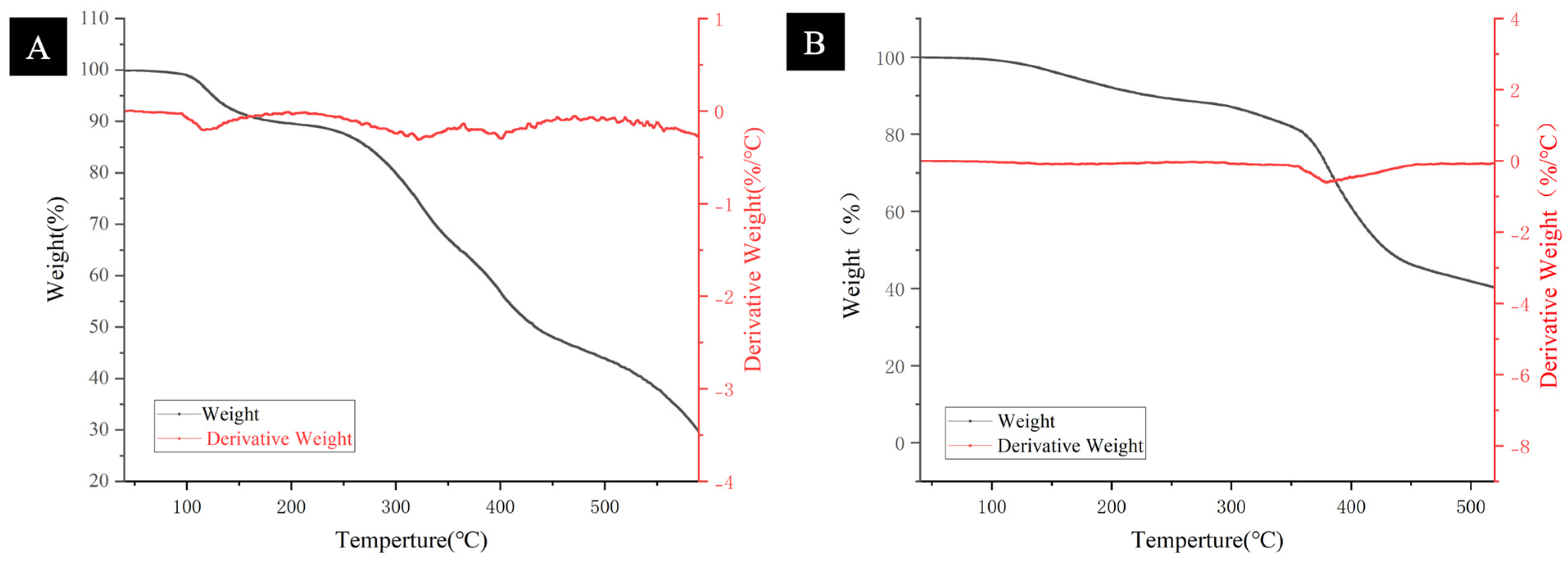
3.2. Thermodynamic Analysis of Polymers
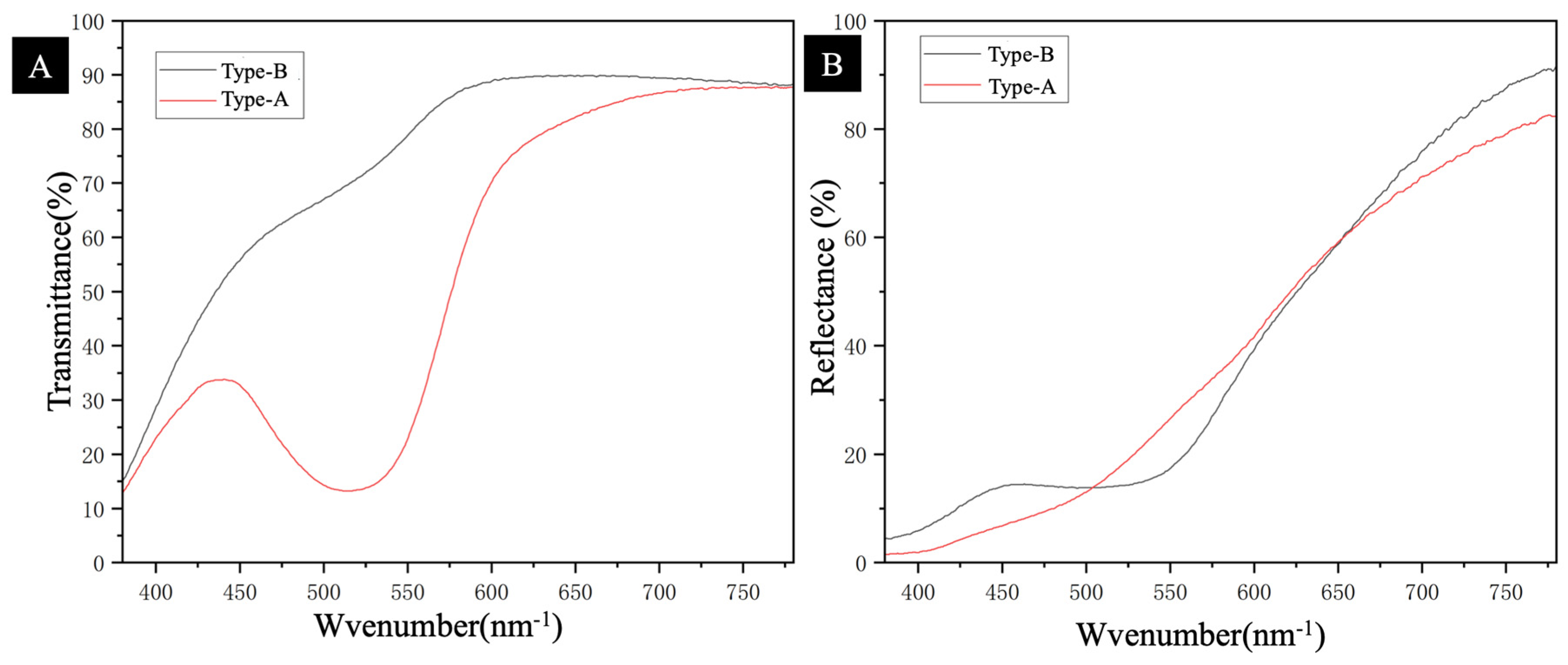
3.3. Coating Optical Property Analysis
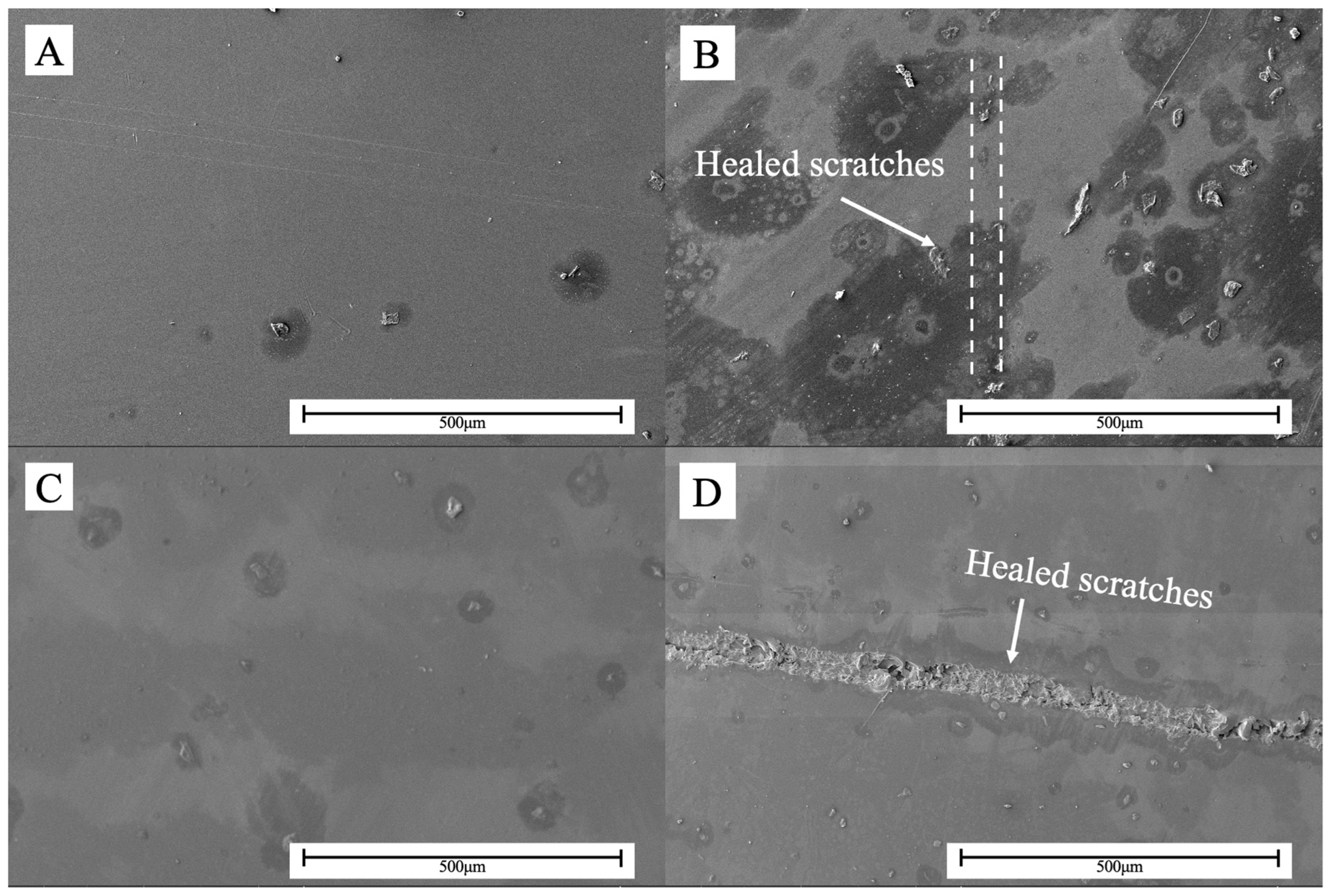
3.4. Self-Healing Properties of Polymers
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cao, Y.; Zhang, J.T.; Zhang, D.D.; Lv, Y.; Li, J.; Xu, Y.T.; He, K.B.; Chen, G.R.; Yuan, C.H.; Zeng, B.R.; et al. A novel shape memory-assisted and thermo-induced self-healing boron nitride/epoxy composites based on Diels–Alder reaction. Atlas J. Mater. Sci. 2020, 55, 11325–11338. [Google Scholar] [CrossRef]
- Kim, H.J.; Baek, S.Y.; Yang, H.C.; Lim, H.S. In-drop capillary spooling and shape-memory behavior of microfiber made from self-healing thermoplastic polyurethane with supramolecular interactions and dynamic covalent bonds. Eur. Polym. J. 2024, 216, 113287. [Google Scholar] [CrossRef]
- Peterson, A.M.; Jensen, R.E.; Palmese, G.R. Room-temperature healing of a thermosetting polymer network using the Diels-Alder reaction (vol 2, pg 1149, 2010). ACS Appl. Mater. Interfaces 2010, 2, 2169. [Google Scholar] [CrossRef]
- Zhou, J.C.; Xu, W. Phase-change thermal storage transparent wood based on optical reversibility. Ind. Crop. Prod. 2025, 223, 120275. [Google Scholar] [CrossRef]
- Wu, S.S.; Na, R.; Avramidis, S.; Xu, W. Effect of pH regulation on the properties of furfuryl alcohol-modified decorative veneer. Eur. J. Wood Wood Prod. 2024, 82, 2085–2098. [Google Scholar] [CrossRef]
- Liu, Q.Q.; Gao, D.; Xu, W. Effect of sanding processes on the surface properties of modified poplar coated by primer compared with mahogany. Coatings 2020, 10, 856. [Google Scholar] [CrossRef]
- Wu, D.; Wang, W.D.; Diaz-Dussan, D.; Peng, Y.Y.; Chen, Y.J.; Narain, R.; Hall, D.G. In situ forming, dual-crosslink network, self-healing hydrogel enabled by a bioorthogonal nopoldiol–benzoxaborolate click reaction with a wide pH range. Chem. Mater. 2019, 31, 4092–4102. [Google Scholar] [CrossRef]
- Ahner, J.; Pretzel, D.; Enke, M.; Geitner, R.; Zechel, S.; Popp, J.; Schubert, U.S.; Hager, M.D. Conjugated oligomers as fluorescence marker for the determination of the self-healing efficiency in mussel-inspired polymers. Chem. Mater. 2018, 30, 2791–2799. [Google Scholar] [CrossRef]
