Cold Spray Preparation of High-Performance Anti-Cavitation Copper Coatings on Steel Substrates
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Coating Preparation
2.3. Microstructure and Phase Analyses
2.4. Mechanical Property Tests
2.5. Cavitation Erosion Test
3. Results and Discussion
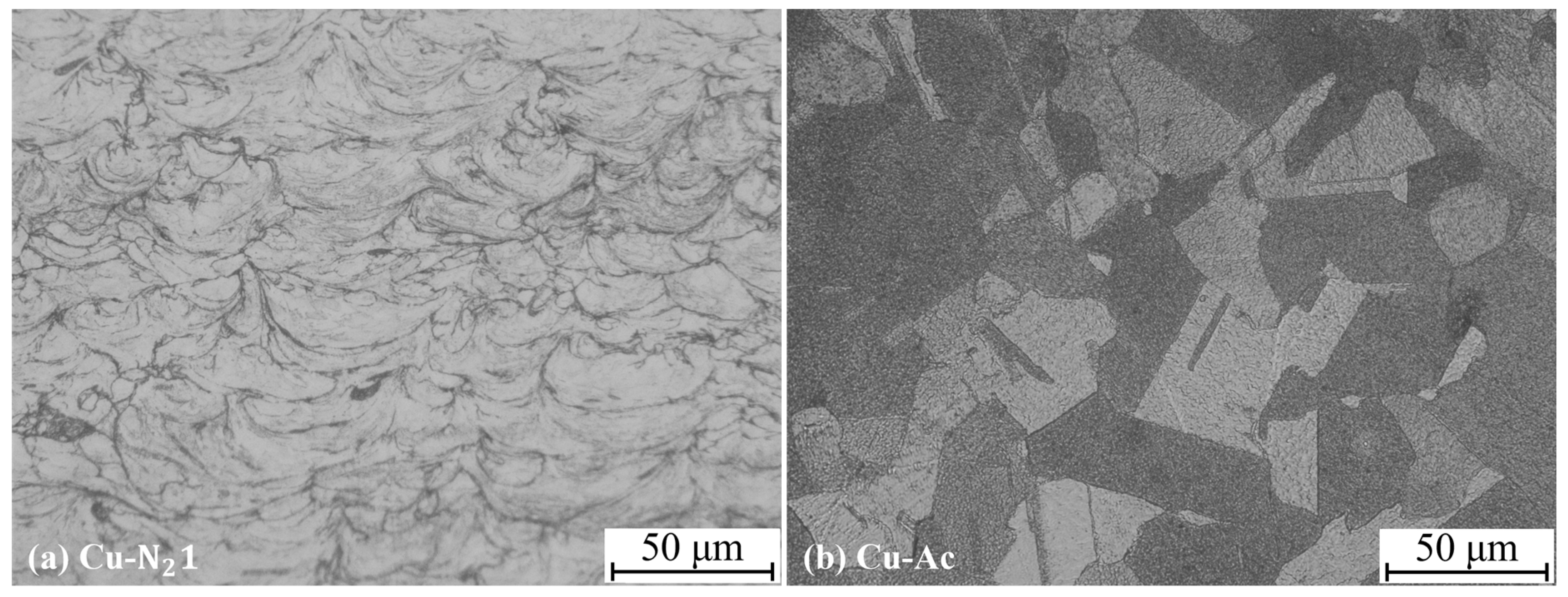
3.1. Microstructure
3.2. Mechanical Properties
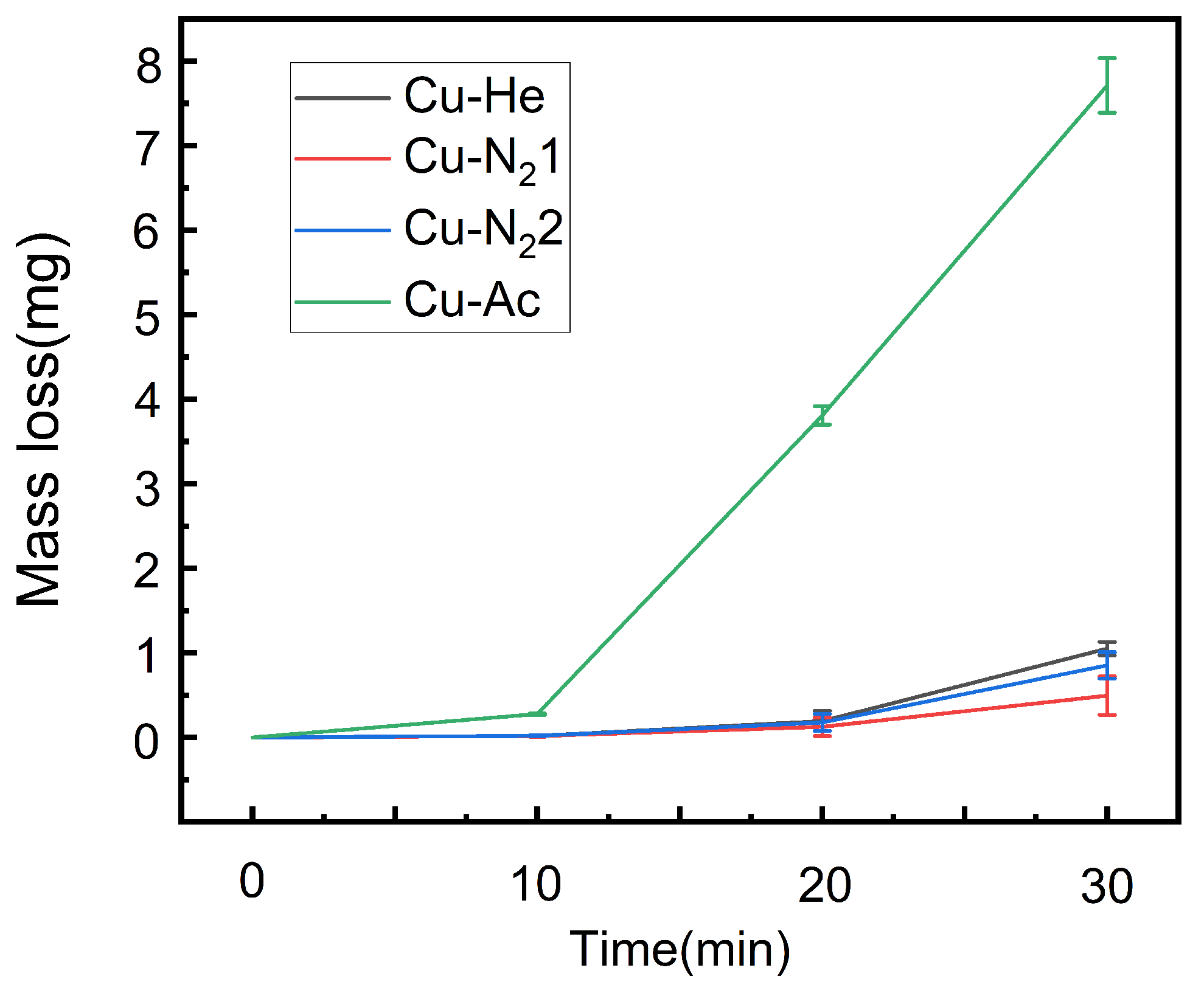
