Preparation and Properties of Micro-Arc Oxidation Coatings on Friction-Stir-Processed ZK60 Mg Alloys with Hydroxyapatite Particles
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. MAO Treatment
2.3. Testing and Characterization
2.3.1. Microstructure and Composition Analysis
2.3.2. Phase Analysis
2.3.3. Tribological and Mechanical Properties
2.3.4. Electrochemical Characterization
2.3.5. Wettability and Antibacterial Evaluation
3. Results and Discussion
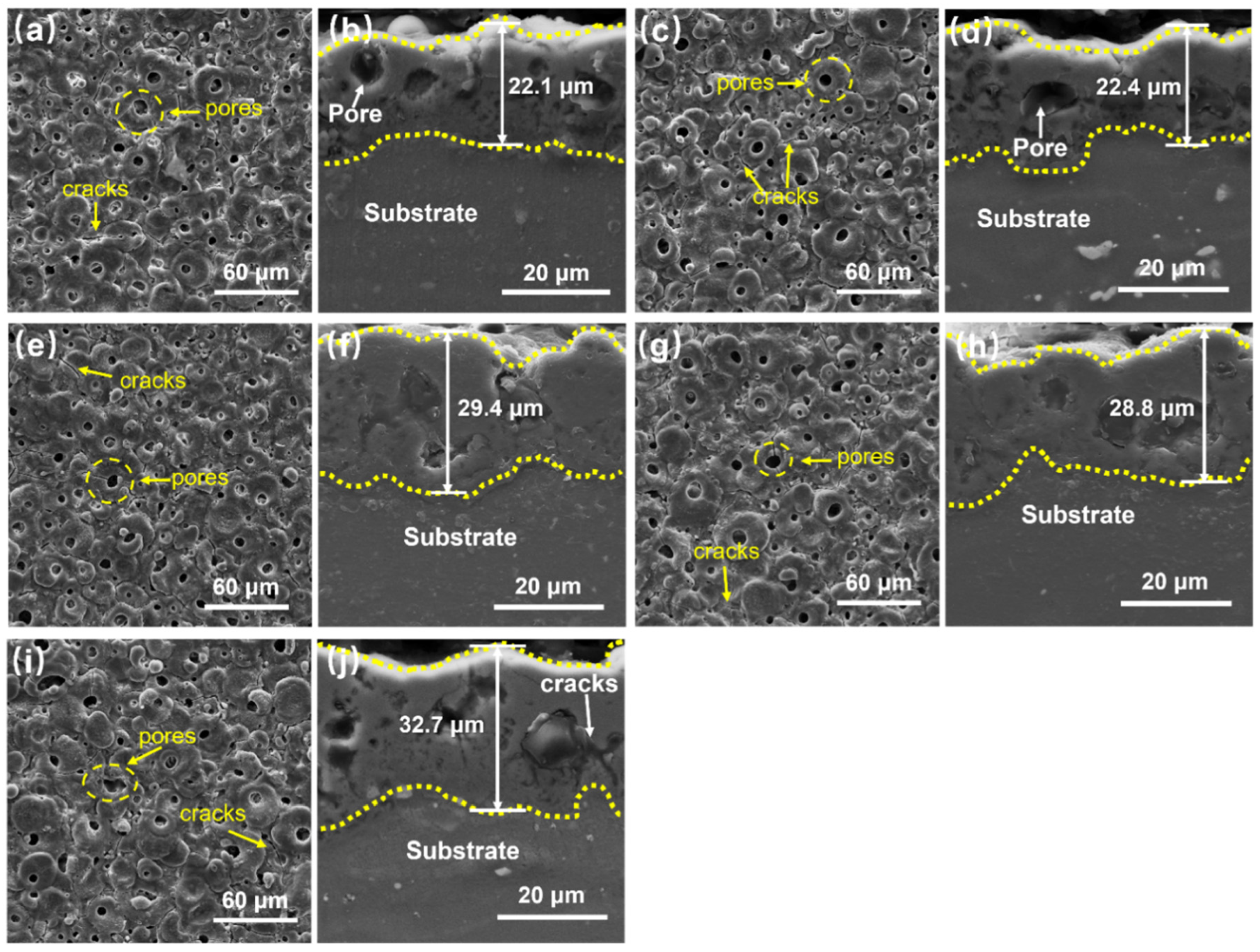
3.1. Macroscopic Morphology and Microstructure of MAO Coating
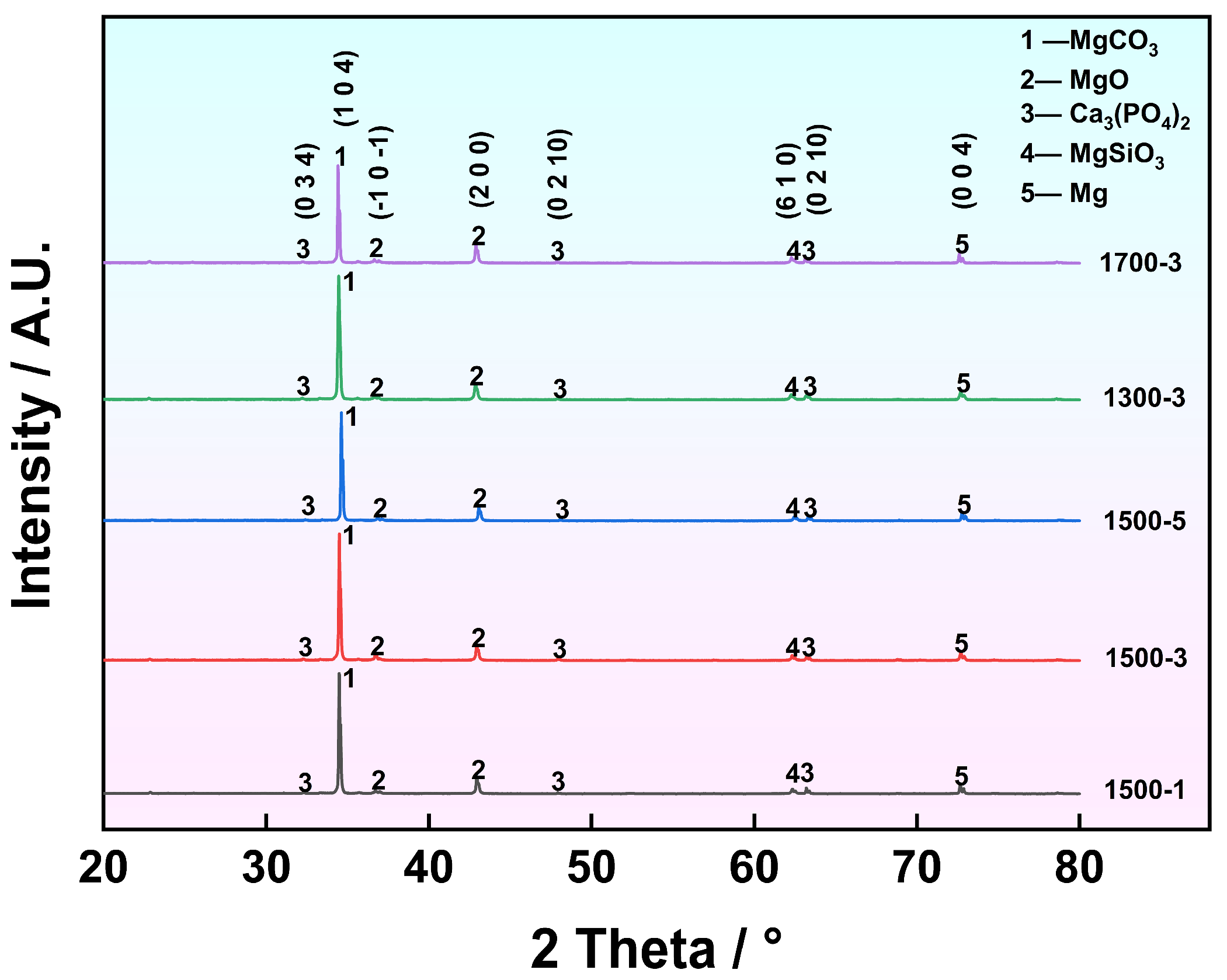
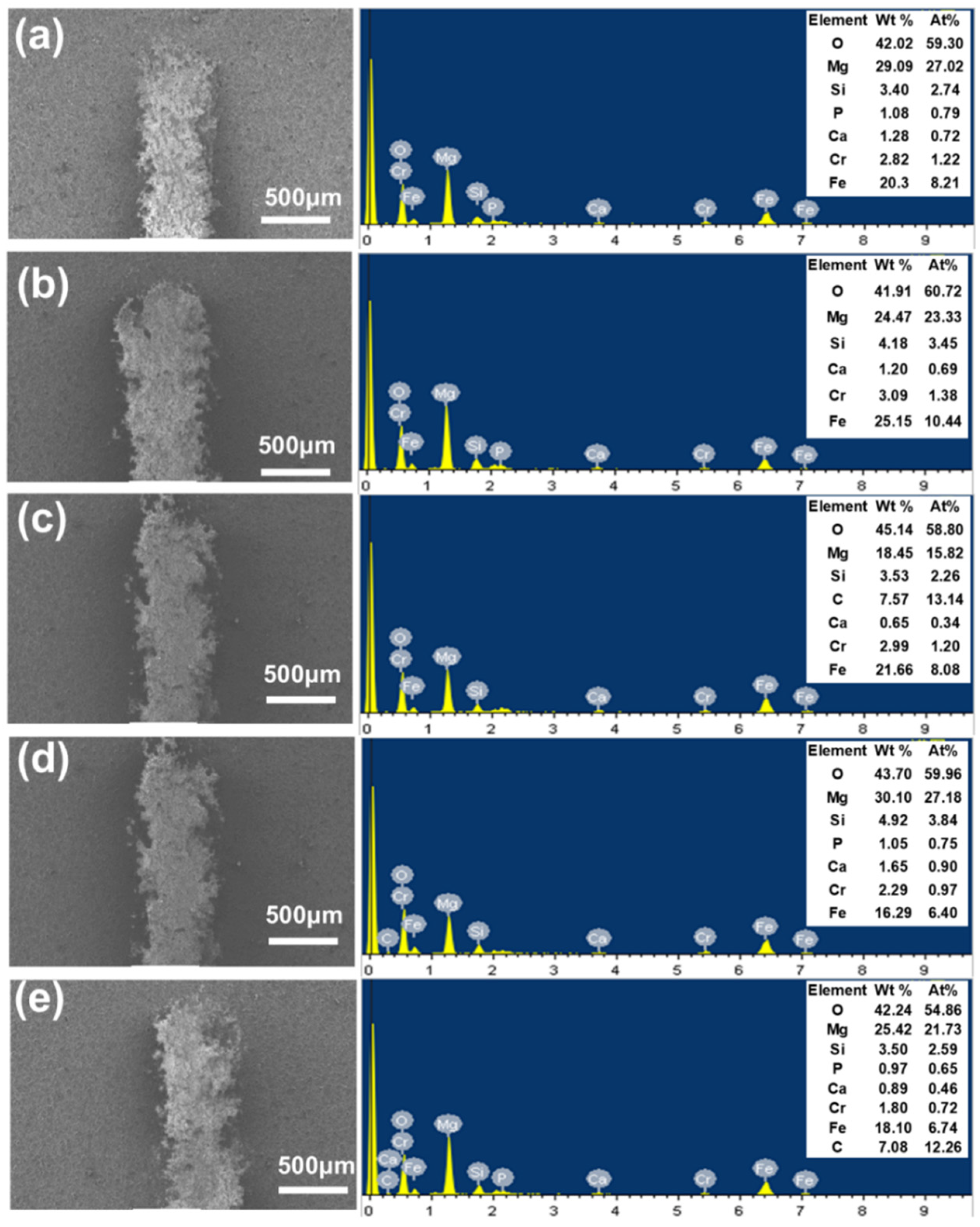
3.2. Phase and Elemental Analysis of MAO Coating
3.3. Properties of MAO Coating
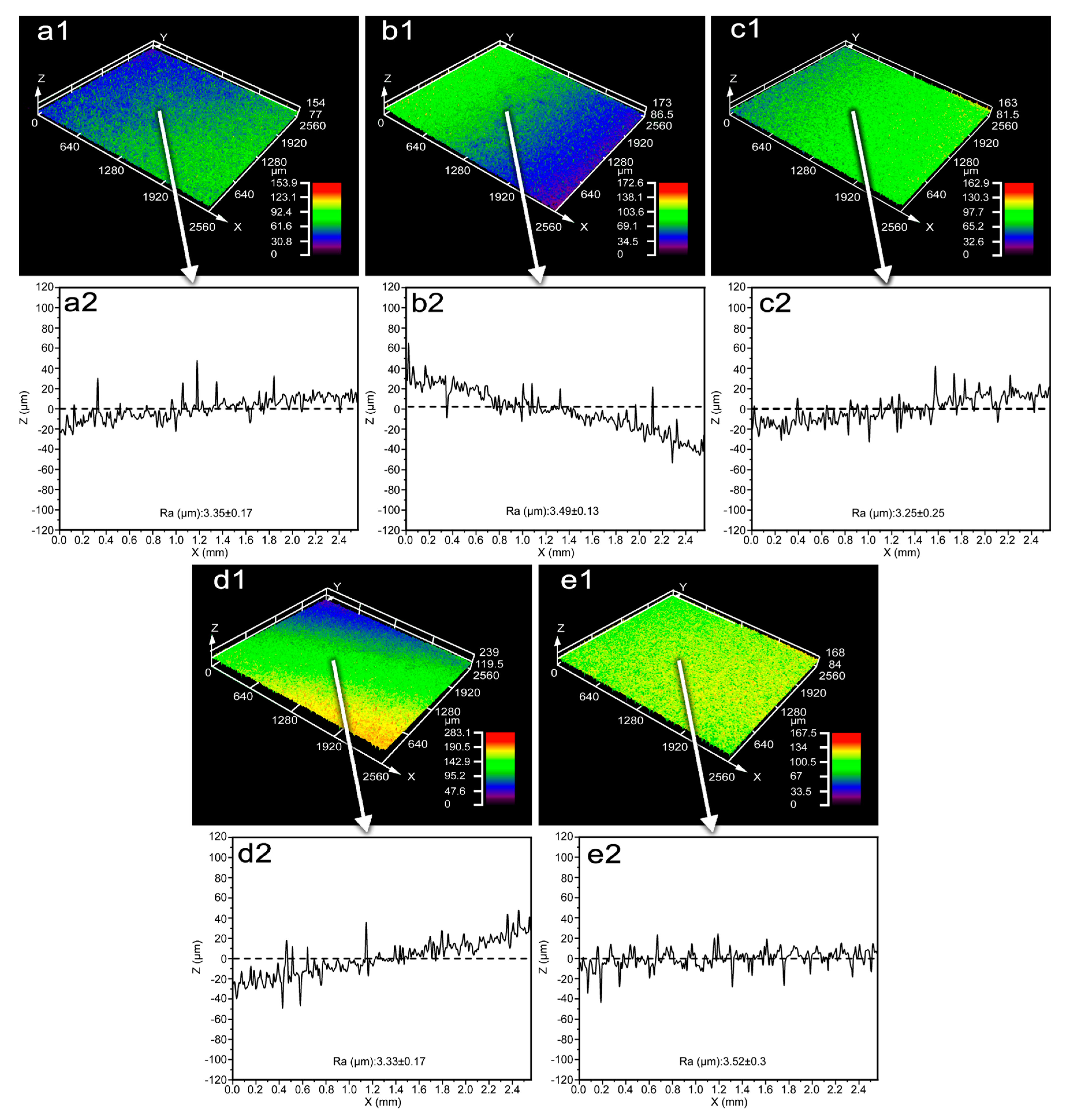
3.3.1. Surface Roughness of MAO Coating
3.3.2. Wear Resistance of MAO Coating
