Process and Mechanism of Surface Brazing of Graphene on Aluminum Nitride
Abstract
1. Introduction
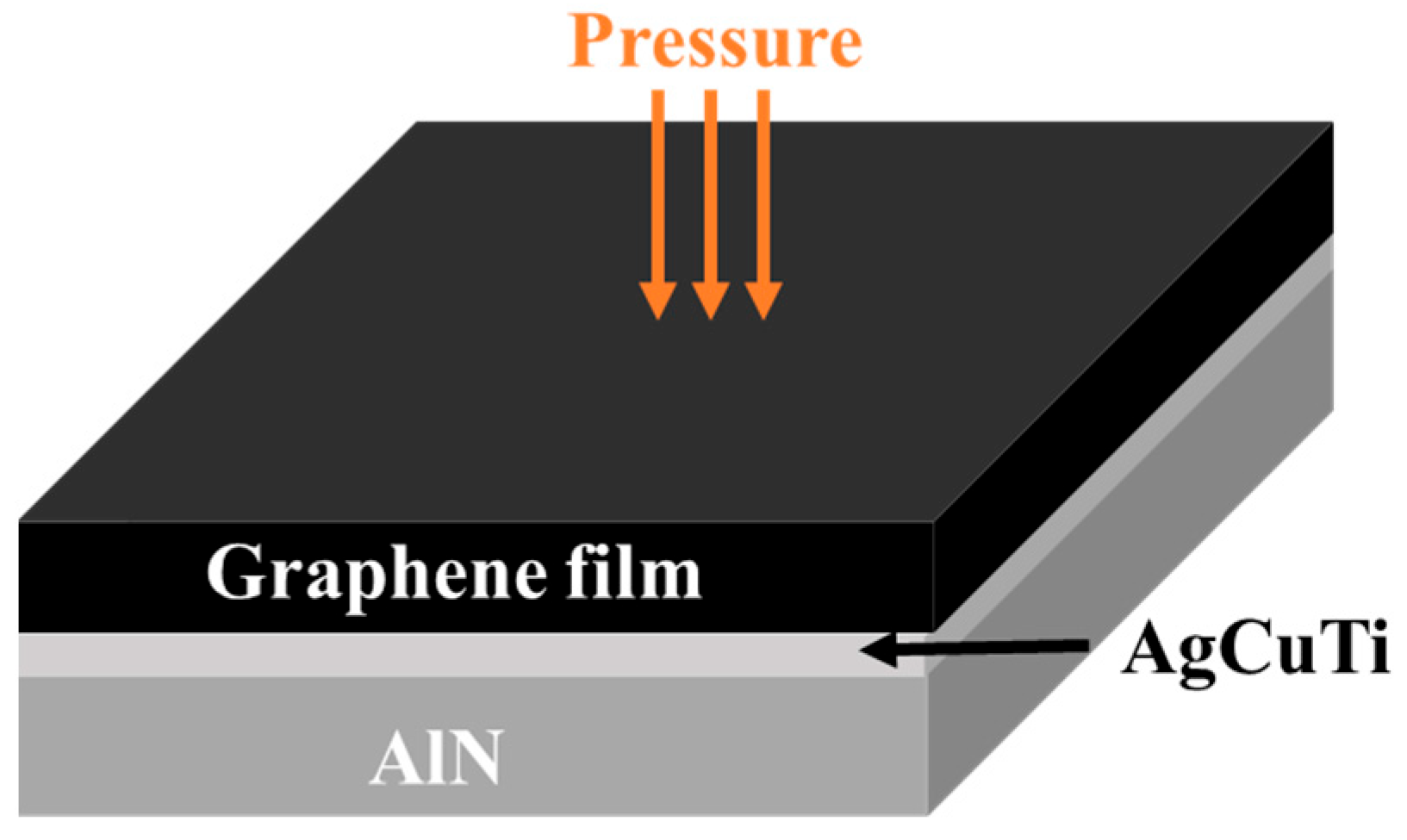
2. Materials and Methods
3. Results and Discussion
3.1. Interfacial Microstructure of the Brazing Joints
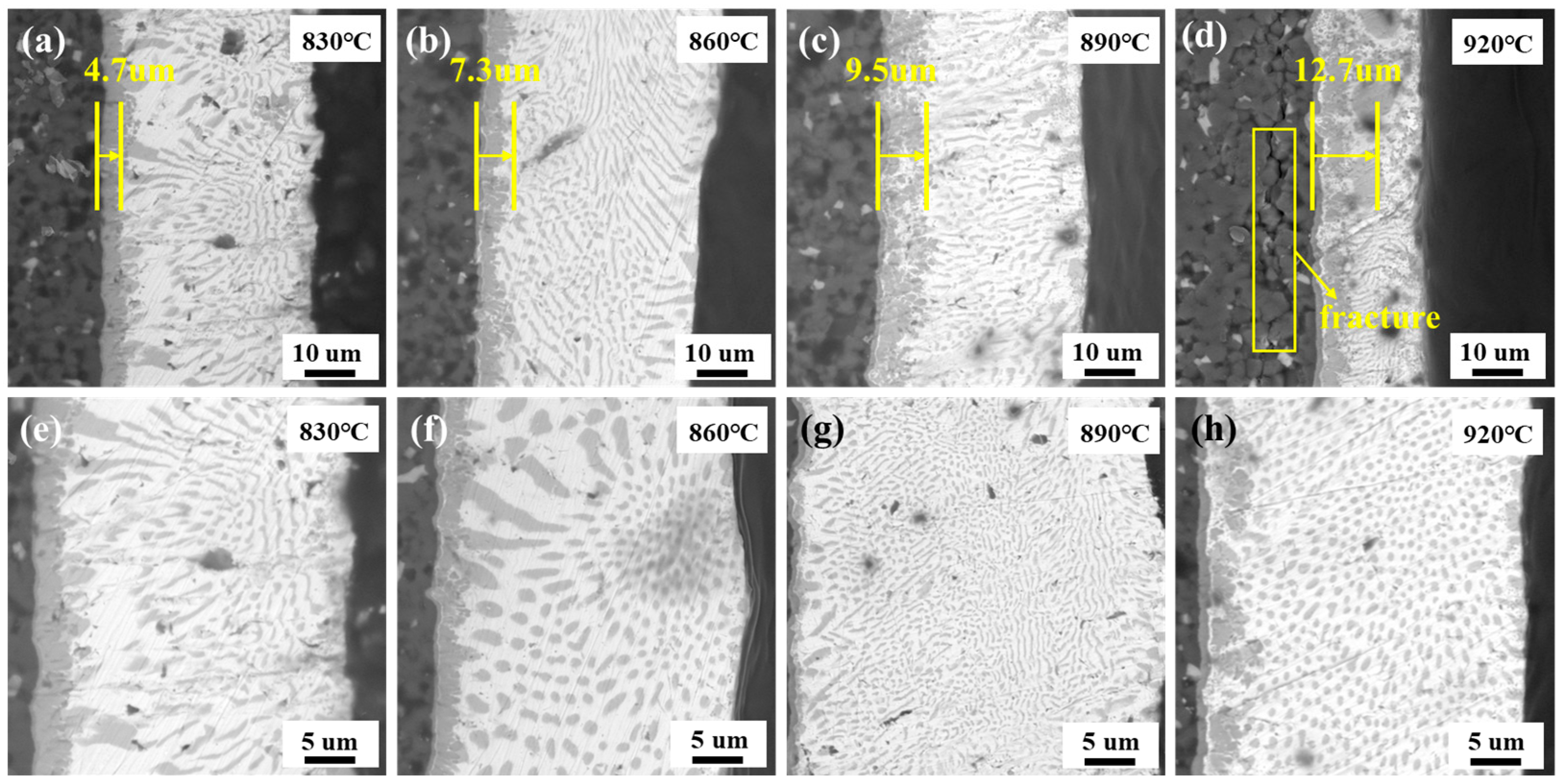
3.2. Effect of Brazing Temperature on the Interfacial Microstructure of Brazed Joint
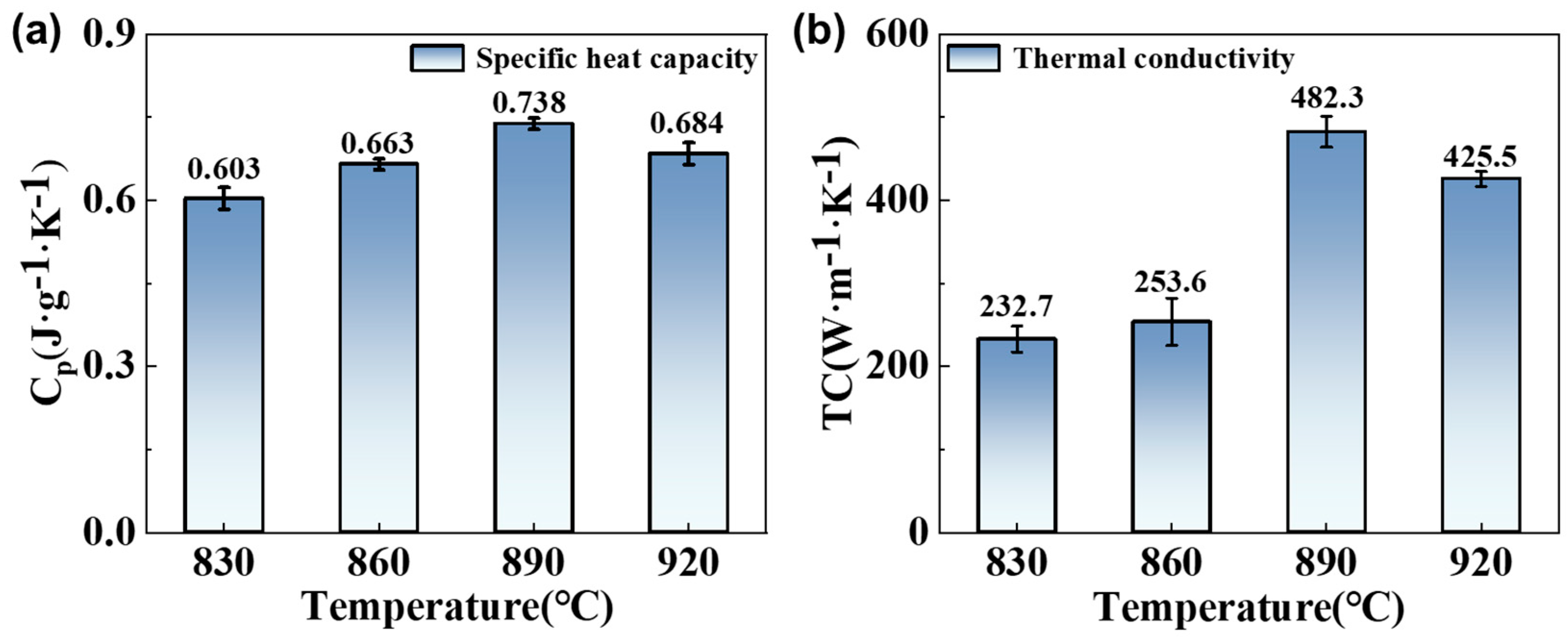
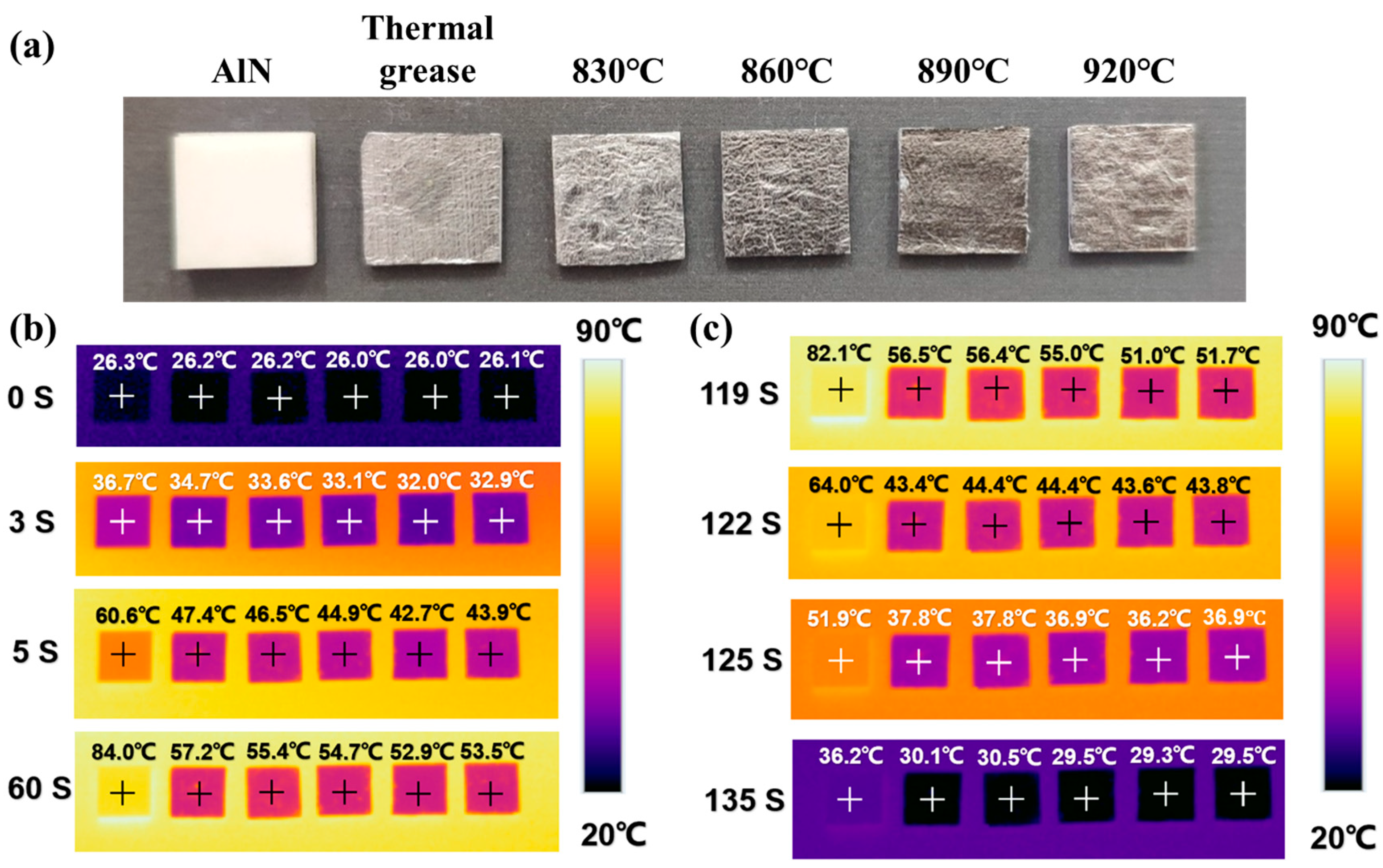
3.3. Effect of Brazing Temperature on the Thermal Conductivity of Brazed Joint
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, Z.; Liu, Z.; Lei, J.; Chen, L.; Li, L.; Zhao, N.; Fang, X.; Ruan, Y.; Tian, B.; Zhao, L. Flexible Thin Film Thermocouples: From Structure, Material, Fabrication to Application. iScience 2023, 26, 107303. [Google Scholar] [CrossRef]
- Gao, X.; Zhang, C.; Zhang, S.; Wang, Y.; Zhao, N.; Yang, Z.; Ding, G. A Heat Flux Sensor Based on Ceramic Thin-Film for Ultra-High Temperature Applications. J. Alloys Compd. 2025, 1020, 179243. [Google Scholar] [CrossRef]
