Abstract
Valve leakage mainly comes from worn sealing surfaces caused by abrasive particles. This study uses plasma beam spraying to create Fe55 alloy coatings with CeO2 and TiN added to improve microstructure and wear resistance. Five coatings were prepared: Fe55 with 0.02% CeO2 (FC2), 0.04% CeO2 (FC4), 1% TiN (FT1), 2% TiN (FT2), and 2% TiN/0.02% CeO2 (FC2T2). These coatings were tested for wear and erosion using wet sand and slurry experiments. Results showed that FC2T2 had the most uniform microstructure with fully equiaxed grains (20.32 μm size) and no columnar grains. This was due to CeO2 and TiN co-working effect: CeO2 was adsorbed onto TiN surfaces, reducing TiN decomposition and acting as nucleation sites. The FC2T2 coating also showed the highest hardness uniformity (no large changes with depth) and the lowest surface roughness after wear (41% lower than pure Fe55). In wear tests, FC2T2’s Cr7C3 hard phases blocked abrasive cutting, while the γ-Fe matrix prevented Cr7C3 from breaking off. Erosion tests confirmed FC2T2’s superior performance, as its uniform structure limited deep grooves. Adding both CeO2 and TiN improved wear resistance by providing a balanced microstructure, reducing leakage risks in valve sealing surfaces.
1. Introduction
Valve leakage primarily stems from the failure of line sealing mechanisms [1,2,3,4]. During slurry transportation, mineral particles induce abrasion on both the pipeline interior and sealing surfaces under fluid pressure. Post-abrasion sealing surfaces develop furrows with varying depths, which subsequently permit pressurized slurry leakage through deeper furrows [5].
Current industrial practices employ two principal techniques for fabricating hard alloy coatings on valve sealing surfaces: laser cladding and plasma spraying. The laser cladding process involves melting pre-deposited alloy powder on metallic substrates to achieve metallurgical bonding. In contrast, plasma spraying generates a plasma arc above the substrate surface, wherein injected alloy particles are simultaneously melted and accelerated by ionized gas toward the substrate [6,7,8,9]. This method facilitates superior metallurgical bonding characteristics, making plasma spraying particularly advantageous for both initial coating preparation and subsequent repair operations on valve sealing components.
Fe-based powders are predominantly employed for fabricating or repairing valve sealing surfaces [10]. However, their limited self-fluxing capacity frequently results in defect formation, including cracks and porosity. This inherent limitation necessitates the application of elevated welding currents, which consequently induces grain coarsening in the microstructure. Although Ni-based and Co-based powders exhibit superior self-fluxing properties, their widespread industrial adoption remains constrained by prohibitive costs [11,12].
Furthermore, while the high hardness of alloy coatings enhances wear resistance, it cannot entirely prevent the formation of differential furrow depths. Coatings with high hardness primarily function by delaying the onset of deep furrow generation rather than eliminating the phenomenon. Consequently, researchers are increasingly focusing on optimizing uniform wear distribution across welding layers as a critical strategy for extending service life.
Chen et al. analyzed the wear morphology of aeronautical spool valves and investigated the failure mechanisms of sealing surfaces [13]. A critical finding indicates that heterogeneity in hardness and elastic modulus inevitably results in microscale variations in wear depth. Furthermore, variations in microscale wear are also influenced by particle size, shape, and type of particles [14]. Meng et al. introduced TiC particles into welding layers, where the added reinforcement phase provided heterogeneous nucleation sites and suppressed grain growth [15]. However, the high surface energy of nano-sized reinforcement particles promotes their agglomeration in molten steel, ultimately constraining the additive concentration in metallic matrices. They demonstrated that surface modification of the reinforcement phase effectively mitigates particle agglomeration. Researchers. demonstrated that the addition of CeO2 effectively suppresses the decomposition of TiC and enhances grain refinement [16]. Furthermore, CeO2 facilitates dispersive distribution of the reinforcement phase, thereby promoting homogeneous nucleation of grains through optimized heterogeneous nucleation dynamics [17].
The incorporation of CeO2 significantly mitigates crack initiation and defect formation induced by Ti(C,N) while concurrently enhancing hardness and wear resistance [18]. This improvement is attributed to grain boundary segregation of CeO2, wherein Ce exert a grain boundary drag effect that impedes grain growth [19]. Concurrently, CeO2 particles generate abundant heterogeneous nucleation sites, thereby refining microstructural homogeneity.
However, it should be noted that the addition of Ce reduces molten pool fluidity, which paradoxically diminishes grain refinement efficacy. This reduced fluidity, while limiting grain size reduction, effectively suppresses agglomeration kinetics of dispersed particles [19]. Consequently, it enables the stable incorporation of ceramic reinforcements without introducing structural defects. This grain boundary drag effect has also been documented in rare earth (RE)-strengthened magnesium alloys. Qian et al. confirmed that the introduction of Y, Gd, and Nd demonstrates that rare earth (RE) elements primarily act to hinder the diffusion of grain boundary segregating elements. Specifically, Ce and Nd form eutectic precipitates with alloying elements at the grain boundaries, generating significant solute drag stresses. This results in restricted diffusion of elements critical to grain boundary formation [20]. Robson et al. observed in their Mg-Nd alloy system that all Nd atoms were immobilized within insoluble precipitates. These Nd-containing particles synergistically interacted with other alloying elements, preferentially enriching at grain boundaries to obstruct solute diffusion pathways [21]. Recent studies have revealed that when NbC and CeO2 are co-incorporated into welding layers, CeO2 particles act as nucleation sites for NbC, effectively suppressing NbC decomposition and its dissolution into the molten steel matrix [22,23,24]. This synergistic mechanism has been validated in studies involving the concomitant addition of CeO2 and TiN [25]. However, the study by Chen et al. focused on enhancing the hardness of the welding layer rather than its uniformity. Ti(C, N) was introduced into the welding layer via in situ generation, resulting in its primary role as a grain refiner and hard phase within their findings. Notably, the decomposition behavior of these particles in molten metal remains underexplored in their research. Their work demonstrated that the coexistence of CeO2 and TiN particles significantly modulates the microstructure of the welding layer, with the grain boundary drag effect employed to explain this phenomenon. To further investigate the relationship between the co-addition of CeO2 and TiN and the grain boundary drag effect, it is essential to utilize this method to prepare welding layers with uniform hardness and microstructure. Additionally, a thorough investigation is required into the impact of this dual-enhancement strategy on wear uniformity, particularly focusing on the interaction mechanism between the distribution of heterogeneous particles during micro-cutting processes and their micro-wear behavior.
In this study, Fe55 with 0.02 wt% CeO2(FC2), 0.04 wt% CeO2(FC4), 1 wt% TiN(FT1), 2 wt% TiN(FT2) and 2 wt% TiN/0.02 wt% CeO2(FC2T2) were fabricated via vibration-assisted mechanical alloying, followed by deposition of hardfacing coatings using plasma spraying. The coatings underwent wet sand wear tests and slurry erosion experiments to evaluate their tribological performance. Post-test characterization via SEM and 3D profilometry was employed to systematically analyze the worn morphology, wear behavior and mechanism. This study integrated the microscopic surface morphology of wear surfaces with 3D wear morphology data, revealing the dominant ploughing-exfoliation mechanism in slurry erosion and the dynamic evolution processes of micro-cutting in wet sand wear, thereby providing a theoretical basis for predictive modeling of coating service reliability.
2. Materials and Methods
Annealed 1025 carbon steel is commonly used as the base material for valve spools matrix. In this work, a 1025 steel plate of size 100 mm × 50 mm × 10 mm was selected as the substrate.
Prior to spraying, substrate sur faces were mechanically descaled to remove oxide layers and preheated to 500 °C under argon shielding. A secondary descaling operation was performed immediately before plasma spraying to ensure surficial purity. Matrix powder: Fe55 alloy (purity > 99%, d50 = 150~200 μm, Hebei Hangba Metal Materials Co., Ltd., Xingtai, China); Reinforcement phases: TiN (purity > 99%, d50 = 180~200 μm, Hebei Hangba Metal Materials Co., Ltd., Xingtai, China). CeO2 (purity > 99%, d50 = 100 nm, Hebei Hangba Metal Materials Co., Ltd., Xingtai, China) employed as a microstructural modifier.
The powder processing protocol as following:
Primary Blending: 2 g Fe55 matrix powder was combined with the designated TiN/CeO2 ratios in a planetary ball mill (YXQM-4L, Changsha MiQi Experimental Equipment Co., Ltd., Changsha, China) (250 rpm, 30 min, ball-to-powder ratio 10:1). The pre-mixed powders were subjected to vortex-assisted mechanical stirring (3000 rpm, 15 Hz frequency) for 60 min to achieve homogeneity of nano-CeO2 dispersion. The compositional profile of the Fe55 alloy powder is provided in Table 1. The Chemical composition of powders is provided in Table 2.

Table 1.
Chemical composition of Fe55.

Table 2.
Chemical composition of powders.
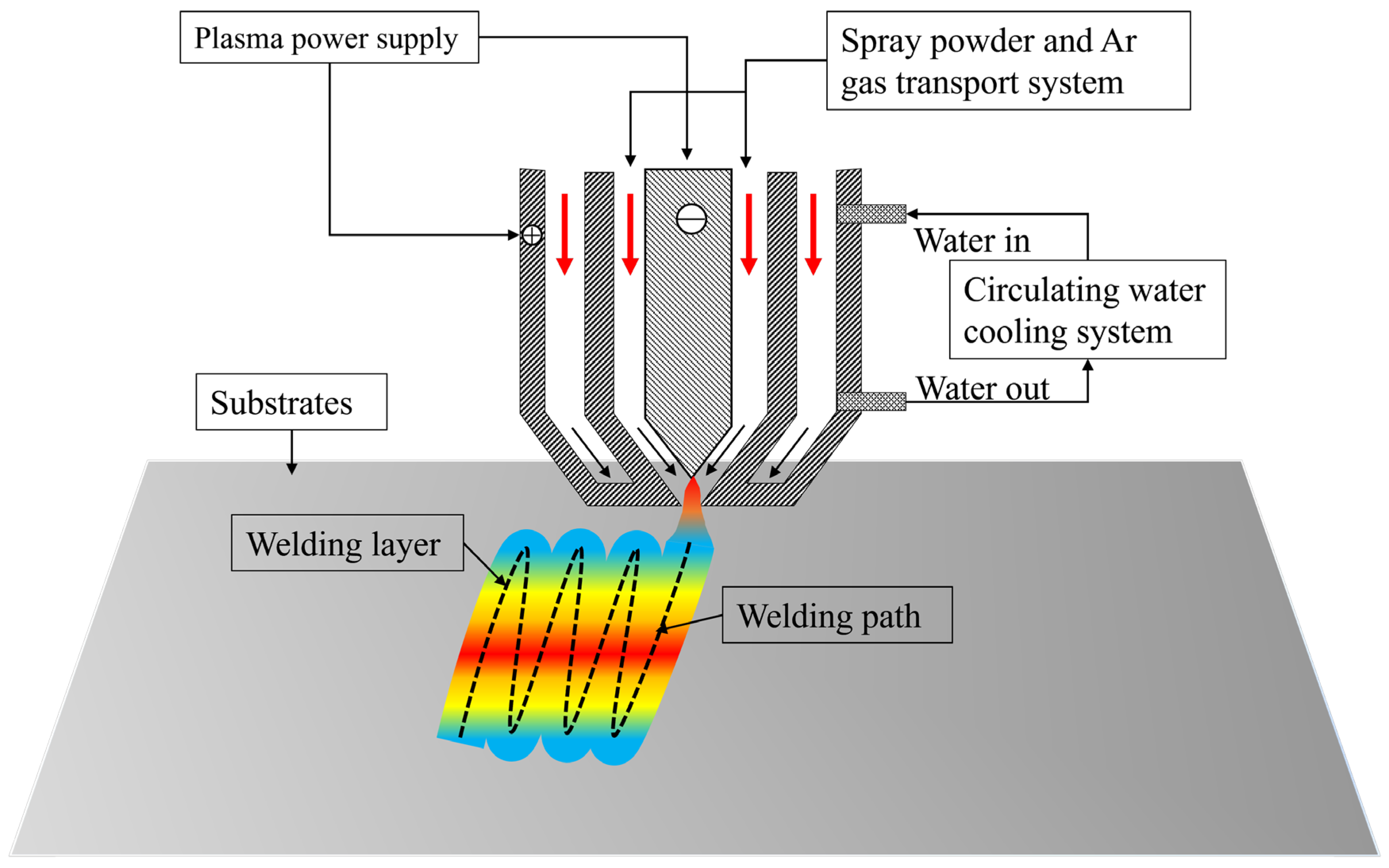
Figure 1 schematically illustrates the plasma spraying system and deposition process (DML-V03BD, Shanghai Duo Mu Industrial Co., Shanghai, China). The coating fabrication protocol comprised the following stages: Mixed powders were loaded into a rotating mixing chamber with continuous agitation to ensure homogeneity; 1025 steel substrates were preheated to 500 °C under inert atmosphere, followed by mechanical descaling to eliminate oxide layers prior to spraying. The plasma spraying parameters as follows: Arc current: 95 A, shielding gas (Ar): 10 L/min, plasma gas (Ar): 3.5 L/min, traverse speed: 6 mm/s, and powder feed rate: 1.5 g/s
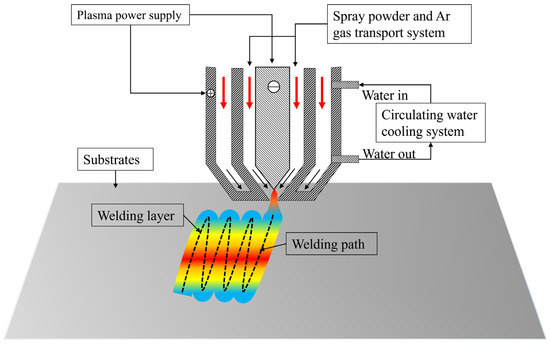
Figure 1.
Schematic diagram of 3D PSM system and process [26].
Specimens with dimensions of 10 × 10 × 8 mm3 were polished and chemically etched with 4 vol.% nital etchant for 15–25 s to reveal microstructural features. Optical microscopy (BMM-420, Shanghai Batuo Instrument Co., Ltd., Shanghai, China) and Zeiss GeminiSEM 300 field-emission scanning electron microscope (Oberkochen, Germany) (Oberkochen, Germany) were employed to characterize grain morphology and phase distribution. Energy-dispersive X-ray spectroscopy (Oberkochen, Germany) provided elemental mapping and semi-quantitative phase composition analysis. Worn surfaces were examined using 3D laser scanning microscopy (μsurf explorer, NanoFocus AG, Oberhausen, Germany) and SEM to quantify wear track topography and identify dominant wear mechanisms. Surface roughness parameters were extracted from 3D profilometry data using Gaussian filtering (ISO 21920-2:2021 [27]). Microhardness measurements were performed using a LECO semi-automatic Vicker’s hardness tester (AMH43, LECO, Laboratory Equipment Co., St. Joseph, MI, USA) with the following parameters: indentation spacing is 100 μm; applied load: 0.3 kgf; dwell time: 15 s.
The wet friction performance of welding layers was tested using an LGM-225 wet sand rubber wheel friction tester (LGM-225, Jinan Liangong Test Technology Co., Jinan, China). Prior to wear testing, the welding layers were polished to a surface roughness Ra < 0.05 μm. SiC particles with a size of 75 μm were employed as abrasives. A wear load of 20 N was applied during testing, with the rubber wheel maintaining a linear velocity of 3 m/s.
