Abstract
Dental caries is a prevalent health condition affecting 87% of the population. The application of fluorinated varnishes to incipient lesions promotes remineralization. To evaluate the remineralizing effect of three fluorinated varnishes through chemical and physical characterization of incipient enamel lesions in vitro, a total of 150 enamel surfaces were randomly divided into five groups (n = 30): healthy enamel, initial lesion, Fluor-Protector, β-Clinpro-White-Varnish, and Duraphat. All groups, except for the healthy enamel, were immersed in a demineralizing solution (pH 4.4) for 96 h. Remineralization was assessed using a pH cycling model over 5, 10, and 15 days. Fluoride release was measured via ISE-F, and enamel was analyzed by Raman spectroscopy (PO43−), roughness, and Vickers hardness. Data were analyzed using ANOVA and a post hoc test (Tukey). Ion Selective Electron-Fluor showed a residual F concentration of 0.40 ppm for the Fluor-Protector remineralizing solution: 40.00 ppm for Clinpro-White-Varnish, and 50.0 ppm for Duraphat. Raman analysis confirmed PO43− at 956 cm−1 mainly in CDu group. Roughness decreased with varnish application: Fluor-Protector (0.36 µm), β-Clinpro-White-Varnish (0.73 µm), and Duraphat (0.65 µm). Hardness increased with Fluor-Protector. Statistically significant differences were found between FP and other types of varnish. Fluorinated varnishes enhance remineralization and reduce enamel roughness and demineralization. Fluor Protector and β-Clinpro-White-Varnish showed the most favorable results, suggesting their recommendation for high-risk pediatric patients.
1. Introduction
Dental enamel is the hardest tissue in the human body [1], highly mineralized and acellular, covering the crown of each tooth, whose microstructure presents a highly complex organization [2]. Dental enamel is mainly composed of hydroxyapatite crystals [3] arranged in a hexagonal disposition; its primary components are calcium and phosphate ions; in the center, we find hydroxyl functional groups. The loss of calcium (Ca2+), phosphate (PO43−), and hydroxyl (OH−) groups in this tissue starts a process of demineralization caused mainly by acids produced by biofilm bacteria. This loss of minerals occurs at a critical pH of 5.5. If demineralization continues, the first clinical manifestation of a carious lesion—a white spot without cavitation—can be observed [4,5]. White spots are described as porous enamel due to demineralization and are observed as opaque spots [6]. In this stage, the lesion can be completely reversed in children and adults [7], i.e., dental enamel can be remineralized by reintegrating these ions. For this to happen, a neutral pH of 7 must be achieved in the [8].
A cyclical pH model in the laboratory helps reproduce incipient lesions, simulating the environment of the oral cavity. A model of this type utilizes a demineralizing solution with a pH of approximately 4, thereby substituting for the acidic attack of biofilm present in the oral cavity. Additionally, remineralizing solutions, such as artificial saliva with a pH of 7, are used to replace the buffering capacity of natural saliva secreted in the mouth [9]. Such a process enables the in vitro evaluation of remineralizing agents for repairing or preventing the development of white spots. The professional application of fluorinated varnishes represents the first line of defense in stopping this process, utilizing minimally invasive therapies.
A popular varnish used in dental practices is the White Varnish Clinpro (3M ESPE), which contains fluoride and calcium phosphate that activate upon contact with saliva. This varnish contains 5% sodium fluoride, tricalcium phosphate, and is sweetened with xylitol. This product prevents the accumulation of Streptococcus mutans and Lactobacillus acidophilus colonies. Another varnish commonly used to remineralize carious lesions is Fluor Protector (Ivoclar), which is used in the treatment of hypersensitivity and protection against dental erosion. This product contains 0.9% difluorosilane with a polyurethane base. The amount of fluoride in the solution is equivalent to 0.1% or 1000 parts per million (ppm). Duraphat (Colgate) is another varnish used in the dental practice. Its composition consists of 5% sodium fluoride. It is used in dentine hypersensitivity caused by the frequent consumption of acidic beverages or in cases of gastric reflux [10].
The efficacy of these products is attributed to the amount of fluoride in their composition. Fluoride interacts with the hydroxyl group found in enamel, causing the formation of fluorohydroxyapatite [Ca10(PO4)6(OH)2(F)] [11], an even more resistant component. The detection of fluoride plays a crucial role in understanding its benefits and potential toxicity. A fluoride ion-specific electrode with a LaF3 membrane is the most commonly used instrument to obtain practical measurements in distinct solutions.
Raman spectroscopy is a chemical technique used to characterize dental enamel by indirectly measuring the light absorption of molecules/solids in response to a monochromatic laser. This method has demonstrated its ability to monitor changes in the mineral content of dental enamel during demineralization. Raman spectroscopy explicitly detects the presence or absence of phosphate and hydroxyl functional groups [12].
In this research, we aimed to determine the amount of fluoride in remineralizing and demineralizing solutions used in a cyclical pH model after treatment with three different fluorinated varnishes, as well as the presence of hydroxyl and phosphate functional groups after treatment, using Raman spectroscopy.
2. Materials and Methods
2.1. Study Design
Enamel samples were collected from the lower third molars of 17- to 22-year-old patients who were scheduled for third molar extraction as part of their orthodontic or preventive treatment. These specific teeth were chosen for their straight mesiodistal surfaces, which provided ideal working surfaces on both the buccal and lingual areas for analysis while minimizing superficial enamel wear. All donors provided written informed consent after a thorough explanation of the procedure.
