Understanding the Chamber Wall-Deposited Thin Film of Plasma Deposition Equipment for the Efficiency of In Situ Dry-Cleaning
Abstract
1. Introduction
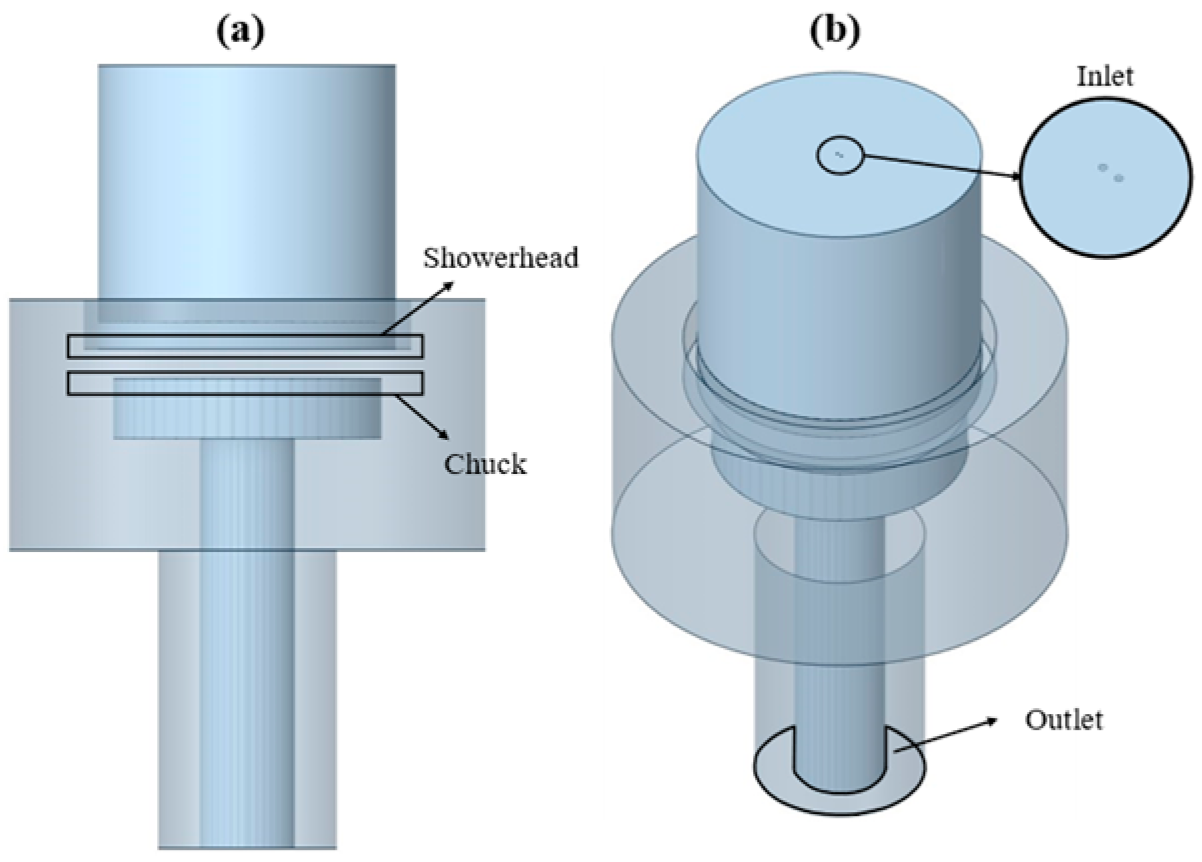
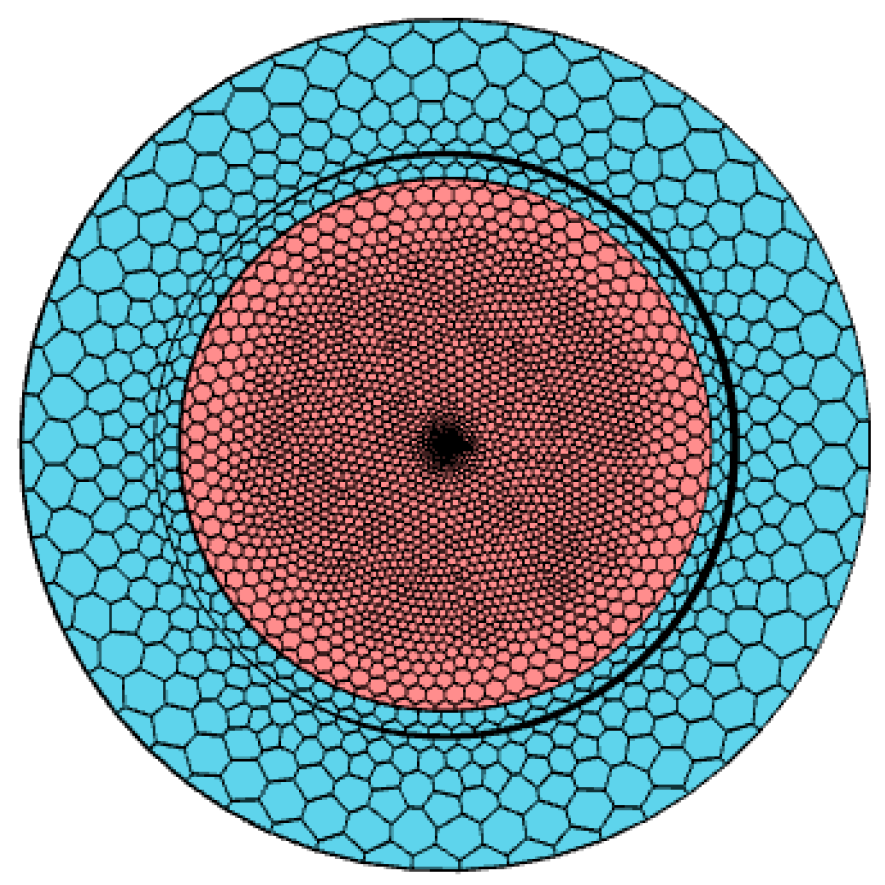
2. Simulation Method
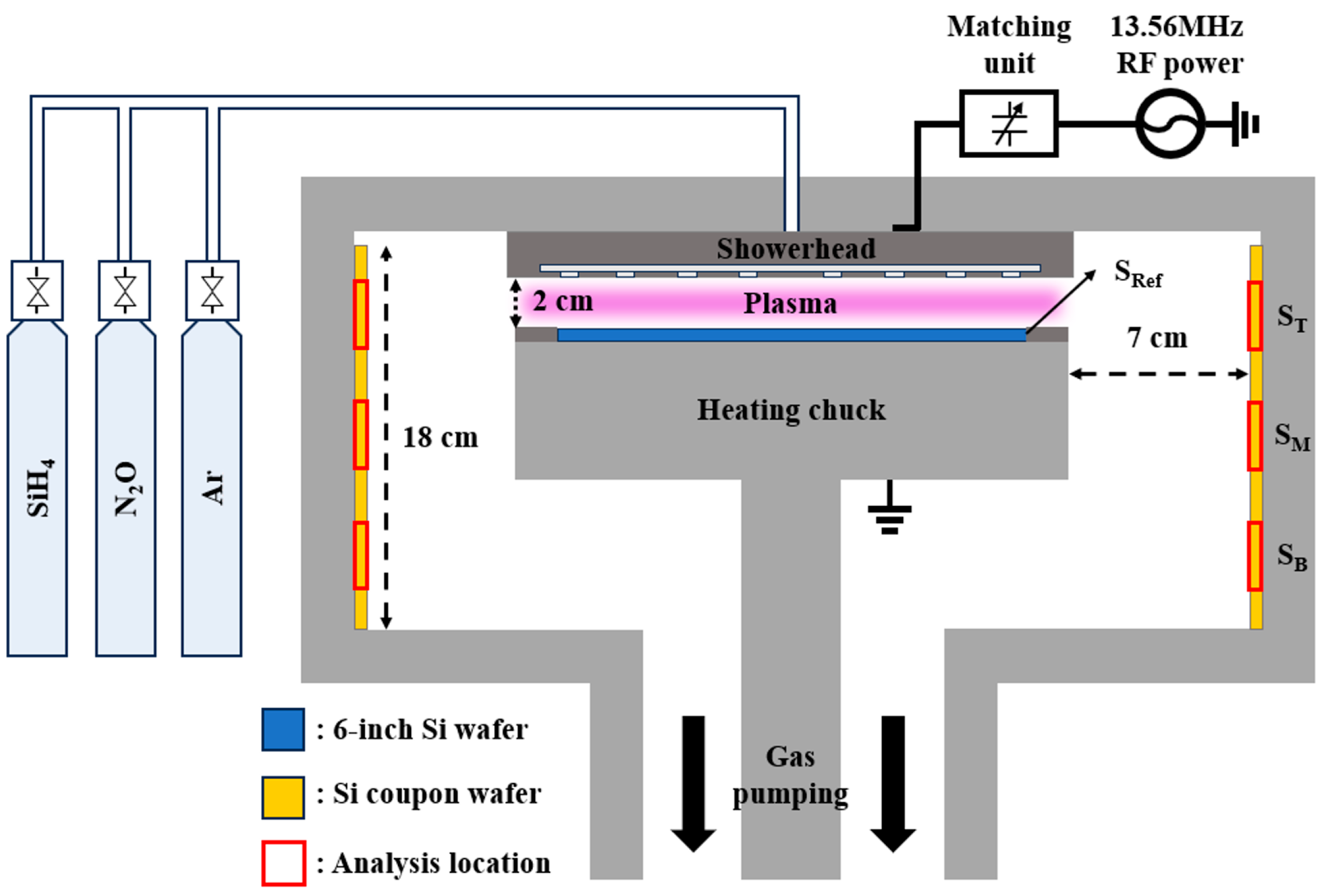
3. Experimental Methods
4. Results and Discussion
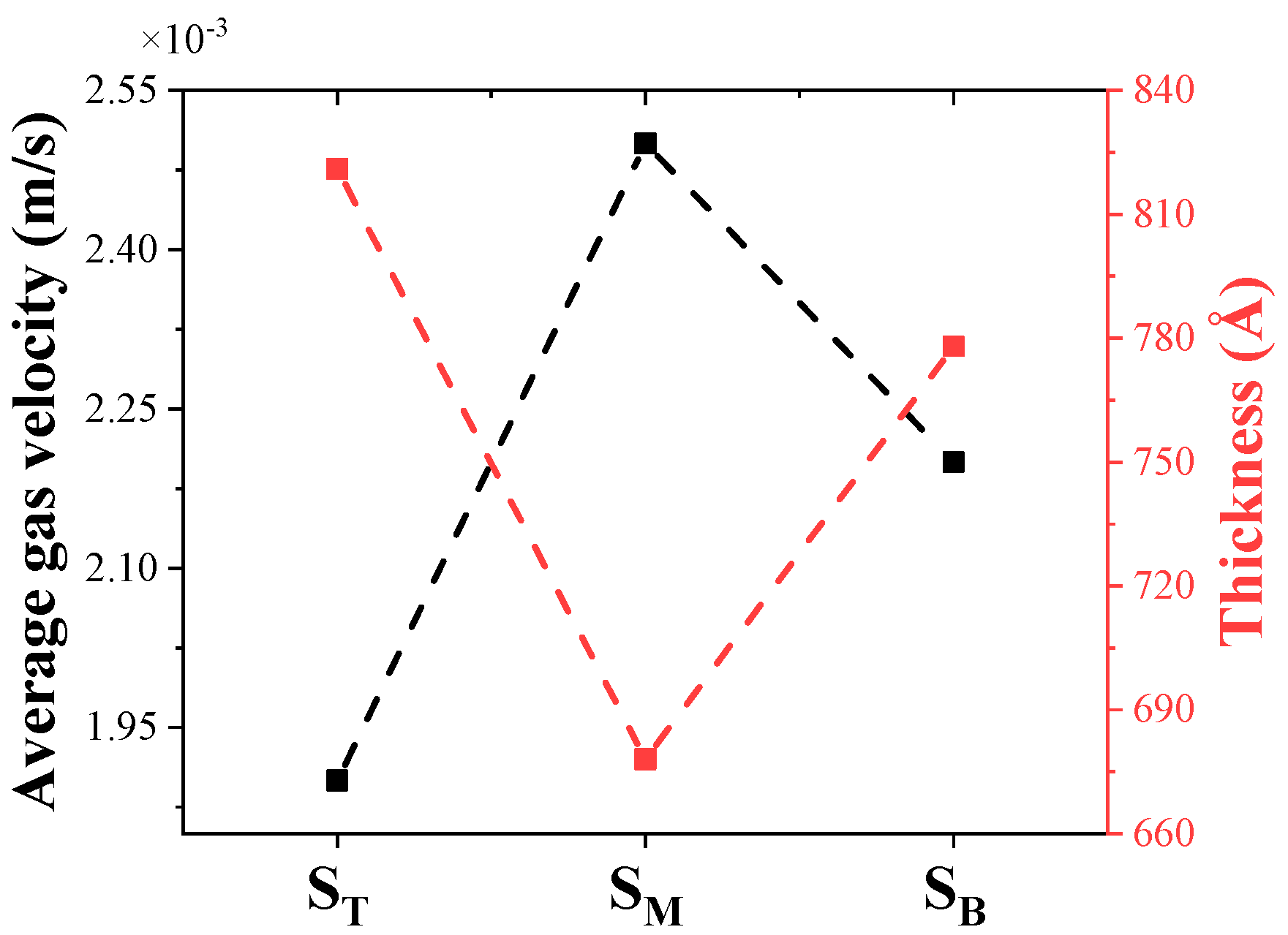
4.1. Thickness Distribution and Gas Flow Dynamics
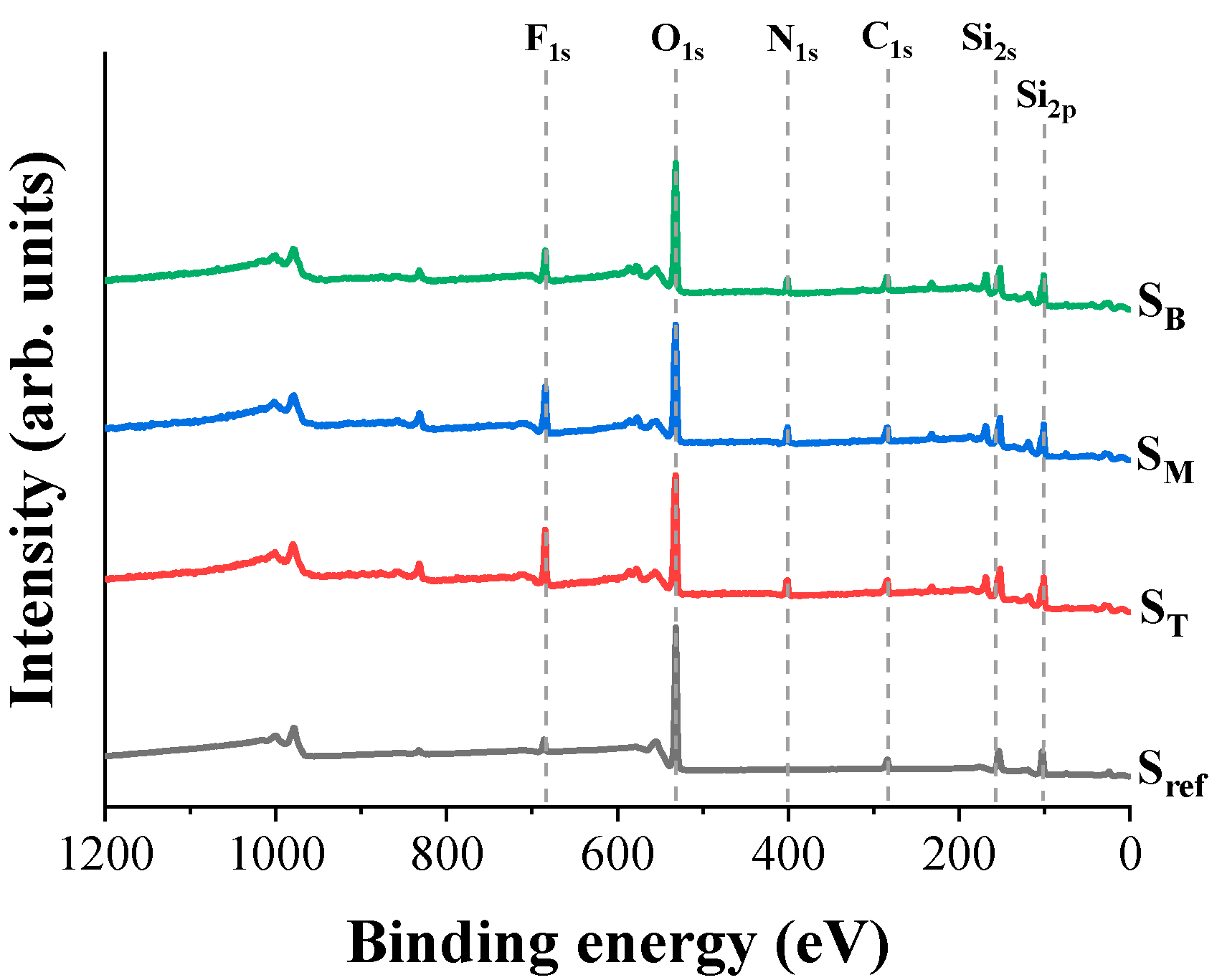
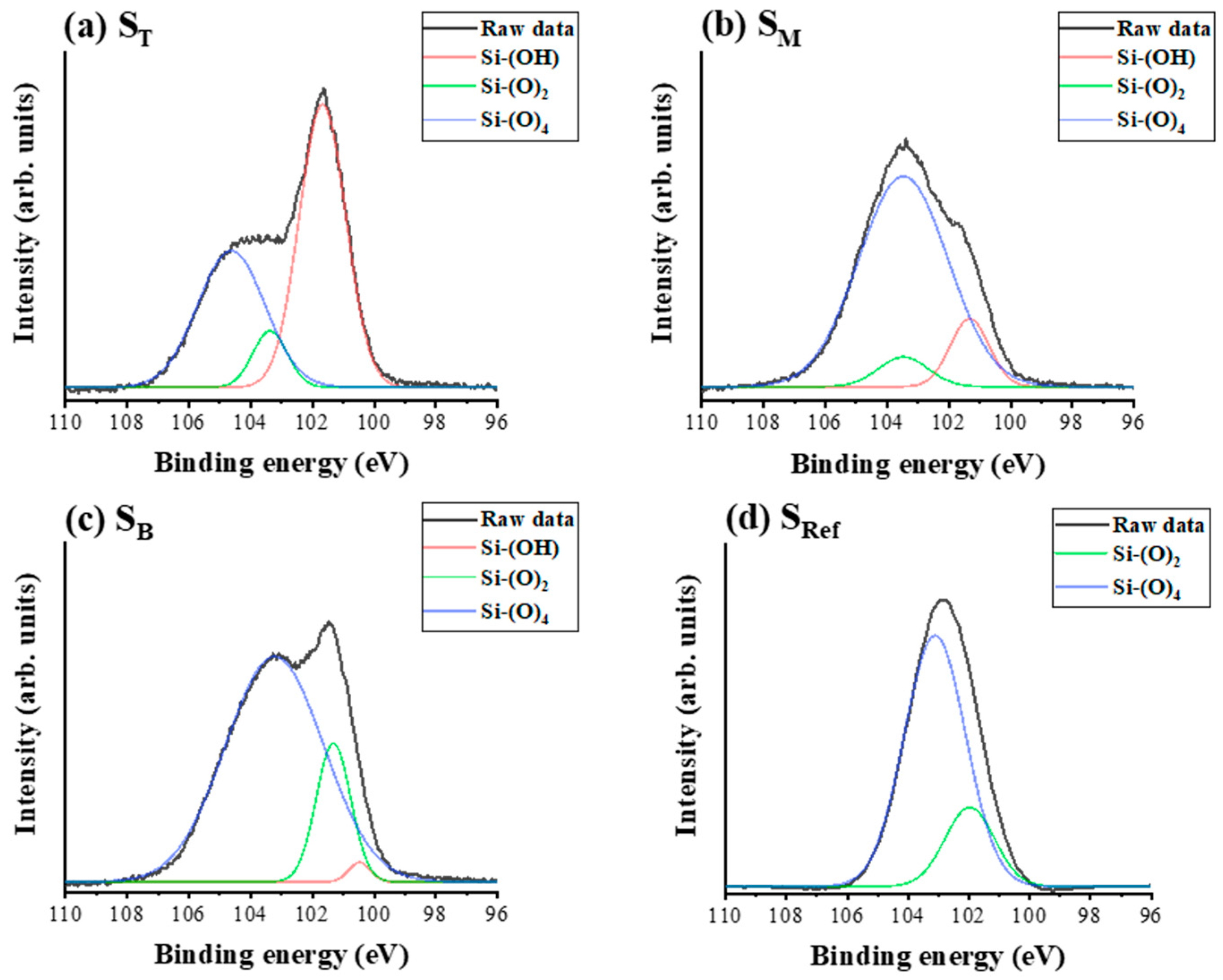
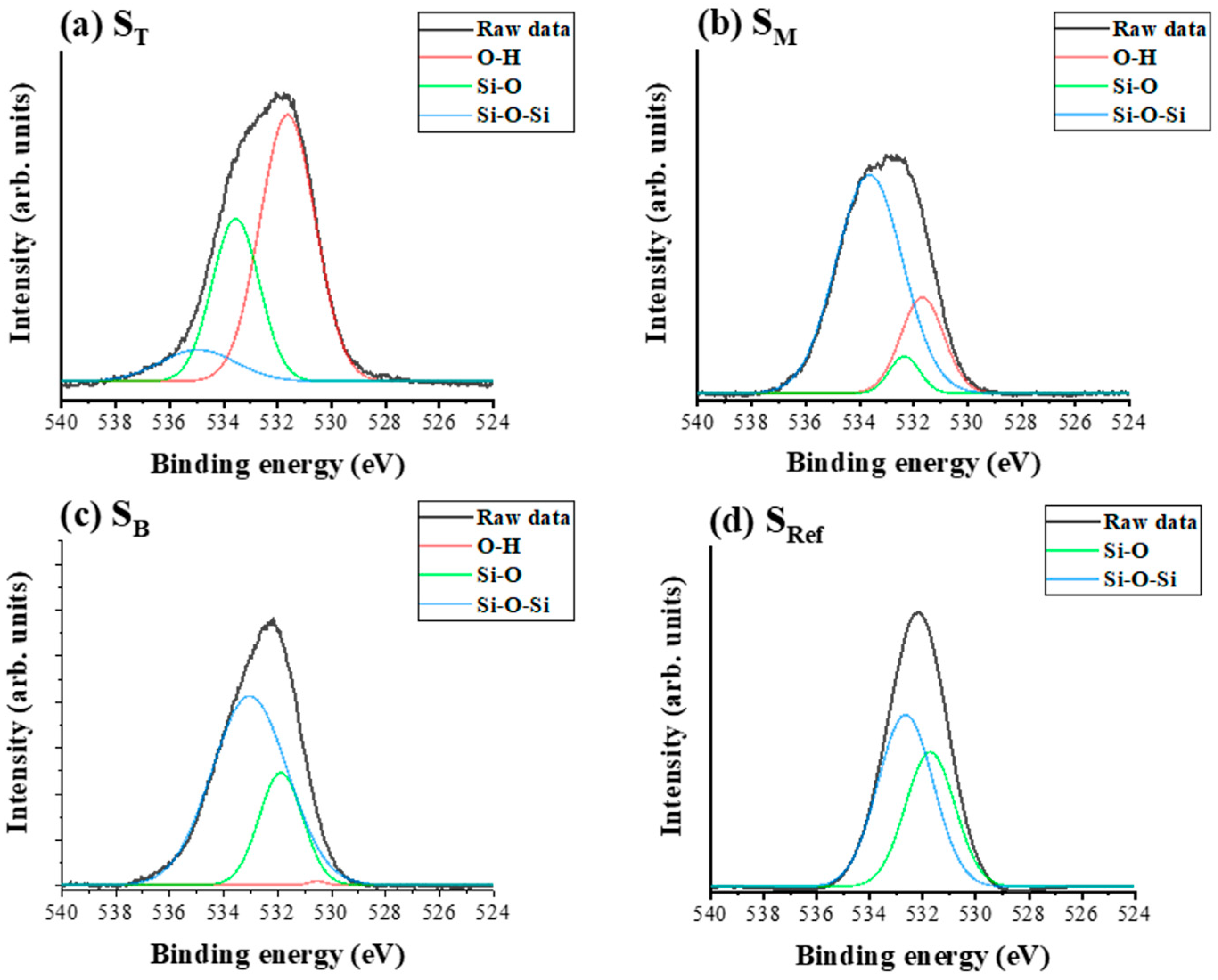
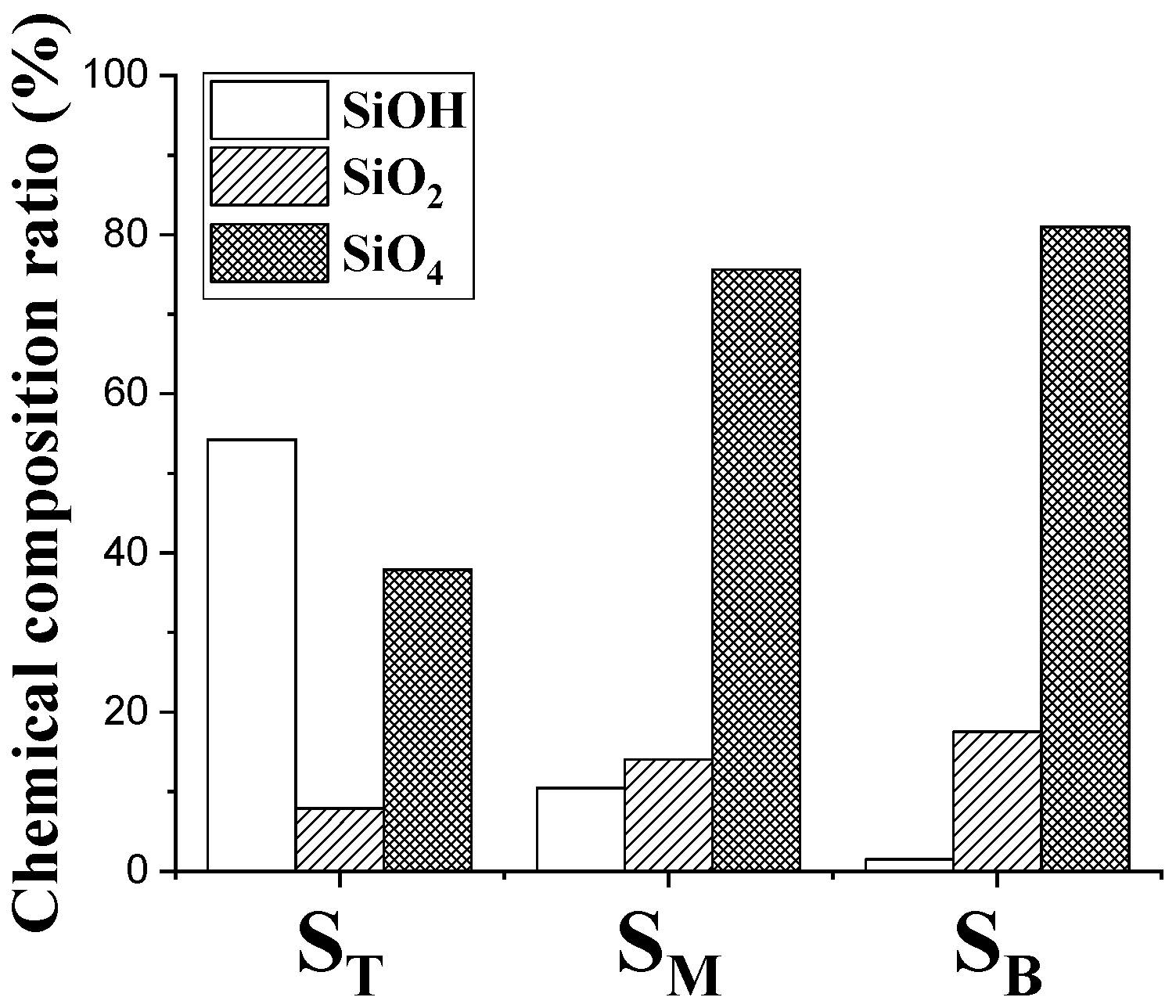
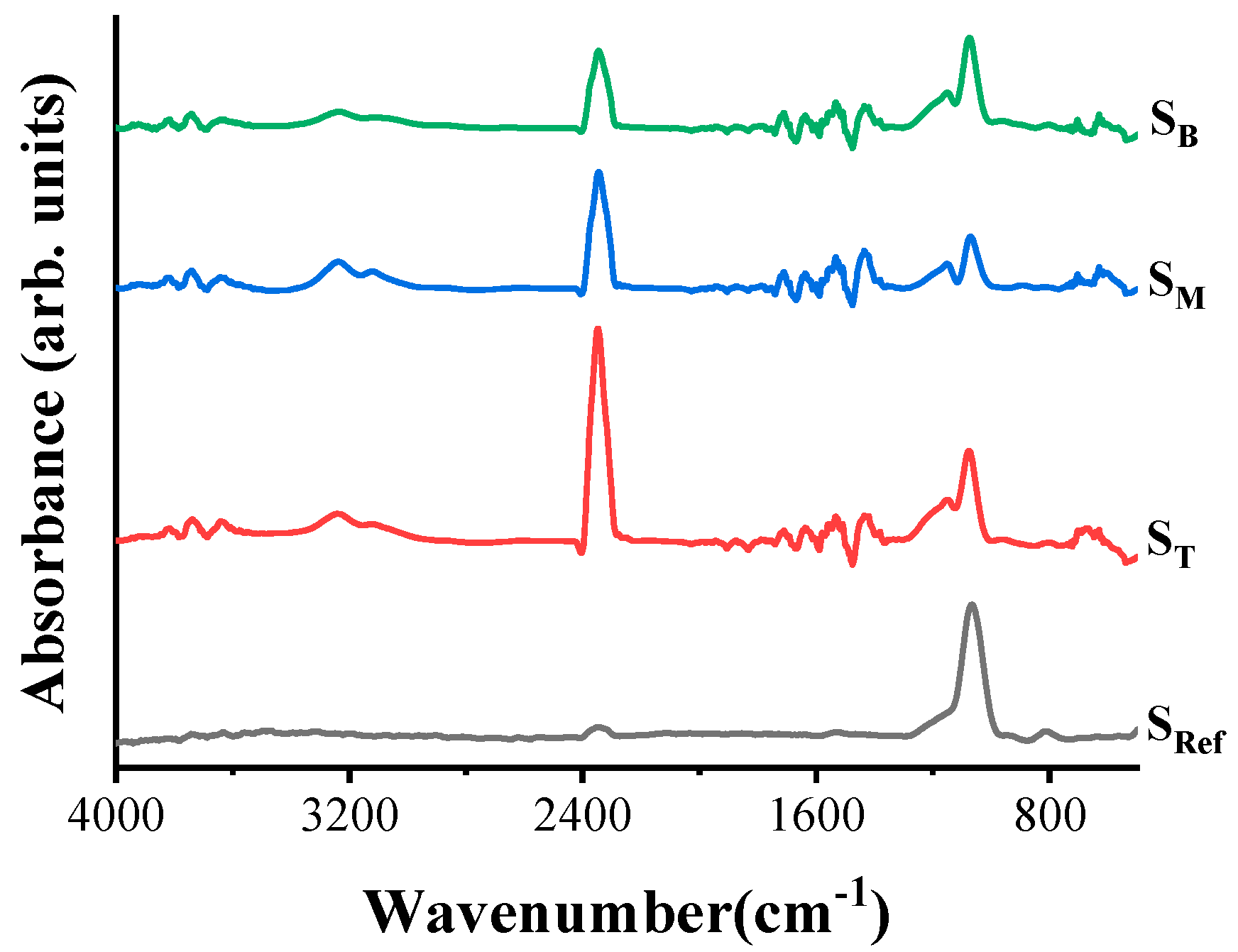
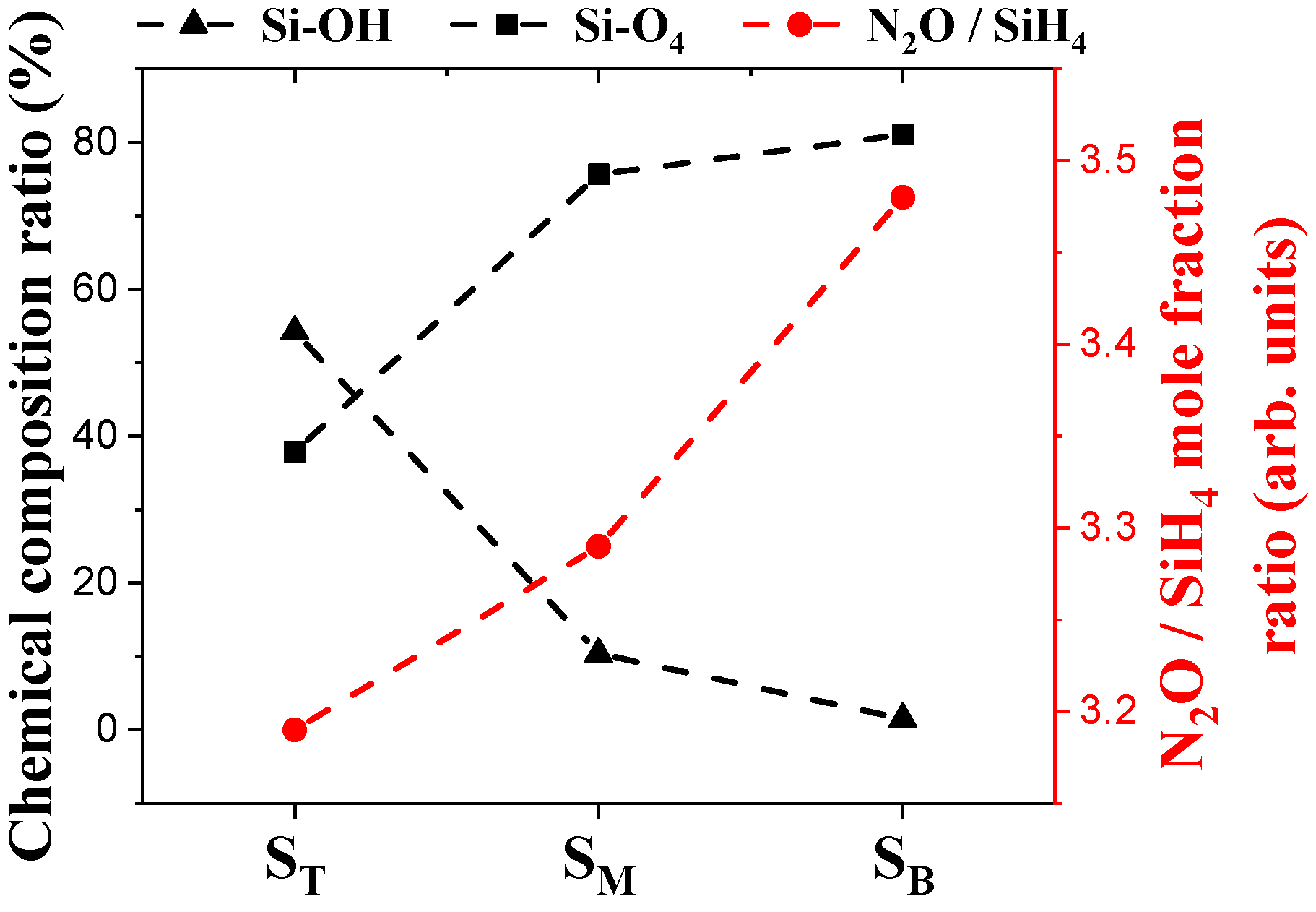
4.2. Chemical Composition and Bonding States
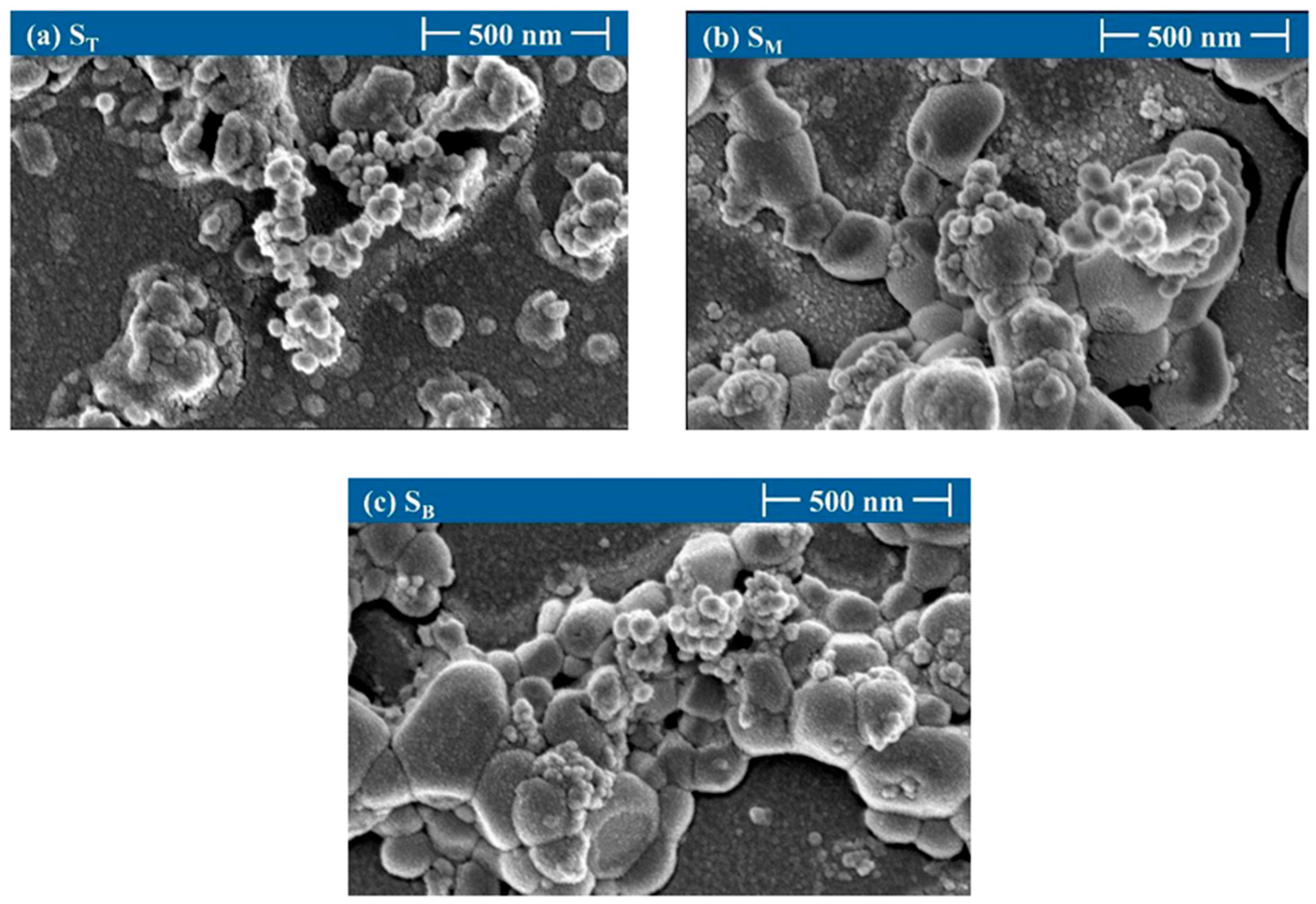
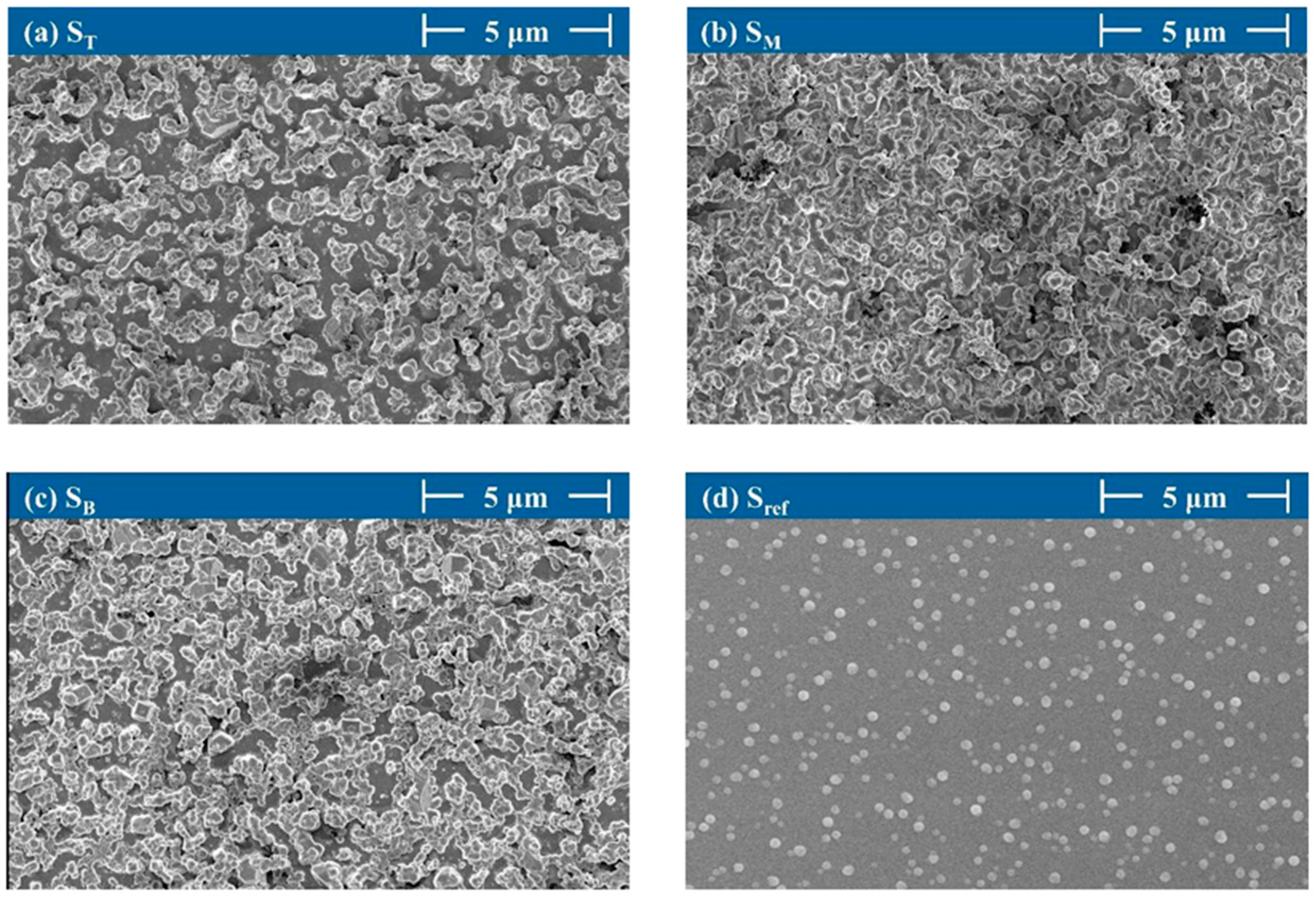
4.3. Surface Morphology and Particle Size
4.4. Implications for ISD Process
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kim, M.; Kwon, H.; Kim, H.; Park, S.; Lee, J.; Na, K.; Kong, C.; Kim, Y. First Wafer Effect of Multiple SiO2/SiN Stack Layers Prepared by using Plasma-enhanced Chemical Vapor Deposition. J. Korean Phys. Soc. 2020, 76, 911. [Google Scholar] [CrossRef]