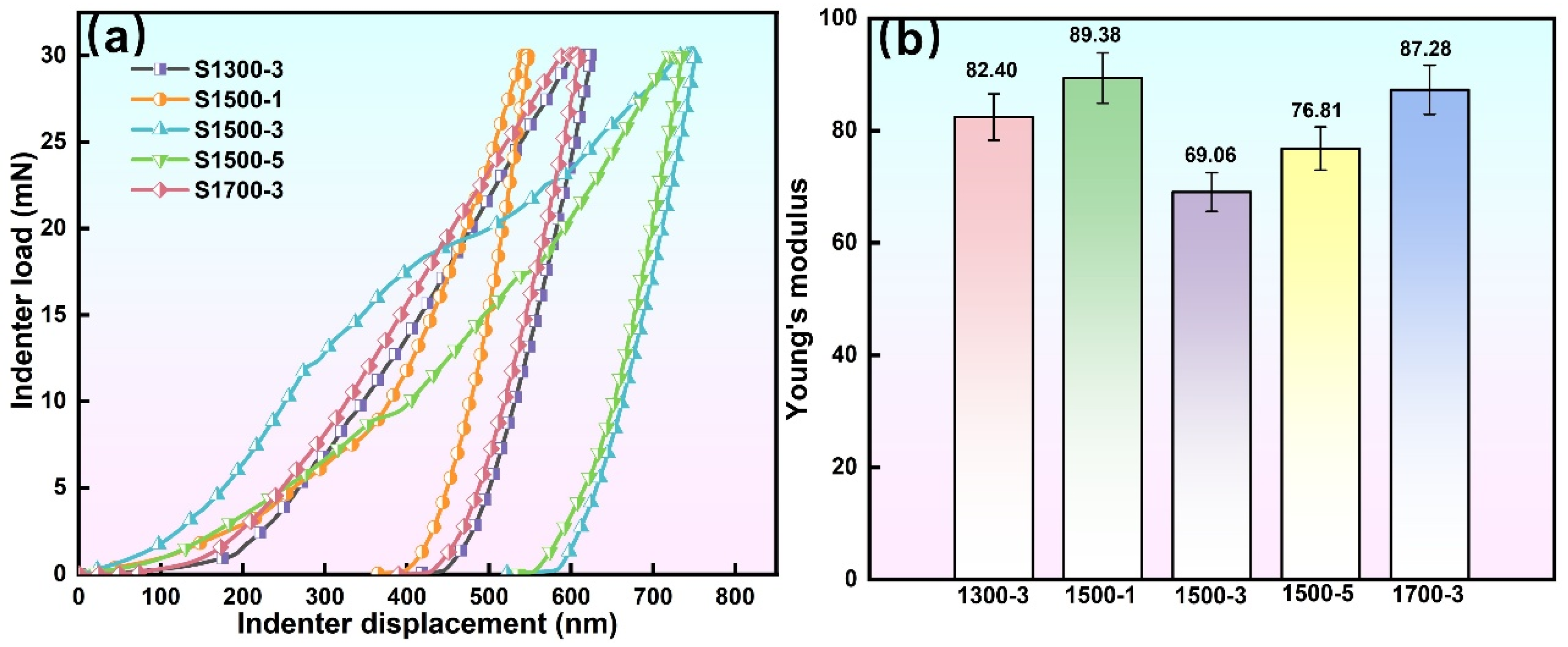
3.3.3. Elastic Modulus and Adhesion Strength of MAO Coating
3.3.4. Corrosion Resistance of MAO Coating
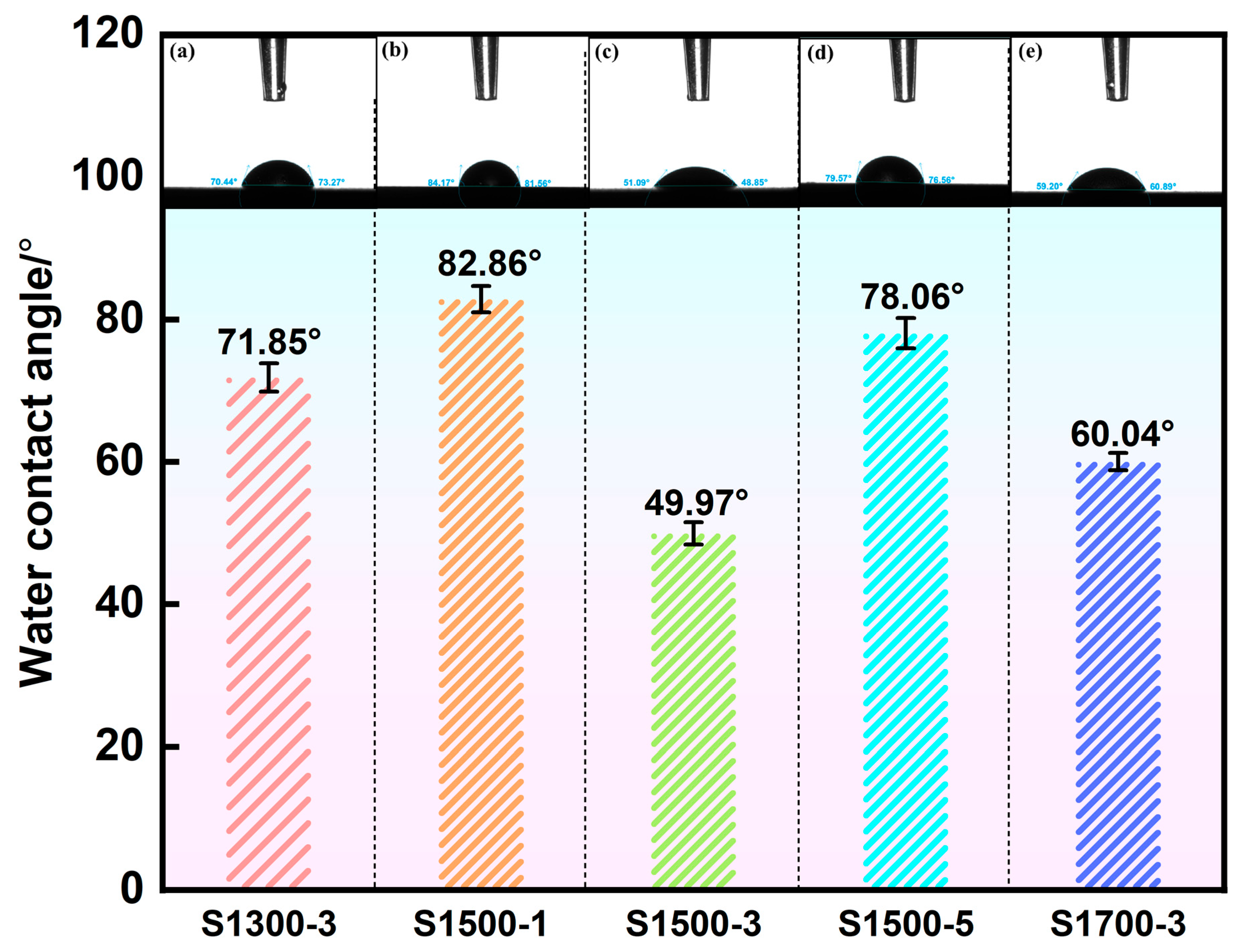
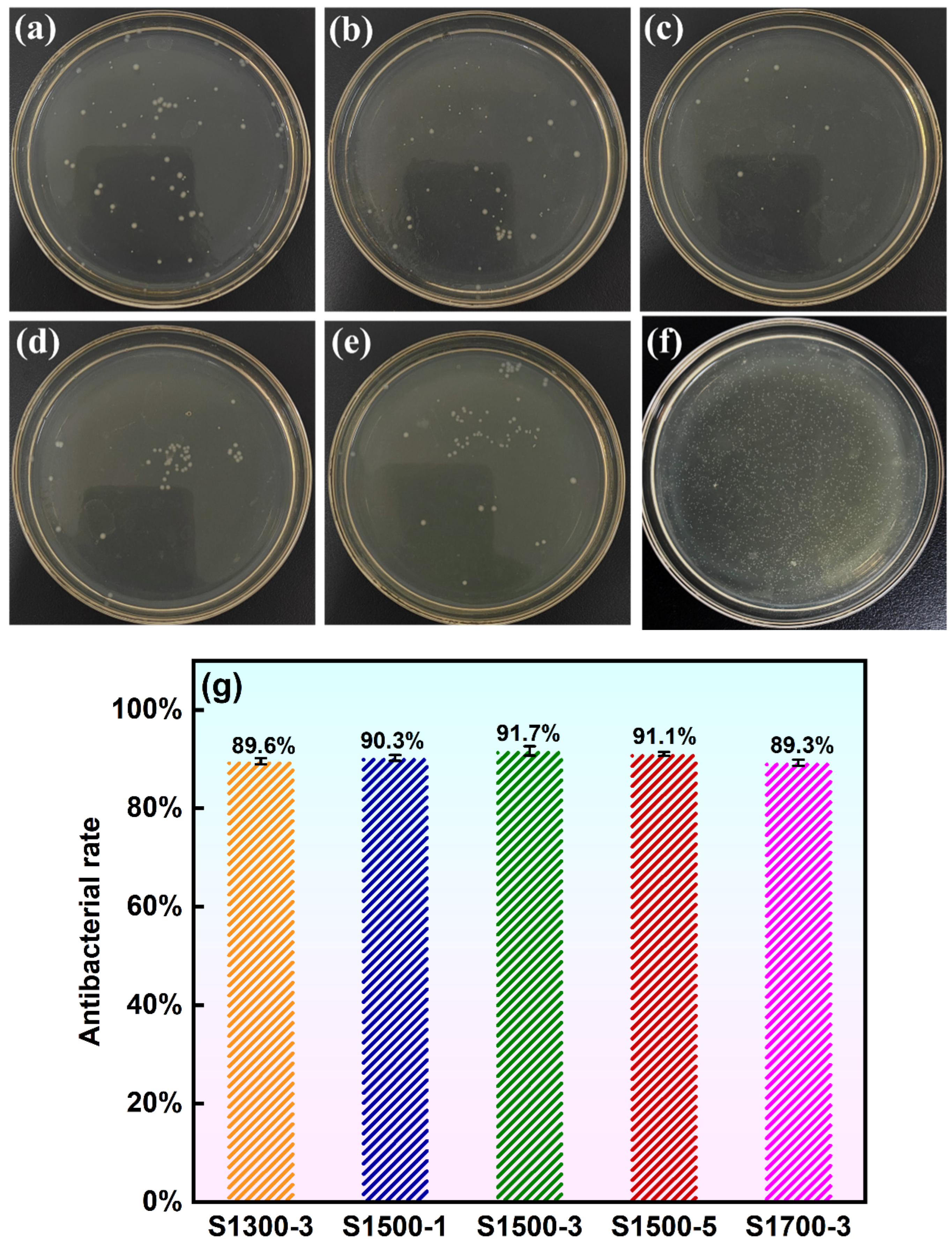
3.3.5. Wettability and Antibacterial Properties of MAO Coating
4. Conclusions
- All MAO coatings fabricated on the magnesium alloy substrates exhibited dense and uniform gray–white appearances. The surface microstructures were characterized by porous “volcanic crater” features with pore diameters of approximately 2.6–12 μm, containing two typical types of micropores, namely “pore-in-pore” and “repaired blind pore” structures, together with a few fine microcracks. Cross-sectional examination demonstrates excellent metallurgical bonding at the coating–substrate interface, with only a small number of blind pores and microcracks observed.