Seventy-five inferior third molars with intact anatomical crowns without structural defects were obtained and stored in deionized water at ~36 °C and 100% humidity. Any remaining gingival tissue was removed with curettes (Hu-Friedy), and the molars were sectioned mesiodistally with a diamond disk (Brasseler) under constant irrigation, obtaining 150 surfaces (buccal and lingual). The pulp chamber was covered with wax to create a smooth working surface; prophylaxis was performed on all surfaces using fluoride-free toothpaste. In the anatomic crown, a 3 × 6 mm2 window was delimited for the application of products; the rest of the tooth was covered with acid-resistant varnish, a different color for each experimental group.
The samples were divided into five experimental groups: initial lesion (n = 30); Flour Protector, Ivoclar (FP, n = 30); Clinpro white varnish, 3M ESPE (β-TCP, n = 30); Duraphat, Colgate (CDu, n = 30); and a healthy enamel group (HE) used as a control without treatment (n = 30). Except for the healthy enamel group, all samples were submerged in a demineralizing solution composed of 2.2 mM CaCl2, 2.2 mM NaH2PO4, and 0.05 M of CH3COOH at a pH of 4.4 at a temperature of ~36 °C for 96 h, to produce an incipient caries lesion. Remineralizing solutions comprised 1.5 mM CaCl2, 0.9 mM NaH2PO4, and 0.15 mM KCl, adjusted to a pH of 7.
2.2. pH Cycling
The experimental groups, CDu, FP, and β-TCP, and the initial lesion group (without treatment), were subjected to the following pH cycle: 3 h of immersion in a demineralizing solution, followed by 60 s of application of the fluoridated varnishes according to the manufacturer’s instructions. After this, 21 h of immersion in a remineralizing solution were performed. The samples were rinsed after each change in solution.
Then, 10 samples were randomly selected from each group during the pH cycle and stored in deionized water at ~36 °C. These 10 samples from each group were mounted on plastic squares with epoxy resin to avoid movement and provide stability during subsequent testing.
2.3. Fluoride Testing
An ion-specific electrode and fluorometer (Orion A-214, Orion Research, Austin, TX, USA) were used to measure the fluoride ion concentration in the solution. Calibration curves were prepared for electrode calibration, ranging from 0.250 to 2 µg/mL, according to the fluoride content. The fluoride concentration in the samples was detected by comparing them to the calibration curves. To avoid interference from other ions (iron, calcium, aluminum, etc.), different 2 mL Tissab ll (Orion, Techno Scientific, Waltham, MA, USA) solutions were mixed, which maintains the stability of ions, elevates the pH, and allows free fluoride ions to merge with metallic ions. The equipment was calibrated daily to ensure the reproducibility of ±2%.
2.4. Raman Spectroscopy
A synthetic hydroxyapatite pellet was used for calibration of the micro-Raman equipment, with monitoring performed before each session. A Nd: YV04 laser (λ = 532 nm, 4 cm−1 resolution) was used on an Enspectr R532 Raman microscope (Emmett, 83646, ID, USA) with an OLYMPUS microscope. Spectra were obtained from 180 to 4000 cm−1 and processed using MicrocalTM Origin v.8.5 graphics program (Microcal Software, Northampton, MA, USA) to identify the characteristic vibrations of the PO43− functional groups (range 1100–900 cm−1). Following calibration, each specimen was mounted on a microscope slide attached to the Raman instrument.
To obtain the spectra of the fluoride varnishes (FP, β-TCP-F, and CDu) used in pediatric dentistry, surface enamel spectra were collected from each sample at 5, 10, and 15 days of the pH cycle.
2.5. Surface Roughness
The roughness of the surfaces was measured using a ZYGO 3D-Nexview (Zygo, Middlefield, CT, USA) non-contact optical profilometer. The average roughness, Ra, which is defined as the arithmetic mean of the absolute values of the surface profile deviations from the mean line, was used to report surface roughness. It measures the average distance between the surface’s peaks and valleys over a given evaluation length and is the most widely reported roughness value in materials science. For this, six roughness measurements (Ra) were randomly recorded on the 3 × 6 mm2 working surface of each sample, and the average of the profilometry data was obtained. Surface roughness measurements were recorded at 0, 5, 10, and 15 days of pH cycling treatment. Significant differences were calculated by comparing the measurements of the experimentally treated groups with those of FP, β-TCP-F, CDu, Initial Lesion (IL), and HE.
2.6. Vickers Hardness
The Vickers hardness of 10 working surfaces from each group, after 0, 5, 10, and 15 days of pH cycling treatment, was determined using a microindenter (Nano-Microindentador, NANOVEA Inc., Headquarters, Irvine, CA, USA; software: Nanovea Micro Indentation Tester v1.8.3). Using a 20X objective lens, the indenter was placed perpendicularly to the working surface of each sample, and the assays were performed with the following conditions: approach speed: 50 μm/min; contact load: 50 mN; load: 10 N; loading rate: 5 N/min; and unloading rate: 5 N/min, with 10 notches per sample. The Vickers hardness number (VHN) was calculated using the Nanovea Micro Indentation Tester software v1.8.3 (NANOVEA Inc., Headquarters, Irvine, CA, USA) [10].