3.3. Influence of Cavitation Time on Coating Weight Loss
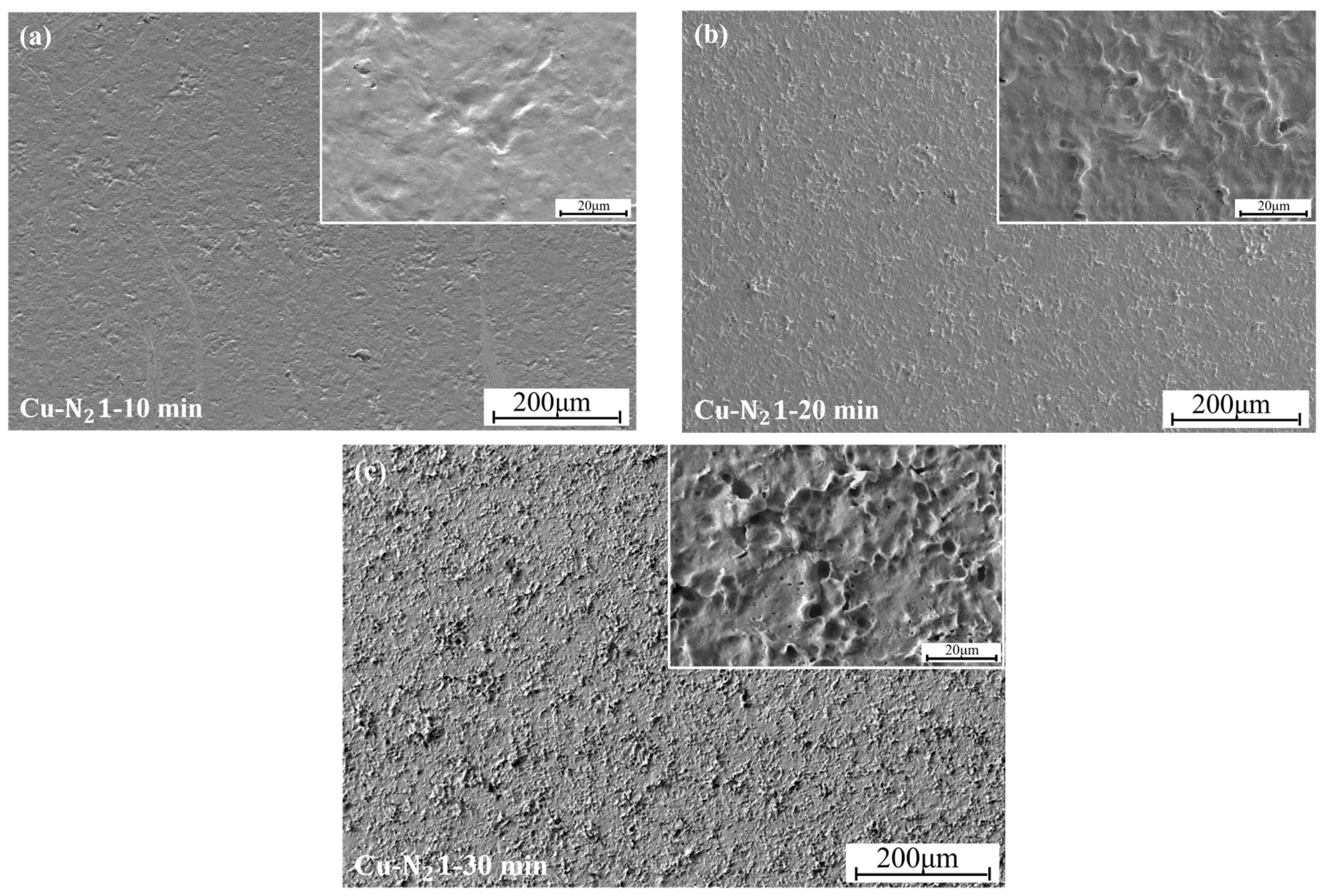
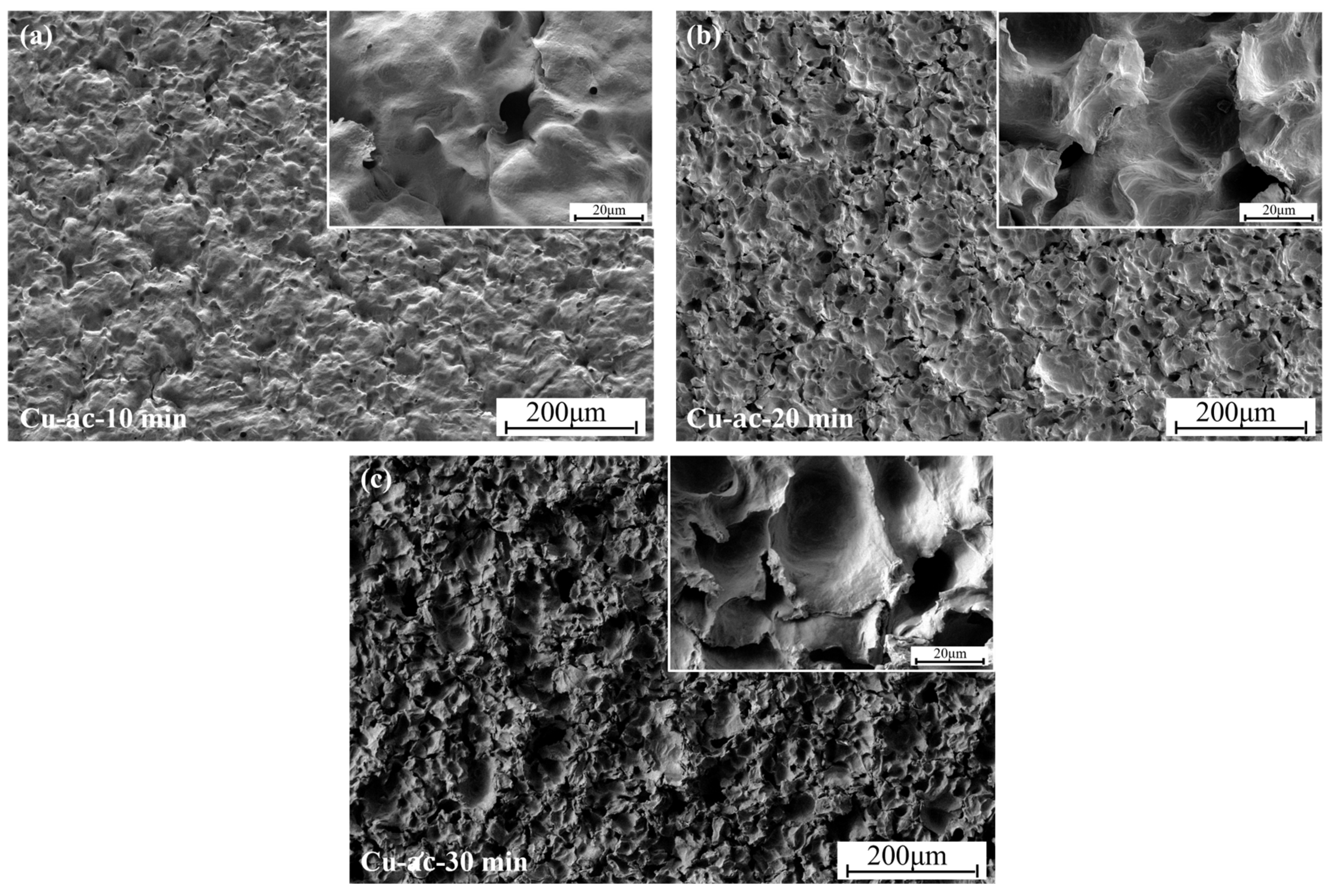
3.4. The Effect of Cavitation on the Microstructure of Coatings
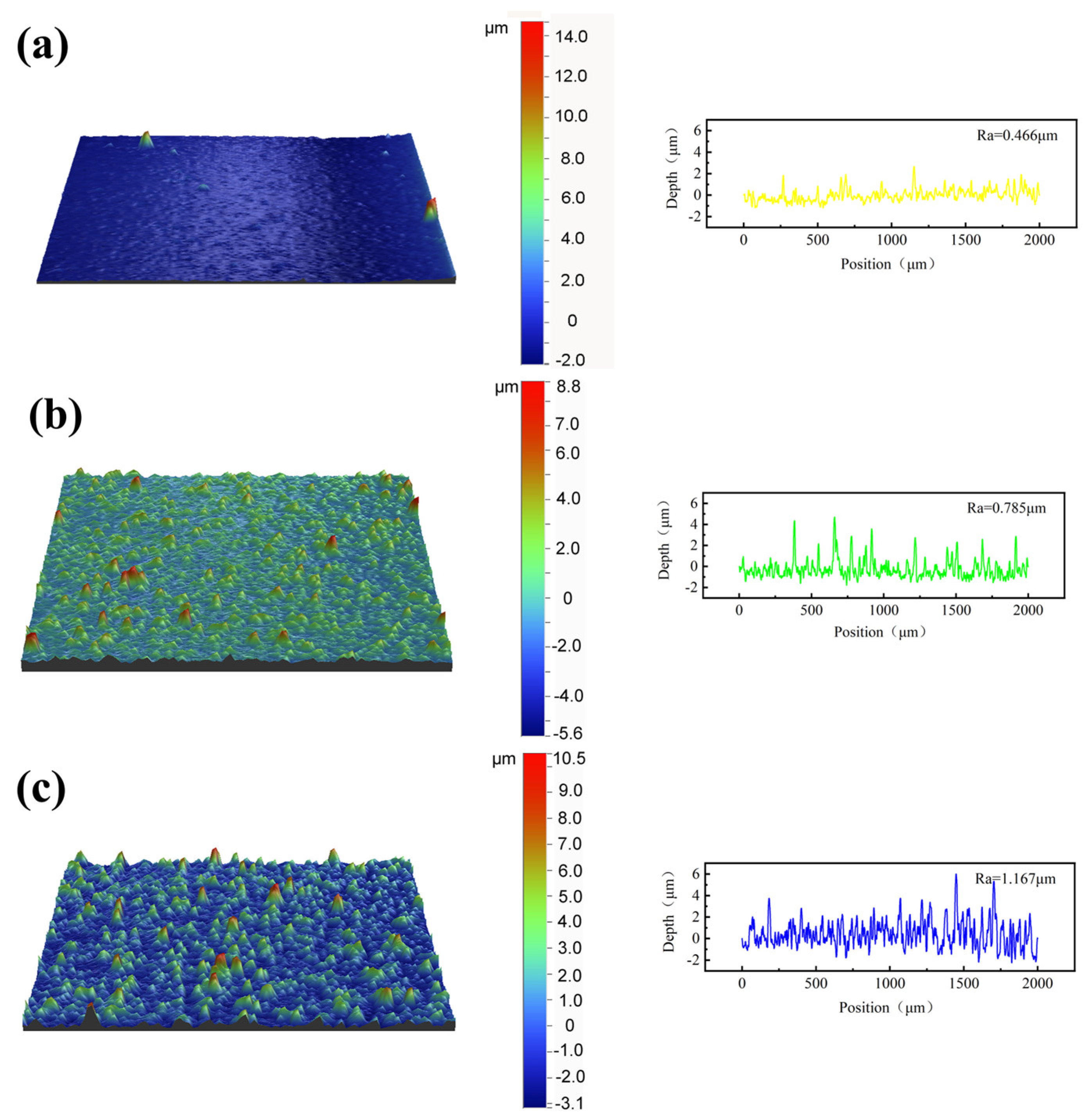
3.5. Three-Dimensional Profile Changes After Coating Cavitation
3.6. Cross-Sectional Morphology of Coating After Cavitation
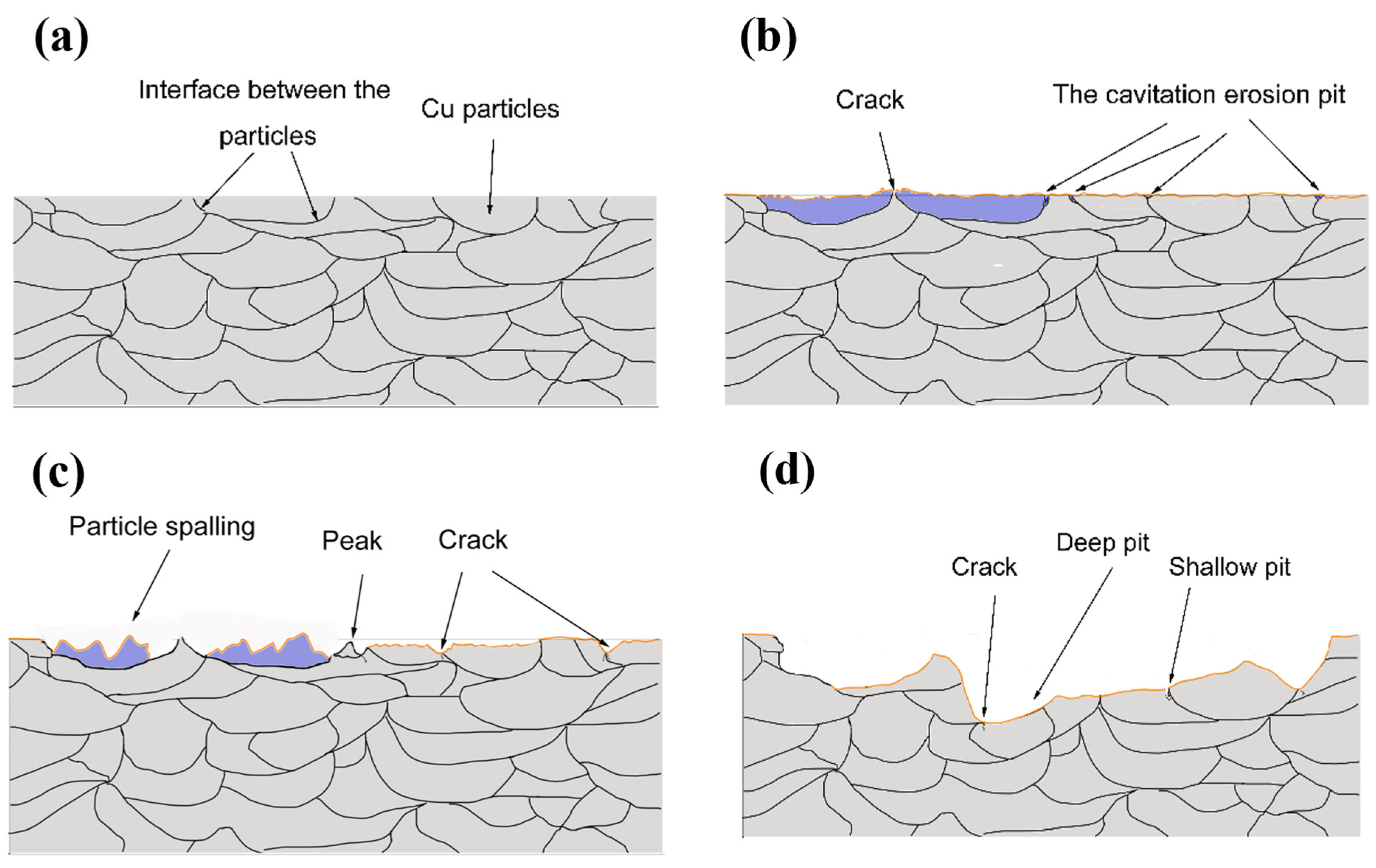
3.7. Analysis of Coating Cavitation Damage Mechanism
4. Conclusions
- (1)
- The Cu coating prepared with nitrogen has the best anti-cavitation performance, and the effect is better and more obvious with the prolongation of time, followed by the Cu coating prepared with helium. The cavitation resistance of as-cast Cu is the weakest, and the cavitation resistance of the Cu coating prepared by cold spray after 30 min is more than 10 times higher than that of the as-cast copper.
- (2)
- Analysis of the coating micromorphology of each group of cavitation samples shows that the degree of damage to the coating surface by cavitation reflects the degree of damage to the material, and the degree of cavitation damage in each time period corresponds to the weight loss curve of the material in the same time period. Therefore, the cavitation resistance of the material can be judged by the damage degree of the microstructure after the material cavitation.
