Investigation of Scaling and Materials’ Performance of EHLA-Fabricated Cladding in Simulated Geothermal Brine
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Laser Cladding Equipment
2.2. Scaling and Corrosion Tests
2.2.1. Test Performed Simulating Geothermal Conditions
2.2.2. Post-Tests Examination
2.2.3. Critical Pitting Temperature
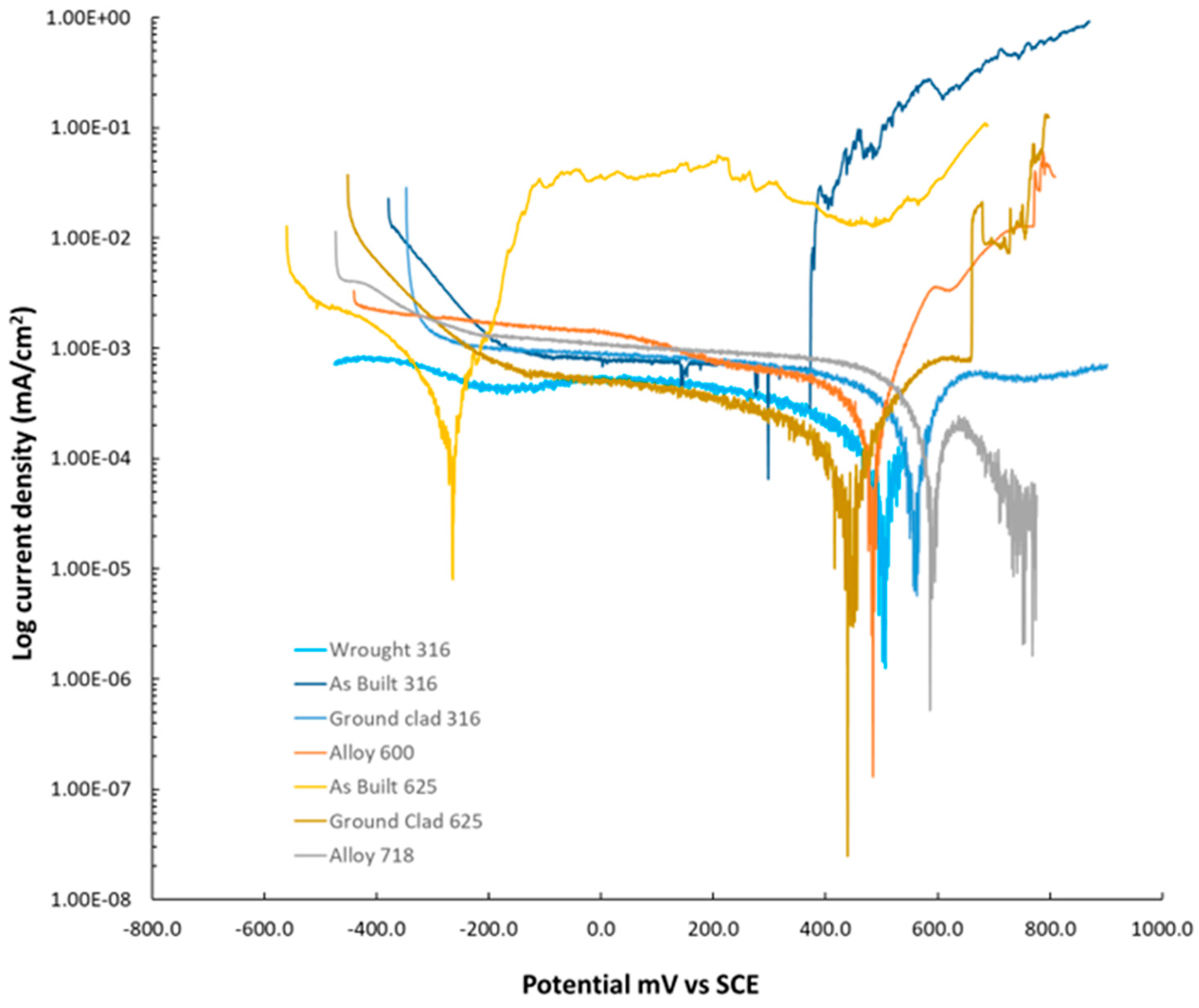
3. Results
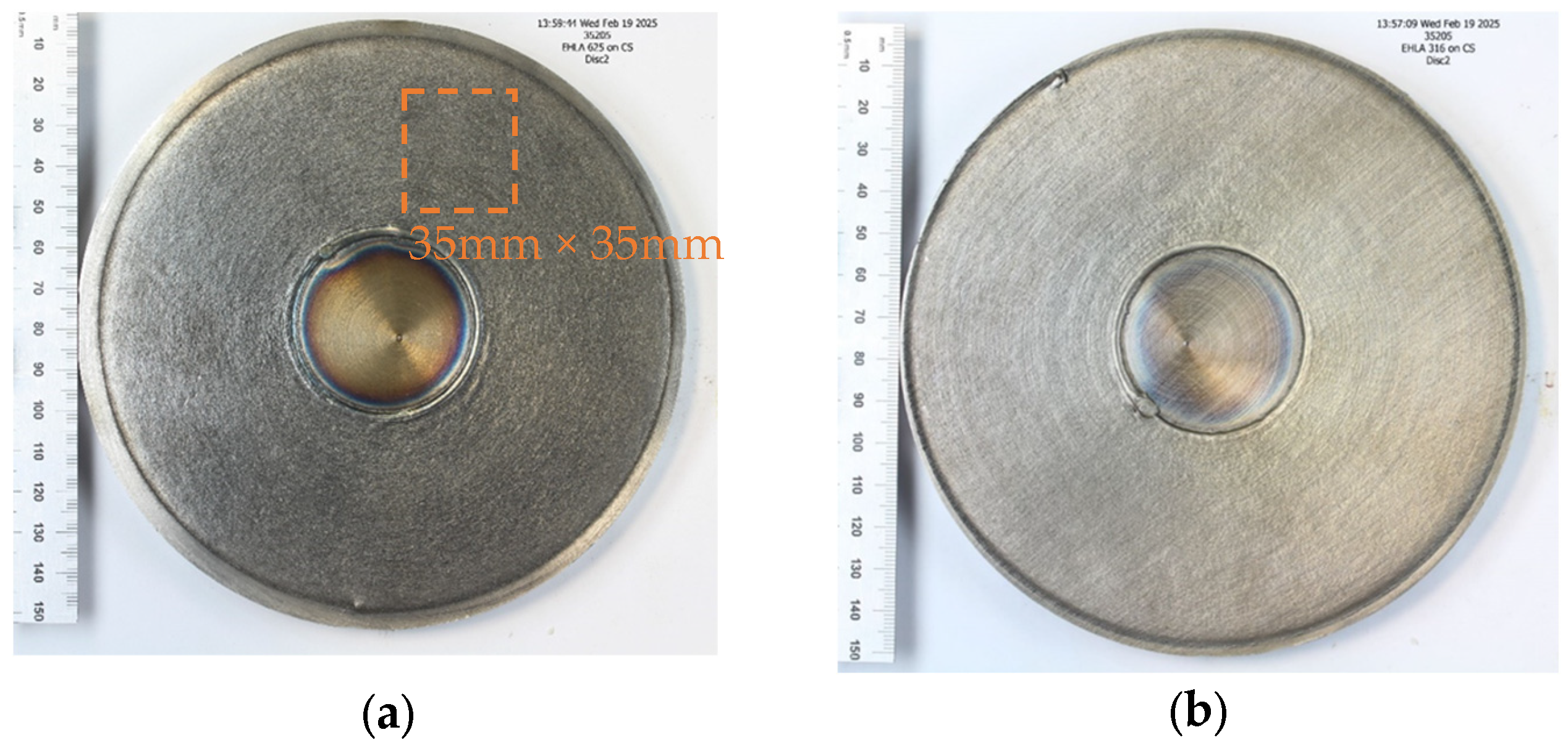
3.1. Sample Manufacturing by EHLA
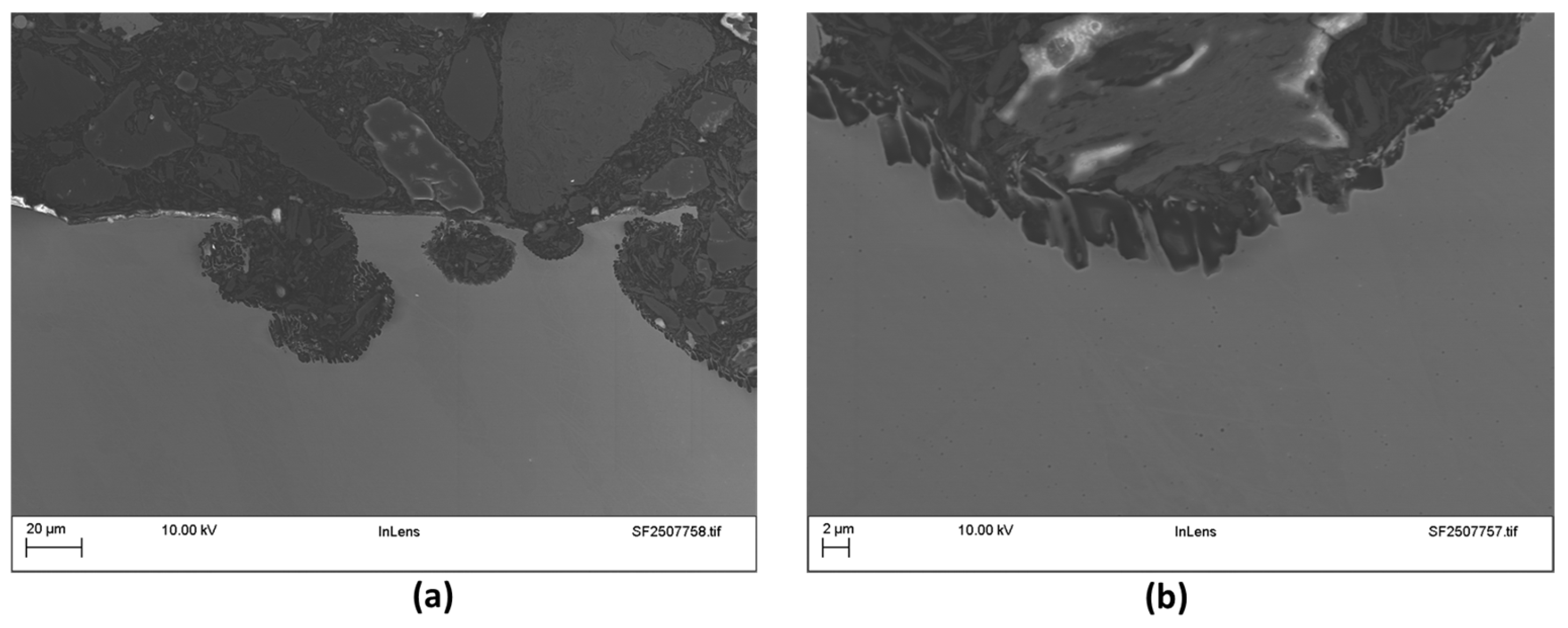
3.1.1. Inconel 625
3.1.2. EHLA 316L
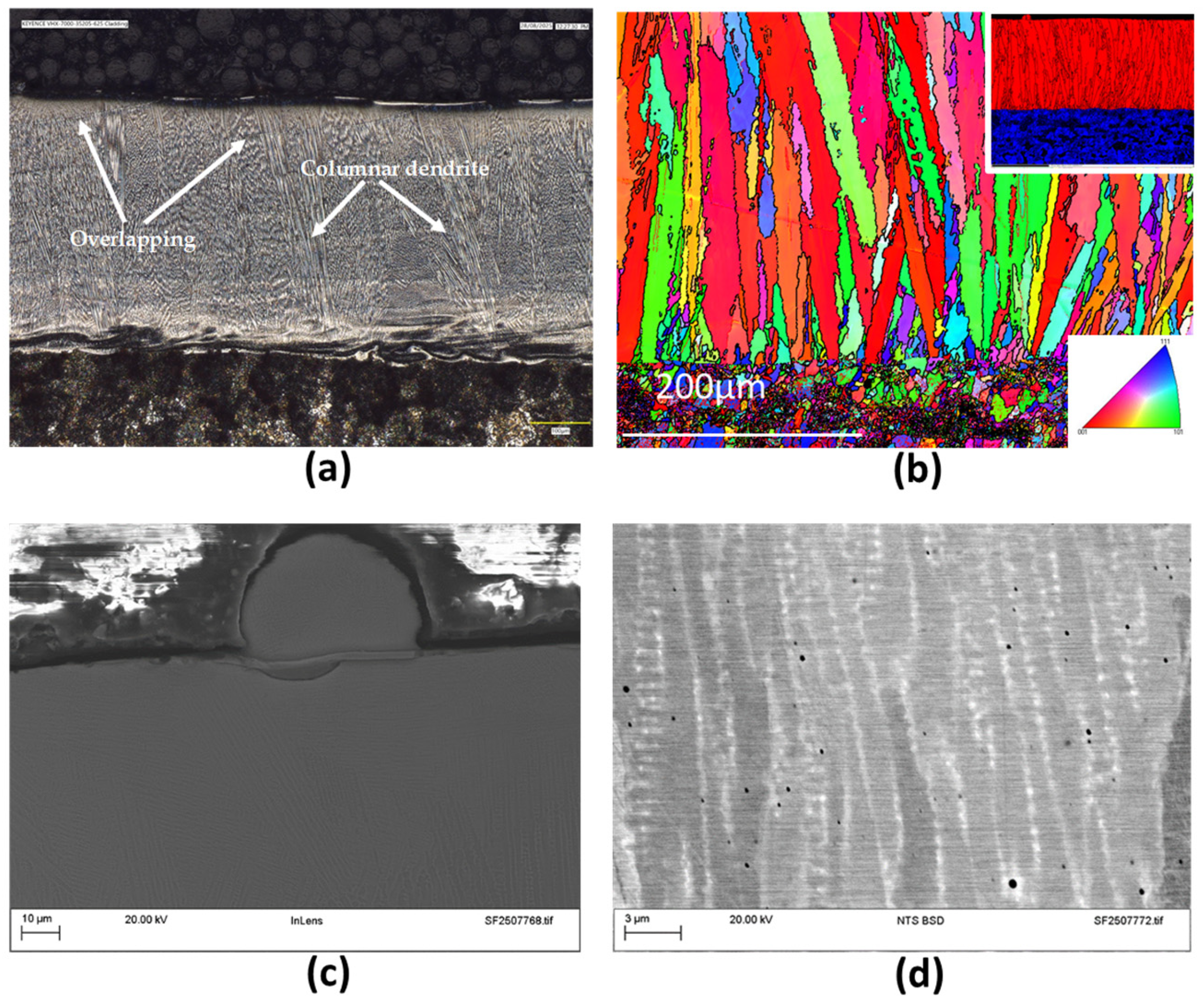