- Li, L.; Tian, B.; Zhang, Z.; Shi, M.; Liu, J.; Liu, Z.; Lei, J.; Li, S.; Lin, Q.; Zhao, L.; et al. Highly Sensitive Flexible Heat Flux Sensor Based on a Microhole Array for Ultralow to High Temperatures. Microsyst. Nanoeng. 2023, 9, 133. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Tang, Y.; Zeng, Y.; Liu, X.; Tang, D. Recent Advances in Flexible Sensors: From Sensing Materials to Detection Modes. TrAC Trends Anal. Chem. 2024, 181, 118027. [Google Scholar] [CrossRef]
- Li, Z.; Tao, B.; Zhao, R.; Yang, K.; Chen, X.; Xie, T.; Zhao, M.; Zhu, H.; Chen, Y.; Xia, Y. Interface Modification Based on the Homoepitaxial MOCVD-MgO Process: Optimizing the Performance of the Flexible Atomic Layer Thermopile Heat Flux Sensor. IEEE Sens. J. 2025, 25, 4218–4226. [Google Scholar] [CrossRef]
- Zhang, W.; Li, J.; Fang, J.; Hui, L.; Qin, L.; Gong, T.; Sun, F.; Feng, H. Atomic Layer Deposited High Quality AlN Thin Films for Efficient Thermal Management. J. Mater. Chem. A 2023, 11, 21846–21856. [Google Scholar] [CrossRef]
- Li, Z.; Tao, B.; Zhao, R.; Yang, K.; Chen, X.; Xie, T.; Xia, Y.; Zhu, H.; Tian, H.; Yu, Y. Novel Flexible Atomic Layer Thermopile Heat Flux Sensor via Orientation-Controlled Growth Technique. Ceram. Int. 2024, 50, 30334–30344. [Google Scholar] [CrossRef]