- Heydari Gharahcheshmeh, M. Fabrication of Conjugated Conducting Polymers by Chemical Vapor Deposition (CVD) Method. Nanomaterials 2025, 15, 452. [Google Scholar] [CrossRef] [PubMed]
- Faggio, G.; Politano, G.G.; Lisi, N.; Capasso, A.; Messina, G. The structure of chemical vapor deposited graphene substrates for graphene-enhanced Raman spectroscopy. J. Phys. Condens. Matter 2024, 36, 195303. [Google Scholar] [CrossRef] [PubMed]
- Raoux, S.; Liu, K.S.; Guo, X.; Silvetti, D. In-situ RF diagnostic for PECVD process control. MRS Online Proc. Libr. 1997, 502, 53. [Google Scholar] [CrossRef]
- Young, B.; Yoo, K.; Chung, C. Real-time observation of the capacitance variation in a surface dielectric layer in radio frequency discharge. J. Appl. Phys. 2012, 111, 036805. [Google Scholar] [CrossRef]
- Jellum, G.M.; Graves, D.B. Particle-plasma interactions in low-pressure discharges. Appl. Phys. Lett. 1990, 57, 2077. [Google Scholar] [CrossRef]
- Kim, J.; Chung, C. Real-time dielectric-film thickness measurement system for plasma processing chamber wall monitoring. Rev. Sci. Instrum. 2015, 86, 123677. [Google Scholar] [CrossRef]