Erosion wear experiments were conducted using an MCF-20 erosion tester (Jinan Hansen Test Technology Co., Jinan, China), wherein 75 μm SiC particles were projected onto the welding layer surface at a 45° angle with a velocity of 10 m/s. The welding layers were similarly polished to Ra < 0.05 μm before erosion testing. The wear duration was systematically set at intervals of 10, 20, 30, 40, 50, and 60 min for comprehensive evaluation.
3. Results and Discussion
3.1. Morphology and Chemical Composition of the Raw Powders
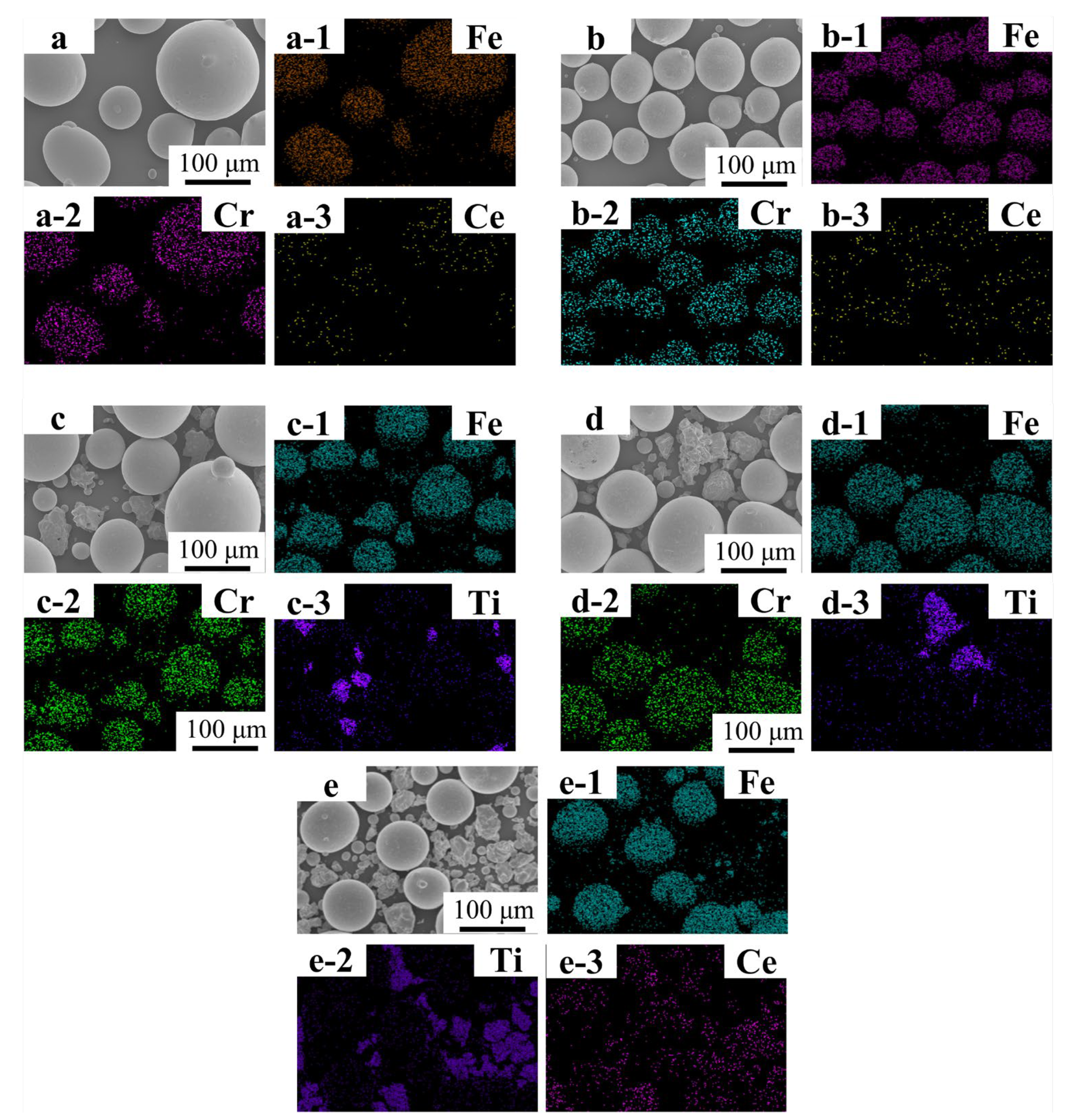
Figure 2(a–a-3,b–b-3) present the SEM micrograph and EDS mapping results of Fe55 powder blended solely with CeO2. The Fe55 particles exhibit a spherical morphology with diameters ranging from 75 to 150 μm, dominated by Fe and Cr signals. Notably, Ce elemental signals colocalize with Fe/Cr-rich regions, indicating uniform dispersion of CeO2 nanoparticles on Fe55 particle surfaces after ball milling. Figure 2(c–c-3,d–d-3) display the Fe55-TiN composite powder. TiN particles exhibit irregular geometries (30–90 μm), with Ti and N signals demonstrating spatial correlation. However, partial TiN particles were obscured by the Fe55 matrix, resulting in localized absence of Ti signals in certain regions. Figure 2(e–e-3) illustrates the co-doped Fe55-TiN-CeO2 system. Ce signals predominantly overlap with Ti-enriched zones, demonstrating preferential adsorption of CeO2 onto TiN particle surfaces during the pre-mixing stage.
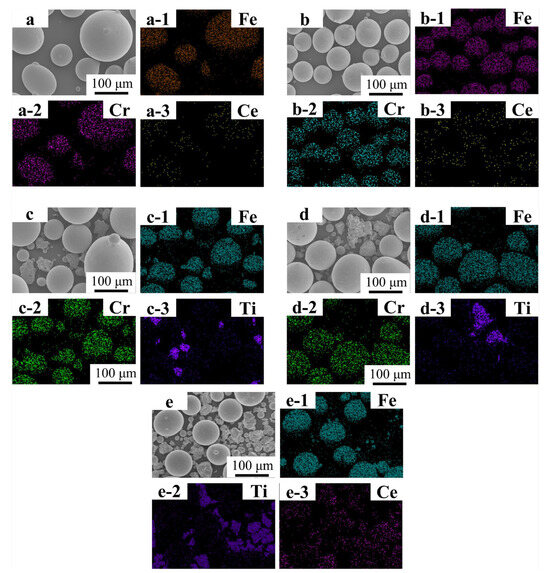
Figure 2.
FT1, FT1, FC2T2 Alloy powder SEM micrographs and EDS mapping results: (a–a-3) SEM micrographs and EDS mapping result of FC1 alloy powder; (b–b-3) SEM micrographs and EDS mapping result of FC2 alloy powder; (c–c-3) SEM micrographs and EDS mapping result of FT1 alloy powder; (d–d-3) SEM micrographs and EDS mapping result of FT2 alloy powder; (e–e-3) SEM micrographs and EDS mapping result of FC2T2 alloy powder.
3.2. Morphology of Welding Layer
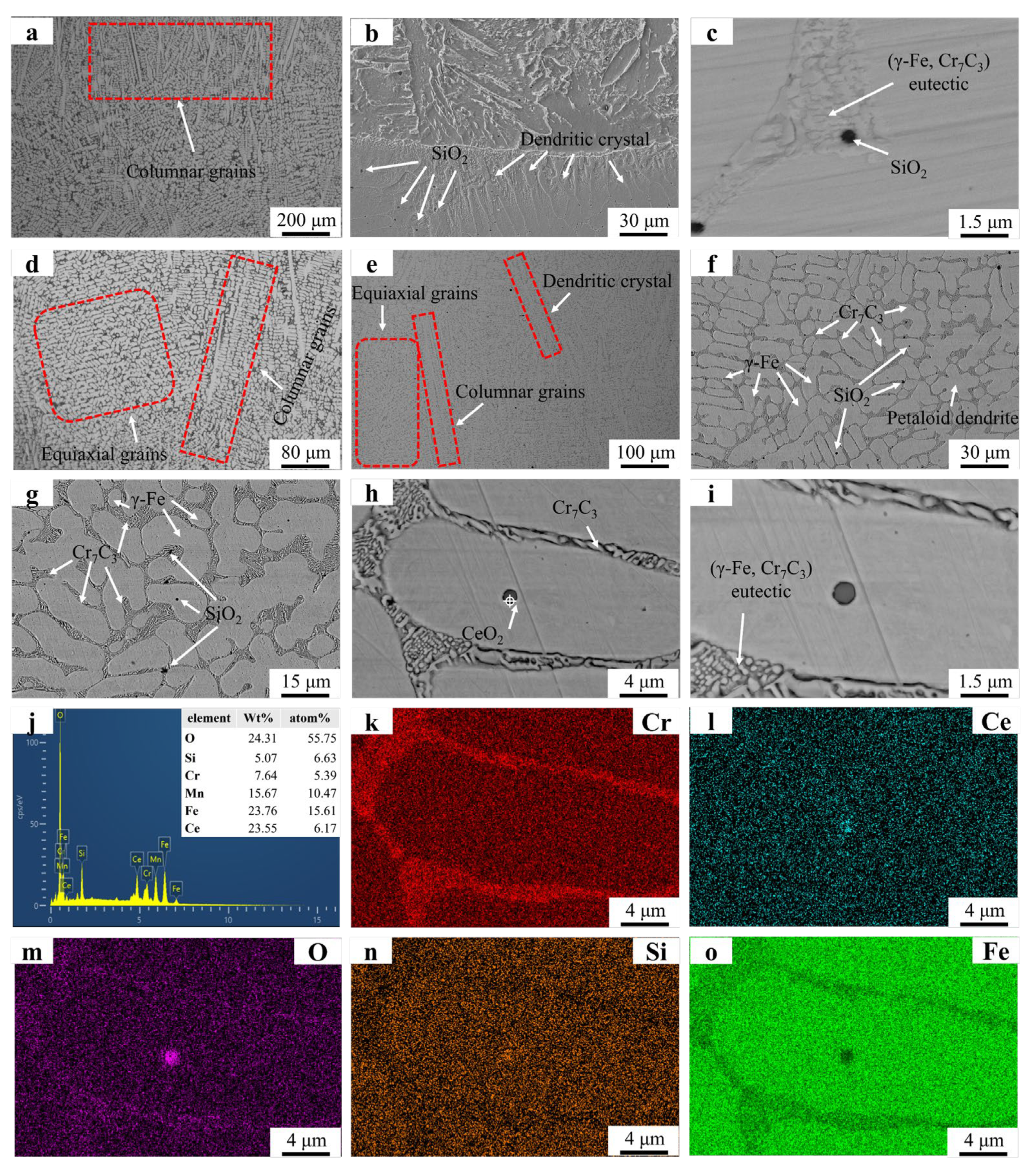
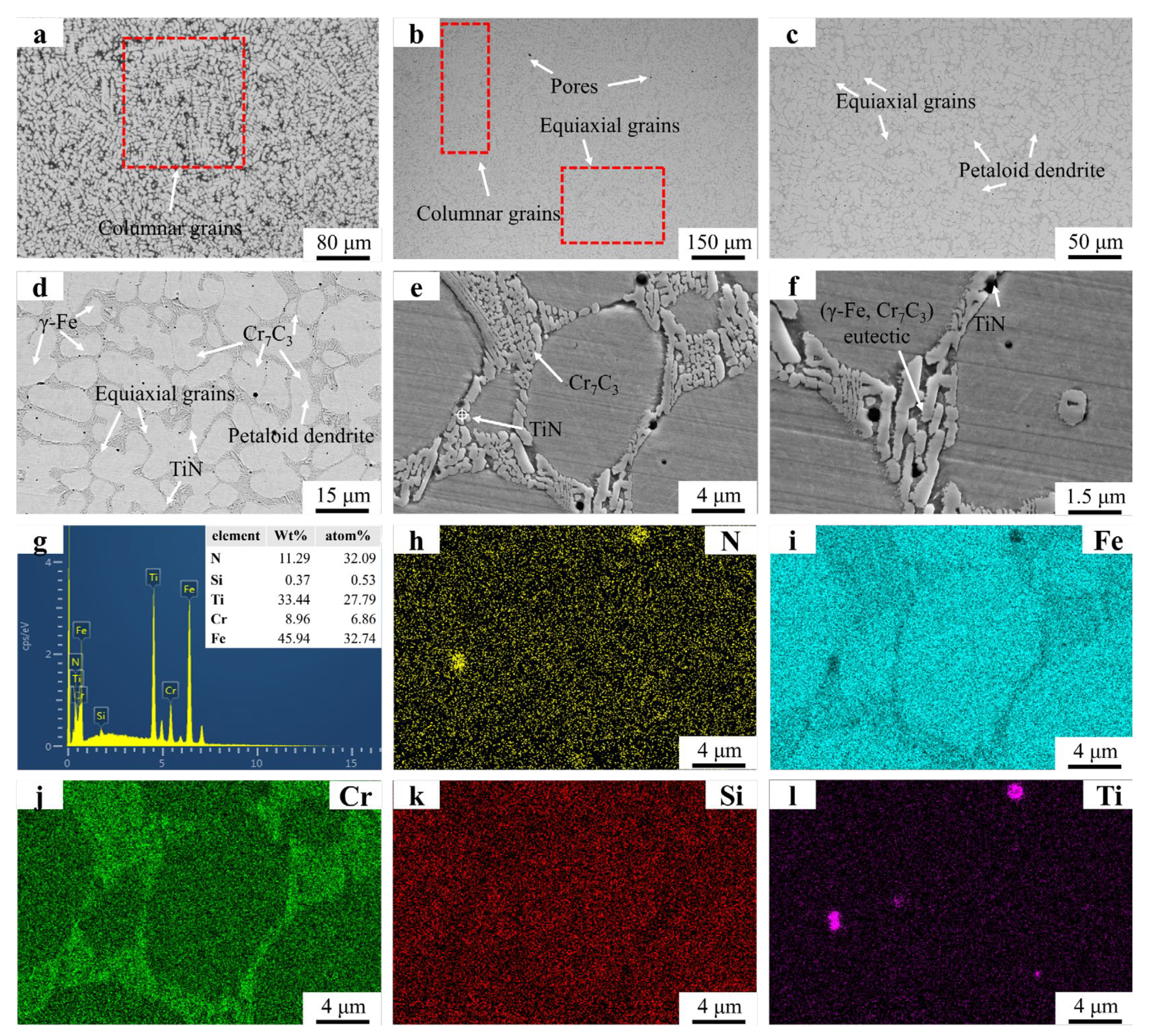
Figure 3 presents the microstructural morphology and EDS mapping results of the FC2 and Fe55 samples. Figure 3a shows the coexistence of columnar grains and irregular equiaxed grains in specific regions. The irregular shape of these equiaxed grains suggests they likely formed through partial coalescence of columnar grains before complete solidification, resulting in incomplete equiaxed grain formation. Figure 3b reveals a predominantly dendritic structure near the substrate–coating interface. The extensive merging of columnar grains during solidification limits the observable columnar grains in this region. The petaloid grains in Figure 3 originated from the coalescence of columnar grains rather than the expansion of equiaxed grains. SiO2 particles are distributed at both grain boundaries and grain interiors. Low-melting-point SiO2 precipitated after Cr7C3 formation, with Cr7C3 acting as nucleation sites for SiO2. In grain interiors, SiO2 particles solidified spontaneously due to the absence of nucleation sites, leading to higher SiO2 amount at grain boundaries. Cr7C3 carbides within the eutectic structure exhibit significant refinement in size compared to those in Fe55. However, the overall eutectic structure dimensions in FC2 are larger than in Fe55, indicating distinct structural differences. Figure 3g confirms CeO2 enrichment in specific regions. Figure 3h–j (EDS mapping of Figure 3e) show O signal clusters in CeO2-rich zones and weak O signals near eutectic structures, suggesting trace CeO2 act as the nucleation sites during solidification.