- (3)
- By comparing the depth of cavitation pits on the cross section of the samples after cavitation, it can be concluded that the cold spray Cu coating has better anti-cavitation performance.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
References
- Dular, M.; Bachert, B.; Stoffel, B.; Širok, B. Relationship between cavitation structures and cavitation damage. Wear 2004, 257, 1176–1184. [Google Scholar]
- Bajracharya, T.R.; Acharya, B.; Joshi, C.B.; Saini, R.P.; Dahlhaug, O.G. Sand erosion of Pelton turbine nozzles and buckets: A case study of Chilime Hydropower Plant. Wear 2008, 264, 177–184. [Google Scholar] [CrossRef]
- Peng, Y.-c.; Chen, X.-y.; Cao, Y.; Hou, G.-x. Numerical Study of Cavitation on the Surface of the Guide Vane in Three Gorges Hydropower Unit. J. Hydrodyn. 2010, 22, 703–708. [Google Scholar]
- Chen, F.; Du, J.; Zhou, S. Cavitation erosion behaviour of incoloy alloy 865 in NaCl solution using ultrasonic vibration. J. Alloys Compd. 2020, 831, 154783. [Google Scholar]
- Howard, R.L.; Ball, A. The solid particle and cavitation erosion of titanium aluminide intermetallic alloys. Wear 1995, 186, 123–128. [Google Scholar]
- Sun, X.; Liu, J.; Ji, L.; Wang, G.; Zhao, S.; Yoon, J.Y.; Chen, S. A review on hydrodynamic cavitation disinfection: The current state of knowledge. Sci. Total. Environ. 2020, 737, 139606. [Google Scholar]
- Nair, R.B.; Arora, H.S.; Grewal, H.S. Microwave Synthesized Complex Concentrated Alloy Coatings: Plausible Solution to Cavitation Induced Erosion -Corrosion. Ultrason. Sonochemistry 2018, 50, 114–125. [Google Scholar]
- Lakkannavar, V.; Yogesha, K.B.; Prasad, C.D.; Phanden, R.K.; Prasad, S.C. Thermal spray coatings on high-temperature oxidation and corrosion applications—A comprehensive review. Results Surf. Interfaces 2024, 16, 100250. [Google Scholar]
- Padmavathi, G.; Sarada, B.N.; Shanmuganathan, S.P.; Padmini, B.V.; Mohan, N. Effects of high velocity oxy fuel thermal spray coating on mechanical and tribological properties of materials—A review. Mater. Today Proc. 2020, 27, 2152–2157. [Google Scholar]
- Koivuluoto, H.; Larjo, J.; Marini, D.; Pulci, G.; Marra, F. Cold-Sprayed Al6061 Coatings: Online Spray Monitoring and Influence of Process Parameters on Coating Properties. Coatings 2020, 10, 348. [Google Scholar] [CrossRef]
