Failure and Damage Analysis of a Polymeric Finish Topcoat on Vinyl Coated Fabrics for Marine Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
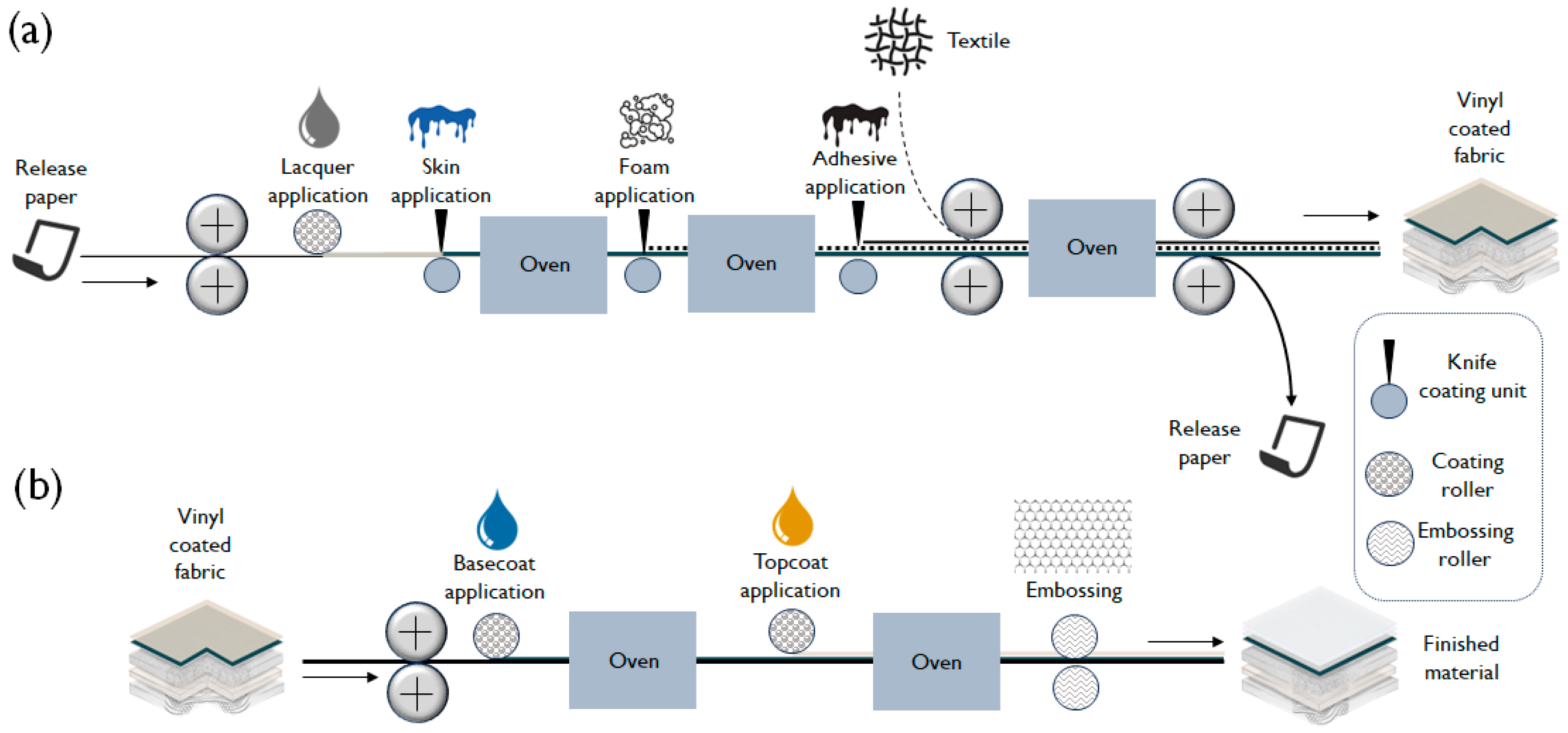
2.2. Vinyl Coated Fabric Construction
2.3. Experimental Design
2.4. Experimental Techniques
2.5. Plasticizer Extraction
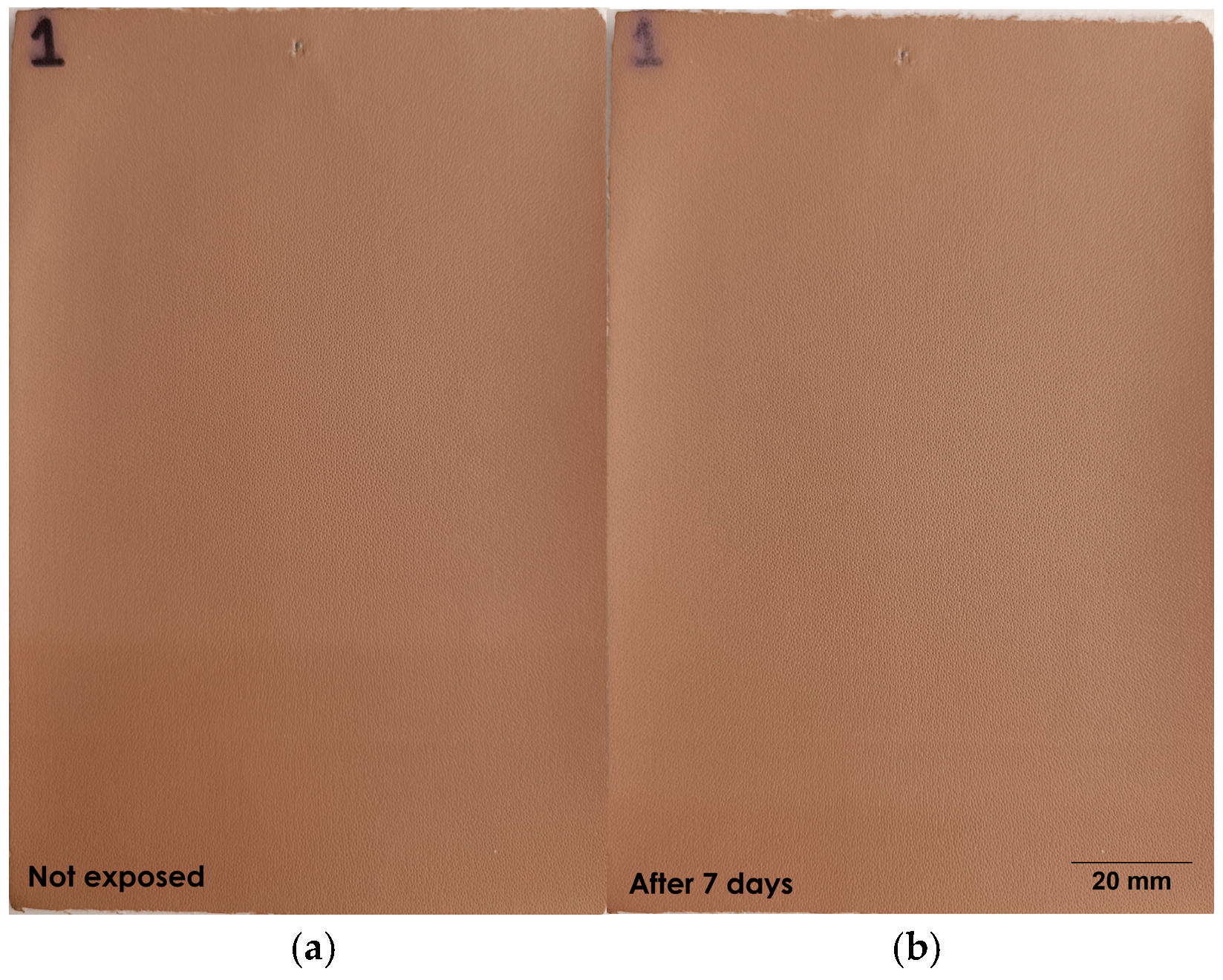
2.6. Accelerated Aging Tests
2.6.1. Salt Fog Chamber Exposure
2.6.2. UV Light Exposure
- •
- ASTM D4329: Performed in a QUV/Spray Accelerated Weathering Tester (Q-Lab Corporation, Westlake, OH, USA) [17].