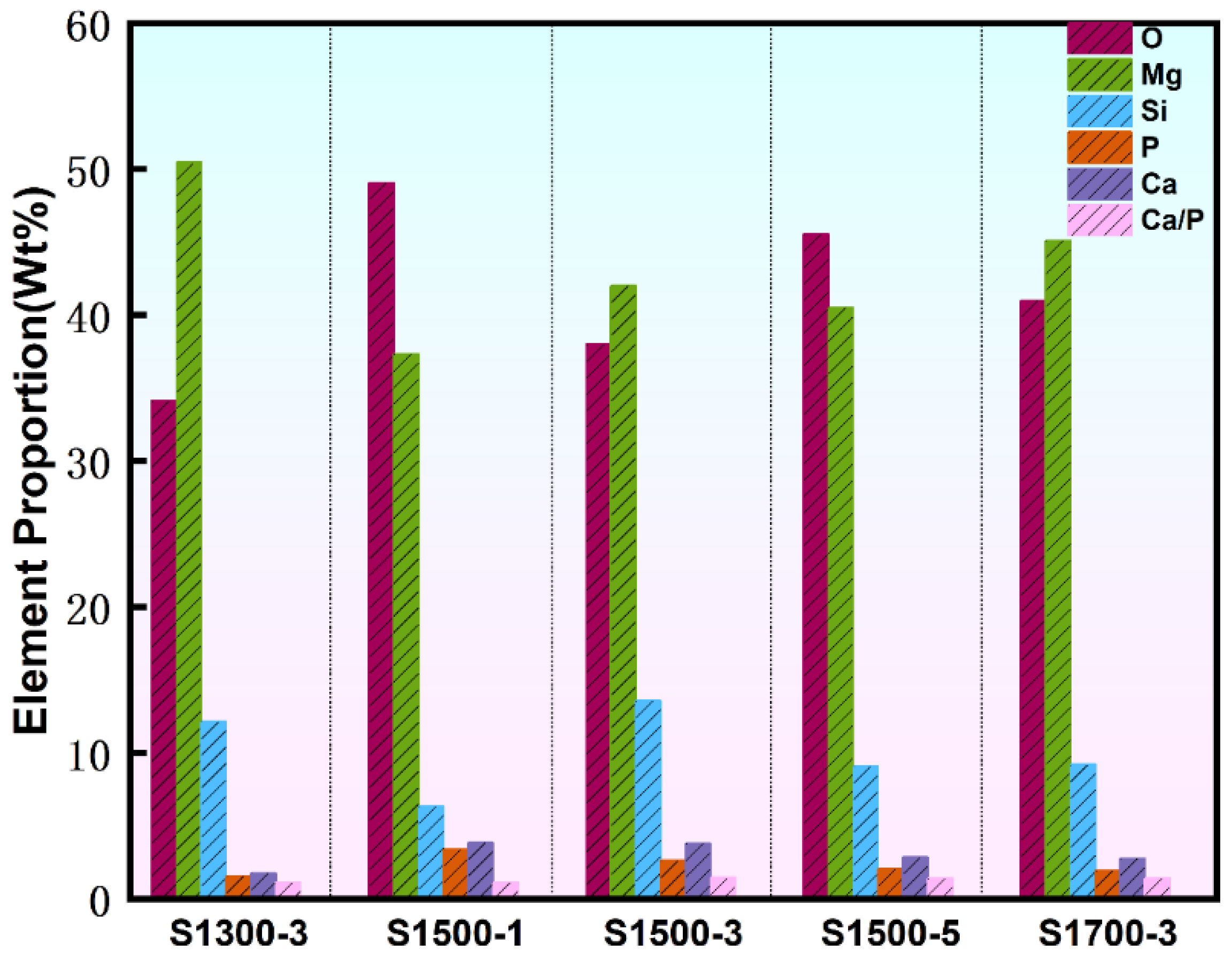
- XRD analysis indicates that Ca3(PO4)2, CaCO3, Mg, Mg2SiO3, MgO, and Ca2P2O7 are the main phases of the coating. MgO and Mg2SiO3 phases form a multiphase composite structure in the MAO coating, enhancing the hardness and resistance to plastic deformation of the surface material by impeding dislocation motion and microcrack propagation. Elemental analysis showed that the coating formed under the S1500-3 condition had the highest RCa/P of 1.44. This indicates that the uniformly dispersed HA particles in the substrate promoted the release of Ca2+ and PO43− ions during the MAO process. The enhanced ion transport increased the Ca/P ratio in the coating, thereby improving its biological activity and promoting osteoblast adhesion and growth on the surface.
- Performance evaluation showed that all MAO coatings possessed comparable surface roughness, with an average value of about 3.25 μm. The coatings exhibited excellent wear resistance and strong adhesion, with the highest adhesion strength reaching 14.485 N. In addition, all coatings demonstrated excellent corrosion resistance. Wettability and antibacterial tests further confirmed their hydrophilic nature, and the coatings produced under the S1500-3 and S1700-3 conditions exhibited moderate hydrophilicity, which is more conducive to cell adhesion and spreading during in vivo implantation. The antibacterial rate of the coatings reached as high as 91.7%, satisfying the short-term antibacterial criterion for biomedical implants.
- Thus, the MAO coating formed on the substrate processed under the S1500-3 condition showed the most balanced and optimal performance, combining excellent mechanical integrity, corrosion resistance, hydrophilicity, and antibacterial activity. This study provides theoretical support for the application of biomedical magnesium alloys as in vivo implant materials and offers data backing for further advancing their clinical translation.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Rajendran, S.; Ranganathan, B.; Karuppasamy, K. Predictive Modeling and Optimization of Abrasive Waterjet Drilling (AWJD) Parameters on AZ31B-Mg Alloy using Neural Networks and Grey Relational Analysis. Rev. Metal. 2025, 60, e261. [Google Scholar] [CrossRef]
- Sharma, S.K.; Gajevic, S.; Sharma, L.K.; Pradhan, R.; Miladinovic, S.; Asonja, A.; Stojanovic, B. Magnesium-Titanium Alloys: A Promising Solution for Biodegradable Biomedical Implants. Materials 2024, 17, 5157. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.z.; Yang, C.l.; Liu, Y.j.; Yuan, F.s.; Liang, S.s.; Li, H.x.; Zhang, J.s. Microstructure, mechanical, and corrosion properties of extruded low-alloyed Mg-xZn-0.2Ca alloys. Int. J. Miner. Metall. Mater. 2019, 26, 1274–1284. [Google Scholar] [CrossRef]
- Eivani, A.R.; Tabatabaei, F.; Khavandi, A.R.; Tajabadi, M.; Mehdizade, M.; Jafarian, H.R.; Zhou, J. The effect of addition of hardystonite on the strength, ductility and corrosion resistance of WE43 magnesium alloy. J. Mater. Res. Technol. 2021, 13, 1855–1865. [Google Scholar] [CrossRef]
- Sharan Krishna, R.S.; Muhammad Rabeeh, V.P.; Rahim, S.A.; Joseph, M.A.; Hanas, T. Effect of grain refinement on biomineralization and biodegradation of Mg–Ca alloy. J. Mater. Res. 2023, 38, 4772–4783. [Google Scholar] [CrossRef]
- Johari, M.; Jabbari, A.H.; Sedighi, M. High cycle fatigue and corrosion behaviors of Mg3Zn/HA biodegradable composite. J. Mater. Res. Technol. 2024, 28, 695–706. [Google Scholar] [CrossRef]
- Liu, D.; Yang, D.; Li, X.; Hu, S. Mechanical properties, corrosion resistance and biocompatibilities of degradable Mg-RE alloys: A review. J. Mater. Res. Technol. 2019, 8, 1538–1549. [Google Scholar] [CrossRef]
- Garimella, A.; Bandhu Ghosh, S.; Bandyopadhyay-Ghosh, S. Composite bone-implant engineered with magnesium and variable degradation for orthopaedics. Mater. Today Proc. 2023, 28. [Google Scholar] [CrossRef]
- Zhang, D.; Zhou, J.; Peng, F.; Tan, J.; Zhang, X.; Qian, S.; Qiao, Y.; Zhang, Y.; Liu, X. Mg-Fe LDH sealed PEO coating on magnesium for biodegradation control, antibacteria and osteogenesis. J. Mater. Sci. Technol. 2022, 105, 57–67. [Google Scholar] [CrossRef]
- Wang, J.; Witte, F.; Xi, T.; Zheng, Y.; Yang, K.; Yang, Y.; Zhao, D.; Meng, J.; Li, Y.; Li, W.; et al. Recommendation for modifying current cytotoxicity testing standards for biodegradable magnesium-based materials. Acta Biomater. 2015, 21, 237–249. [Google Scholar] [CrossRef]
- Kumar, R.; Katyal, P. Effects of alloying elements on performance of biodegradable magnesium alloy. Mater. Today Proc. 2022, 56, 2443–2450. [Google Scholar] [CrossRef]
- Bairagi, D.; Mandal, S. A comprehensive review on biocompatible Mg-based alloys as temporary orthopaedic implants: Current status, challenges, and future prospects. J. Magnes. Alloys 2022, 10, 627–669. [Google Scholar] [CrossRef]
- Guo, L.; Yu, B.; Zhou, P.; Zhang, T.; Wang, F. Fabrication of low-cost Ni-P composite coating on Mg alloys with a significant improvement of corrosion resistance: Critical role of mitigating the galvanic contact between the substrate and the coating. Corros. Sci. 2021, 183, 109329. [Google Scholar] [CrossRef]