2.7. Statistical Analysis
The normality of the datasets was evaluated using the Shapiro–Wilk test. Surface roughness (Ra) and Vickers microhardness data adhered to a normal distribution; therefore, a two-way analysis of variance (two-way ANOVA) was conducted, followed by Tukey’s post hoc test for multiple comparisons. In contrast, fluoride release data (ISE, ppm F−) did not follow a normal distribution. They were analyzed with the Kruskal–Wallis test, and pairwise comparisons were performed using the Mann–Whitney (Wilcoxon rank-sum) test.
All statistical analyses and graphical representations were performed with GraphPad Prism software (version 8.0, 225 Franklin Street. Fl. 26, Boston, MA, USA), while Raman spectra were processed and analyzed using OriginPro v.8.5 software (OriginLab, Northampton, MA, USA).
3. Results
3.1. Ion-Selective Fluoride Test (ISE-F)
During the 15-day pH cycle, the amount of the fluorine element (ppm) in the demineralizing and remineralizing solutions was measured daily using the ISE-F. The results obtained did not have a normal distribution, and the median value was considered as presented in (Chi2 = 105.1, DF = 6, p < 0.05). Figure 1 shows the box and whisker plot of the ISE-F measurements results during the 15 days of cyclical pH treatment with the different experimental groups, where statistically significant differences are observed. (*) (p < 0.05).
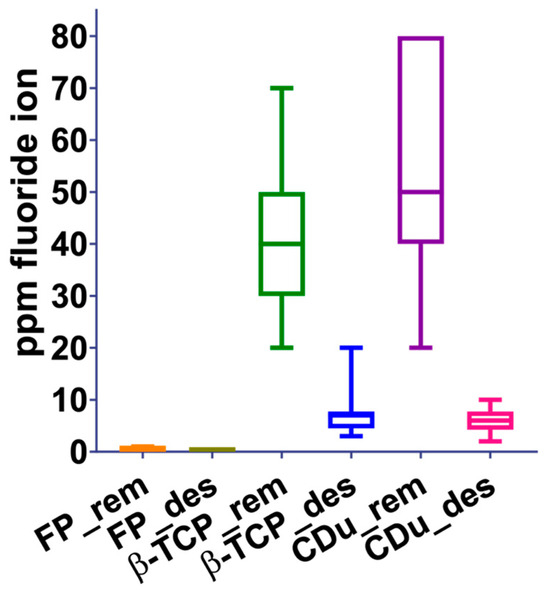
Figure 1.
Behavior of the three experimental groups with the different solutions (remineralizing and demineralizing). Significant differences are observed between the ppm of F− in the groups during the 15 days of cyclical pH.
3.2. Raman Spectroscopy Results
Ten spectra were taken from each experimental group. Noise was reduced through a filter based on the least squares smoothing algorithm; all the above was performed using Origin v8.5.
The Raman spectra showed the characteristic band for the PO43− functional group at 956 cm−1. Figure 2 displays the spectra from 850 to 1150 cm−1 for the Hydroxyapatite calibration standard, followed by the healthy Enamel and initial Lesion control groups. The full Raman spectrum of the experimental sample is shown in the Supplementary Material.
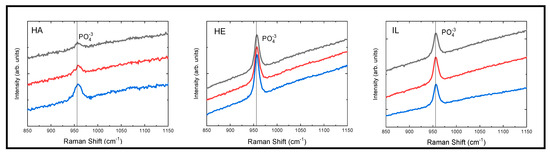
Figure 2.
Raman spectra of Hydroxyapatite (HA), Healthy Enamel (HE), and Initial Lesion (IL) groups. This figure presents three representative spectra for each group. The characteristic band for the PO43− group is centered at 956 cm−1. The black, red, and blue spectra correspond to specific dental samples after the application of remineralizing protective varnishes. The spectra have been vertically offset in arbitrary units to improve visualization.
The results obtained from the Raman spectroscopy on tooth enamel are shown in Figure 3. It can be observed that the three experimental treatments presented PO43− groups, which were detected with the distinctive vibrational bands at a shift of 956 cm−1. However, these treatments behaved differently, as the CDu paste showed a greater presence of groups at 10 and 15 days after application of the product. Meanwhile, the FP and β-TCP-F varnishes recorded PO43− groups at the beginning of treatment and a decrease over time. Therefore, on day 15, the detection of PO43− groups was lower, and the band intensity decreased compared to the CDu group. This behavior in the varnish-treated groups may be due to a physical-mechanical effect caused by the constant coating of the enamel with these products, which interferes with the detection of these phosphate groups.
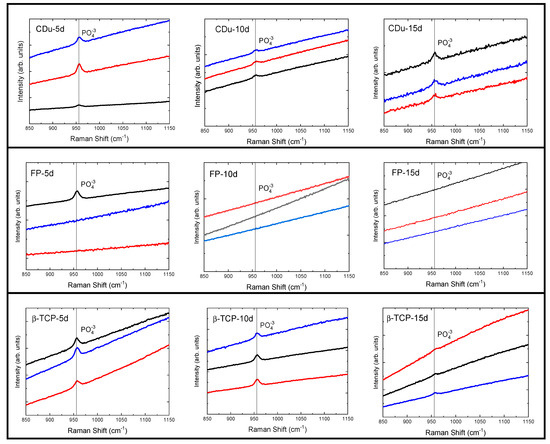
Figure 3.