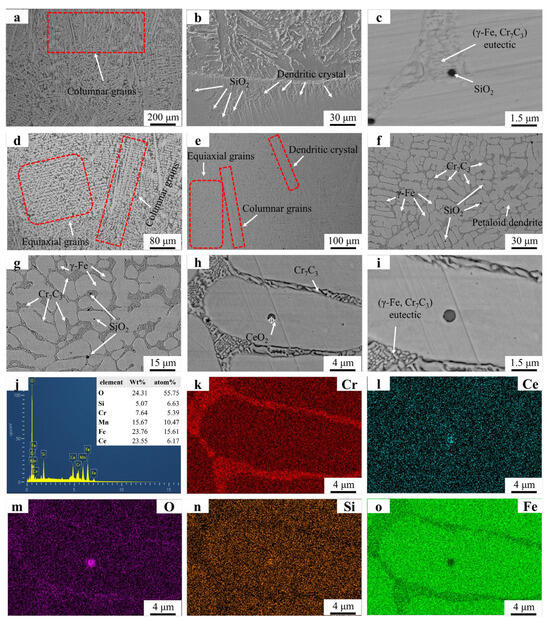
Figure 3.
SEM micrographs and EDS mapping results of Fe55 and FC2: (a) Fe55 OM graph (b) ×500; (c) ×8000; (d) FC2 OM graph; (e) ×150, (f) ×500; (g) ×2000; (h) ×5000; (i) ×8000; (j) EDS result; (k–o) EDS mapping.
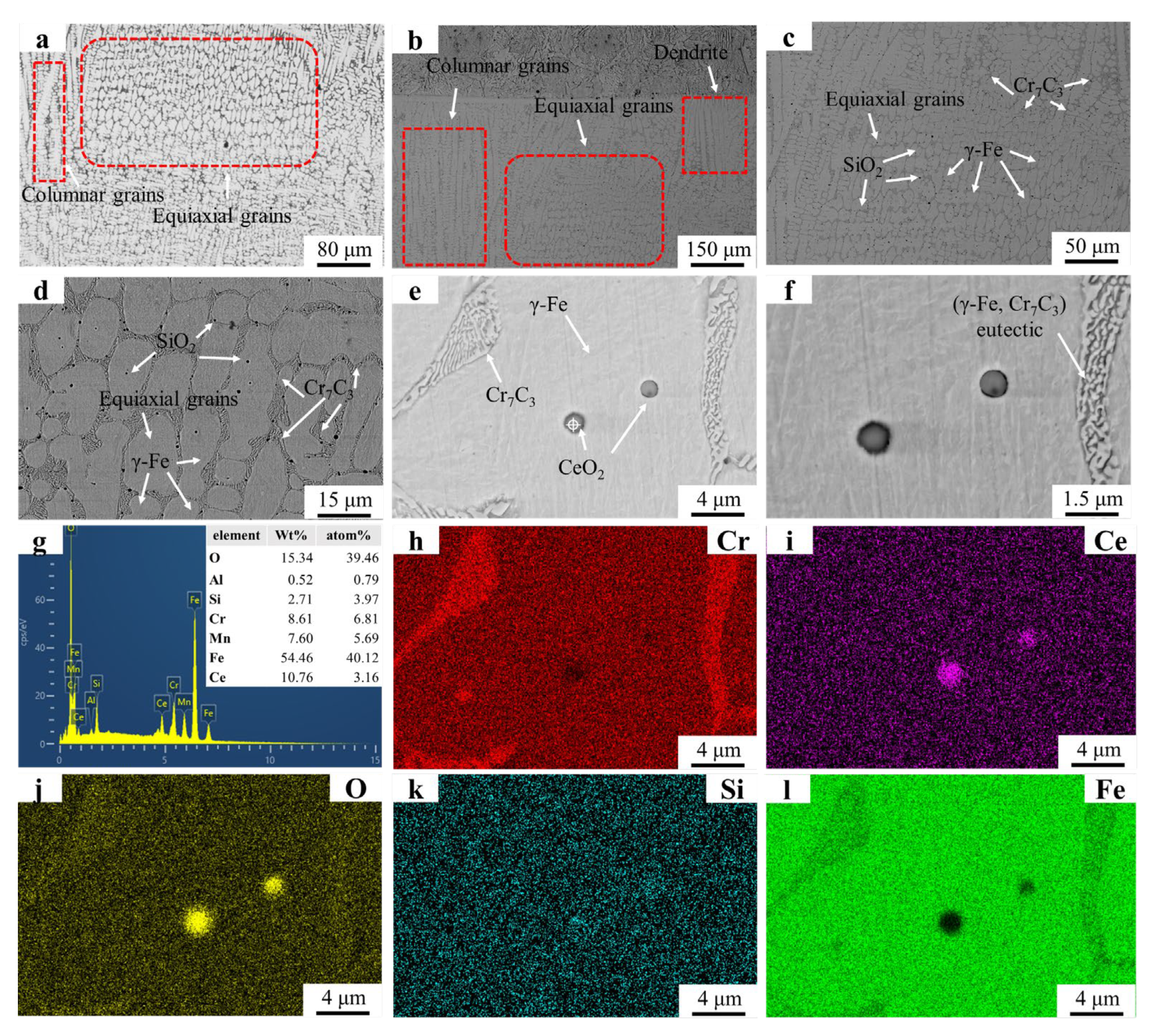
Figure 4 displays the microstructural morphology and EDS mapping results of the FC4 sample. The columnar grain coalescence has led to the formation of equiaxed grains were observed in Figure 4a, which are larger in size compared to those observed in Figure 4a. The elongated morphology of these equiaxed grains along the temperature gradient direction aligns with the grain boundary drag effects reported in prior studies [28]. Figure 4b shows areas where columnar grains remain unmerged, suggesting lower CeO2 content in these regions compared to equiaxed grain-dominated zones. Figure 4c,d demonstrate an increased density of SiO2 particles relative to the FC2 sample. SiO2 is distributed not only at grain boundaries but also extensively within grain interiors. Figure 4g confirms localized CeO2 enrichment in the region shown in Figure 4e. Figure 4h–j (EDS mapping of Figure 4e) exhibit overlapping signals of O, Si, and Ce in CeO2-rich zones, indicating that SiO2 particles may utilize CeO2 as nucleation sites during solidification. Cr7C3 carbides within the eutectic structure retain their refined characteristics. However, the increase in grains amount disperses eutectic structures along grain boundaries, resulting in a smaller eutectic structure compared to FC2.
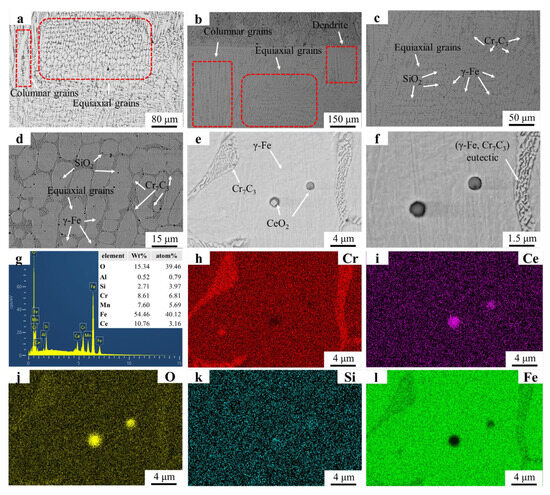
Figure 4.
SEM micrographs and EDS mapping results of FC4: (a) FC4 OM graph; (b) ×150; (c) ×500; (d) ×2000; (e) ×5000; (f) ×8000; (g) EDS result; (h–l) EDS mapping.
Based on the aforementioned findings, it is reasonable to conclude that the addition of CeO2 promotes the coalescence of columnar grains into equiaxed grains. Increased CeO2 content further enhances equiaxed grain growth. However, the grain boundary drag effect induced by CeO2 in molten steel may account for the elongation of equiaxed grains along thermal gradients.
CeO2 facilitates both the coarsening of eutectic structures and the dispersion of Cr7C3 carbides. Concurrently, the increase in grain amounts due to equiaxed grain growth leads to reduced eutectic structure dimensions. Consequently, an optimal CeO2 concentration effectively suppresses columnar grain development and promotes their transition to equiaxed morphology. Nevertheless, excessive CeO2 additions may exacerbate grain boundary drag effects and result in inhomogeneous Ce distribution, ultimately degrading microstructural uniformity.
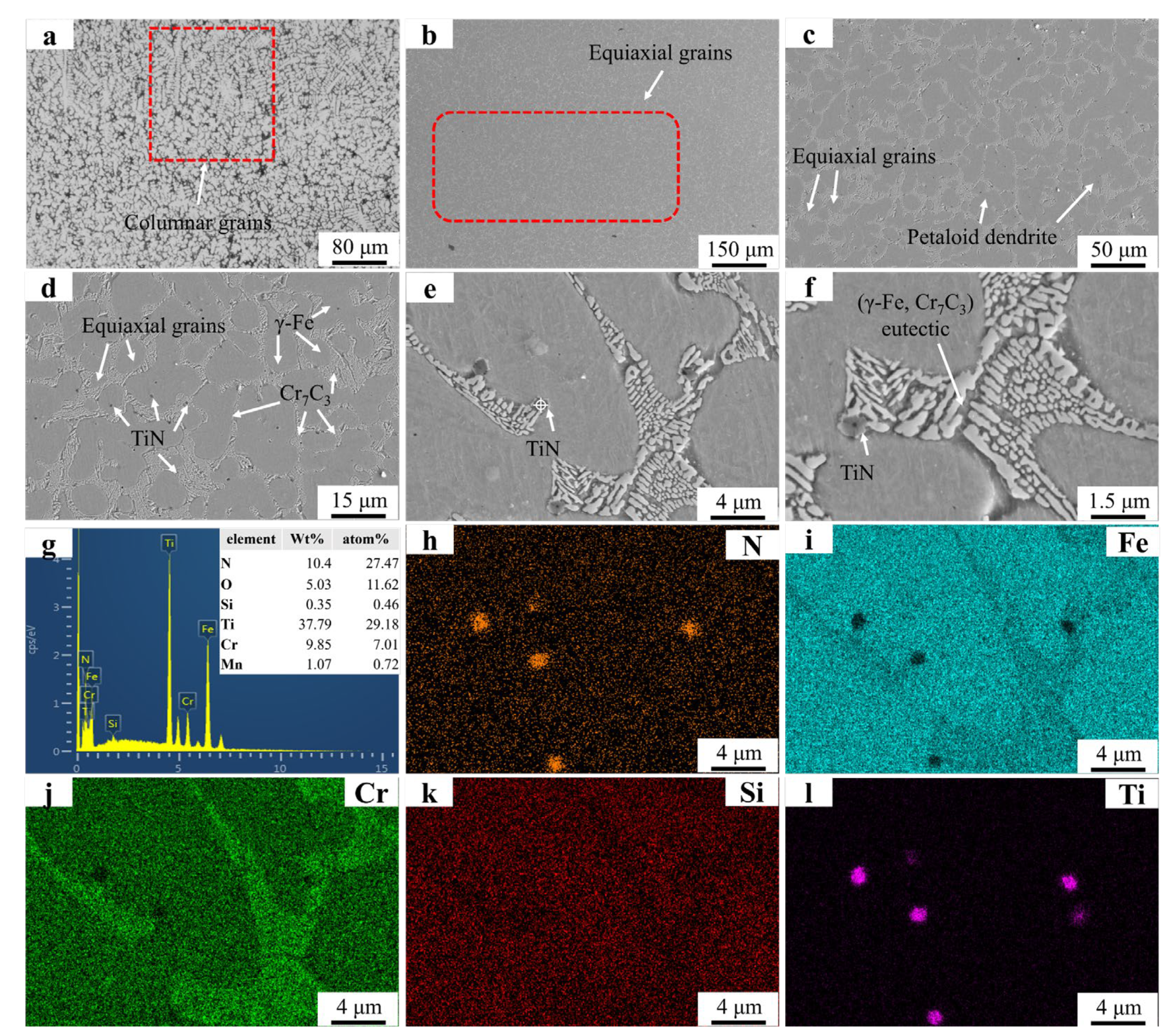
Figure 5 presents the microstructural morphology and EDS mapping results of the FT1 sample. Figure 5a shows the absence of dendritic structures, with a notable reduction in columnar grain size, indicating TiN’s significant inhibitory effect on columnar grain growth. Figure 5b reveals the presence of pores, likely caused by N2 gas release from partial decomposition of TiN particles [29]. Although TiN’s melting point exceeds the molten pool temperature, smaller TiN particles may still undergo decomposition. Figure 5c,d exhibit equiaxed grains and petaloid grains. No TiN particles are observed within the petaloid grains. The addition of TiN promotes equiaxed grain nucleation. When the equiaxed grain density reaches a critical threshold and TiN particles no longer impede growth, these grains spontaneously coalesce into petaloid configurations to minimize grain boundary energy. Figure 5g (EDS of the particle in Figure 5e) confirms the presence of TiN within eutectic structures. Figure 5 h–j (EDS mapping of Figure 5e) show limited Ti signal enrichment due to the low concentration and fine size of precipitated TiN particles.
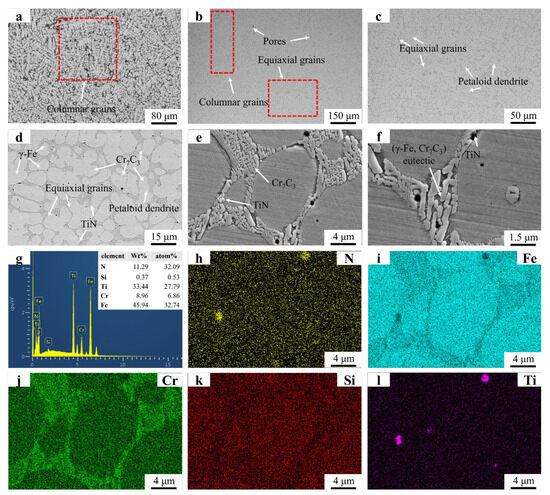
Figure 5.
FT1 SEM micrographs and EDS mapping results of FT1: (a) FT1 OM graph; (b) ×150; (c) ×500; (d) ×2000; (e) ×5000; (f) ×8000; (g) EDS result; (h–l) EDS mapping.
Figure 6 presents the microstructural morphology and EDS mapping results of the FT2 sample. Figure 6a reveals a microstructure dominated by equiaxed grains, with residual dendritic structures observed near the substrate–coating interface. This contrasts with the FT1 sample, where partially suppressed dendritic remnants persist in the mid-coating region. The complete absence of dendrites in the central zone of FT2 highlights the enhanced inhibitory effect of increased TiN addition on dendritic growth. Figure 6b,c display petaloid grains, whose formation mechanism aligns with observations in the FT1 sample and parallels findings reported in prior studies. TiN particles in Figure 6e exhibit significantly larger dimensions compared to those in Figure 6e. The elevated TiN content increases [Ti] and [N] concentrations in the molten pool, promoting the growth of precipitated TiN during solidification. As demonstrated in previous studies, coarsened precipitates exert stronger grain boundary drag effects, explaining the increased equiaxed grain amount with higher TiN addition. Overlapping Ti and N signals in the EDS maps confirm TiN predominantly distributes along grain boundaries, where it pins boundaries to facilitate equiaxed grain formation. In regions devoid of TiN particles, unimpeded grain boundary migration enables petaloid grain development through spontaneous coalescence.