- Hung, S.-W.; Chen, T.-K. Disclosing AlN Ceramic Substrate Process Failure Mode and Effect Analysis. Microelectron. Reliab. 2019, 103, 113508. [Google Scholar] [CrossRef]
- Yan, Z.; Nika, D.L.; Balandin, A.A. Thermal Properties of Graphene and Few-layer Graphene: Applications in Electronics. IET Circuits Devices Syst. 2015, 9, 4–12. [Google Scholar] [CrossRef]
- Liu, L.; Xu, C.; Yang, Y.; Fu, C.; Ma, F.; Zeng, Z.; Wang, G. Graphene-Based Polymer Composites in Thermal Management: Materials, Structures and Applications. Mater. Horiz. 2025, 12, 64–91. [Google Scholar] [CrossRef]
- Bae, S.-H.; Shabani, R.; Lee, J.-B.; Baeck, S.-J.; Cho, H.J.; Ahn, J.-H. Graphene-Based Heat Spreader for Flexible Electronic Devices. IEEE Trans. Electron. Devices 2014, 61, 4171–4175. [Google Scholar] [CrossRef]
- Liu, D.; Liu, K.; Song, Y.; Li, X.; Liu, R.; Li, Y.; Hu, S.; Song, X.G. Brazing of Graphite to Tungsten Using Graphene Nanoplates Reinforced Tinicu Composite Filler. J. Alloys Compd. 2023, 968, 172107. [Google Scholar] [CrossRef]