Raman spectra of enamel surfaces after treatment with remineralizing protective varnishes (CDu, FP, and β-TCP-F). In some of the treatments, the specific vibrational band corresponding to the detection of the phosphate functional group is detected at approximately 960 cm−1. The black, red, and blue lines correspond to representative zones measured from each sample. These recordings are separated on the Y axis by arbitrary units for improved visualization. The Functional group (PO43−) at 956 cm−1 was measured on days 5, 10, and 15 post-treatments. Notably, the detection of this group decreases by day 15, which we attribute to a physical insulating effect caused by the thickness of the FP and β-TCP-F varnishes.
3.3. Roughness Surface
Three-dimensional optical profilometry was performed to analyze surface roughness using an optical profilometer at 100X magnification. Representative images (Figure 4) were obtained for each experimental group after 5, 10, and 15 days of treatment with different remineralizing agents, as well as for healthy enamel (positive control) and initially demineralized enamel (negative control). The surface topography was evaluated in areas measuring 83.139 × 83.139 µm2 per sample, with peaks and valleys visualized using a color scale that reflects height variations (µm). Warm colors (red/yellow) represent elevated regions, while cool colors (blue/green) indicate depressions or valleys. The images clearly illustrate microstructural differences among the treatments and evaluation times, highlighting the effects of the remineralization process on enamel morphology.
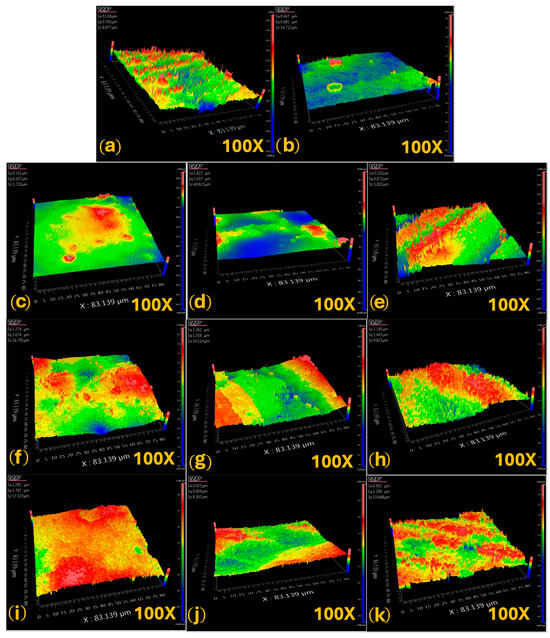
Figure 4.
Representative optical profilometry images of each sample: (a) healthy enamel (HE); (b) initial lesion (IL); (c) FP, 5-day treatment; (d) FP, 10-day treatment; (e) FP, 15-day treatment; (f) β-TCP-F, 5-day treatment; (g) β-TCP-F, 10-day treatment; (h) β-TCP-F, 15-day treatment; (i) CDu, 5-day treatment; (j) CDu, 10-day treatment and (k) CDu, 15-day treatment.
The surface roughness analysis (Ra) obtained from the average value of six different areas revealed significant differences between the groups treated with remineralizing agents and the controls. HE exhibited a low baseline roughness (Ra = 0.71 ± 0.06 µm) (Table 1). In contrast, the IL group showed a marked increase in roughness (Ra = 6.29 ± 1.85 µm), demonstrating the destructive effect of demineralization. In the group treated with FP, a notable reduction in roughness was observed at day 5 (Ra = 0.609 ± 0.18 µm), followed by an increase at day 10 (Ra = 1.60 ± 0.13 µm), and a more pronounced decrease by day 15 (Ra = 0.358 ± 0.12 µm). Statistical analysis showed significant differences between days 5 and 10 (p = 0.0057) and between days 10 and 15 (p = 0.0001), with no significant difference between days 5 and 15 (p = 0.3014).

Table 1.
Roughness media (Ra) results (mean ± SD) of human enamel specimens according to different groups.
The β-TCP-F treatment group exhibited a different pattern, with higher roughness at day 5 (Ra = 1.363 ± 0.21 µm), which significantly decreased by day 10 (Ra = 0.73 ± 0.09 µm) and remained stable through day 15 (Ra = 0.776 ± 0.03 µm). Significant differences were found between days 5 and 10 (p = 0.0180) and between days 5 and 15 (p = 0.0067), with no significant changes between days 10 and 15 (p = 0.6860). Regarding the group treated with the commercial paste CDu, roughness decreased from 1.55 ± 0.09 µm (day 5) to 0.65 ± 0.09 µm (day 10), followed by a slight increase at day 15 (Ra = 0.99 ± 0.11 µm). Significant differences were observed between days 5 and 10 (p = 0.0009) and between days 5 and 15 (p = 0.0199), with no significant difference between days 10 and 15 (p = 0.1418).
These findings suggest that all evaluated remineralizing treatments induced a progressive reduction in roughness on demineralized enamel surfaces, with variations in the temporal response. Notably, FP varnish achieved roughness values lower than sound enamel by day 15, while β-TCP-F maintained a stable roughness close to the baseline from day 10 onward. Overall, these results support the efficacy of FP and β-TCP-F in restoring the topographic integrity of dental enamel, positioning them as viable options in the clinical management of early carious lesions.