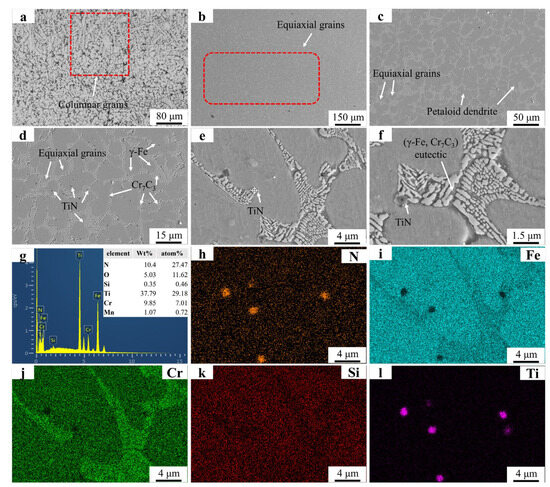
Figure 6.
SEM micrographs and EDS mapping results of FT2: (a) FT2 OM graph: (b) ×150; (c) ×500; (d) ×2000; (e) ×5000; (f) ×8000; (g) EDS result; (h–l) EDS mapping.
These results indicate that TiN primarily contributes to grain refinement by providing additional nucleation sites, resulting in an increased number of grains and grain boundaries [30]. As the quantity of grain boundaries increases, the Cr7C3 within the eutectic structure, serving as a constituent of grain boundaries, diminishes in size to accommodate the formation of additional boundaries. Furthermore, TiN also acts as nucleation sites for Cr7C3 in the eutectic structure, thereby promoting grain boundary formation. However, when the TiN addition level is low, dendritic growth remains not fully suppressed, leading to the observed substantial residual dendrites in the figure. When the TiN content increases to 2%, the precipitated TiN particles exhibit enlarged dimensions, which enhances their grain boundary inhibition effectiveness and consequently reduces dendritic formation.
The experimental findings demonstrate that with 0.02% CeO2 addition, dendritic coalescence in the welding layer is promoted. However, when the CeO2 content elevates to 0.04%, although equiaxed grains emerge, significant grain boundary dragging effects become apparent and lead to grain expansion. The co-addition of CeO2 and TiN may potentially induce unnecessary grain expansion, thus the optimal CeO2 content is determined as 0.02% to achieve a structurally homogeneous welding layer. Concurrently, when TiN addition reaches 2%, effective suppression of dendritic growth is observed, thereby establishing 2% as the optimal TiN addition level.
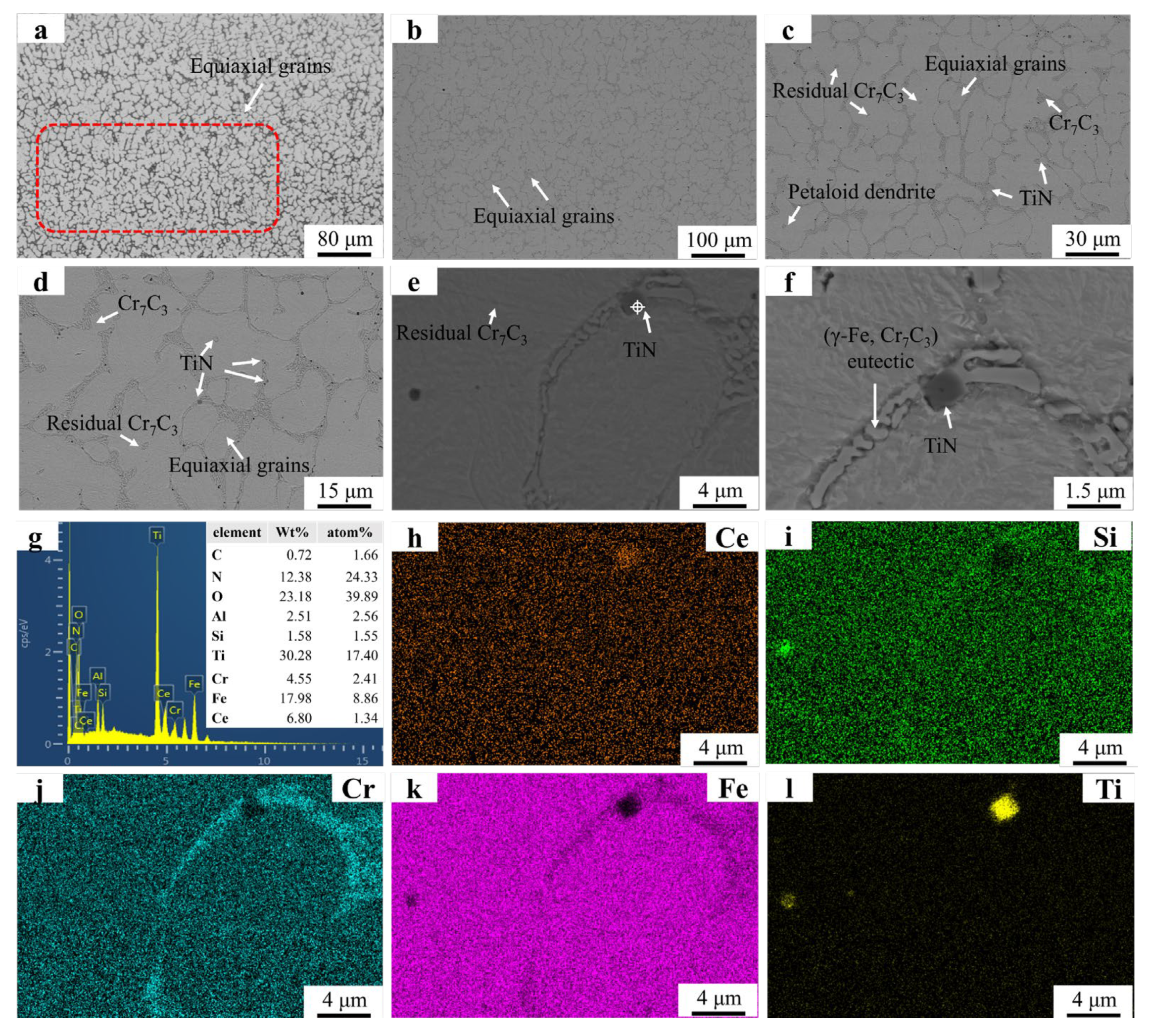
The microstructure and EDS mapping results of the FC2T2 sample are illustrated in Figure 7. In Figure 7a, only a small number of dendrites are observed near the substrate region. These dendritic formations are difficult to avoid due to the significant temperature gradient between the substrate and the welding layer. Figure 7b,c reveal the presence of petaloid grains and equiaxed grains, whose formation mechanisms align with those described earlier. In Figure 7d, residual eutectic structures are observed at the centers of the petaloid grains. The grain boundary dragging effect induced by CeO2 prevents complete contraction of the grain boundaries formed during the coalescence of equiaxed grains, resulting in spherical remnants of these boundaries under surface tension. Figure 7e,f demonstrate the distribution of TiN particles within the grains and along grain boundaries. Notably, the TiN particles exhibit significantly smaller dimensions compared to those in the FT2 sample, indicating that CeO2 addition promotes both spheroidization and dispersion of TiN particles. This enhanced distribution enables the precipitated TiN to generate more nucleation sites and grain boundary pinning points, consistent with previous reports. These mechanisms collectively explain why the FC2T2 sample exhibits markedly superior dendritic suppression compared to the FT2 sample.
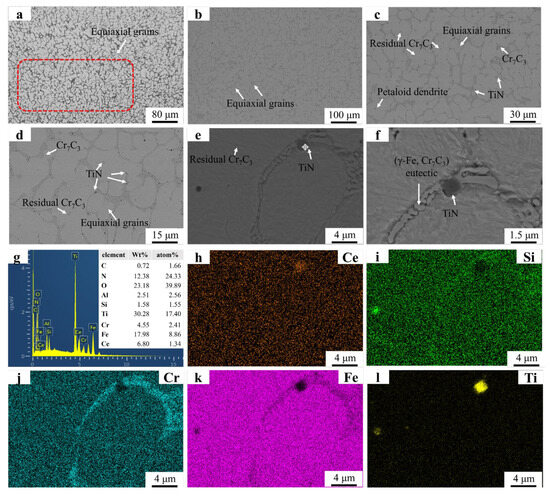
Figure 7.
SEM micrographs and EDS mapping results of FC2T2: (a) FC2T2 OM graph; (b) ×150; (c) ×500; (d) ×2000; (e) ×5000; (f) ×8000; (g) EDS result; (h–l) EDS mapping.
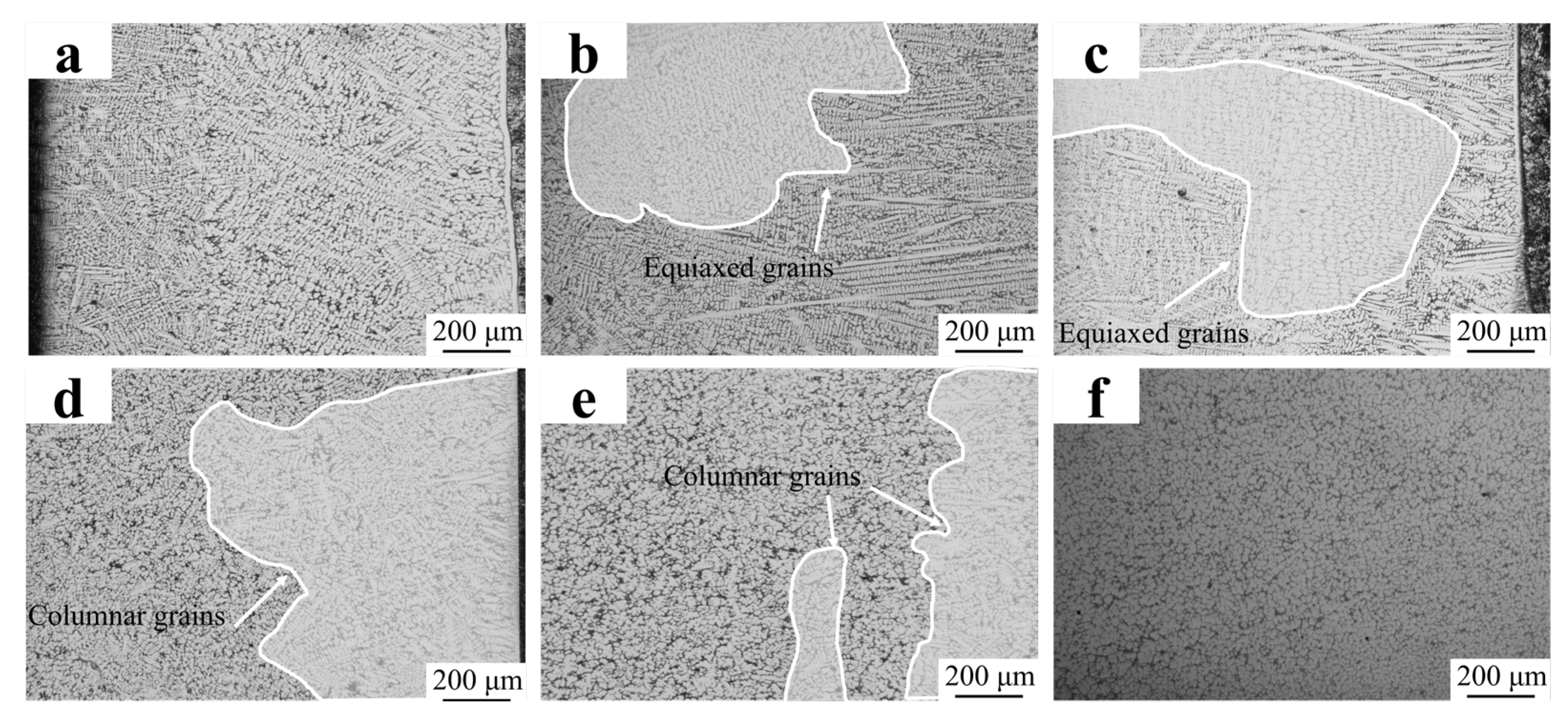
The above study has identified the FC2T2 sample as possessing the most uniform microstructure. To further determine the influence of the co-addition of CeO2 and TiN on the equiaxed/columnar grain ratio, low-magnification optical microscopy (OM) images were obtained to statistically quantify this ratio across different compositional conditions, as shown in Figure 8. The equiaxed/columnar grain ratio(Table 3) was quantified using ImageJ(1.8.0) software. As shown in Figure 8a, columnar grains accounted for 100%, indicating that the columnar grain growth in the Fe55 welding layer was not inhibited during solidification. The fine columnar grains observed at the top of the welding layer originated from air convection caused by the protective gas, which stirred the molten steel and increased the nucleation rate.
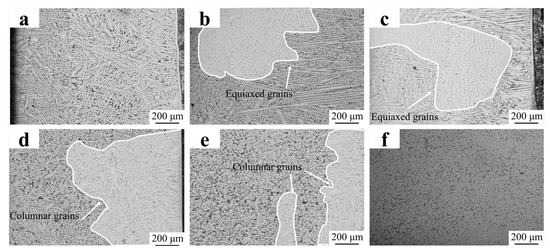
Figure 8.
OM micrographs of welding layer: (a) Fe55; (b) FC2; (c) FC4; (d) FT1; (e) FT2; (f) FC2T2.

Table 3.
The ratio of equiaxed grains and grain size in different samples.
At a CeO2 addition of 0.02%, the proportion of columnar grains decreased slightly to approximately 81.38%. However, the subgrain size within the columnar grains was significantly reduced, dropping from 45 μm in the Fe55 sample to approximately 20 μm. Concurrently, some columnar grain boundaries fused and began to transition toward equiaxed grain formation. Despite these structural adjustments, the microstructure of the welding layer remained highly non-uniform at this stage.
At a CeO2 addition of 0.04%, the columnar grain proportion dropped to 64.23%, marking a significant reduction. Concurrently, prominent grain drag phenomena were observed in the equiaxed region, confirming the manifestation of the grain boundary drag effect. The equiaxed grains reached approximately 60 μm in size, significantly larger than those of the FC2 sample. Near the equiaxed region, merging columnar grains were observed, though their grain size exceeded that of the FC2 sample’s grains.
The results depicted in Figure 8d reveal that the columnar grain proportion in the FT1 sample decreased to 51.08%, with an average grain size of 32.5 μm. Residual columnar grains were observed at the top and bottom of the welding layer, attributed to insufficient TiN particle addition, which limited nucleation sites required for equiaxed grain formation. As shown in Figure 8e, increasing the TiN addition to 2% further reduced the columnar grain proportion to 27.48%, with equiaxed grains averaging approximately 27.6 μm in size. The notable reduction in columnar grain count and grain size can be attributed to the increase in nucleation sites provided by increased TiN content, which simultaneously suppressed grain coarsening. However, residual columnar grains remained identifiable in the microstructure. Notably, the columnar regions in both FT1 and FT2 samples are predominantly localized at the bottom, where elevated superheat levels impeded TiN precipitation, thereby limiting its grain-refining effect.
The results depicted in Figure 8e indicate that the FC2T2 sample exhibited a non- columnar grain structure and the smallest equiaxed grain size (20.32 μm). The disappearance in columnar equiaxed grains at the bottom can be attributed to the combined effects of CeO2 addition and TiN precipitation mechanisms. Specifically, CeO2 promoted the formation of composite inclusions with TiN, thereby enhancing TiN precipitation at high temperature, which forced the residual columnar grains to transform into equiaxed grains—a phenomenon consistent with the findings of Chen et al. [31].