- •
- ISO 105B 04: Performed in a Ci4400 Xenon Weather-Ometer aging chamber (Atlas, Mount Prospect, IL, USA) [18].
- •
- NTC 1479: Performed in a 220+ Xenotest aging chamber (Atlas, Mount Prospect, IL, USA) [19].
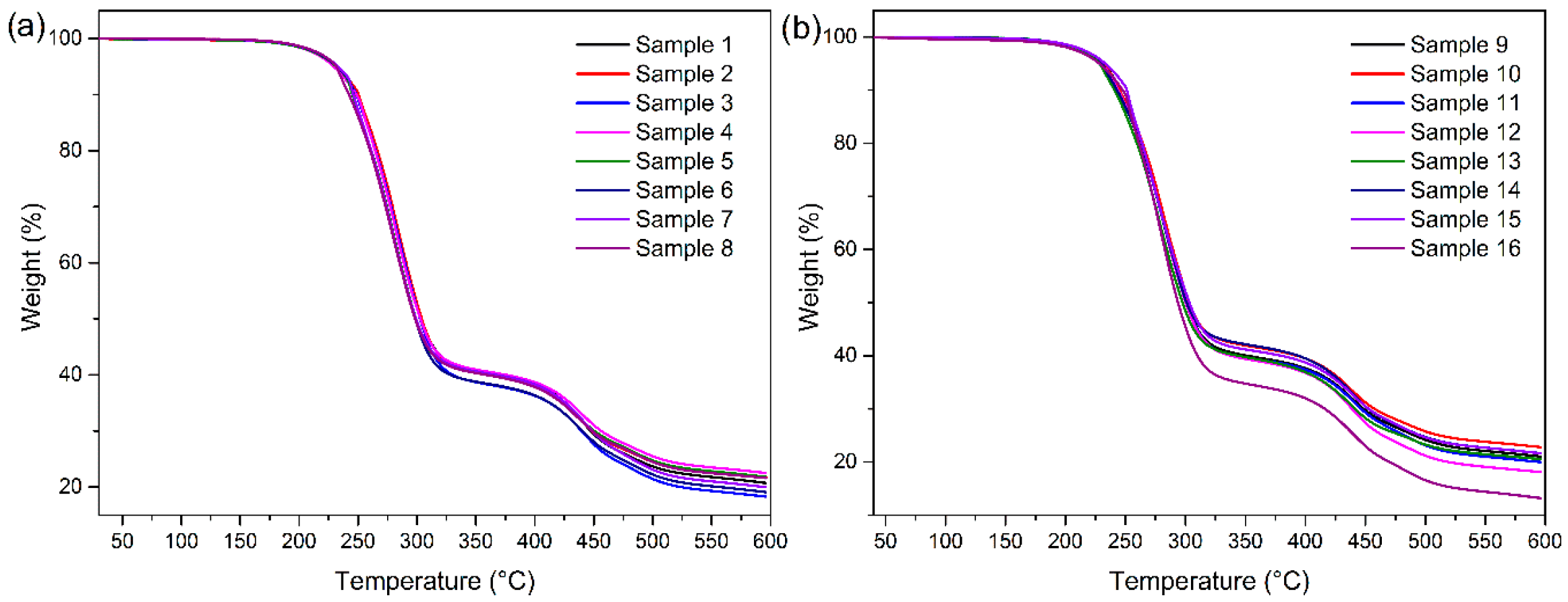
2.6.3. Thermal Exposure
3. Results and Discussion
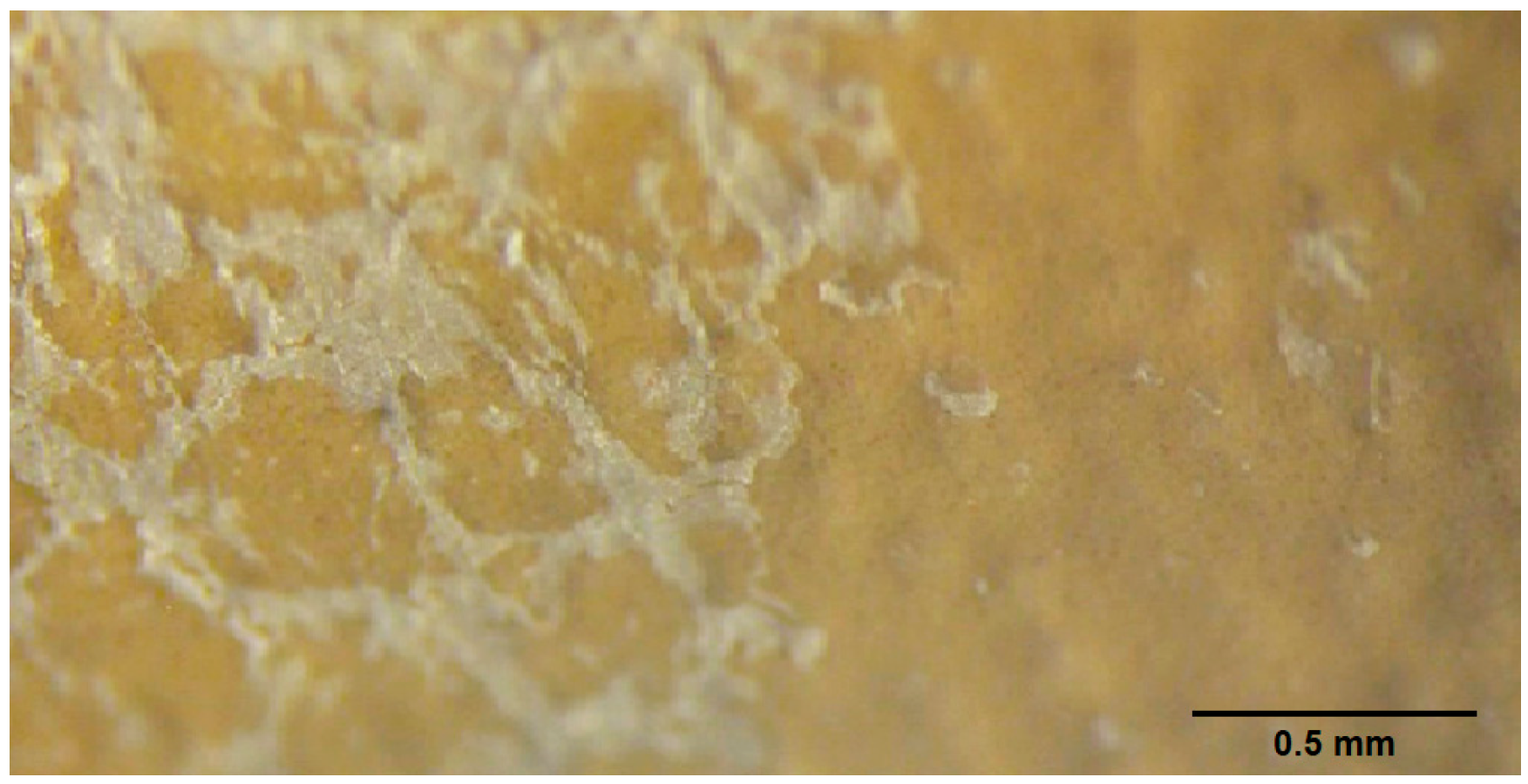
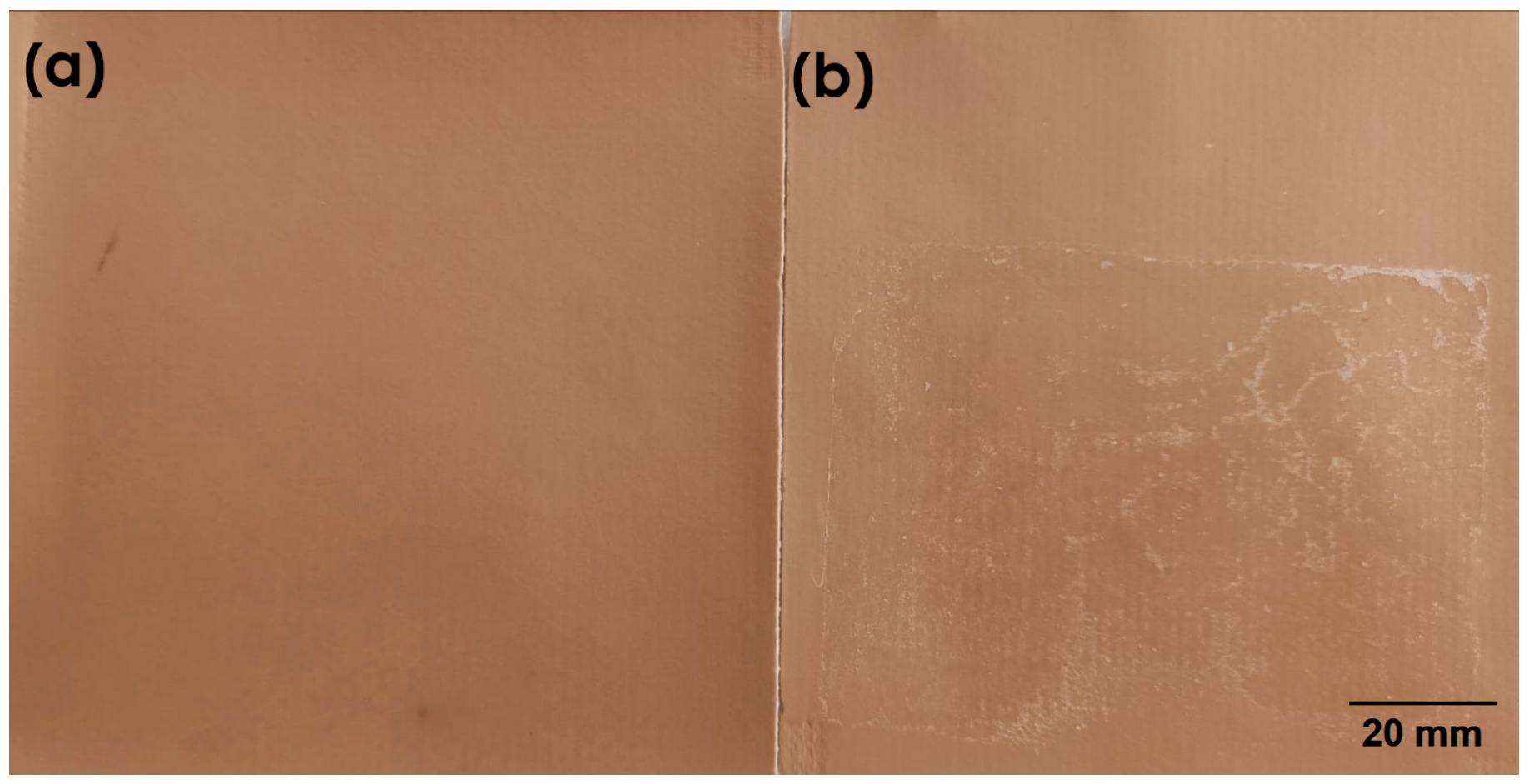
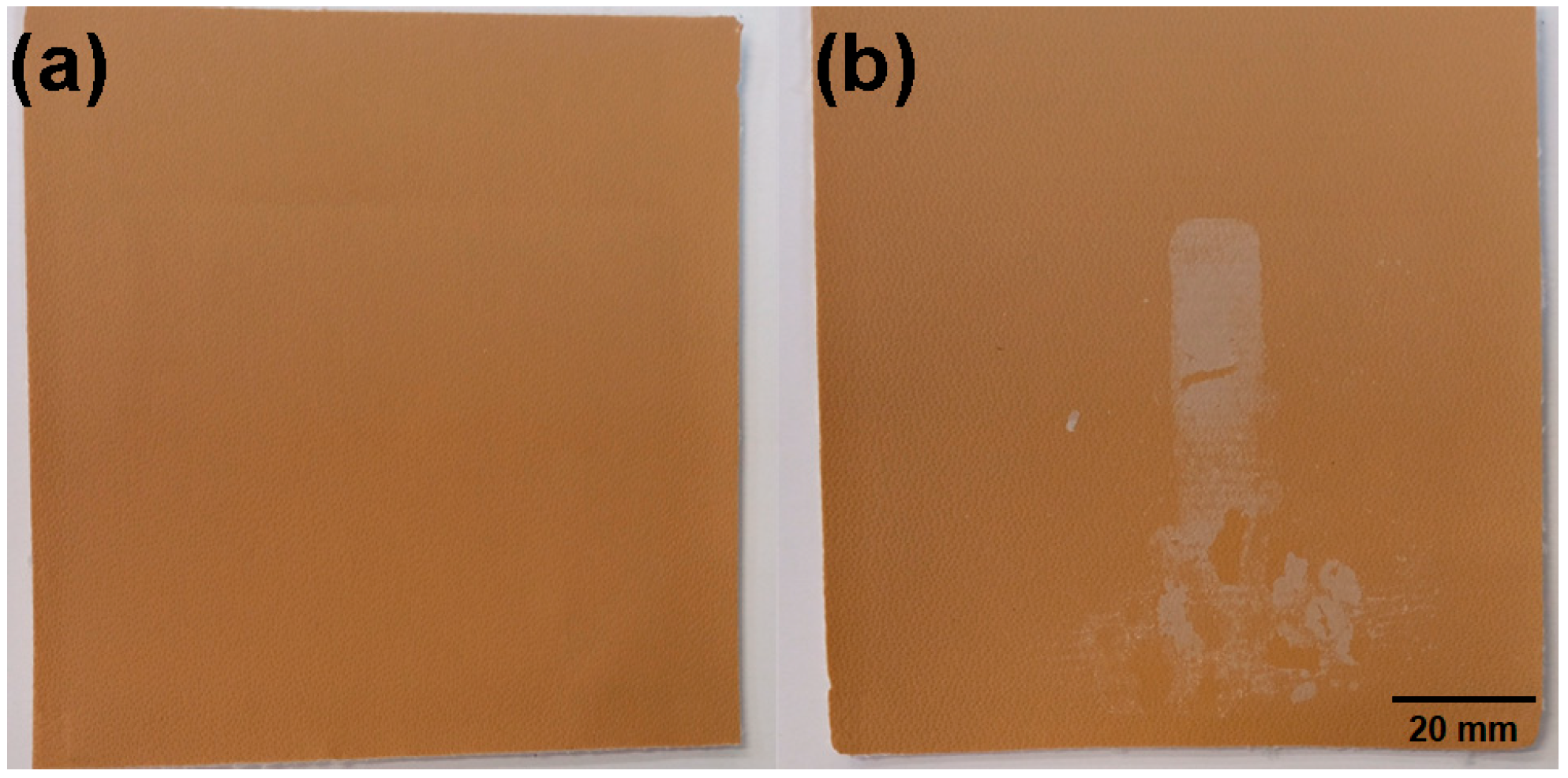
3.1. Preliminary Analysis of the Delaminated Vinyl Coated Fabric Identified During the Development Process
3.2. Experimental Design and Effect of Salt Fog and Temperature on the Vinyl Coated Fabric Samples
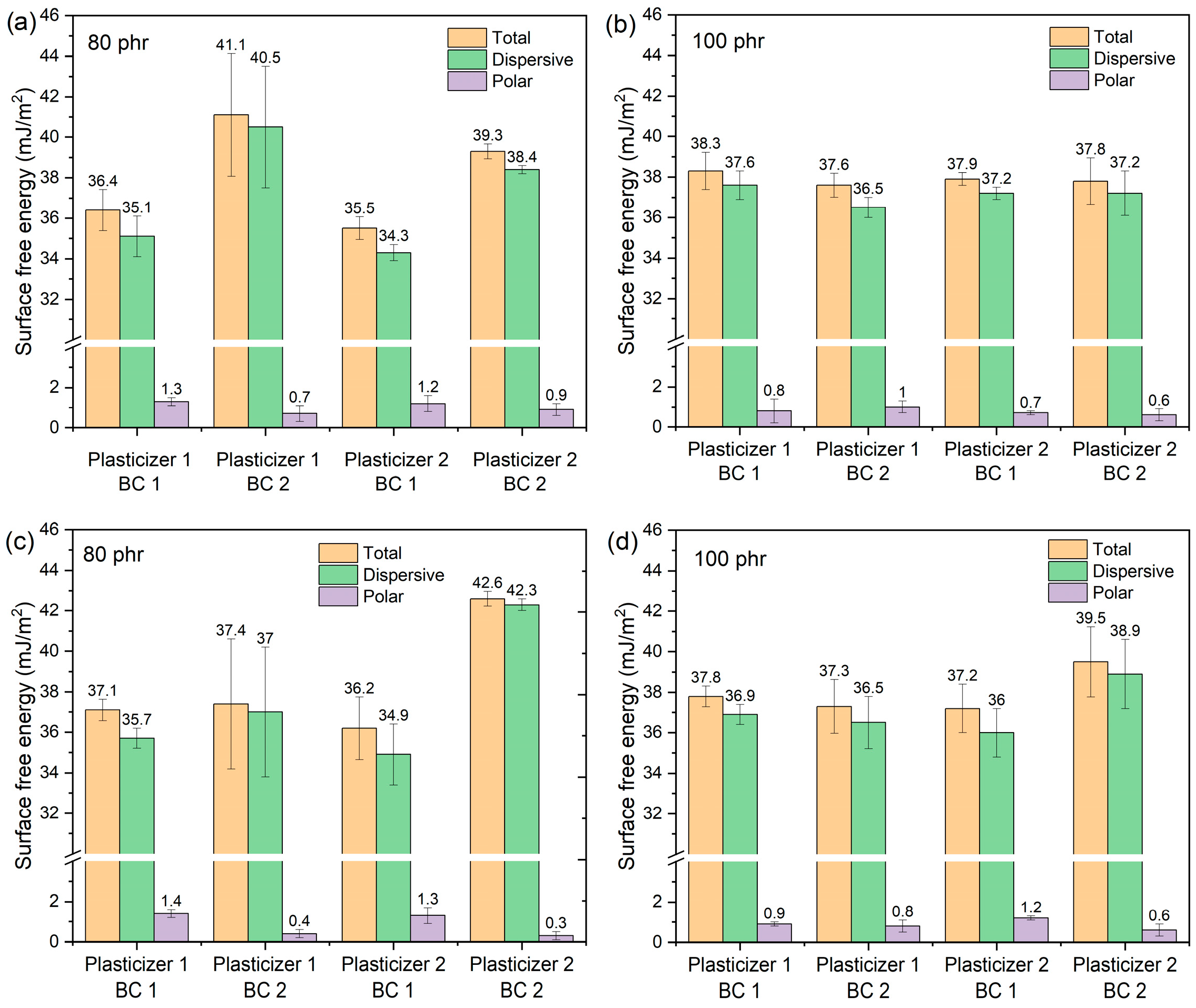
3.3. Study of the Surface Free Energy of Coatings on Vinyl Fabrics
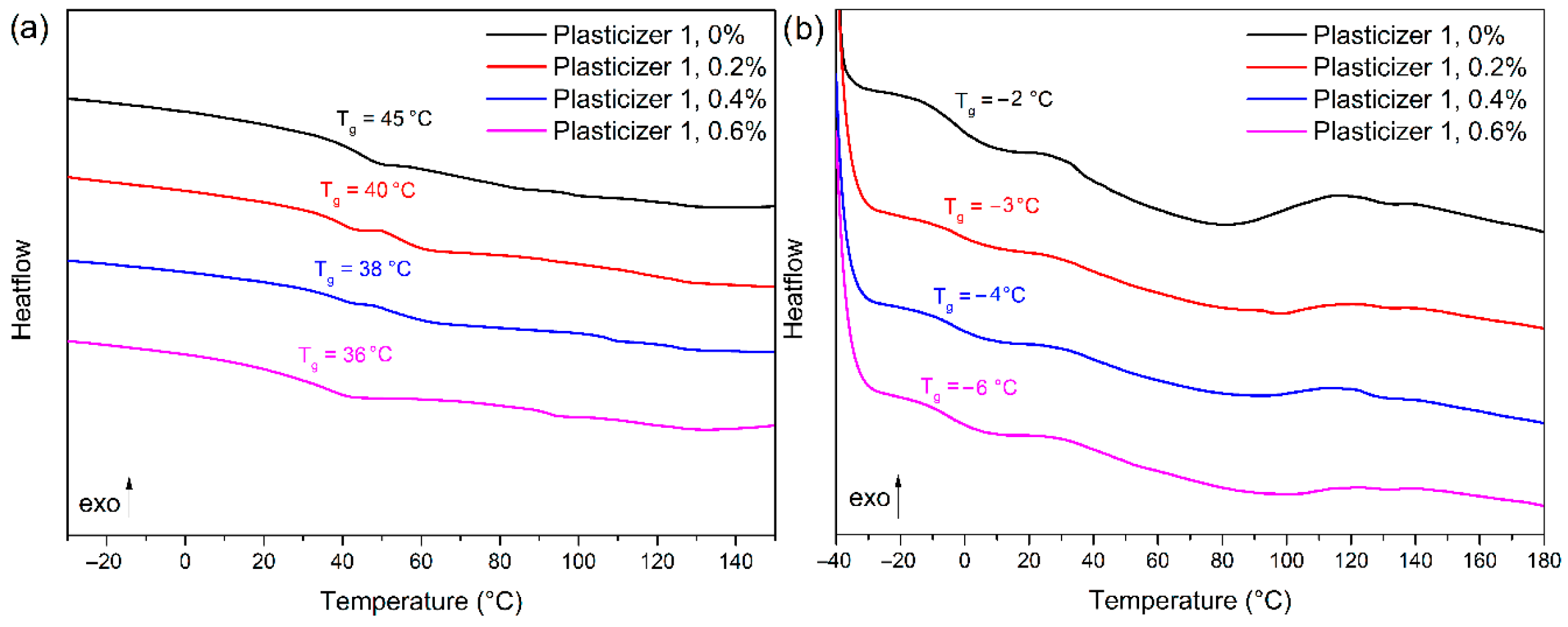
3.4. Plasticizer Extraction and Barrier Properties of the Coating on Vinyl Coated Fabrics
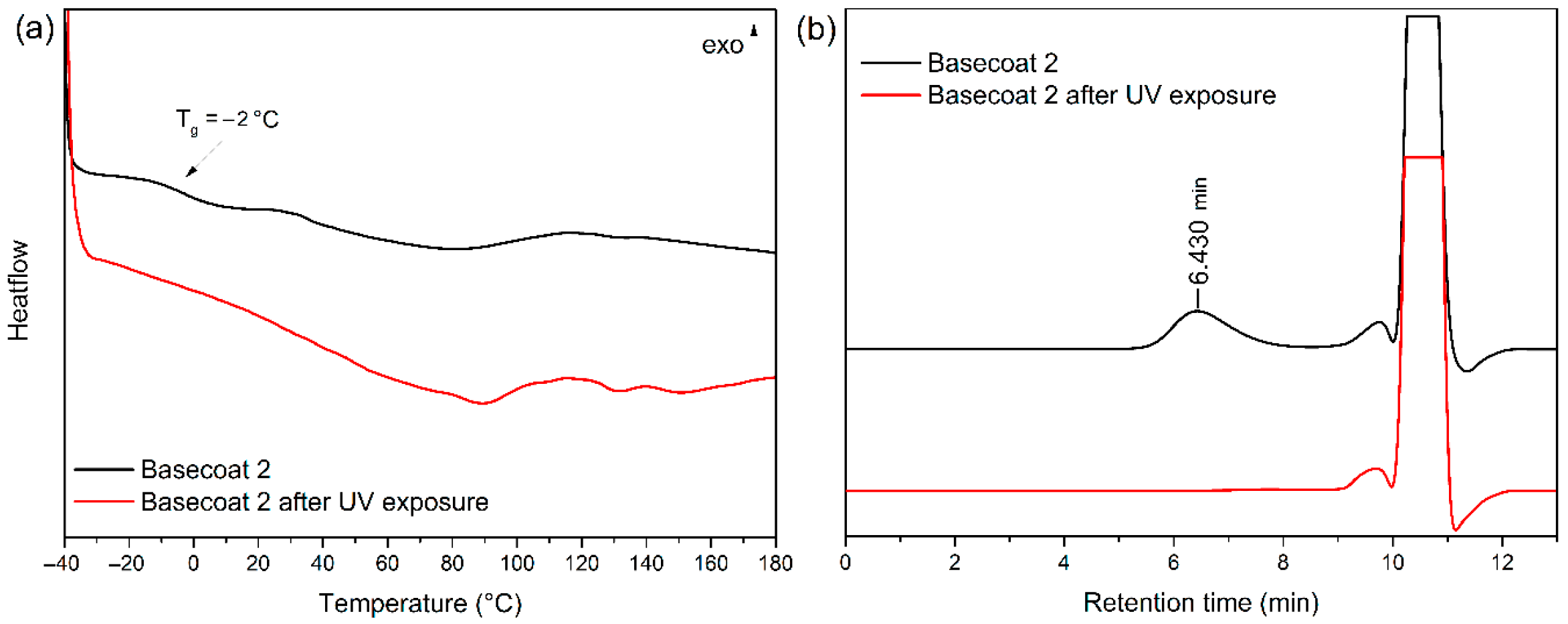
3.5. Effect of UV Light on the Vinyl Coated Fabric and the Coating
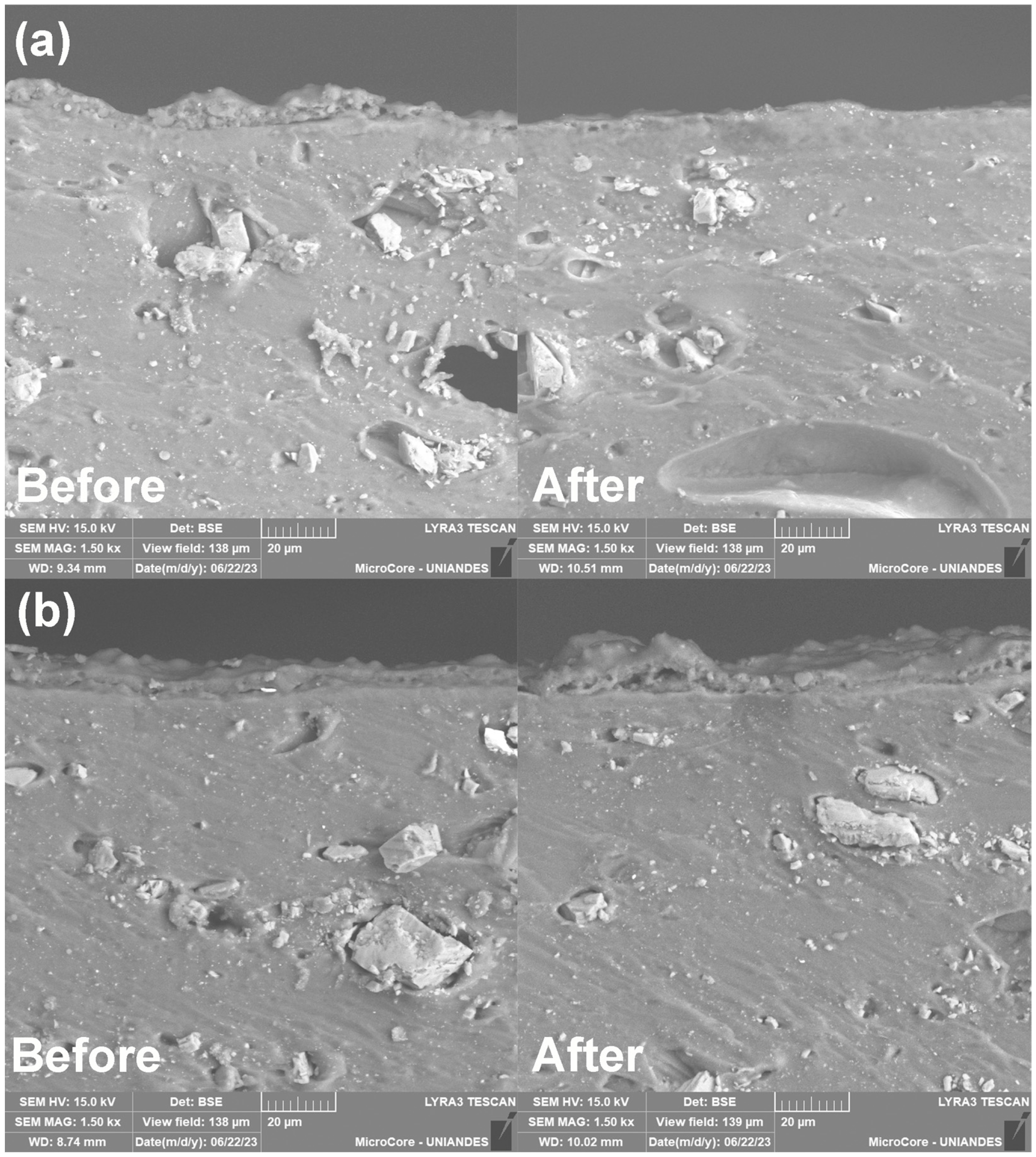
3.6. Microscopy Evaluation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PVC | Poly(vinyl chloride) |
| UV | Ultraviolet radiation |
| SEM | Scanning electron microscopy |
| γs | Total surface free energy |
| γsd | Dispersive free energy component |
| γsp | Polar free energy component |
| SFE | Surface free energy |
| DSC | Differential scanning calorimetry |
| TGA | Thermogravimetric analysis |
| Tg | Glass transition temperature |
| FTIR | Fourier Transform Infrared Spectroscopy |
| ATR | Attenuated Total Reflectance |
| GPC | Gel permeation chromatography |
| HPLC | High-Performance Liquid Chromatography |
| BC | Basecoat |
| phr | Parts per hundred resin |
References