- Masuda, A.; Ishibashi, Y.; Uchida, K.; Kamesaki, K.; Izumi, A.; Matsumura, H. In situ chamber cleaning using atomic H in catalytic-CVD apparatus for mass production of a-Si: H solar cells. Sol. Energy Mater. Sol. Cells 2002, 74, 373–381. [Google Scholar] [CrossRef]
- Xu, S.; Sun, Z.; Qian, X.; Holland, J.; Podlesnik, D. Characteristics and mechanism of etch process sensitivity to chamber surface condition. J. Vac. Sci. Technol. B 2001, 19, 166–171. [Google Scholar] [CrossRef]
- Park, H.K.; Song, W.S.; Hong, S.J. In situ plasma impedance monitoring of the oxide layer PECVD process. Coatings 2023, 13, 559. [Google Scholar] [CrossRef]
- Cunge, G.; Pelissier, B.; Joubert, O.; Ramos, R.; Maurice, C. New chamber walls conditioning and cleaning strategies to improve the stability of plasma processes. Plasma Sources Sci. Technol. 2005, 14, 599–606. [Google Scholar] [CrossRef]
- Boudaden, J.; Altmannshofer, S.; Wieland, R.; Pittroff, M.; Eisele, I. An Approach to Reduce Greenhouse Gases in the Semiconductor Industry Using F2 Dissociated in Plasma for CVD Chamber Cleaning. Appl. Sci. 2018, 8, 846. [Google Scholar] [CrossRef]