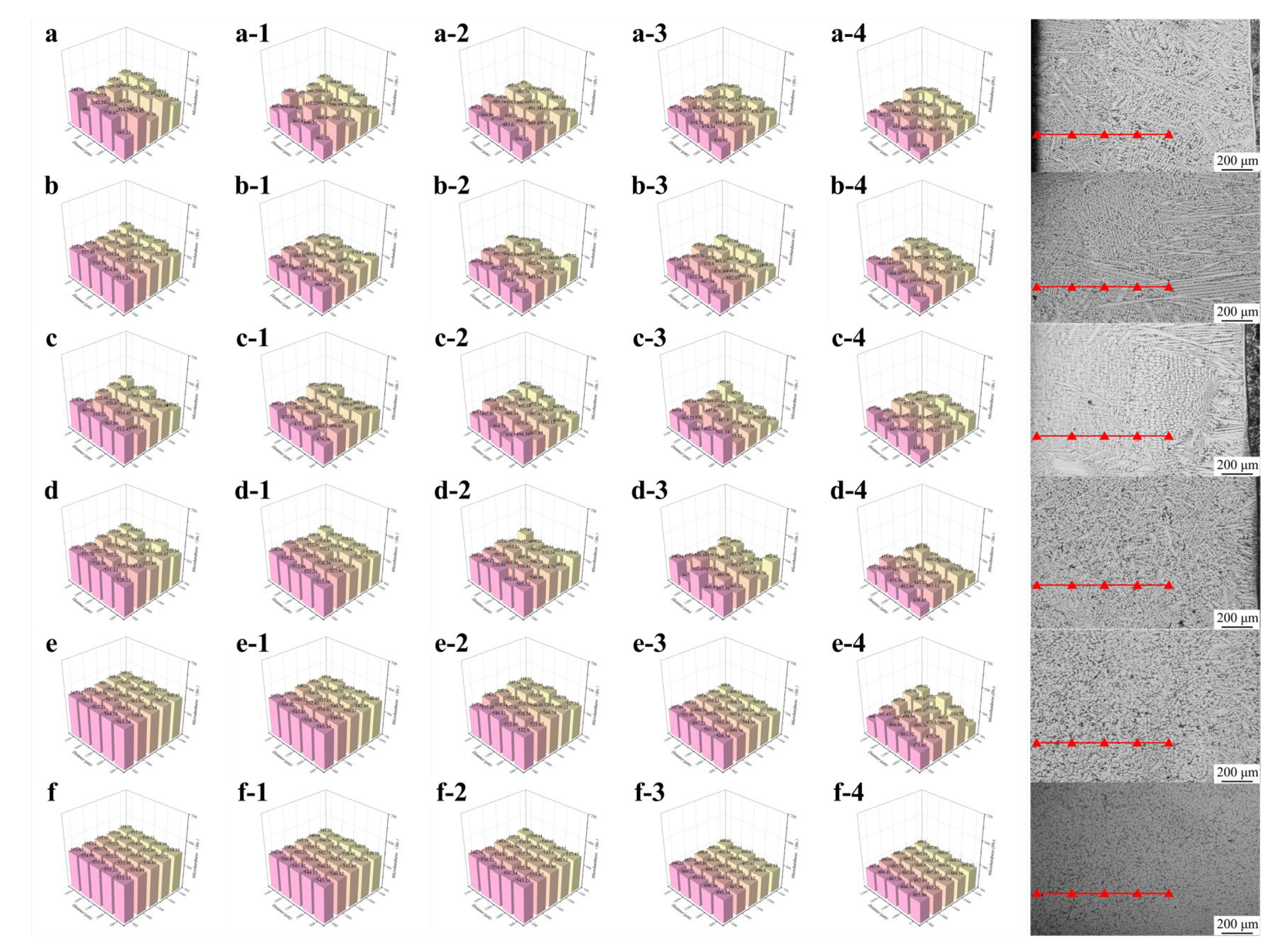
The results presented in Figure 9 and Table 4 illustrate the surface hardness uniformity at varying depths from the welding layer surface. As shown in Figure 9a, the surface exhibited a highly non-uniform hardness distribution due to the entirely columnar grain structure within the welding layer. The maximum hardness reached approximately 540, while the minimum value was approximately 480. Notably, this trend of non-uniform hardness distribution persisted without significant changes as the depth increased. High-hardness regions were concentrated in zones enriched with Cr7C3, whereas low-hardness areas corresponded to regions dominated by γ-Fe. The non-uniform distribution of hard and soft phases directly resulted in substantial fluctuations in hardness values across the welding layer.
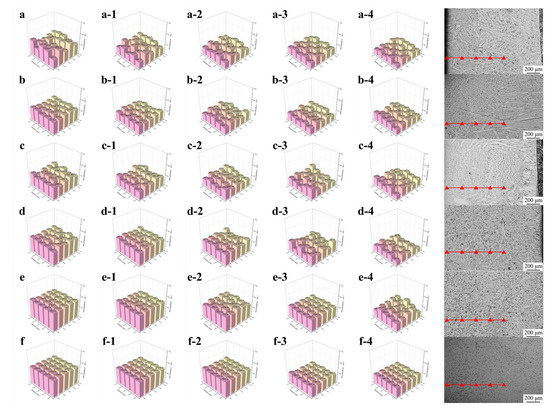
Figure 9.
The surface hardness uniformity at varying depths from the welding layer surface: (a) Fe55; (b) FC2; (c) FC4; (d) FT1; (e) FT2; (f) FC2T2.

Table 4.
Microhardness test results of all samples.
The hardness uniformity test results for welding layers with varying CeO2 contents are presented in Figure 9b,c. Microstructural analyses revealed that CeO2 addition promoted grain refinement and increased the proportion of equiaxed grains. These structural changes lead to a more uniform distribution of hard and soft phases, thereby reducing hardness fluctuations in the welding layer. However, the persistent high columnar grain proportion and non-uniform grain distribution still resulted in significant hardness variations.
Figure 9d,e show the microhardness test results of welding layers with different TiN contents. The top regions of all welding layers were composed of equiaxed grains, exhibiting uniform distributions of hard and soft phases. This outcome suggests that the prevalence of equiaxed grains is the primary factor enhancing hardness uniformity. However, FT1 samples displayed significant hardness fluctuations at shallower depths than FT2 samples. This disparity arises from the higher proportion of columnar grain regions in FT1 compared to FT2. Consequently, the hypothesis that columnar grains induce substantial hardness fluctuations is justified. In FT2 samples, the columnar regions mainly formed at the bottom of the welding layer, resulting in low hardness fluctuations across most areas. As proximity to the heat-affected zone (HAZ) increases, the number of residual columnar grains rises, which accounts for the onset of hardness fluctuations observed in FT2 samples at a test depth of 1000 μm.
Figure 9f demonstrates that the FC2T2 sample exhibited the highest hardness uniformity, which remained consistent without significant variation with increasing depth. This can be attributed to its fully equiaxed microstructure, characterized by refined grain sizes and the absence of columnar grains in the welding layer. The uniform distribution of hard and soft phases significantly enhanced this hardness uniformity. Consequently, in subsequent friction and wear experiments, only the wear behaviors of the FC2T2 and Fe55 samples will be compared to investigate the relationship between their substantial differences in hardness uniformity and wear performance.
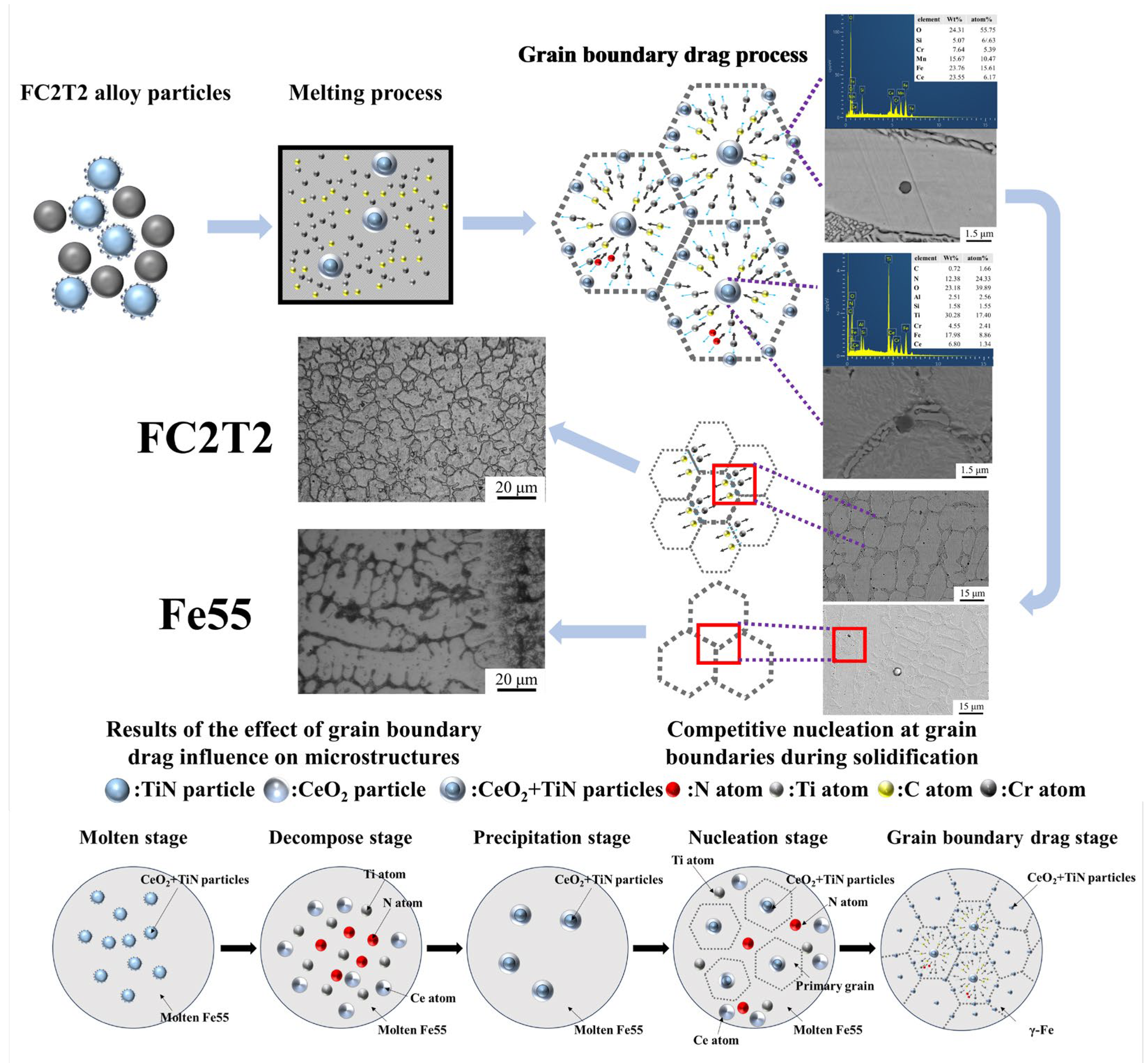
Figure 10 illustrates the process and mechanism of grain boundary drag effects on the microstructure of the welding layer. Initially, CeO2 adheres to the surfaces of TiN particles and becomes uniformly mixed with Fe55 alloy powder. When the powder is subjected to a high-temperature plasma arc, it melts into molten steel liquid containing alloying elements such as Cr, C and Si. Under elevated temperatures, TiN reacts with and decomposes in the molten steel. However, due to the encapsulation by CeO2 on TiN surfaces, a portion of TiN particles survives and subsequently acts as nucleation sites or grain boundary pinning points during solidification.
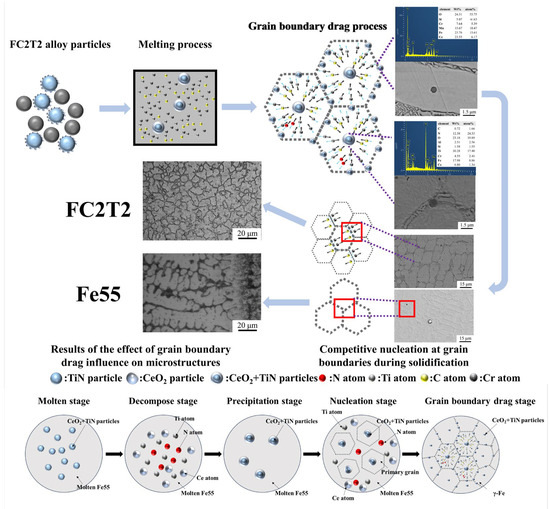
Figure 10.
Process and mechanism of grain boundary drag effect on the microstructure of welding layer.
As the welding layer initiates solidification, the high chemical activity of Ce drives CeO2-encapsulated TiN particles within grains to drag solute atoms (e.g., C, Cr, Si) from the molten steel to form new intermetallic compounds. This process reduces atomic diffusion coefficients and increases the viscosity of the molten steel. Since Cr and C are essential constituents of grain boundaries, a competitive mechanism emerges between CeO2-encapsulated TiN particles within grains and the grain boundaries. The expansion of grain boundaries requires additional solute atoms to form the (γ-Fe, Cr7C3) eutectic structure, and this competition impedes grain expansion. However, the high activity of Ce simultaneously promotes the formation of Cr7C3 hard phases, resulting in smaller Cr7C3 dimensions within the eutectic structure.
Furthermore, the CeO2-encapsulated TiN particles exert drag effects on solute atoms across all directions, favoring the development of equiaxed grains. Although grain refinement increases the number of grain boundaries, insufficient solute atoms are available in the welding layer to sustain boundary formation. Consequently, solute atoms are competitively consumed during grain boundary formation, leading to a reduction in eutectic structure dimensions. Thus, the addition of CeO2 introduces two competitive mechanisms: (1) competition between CeO2-encapsulated TiN particles and grain boundaries; (2) competition among grain boundaries for solute atoms to form eutectic structures. Under the combined effects of these mechanisms, the microstructure becomes more homogeneous and preferentially evolves into equiaxed grains. In contrast, the Fe55 welding layer lacks such competitive mechanisms, allowing sufficient solute atoms for unrestricted grain boundary formation during nucleation. This results in a predominantly columnar grain structure rather than equiaxed grains in Fe55.
The FC2T2 sample underwent five distinct stages: melting, decomposition, precipitation, grain nucleation, and grain boundary drag. During the melting stage, CeO2 was adsorbed onto the surface of TiN particles. When the plasma arc further elevated the temperature of the molten steel, TiN and CeO2 decomposed into [Ce], [Ti], and [N]. In the cooling stage, complex inclusions containing Ti, N, and Ce began to precipitate from the molten steel and subsequently acted as nucleation sites for primary grains during the nucleation phase. As the grains continued to grow, these Ti-N-Ce-containing complex inclusions dragged grain boundaries, ultimately promoting the formation of equiaxed grains.
