1. Introduction
With the continuous advancement of internal combustion engine (ICE) technologies, the durability, working-temperature wear characteristics, mechanical properties, and oxidation resistance of valves and their assemblies operating under severe tribological and thermal conditions have become increasingly vital. In ICE systems, materials employed in valves and their corresponding seat inserts comprise a tribological pair exposed to combined cyclic impact and sliding contact, resulting from combustion pressure and valve motion dynamics [
1,
2,
3,
4]. Particularly, exhaust valves are exposed to combustion pressures exceeding 20 MPa and temperatures reaching up to 650 °C, while intake valves operate at temperatures up to 350 °C [
5,
6,
7,
8]. Under these elevated thermal and mechanical loads, repeated stress and thermal cycling significantly accelerate surface degradation, ultimately leading to the failure of the valve–seat assembly and a consequential decline in engine performance and efficiency [
3,
9,
10,
11].
The high-temperature performance of valve materials is largely governed by their stability, adherence, and protective surface oxides and to retain microstructural stability under thermal cycling. Therefore, ferritic and austenitic stainless steels are frequently employed in valve applications thanks to their favorable integrated oxidation-resistant performance, mechanical strength, and cost-effectiveness [
12,
13,
14,
15,
16,
17]. Among these, 1.4718 ferritic stainless steel, with its chromium and silicon content, supports the development of Cr-rich and Si-rich oxide layers, whereas 1.4871 austenitic stainless steel, with high chromium (~21 wt.%) and manganese (~10 wt.%) levels, facilitates the formation of dense, multi-layered oxide scales that enhance oxidation resistance in high-temperature environments [
1,
4,
18,
19,
20]. The oxidation behavior of these alloys is strongly governed by their elemental composition and the type of oxides they promote. Chromium is well recognized for promoting the formation of a stable and adherent Cr
2O
3 scale, which serves as an effective barrier against further oxidation [
12,
13,
14,
20,
21,
22,
23]. Manganese in austenitic steels contributes to the formation of Mn-rich oxides such as MnCr
2O
4 spinel and Mn
2O
3 (corundum structure), which improve scale integrity and adhesion, especially during cyclic conditions [
24,
25,
26,
27]. Silicon, on the other hand, enhances oxidation resistance in ferritic steels by forming Si-containing oxide sublayers at the metal/oxide interface, which suppress outward diffusion of metal cations and inward diffusion of oxygen. These synergistic effects contribute significantly to the long-term cyclic oxidation resistance of high-temperature steels [
28,
29].
Numerous studies have highlighted the influence of these alloying elements on high-temperature oxidation behavior. Lins et al. [
30] observed that Fe–Cr ferritic alloys formed Cr
2O
3 scales with good spallation resistance under cyclic oxidation. Kumar and Mahobia [
24] demonstrated that the presence of high Mn content in Fe–Cr–Mn–N austenitic steels promoted the formation of protective Mn-containing spinels. Similarly, da Silva et al. [
31] announced that the implementation of Si to Fe–Cr alloys improved oxide layer adherence and reduced the oxidation rate. Singh et al. [
32] found that low-Ni austenitic steels formed multilayered M
2O
3 and M
3O
4 oxides under cyclic oxidation, thereby enhancing thermal stability.
Based on the aforementioned literature review, various studies have investigated the individual oxidation behavior of heat-resistant steels, focusing on factors such as oxide layer adhesion, spallation resistance, and oxide phase composition. Nevertheless, the majority of these investigations focus on individual alloys or general alloy categories, without providing a direct, systematic comparison of these two widely utilized grades under uniform testing conditions. In this regard, a direct and comprehensive comparison between the oxidation resistance of 1.4871 and 1.4718 stainless steel remains unexplored in the current literature. This study uniquely addresses this gap by systematically investigating and comparing the cyclic oxidation behavior of 1.4871 and 1.4718 stainless steels under identical thermal conditions (550 °C, 650 °C and 750 °C) over 25 thermal cycles. Advanced microstructural characterization techniques, including SEM, EDS (point, line, and mapping), and XRD, were employed to examine the microstructural developments, composition, and phase evolution of the formed oxide scales. The outcomes are expected to provide valuable insights into the relationship between alloy composition and oxidation mechanisms, thereby aiding in the selection of valve materials for high-temperature ICE applications.
3. Results and Discussion
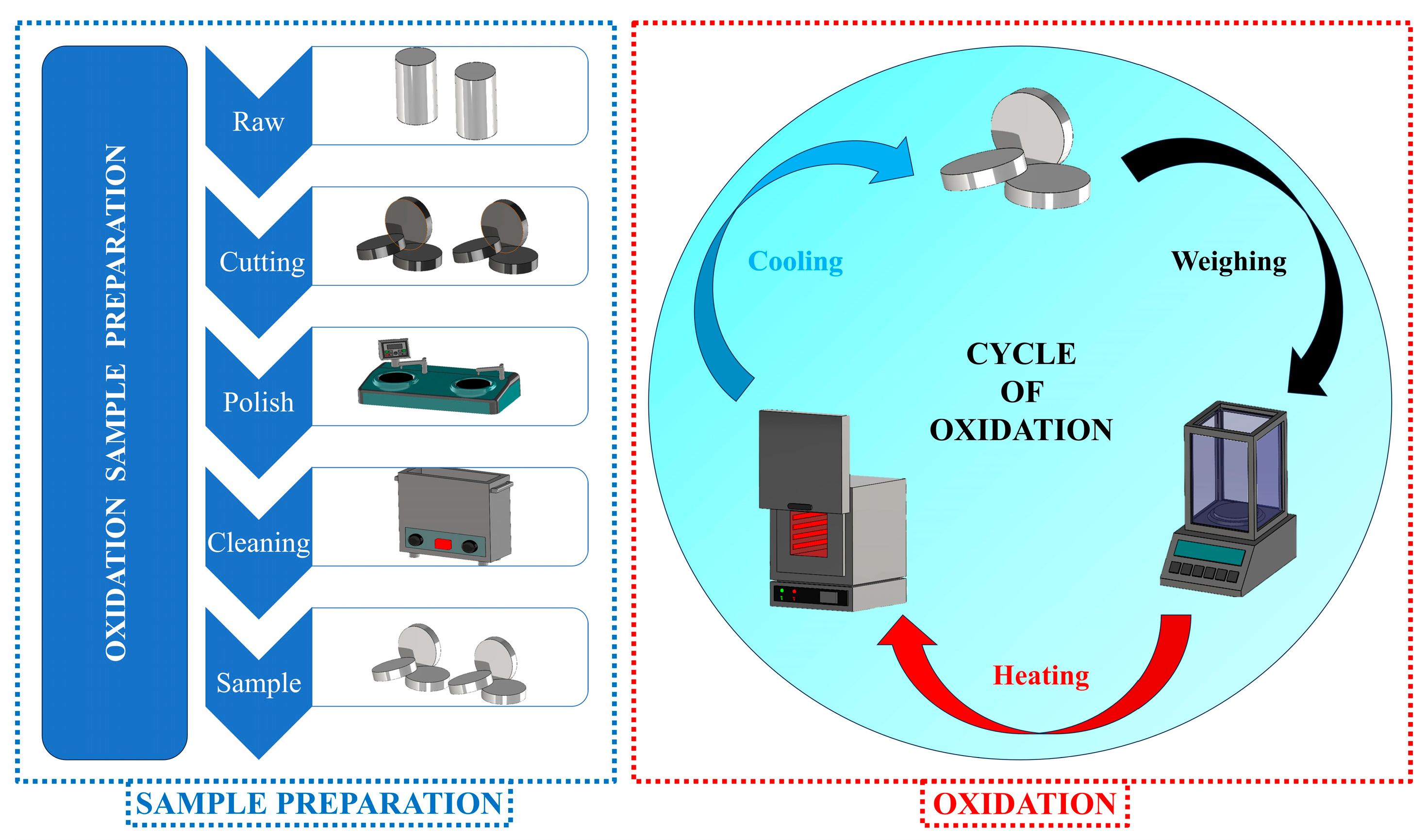
In this study, the high-temperature oxidation behavior of two candidate alloys, 1.4718 and 1.4871, was systematically evaluated through cyclic oxidation testing. The experiments were conducted under laboratory conditions, with each cycle consisting of 1 h of isothermal exposure at 550 °C, 650 °C or 750 °C, followed by cooling to room temperature. All oxidation tests were performed in triplicate for each specimen to ensure reproducibility. The consistency of the oxidation kinetics is illustrated in
Figure 2, where the mass-gain curves are presented together with their standard deviations. The curves revealed two different oxidation stages: an accelerated oxidation phase occurring during cycles 0–9, followed by a more gradual, steady-state growth phase from cycles 10–25. For both alloys, the total mass gain increased with the number of cycles and the test temperature, demonstrating the temperature dependence of the oxidation kinetics. The ferritic stainless steel 1.4718 showed a moderate increase in mass gain across the temperature range, with oxidation becoming more pronounced at higher temperatures. Notably, at 550 °C and 650 °C, the mass gain values of 1.4718 were comparable to those of 1.4871, suggesting similar oxidation behavior in the early to intermediate stages. However, at 750 °C, more pronounced divergence in oxidation resistance was observed between the two alloys, likely due to compositional differences, particularly in such as chromium, silicon and manganese, which strongly influence the development and stability of protective oxide scales.