- Wong, H.M.; Zhao, Y.; Tam, V.; Wu, S.; Chu, P.K.; Zheng, Y.; To, M.K.T.; Leung, F.K.L.; Luk, K.D.K.; Cheung, K.M.C.; et al. In vivo stimulation of bone formation by aluminum and oxygen plasma surface-modified magnesium implants. Biomaterials 2013, 34, 9863–9876. [Google Scholar] [CrossRef] [PubMed]
- Campo, R.d.; Savoini, B.; Muñoz, A.; Monge, M.A.; Garcés, G. Mechanical properties and corrosion behavior of Mg–HAP composites. J. Mech. Behav. Biomed. Mater. 2014, 39, 238–246. [Google Scholar] [CrossRef] [PubMed]
- Khalajabadi, S.Z.; Abdul Kadir, M.R.; Izman, S.; Ebrahimi-Kahrizsangi, R. Fabrication, bio-corrosion behavior and mechanical properties of a Mg/HA/MgO nanocomposite for biomedical applications. Mater. Des. 2015, 88, 1223–1233. [Google Scholar] [CrossRef]
- Xiong, G.; Nie, Y.; Ji, D.; Li, J.; Li, C.; Li, W.; Zhu, Y.; Luo, H.; Wan, Y. Characterization of biomedical hydroxyapatite/magnesium composites prepared by powder metallurgy assisted with microwave sintering. Curr. Appl. Phys. 2016, 16, 830–836. [Google Scholar] [CrossRef]
- Zhao, Y.q.; Yang, H.k.; Andriia, A.; Lo, H.h.; Li, J.x. Refill friction stir spot welding (RFSSW): A review of processing, similar/dissimilar materials joining, mechanical properties and fracture mechanism. J. Iron Steel Res. Int. 2024, 31, 1825–1839. [Google Scholar] [CrossRef]
- Wu, D.c.; Wang, F.f.; Li, S.; Wang, W.q.; Wang, D.; Li, Y.l.; Miao, T. Microstructural and nanomechanical behavior of friction stir welded dissimilar joint of AA2219-T6/AA2195-T8 alloys. J. Iron Steel Res. Int. 2024, 31, 3080–3094. [Google Scholar] [CrossRef]
- Karageorgiou, V.; Kaplan, D. Porosity of 3D biomaterial scaffolds and osteogenesis. Biomaterials 2005, 26, 5474–5491. [Google Scholar] [CrossRef]
- Vandana, B.; Syamala, P.; Venugopal, D.S.; Sk, S.R.K.I.; Venkateswarlu, B.; Jagannatham, M.; Kolenčík, M.; Ramakanth, I.; Dumpala, R.; Sunil, B.R. Magnesium/fish bone derived hydroxyapatite composites by friction stir processing: Studies on mechanical behaviour and corrosion resistance. Bull. Mater. Sci. 2019, 42, 1799. [Google Scholar] [CrossRef]
- Ratna Sunil, B.; Sampath Kumar, T.S.; Chakkingal, U.; Nandakumar, V.; Doble, M. Nano-hydroxyapatite reinforced AZ31 magnesium alloy by friction stir processing: A solid state processing for biodegradable metal matrix composites. J. Mater. Sci. Mater. Med. 2013, 25, 975–988. [Google Scholar] [CrossRef] [PubMed]
- Hanas, T.; Sampath Kumar, T.S.; Perumal, G.; Doble, M.; Ramakrishna, S. Electrospun PCL/HA coated friction stir processed AZ31/HA composites for degradable implant applications. J. Mater. Process. Technol. 2018, 252, 398–406. [Google Scholar] [CrossRef]
- Ratna Sunil, B.; Sampath Kumar, T.S.; Chakkingal, U.; Nandakumar, V.; Doble, M. Friction stir processing of magnesium–nanohydroxyapatite composites with controlled in vitro degradation behavior. Mater. Sci. Eng. C 2014, 39, 315–324. [Google Scholar] [CrossRef]
- Zhao, C.; Wu, H.; Hou, P.; Ni, J.; Han, P.; Zhang, X. Enhanced corrosion resistance and antibacterial property of Zn doped DCPD coating on biodegradable Mg. Mater. Lett. 2016, 180, 42–46. [Google Scholar] [CrossRef]
- Amaravathy, P.; Kumar, T.S.S. Bioactivity enhancement by Sr doped Zn-Ca-P coatings on biomedical magnesium alloy. J. Magnes. Alloys 2019, 7, 584–596. [Google Scholar] [CrossRef]
- Xie, Y.; Wang, X.; Cui, S.; Hu, J.; Wei, Y.; Lian, Y.; Xuan, A.; Yu, B.; Zhang, E. Effect of micro-arc oxidation on antimicrobial properties and biocompatibility of biomedical Ti-xFe alloys. Surf. Coat. Technol. 2024, 476, 130174. [Google Scholar] [CrossRef]
- Tian, P.; Xu, D.; Liu, X. Mussel-inspired functionalization of PEO/PCL composite coating on a biodegradable AZ31 magnesium alloy. Colloids Surf. B Biointerfaces 2016, 141, 327–337. [Google Scholar] [CrossRef]
- Wang, Z.X.; Zhang, J.W.; Ye, F.; Lv, W.G.; Lu, S.; Sun, L.; Jiang, X.Z. Properties of Micro-Arc Oxidation Coating Fabricated on Magnesium Under Two Steps Current-Decreasing Mode. Front. Mater. 2020, 7, 261. [Google Scholar] [CrossRef]
- Li, W.; Liu, X.; Deng, Z.; Chen, Y.; Yu, Q.; Tang, W.; Sun, T.L.; Zhang, Y.S.; Yue, K. Tough Bonding, On-Demand Debonding, and Facile Rebonding between Hydrogels and Diverse Metal Surfaces. Adv. Mater. 2019, 31, 732. [Google Scholar] [CrossRef]
- Yang, X.; Li, M.; Lin, X.; Tan, L.; Lan, G.; Li, L.; Yin, Q.; Xia, H.; Zhang, Y.; Yang, K. Enhanced in vitro biocompatibility/bioactivity of biodegradable Mg–Zn–Zr alloy by micro-arc oxidation coating contained Mg2SiO4. Surf. Coat. Technol. 2013, 233, 65–73. [Google Scholar] [CrossRef]
- Chen, Y.; Dou, J.; Pang, Z.; Zheng, Z.; Yu, H.; Chen, C. Ag-containing antibacterial self-healing micro-arc oxidation coatings on Mg–Zn–Sr alloys. Surf. Eng. 2020, 37, 926–941. [Google Scholar] [CrossRef]
- Xiong, Y.; Yu, Q.; Jiang, Y. An experimental study of cyclic plastic deformation of extruded ZK60 magnesium alloy under uniaxial loading at room temperature. Int. J. Plast. 2014, 53, 107–124. [Google Scholar] [CrossRef]