- Sun, W.; Tan, A.W.-Y.; Wu, K.; Yin, S.; Yang, X.; Marinescu, I.; Liu, E. Post-Process Treatments on Supersonic Cold Sprayed Coatings: A Review. Coatings 2020, 10, 123. [Google Scholar] [CrossRef]
- Rokni, M.R.; Nutt, S.R.; Widener, C.A.; Champagne, V.K.; Hrabe, R.H. Review of Relationship Between Particle Deformation, Coating Microstructure, and Properties in High-Pressure Cold Spray. J. Therm. Spray Technol. 2017, 26, 1308–1355. [Google Scholar] [CrossRef]
- Sun, C.; Gui, W.; Lu, J.; Xie, L.; Wu, Y. Comparison of the Deposition Process for Metallic and MG Coatings Using Cold Spraying. J. Therm. Spray Technol. 2022, 31, 1155–1172. [Google Scholar]
- Chen, J.; An, Y.; Liu, G.; Chen, G.; Zhao, X.; Jia, L. Tribological Performance and Thermal Stability of a Novel Cold Sprayed Nanostructured Ni-based Lubrication Coating. J. Therm. Spray Technol. 2022, 31, 1702–1711. [Google Scholar]
- Silvello, A.; Cavaliere, P.; Yin, S.; Lupoi, R.; Garcia Cano, I.; Dosta, S. Microstructural, Mechanical and Wear Behavior of HVOF and Cold-Sprayed High-Entropy Alloys (HEAs) Coatings. J. Therm. Spray Technol. 2022, 31, 1184–1206. [Google Scholar]
- Nikbakht, R.; Jodoin, B. Thick Cu-hBN Coatings Using Pulsed Gas Dynamic Spray Process: Coating Formation Analysis and Characterization. J. Therm. Spray Technol. 2022, 31, 609–622. [Google Scholar]
- Mahaffey, J.; Vackel, A.; Whetten, S.; Melia, M.; Kustas, A.B. Structure Evolution and Corrosion Performance of CoCrFeMnNi High Entropy Alloy Coatings Produced Via Plasma Spray and Cold Spray. J. Therm. Spray Technol. 2022, 31, 1143–1154. [Google Scholar]
- Assadi, H.; Schmidt, T.; Richter, H.; Kliemann, J.O.; Binder, K.; Gärtner, F.; Klassen, T.; Kreye, H. On Parameter Selection in Cold Spraying. J. Therm. Spray Technol. 2011, 20, 1161–1176. [Google Scholar]
- Zheng, L.; Haché, M.J.R.; Poirier, D.; Legoux, J.G.; Giallonardo, J.D.; Zou, Y.; Howe, J.Y.; Erb, U. Submicrometer scale mapping of microstructure and mechanical properties of cold sprayed copper. Mater. Sci. Eng. A 2025, 920, 147556. [Google Scholar]
- Hucińska, J.; Głowack, M. Cavitation Erosion of Copper and Copper-Based Alloys. Metall. Mater. Trans. A 2001, 32, 1325–1333. [Google Scholar]