- Apebende, C.G.; Amodu, I.O.; Ogbogu, M.N.; Unimuyi, U.P.; Raimi, M.A.; Igomah, G.O. Computational Modelling of Graphene/Aluminum Nitride (GP/AlN) Hybrid Materials for the Detection of 2,4 Dichlorophenoxyacetic Acid (DCP) Pollutant. RSC Adv. 2024, 14, 21901–21914. [Google Scholar] [CrossRef]
- Yun, C.; Feng, Y.; Qiu, T.; Yang, J.; Li, X.; Yu, L. Mechanical, Electrical, and Thermal Properties of Graphene Nanosheet/Aluminum Nitride Composites. Ceram. Int. 2015, 41, 8643–8649. [Google Scholar] [CrossRef]
- Huang, P.; Li, Y.; Yang, G.; Li, Z.-X.; Li, Y.-Q.; Hu, N.; Fu, S.-Y.; Novoselov, K.S. Graphene Film for Thermal Management: A Review. Nano Mater. Sci. 2021, 3, 1–16. [Google Scholar] [CrossRef]
- Chen, Y.; Pang, K.; Liu, X.; Li, K.; Lu, J.; Cai, S.; Liu, Y.; Xu, Z.; Gao, C. Environment-Adaptive, Anti-Fatigue Thermal Interface Graphene Foam. Carbon 2023, 212, 118142. [Google Scholar] [CrossRef]
- Mondal, S.; Kolya, H.; Pagidi, S.; Kang, C.-W.; Nah, C. Thermal Conductivity of Graphene-Polymer Composites. In Polymer Nanocomposites Containing Graphene; Woodhead Publishing: Cambridge, UK, 2022; pp. 245–273. [Google Scholar]