Alloy 1.4871 displayed the lowest mass gain at all temperatures tested (
Figure 2b), which is attributed to its relatively high Cr (>20 wt.%) and Mn (~10 wt.%) contents. These compositional features are known to enhance the formation of dense, stable, and adherent oxide layers that suppress further oxidation. The results suggest that increasing the Cr and Mn content significantly improves oxidation and spallation resistance by promoting the formation of protective Cr
2O
3-based oxide scales in both 1.4718 and 1.4871 alloys.
Further analysis of the cyclic oxidation behavior of alloys 1.4718 and 1.4871 was conducted using diffusion-controlled kinetics. The mass gain trends were interpreted using Wagner’s parabolic rate law, and
Table 2 summarizes the related 1/
n and parabolic rate constant (
kp) values. The oxidation of 1.4871 alloy follows parabolic kinetics since the 1/
n values (0.53, 0.60, and 0.50 at 550 °C, 650 °C and 750 °C, respectively) are around the optimal parabolic value of 0.5. While the general trend in 1.4718 is also diffusion-controlled, it shows consistently higher 1/
n values (0.78, 0.76, 0.76), indicating slight deviations from perfect parabolic behavior. The temperature dependence of the oxidation rate is confirmed by the increase in
kp values for both alloys with elevating temperature. EDS elemental mapping enabled the identification of distinct oxide scale layers. A continuous Cr
2O
3 layer developed at the metal-oxide interface, while the outer scale consisted primarily of Fe and Mn-containing spinel phases. This compositional stratification aligns with classical oxidation models, where Cr diffusion sustains the formation of a protective inner layer and the outward diffusion Fe and Mn drives the growth of spinel phases in the outer scale. Grain boundary and lattice diffusion paths were also compared to identify potential rate-limiting steps. The result, supported by literature-reported diffusion coefficients of Cr, Fe, and Mn, suggests that lattice diffusion promotes outer spinel formation, whereas Cr diffusion through the chromia layer probably regulates the overall oxidation rate [
35]. Overall, these factors link the experimental results to established diffusion theories and provide a mechanistic explanation for the observed mass gain trends.
Figure 3 and
Table 3 illustrate the surface oxide morphologies and corresponding chemical compositions of alloy 1.4718 following cyclic oxidation at three different temperatures. At 550 °C, only a very thin oxide layer developed on the surface, and no distinct morphological features were observed. Point EDS analysis indicated that the oxide scale was primarily composed of iron (Fe), with minor contributions from chromium (Cr) and oxygen (O) (
Table 3). This suggests the initial formation of a simple, Fe-rich oxide scale under moderate thermal conditions. The minimal oxide growth at this temperature reflects the alloy’s high oxidation resistance at lower temperatures. At 650 °C, specimen surface was fully covered by a uniform, compact, and adherent oxide scale, as seen in the SEM images of
Figure 3. From the examined surface region, the oxide layer appeared relatively dense and free of evident porosity, delamination, or spallation. While top-view SEM provides useful qualitative information, a more comprehensive assessment of porosity would ideally require complementary cross-sectional analysis. Across all analyzed regions, the morphology of the oxide scale was consistent, characterized by irregular, platelet-like structures with sharp edges and flat surfaces. EDS analysis confirmed that the oxide scale was composed of Fe, Cr and O, indicating the formation of mixed iron–chromium oxides (
Table 3). Following oxidation at 750 °C, a more substantial growth of surface oxides was observed. These oxide structures became increasingly irregular and porous but remained uniformly distributed across the surface. Despite the porous nature of the oxide layer, it exhibited strong adhesion to the substrate, with no evidence of cracking or flaking. The chemical composition of the oxide scale, again consisting of Fe, Cr and O, suggested the formation of spinel-type oxides such as FeCr
2O
4 [
21,
22,
36].
Alloy 1.4718 exhibited distinct oxidation behavior characterized by the formation of porous and morphologically heterogeneous oxide structures, particularly at 750 °C. Nevertheless, the oxide layers remained strongly adherent and free of cracks, thereby contributing to the overall oxidation stability of the alloy under cyclic exposure. The presence of spinel-type oxides such as FeCr2O4 at elevated temperatures indicates the formation of a Cr-rich protective scale. This spinel formation plays a critical role in enhancing the structural integrity and adhesion of the oxide scale, thereby improving the alloy’s resistance to thermal cycling.