- Shi, Z.-P.; Wang, Z.-B.; Wang, J.-Q.; Qiao, Y.-X.; Chen, H.-N.; Xiong, T.-Y.; Zheng, Y.-G. Effect of Ni Interlayer on Cavitation Erosion Resistance of NiTi Cladding by Tungsten Inert Gas (TIG) Surfacing Process. Acta Metall. Sin. Engl. Lett. 2019, 33, 415–424. [Google Scholar] [CrossRef]
- Sun, W.; Chu, X.; Lan, H.; Huang, R.; Huang, J.; Xie, Y.; Huang, J.; Huang, G. Current Implementation Status of Cold Spray Technology: A Short Review. J. Therm. Spray Technol. 2022, 31, 848–865. [Google Scholar] [PubMed]
- Zhang, X.F.; Fang, L. The effect of stacking fault energy on the cavitation erosion resistance of alpha-phase aluminum bronzes. Wear 2002, 253, 1105–1110. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, Y.; Hong, S.; Cheng, J.; Zhu, S. Cavitation erosion behavior of HVAF-sprayed Cu-based glassy composite coatings in NaCl solution. Intermetallics 2024, 168, 108266. [Google Scholar] [CrossRef]
- Chang, Y.; Liu, Z.; Gan, R.; Xiao, H.; Shen, Y. Cavitation erosion characterization of Cu-Ni alloy laser cladding layer under the action of high-pressure water jet. J. Alloys Compd. 2025, 1020, 179325. [Google Scholar] [CrossRef]
- Prashar, G.; Vasudev, H. A comprehensive review on the analysis of adhesion strength of cold spray deposits. Results Surf. Interfaces 2024, 16, 100263. [Google Scholar]
- Khmelev, V.N.; Barsukov, R.V.; Golykh, R.N.; Abramenko, D.S.; Genne, D.V.; Tertishnikov, P.P. Method and means of cavitation erosion tests under abnormal conditions. J. Phys. Conf. Ser. 2020, 1679, 042041. [Google Scholar] [CrossRef]
- Steller, J. International Cavitation Erosion Test and quantitative assessment of material resistance to cavitation. Wear 1999, 233, 51–64. [Google Scholar]
- Hong, S.; Wu, Y.; Zhang, J.; Zheng, Y.; Qin, Y.; Lin, J. Ultrasonic cavitation erosion of high-velocity oxygen-fuel (HVOF) sprayed near-nanostructured WC–10Co–4Cr coating in NaCl solution. Ultrason. Sonochem. 2015, 26, 87–92. [Google Scholar] [CrossRef]
- Kazasidis, M.; Yin, S.; Cassidy, J.; Volkov-Husović, T.; Vlahović, M.; Martinović, S.; Kyriakopoulou, E.; Lupoi, R. Microstructure and cavitation erosion performance of nickel-Inconel 718 composite coatings produced with cold spray. Surf. Coat. Technol. 2020, 382, 125195. [Google Scholar] [CrossRef]