- Liu, C.Z.; McClements, D.J.; Li, M.; Xiong, L.; Sun, Q.J. Development of self-healing double-network hydrogels: Enhancement of the strength of wheat gluten hydrogels by in situ metal–catechol coordination. J. Agric. Food Chem. 2019, 67, 6508–6516. [Google Scholar] [CrossRef]
- Mordvinkin, A.; Suckow, M.; Böhme, F.; Colby, R.H.; Creton, C.; Saalwächter, K. Hierarchical sticker and sticky chain dynamics in self-healing butyl rubber ionomers. Macromolecules 2019, 52, 4169–4184. [Google Scholar] [CrossRef]
- Romo-Uribe, A.; Albanil, L. POSS-induced dynamic cross-links produced self-healing and shape memory physical hydrogels when copolymerized with N-isopropyl acrylamide. ACS Appl. Mater. Interfaces 2019, 11, 24447–24458. [Google Scholar] [CrossRef]
- Guo, W.C.; Jia, Y.; Tian, K.S.; Xu, Z.P.; Jiao, J.; Li, R.F.; Wu, Y.H.; Cao, L.; Wang, H.Y. UV-triggered self-healing of a single robust SiO2 microcapsule based on cationic polymerization for potential application in aerospace coatings. ACS Appl. Mater. Interfaces 2016, 8, 21046–21054. [Google Scholar] [CrossRef]
- Shchukina, E.; Wang, H.; Shchukin, D.G. Nanocontainer-based self-healing coatings: Current progress and future perspectives. Chem. Commun. 2019, 55, 3859–3867. [Google Scholar] [CrossRef]
- Fu, S.D.; Zhou, H.; Wang, H.X.; Niu, H.T.; Yang, W.D.; Shao, H.; Lin, T. Amphibious superamphiphilic fabrics with self-healing underwater superoleophilicity. Mater. Horiz. 2019, 6, 122–129. [Google Scholar] [CrossRef]
- Qin, J.X.; Lin, F.; Hubble, D.; Wang, Y.J.; Li, Y.; Murphy, I.A.; Jang, S.H.; Yang, J.H.; Jen, A.K.Y. Tuning self-healing properties of stiff, ion-conductive polymers. J. Mater. Chem. A 2019, 7, 6773–6783. [Google Scholar] [CrossRef]
- Mo, R.B.; Hu, J.; Huang, H.W.; Sheng, X.X.; Zhang, X.Y. Tunable, self-healing and corrosion inhibiting dynamic epoxy–polyimine network built by post-crosslinking. J. Mater. Chem. A 2019, 7, 3031–3038. [Google Scholar] [CrossRef]
- Li, W.; Wu, G.; Tan, J.H.; Yu, X.F.; Sun, G.; You, B. Transparent surfactant/epoxy composite coatings with self-healing and superhydrophilic properties. Macromol. Mater. Eng. 2019, 304, 1800765. [Google Scholar] [CrossRef]
- Bossion, A.; Olazabal, I.; Aguirresarobe, R.H.; Marina, S.; Martín, J.; Irusta, L.; Taton, D.; Sardon, H. Synthesis of self-healable waterborne isocyanate-free poly(hydroxyurethane)-based supramolecular networks by ionic interactions. Polym. Chem. 2019, 10, 2723–2733. [Google Scholar] [CrossRef]