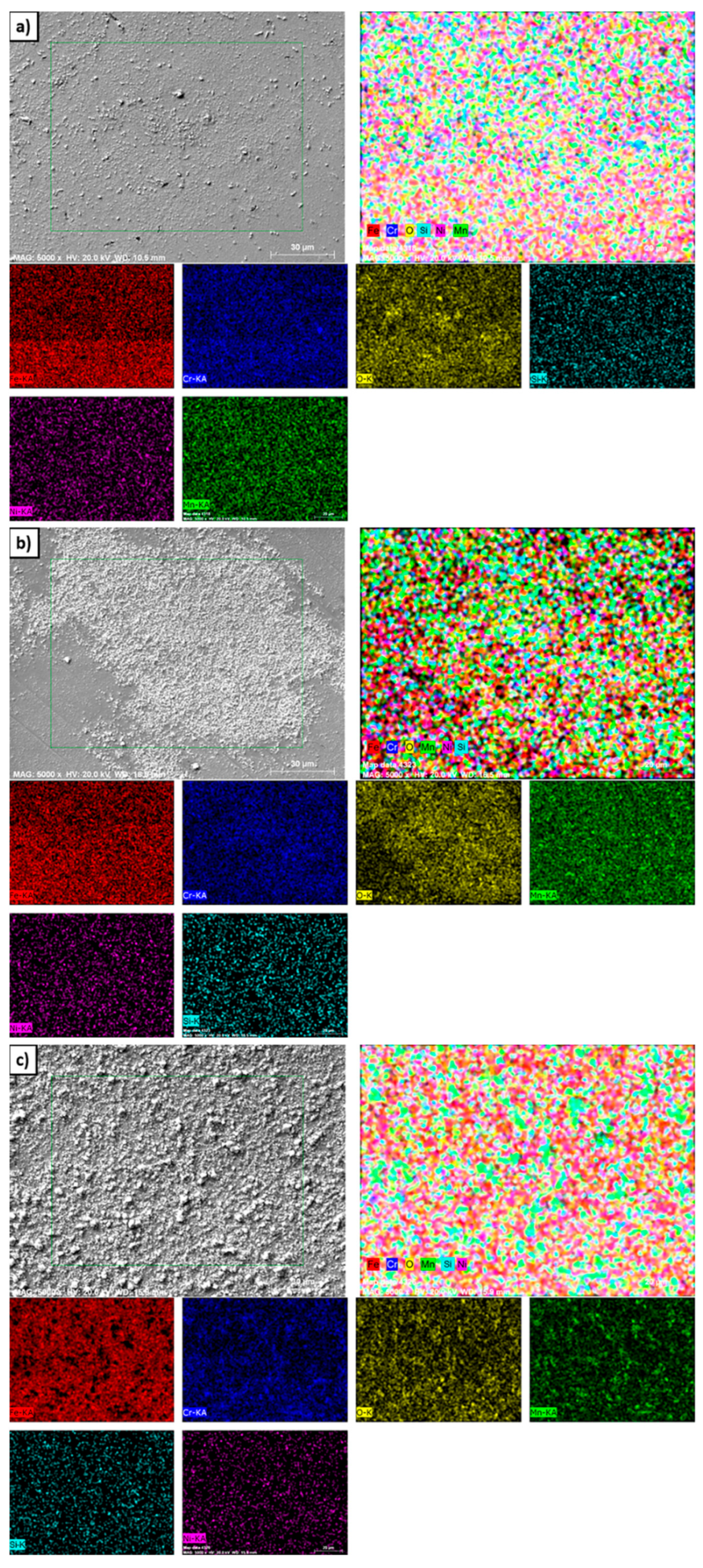
Surface SEM-EDS mapping analyses of the 1.4718 alloy after cyclic oxidation at different temperatures are presented in
Figure 4. Examination of the specimen oxidized at 550 °C reveals that Fe and Cr are widely distributed within the surface oxide layer, and O is also prominently detected. Consistent with the EDS results shown in
Table 3, the mapping images confirm the existence of a Fe-rich oxide scale on the surface. These observations indicate that oxidation is at an early stage at this temperature. At 650 °C, although the oxide layer was relatively thin, it exhibited strong adhesion to the substrate. The observed increase in surface roughness was primarily attributed to the progressive thickening of the oxide layer formed during the oxidation process [
37]. It has also been reported that Cr diffusion facilitated the formation of a more pronounced Cr
2O
3 layer, contributing to the thickening of the surface oxide scale. In the specimen subjected to cyclic oxidation at 750 °C, localized enrichment of Fe−O compounds were observed, while Cr−O species appeared to be uniformly and strongly distributed across the entire surface. This suggests the formation of a Cr-rich oxide sublayer at this temperature, consistent with Cr
2O
3. Although XRD did not reveal distinct Cr
2O
3 peaks, likely due to the thinness or poor crystallinity of this layer, EDS mapping indicates that Cr enrichment becomes more pronounced with increasing oxidation temperature.
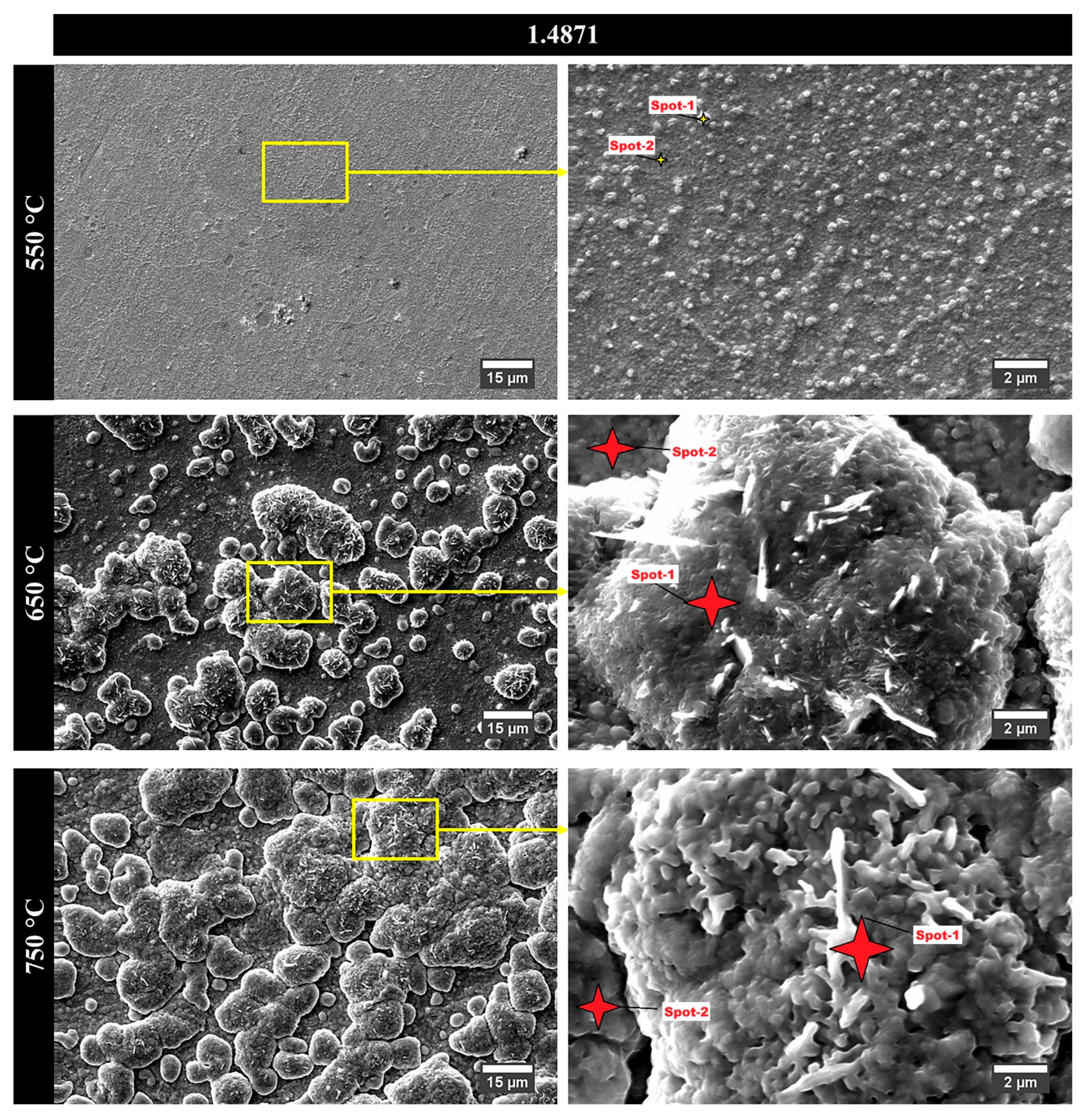
Figure 5 and
Table 4 illustrate the surface oxide morphologies and corresponding elemental compositions of alloy 1.4871 after cyclic oxidation at three different temperatures.