3.4. Vickers Hardness
Vickers hardness was measured using a load of 10 N, equivalent to 1019 g force (Figure 5). The specimens were mounted on glass slides using epoxy resin to ensure proper fixation and avoid unwanted movement during testing. Subsequently, the working surface was selected by identifying areas with minimal convexity to facilitate precise and reliable indentation. Panels a–c in Figure 5 correspond to the FP group after 5, 10, and 15 days of treatment, respectively. The indentations display well-defined rhomboid geometries, suggesting improved mechanical resistance over time. Panels d–f show the β-TCP group at the same time intervals. The rhomboid indentations are less distinct, indicating intermediate levels of enamel remineralization. Panels g–i depict the CDu group. The indentations appear more diffused and less sharply defined, particularly at day 10 (h), which may reflect variability in surface hardness or light scattering due to surface roughness.
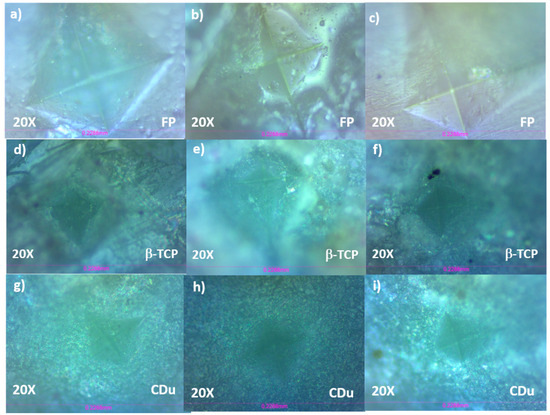
Figure 5.
Representative profilometry images of each experimental group: (a) FP, 5-day treatment; (b) FP, 10-day treatment; (c) FP, 15-day treatment; (d) β-TCP-F, 5-day treatment; (e) β-TCP-F, 10-day treatment; (f) β-TCP-F, 15-day treatment; (g) CDu, 5-day treatment; (h) CDu, 10-day treatment; and (i) 15-day treatment.
Each indentation was produced under a 10 N load, and the characteristic rhomboid shape results from the intersection of vertical and horizontal diagonals formed by the diamond-shaped indenter.
The results were non-parametric, and the median was used to determine the hardness of the healthy enamel group (HE = 533.8 ± 3.12 HV), which was significantly reduced after the initial demineralizing lesion (IL = 281.0 ± 4.1 HV) (Figure 6). The hardness of the FP group was unexpectedly very low: 80 ± 9.3, 62.50 ± 6.15, and 40 ± 8.30 at 5, 10, and 15 days, respectively. Residual varnish was likely present during testing. Meanwhile, the group treated with β-TCP showed a median of 448.2 ± 9.82 at 5 days; 406.3 ± 22.09 at 10 days; and 473.6 ± 12.78 at 15 days. The group treated with CDu had medians of 344.5 ± 6.28, 352.5 ± 11.66, and 483.0 ± 13.00 at 5, 10, and 15 days, respectively, with a p-value < 0.05.
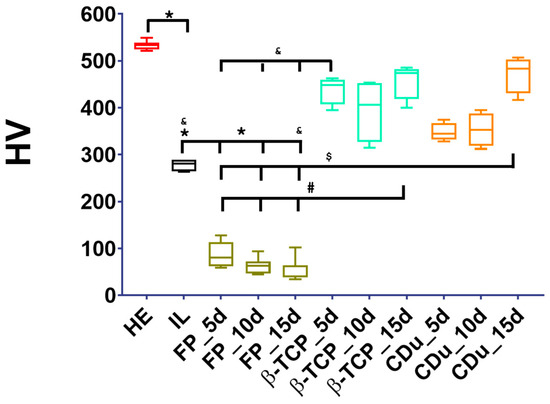
Figure 6.
Vickers microhardness (HV) values by experimental group. The figure highlights statistically significant differences in HV values. Key findings include: a significant difference between Healthy Enamel (HE) and both Incipient Lesion (IL) and FP groups at 5, 10, and 15 days; a significant difference between Incipient Lesion (IL) and the FP and CDu groups at 15 days; and the β-TCP group showing a significant difference when compared to all FP groups at all time points.
4. Discussion
Dental cavities, or caries, are a significant global health issue, especially in developing countries like Mexico, where over 86% of the population is affected. Changes to tooth enamel are the main cause of this condition [13]. Minimally invasive dentistry emphasizes preventing and reversing early carious lesions. To do this, various effective treatments are available for enamel remineralization, ranging from daily products like fluoridated mouthwashes and toothpastes to professional treatments such as fluoride varnishes.
Multiple studies have shown that fluoride varnishes, combined with other preventive measures like biofilm control and dietary management, can effectively remineralize early caries lesions [14,15,16,17,18]. This in vitro study assessed the effectiveness of three fluoride varnishes—FP, CDu, and β-TCP-F—on the surface of a developing carious lesion. We employed a well-established pH-cycling protocol to simulate the initiation and progression of these lesions. This model enabled us to replicate the demineralization and remineralization phases of a white spot lesion, which is essential for testing the potential of remineralizing agents to prevent enamel loss [19,20].
Fluoride detection is crucial for understanding both the beneficial and toxic effects of naturally occurring fluoride and various fluorinated compounds. Our results showed high levels of fluoride ions in solutions from the Duraphat and Clinpro varnish treatments. This indicates a need to limit their use in very young patients to prevent dental fluorosis or systemic toxicity [21].