3.3. Wear Morphology
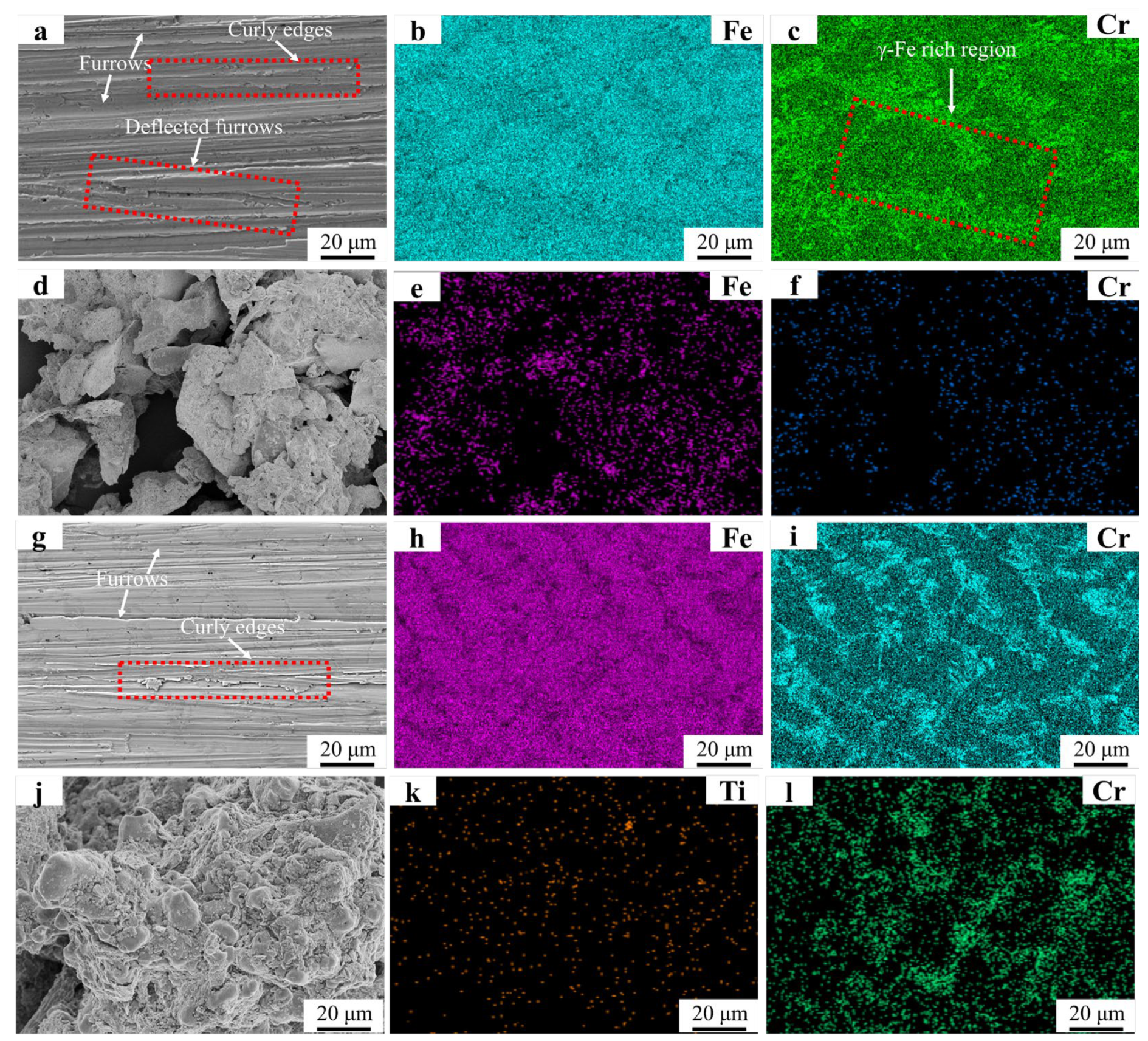
Figure 11 displays the surface morphology of the FC2T2 welding layer after wet friction testing. Densely distributed plowing furrows with curled edges are observed in Figure 11a, while deflected furrows are absent. This phenomenon can be attributed to the homogeneous distribution of Cr7C3 hard phases shown in Figure 11i. As Cr7C3 uniformly resists abrasive particle cutting on the worn surface, no microscale wear heterogeneity occurs, a result corroborated by the significantly enhanced Cr signal in Figure 11i compared to Figure 11c. In the FC2T2 welding layer, both Cr7C3 and γ-Fe are simultaneously removed during wear, whereas the Fe55 welding layer primarily loses γ-Fe in worn regions. This explains the markedly higher Cr signal intensity in FC2T2 wear debris compared to Fe55. In the red dashed area of Figure 11c γ-Fe-rich zones align with the deflection direction of particles in Figure 11a. The deflected plowing furrows follow the distribution orientation of Cr7C3, suggesting that SiC abrasives preferentially cut γ-Fe-rich regions during wear. As the kinetic energy of SiC abrasives dissipates progressively during cutting, they become incapable of removing adjacent Cr7C3 hard phase-rich zones, ultimately being redirected along and blocked by these regions.
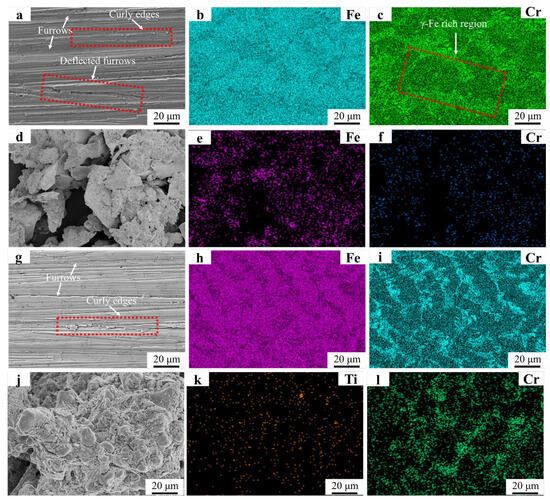
Figure 11.
SEM micrographs and EDS mapping results of Fe55 and FC2T2 worn surfaces and debris: (a) micrograph of Fe55 worn surface; (b,c) EDS mapping results of Fe55 worn surface; (d) micrographs of Fe55 debris; (e,f) debris EDS mapping results of Fe55; (g) micrograph of FC2T2 worn surface; (h,i) EDS mapping results of FC2T2 worn surface; (j) micrographs of FC2T2 debris; (k,l) debris EDS mapping results of FC2T2.
Our previous work demonstrated that γ-Fe functions as a supportive matrix for Cr7C3 [26]. Once the ductile γ-Fe is worn away, Cr7C3 undergoes brittle fracture under abrasive particle impact, ultimately leading to material surface degradation. In Fe55 welding layers, the absence of Cr7C3 reinforcement in γ-Fe-rich regions results in more severe abrasive cutting, explaining the largest wear debris dimensions in Fe55. In contrast, Figure 11j shows significantly reduced debris sizes compared to Figure 11d, primarily due to FC2T2’s dominant equiaxed microstructure with uniform distribution of high-toughness γ-Fe and high-hardness Cr7C3. When SiC abrasives attempt to cut soft γ-Fe regions, Cr7C3 hard phases obstruct the abrasives, mitigating γ-Fe wear and preserving the ductile matrix. This protective mechanism conversely prevents Cr7C3 hard phases from detaching under SiC impact. The synergistic interaction between γ-Fe and Cr7C3 mutually reduces material loss: Cr7C3 mitigates γ-Fe cutting, while γ-Fe retention prevents Cr7C3 dislodgement. Consequently, each abrasive cutting event produces substantially less material removal, explaining the markedly smaller debris sizes in FC2T2.
Furthermore, Ti signals detected in Figure 11k indicate that TiN not only serves as grain nucleation sites but also partially bears the frictional load during wear. Comparative analysis of Figure 11d,j reveals distinct debris morphologies: Fe55 debris primarily exhibits lamellar structures (~20 μm), whereas FC2T2 debris contains spherical particles (~10 μm) that agglomerate due to high surface energy and undergo corrosion. Lamellar debris suggests brittle spallation, while spherical debris implies uniform micro-cutting with consistent material removal per abrasive event. This limited the micro-cutting on FC2T2 surfaces generates finer debris, reflecting enhanced wear resistance.
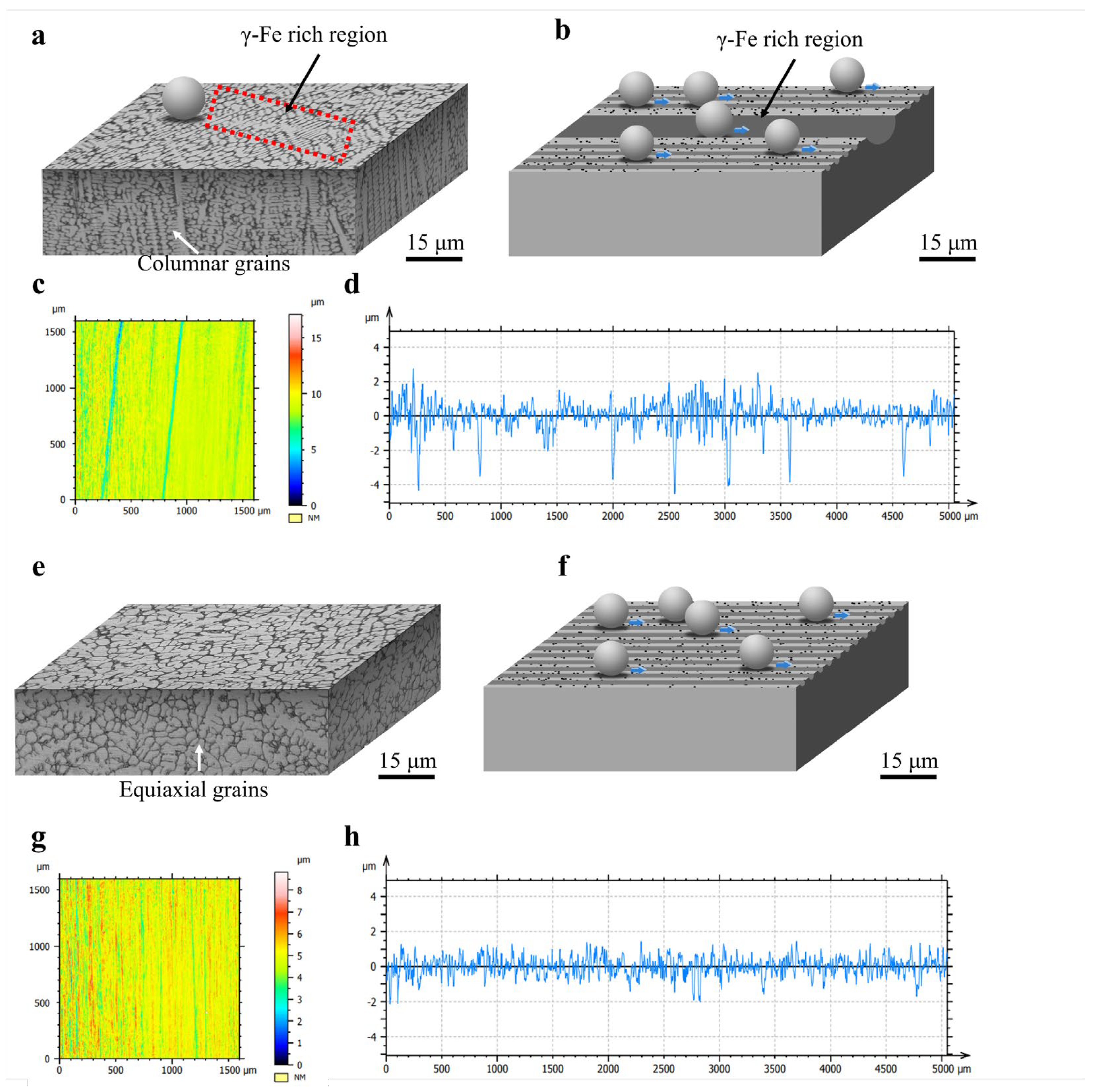
Figure 12a,b present schematic diagrams of the three-dimensional microstructure and wear process of Fe55. In Figure 12a, the cross-sectional structure of Fe55 predominantly consists of columnar grains, with numerous γ-Fe-rich zones existing on the wear surface. These γ-Fe-rich zones are susceptible to cutting, forming deep furrows. Research by Zum et al. demonstrated that micro-cutting generates additional abrasive particles [32]. However, during wet friction processes, these secondary abrasives may be carried away by water, partially mitigating three-body abrasion. Notably, owing to the high toughness of γ-Fe, friction energy is dissipated not only through micro-cutting but also via micro-ploughing-induced plastic deformation. The curled edges observed in Figure 11g result from high-energy abrasives interacting with the surface at shallow attack angles. With water acting as a boundary lubricant, insufficient energy remains to cause micro-cutting, leading kinetic energy dissipation through micro-ploughing.
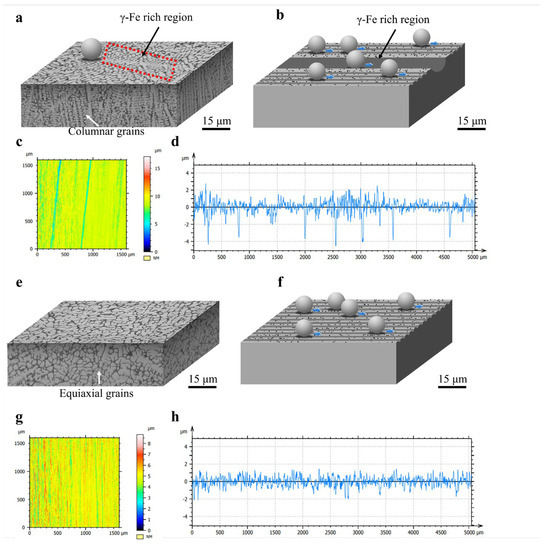
Figure 12.
Schematic of Fe55 and FC2T2 3D microstructure and wear process, 3D morphology of worn surface and worn surface roughness test results: (a) schematic diagram of Fe55 3D microstructure; (b) schematic diagram of Fe55 wear process; (c) 3D morphology of Fe55 wear surface; (d,h) Fe55 worn surface roughness; (e) schematic diagram of FC2T2 3D microstructure; (f) schematic diagram of FC2T2 wear process; (g) 3D morphology of FC2T2 wear surface.
Figure 12b illustrates the mechanism of non-uniform wear in Fe55. In regions with homogeneous Cr7C3 hard phase distribution, micro-cutting depths remain consistent. In contrast, γ-Fe undergoes plastic deformation in micro-ploughing-dominated regions, but excessive deformation is constrained by Cr7C3. These γ-Fe zones neither detach from the surface nor contribute to wear loss. The γ-Fe-rich zones typically correspond to columnar grain cores, where Cr7C3 hard phases are absent. Consequently, the wear depth in these regions matches columnar grain lengths, resulting in unmitigated severe wear.
Figure 12c,d display the surface morphology and roughness profile of Fe55 after wet friction testing. Blue regions in Figure 12c represent γ-Fe-rich zones exhibiting deeper plowing furrows compared to yellow regions with uniform Cr7C3 distribution. The minimal color variation in yellow zones indicates homogeneous wear. The roughness profile in Figure 12d confirms Fe55’s non-uniform wear characteristics through observed deep furrow formations.