Table 4 presents two distinct sets of data for each temperature: one corresponding to the surface oxide particles and the other to the suboxide layer found between these particles. At 550 °C, the oxidized surface exhibited a thin suboxide layer overlaid with spherical oxide particles. EDS analysis indicated that the particles were primarily composed of Fe and O, while the underlying sublayer contained Fe, Cr, and O. This suggests that Cr actively contributed to the formation of the suboxide layer, thereby improving oxidation resistance at low temperature. At 650 °C, the oxide scale fully covered the specimen surface, and the development of oxide particles with a distinct cauliflower-like morphology was observed. These particles were separated by visible inter-particle gaps, and internal rod- or beam-shaped structures were detected within the cauliflower clusters. At 550 °C, the oxide layer was composed predominantly of fine spherical particles. With increasing temperature, these features gradually coalesced into more complex cauliflower-like structures. This morphological evolution was consistently observed across all specimens, with the oxides generally exhibiting irregular, plate-like characteristics at higher magnifications, reflecting the typical growth behavior of mixed oxide scales. EDS results showed that the oxide particles were composed of Fe and O, whereas the suboxide regions contained Fe, Cr, Mn, and O, suggesting the presence of complex mixed oxides. Following oxidation at 750 °C, the general surface morphology remained similar to that at lower temperatures; however, a notable increase in the size of the cauliflower-like oxide particles was observed. This growth was attributed to enhanced diffusion kinetics at elevated temperatures, which facilitated the outward migration of elements with high oxygen affinity [
26,
38]. EDS analysis confirmed that these larger surface particles consisted entirely of Fe and O, corresponding to Fe
2O
3. The suboxide regions, in contrast, showed high concentrations of Fe, Cr, Mn, and O, consistent with the formation of spinel-type mixed oxides, possibly of the (Fe, Cr, Mn)
3O
4 type.
Compared to alloy 1.4718, and alloy 1.4871 exhibited distinct oxidation behavior in both surface morphology and chemical composition. While alloy 1.4718 developed porous but strongly adherent oxide layers primarily composed of Fe- and Cr-rich oxides, including spinel-type FeCr2O4, alloy 1.4871 formed more complex, multiphase oxide scales especially at 650 °C and 750 °C. These layers were characterized by Fe2O3-rich cauliflower-like structures on the surface and sublayers enriched in chromium (Cr) and manganese (Mn). The presence of Mn in 1.4871 played a significant role in the oxidation mechanism by promoting the formation of Mn-containing spinel phases, which may contribute to improved scale adherence and structural integrity under thermal cycling conditions. In summary, although higher temperatures led to thicker and more complex oxide structures in both alloys, the specific morphology and phases of the oxides were primarily governed by the elemental composition of each alloy.
Figure 6 illustrates the surface SEM-EDS elemental distribution maps for the 1.4871 alloy following cyclic oxidation tests conducted at three different temperatures. At 550 °C, a thin and discontinuous oxide layer suggested a relatively low oxidation rate. Surface analysis reveals the presence of oxide-forming elements such as Fe, Cr, and Mn. However, the dominance of Fe−O compounds suggested that Fe primarily contributed to the initial oxidation process. This behavior can be attributed to the faster outward diffusion kinetics of Fe relative to Cr, which promotes the preferential formation of Fe-rich oxides under the given conditions. Consequently, the preferential formation of Fe-rich oxides was more pronounced under these conditions. At 650 °C, the oxide particles showed significant growth, indicating a thermally accelerated oxidation mechanism. SEM-EDS mapping demonstrates that the diffusion rates of Fe, Mn and Cr were markedly enhanced, facilitating the development of complex oxide phases across the surface. In particular, manganese-based oxides became more distinguishable at this temperature, indicating a greater thermodynamic driving force for Mn migration and subsequent oxidation. At 750 °C, the oxidation process progresses further and resulted in the agglomeration and coalescence of oxide particles into a more continuous and compact scale. This morphological evolution was indicative of enhanced cationic mobility and increased reactivity of surface-active elements. The larger oxide particles observed on the surface of the 1.4871 alloy were primarily composed of Fe−O compounds, whereas the overall oxide scale exhibited an outer layer rich in MnO
3, underlain by a sublayer enriched in CrO
3. These thermodynamically stable oxide sublayers were primarily produced through the preferred migration of elements with high oxygen affinity, such as Cr and Mn, rather than the larger surface Fe-O particles. As also observed by Singh et al. [
39], this behavior was consistent with SEM-EDS mapping and XRD analysis, demonstrating that the distribution and composition of the oxide scale are controlled by temperature-driven diffusion of reactive elements.
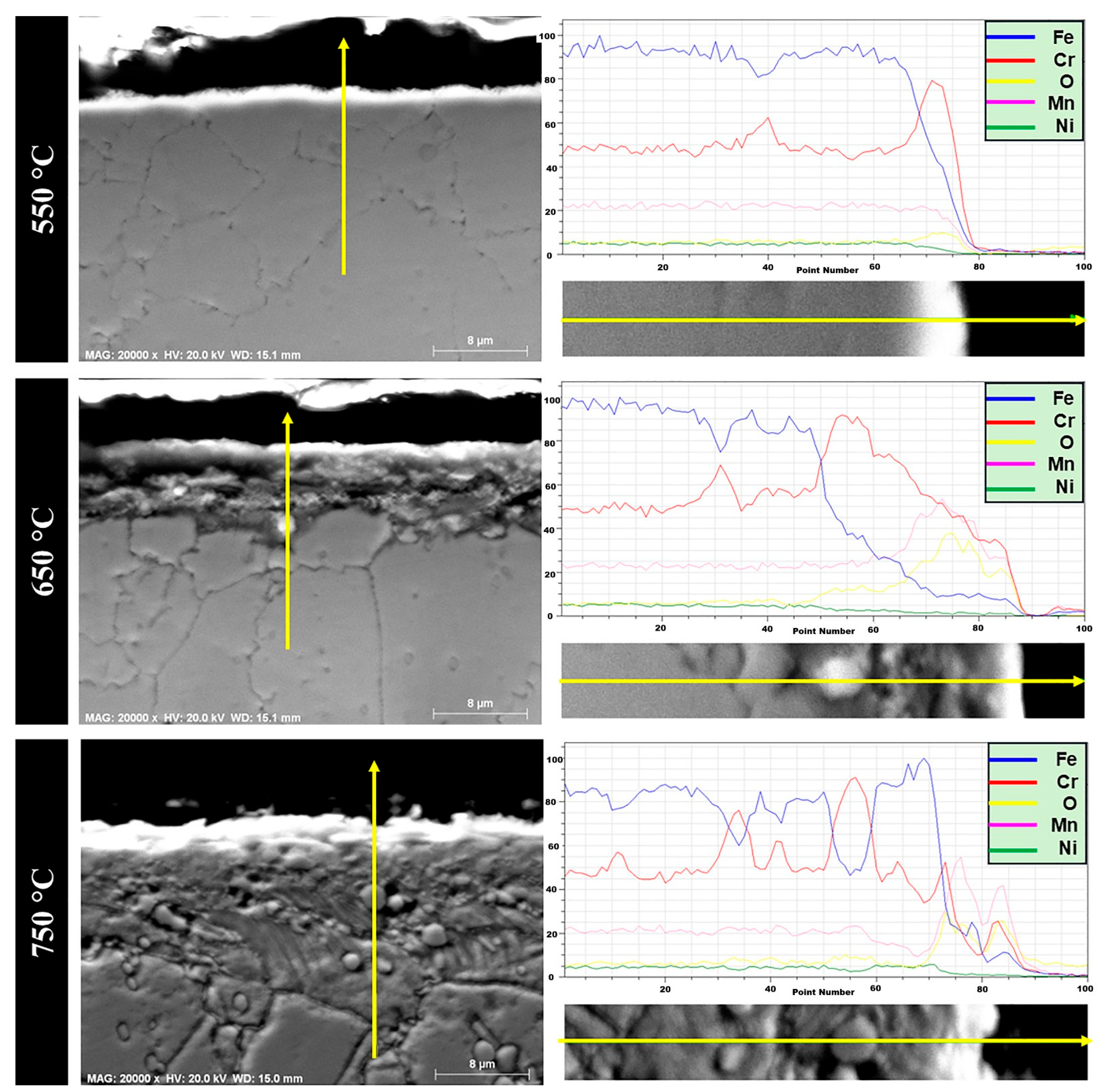
The investigation of surface oxides alone does not typically reflect the entire oxidation mechanism. In addition to surface oxide layers, internal oxidation products may also form beneath the surface oxide scale or just above the substrate alloy. In this study, cross-sectional analyses of the 1.4718 and 1.4871 alloys after oxidation at three different temperatures were performed using line scan, point and mapping EDS techniques. Point and mapping EDS were applied only at 750 °C to characterize the oxidation behavior of both alloys.