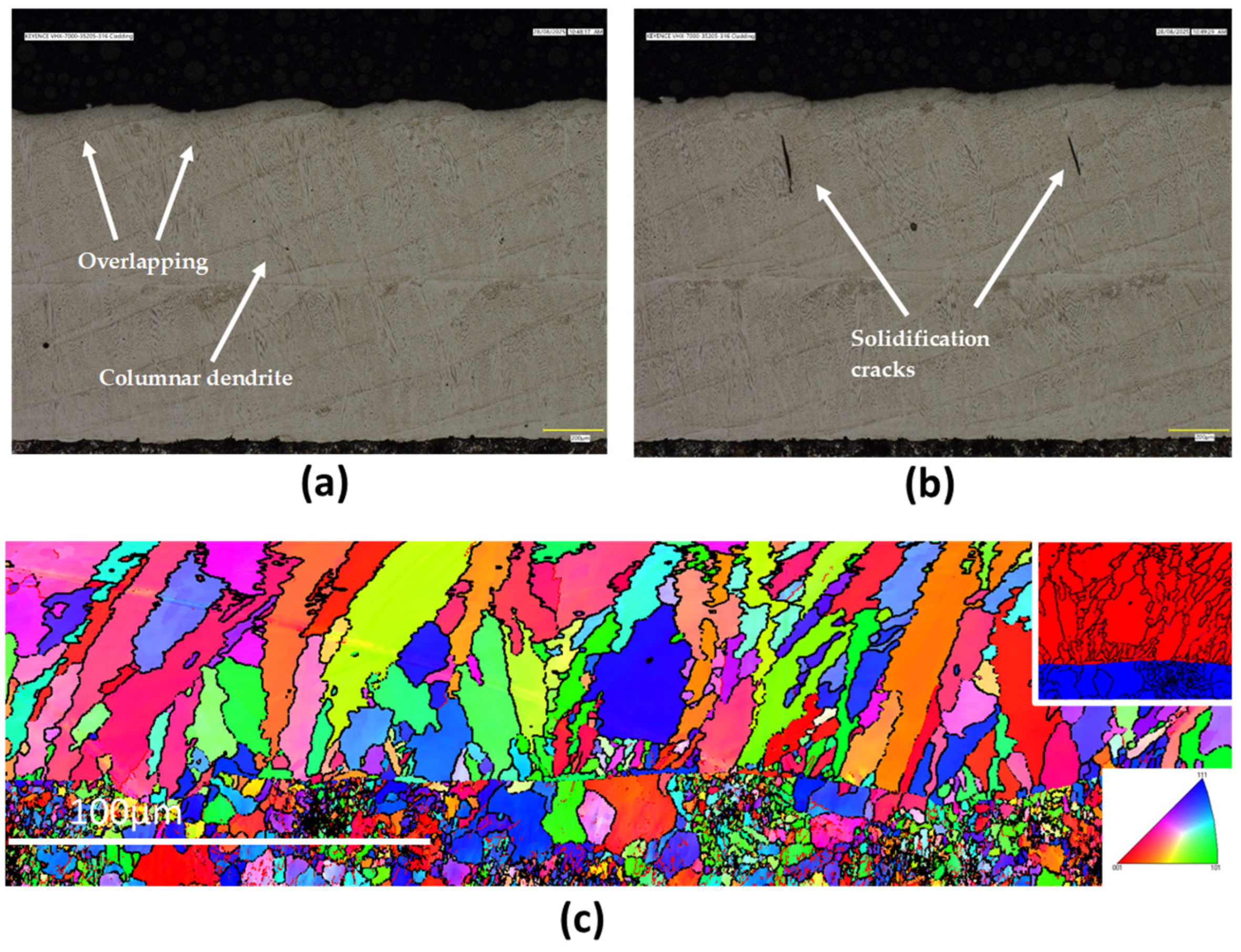
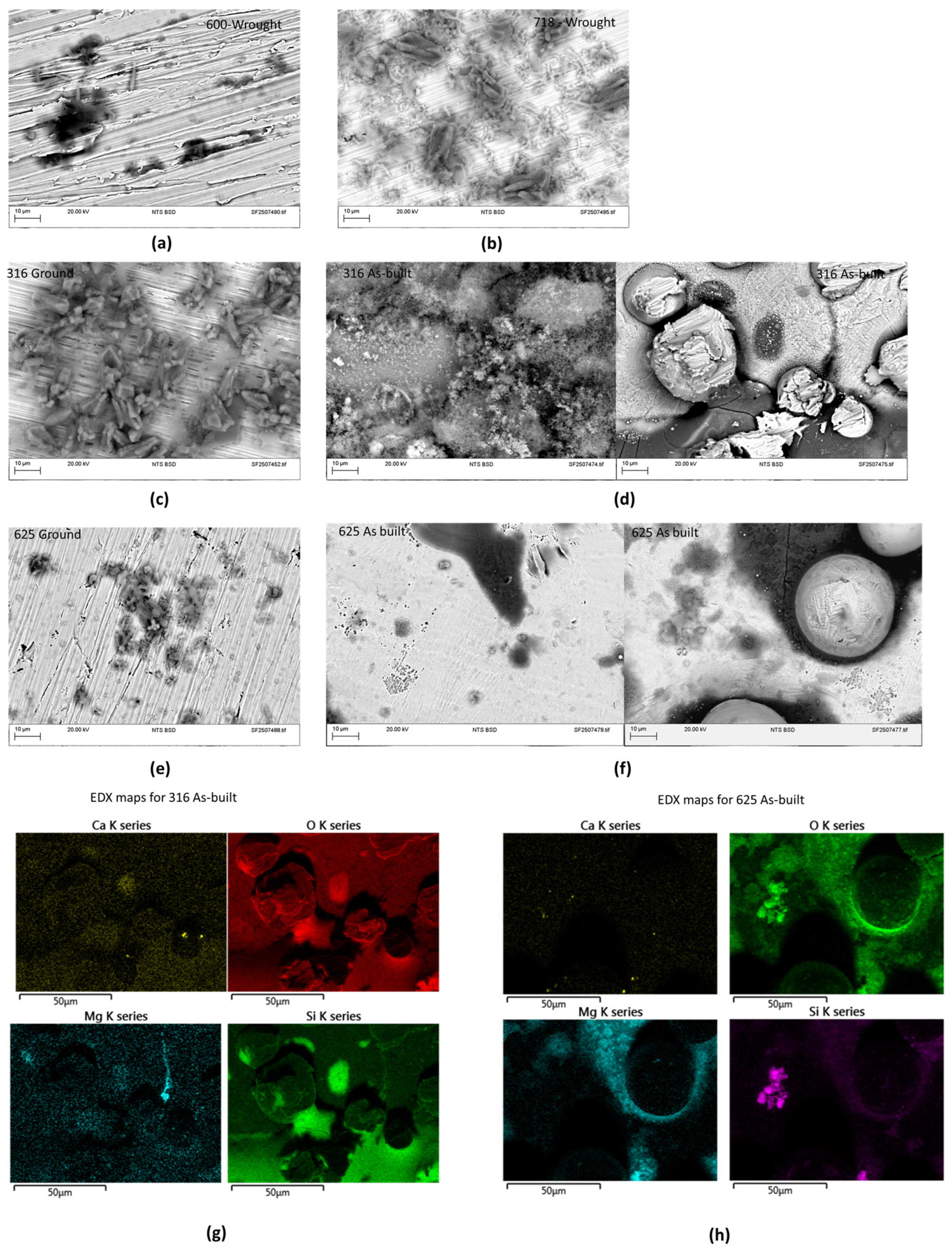
3.2. Microstructural Examinations
3.2.1. Inconel 625
3.2.2. 316L Stainless Steel
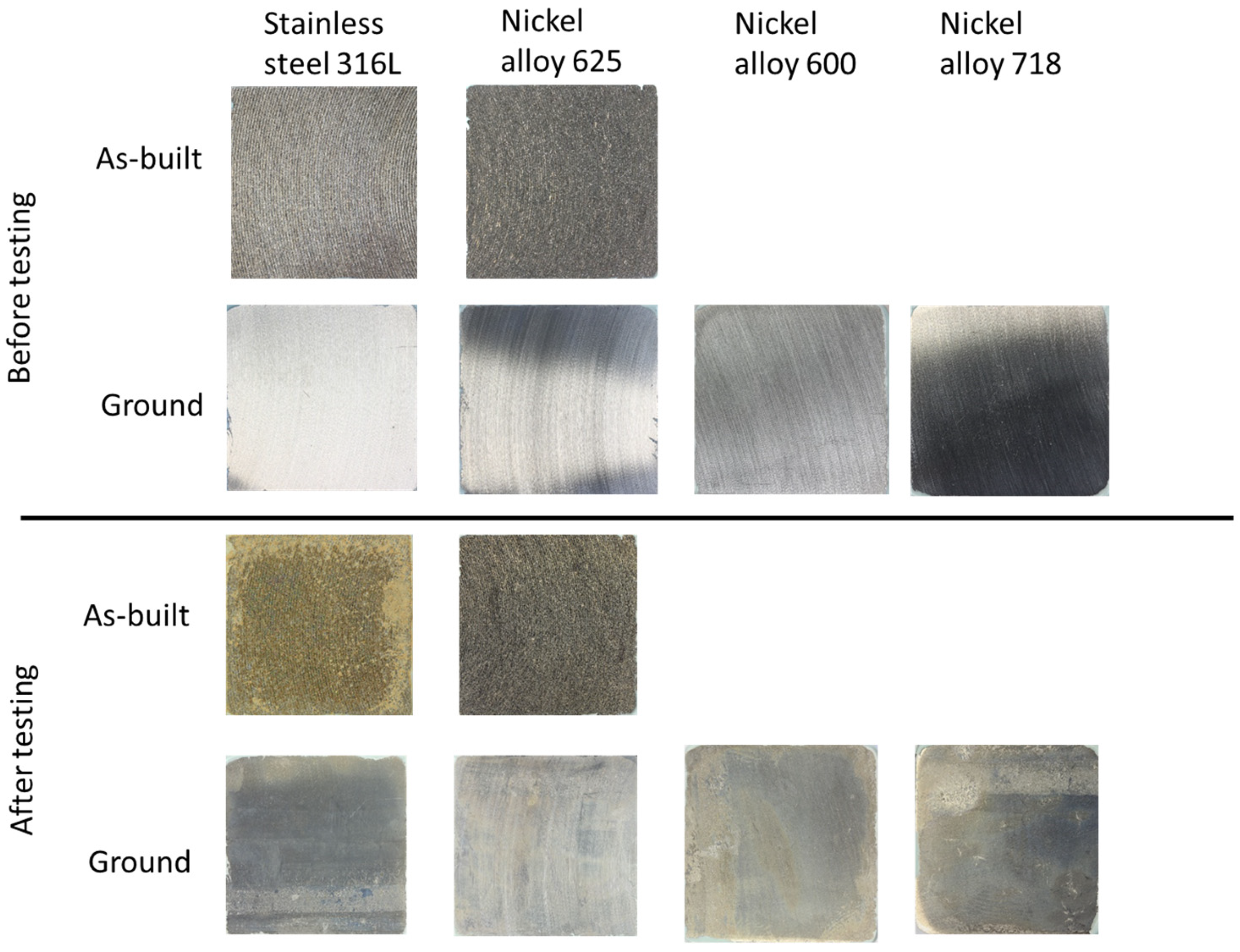
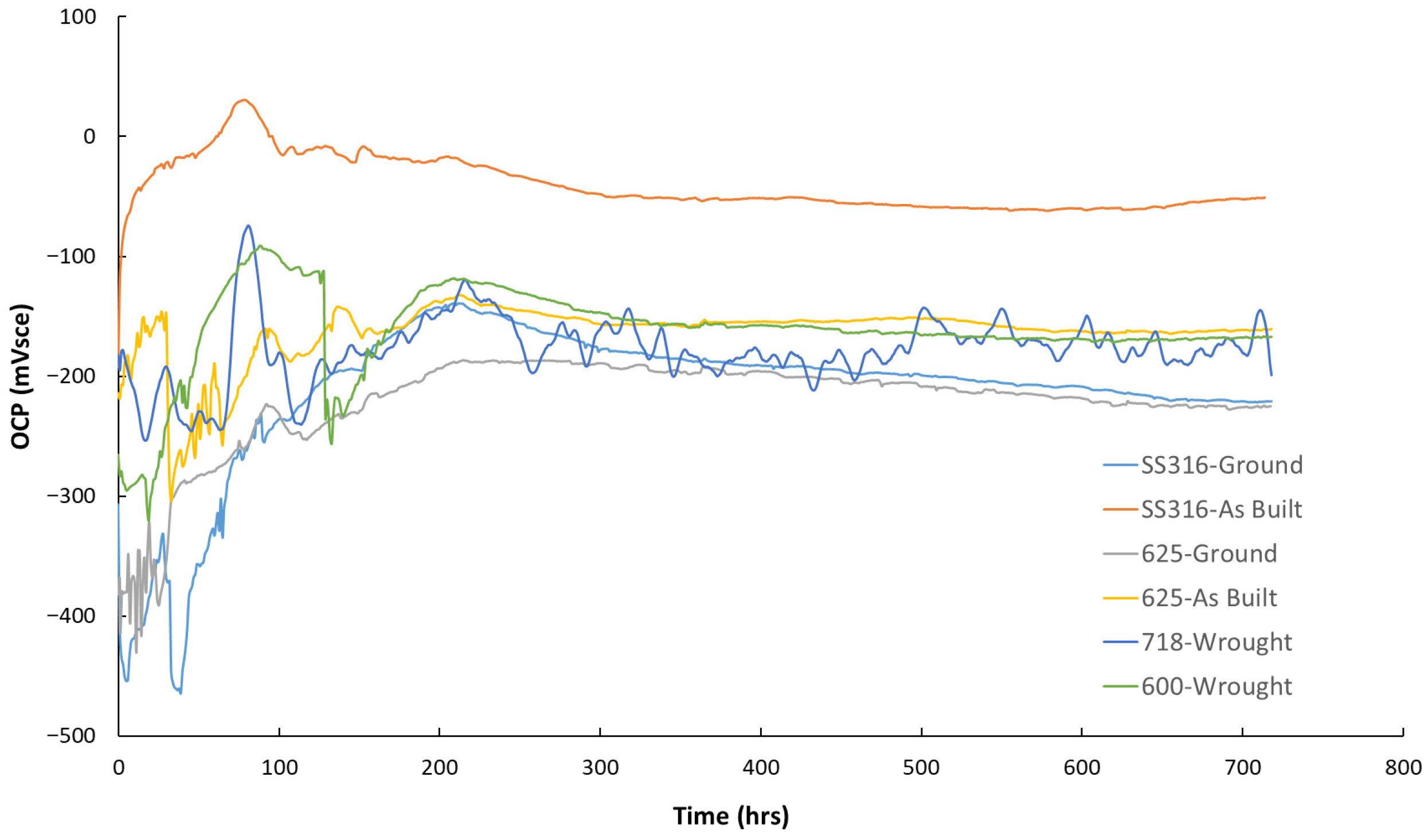
3.3. Flow Rig Test
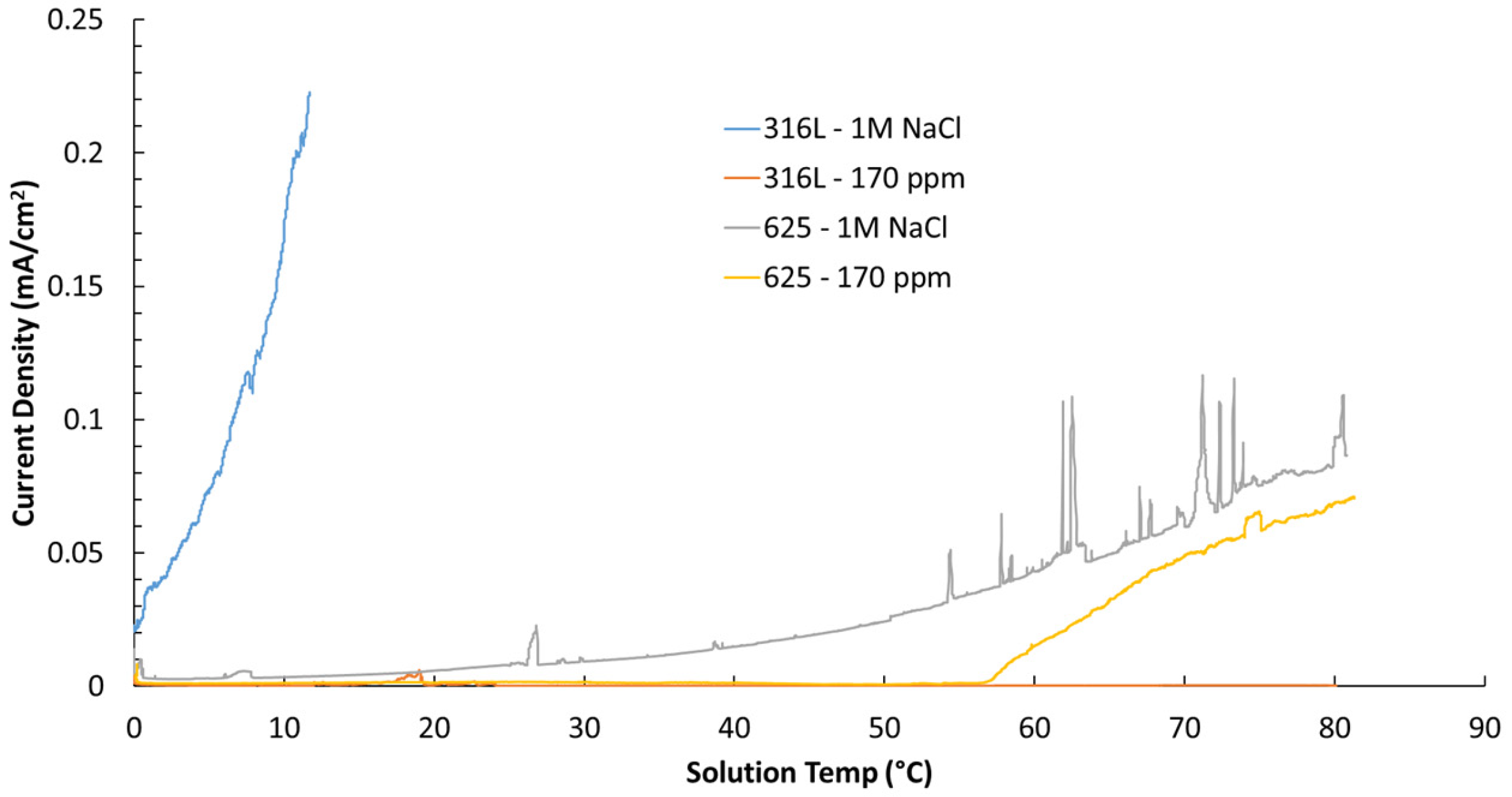
3.4. Critical Pitting Temperature
4. Discussion
5. Conclusions
- •
- EHLA-fabricated coatings exhibited dense microstructures with excellent substrate bonding and minimal porosity, confirming the potential of the process to produce coatings suitable for corrosion-resistant applications.
- •
- Although the findings reported in this investigation are only indicative of the corrosion performance under the tested laboratory conditions and further investigation would be required to confirm equivalence in industrial environments, ground 316L EHLA shows performance comparable to wrought 316L.
- •
- EHLA alloy 625 showed better corrosion and scaling resistance than EHLA 316L in simulated geothermal brine due to its stable passive film and higher alloying content. However, in the ground condition, after removal of the as-deposited surface the differences in corrosion performance are not significant.