Several studies support the effectiveness of the treatments used in this work. For instance, Clinpro White Varnish and Enamel Pro Varnish have been shown to heal early enamel caries lesions on premolars in vitro [15]. In a similar effort to find new strategies for enamel protection, Farooq et al. [22] evaluated the remineralizing effects of other treatments, including Plax Kids, Colgate, and Listerine Smart Rinse, using a white spot caries lesion model. While our results show a stronger remineralizing impact compared to their findings, it is important to note that their demineralizing model used a 35% phosphoric acid for only 30 s, which represents a different methodology. Mohd Said et al. [23] conducted a similar study assessing the remineralizing effects of several fluoride varnishes, including Duraphat, MI Varnish, Embrace Varnish, Enamel Pro Varnish, and Clinpro White Varnish. Their research involved creating an artificial carious lesion through immersion in a demineralizing solution, applying a cyclical pH model, and performing physical characterization. The Knoop microhardness test concluded that Duraphat, which contains 5% sodium fluoride, achieved significant remineralization of enamel carious lesions. Clinpro White Varnish also demonstrated a similar remineralization effect, while the other varnishes were less effective. Our findings align with their results.
A key difference in our research, however, is the methodology. While Mohd Said et al. [22] utilized a microhardness test for physical characterization, we chose chemical characterization by analyzing the functional groups on the treated enamel surfaces. Our results show that the PO43− group significantly oversaturates the enamel. With continued treatment, the characteristic bands of the OH− group become imperceptible, as the high-viscosity varnishes fully cover them.
While various physical characterization techniques, such as scanning electron microscopy (SEM) and Vickers microhardness, have been used to evaluate remineralization, our study employed Raman spectroscopy. This non-destructive and highly effective chemical characterization method is emerging as a valuable tool for early caries detection. It enabled us to specifically identify the PO43− functional group. Our findings align with the research of Barrera-Ortega et al. [24], who also used Raman spectroscopy to assess the remineralizing effects of three different fluorinated compounds (silver diamine fluoride, difluorosilane, and acidulated phosphate fluoride) on demineralized enamel. They observed characteristic bands for the PO43− and OH− groups at 3621 cm−1. However, our approach differed in that we evaluated the surface of an incipient carious lesion in vitro. We only detected the PO43− functional group and did not observe the OH− group in our three experimental groups. The absence of the OH− signal is attributed to the high viscosity of the fluoride varnishes, which completely cover and saturate the enamel surface. Given these differences, we believe it is essential to conduct multiple assessments to fully understand the effects of these treatments on enamel. For future research, it is advisable to conduct additional experimental studies on Raman spectroscopy, as information on its application in enamel is currently limited. In our current study, only the PO43− group was observed in fluorinated varnishes; it is recommended that this method be applied to dental enamel treated with different remineralizing agents.
The initial increase in surface roughness of demineralized enamel (Ra = 1.33 µm) compared to healthy enamel (Ra = 0.88 µm) confirms the destructive effect of acidic pH (4.4) on enamel integrity, consistent with prior findings on enamel erosion mechanisms [21,25]. Following 15 days of treatment, β-TCP-F significantly restored surface smoothness to Ra = 0.76 µm, matching healthy enamel levels. This outcome aligns closely with Saady et al. [26], who reported comparable roughness recovery following β-TCP-F application under pH cycling. The underlying mechanism involves β-TCP-F acting as a bioavailable reservoir of calcium, phosphate, and fluoride, which adsorb into surface defects and promote the formation of fluorapatite, thereby enhancing roughness and hardness [27]. The application of CDu resulted in a notable reduction in surface roughness, from 1.55 ± 0.09 µm at day 5 to 0.65 ± 0.09 µm at day 10, indicating a swift remineralizing effect. High-fluoride varnishes are well-documented for enhancing enamel microhardness and preventing demineralization, outperforming lower-fluoride formulations, particularly in patients with elevated caries risk [28]. The slight rebound in roughness to 0.99 ± 0.11 µm by day 15, with no significant difference from day 10 (p = 0.1418), likely reflects a natural surface reorganization rather than diminished therapeutic efficacy. This stabilization phase is consistent with previous findings, which show that minor microtopographic adjustments may occur once fluoride-induced remineralization has taken place without compromising structural integrity [28,29].
The findings of this in vitro study suggest that the tested varnishes have a protective and remineralizing effect. Our results are promising and set a precedent for understanding the benefits and efficacy of these remineralizing products. However, these data should be interpreted with caution for daily dental care. The excessive use of fluoride in patients aged 3 to 5 years can directly impact the development of dental fluorosis in their permanent dentition. This effect has been reported in various populations and is often associated with specific dietary habits.
5. Conclusions
This research shows that all three tested varnishes—CDu, FP, and β-TCP—effectively contributed to the partial recovery of enamel microhardness. The FP and β-TCP varnishes were the most effective, supporting their widespread use in clinical practice.
Raman spectroscopy analysis detected phosphate groups (PO43−) on the dental enamel surface, with the CDu and β-TCP groups showing an increase in phosphate presence throughout the treatment. The PO43− functional group was not observed on days 10 and 15, which is not a sign of ineffectiveness. Instead, the high viscosity and low solubility of the varnishes likely caused them to cover the enamel excessively, obscuring the signal of the phosphate groups from the Raman test.
Fluoride ion measurements showed that the Duraphat varnish remineralizing solution liberated up to 50 ppm of fluoride, while the Clinpro White Varnish solution reached 40 ppm over the 15-day treatment period. Due to these high levels, the clinical application of these products should be limited in patients aged 3 to 5 years to prevent dental fluorosis.