- Weng, M.Y.; Fu, Y.C.; Xu, W. Flame-Retardant Coating on Wood Surface by Natural Biomass Polyelectrolyte via a Layer-by-Layer Self-Assembly Approach. Forests 2024, 15, 1362. [Google Scholar] [CrossRef]
- Zhou, J.C.; Xu, W. A fast method to prepare highly isotropic and optically adjustable transparent wood-based composites based on interface optimization. Ind. Crops Prod. 2024, 218, 118898. [Google Scholar] [CrossRef]
- Liu, Q.Q.; Gao, D.; Xu, W. Effect of paint process on the performance of modified poplar wood antique. Coatings 2021, 11, 1174. [Google Scholar] [CrossRef]
- Chen, Y.L.; Kushner, A.M.; Williams, G.A.; Guan, Z.B. Multiphase design of autonomic self-healing thermoplastic elastomers. Nat. Chem. 2012, 4, 467–472. [Google Scholar] [CrossRef]
- Hou, R.; Li, G.Q.; Zhang, Y.; Li, M.J.; Zhou, G.M.; Chai, X.M. Self-healing polymers materials based on dynamic supramolecular motifs. Prog. Chem. 2019, 31, 690–698. [Google Scholar]
- Lai, J.C.; Mei, J.F.; Jia, X.Y.; Li, C.H.; You, X.Z.; Bao, Z.A. A stiff and healable polymer based on dynamic-covalent boroxine bonds. Adv. Mater. 2016, 28, 8277–8282. [Google Scholar] [CrossRef]
- Liu, Q.Q.; Gao, D.; Xu, W. Influence of the bottom color modification and material color modification process on the performance of modified poplar. Coatings 2021, 11, 660. [Google Scholar] [CrossRef]
- Wu, S.S.; Xu, W. Sound Insulation performance of furfuryl alcohol-modified poplar veneer used in functional plywood. Materials 2022, 15, 6187. [Google Scholar] [CrossRef]
- Liu, Q.Q.; Gao, D.; Xu, W. Effect of polyurethane non-transparent coating process on paint film performance applied on modified poplar. Coatings 2021, 12, 39. [Google Scholar] [CrossRef]
- Wu, T.; Xu, W. Preparation of Tung Oil-Modified Raw Lacquer Films and Application for Mechanical Carving Technique. Coatings 2024, 14, 1264. [Google Scholar] [CrossRef]
- Liu, C.; Xu, W. Effect of Coating Process on Properties of Two-Component Waterborne Polyurethane Coatings for Wood. Coatings 2022, 12, 1857. [Google Scholar] [CrossRef]
- Xu, W.; Chen, P.; Yang, Y.; Wang, X.; Liu, X. Effects of freezing and steam treatments on the permeability of Populus tomentosaEinfluss von Vereisungs- und Dampfbehandlung auf die Permeabilitat von Populus tomentosa. Mater. Werkst. 2021, 52, 907–915. [Google Scholar] [CrossRef]