- Ryu, J.; Hao, L.T.; Kim, H.; Lee, S.; Jeon, H.; Hwang, D.S.; Park, J.; Park, C.H.; Oh, D.X.; Koo, J.M.; et al. Biobased poly(ester amide)s as sustainable coating materials for vegan leather with improved haptic sensation. ACS Sustain. Chem. Eng. 2025, 13, 7585–7597. [Google Scholar] [CrossRef]
- Meyer, M.; Dietrich, S.; Schulz, H.; Mondschein, A. Comparison of the Technical Performance of Leather, Artificial Leather, and Trendy Alternatives. Coatings 2021, 11, 226. [Google Scholar] [CrossRef]
- Cheng, J.; Cao, Y.; Jiang, S.; Gao, Y.; Nie, J.; Sun, F. Synthesis and Performances of UV-Curable Polysiloxane-Polyether Block Polyurethane Acrylates for PVC Leather Finishing Agents. Ind. Eng. Chem. Res. 2015, 54, 5635–5642. [Google Scholar] [CrossRef]
- Bautista, M.; Lasprilla-Botero, J.; Mendez, J.; Alvarez, J.; Forero, A.; Santana, R. Investigación y Tecnología en Telas de Tapicería con Altos Niveles de Desempeño Recubiertas con Polímeros por Transferencia. Rev. Colomb. Mater. 2020, 15, 1–7. [Google Scholar]
- Liu, Z.; Zheng, X.; Zhang, H.; Li, W.; Jiang, R.; Zhou, X. Review on Formation of Biofouling in the Marine Environment and Functionalization of New Marine Antifouling Coatings. J. Mater. Sci. 2022, 57, 18221–18242. [Google Scholar] [CrossRef]
- Sun, Z.; Ren, S.; Wu, T.; Wen, J.; Fang, J.; Fan, H. A Self-Matting Waterborne Polyurethane Coating for PVC Artificial Leather. Polymers 2023, 15, 127. [Google Scholar] [CrossRef]
- Ammar, S.; Ma, I.A.W.; Ramesh, K.; Ramesh, S. Polymers-Based Nanocomposite Coatings. In Nanomaterials-Based Coatings: Fundamentals and Applications; Elsevier: Amsterdam, The Netherlands, 2019; pp. 9–39. ISBN 9780128158845. [Google Scholar]