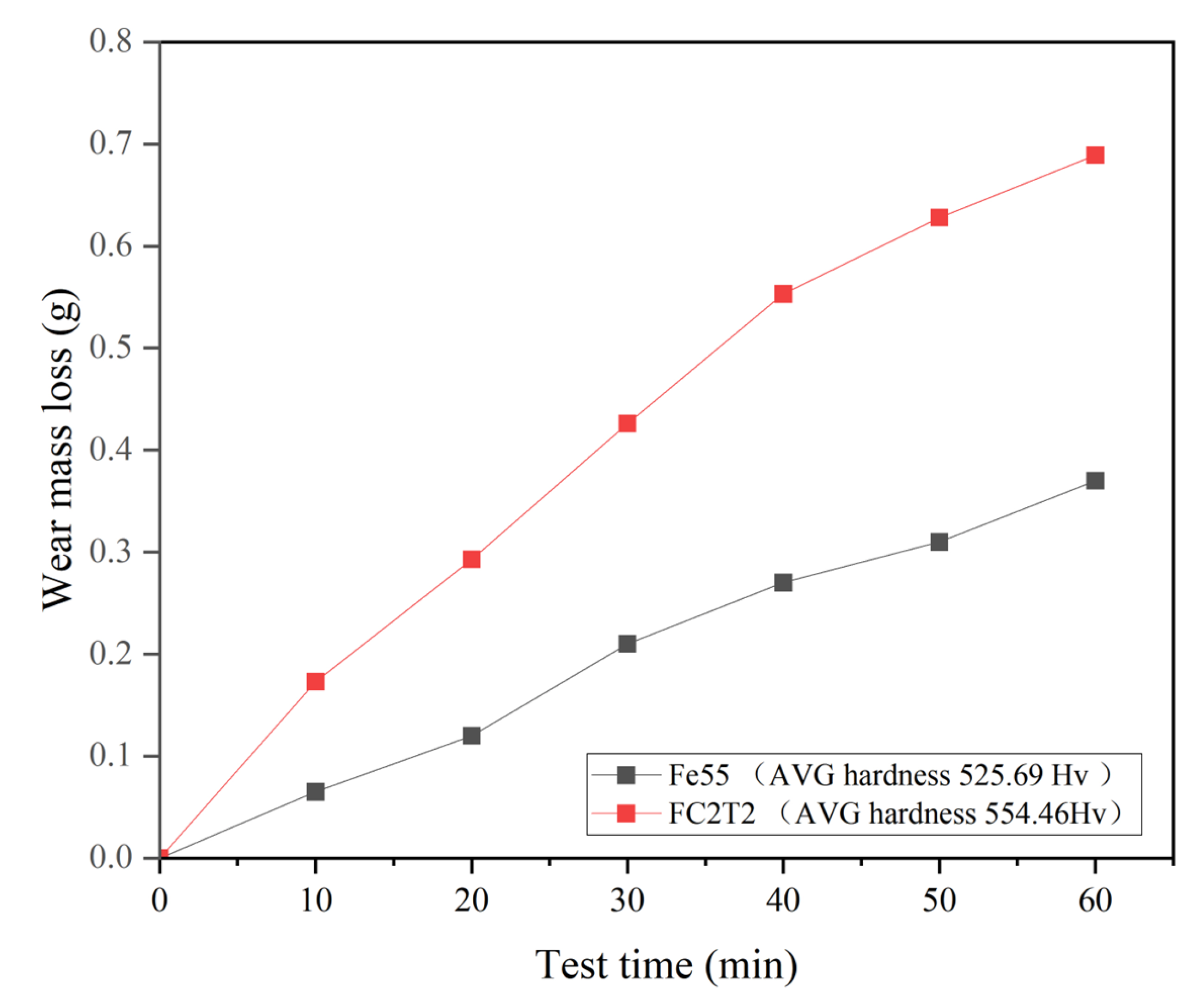
Figure 13 presents the erosion mass loss and surface morphology of the Fe55 welding layer. At each erosion stage, the erosion mass loss of Fe55 consistently exceeds that of FC2T2, which can be partially attributed to Fe55’s lower average hardness compared to FC2T2. However, the hardness discrepancy alone cannot fully account for the substantial wear loss difference. Additionally, comparative analysis of Figure 14a,b,g,h reveals significantly higher surface roughness in Fe55 than in FC2T2. Beyond micro-cutting, the primary wear mechanisms during erosion include micro-ploughing induced by high-velocity particle impacts. Unlike wet friction processes, erosion continuously removes soft γ-Fe regions without the secondary embedding of wear debris into the surface (as observed in wet friction tests with rubber wheels). All generated debris is flushed away by slurry flow. Thus, no debris is embedded on worn surface.
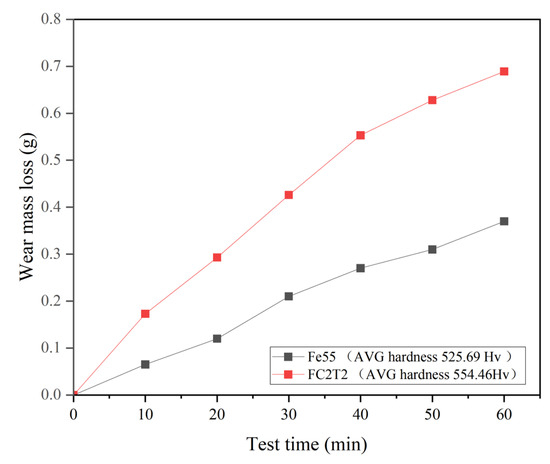
Figure 13.
Fe55 and FC2T2 erosion weight loss and surface hardness results.
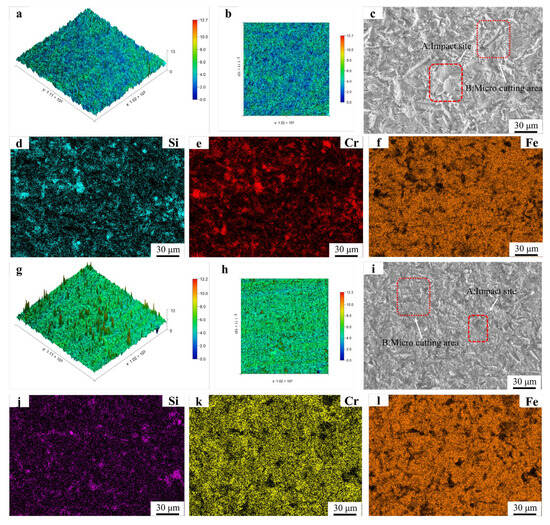
Figure 14.
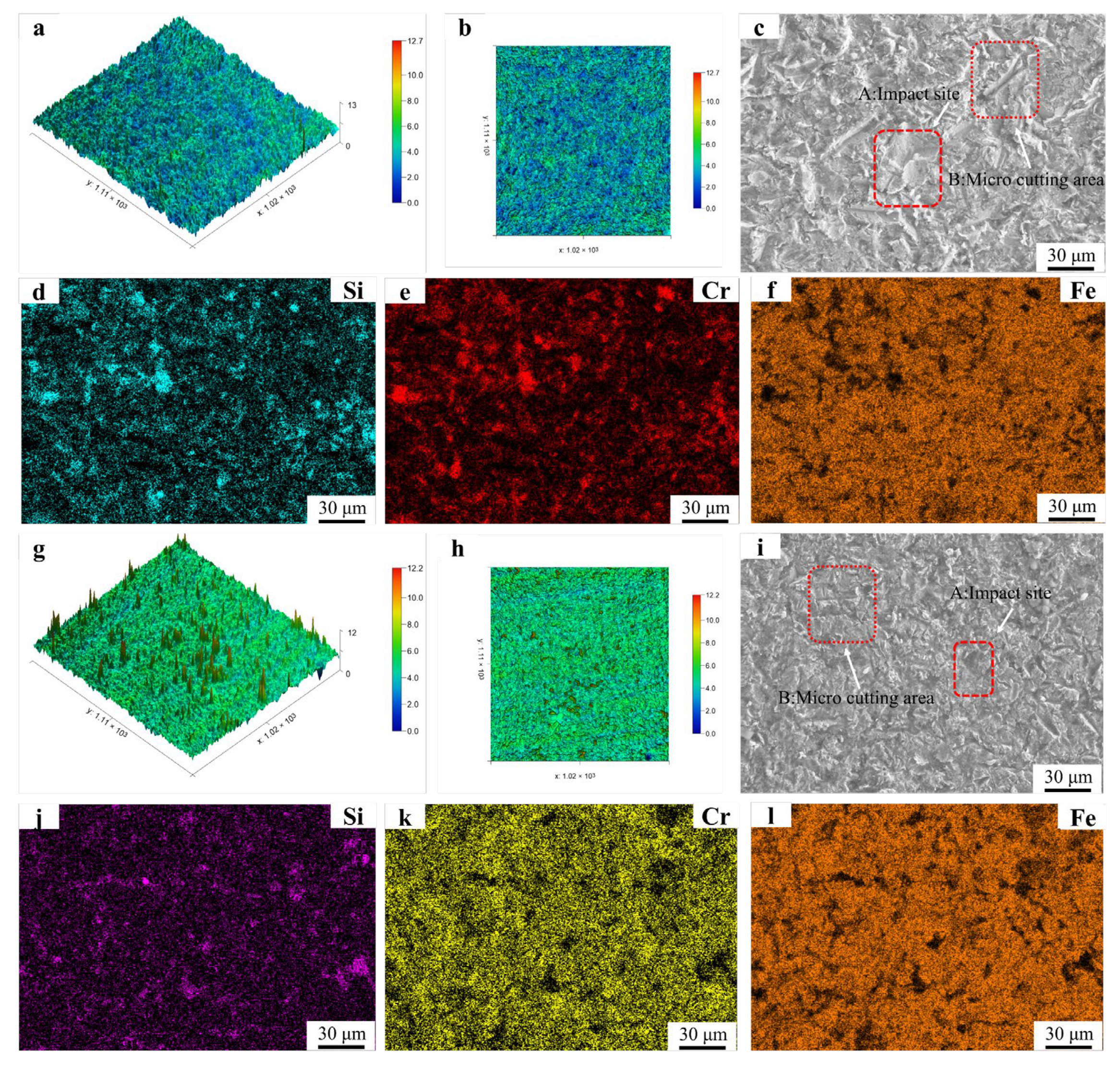
Erosion surface morphology and EDS mapping results of Fe55 and FC2T2: (a,b) 3D morphology of Fe55 eroded surface; (c) micrograph of Fe55 eroded surface; (d–f) EDS mapping results of Fe55 eroded surface; (g,h) 3D morphology of FC2T2 eroded surface; (i) micrograph of FC2T2 eroded surface; (j,k,l) EDS mapping results of FC2T2 eroded surface.
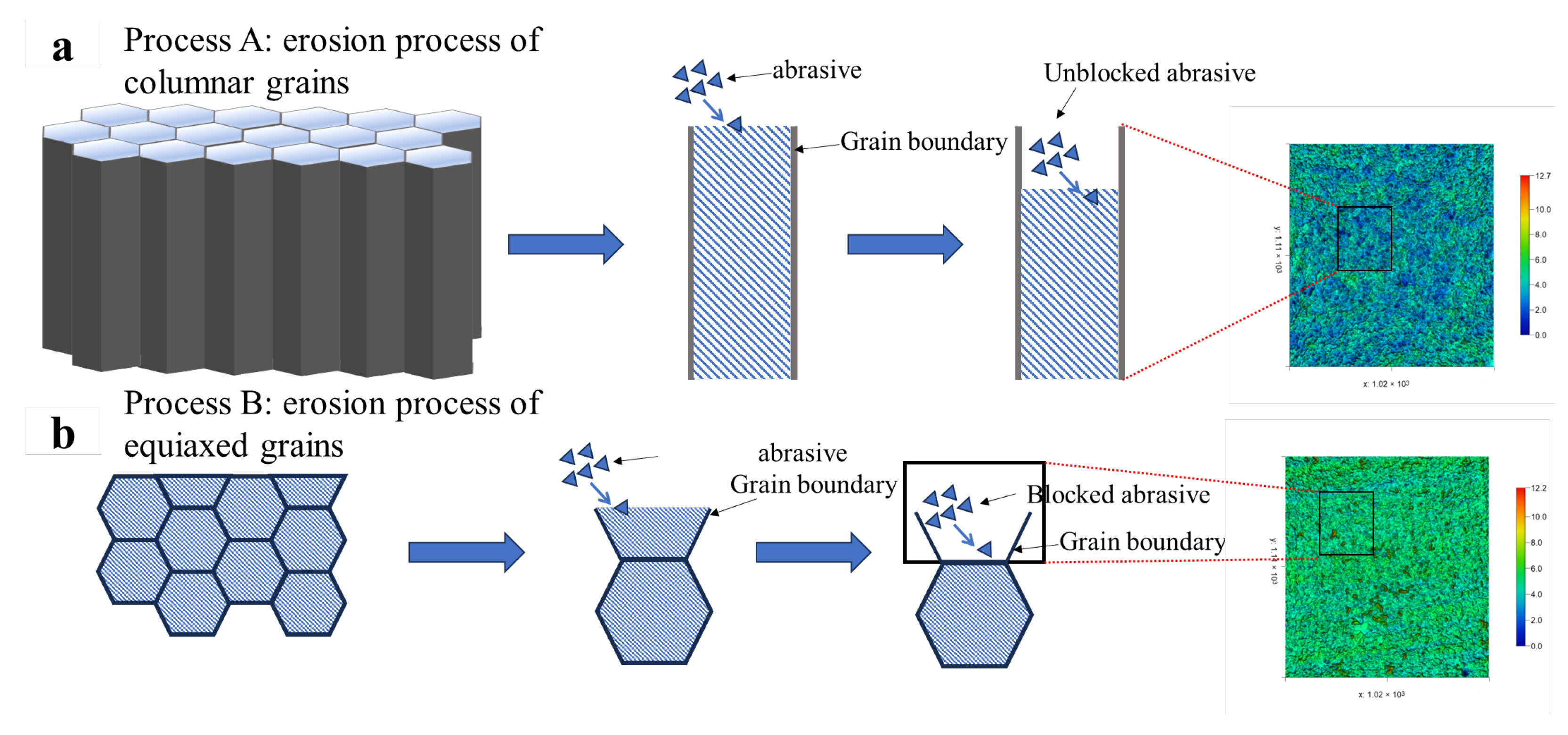
The columnar grain-dominated microstructure of Fe55 further exacerbates wear. Once the upper soft γ-Fe regions are removed, the absence of grain boundary Cr7C3 hard phases beneath allows SiC abrasives to progressively deepen their cutting into γ-Fe-rich zones, ultimately forming deep cutting pores and high wear loss, as illustrated in Process A of Figure 15.
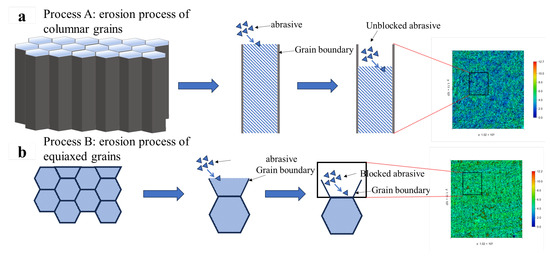
Figure 15.
Wear mechanism of different microstructures on the erosion process and roughness test results; (a) erosion process of columnar grains; (b) erosion process of equiaxed grains;.
Moreover, high-velocity SiC particles in the slurry induce plastic deformation in unprotected γ-Fe regions (lacking Cr7C3 constraints), leading to plastic fatigue wear [33]. The combined effects of these two wear mechanisms explain Fe55’s substantially higher mass loss compared to FC2T2.
Figure 14g displays the three-dimensional erosion morphology of FC2T2. The observed sharp protrusions originate from brittle SiC particles fracturing and embedding into the surface during impacts. These embedded fragments exhibit higher reflectivity during laser scanning, creating topographic peaks. The uniform color distribution across FC2T2’s surface indicates homogeneous wear resistance, benefiting from its equiaxed grains microstructure. When surface γ-Fe undergoes micro-cutting by SiC abrasives, Cr7C3 hard phases inhibit excessive material removal. Even if γ-Fe within grains is completely damaged, underlying Cr7C3-rich grain boundaries continue to protect against further wear, as shown in Process B of Figure 15.
Furthermore, Cr7C3 restricts excessive plastic deformation of γ-Fe under abrasive impacts, mitigating plastic fatigue wear. This uniform Cr7C3 distribution effectively suppresses wear progression. EDS mapping in Figure 14j–l reveals minimal Si signals but strong Cr signals on FC2T2’s worn surface. This confirms that after γ-Fe removal, exposed Cr7C3 from eutectic structures bears the erosive loads. The predominant Cr7C3 coverage prevents SiC abrasives from embedding into the surface, explaining the scarce Si detection. However, in Figure 14d–f, the Si signal exhibit distinct clustering, indicating that SiC particles have embedded into the surface of the Fe55 sample during erosion. This provides experimental evidence of the inferior erosion resistance of Fe55.
Figure 14i(A) displays traces left by SiC particles during erosion. As the particles engage the surface at a 45° attack angle with high velocity, their trajectories are truncated (approximately 20 μm) upon encountering Cr7C3 hard phases, which either deflect or block the abrasives. In contrast, regions subjected to micro-ploughing (e.g., Figure 14i(B)) exhibit flatter topography due to Cr7C3’s suppression of γ-Fe plastic deformation.