Figure 7 presents the cross-sectional micrographs of the 1.4718 alloy after cyclic oxidation at three different temperatures. The oxide scale formed on 1.4718 remained relatively thin across all tested conditions. According to our findings, the 1.4718 alloy’s XRD analysis primarily revealed Fe peaks, with no clearly detectable oxide phases. However, line scan profiles and cross-sectional SEM-EDS confirmed that the oxide scale consists of Fe, Cr and O elements. Furthermore, increasing the oxidation temperature from 550 °C to 750 °C resulted in a roughly twofold increase in oxide layer thickness. This growth is attributed to the acceleration of diffusion processes at higher temperatures. Specifically, the formation and continuity of the oxide layer were promoted through the simultaneous diffusion of oxygen inward and the outward diffusion of Fe and Cr cations. These findings suggest that a temperature-dependent, diffusion-controlled mechanism governs the oxidation behavior of the 1.4718 alloy [
15,
16]. At 650 °C, the EDS results showed the presence of Fe, Cr, Si and O elements in the oxide layer. This observation is consistent with previous reports indicating the formation of Fe
2O
3 and complex spinel-type oxides such as Fe
2SiO
4 and Cr
2SiO
4, which are thermodynamically stable in this temperature range [
28]. Similarly, EDS line scan analysis after oxidation at 750 °C revealed the presence of Fe, Cr and O, suggesting the continuation of spinel and chromia-based oxide formation at elevated temperatures. In order to more precisely identify the oxide phases and clearly differentiate the layered structure, EDS mapping was employed. This technique enabled the determination of oxide species present in the subsurface and at the metal/oxide interface with greater spatial resolution. At 750 °C, cross-sectional EDS mapping analysis was applied to the oxidized 1.4718 alloy to better characterize the oxide layers and identify the oxidation products as shown in
Figure 8. The mapping results revealed a two-layered oxide structure. A composition dominated by Fe, Cr, Si and O was detected in the outer oxide region, while the interface between the oxide and base metal showed a thin layer rich in Fe and O.
According to the literature, iron’s strong chemical interaction with oxygen facilitates the initial nucleation and growth of Fe-oxides ahead of other alloying constituents during the early stages of the oxidation process [
15,
16,
29,
40]. This explains the observed enrichment of Fe at the metal/oxide interface, where Fe
2O
3 likely forms first. Subsequently, Cr and Si in the alloy contribute to the formation of a Fe−Cr−Si−O rich oxide scale in the outer region. This is indicative of the formation of a spinel-type oxide, most likely Cr
2SiO
4, which is known to form in Fe−Cr−Si alloys during high-temperature oxidation [
41,
42,
43]. In agreement with these findings, Song’s thesis work also reported that Si-containing ferritic alloys tend to form spinel oxides such as Fe
2SiO
4 or Cr
2SiO