- Yang, B.; Peng, C.; Song, M.; Tang, Y.; Wu, Y.; Wu, X.; Zheng, H. Thermal Transport of AlN/Graphene/3C-SiC Typical Heterostructures with Different Crystallinities of Graphene. ACS Appl. Mater. Interfaces 2022, 15, 2384–2395. [Google Scholar] [CrossRef]
- Cao, H.; Tan, Z.; Fan, G.; Guo, Q.; Su, Y.; Li, Z.; Xiong, D.-B. Wide and Fine Alignment Control and Interface Modification for High-Performance Thermally Conductive Graphite/Copper Composite. Compos. Part B Eng. 2020, 191, 107965. [Google Scholar] [CrossRef]
- Li, D.; Zhang, B.; Zhang, L.; Chang, Q.; Sun, Z. Interfacial Microstructure and Mechanical Properties of Y2O3-MgO Nanocomposite Ceramic and Ti6Al4V Joint Brazed with AgCuTi Filler. J. Eur. Ceram. Soc. 2025, 45, 117181. [Google Scholar] [CrossRef]
- Wang, N.; Wang, D.P.; Yang, Z.W.; Wang, Y. Interfacial Microstructure and Mechanical Properties of Zirconia Ceramic and Niobium Joints Vacuum Brazed with Two Ag-Based Active Filler Metals. Ceram. Int. 2016, 42, 12815–12824. [Google Scholar] [CrossRef]