- Zhou, S.f.; Chen, L.y.; Lv, W.g.; Gu, J.j.; Ye, F.; Oleksandr, D.; Lu, S.; Wang, Z.x. Growth pattern of MAO coating under constant voltage–current two-step power mode. J. Iron Steel Res. Int. 2025, 32, 1245–1262. [Google Scholar] [CrossRef]
- Zhou, S.F.; Lu, S.; Lv, W.G.; Wang, Z.X.; Oleksandr, D.; Gu, J.J.; Zhang, J.W.; Chen, L.Y. Influence of NaAlO2 Concentration on the Characteristics of Micro-Arc Oxidation Coating Fabricated on a ZK60 Magnesium Alloy. Coatings 2024, 14, 353. [Google Scholar] [CrossRef]
- Hussein, R.O.; Nie, X.; Northwood, D.O. An investigation of ceramic coating growth mechanisms in plasma electrolytic oxidation (PEO) processing. Electrochim. Acta 2013, 112, 111–119. [Google Scholar] [CrossRef]
- Veys-Renaux, D.; Rocca, E.; Martin, J.; Henrion, G. Initial stages of AZ91 Mg alloy micro-arc anodizing: Growth mechanisms and effect on the corrosion resistance. Electrochim. Acta 2014, 124, 36–45. [Google Scholar] [CrossRef]
- He, L.J.; Shao, Y.; Li, S.Q.; Cui, L.Y.; Ji, X.J.; Zhao, Y.B.; Zeng, R.C. Advances in layer-by-layer self-assembled coatings upon biodegradable magnesium alloys. Sci. China Mater. 2021, 64, 2093–2106. [Google Scholar] [CrossRef]
- Barati Darband, G.; Aliofkhazraei, M.; Hamghalam, P.; Valizade, N. Plasma electrolytic oxidation of magnesium and its alloys: Mechanism, properties and applications. J. Magnes. Alloys 2017, 5, 74–132. [Google Scholar] [CrossRef]
- Pillai, A.M.; Ghosh, R.; Dey, A.; Prajwal, K.; Rajendra, A.; Sharma, A.K.; Sampath, S. Crystalline and amorphous PEO based ceramic coatings on AA6061: Nanoindentation and corrosion studies. Ceram. Int. 2021, 47, 14707–14716. [Google Scholar] [CrossRef]
- Liu, X.; Zhu, L.; Liu, H.; Li, W. Investigation of MAO coating growth mechanism on aluminum alloy by two-step oxidation method. Appl. Surf. Sci. 2014, 293, 12–17. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, M.; Zhao, Y. Preparation and Corrosion Resistance of Microarc Oxidation-Coated Biomedical Mg–Zn–Ca Alloy in the Silicon–Phosphorus-Mixed Electrolyte. ACS Omega 2019, 4, 20937–20947. [Google Scholar] [CrossRef]
- Pratama, Y.A.; Marhaeny, H.D.; Deapsari, F.; Budiatin, A.S.; Rahmadi, M.; Miatmoko, A.; Taher, M.; Khotib, J. Development of Hydroxyapatite as a Bone Implant Biomaterial for Triggering Osteogenesis. Eur. J. Dent. 2025, ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Gokcekaya, O.; Ergun, C.; Webster, T.J.; Bahadir, A.; Ueda, K.; Narushima, T.; Nakano, T. Effect of Precursor Deficiency Induced Ca/P Ratio on Antibacterial and Osteoblast Adhesion Properties of Ag-Incorporated Hydroxyapatite: Reducing Ag Toxicity. Materials 2021, 14, 3158. [Google Scholar] [CrossRef]
- Fazel, M.; Shamanian, M.; Salimijazi, H.R. Enhanced corrosion and tribocorrosion behavior of Ti6Al4V alloy by auto–sealed micro-arc oxidation layers. Biotribology 2020, 23, 100131. [Google Scholar] [CrossRef]
- Atkins, D.J.; Rogers, A.E.; Shaffer, K.E.; Moore, I.; Miller, W.D.; Morrissey, M.A.; Pitenis, A.A. Pro-Inflammatory Response to Macrotextured Silicone Implant Wear Debris. Tribol. Lett. 2025, 73, 1965. [Google Scholar] [CrossRef]
- Xie, J.; Zhang, T.; Jiang, J.; Xue, W.; Wang, W.; Ni, J.; Zhang, X.; Liu, X. Advances in magnesium-based implants for biomedical applications: A comprehensive review and future perspectives. J. Magnes. Alloys 2025, 13, 2978–3003. [Google Scholar] [CrossRef]
- Ma, H.; Miao, Q.; Liang, W.; Sun, S.; Qi, Y.; Jia, F.; Chang, X. Wear Behavior of TiN/TiAlSiN Nanocomposite Multilayer Coatings from Ambient Temperature to Medium Temperature. Coatings 2024, 14, 1139. [Google Scholar] [CrossRef]
- Zeng, R.C.; Cui, L.y.; Jiang, K.; Liu, R.; Zhao, B.D.; Zheng, Y.F. In Vitro Corrosion and Cytocompatibility of a Microarc Oxidation Coating and Poly(l-lactic acid) Composite Coating on Mg–1Li–1Ca Alloy for Orthopedic Implants. ACS Appl. Mater. Interfaces 2016, 8, 10014–10028. [Google Scholar] [CrossRef]
- Curioni, M.; Salamone, L.; Scenini, F.; Santamaria, M.; Di Natale, M. A mathematical description accounting for the superfluous hydrogen evolution and the inductive behaviour observed during electrochemical measurements on magnesium. Electrochim. Acta 2018, 274, 343–352. [Google Scholar] [CrossRef]
- Akbari, E.; Di Franco, F.; Ceraolo, P.; Raeissi, K.; Santamaria, M.; Hakimizad, A. Electrochemically-induced TiO2 incorporation for enhancing corrosion and tribocorrosion resistance of PEO coating on 7075 Al alloy. Corros. Sci. 2018, 143, 314–328. [Google Scholar] [CrossRef]
- Qiao, Y.X.; Zheng, Y.G.; Ke, W.; Okafor, P.C. Electrochemical behaviour of high nitrogen stainless steel in acidic solutions. Corros. Sci. 2009, 51, 979–986. [Google Scholar] [CrossRef]
- Zhu, H.j.; Han, J.y.; Fang, K.w.; Zheng, Z.b.; Zhou, H.l.; Yang, L.l.; Qiao, Y.x.; Chen, J.; Cui, J.; Wang, Q. Corrosion behavior of CoCrCu0.1FeMoNi high entropy alloy in 0.5 mol/L NaOH solution. J. Iron Steel Res. Int. 2025, 32, 1163–1175. [Google Scholar] [CrossRef]