- Chang, Y.J.; Liu, E.W.; Wu, Z.H. Constructing chitosan microcapsules using hydroxypropyl methylcellulose for self-healing antibacterial wood coating. Int. J. Biol. Macromol. 2025, 308, 142300. [Google Scholar] [CrossRef]
- Dello Iacono, S.; Martone, A.; Pastore, A.; Filippone, G.; Acierno, D.; Zarrelli, M.; Giordano, M.; Amendola, E. Thermally activated multiple self-healing diels-alder epoxy system. Polym. Eng. Sci. 2017, 57, 674–679. [Google Scholar] [CrossRef]
- Chang, Y.J.; Yan, X.X.; Wu, Z.H. Application and prospect of self-healing microcapsules in surface coating of wood. Colloids Interface Sci. Commun. 2023, 56, 100736. [Google Scholar] [CrossRef]
- Wang, C.L.; Wu, Z.H.; Wang, J.X.; Liu, Y. Theory and Method for Rapid Carbon Footprint Accounting of Solid Wood Furniture. For. Prod. J. 2025, 75, 155–163. [Google Scholar] [CrossRef]
- Zhang, H.Q.; Feng, X.H.; Wu, Y.; Wu, Z.H. Effect of photoinitiator concentration and film thickness on the properties of UV-curable self-matting coating for wood-based panels. Forests 2023, 14, 1189. [Google Scholar] [CrossRef]
- Shi, L.Y.; Liu, Y.; Hu, J.; Chen, H.; Ji, J.G. Life-Cycle Assessment of Bicycles Based on Bamboo Bending Technology. For. Prod. J. 2025, 75, 179–188. [Google Scholar] [CrossRef]
- Sun, Y.X.; Man, C.; Kong, D.C.; Cui, Z.Y.; Wang, X.; Dong, C.F.; Cui, H.Z. Correlation between low-temperature anticorrosion performance and mechanical properties of composite coatings reinforced by modified Fe3O4. Prog. Org. Coat. 2022, 165, 106737. [Google Scholar] [CrossRef]
- Wu, J.; Liang, N.; Liu, X.; Cheng, J.; Xu, Z.J.; Li, T.C.; Miao, M.H.; Zhang, D.H. Controllability on topological structures and properties of hyperbranched epoxy resins. Prog. Org. Coat. 2022, 165, 106735. [Google Scholar] [CrossRef]
- Liang, X.; Li, X.J.; Tang, Y.; Zhang, X.Y.; Wei, W.; Liu, X.Y. Hyperbranched epoxy resin-grafted graphene oxide for efficient and allpurpose epoxy resin modification. J. Colloid Interface Sci. 2022, 611, 105–117. [Google Scholar] [CrossRef]
- He, J.; Wang, H.; Gong, Y.; Tian, X.Y.; Zhang, Z.L.; He, J.Y. A novel three-dimensional boron phosphide network for thermal management of epoxy composites. Compos. Part B Eng. 2022, 233, 109662. [Google Scholar] [CrossRef]
- Guo, F.; Zhang, Y.Z.; Cai, L.; Li, L.J. NiFe prussian blue analogue nanocages decorated magnesium hydroxide rod for enhancing fire safety and mechanical properties of epoxy resin. Compos. Part B Eng. 2022, 233, 109650. [Google Scholar] [CrossRef]