- Liu, J.; Recupido, F.; Lama, G.C.; Oliviero, M.; Verdolotti, L.; Lavorgna, M. Recent Advances Concerning Polyurethane in Leather Applications: An Overview of Conventional and Greener Solutions. Collagen Leather 2023, 5, 8. [Google Scholar] [CrossRef]
- Gardi, S.; Giannone, L.; Sarti, G.; Sarti, G. Surface Chalking upon Weathering of Dark-Colored PVC Articles and Relevant Stabilizers. Polymers 2024, 16, 1047. [Google Scholar] [CrossRef] [PubMed]
- Gardi, S.; Giannone, L.; Sarti, G.; Sarti, G.; Costa, M. Influence of Initial Season on PVC Weathering. Polym. Test. 2023, 125, 108123. [Google Scholar] [CrossRef]
- Al-Dossary, A.K.; Gilbert, M.; Hitt, D. Evaluating PVC Degradation Using UV and Raman Spectroscopies. Adv. Mater. Res. 2010, 83–86, 923–930. [Google Scholar] [CrossRef]
- Palaniappan, E. Natural and Artificial Weathering of Automotive Leather, Leatherette and Textile; SAE Technical Paper 2019-28-0091; SAE International: Warrendale, PA, USA, 2019. [Google Scholar] [CrossRef]
- Dobilaitė, V.; Jucienė, M.; Bliūdžius, R.; Šveikauskaitė, L. Investigation of some weathering impacts on tearing properties of PVC-coated fabrics used for architectural purposes. J. Ind. Text. 2020, 51, 5328S–5346S. [Google Scholar] [CrossRef]
- Owens, D.K.; Wendt, R.C. Estimation of the Surface Free Energy of Polymers. J. Appl. Polym. Sci. 1969, 13, 1741–1747. [Google Scholar] [CrossRef]
- Peršin Fratnik, Z.; Plohl, O.; Kokol, V.; Fras Zemljič, L. Using Different Surface Energy Models to Assess the Interactions between Antiviral Coating Films and Phi6 Model Virus. J. Funct. Biomater. 2023, 14, 232. [Google Scholar] [CrossRef]
- ASTM B117-19; Standard Practice for Operating Salt Spray (Fog) Apparatus. American Society for Testing and Materials: West Conshohocken, PA, USA, 2019.