- Wang, Z.X.; Zhang, Z.Y.; Lv, W.G.; Gan, J.J.; Lu, S. Optimization of Duty Cycle and Frequency Parameters of ZK60 Magnesium Alloy under Two-Step Voltage-Increasing Mode. J. Mater. Eng. Perform. 2022, 32, 2084–2096. [Google Scholar] [CrossRef]
- Parfenov, E.V.; Parfenova, L.V.; Dyakonov, G.S.; Danilko, K.V.; Mukaeva, V.R.; Farrakhov, R.G.; Lukina, E.S.; Valiev, R.Z. Surface functionalization via PEO coating and RGD peptide for nanostructured titanium implants and their in vitro assessment. Surf. Coat. Technol. 2019, 357, 669–683. [Google Scholar] [CrossRef]
- Weldemhret, T.G.; Park, Y.T.; Song, J.I. Recent progress in surface engineering methods and advanced applications of flexible polymeric foams. Adv. Colloid Interface Sci. 2024, 326, 103132. [Google Scholar] [CrossRef]
- Greiner, A.M.; Sales, A.; Chen, H.; Biela, S.A.; Kaufmann, D.; Kemkemer, R. Nano- and microstructured materials for in vitro studies of the physiology of vascular cells. Beilstein J. Nanotechnol. 2016, 7, 1620–1641. [Google Scholar] [CrossRef]
- Jiang, H.; Wang, J.; Chen, M.; Liu, D. Biological activity evaluation of magnesium fluoride coated Mg-Zn-Zr alloy in vivo. Mater. Sci. Eng. C 2017, 75, 1068–1074. [Google Scholar] [CrossRef]
- Yirijor, J.; Danyuo, Y.; Salifu, A.A.; Ezenwafor, T.; McBagonluri, F. Surface coating and wettability study of PDMS-based composites: Effect on contact angle and cell-surface interaction. MRS Adv. 2022, 7, 656–662. [Google Scholar] [CrossRef]
- Gu, Y.; Bandopadhyay, S.; Chen, C.f.; Ning, C.y.; Guo, Y.j. Long-term corrosion inhibition mechanism of microarc oxidation coated AZ31 Mg alloys for biomedical applications. Mater. Des. (1980–2015) 2013, 46, 66–75. [Google Scholar] [CrossRef]
- Chen, X.; Chen, Y.; Zhang, L.; Liu, Z.; Qiu, E.; Liu, Q.; Regulacio, M.D.; Lin, C.; Yang, D.-P. Hydrophilic ZnO/C nanocomposites with superior adsorption, photocatalytic, and photo-enhanced antibacterial properties for synergistic water purification. J. Colloid Interface Sci. 2023, 648, 535–550. [Google Scholar] [CrossRef] [PubMed]
- Xue, K.; Li, Y.J.; Ma, T.H.; Cui, L.Y.; Liu, C.b.; Zou, Y.h.; Li, S.Q.; Zhang, F.; Zeng, R.C. In vitro corrosion resistance and dual antibacterial ability of curcumin loaded composite coatings on AZ31 alloy: Effect of amorphous calcium carbonate. J. Colloid Interface Sci. 2023, 649, 867–879. [Google Scholar] [CrossRef] [PubMed]
- Munir, M.U.; Ihsan, A.; Javed, I.; Ansari, M.T.; Bajwa, S.Z.; Bukhari, S.N.A.; Ahmed, A.; Malik, M.Z.; Khan, W.S. Controllably Biodegradable Hydroxyapatite Nanostructures for Cefazolin Delivery against Antibacterial Resistance. ACS Omega 2019, 4, 7524–7532. [Google Scholar] [CrossRef]





| Sample | Tool Rotational Speed (mm/min) | FSP Pass | Tool Traverse Speed (mm/min) | Tool Tilt Angle |
|---|---|---|---|---|
| S1300-3 | 1300 | 3 | 100 mm/min | 2.5° |
| S1500-3 | 1500 | 3 | 100 mm/min | 2.5° |
| S1700-3 | 1700 | 3 | 100 mm/min | 2.5° |
| S1500-1 | 1500 | 1 | 100 mm/min | 2.5° |
| S1500-3 | 1500 | 3 | 100 mm/min | 2.5° |
| S1500-5 | 1500 | 5 | 100 mm/min | 2.5° |
| Electrical Parameters | Constant Voltage Mode | Constant Current Mode |
|---|---|---|
| Time | 0–5 min | 6–15 min |
| Current | - | 1.5 A |
| Voltage | 400 V | - |
| Positive Duty Cycle | 30% | 30% |
| Negative Duty Cycle | 70% | 70% |
| Frequency | 600 Hz | 600 Hz |
| Processing Condition/Elemental Composition | O | Mg | Si | P | Ca | Ca/P |
|---|---|---|---|---|---|---|
| S1300-3 | 34.13 | 50.44 | 12.13 | 1.55 | 1.75 | 1.13 |
| S1500-1 | 49.07 | 37.33 | 6.33 | 3.40 | 3.86 | 1.14 |
| S1500-3 | 37.99 | 42.01 | 13.58 | 2.63 | 3.79 | 1.44 |
| S1500-5 | 45.54 | 40.50 | 9.07 | 2.05 | 2.85 | 1.39 |
| S1700-3 | 41.01 | 45.10 | 9.19 | 1.93 | 2.76 | 1.43 |
| FSP | Rs (Ω·cm2) | Rc (Ω·cm2) | Rct (Ω·cm2) |
|---|---|---|---|
| S1300-3 | 35.81 | 136.3 | 1.50 × 104 |
| S1500-1 | 20.15 | 100 | 1.03 × 104 |
| S1500-3 | 30.36 | 1000 | 2.22 × 104 |
| S1500-5 | 10 | 297.6 | 1.98 × 104 |
| S1700-3 | 10 | 502.7 | 2.05 × 104 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lv, W.; Wang, Z.; Xiao, Z.; Zhao, Y.; Ma, J.; Chen, L.; Lu, S.; Oleksandr, D. Preparation and Properties of Micro-Arc Oxidation Coatings on Friction-Stir-Processed ZK60 Mg Alloys with Hydroxyapatite Particles. Coatings 2025, 15, 1362. https://doi.org/10.3390/coatings15121362
Lv W, Wang Z, Xiao Z, Zhao Y, Ma J, Chen L, Lu S, Oleksandr D. Preparation and Properties of Micro-Arc Oxidation Coatings on Friction-Stir-Processed ZK60 Mg Alloys with Hydroxyapatite Particles. Coatings. 2025; 15(12):1362. https://doi.org/10.3390/coatings15121362
Chicago/Turabian StyleLv, Weigang, Zexin Wang, Zimeng Xiao, Youna Zhao, Jun Ma, Liangyu Chen, Sheng Lu, and Dubovyy Oleksandr. 2025. "Preparation and Properties of Micro-Arc Oxidation Coatings on Friction-Stir-Processed ZK60 Mg Alloys with Hydroxyapatite Particles" Coatings 15, no. 12: 1362. https://doi.org/10.3390/coatings15121362
APA StyleLv, W., Wang, Z., Xiao, Z., Zhao, Y., Ma, J., Chen, L., Lu, S., & Oleksandr, D. (2025). Preparation and Properties of Micro-Arc Oxidation Coatings on Friction-Stir-Processed ZK60 Mg Alloys with Hydroxyapatite Particles. Coatings, 15(12), 1362. https://doi.org/10.3390/coatings15121362