- Rößler, T.; Albert, M.; Terasa, R.; Bartha, J.W. Alternative etching gases to SF6 for plasma enhanced chamber cleaning in silicon deposition systems. Surf. Coat. Technol. 2005, 200, 552–556. [Google Scholar] [CrossRef]
- An, S.; Hong, S.J. Spectroscopic analysis of NF3 plasmas with oxygen additive for PECVD chamber cleaning. Coatings 2023, 13, 91. [Google Scholar] [CrossRef]
- Jang, H.; Lee, J.; Kim, Y.; Lee, S.; Hong, S.J. Real-time endpoint detection of small exposed area SiO2 films in plasma etching using plasma impedance monitoring with modified principal component analysis. Plasma Process. Polym. 2013, 10, 850–856. [Google Scholar] [CrossRef]
- Lee, H.; Seo, D.; Hong, S.; May, G. PECVD Chamber cleaning end point detection (EPD) using optical emission spectroscopy data. Trans. Electr. Electron. Mater. 2013, 14, 254–258. [Google Scholar] [CrossRef]
- Lucken, R.; Croes, V.; Lafleur, T.; Raimbault, J.L.; Bourdon, A.; Chabert, P. Edge-to-center plasma density ratios in two-dimensional plasma discharges. Plasma Sources Sci. Technol. 2018, 27, 035004. [Google Scholar] [CrossRef]