- ASTM D4329-21; Standard Practice for Fluorescent Ultraviolet (UV) Lamp Apparatus Exposure of Plastics. American Society for Testing and Materials: West Conshohocken, PA, USA, 2021.
- ISO 105-B04:2024; Textiles—Tests for Colour Fastness—Part B04: Colour Fastness to Artificial Weathering: Xenon Arc Fading Lamp Test. International Standard Organization: Geneva, Switzerland, 2024.
- NTC 1479-2; Texitles. Test for Colorfastness. Part 2: Colorfastness to Artificial Ligth. Zenon Arc Fading Lamp Test. ICONTEC—Instituto Colombiano de Normas Técnicas: Bogotá, Colombia, 2016.
- Coltro, L.; Pitta, J.B.; Madaleno, E. Performance Evaluation of New Plasticizers for Stretch PVC Films. Polym. Test. 2013, 32, 272–278. [Google Scholar] [CrossRef]
- Mallikarjunachari, G.; Ghosh, P. Analysis of Strength and Response of Polymer Nano Thin Film Interfaces Applying Nanoindentation and Nanoscratch Techniques. Polymer 2016, 90, 53–66. [Google Scholar] [CrossRef]
- Peng, H. Synthesis and Application of Fluorine-Containing Polymers with Low Surface Energy. Polym. Rev. 2019, 59, 739–757. [Google Scholar] [CrossRef]
- Gong, L.; Xiang, L.; Zhang, J.; Chen, J.; Zeng, H. Fundamentals and Advances in the Adhesion of Polymer Surfaces and Thin Films. Langmuir 2019, 35, 15914–15936. [Google Scholar] [CrossRef]
- Ivorra-Martinez, J.; Peydro, M.A.; Gomez-Caturla, J.; Boronat, T.; Balart, R. The Potential of an Itaconic Acid Diester as Environmentally Friendly Plasticizer for Injection-Molded Polylactide Parts. Macromol. Mater. Eng. 2022, 307, 2200360. [Google Scholar] [CrossRef]
- Stachowiak, N.; Kowalonek, J.; Kozlowska, J. Effect of Plasticizer and Surfactant on the Properties of Poly(Vinyl Alcohol)/Chitosan Films. Int. J. Biol. Macromol. 2020, 164, 2100–2107. [Google Scholar] [CrossRef] [PubMed]