- Shi, X.H.; Li, X.L.; Li, Y.M.; Li, Z.; Wang, D.Y. Flame-retardant strategy and mechanism of fiber reinforced polymeric composite: A review. Compos. Part B Eng. 2022, 233, 109663. [Google Scholar] [CrossRef]
- Rebizant, V.; Venet, A.S.; Tournilhac, F.; Girard-Reydet, E.; Navarro, C.; Pascault, J.P.; Leibler, L. Chemistry and mechanical properties of epoxy-based thermosets reinforced by reactive and nonreactive SBMX block copolymers. Macromolecules 2004, 37, 8017–8027. [Google Scholar] [CrossRef]
- Zhang, H.Q.; Wu, Z.H. UV-curable self-matting waterborne polyurethane acrylate coating via self-wrinkled surface during curing in open-air. RSC Adv. 2022, 12, 33945–33954. [Google Scholar] [CrossRef]
- Soldatov, M.; Liu, H.Z. Hybrid porous polymers based on cage-like organosiloxanes: Synthesis, properties and applications. Prog. Polym. Sci. 2021, 119, 101419. [Google Scholar] [CrossRef]
- Thapa, S.; Dickie, D.A.; Qin, Y. Charge-separated metal–organic frameworks derived from boron-centered tetrapods. Cryst. Growth Des. 2020, 20, 1598–1608. [Google Scholar] [CrossRef]
- Ratwani, C.R.; Kamali, A.R.; Abdelkader, A.M. Self-healing by Diels-Alder cycloaddition in advanced functional polymers: A review. Prog. Mater. Sci. 2023, 131, 101001. [Google Scholar] [CrossRef]
- Chang, Y.J.; Wu, Z.H. Effect of Sandpaper Meshes on the Performance of Tilia Sp. Self-Repairing Coatings. Polymers 2023, 15, 2835. [Google Scholar] [CrossRef]
- Fortunato, G.; van den Tempel, P.; Bose, R.K. Advances in self-healing coatings based on Diels-Alder chemistry. Polymer 2024, 294, 126693. [Google Scholar] [CrossRef]
- Urdl, K.; Weiss, S.; Christöfl, P.; Kandelbauer, A.; Müller, U.; Kern, W. Diels-Alder modified self-healing melamine resin. Eur. Polym. J. 2020, 127, 109601. [Google Scholar] [CrossRef]
- Li, J.H.; Zhang, G.P.; Deng, L.B.; Jiang, K.; Zhao, S.F.; Gao, Y.J.; Sun, R.; Wong, C.P. Thermally reversible and self-healing novolac epoxy resins based on Diels–Alder chemistry. J. Appl. Polym. Sci. 2015, 132, 42167. [Google Scholar] [CrossRef]
- Liu, Y.; Hu, J.; Xu, W. Surface Roughness of Wood Substrates after Grinding and Its Influence on the Modification Effect of Structural Color Layers. Forests 2023, 14, 2213. [Google Scholar] [CrossRef]
- Aubert, J.H. Thermally removable epoxy adhesives incorporating thermally reversible diels-alder adducts. J. Adhes. 2003, 79, 609–616. [Google Scholar] [CrossRef]