- You, Y.M.; Lee, J.S.; Kim, M.H.; Hong, S.J. Use of plasma process diagnostic sensors for the monitoring of in situ dry cleaning of plasma enhanced chemical vapor deposition chamber. J. Vac. Sci. Technol. B. 2024, 42, 042203. [Google Scholar] [CrossRef]
- Le Gouil, A.; Pargon, E.; Cunge, G.; Joubert, O.; Pelissier, B. Chemical analysis of deposits formed on the reactor walls during silicon and metal gate etching processes. J. Vac. Sci. Technol. B 2006, 24, 2191–2197. [Google Scholar] [CrossRef]
- Xu, X.; Feng, J.; Liu, X.M.; Wang, Y.N.; Yan, J. Study of the neutral gas flow on discharges of capacitively coupled plasma in a PECVD reactor. Vacuum 2013, 92, 1–6. [Google Scholar] [CrossRef]
- Gusev, E.Y.; Jityaeva, J.Y.; Ageev, O.A. Effect of PECVD conditions on mechanical stress of silicon films. Mater. Phys. Mech. 2018, 37, 67–73. [Google Scholar] [CrossRef]
- Pan, P.; Nesbit, L.A.; Douse, R.W.; Gleason, R.T. The Composition and Properties of PECVD Silicon Oxide Films. J. Electrochem. Soc. 1985, 132, 2012. [Google Scholar] [CrossRef]