- •
- Localised Nb- and Mo-rich segregation in EHLA 625 had some influence on the corrosion resistance under the tested conditions. Surface conditions strongly influenced performance. Processes such as grinding reduced scale deposition and enhanced electrochemical stability. Post-deposition surface engineering is required to enhance the performance of the deposits. However, with the limited studies carried out in this project it is difficult to state with certainty if further process parameter optimisation could achieve this.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Elders, W.A.; Friðleifsson, G.Ó.; Albertsson, A. Drilling into magma and the implications of the Iceland Deep Drilling Project (IDDP) for high-temperature geothermal systems worldwide. Geothermics 2014, 49, 111–118. [Google Scholar] [CrossRef]
- Kruszewski, M.; Wittig, V. Review of failure modes in supercritical geothermal drilling projects. Geotherm. Energy 2018, 6, 28. [Google Scholar] [CrossRef]
- Nogara, J.; Zarrouk, S.J.; Seastres, J. Surface analysis of metal alloys exposed to geothermal fluids with high non-condensable gas content. Geothermics 2018, 72, 372–399. [Google Scholar] [CrossRef]
- Karlsdottir, S.N.; Thorhallson, A.I.; Prikryl, J.; Pekyavas, M.; Asbjornsson, S.; Wallevik, S.O.; Alexandersson, K.F. Material challenges for deep superhot geothermal wells. Geotherm. Resour. Counc. Trans. 2022, 46, 271–284. [Google Scholar]
- Gallup, D.L. Unusual adherence of manganese silicate scale to metal substrates. Geotherm. Resour. Counc. Trans. 2004, 28, 529–532. [Google Scholar]
- Penot, C.; Martelo, D.; Paul, S. Corrosion and scaling in geothermal heat exchangers. Appl. Sci. 2023, 13, 11549. [Google Scholar] [CrossRef]
- Brownlie, F.; Hodgkiess, T.; Pearson, A.; Galloway, A.M. A study on the erosion-corrosion behaviour of engineering materials used in the geothermal industry. Wear 2021, 477, 203821. [Google Scholar] [CrossRef]
- Karlsdóttir, S.N.; Hjaltason, S.M.; Ragnarsdóttir, K.R. Corrosion behavior of materials in hydrogen sulfide abatement system at Hellisheiði geothermal power plant. Geothermics 2017, 70, 222–229. [Google Scholar] [CrossRef]
- Mundhenk, N.; Huttenloch, P.; Bäßler, R.; Kohl, T.; Steger, H.; Zorn, R. Electrochemical study of the corrosion of different alloys exposed to deaerated 80 C geothermal brines containing CO2. Corros. Sci. 2014, 84, 180–188. [Google Scholar] [CrossRef]
- Liu, M.; Cai, Y.; Duan, C.; Li, G. Key techniques in parts repair and remanufacturing based on laser cladding: A review. J. Manuf. Process. 2024, 132, 994–1014. [Google Scholar] [CrossRef]
- Wang, K.; Du, D.; Liu, G.; Chang, B.; Ju, J.; Sun, S.; Fu, H. Microstructure and property of laser clad Fe-based composite layer containing Nb and B4C powders. J. Alloys Compd. 2019, 802, 373–384. [Google Scholar] [CrossRef]
- Ranjan, R.; Das, A.K. Protection from corrosion and wear by different weld cladding techniques: A review. Mater. Today Proc. 2022, 57, 1687–1693. [Google Scholar] [CrossRef]
- Syed, W.U.H.; Pinkerton, A.J.; Li, L. A comparative study of wire feeding and powder feeding in direct diode laser deposition for rapid prototyping. Appl. Surf. Sci. 2005, 247, 268–276. [Google Scholar] [CrossRef]
- Wang, K.; Liu, W.; Hong, Y.; Sohan, H.S.; Tong, Y.; Hu, Y.; Ju, J. An overview of technological parameter optimization in the case of laser cladding. Coatings 2023, 13, 496. [Google Scholar] [CrossRef]
- Li, X.; Zhang, S.; Liu, W.; Pang, X.; Tong, Y.; Zhang, M.; Wang, K. Effect of WC content on the wear and corrosion properties of oscillating laser-cladding-produced nickel-based coating. Coatings 2023, 13, 1614. [Google Scholar] [CrossRef]
- Mahmoud, E.R.; Khan, S.Z.; Ejaz, M. Laser surface cladding of mild steel with 316L stainless steel for anti-corrosion applications. Mater. Today Proc. 2021, 39, 1029–1033. [Google Scholar] [CrossRef]
- Ding, H.; Yang, T.; Wang, W.; Zhu, Y.; Lin, Q.; Guo, J.; Liu, Q. Optimization and wear behaviors of 316L stainless steel laser cladding on rail material. Wear 2023, 523, 204830. [Google Scholar] [CrossRef]
- Du, J.; Xu, X.; Zhang, H.M.; Lu, M.W.; Sun, J.F.; Luo, K.Y.; Lu, J.Z. Microstructure and wear resistance of CoCrFeNiMn coatings prepared by extreme-high-speed laser cladding. Surf. Coat. Technol. 2023, 470, 129821. [Google Scholar] [CrossRef]
- Pacheco, J.T.; Veiga, M.T.; dos Santos, M.T.; Trabasso, L.G. Status of high-speed laser cladding process: An up-to-date review. Progress. Addit. Manuf. 2024, 9, 1857–1868. [Google Scholar] [CrossRef]
- Lampa, C.; Smirnov, I. High speed laser cladding of an iron-based alloy developed for hard chrome replacement. J. Laser Appl. 2019, 31, 2. [Google Scholar] [CrossRef]
- Li, L.; Shen, F.; Zhou, Y.; Tao, W. Comparative study of stainless steel AISI 431 coatings prepared by extreme-high-speed and conventional laser cladding. J. Laser Appl. 2019, 31, e5094378. [Google Scholar] [CrossRef]
- Conover, M.; Ellis, P.; Curzon, A. Material selection guidelines for geothermal power systems: An overview. In Geothermal Scaling and Corrosion; Casper, L.A., Pinchback, T.R., Eds.; STP717-EB; ASTM International: West Conshohocken, PA, USA, 1980; pp. 24–40. [Google Scholar]
- Abedi Esfahani, E.; Maccio, T. Corrosion Performance Testing of Cladding—D4.3. In Compass Deliverable; Data Management Plan; COMPASS Consortium—European Commission: Brussels, Belgium, 2025. [Google Scholar]
- Martelo, D.F.; Holmes, B.; Kale, N.; Scott, S.W.; Paul, S. Investigation of scaling and materials’ performance in simulated geothermal brine. Materials 2024, 17, 5250. [Google Scholar] [CrossRef]
- Galeczka, I.M.; Stefansson, A.; Kleine, B.I.; Gunnarsson-Robin, J.; Snæbjörnsdóttir, S.Ó.; Sigfusson, B.; Hlíf Gunnarsdóttir, S.; Weisenberger, T.G.; Oelkers, E. H pre-injection assessment of CO2 and H2S mineralization reactions at the Nesjavellir (Iceland) geothermal storage site. Int. J. Greenh. Gas. Control 2022, 115, 103610. [Google Scholar] [CrossRef]
- ASTM G150-18; Standard Test Method for Electrochemical Critical Pitting Temperature Testing of Stainless Steels and Related Alloys. ASTM International: West Conshohocken, PA, USA, 2018.