4, depending on the alloy composition and oxidation environment [
44]. Thus, the presence of Cr and Si together with oxygen in the outer oxide layer strongly supports the formation of spinel-type Cr
2SiO
4 as the dominant oxidation product in this region.
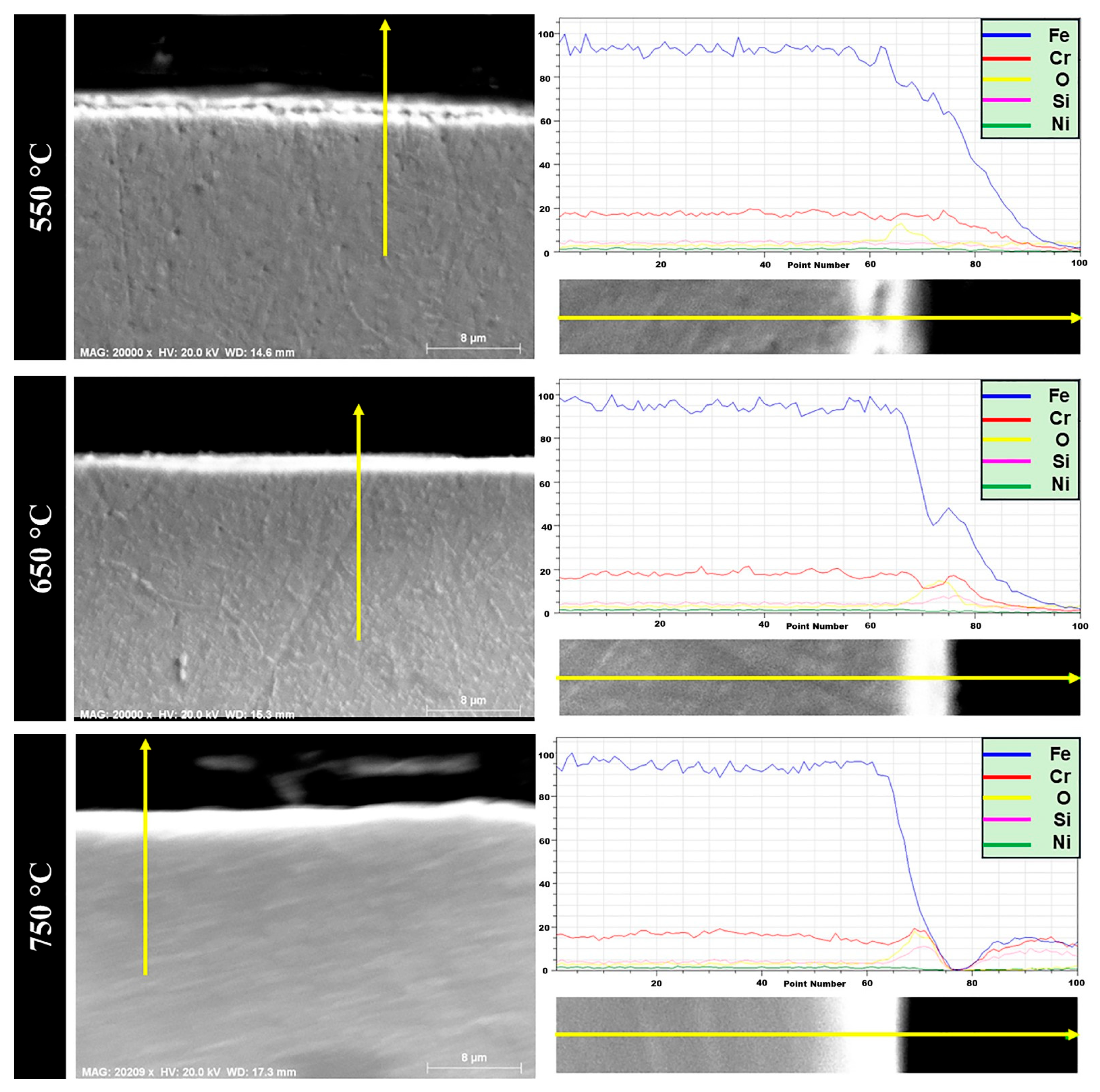
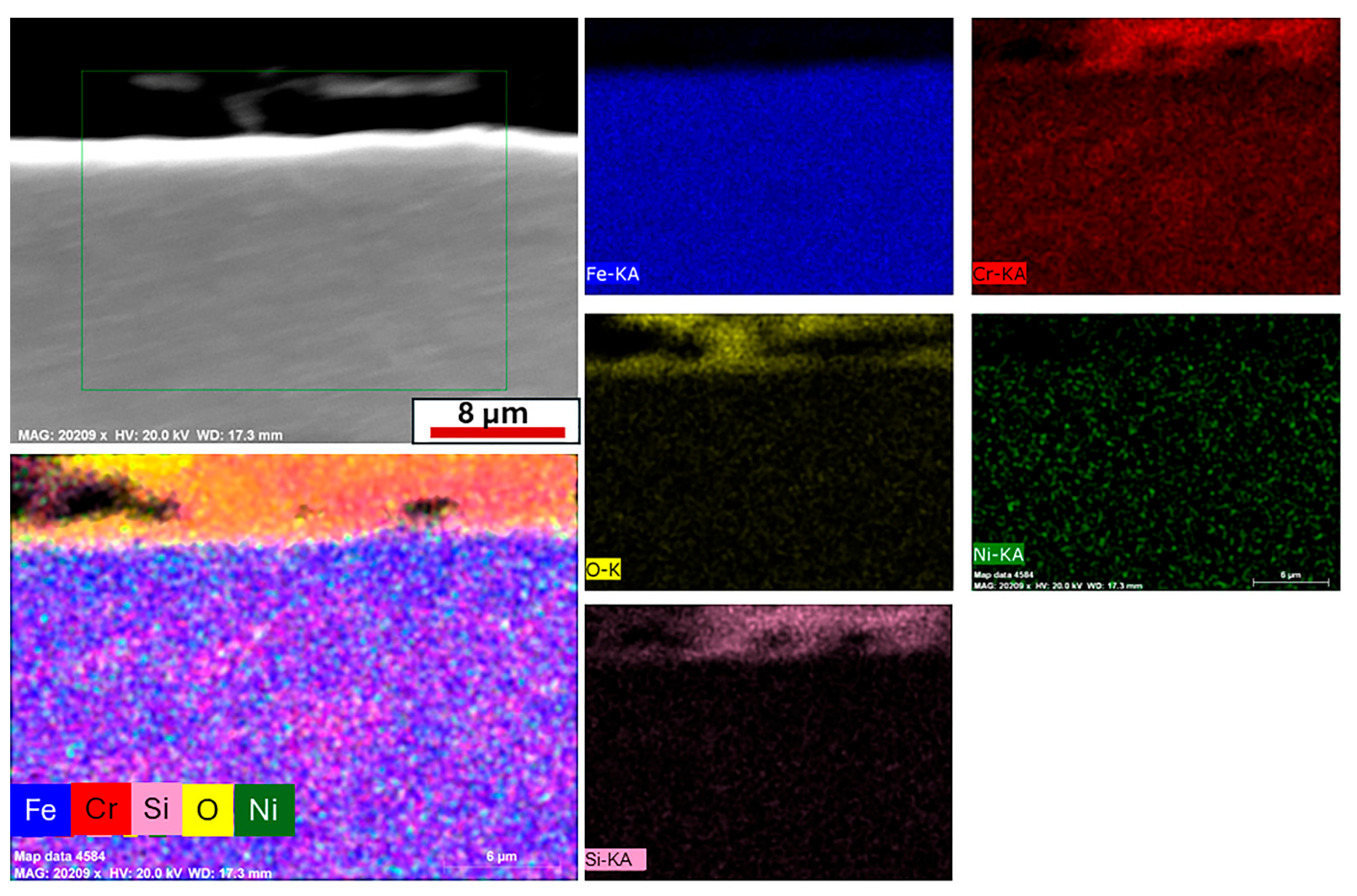
The austenitic 1.4871 alloy was subjected to cyclic oxidation at 550 °C, 650 °C and 750 °C, and cross-sectional analyses are presented in
Figure 9. EDS line scan results revealed that the oxide layer thickness at all three temperatures was significantly thinner compared to that of the 1.4718 alloy. This observation is consistent with the mass gain data obtained from thermogravimetric measurements, as earlier shown in
Figure 2. With increasing temperature, microstructural changes were observed in the oxide scale, accompanied by a gradual increase in oxide thickness. Notably, at 650 °C and 750 °C, unlike in 1.4718, a distinct affected zone appeared at the metal/oxide interface. As described in the study by Pei et al., such oxidation-affected zones typically consist of two distinct regions: (i) a well-defined outer oxidation zone and (ii) a broader internal oxidation zone [
45]. The internal oxidation region generally contains a mixture of fine oxide precipitates and a diffusion-affected metal matrix. In the current study, this two-region structure was clearly observed in the 1.4871 alloy, particularly at 650 °C and 750 °C.
At 550 °C, EDS line analysis of the oxidized 1.4871 alloy revealed the formation of a surface oxide consisting of Fe, Cr and O, which is attributed to the development of a protective FeCr2O4 layer. Unlike the 1.4718 alloy, where Fe-rich oxides predominate and the Cr contribution remains limited, the higher Cr content in 1.4871 promotes the formation of a more continuous and stable spinel oxide scale, representing a key distinction in oxidation behavior between the two alloys.
At 650 °C, the EDS results showed the presence of Fe, Cr, Mn and O elements within the oxide scale. This suggests the formation of thermodynamically stable oxides such as Fe
2O
3, Cr
2O
3 and Mn
2O
3, or the development of complex spinel phases such as FeCr
2O
4, Fe
2MnO
4, MnCr
2O
4, as well as mixed oxides formed from their combinations [
46]. The presence of approximately 9 wt. % Mn in the alloy significantly influences its oxidation behavior. It is well established that in Fe-based austenitic alloys, manganese exhibits preferential oxidation behavior. Accordingly, oxide products such as Mn oxides, Fe oxides, Mn-Cr or Mn-Fe spinels have been reported under similar conditions [
24,
25,
27,
47,
48].
At 750 °C, the EDS line scan analysis of the oxidized 1.4871 alloy exhibited a similar trend to that observed at 650 °C. However, to more precisely identify the oxide phases and distinguish the layered oxide structure, both EDS mapping and point analysis were conducted. These techniques enabled the identification of oxide species within the subsurface region and at the metal/oxide interface with improved spatial resolution.