- Overzet, L.J.; Verdeyen, J.T. Enhancement of the plasma density and deposition rate in rf discharges. Appl. Phys. Lett. 1986, 48, 695–697. [Google Scholar] [CrossRef]
- Greczynski, G.; Hultman, L. X-ray photoelectron spectroscopy: Towards reliable binding energy referencing. Prog. Mater. Sci. 2020, 107, 100591. [Google Scholar] [CrossRef]
- Pan, Y.; Liu, H.; Wang, Z.; Jia, J.; Zhao, J. Optical constant and conformality analysis of SiO2 thin films deposited on linear array microstructure substrate by PECVD. Coatings 2021, 11, 510. [Google Scholar] [CrossRef]
- Jeong, H.; Cho, J. Fabrication and evaluation of protective SiOx layers using plasma-enhanced chemical vapor deposition. Surf. Coat. Technol. 2017, 330, 71–78. [Google Scholar] [CrossRef]
- Alam, A.U.; Howlader, M.M.R.; Deen, M.J. Oxygen Plasma and Humidity Dependent Surface Analysis of Silicon, Silicon Dioxide and Glass for Direct Wafer Bonding. ECS J. Solid State Sci. Technol. 2013, 2, P515–P523. [Google Scholar] [CrossRef]
- Alexander, M.R.; Short, R.D.; Jones, F.R.; Michaeli, W.; Blomfield, C.J. A study of HMDSO/O2 plasma deposits using a high-sensitivity and -energy resolution XPS instrument: Curve fitting of the Si 2p core level. Appl. Surf. Sci. 1999, 137, 179–185. [Google Scholar] [CrossRef]