- Nguejio, J.; Szmytka, F.; Hallais, S.; Tanguy, A.; Nardone, S.; Martinez, M.G. Comparison of microstructure features and mechanical properties for additive-manufactured and wrought nickel alloy 625. Mater. Sci. Eng. A 2019, 764, 138214. [Google Scholar] [CrossRef]
- Scott, S.; Galeczka, I.M.; Gunnarsson, I.; Arnorsson, S.; Stefansson, A. Silica polymerization and nanocolloid nucleation and growth kinetics in aqueous solutions. Geochim. Cosmochim. Acta 2024, 371, 78–94. [Google Scholar] [CrossRef]
- Teo, A.Q.A.; Yan, L.; Chaudhari, A.; O’Neill, G.K. Post-processing and surface characterization of additively manufactured stainless steel 316L lattice: Implications for biomedical use. Materials 2021, 14, 1376. [Google Scholar] [CrossRef] [PubMed]
- Clark, C.L.; Karasz, E.K.; Melia, M.; Hooks, D.E.; Hackenberg, R.; Colon-Mercado, H.; Stull, J.A. Machine-to-machine variability of roughness and corrosion in additively manufactured 316L stainless steel. J. Manuf. Process. 2023, 106, 380–392. [Google Scholar] [CrossRef]
- Ansari, N.; Alabtah, F.G.; Khraisheh, M. Influence of temperature on corrosion behavior of additively manufactured SS316L stainless steel in CO2-saturated brine solution. Mater. Lett. 2024, 376, 137272. [Google Scholar] [CrossRef]
- Ezuber, H.M. Influence of temperature on the pitting corrosion behavior of AISI 316L in chloride–CO2 (sat.) solutions. Mater. Des. 2014, 59, 339–343. [Google Scholar] [CrossRef]
- Voisin, T.; Shi, R.; Zhu, Y.; Qi, Z.; Wu, M.; Sen-Britain, S.; Wood, B.C. Pitting corrosion in 316L stainless steel fabricated by laser powder bed fusion additive manufacturing: A review and perspective. JOM 2022, 74, 1668–1689. [Google Scholar] [CrossRef]
- Wang, Y.M.; Voisin, T.; McKeown, J.T.; Ye, J.; Calta, N.P.; Li, Z.; Zhu, T. Additively manufactured hierarchical stainless steels with high strength and ductility. Nat. Mater. 2018, 17, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Chao, Q.; Cruz, V.; Thomas, S.; Birbilis, N.; Collins, P.; Taylor, A.; Fabijanic, D. On the enhanced corrosion resistance of a selective laser melted austenitic stainless steel. Scr. Mater. 2017, 141, 94–98. [Google Scholar] [CrossRef]
- Gola, K.; Ledwig, P.; Dubiel, B. Effect of microstructure of additively manufactured Inconel 625 on long-term corrosion behaviour in sulfuric acid media. JOM 2023, 75, 1242–1250. [Google Scholar] [CrossRef]
- Marchese, G.; Lorusso, M.; Parizia, S.; Bassini, E.; Lee, J.W.; Calignano, F.; Biamino, S. Influence of heat treatments on micro-structure evolution and mechanical properties of Inconel 625 processed by laser powder bed fusion. Mater. Sci. Eng. A 2018, 729, 64–75. [Google Scholar]
- Cabrini, M.; Lorenzi, S.; Testa, C.; Pastore, T.; Brevi, F.; Biamino, S.; Fino, P.; Manfredi, D.; Marchese, G.; Calignano, F.; et al. Evaluation of corrosion resistance of alloy 625 obtained by laser powder bed fusion. J. Electrochem. Soc. 2019, 166, C3399–C3408. [Google Scholar] [CrossRef]

| Material | Spot Size (mm) | Laser Power (kW) | Speed (m/min) | Powder Feed Rate (g/min) | Feeder (RPM) | Pitch % | Pitch (mm) |
|---|---|---|---|---|---|---|---|
| 625 | 3 | 2 | 10 | 15 | 1.1 | 80 | 0.4 |
| 316L | 3 | 2.5 | 10 | 25 | 4.6 | 70 | 0.48 |
| Element | 316L wt% | Inconel 625 wt% | CS Substrate wt% |
|---|---|---|---|
| Fe | Balance | ≤5 | Balance |
| Cr | 16.0–18.0 | 20.0–23.0 | - |
| Ni | 10.0–14.0 | 58.0 min | - |
| Mo | 2.0–3.0 | 8.0–10.0 | - |
| Nb | - | 3.15–4.15 | - |
| Al | - | ≤0.40 | - |
| Ti | - | ≤0.4 | - |
| Mn | ≤2.0 | ≤0.50 | 0.8 |
| Si | ≤1.0 | ≤0.50 | 0.2 |
| C | ≤0.03 | ≤0.10 | ≤0.03 |
| Material | Environment | CPT Value |
|---|---|---|
| 316L | 1 M NaCl | 7.7 °C |
| 316L | 170 ppm chloride | >80 °C |
| 625 | 1 M NaCl | >80 °C |
| 625 | 170 ppm chloride | >80 °C |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martelo, D.; Abedi Esfahani, E.; Kale, N.; Maccio, T.; Paul, S. Investigation of Scaling and Materials’ Performance of EHLA-Fabricated Cladding in Simulated Geothermal Brine. Coatings 2025, 15, 1366. https://doi.org/10.3390/coatings15121366
Martelo D, Abedi Esfahani E, Kale N, Maccio T, Paul S. Investigation of Scaling and Materials’ Performance of EHLA-Fabricated Cladding in Simulated Geothermal Brine. Coatings. 2025; 15(12):1366. https://doi.org/10.3390/coatings15121366
Chicago/Turabian StyleMartelo, David, Erfan Abedi Esfahani, Namrata Kale, Tomaso Maccio, and Shiladitya Paul. 2025. "Investigation of Scaling and Materials’ Performance of EHLA-Fabricated Cladding in Simulated Geothermal Brine" Coatings 15, no. 12: 1366. https://doi.org/10.3390/coatings15121366
APA StyleMartelo, D., Abedi Esfahani, E., Kale, N., Maccio, T., & Paul, S. (2025). Investigation of Scaling and Materials’ Performance of EHLA-Fabricated Cladding in Simulated Geothermal Brine. Coatings, 15(12), 1366. https://doi.org/10.3390/coatings15121366