- Li, P.; Wang, Z.; Chen, Y.; Butt, H.A.; Zhang, Z.; Huang, Z.; Zhang, T.; Qiao, L.; Zhong, Y.; Krasnikov, D.; et al. Mechanical Strength Enhancement of C/SiC–Nb Brazed Joints through Ultrafast High-Temperature Non-Equilibrium Surface High-Entropy Metallization. Carbon 2025, 242, 120416. [Google Scholar] [CrossRef]
- Chou, T.; Tuan, W.; Nishikawa, H.; Weng, B. Brazing Graphite to Aluminum Nitride for Thermal Dissipation Purpose. Adv. Eng. Mater. 2017, 19, 1600876. [Google Scholar] [CrossRef]
- Sheeja, D.; Tay, B.K.; Leong, K.W.; Lee, C.H. Effect of Film Thickness on the Stress and Adhesion of Diamond-like Carbon Coatings. Diam. Relat. Mater. 2002, 11, 1643–1647. [Google Scholar] [CrossRef]
- Li, H.; Sun, P.; Cheng, D.; Liu, Z. Effects of Deposition Temperature on Structure, Residual Stress and Corrosion Behavior of Cr/TiN/Ti/TiN Films. Ceram. Int. 2021, 47, 34909–34917. [Google Scholar] [CrossRef]
- Su, J.-F.; Zhao, Y.-H.; Wang, X.-Y.; Dong, H.; Wang, S.-B. Effect of Interface Debonding on the Thermal Conductivity of Microencapsulated-Paraffin Filled Epoxy Matrix Composites. Compos. Part A Appl. Sci. Manuf. 2012, 43, 325–332. [Google Scholar] [CrossRef]