- Ma, H.; Yang, J.; Yang, J.; Zhu, L.; Huang, W.; Yuan, G.; Feng, J.; Jen, T.; Lu, H. Systematic Study of the SiOx Film with Different Stoichiometry by Plasma-Enhanced Atomic Layer Deposition and Its Application in SiOx/SiO2 Super-Lattice. Nanomaterials 2019, 9, 55. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhu, L.; Chen, L.; Liu, L.; Ye, G. Influence of Magnesia on Demoulding Strength of Colloidal Silica-Bonded Castables. Rev. Adv. Mater. Sci. 2019, 58, 32–38. [Google Scholar] [CrossRef]
- Deshmukh, S.C.; Aydil, E.S. Investigation of low temperature SiO2 plasma enhanced chemical vapor deposition. J. Vac. Sci. Technol. B 1996, 14, 738–745. [Google Scholar] [CrossRef]
- Peng, Z.W.; Hsieh, P.T.; Lin, Y.J.; Huang, C.J.; Li, C.C. Investigation on Blistering Behavior for n-type Silicon Solar Cells. Energy Procedia 2015, 77, 827–832. [Google Scholar] [CrossRef]




| Boundary Conditions | ||
|---|---|---|
| Pressure (mTorr) | 500 | |
| Chuck temperature (°C) | 300 | |
| Mass flow rate [sccm] | SiH4 | 9 |
| N2O | 35 | |
| Ar | 2 | |