- Massard, C.; Bernard, L.; Cueff, R.; Raspal, V.; Feschet-Chassot, E.; Sibaud, Y.; Sautou, V.; Awitor, K.O. Photopolymerizable Hybrid Sol Gel Coating as a Barrier against Plasticizer Release. Prog. Org. Coat. 2012, 75, 116–123. [Google Scholar] [CrossRef]
- Dedov, A. V Simulation of the Kinetics of Plasticizer Extraction with Ethanol. Russ. J. Appl. Chem. 2003, 76, 2033–2037. [Google Scholar] [CrossRef]
- Xu, Y.; Morado, E.G.; Zimmerman, S.C. Construction from Destruction Using a Photo-Triggered Self-Propagating Degradable Polyurethane as a One-Pot Epoxy. Polym. Chem. 2020, 11, 6215–6220. [Google Scholar] [CrossRef]




| Sample | Type of Basecoat | Plasticizer | Plasticization Level (phr) | Layers of Demolding Lacquer b |
|---|---|---|---|---|
| 1 | Solvent-based vinyl acrylic (BC 1) | Terephtalate (plasticizer 1) | 100 | 1 |
| 2 | 2 | |||
| 3 | 80 | 1 | ||
| 4 | 2 | |||
| 5 | Phtalate (plasticizer 2) | 100 | 1 | |
| 6 | 2 | |||
| 7 | 80 | 1 | ||
| 8 | 2 | |||
| 9 a | Solvent-based aliphatic polyurethane (BC 2) | Terephtalate (plasticizer 1) | 100 | 1 |
| 10 | 2 | |||
| 11 | 80 | 1 | ||
| 12 | 2 | |||
| 13 | Phtalate (plasticizer 2) | 100 | 1 | |
| 14 | 2 | |||
| 15 | 80 | 1 | ||
| 16 | 2 |
| Time (min) | Volume Fraction | ||
|---|---|---|---|
| CH3CN (%) | CH3OH (%) | H2O (%) | |
| 0–8 | 60 | 35 | 5 |
| 8–11 | 70 | 30 | 0 |
| 11–13 | 75 | 25 | 0 |
| 13–15 | 80 | 20 | 0 |
| 15–20 | 60 | 35 | 5 |
| Sample | Type of Basecoat Layer | Thermal Exposure at 100 °C | ||
|---|---|---|---|---|
| 0 Days | 7 Days | 15 Days | ||
| 1 | BC 1 |  |  |  |
| 9 | BC 2 |  |  |  |