- Chang, G.; Sun, F.; Duan, J.; Che, Z.; Wang, X.; Wang, J.; Kim, M.J.; Zhang, H. Effect of Ti Interlayer on Interfacial Thermal Conductance between Cu and Diamond. Acta Mater. 2018, 160, 235–246. [Google Scholar] [CrossRef]
- Zhou, W.; Cheng, Y.; Chen, K.; Xie, G.; Wang, T.; Zhang, G. Thermal Conductivity: Thermal Conductivity of Amorphous Materials (Adv. Funct. Mater. 8/2020). Adv. Funct. Mater. 2020, 30, 2070048. [Google Scholar] [CrossRef]
- Chason, E.; Shin, J.W.; Hearne, S.J.; Freund, L.B. Kinetic Model for Dependence of Thin Film Stress on Growth Rate, Temperature, and Microstructure. J. Appl. Phys. 2012, 111, 083520. [Google Scholar] [CrossRef]
- Matsumae, T.; Kurashima, Y.; Takagi, H.; Nishizono, K.; Amano, T.; Higurashi, E. Room-Temperature Bonding of AlN Ceramic and Si Semiconductor Substrates for Improved Thermal Management. In Proceedings of the 2021 International Conference on Electronics Packaging (ICEP), Tokyo, Japan, 12–14 May 2021; pp. 5–6. [Google Scholar]
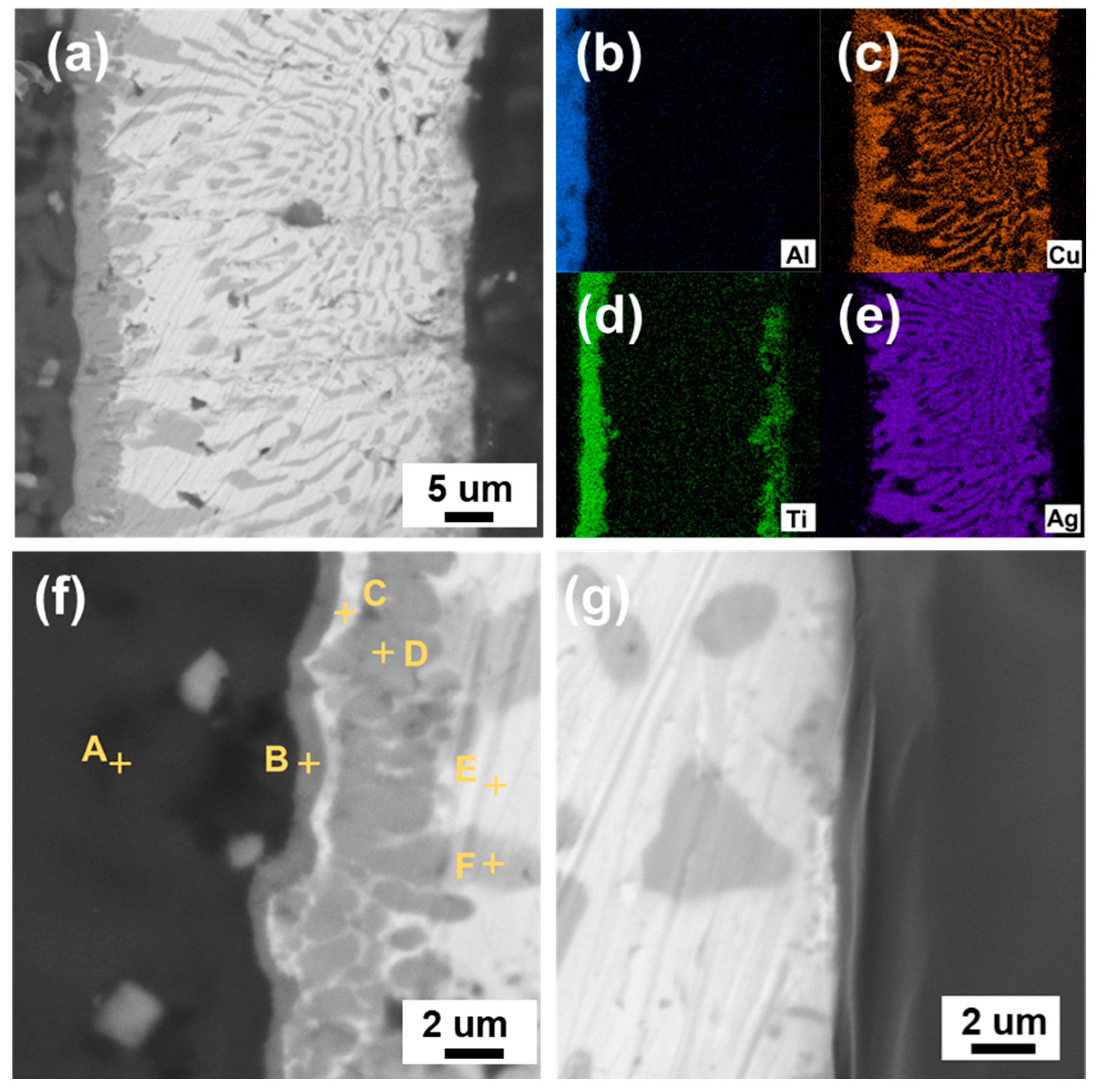
| Spots | N (at%) | Al (at%) | Ti (at%) | Cu (at%) | Ag (at%) | Possible Phase |
|---|---|---|---|---|---|---|
| A | 7.57 | 92.10 | 0.06 | 0.28 | 0.00 | AlN |
| B | 11.43 | 16.04 | 62.47 | 4.56 | 5.53 | Ti3Al |