The application of remineralizing varnishes helps prevent the demineralization of dental enamel. Given a patient’s age, oral hygiene, and diet, these varnishes are recommended not only for pediatric patients at high risk of caries but also for a broader population.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/coatings15091091/s1. Figure S1: Full Raman spectra of the experimental samples; Figure S2: Full Raman spectra of the Duraphat, Colgate; Figure S3: Full Raman spectra of the Flour Protector, Ivoclar; Figure S4: Full Raman spectra of the Clinpro white varnish, 3M ESPE.
Author Contributions
Conceptualization, C.C.B.-O. and S.E.R.; methodology, K.I.P.-D., S.E.M.-G. and N.R.-Y.; software, S.E.M.-G. and K.I.P.-D.; validation, C.C.B.-O. and O.N.-Y.; formal analysis, C.C.B.-O., K.I.P.-D., N.R.-Y. and O.N.-Y.; investigation, C.C.B.-O., S.E.M.-G. and I.L.A.-V.; resources, C.C.B.-O. and I.L.A.-V.; data curation, O.N.-Y. and C.A.C.-G.; writing—original draft preparation, C.C.B.-O., K.I.P.-D. and C.A.C.-G.; writing—review and editing, C.C.B.-O., O.N.-Y., I.L.A.-V., N.R.-Y. and S.E.R.; visualization, O.N.-Y. and C.A.C.-G.; supervision, C.C.B.-O. and S.E.R.; project administration, C.C.B.-O.; funding acquisition, C.C.B.-O. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This study was approved by the Ethics Committee of the Faculty of Higher Studies (FES) Iztacala (CE/FESI/032022/1499, approved on 1 March 2022), in accordance with the Declaration of Helsinki.
Informed Consent Statement
The third molars studied were obtained from donors undergoing dental extraction procedures through donations from maxillofacial surgeons from different private clinics. Each participant was explained the procedures and given an “informed consent” form, which they signed. This study followed the Declaration of Helsinki.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Acknowledgments
This work was supported by UNAM, DGAPA, and PAPIIT IA-202423—Department of Low-Dimensional Materials, Institute of Materials Research, National Autonomous University of Mexico (UNAM).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Hegedűs, M.; Kovács, Z.; Vásárhelyi, L.; Kukovecz, Á.; Illés, L.; Szász, N.; Mlinkó, É.; Katinka, N.R.; Kis, V.K. Ribbon-like hypomineralization in human dental enamel. Acta Biomater. 2025, 196, 281–292. [Google Scholar] [CrossRef]
- Arola, D.; Gao, S.; Zhang, H.; Masri, R. The tooth: Its structure and properties. Dent. Clin. N. Am. 2017, 61, 651. [Google Scholar] [CrossRef]
- Webb, E.C.; White, C.D.; Longstaffe, F.J. Investigating inherent differences in isotopic composition between human bone and enamel bioapatite: Implications for reconstructing residential histories. J. Archaeol. Sci. 2014, 50, 97–107. [Google Scholar] [CrossRef]
- Machiulskiene, V.; Campus, G.; Carvalho, J.C.; Dige, I.; Ekstrand, K.R.; Jablonski-Momeni, A.; Maltz, M.; Manton, D.J.; Martignon, S.; Martinez-Mier, E. Terminology of dental caries and dental caries management: Consensus report of a workshop organized by ORCA and Cariology Research Group of IADR. Caries Res. 2020, 54, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Roopa, K.B.; Pathak, S.; Poornima, P.; Neena, I.E. White spot lesions: A literature review. J. Pediatr. Dent. 2015, 3, 1–7. [Google Scholar] [CrossRef]
- Bishara, S.E.; Ostby, A.W. White spot lesions: Formation, prevention, and treatment. Semin. Orthod. 2008, 14, 174–182. [Google Scholar] [CrossRef]
- Belli, R.; Rahiotis, C.; Schubert, E.W.; Baratieri, L.N.; Petschelt, A.; Lohbauer, U. Wear and morphology of infiltrated white spot lesions. J. Dent. 2011, 39, 376–385. [Google Scholar] [CrossRef]
- de Dios Teruel, J.; Alcolea, A.; Hernández, A.; Ruiz, A.J.O. Comparison of chemical composition of enamel and dentine in human, bovine, porcine and ovine teeth. Arch. Oral Biol. 2015, 60, 768–775. [Google Scholar] [CrossRef]
- Batista, G.R.; Torres, C.R.G.; Sener, B.; Attin, T.; Wiegand, A. Artificial saliva formulations versus human saliva pretreatment in dental erosion experiments. Caries Res. 2016, 50, 78–86. [Google Scholar] [CrossRef]
- Manchanda, S.; Liu, P.; Sardana, D.; Peng, S.; Lo, E.C.; Yiu, C.K. Randomized clinical trial to compare three fluoride varnishes in preventing early childhood caries. J. Dent. 2024, 147, 105141. [Google Scholar] [CrossRef]