| Plasticizer Content (phr) | Layers of Demolding Lacquer | Plasticizer | Evaluated Surface | γs (mJ/m2) | γsd (mJ/m2) | γsp (mJ/m2) |
|---|---|---|---|---|---|---|
| 100 | 1 | Type 1 | Skin layer | 45.4 ± 0.4 | 43.9 ± 0.2 | 1.5 ± 0.4 |
| Basecoat 1 | 38.3 ± 0.9 | 37.6 ± 0.7 | 0.8 ± 0.6 | |||
| Basecoat 2 | 37.6 ± 0.6 | 36.5 ± 0.5 | 1.0 ± 0.3 | |||
| Finish Topcoat | 14.9 ± 0.9 | 12.8 ± 0.8 | 2.1 ± 0.5 | |||
| Type 2 | Skin layer | 42.4 ± 0.6 | 39.2 ± 0.5 | 3.1 ± 0.3 | ||
| Basecoat 1 | 37.9 ± 0.3 | 37.2 ± 0.3 | 0.7 ± 0.1 | |||
| Basecoat 2 | 37.8 ± 1.1 | 37.2 ± 1.1 | 0.6 ± 0.3 | |||
| Finish Topcoat | 15.4 ± 1.2 | 13.8 ± 1.1 | 1.7 ± 0.5 | |||
| 2 | Type 1 | Skin layer | 44.3 ± 0.9 | 42.8 ± 0.8 | 1.5 ± 0.4 | |
| Basecoat 1 | 37.8 ± 0.5 | 36.9 ± 0.5 | 0.9 ± 0.1 | |||
| Basecoat 2 | 37.3 ± 1.3 | 36.5 ± 1.3 | 0.8 ± 0.3 | |||
| Finish Topcoat | 15.0 ± 2.4 | 13.5 ± 2.3 | 1.5 ± 0.6 | |||
| Type 2 | Skin layer | 40.5 ± 0.4 | 38.1 ± 0.1 | 2.5 ± 0.4 | ||
| Basecoat 1 | 37.2 ± 1.2 | 36.0 ± 1.2 | 1.2 ± 0.1 | |||
| Basecoat 2 | 39.5 ± 1.7 | 38.9 ± 1.7 | 0.6 ± 0.3 | |||
| Finish Topcoat | 15.9 ± 1.5 | 14.0 ± 1.3 | 1.9 ± 0.7 | |||
| 80 | 1 | Type 1 | Skin layer | 39.6 ± 1.2 | 38.1 ± 1.1 | 1.5 ± 0.4 |
| Basecoat 1 | 36.4 ± 1.0 | 35.1 ± 1.0 | 1.3 ± 0.2 | |||
| Basecoat 2 | 41.1 ± 3.0 | 40.5 ± 3.0 | 0.7 ± 0.4 | |||
| Finish Topcoat | 16.2 ± 1.9 | 13.3 ± 1.8 | 2.9 ± 0.7 | |||
| Type 2 | Skin layer | 39.0 ± 0.6 | 37.5 ± 0.4 | 1.5 ± 0.4 | ||
| Basecoat 1 | 35.5 ± 0.6 | 34.3 ± 0.4 | 1.2 ± 0.4 | |||
| Basecoat 2 | 39.3 ± 0.4 | 38.4 ± 0.2 | 0.9 ± 0.3 | |||
| Finish Topcoat | 14.8 ± 0.9 | 13.0 ± 0.8 | 1.8 ± 0.3 | |||
| 2 | Type 1 | Skin layer | 39.2 ± 0.6 | 38.0 ± 0.6 | 1.2 ± 0.1 | |
| Basecoat 1 | 37.1 ± 0.5 | 35.7 ± 0.5 | 1.4 ± 0.2 | |||