| Power (W) | Pressure (mTorr) | Gas Flow Rate (sccm) | Temp (°C) | ||
|---|---|---|---|---|---|
| SiH4 | N2O | Ar | |||
| 350 | 500 | 9 | 35 | 2 | 300 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.; Jang, J.; Hong, S.J. Understanding the Chamber Wall-Deposited Thin Film of Plasma Deposition Equipment for the Efficiency of In Situ Dry-Cleaning. Coatings 2025, 15, 563. https://doi.org/10.3390/coatings15050563
Lee J, Jang J, Hong SJ. Understanding the Chamber Wall-Deposited Thin Film of Plasma Deposition Equipment for the Efficiency of In Situ Dry-Cleaning. Coatings. 2025; 15(5):563. https://doi.org/10.3390/coatings15050563
Chicago/Turabian StyleLee, Jiseok, Jiwon Jang, and Sang Jeen Hong. 2025. "Understanding the Chamber Wall-Deposited Thin Film of Plasma Deposition Equipment for the Efficiency of In Situ Dry-Cleaning" Coatings 15, no. 5: 563. https://doi.org/10.3390/coatings15050563
APA StyleLee, J., Jang, J., & Hong, S. J. (2025). Understanding the Chamber Wall-Deposited Thin Film of Plasma Deposition Equipment for the Efficiency of In Situ Dry-Cleaning. Coatings, 15(5), 563. https://doi.org/10.3390/coatings15050563