| C | 0.00 | 10.02 | 34.64 | 17.38 | 37.96 | AgTi |
| D | 0.00 | 12.98 | 48.39 | 35.54 | 3.10 | CuTi |
| E | 0.00 | 0.36 | 0.13 | 9.05 | 90.46 | Ag (s, s) |
| F | 0.00 | 5.43 | 0.90 | 90.91 | 2.76 | Cu (s, s) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, W.; Wang, Z.; Wu, X.; Kong, D.; Xu, C.; Yin, Y.; Lv, J. Process and Mechanism of Surface Brazing of Graphene on Aluminum Nitride. Coatings 2025, 15, 1011. https://doi.org/10.3390/coatings15091011
Li W, Wang Z, Wu X, Kong D, Xu C, Yin Y, Lv J. Process and Mechanism of Surface Brazing of Graphene on Aluminum Nitride. Coatings. 2025; 15(9):1011. https://doi.org/10.3390/coatings15091011
Chicago/Turabian StyleLi, Wenbo, Zijia Wang, Xinyun Wu, Deren Kong, Chundong Xu, Yugang Yin, and Jing Lv. 2025. "Process and Mechanism of Surface Brazing of Graphene on Aluminum Nitride" Coatings 15, no. 9: 1011. https://doi.org/10.3390/coatings15091011
APA StyleLi, W., Wang, Z., Wu, X., Kong, D., Xu, C., Yin, Y., & Lv, J. (2025). Process and Mechanism of Surface Brazing of Graphene on Aluminum Nitride. Coatings, 15(9), 1011. https://doi.org/10.3390/coatings15091011