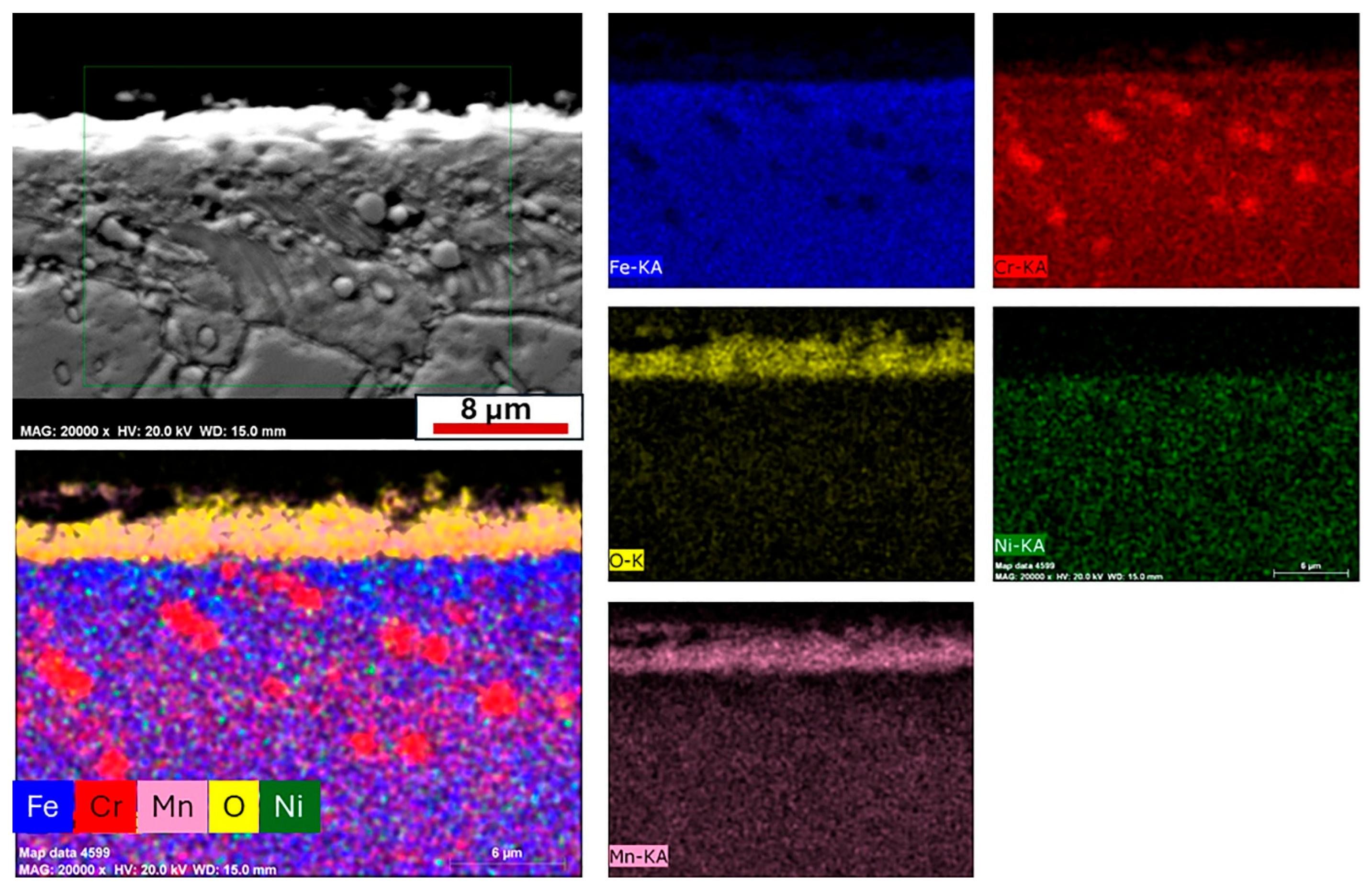
Cross-sectional EDS mapping of the alloy oxidized at 750 °C was performed to better characterize the oxide layers and oxidation products (
Figure 10). In addition, the oxide scale thickness of both alloys was quantitatively determined from SEM micrographs using the ImageJ 1.8.0 software. To obtain representative values, ten measurements were taken from different locations on each specimen. The mean values and standard deviations were calculated to evaluate the uniformity and reproducibility of the oxide scale thickness. The results are presented in
Table 5, provided to aid in the interpretation of the cross-sectional EDS mapping results. The mapping results revealed a two-layered oxide structure. The outer oxide layer consisted mainly of Mn and O, while the thin inner layer located at the metal/oxide interface exhibited higher concentrations of Cr and O. The presence of Cr
2O
3 in the inner layer indicates the formation of a protective and thermodynamically stable oxide, which serves as a diffusion barrier and contributes to the alloy’s high-temperature oxidation resistance. The formation of Mn
2O
3 in the outer layer, together with Cr
2O
3 in the inner region, appears to enhance mechanical adhesion and mitigate thermal stresses at the metal/oxide interface due to the difference in thermal expansion coefficients between the oxide scale and the base metal [
15,
49,
50,
51]. This layered oxide morphology suggests that the coexistence of different oxide phases not only affects oxidation kinetics but also plays a critical role in stabilizing the metal/oxide interface under thermal cycling conditions.
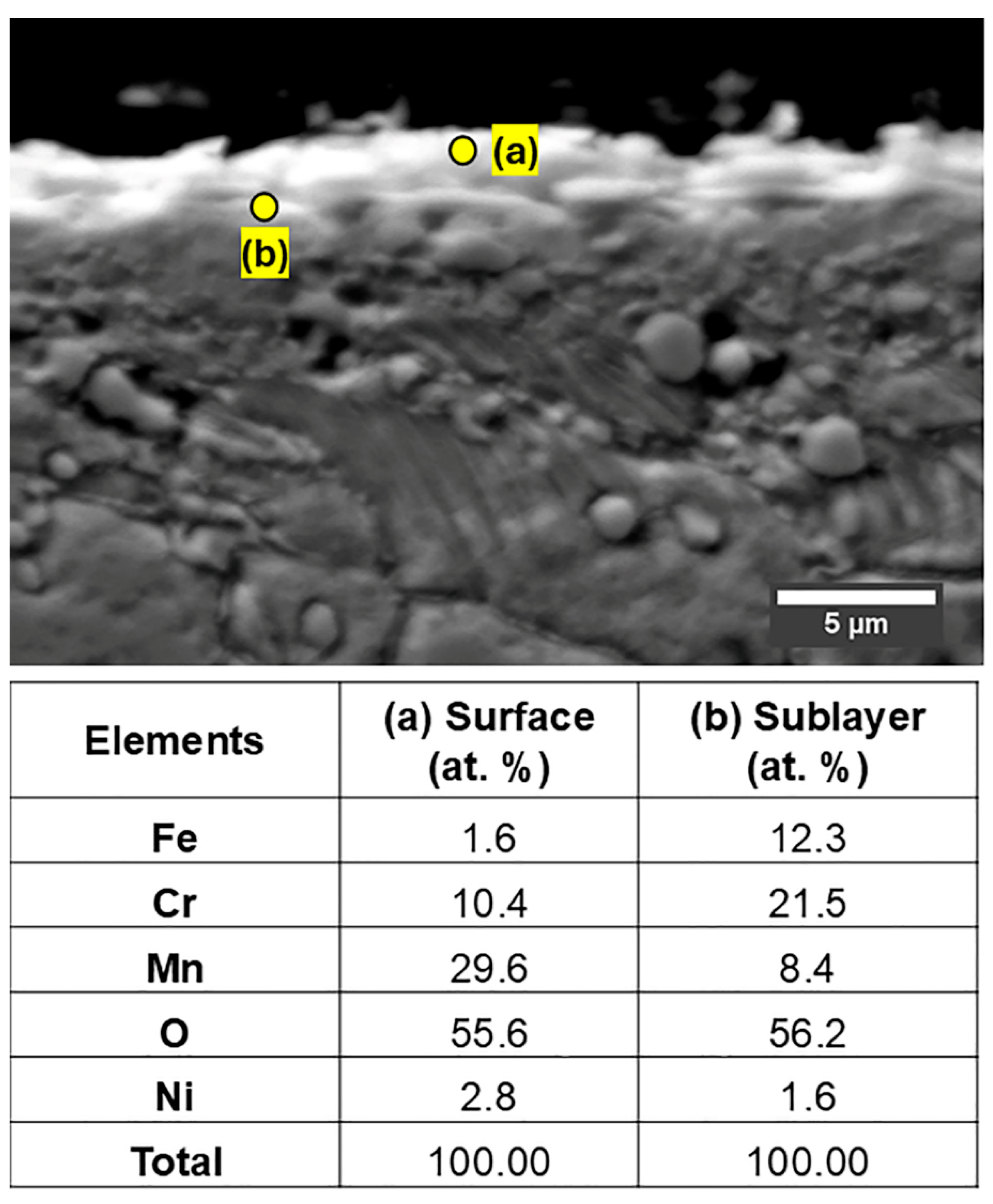
Figure 11 presents the EDS point analysis results for the 1.4871 alloy. The measurement taken from the surface oxide layer revealed a high concentration of Mn and O, together with a considerable amount of Cr, indicating that the outer oxide layer is predominantly composed of a mixed oxide, (Mn,Cr)
2O
3. This observation is consistent with and further supports the results obtained from the EDS line scan and mapping analyses. The measurement performed at the metal/oxide interface showed a significant enrichment of Cr, suggesting the presence of Cr
2O
3 as the primary oxide phase in the inner layer. This finding confirms the formation of a Cr-rich protective oxide layer adjacent to the metal substrate. Both EDS point measurements corroborate the earlier line scan and elemental mapping data, clearly demonstrating that the oxide scale is composed of two distinct compositional layers. This layered oxide structure highlights the complex oxidation behavior of the alloy at elevated temperatures and provides valuable insights into the phase distribution across the oxide scale.