The wear surface morphology of Fe55 in Figure 14c demonstrates concurrent micro-cutting and micro-ploughing. The abrasive trajectories in Fe55 (e.g., Figure 14c(A)) extend up to 50 μm, significantly longer than those in FC2T2, corresponding to greater micro-cutting depths and consistent with Fe55’s higher friction mass loss. Plastic deformation zones induced by particle impacts are observed in Figure 14c(B), measuring ~40 μm in width. Comparable regions in FC2T2 (Figure 14i(B)) show substantially reduced widths (~20 μm). This improvement stems from two mechanisms: (1) Cr7C3 hard phases restrict excessive γ-Fe plastic deformation; (2) TiN addition enhances surface hardness.
EDS mapping reveals intensified Cr signals in FC2T2 (Figure 14k) compared to Fe55 (Figure 14e), indicating preferential removal of γ-Fe from grains during erosion that exposes Cr7C3-rich grain boundaries. Notably, Cr signal absence in multiple Fe55 regions under identical erosion durations confirms the lack of Cr7C3 support beneath columnar grains, directly explaining the deep crater formation in Figure 14b. These observations align with Verma et al.’s findings on erosion morphology in dual-phase steels [34,35].
Analysis of the erosion behavior reveals that the FC2T2 welding layer exhibits the lowest material removal rate and roughness during wet friction. When SiC abrasives induce micro-cutting and micro-ploughing on the wear surface, the homogeneous distribution of hard (Cr7C3) and soft (γ-Fe) phases synergistically mitigates material loss: Cr7C3 blocks abrasive progression during micro-cutting, while γ-Fe’s plastic deformation is suppressed during micro-ploughing, thereby avoiding mass loss and roughness elevation caused by abrasive wear and plastic fatigue.
Table 5 compares surface roughness results of Fe55 and FC2T2 after erosion testing. The Ra value of FC2T2 measures approximately 41% of Fe55, consistent with the three-dimensional morphologies in Figure 14a,g. This demonstrates that welding layers with uniform microstructures maintain stable surface roughness under abrasive or erosive wear conditions, as localized wear intensification is prevented. In addition, it is worth noting that Rt and Rv showed significant reductions. The reduction in the Rt value indicates a significant decrease in the value between the highest contour peaks and lowest valleys on the sample surface. This can be explained by the microstructural changes in the worn surface. In Fe55, the γ-Fe-enriched regions of columnar grains underwent preferential wear, while some Cr7C3-enriched regions with lower wear became contour peaks. This may become the starting point of leakage during the service of valve sealing surfaces. The Rt value of the FC2T2 sample decreased by 74% compared to Fe55, which proves that the difference between the highest point and lowest point in the FC2T2 sample is only 2.094 μm, proving the occurrence of Process B in Figure 15 during erosion. The particle diameter in slurry transportation is generally approximately 5–100 μm. The Rt value being lower than the slurry particle diameter means that the FC2T2 sample reduces the potential leakage risk when used as a valve sealing surface. The reduction in the Rv value indicates a significant decrease in the cutting depth of the sample surface. This test result also proves the separate occurrence of Processes A and B in Figure 15. The uniform distribution of soft phases and hard phases acts an important role in resisting erosion wear.

Table 5.
The surface roughness results of Fe55 and FC2T2.
4. Conclusions
- (1)
- The grain boundary drag effects mainly modify the microstructure of the welding layer by two competitive mechanisms: competition between CeO2-encapsulated TiN particles and grain boundaries as well as competition among grain boundaries for solute atoms to form eutectic structures. The variation in the fluidity of molten steel is primarily attributed to the absorption of Cr and Si elements from the steel by CeO2 within TiN particles, which reduces the atomic diffusion coefficient.
- (2)
- During the wet friction process, the Cr7C3 hard phase in the welding layer primarily functions to impede micro-cutting by abrasive particles and prevent plastic deformation of γ-Fe caused by micro-ploughing. Conversely, γ-Fe in the welding layer serves to provide toughness, thereby avoiding spalling of the Cr7C3 hard phase. In the absence of hard phase support, γ-Fe undergoes micro-cutting first, while spalled Cr7C3 hard phases induce three-body wear. Uniform distribution of the hard phase effectively prevents the formation of deep ploughing grooves.
- (3)
- During the erosion process, the superior wear resistance of equiaxed grains compared to columnar grains can be attributed to the following mechanism: When the soft γ-Fe region above columnar grains is worn away, the absence of Cr7C3 hard phases at grain boundaries beneath this region fails to prevent further erosion by abrasive SiC particles. This enables continuous deepening of micro-cutting in γ-Fe-enriched zones within the Fe55 welding layer. However, when the welding layer exhibits a surface microstructure dominated by equiaxed grains, even if γ-Fe within individual grains is completely destroyed by micro-cutting, the underlying grain boundaries containing high-hardness Cr7C3 hard phases remain effective in mitigating further wear of the welding layer surface by abrasive.
- (4)
- The Ra value of FC2T2 measures approximately 41% of Fe55, the uniform distribution of Cr7C3 can maintain a low wear surface roughness during wet frictional wear and erosion. The hardness and toughness of FC2T2 were well matched.
Author Contributions
Conceptualization, F.H., Z.M., X.L. and L.Y.; writing—original draft preparation, F.H., X.L. and Z.M.; writing—review and editing, T.Z., G.Y., Y.T., X.L., F.H., Z.M. and L.Y.; supervision, X.L., Z.M. and L.Y.; project administration, T.Z., X.H., G.Y. and Y.T.; funding acquisition, T.Z., Z.M. and L.Y. All authors have read and agreed to the published version of the manuscript.
Funding
The work was supported by Natural Science Foundation of Hunan Province, China (Grant No.2025JJ70178), the foundation of Guangxi Science and Technology Major Special Project (Guike A22067081), the foundation of Guangxi Innovation Driven Development Project (Grant No.AA17204021) and the Foundation of Hunan Engineering Research Center for Deep Processing Technology of Special Steel Pipes (KFB24027).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
Yongfu Tang, Gaofei Yan, Fuming He and Xianli Huang were employed by the company Shenglong Metallurgical Co. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Ding, Y.; Liu, R.; Yao, J.; Zhang, Q.; Wang, L. Stellite alloy mixture hardfacing via laser cladding for control valve seat sealing surfaces. Surf. Coat. Technol. 2017, 329, 97–108. [Google Scholar] [CrossRef]
- Li, S.; Zhang, B.; Yang, L.; Zhang, J.; Wang, Y.; Kang, W. Study on Wear Properties of the Graphite-Sealing Surfaces in a Triple Eccentric Butterfly Valve Based on EDEM-Fluent Coupling. Machines 2023, 11, 463. [Google Scholar] [CrossRef]
- Lin, Z.; Yu, H.; Yu, T.; Zhu, Z. Numerical study of solid-liquid two-phase flow and erosion in ball valves with different openings. Adv. Powder Technol. 2022, 33, 103542. [Google Scholar] [CrossRef]
- Moses, D.; Haider, G.; Henshaw, J. An investigation of the failure of a 1/4” ball valve. Eng. Fail. Anal. 2019, 100, 393–405. [Google Scholar] [CrossRef]
- Khan, R.; Petru, J.; Seikh, A.H. Erosion prediction due to micron-sized particles in the multiphase flow of T and Y pipes of oil and gas fields. Int. J. Press. Vessel. Pip. 2023, 206, 105041. [Google Scholar] [CrossRef]
- Faridi, M.A.; Nayak, S.K.; Prasad, D.K.V.D.; Kumar, A.; Laha, T. Effect of Microstructure and Phase Evolution on the Wear Behavior of Fe-Based Amorphous/Nanocrystalline Composite Coatings Synthesized by Plasma Spraying. J. Therm. Spray Technol. 2023, 32, 2054–2067. [Google Scholar] [CrossRef]
- Grain, A.K.; Rahman, O.S.A.; Kumari, S.; Maurya, S.S.; Kumar, K.V.; Islam, A.; Ghosh, S.K.; Keshri, A.K. Plasma-Sprayed Nanodiamond-Reinforced NiCrBSi Composite Coating for Improved Wear and Corrosion Resistance. J. Therm. Spray Technol. 2024, 33, 1055–1074. [Google Scholar] [CrossRef]
- Yarramilli, V.A.; Vadani, M.; Karan, B.; Bhakar, A.; Rai, S.; Maharjan, N.; Bhowmik, A. Investigation of surface structural modifications caused by the influence of the ablative layer in Inconel718 Ni-base superalloy through laser shock peening. Mater. Lett. 2024, 354, 135332. [Google Scholar] [CrossRef]
- Hu, Y.; Wang, Z.; Pang, M. Effect of WC content on laser cladding Ni-based coating on the surface of stainless steel. Mater. Today Commun. 2022, 31, 103357. [Google Scholar] [CrossRef]
- Eremin, E.N.; Losev, A.S.; Akimov, V.V. The Properties of Chromium Steel Overlaying Used as a Hardening Coating for Stop Valve Sealing Surface. Procedia Eng. 2016, 152, 582–588. [Google Scholar] [CrossRef]
- Huang, X.; Chen, Y.; Jiang, J.; Lian, G.; Chen, C. The Influences of Ultrasonic Vibrations on Laser Cladding Ni60/WC-TiO2 + La2O3 Composite Coating. Materials 2023, 16, 6356. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Li, H.; Xiao, Z. Microstructures and performances of Ni-SiC coatings manufactured by laser cladding deposition. Int. J. Electrochem. Sci. 2023, 18, 100030. [Google Scholar] [CrossRef]
- Yunxia, C.; Wenjun, G.; Rui, K. Coupling behavior between adhesive and abrasive wear mechanism of aero-hydraulic spool valves. Chin. J. Aeronaut. 2016, 29, 1119–1131. [Google Scholar] [CrossRef]
- Tan, M.; Lian, Y.; Liu, H.; Wu, X.; Ding, R. Visualizing test on the pass-through and collision characteristics of coarse particles in a double blade pump. Int. J. Nav. Archit. Ocean. Eng. 2018, 10, 1–8. [Google Scholar] [CrossRef]
- Meng, Q.; Wang, C.; Liu, T.; Song, Q.; Xue, B.; Cui, H. Microstructure and performance optimization of laser cladding nano-TiC modified nickel-based alloy coatings. Surf. Coat. Technol. 2024, 479, 130583. [Google Scholar] [CrossRef]
- Zhang, H.; Zou, Y.; Zou, Z.; Shi, C. Effects of CeO2 on microstructure and corrosion resistance of TiC-VC reinforced Fe-based laser cladding layers. J. Rare Earths 2014, 32, 1095–1100. [Google Scholar] [CrossRef]
- Cai, Y.; Luo, Z.; Chen, Y.; Ao, S. Influence of CeO2 on tribological behaviour of TiC/Fe-based composite coating. Surf. Eng. 2017, 33, 936–943. [Google Scholar] [CrossRef]
- Chen, L.; Zhao, Y.; Guan, C.; Yu, T. Effects of CeO2 addition on microstructure and properties of ceramics reinforced Fe-based coatings by laser cladding. Int. J. Adv. Manuf. Technol. 2021, 115, 2581–2593. [Google Scholar] [CrossRef]
- Gao, Z.; Ren, H.; Geng, H.; Yu, Y.; Gao, Z.; Zhang, C. Effect of CeO2 on Microstructure and Wear Property of Laser Cladding Ni-Based Coatings Fabricated on 35CrMoV Steel. J. Mater. Eng. Perform. 2022, 31, 9534–9543. [Google Scholar] [CrossRef]
- Qian, X.; Dong, Z.; Jiang, B.; Lei, B.; Yang, H.; He, C.; Liu, L.; Wang, C.; Yuan, M.; Yang, H.; et al. Influence of alloying element segregation at grain boundary on the microstructure and mechanical properties of Mg-Zn alloy. Mater. Des. 2022, 224, 111322. [Google Scholar] [CrossRef]
- Robson, J.D.; Haigh, S.J.; Davis, B.; Griffiths, D. Grain Boundary Segregation of Rare-Earth Elements in Magnesium Alloys. Metall. Mater. Trans. A 2016, 47, 522–530. [Google Scholar] [CrossRef]
- Liu, H.; Yuan, X.; Rao, L.; Liu, S.; Shi, Z.; Zhou, Y.; Yang, Q. Refinement mechanism of NbC by CeO2 in hypereutectic Fe-Cr-C hardfacing coating. J. Alloys Compd. 2019, 770, 1016–1028. [Google Scholar] [CrossRef]
- Li, Q.; Lei, Y.; Fu, H. Laser cladding in-situ NbC particle reinforced Fe-based composite coatings with rare earth oxide addition. Surf. Coat. Technol. 2014, 239, 102–107. [Google Scholar] [CrossRef]
- Chen, L.; Yu, T.; Xu, P.; Zhang, B. In-situ NbC reinforced Fe-based coating by laser cladding: Simulation and experiment. Surf. Coat. Technol. 2021, 412, 127027. [Google Scholar] [CrossRef]
- Chen, T.; Wu, F.; Wang, H.; Liu, D. Laser Cladding In-Situ Ti(C,N) Particles Reinforced Ni-Based Composite Coatings Modified with CeO2 Nanoparticles. Metals 2018, 8, 601. [Google Scholar] [CrossRef]
- Yu, L.; He, F.; Liu, X.; Jiang, Y.; Sui, M.; Cao, X.; Meng, Z. The Preparation, Microstructure, and Wet Wear Properties of an Fe55-Based Welding Layer with the Co-Addition of 0.01 wt% CeO2 and 1.5 wt% SiC Particles Using the Plasma Beam Spraying Method. Materials 2023, 16, 7439. [Google Scholar] [CrossRef]
- ISO 21920-2:2021; Geometrical Product Specifications (GPS)—Surface Texture: Profile—Part 2: Terms, Definitions and Surface Texture Parameters. ISO: Geneva, Switzerland, 2021.
- Gou, J.; Wang, Y.; Zhang, Y.; Wang, C.; Wang, G. Dry sliding wear behavior of Fe-Cr-C-B hardfacing alloy modified with nano-CeO2 and its mechanisms of modification. Wear 2021, 484-485, 203756. [Google Scholar] [CrossRef]
- Chu, Z.; Wei, F.; Zheng, X.; Zhang, C.; Yang, Y. Microstructure and properties of TiN/Fe-based amorphous composite coatings fabricated by reactive plasma spraying. J. Alloys Compd. 2019, 785, 206–213. [Google Scholar] [CrossRef]
- He, J.; He, Z.; Qin, Y.; Zhao, H.; Bi, Y. A Review of TiCN Coating Prepared by Reaction Plasma Spraying. J. Therm. Spray Technol. 2022, 31, 2280–2299. [Google Scholar] [CrossRef]
- Ou, W.; Xiong, J.; Peng, Y.; Xu, J.; Chen, Z.; Zhang, H.; Li, X.; Yang, J.; Zhang, L.; Mao, G.; et al. Transformation of carbides and mechanisms for cracking in Co-based alloy surfacing layer during thermal fatigue. Mater. Charact. 2023, 201, 112971. [Google Scholar] [CrossRef]
- Bitter, J.G.A. A study of erosion phenomena: Part II. Wear 1963, 6, 169–190. [Google Scholar] [CrossRef]
- Su, C.; Feng, G.; Zhi, J.; Zhao, B.; Wu, W. The Effect of Rare Earth Cerium on Microstructure and Properties of Low Alloy Wear-Resistant Steel. Metals 2022, 12, 1358. [Google Scholar] [CrossRef]
- Verma, P.; Tyagi, R.; Mohan, S. Erosive Wear of Dual Phase Steels Containing Different Amount of Martensite. J. Mater. Eng. Perform. 2023, 32, 314–325. [Google Scholar] [CrossRef]
- Verma, P.; Tyagi, R.; Mohan, S. Effect of microstructure, impact velocity and angle on erosive wear of medium carbon, dual phase and fully martensitic steels. Wear 2023, 518-519, 204645. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).