- Chen, X.X.; Wudl, F.; Mal, A.K.; Shen, H.B.; Nutt, S.R. New thermally remendable highly cross-linked polymeric materials. Macromolecules 2003, 36, 1802–1807. [Google Scholar] [CrossRef]
- Araya-Hermosilla, E.; Giannetti, A.; Lima, G.M.R.; Orozco, F.; Picchioni, F.; Mattoli, V.; Bose, R.K.; Pucci, A. Thermally switchable electrically conductive thermoset rGO/PK self-healing composites. Polymers 2021, 13, 339. [Google Scholar] [CrossRef] [PubMed]
- Pratama, P.A.; Peterson, A.M.; Palmese, G.R. Diffusion and reaction phenomena in solution-based healing of polymer coatings using the Diels–Alder reaction. Macromol. Chem. Phys. 2012, 213, 173–181. [Google Scholar] [CrossRef]
- Terryn, S.; Mathijssen, G.; Vanderborght, B. Development of a self-healing soft pneumatic actuator: A first concept. Bioinspir. Biomim. 2015, 10, 046007. [Google Scholar] [CrossRef] [PubMed]
- Postiglione, G.; Turri, S.; Levi, M. Effect of the plasticizer on the self-healing properties of a polymer coating based on the thermoreversible Diels–Alder reaction. Prog. Org. Coat. 2015, 78, 526–531. [Google Scholar] [CrossRef]
- Tortelli, A.; Manarin, E.; Corsini, F.; Griffini, G.; Turri, S. Water-reducible and self-healing acrylic coatings based on Diels-Alder reversible reaction. Prog. Org. Coat. 2022, 171, 107012. [Google Scholar] [CrossRef]
- Coope, T.S.; Turkenburg, D.H.; Fischer, H.R.; Luterbacher, R.; Van Bracht, H.; Bond, I.P. Novel Diels-Alder based self-healing epoxies for aerospace composites. Smart Mater. Struct. 2016, 25, 084010. [Google Scholar] [CrossRef]
- Zolghadr, M.; Shakeri, A.; Zohuriaan-Mehr, M.J.; Salimi, A. Self-healing semi-IPN materials from epoxy resin by solvent-free furan–maleimide Diels–Alder polymerization. J. Appl. Polym. Sci. 2019, 136, 48015. [Google Scholar] [CrossRef]
- Wen, N.; Song, T.T.; Ji, Z.H.; Jiang, D.W.; Wu, Z.J.; Wang, Y.; Guo, Z.H. Recent advancements in self-healing materials: Mechanicals, performances and features. React. Funct. Polym. 2021, 168, 105041. [Google Scholar] [CrossRef]
- Marref, M.; Mignard, N.; Jegat, C.; Taha, M.; Belbachir, M.; Meghabar, R. Epoxy-amine based thermoresponsive networks designed by Diels–Alder reactions. Polym. Int. 2013, 62, 87–98. [Google Scholar] [CrossRef]