| Basecoat 2 | 37.4 ± 3.2 | 37.0 ± 3.2 | 0.4 ± 0.2 | |||
| Finish Topcoat | 16.8 ± 0.4 | 15.1 ± 0.2 | 1.70 ± 0.4 | |||
| Type 2 | Skin layer | 37.9 ± 1.8 | 36.3 ± 1.5 | 1.6 ± 1.0 | ||
| Basecoat 1 | 36.2 ± 1.6 | 34.9 ± 1.5 | 1.3 ± 0.4 | |||
| Basecoat 2 | 42.6 ± 0.4 | 42.3 ± 0.3 | 0.3 ± 0.2 | |||
| Finish Topcoat | 15.5 ± 0.4 | 12.3 ± 0.2 | 3.1 ± 0.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Acelas, M.; Combariza, C.; Zapata-Gallego, N.T.; Pinto, P.; Peláez, G.J.; Santana, R.; Lasprilla-Botero, J.; Martín-Martínez, J.M. Failure and Damage Analysis of a Polymeric Finish Topcoat on Vinyl Coated Fabrics for Marine Applications. Coatings 2025, 15, 1365. https://doi.org/10.3390/coatings15121365
Acelas M, Combariza C, Zapata-Gallego NT, Pinto P, Peláez GJ, Santana R, Lasprilla-Botero J, Martín-Martínez JM. Failure and Damage Analysis of a Polymeric Finish Topcoat on Vinyl Coated Fabrics for Marine Applications. Coatings. 2025; 15(12):1365. https://doi.org/10.3390/coatings15121365
Chicago/Turabian StyleAcelas, Mauricio, Cristina Combariza, Natalia Trinidad Zapata-Gallego, Paula Pinto, Gabriel J. Peláez, Ricardo Santana, Juliana Lasprilla-Botero, and José Miguel Martín-Martínez. 2025. "Failure and Damage Analysis of a Polymeric Finish Topcoat on Vinyl Coated Fabrics for Marine Applications" Coatings 15, no. 12: 1365. https://doi.org/10.3390/coatings15121365
APA StyleAcelas, M., Combariza, C., Zapata-Gallego, N. T., Pinto, P., Peláez, G. J., Santana, R., Lasprilla-Botero, J., & Martín-Martínez, J. M. (2025). Failure and Damage Analysis of a Polymeric Finish Topcoat on Vinyl Coated Fabrics for Marine Applications. Coatings, 15(12), 1365. https://doi.org/10.3390/coatings15121365