- Sun, J.; Wu, T.; Fan, Q.; Hu, Q.; Shi, B. Comparative study of hydroxyapatite, fluor-hydroxyapatite and Si-substituted hydroxyapatite nanoparticles on osteogenic, osteoclastic and antibacterial ability. RSC Adv. 2019, 9, 16106–16118. [Google Scholar] [CrossRef]
- Barrera-Ortega, C.C.; Olmos, A.R.V.; Berrú, R.I.S.; Itzel, P.D.K. Application of Raman Spectroscopy for Dental Enamel Surface Characterization. In Infrared Spectroscopy-Perspectives and Applications; IntechOpen: London, UK, 2022. [Google Scholar]
- Fernandez-de-Quezada, R.B.E.; Escobar, G.A.A.; de González, W.Y.E.; Cartagena, F.J.R.; Anaya, S.M.S. Vigilancia epidemiológica de enfermedades bucales de población atendida en Unidades de Salud en el año 2021. Rev. Minerva 2023, 6, 37–53. [Google Scholar] [CrossRef]
- Guido, P.M.P.; Aguilar, G.D.; Torres, S.C. Developments in the use of varnish flúor. Reporte case. Rev. Odontopediatría Latinoam. 2021, 3, 111–117. [Google Scholar]
- Malcangi, G.; Patano, A.; Morolla, R.; De Santis, M.; Piras, F.; Settanni, V.; Mancini, A.; Di Venere, D.; Inchingolo, F.; Inchingolo, A.D. Analysis of dental enamel remineralization: A systematic review of technique comparisons. Bioengineering 2023, 10, 472. [Google Scholar] [CrossRef] [PubMed]
- Juárez-López, M.L.A.; Adriano-Anaya, M.P.; Molina-Frechero, N.; Murrieta-Pruneda, F. Remineralization effect on incipient carious lesions of a sodium fluoride with tricalcium phosphate varnish. Acta Pediátrica México 2019, 39, 263–270. [Google Scholar]
- Seppä, L. Fluoride varnishes in caries prevention. Med. Princ. Pract. 2004, 13, 307–311. [Google Scholar] [CrossRef] [PubMed]
- Bijle, M.N.; Abdalla, M.M.; Ashraf, U.; Ekambaram, M.; Yiu, C.K.Y. Enamel remineralization potential of arginine-fluoride varnish in a multi-species bacterial pH-cycling model. J. Dent. 2021, 104, 103528. [Google Scholar] [CrossRef]
- Skucha-Nowak, M.; Gibas, M.; Tanasiewicz, M.; Twardawa, H.; Szklarski, T. Natural and Controlled Demineralization for Study Purposes in Minimally Invasive Dentistry. Adv. Clin. Exp. Med. Off. Organ Wroc. Med. Univ. 2015, 24, 891–898. [Google Scholar] [CrossRef]
- Ten Cate, J.M. Models and role models. Caries Res. 2015, 49, 3–10. [Google Scholar] [CrossRef]
- Barrera-Ortega, C.C.; Rodil, S.E.; Silva-Bermudez, P.; Delgado-Cardona, A.; Almaguer-Flores, A.; Prado-Prone, G. Fluoride Casein Phosphopeptide and Tri-Calcium Phosphate Treatments for Enamel Remineralization: Effects on Surface Properties and Biofilm Resistance. Dent. J. 2025, 13, 246. [Google Scholar] [CrossRef]
- Farooq, I.; Bugshan, A. The role of salivary contents and modern technologies in the remineralization of dental enamel: A narrative review. F1000Research 2021, 9, 171. [Google Scholar] [CrossRef]
- Mohd Said, S.N.; Ekambaram, M.; Yiu, C.K. Effect of different fluoride varnishes on remineralization of artificial enamel carious lesions. Int. J. Paediatr. Dent. 2017, 27, 163–173. [Google Scholar]
- Barrera-Ortega, C.; Vázquez-Olmos, A.; Sato-Berrú, R.; Araiza-Téllez, M. Study of demineralized dental enamel treated with different fluorinated compounds by Raman spectroscopy. J. Biomed. Phys. Eng. 2020, 10, 635. [Google Scholar] [CrossRef]
- Magalhães, G.D.A.; Fraga, M.A.A.; de Souza Araújo, I.J.; Pacheco, R.R.; Correr, A.B.; Puppin-Rontani, R.M. Effect of a self-assembly peptide on surface roughness and hardness of bleached enamel. J. Funct. Biomater. 2022, 13, 79. [Google Scholar] [CrossRef]
- Al Saady, D.; Hall, C.; Edwards, S.; Reynolds, E.C.; Richards, L.C.; Ranjitkar, S. Erosion-inhibiting potential of the stannous fluoride-enriched CPP-ACP complex in vitro. Sci. Rep. 2023, 13, 7940. [Google Scholar]
- Wakwak, M.A.; Alaggana, N.A.A.A.; Morsy, A.S. Evaluation of surface roughness and microhardness of enamel white spot lesions treated by resin infiltration technique (icons): An: In-vitro: Study. Tanta Dent. J. 2021, 18, 88–91. [Google Scholar] [CrossRef]
- Suwannapong, N.; Chantarangsu, S.; Kamnoedboon, P.; Srinivasan, M.; Pianmee, C.; Bunsong, C.; Sivavong, P.; Nantanapiboon, D. Effect of different protocols in preventing demineralization in irradiated human enamel, in vitro study. BMC Oral Health 2025, 25, 46. [Google Scholar] [CrossRef] [PubMed]
- Enerbäck, H.; Lingström, P.; Möller, M.; Nylén, C.; Bresin, C.Ö.; Ros, I.Ö.; Westerlund, A. Effect of a mouth rinse and a high-fluoride toothpaste on caries incidence in orthodontic patients: A randomized controlled trial. Am. J. Orthod. Dentofac. Orthop. 2022, 162, 6–15.e3. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).