- Polgar, L.M.; Fortunato, G.; Araya-Hermosilla, R.; van Duin, M.; Pucci, A.; Picchioni, F. Cross-linking of rubber in the presence of multi-functional cross-linking aids via thermoreversible Diels-Alder chemistry. Eur. Polym. J. 2016, 82, 208–219. [Google Scholar] [CrossRef]
- Chiniwalla, P.; Bai, Y.Q.; Elce, E.; Shick, R.; McDougall, W.C.; Allen, S.A.B.; Kohl, P.A. Crosslinking and decomposition reactions of epoxide functionalized polynorbornene. Part I. FTIR and thermogravimetric analysis. J. Appl. Polym. Sci. 2003, 89, 568–577. [Google Scholar] [CrossRef]
- Liu, Y.L.; Hsieh, C.Y. Crosslinked epoxy materials exhibiting thermal remendablility and removability from multifunctional maleimide and furan compounds. J. Polym. Sci. A Polym. Chem. 2006, 44, 905–913. [Google Scholar] [CrossRef]
- Turkenburg, D.H.; van Bracht, H.; Funke, B.; Schmider, M.; Janke, D.; Fischer, H.R. Polyurethane adhesives containing Diels–Alder-based thermoreversible bonds. J. Appl. Polym. Sci. 2017, 134, 44972. [Google Scholar] [CrossRef]
- Cao, Y.; Wang, X.Y.; Wu, J.H.; Xu, Y.T.; Gerard, J.F.; Jiang, L.Y.; Zeng, B.R.; Dai, L.Z. A novel self-healing and removable hex-agonal boron nitride/epoxy coating with excellent anti-corrosive property based on Diels-Alder reaction. Prog. Org. Coat. 2022, 173, 107209. [Google Scholar] [CrossRef]



| Test Material | Purity | Manufacturer |
|---|---|---|
| Diglycidyl ether bisphenol A | epoxy value 0.38–0.45 | Shanghai Maclin Biochemical Technology Co., Ltd., Shanghai, China. |
| Furfuryl alcohol | 98% | Shanghai Maclin Biochemical Technology Co., Ltd., Shanghai, China. |
| Triethylamine | 95.5% | Shanghai Maclin Biochemical Technology Co., Ltd., Shanghai, China. |
| Bismaleimide diphenylmethane | AR | Shanghai Maclin Biochemical Technology Co., Ltd., Shanghai, China. |
| 4,4-diaminodiphenylmethane | 98% | Shanghai Maclin Biochemical Technology Co., Ltd., Shanghai, China. |
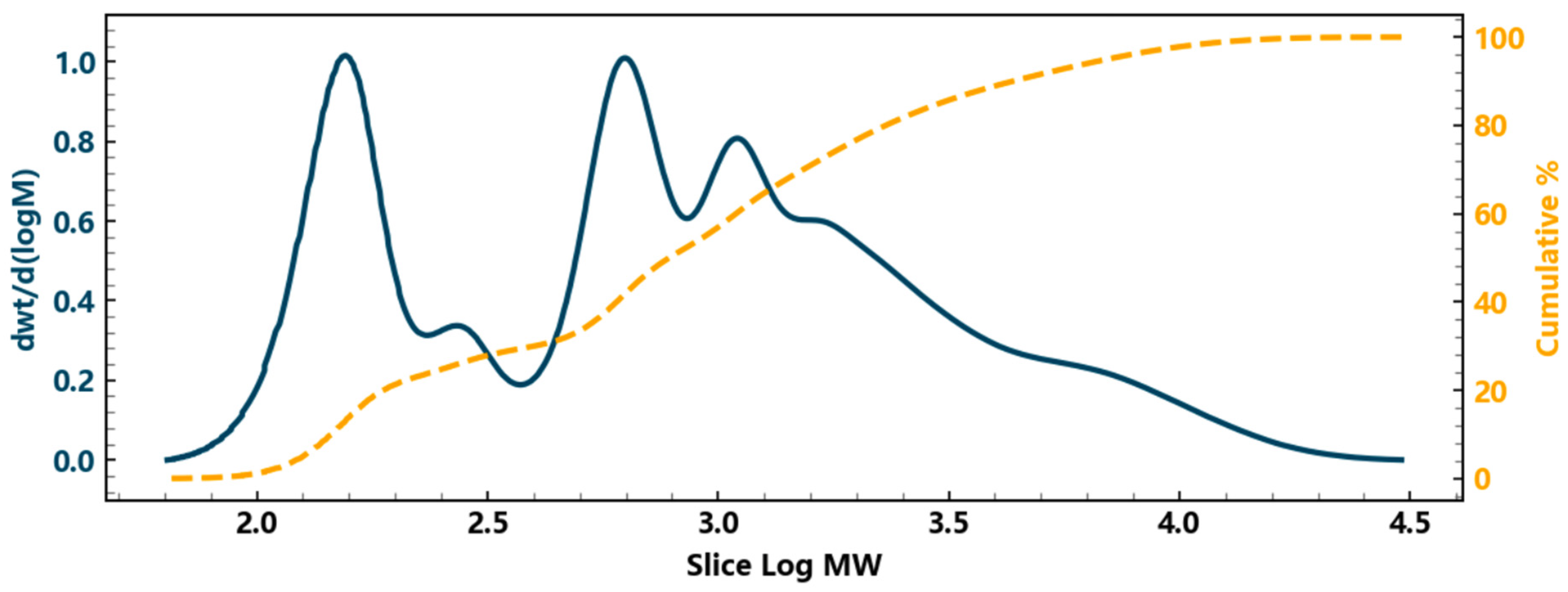
| Number Average Molecular Weight Mn | Weight Average Molecular Weight Mw | Peak Molecular Weight MP | Z-Mean Molecular Weight Mz | Z + 1 Average Molecular Weight Mz + 1 |
|---|---|---|---|---|
| 409 | 1717 | 155 | 5564 | 9989 |
| Coating | Different Position | L | a | b | ΔE |
|---|---|---|---|---|---|
| Type-A resin surface coating on glass plate | Point 1 | 40.6 | 44.9 | 28.4 | - |
| Point 2 | 39.9 | 45.3 | 27.9 | - | |
| chromatic aberration | - | - | - | 0.8 | |
| Type-B resin surface coating on glass plate | Point 1 | 63.3 | 14.4 | 38.9 | - |