In the case of the 1.4718 alloy, no distinct oxide peaks were observed in the XRD patterns, which is likely due to the very thin nature of the oxide layer or its low crystallinity. The oxide phases in this alloy were therefore identified by SEM-EDS analysis. The oxidation behavior and phase evolution of 1.4718 ferritic stainless steel under cyclic and isothermal oxidation conditions have been extensively studied in the literature. Different reports [
52,
53,
54] have confirmed that the Cr and Si content in this alloy promotes the formation of Cr
2O
3 and internal SiO
2 phases, depending on temperature and atmosphere. As for the 1.4871 alloy, it exhibits complex oxide formation involving Cr
2O
3, spinel-type oxides such as (Mn,Fe)Cr
2O
4, and possible hematite (Fe
2O
3), whose relative distribution and crystallographic identification are critical for performance assessment. Given this well-established oxidation mechanism, this paper focuses on the XRD analysis of the 1.4871 austenitic steel, for which phase evolution under cyclic oxidation conditions is comparatively less reported, particularly in the context of long-term exposure.
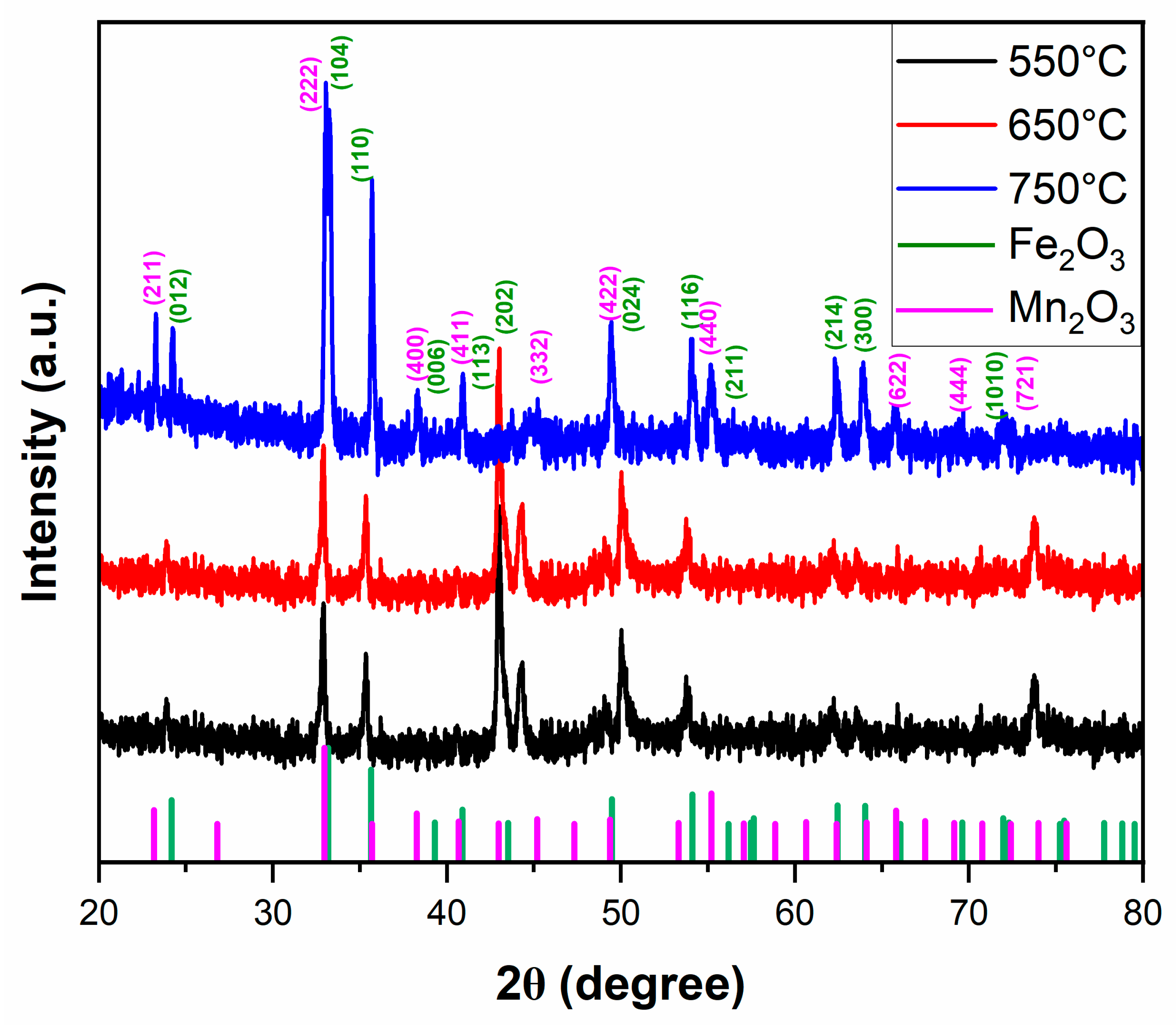
The XRD analysis results employed to identify the surface phases formed on the oxide layer of the 1.4871 alloy after cyclic oxidation are presented in
Figure 12. In the patterns, the principal diffraction peaks were annotated with their specific 2θ positions and Miller indices, providing clearer evidence for phase identification. The findings indicate that both the type and relative amount of oxide phases that formed were strongly influenced by the oxidation temperature. Similar trends were also noted in the study by Liu et al. [
54]. To support phase identification and enable a more rigorous evaluation, the principal 2θ positions and corresponding relative intensities of the detected main oxide phases are summarized in
Table 6. As for the 1.4871 alloy subjected to oxidation at different temperatures, Fe
2O
3 and Mn
2O
3 were identified as the dominant oxide phases. It was observed that these phases progressively developed with increasing oxidation temperature. At lower temperatures, Fe
2O
3 was the predominant phase. Similar results were reported in the works of Liu [
54] and Aryanto [
52], where Fe
2O
3 was identified as the dominant oxide phase at relatively lower oxidation temperatures. However, at 750 °C, the intensity of Fe
2O
3 peaks decreases while reflections corresponding to Mn
2O
3 become more pronounced. This phase transition is associated with the accelerated outward diffusion of Mn at elevated temperatures. SEM–EDS line scan results (
Figure 9) further confirm that the oxide scale thickens with increasing temperature, a trend that is particularly pronounced at 750 °C due to the contribution of the Mn-enriched Mn
2O
3 outer layer. In addition, while only Fe
2O
3 was observed at 650 °C, the simultaneous presence of Fe
2O
3 and Cr
2O
3 in the inner layer and Mn
2O
3 in the outer layer at 750 °C enhances the diversity of oxide products, thereby leading to an increase in overall scale thickness. Accordingly, the intensified Mn
2O
3 reflections observed in the XRD spectrum at 750 °C not only indicate a phase transformation but also reflect the development of a thicker and thermodynamically more stable oxide scale structure resulting from phase diversification and volumetric contributions.