- Wei, Z.; Wu, Y.; Hong, S.; Cheng, J.; Qiao, L.; Cheng, J.; Zhu, S. Ultrasonic cavitation erosion behaviors of high-velocity oxygen-fuel (HVOF) sprayed AlCoCrFeNi high-entropy alloy coating in different solutions. Surf. Coat. Technol. 2021, 409, 126899. [Google Scholar] [CrossRef]
- Kanno, A.; Takagi, K.; Arai, M. Influence of chemical composition, grain size, and spray condition on cavitation erosion resistance of high-velocity oxygen fuel thermal-sprayed WC cermet coatings. Surf. Coat. Technol. 2020, 394, 125881. [Google Scholar] [CrossRef]
- Cheng, J.; Wu, Y.; Hong, S.; Cheng, J.; Qiao, L.; Wang, Y.; Zhu, S. Cavitation-erosion behavior and mechanism of high-velocity oxygen-fuel sprayed CuAlNiTiSi medium-entropy alloy coating. Surf. Coat. Technol. 2022, 432, 128096. [Google Scholar] [CrossRef]
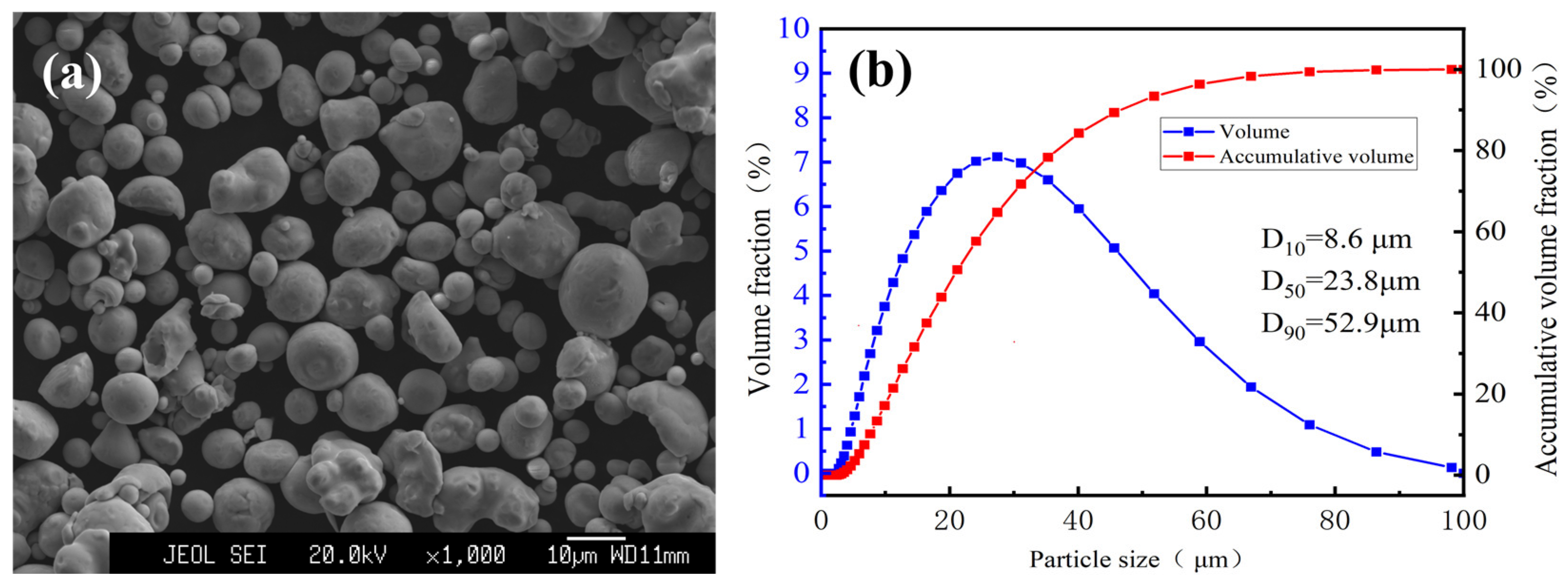
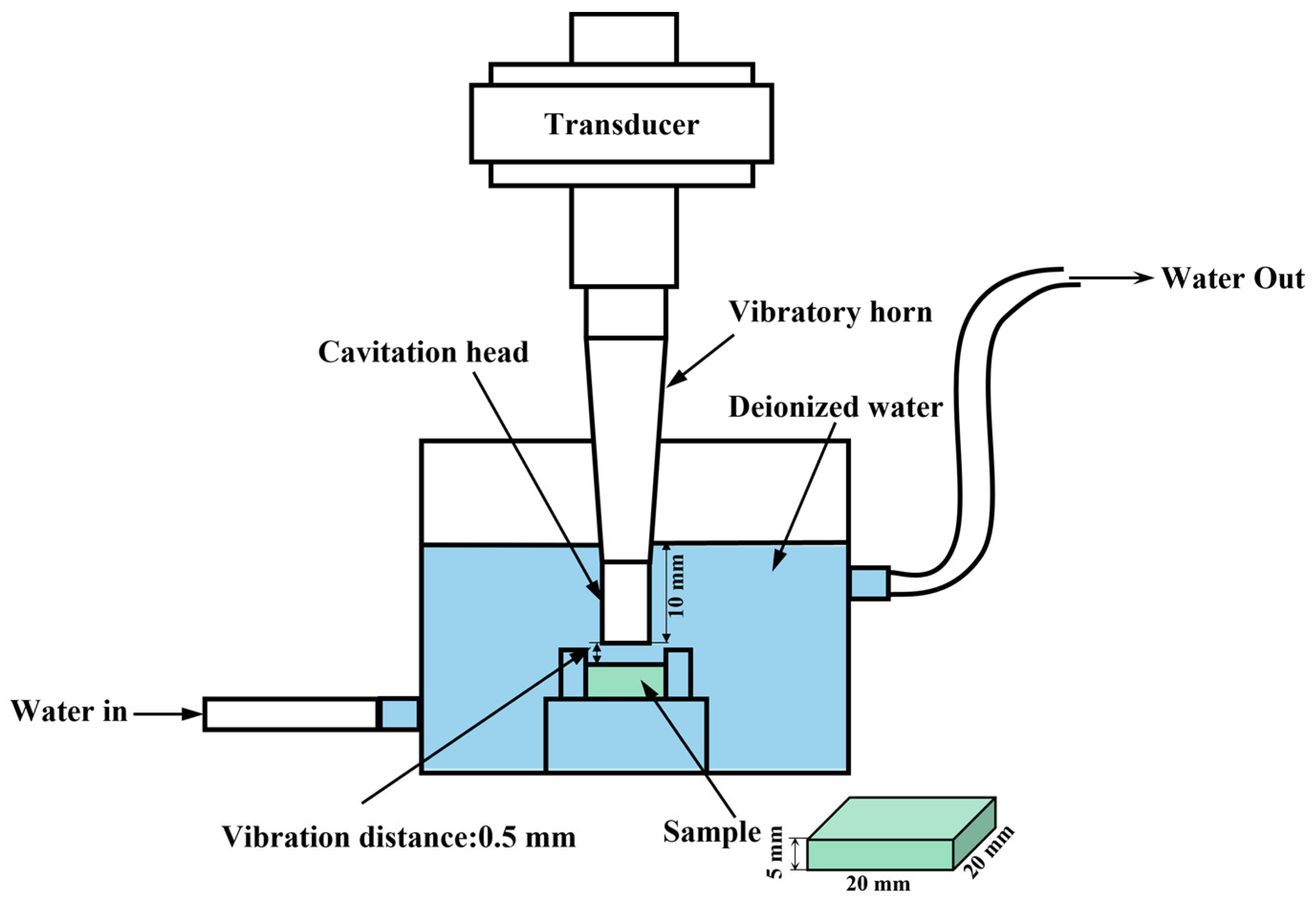

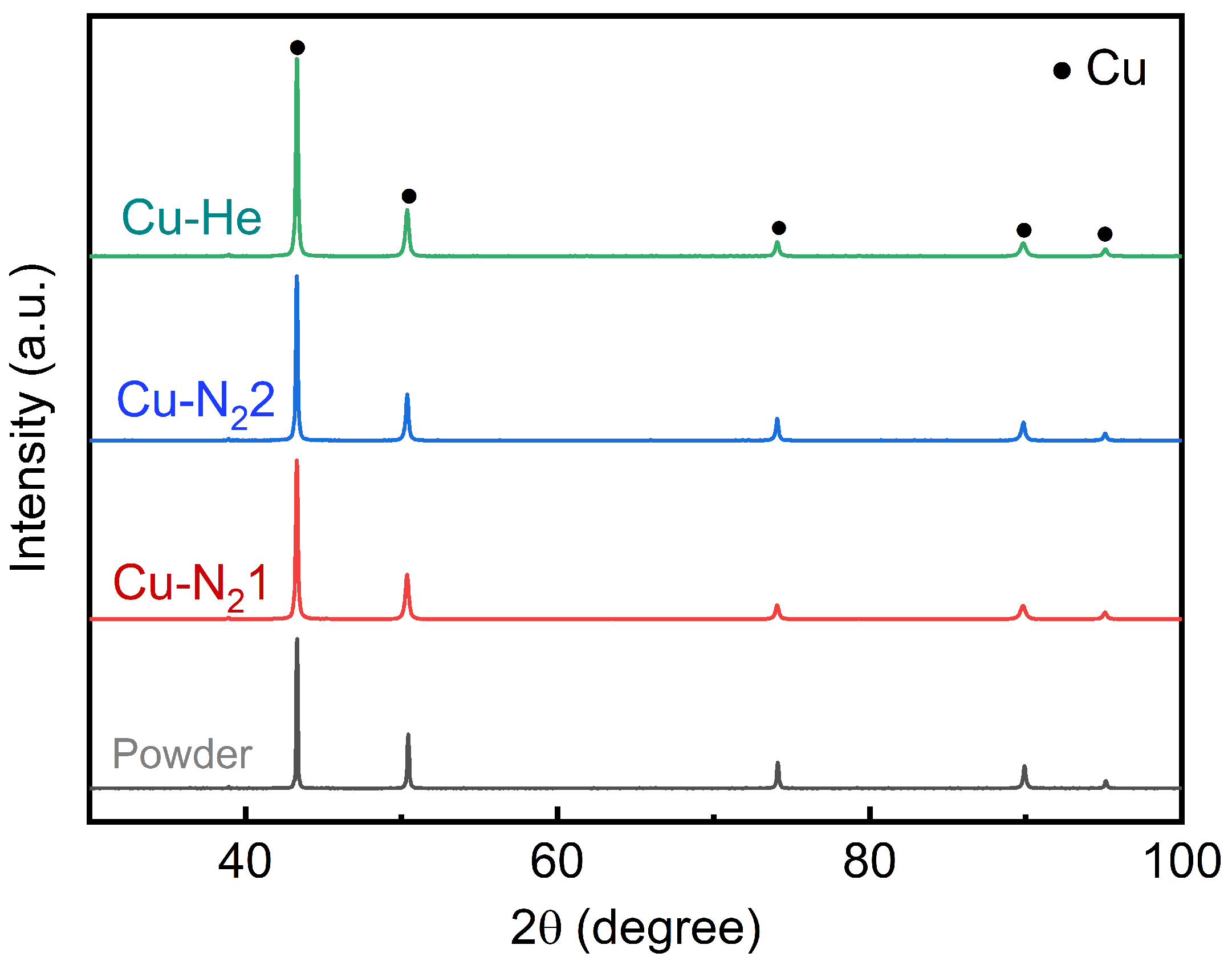
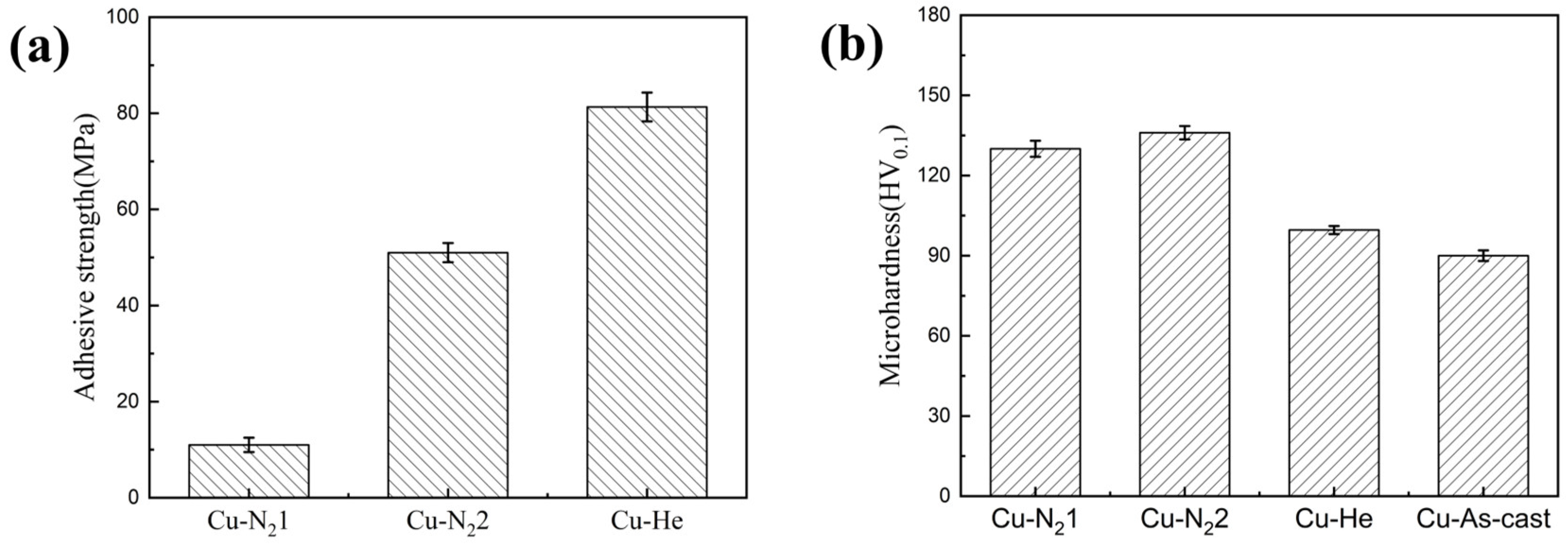


| Gas Type | Denomination | Nozzle Type | Gas Temperature /°C | Gas Pressure/MPa | Spray Distance/mm | Powder Feed Rate (g/min) |
|---|---|---|---|---|---|---|
| N2 | N21 | 1 | 800 | 5 | 30 | 100 |
| N2 | N22 | 2 | 800 | 5 | 30 | 100 |
| He | He | 1 | 600 | 3 | 30 | 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pei, Y.; Sun, Z.; Liu, W.; Deng, C.; Ma, J.; Lan, H.; Chu, X.; Xie, Y. Cold Spray Preparation of High-Performance Anti-Cavitation Copper Coatings on Steel Substrates. Coatings 2025, 15, 381. https://doi.org/10.3390/coatings15040381
Pei Y, Sun Z, Liu W, Deng C, Ma J, Lan H, Chu X, Xie Y. Cold Spray Preparation of High-Performance Anti-Cavitation Copper Coatings on Steel Substrates. Coatings. 2025; 15(4):381. https://doi.org/10.3390/coatings15040381
Chicago/Turabian StylePei, Yunzhen, Zhongwu Sun, Weijie Liu, Chunming Deng, Jiayan Ma, Haiming Lan, Xin Chu, and Yingchun Xie. 2025. "Cold Spray Preparation of High-Performance Anti-Cavitation Copper Coatings on Steel Substrates" Coatings 15, no. 4: 381. https://doi.org/10.3390/coatings15040381
APA StylePei, Y., Sun, Z., Liu, W., Deng, C., Ma, J., Lan, H., Chu, X., & Xie, Y. (2025). Cold Spray Preparation of High-Performance Anti-Cavitation Copper Coatings on Steel Substrates. Coatings, 15(4), 381. https://doi.org/10.3390/coatings15040381