| Point 2 | 64.2 | 16.6 | 37.5 | - | |
| chromatic aberration | - | - | - | 2.7 | |
| Type-A resin surface coating on poplar boards | Point 1 | 46.1 | 41.2 | 22.1 | |
| Point 2 | 46.6 | 41.4 | 24.1 | ||
| chromatic aberration | 2.0 | ||||
| Type-B resin surface coating on poplar boards | Point 1 | 52.1 | 30.1 | 43.7 | |
| Point 2 | 53.2 | 29.2 | 44.5 | ||
| chromatic aberration | 1.6 |
| Materials | 20° Gloss (%) | 60° Gloss (%) | 85° Gloss (%) |
|---|---|---|---|
| Poplar board with Type-A resin | 15.2 | 52.8 | 48.8 |
| Glass pane with Type-A resin | 97.9 | 115.3 | 91.6 |
| Poplar board with Type-B resin | 96.8 | 106.7 | 97.4 |
| Glass plate with Type-B resin | 152.2 | 140.4 | 99.4 |
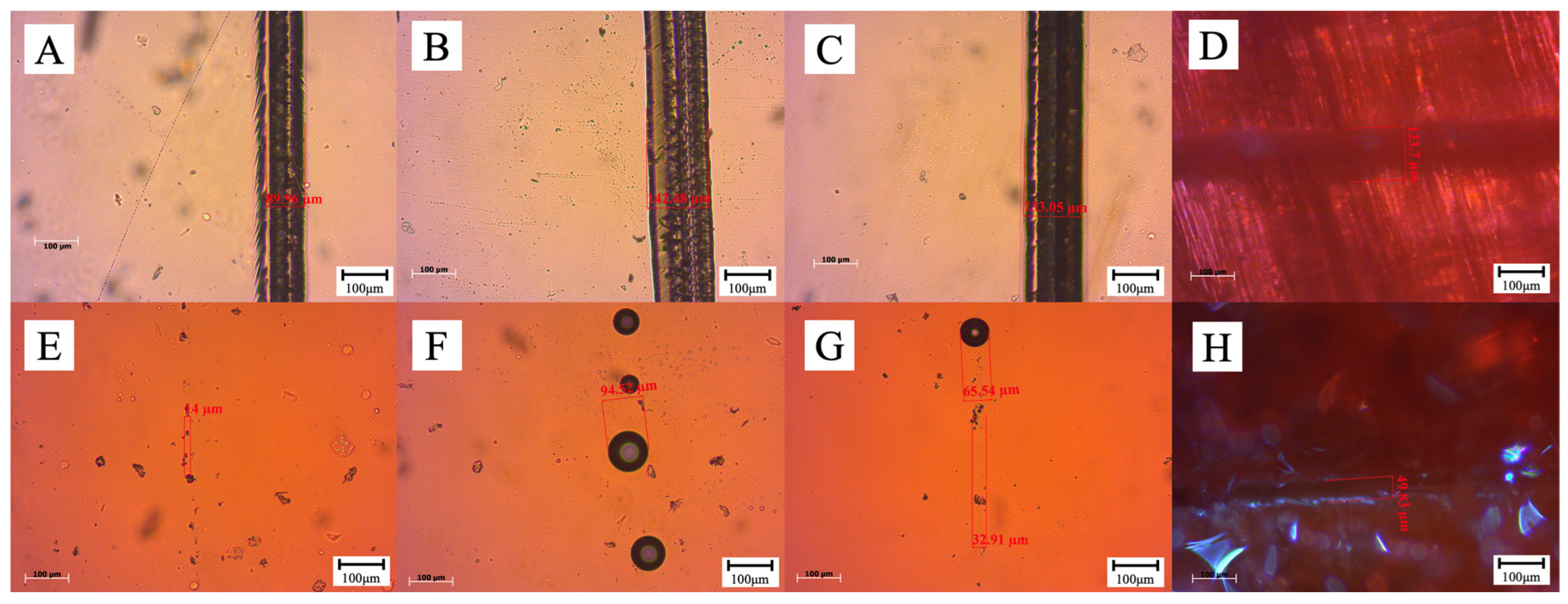
| Scratch Number | Scratch Width (μm) | Scratch Width After 30 min (μm) | Self-Healing Rate (%) |
|---|---|---|---|
| Slide 1 on glass plate | 89.96 | 14.00 | 84.43 |
| Slide 2 on glass plate | 142.48 | 94.52 | 33.66 |
| Slide 3 on glass plate | 133.05 | 32.91 | 75.26 |
| Slide 1 on poplar boards | 123.70 | 49.83 | 59.71 |
| Scratch Number | Scratch Width (μm) | Scratch Width After 30 min (μm) | Self-Healing Rate (%) |
|---|---|---|---|
| Slide 1 | 131.81 | 40.64 | 69.17 |
| Slide 2 | 164.56 | 55.24 | 66.43 |
| Slide 3 | 115.55 | 45.97 | 60.22 |
| Poplar plank | 52.97 | 23.62 | 55.41 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, Y.; Yan, X. Preparation of High Self-Healing Diels–Alder (DA) Synthetic Resin and Its Influence on the Surface Coating Properties of Poplar Wood and Glass. Coatings 2025, 15, 988. https://doi.org/10.3390/coatings15090988
Dong Y, Yan X. Preparation of High Self-Healing Diels–Alder (DA) Synthetic Resin and Its Influence on the Surface Coating Properties of Poplar Wood and Glass. Coatings. 2025; 15(9):988. https://doi.org/10.3390/coatings15090988
Chicago/Turabian StyleDong, Yang, and Xiaoxing Yan. 2025. "Preparation of High Self-Healing Diels–Alder (DA) Synthetic Resin and Its Influence on the Surface Coating Properties of Poplar Wood and Glass" Coatings 15, no. 9: 988. https://doi.org/10.3390/coatings15090988
APA StyleDong, Y., & Yan, X. (2025). Preparation of High Self-Healing Diels–Alder (DA) Synthetic Resin and Its Influence on the Surface Coating Properties of Poplar Wood and Glass. Coatings, 15(9), 988. https://doi.org/10.3390/coatings15090988





