Electrodeposition of Copper-Based Nickel–Graphene Coatings: Effect of Current Density on Microstructure and Properties
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
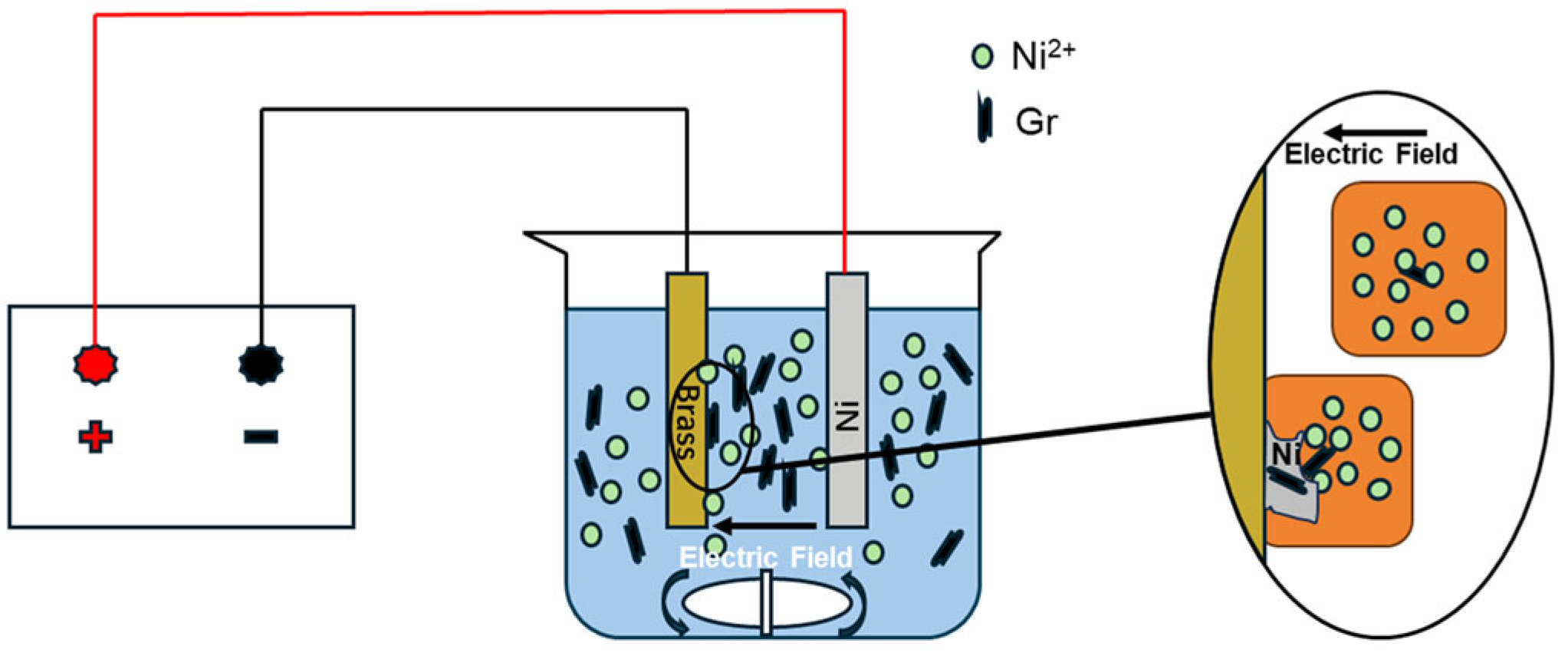
2.2. Preparation
2.3. Characterization
3. Results and Discussion
3.1. Effect of Current Density on Coating Microstructure
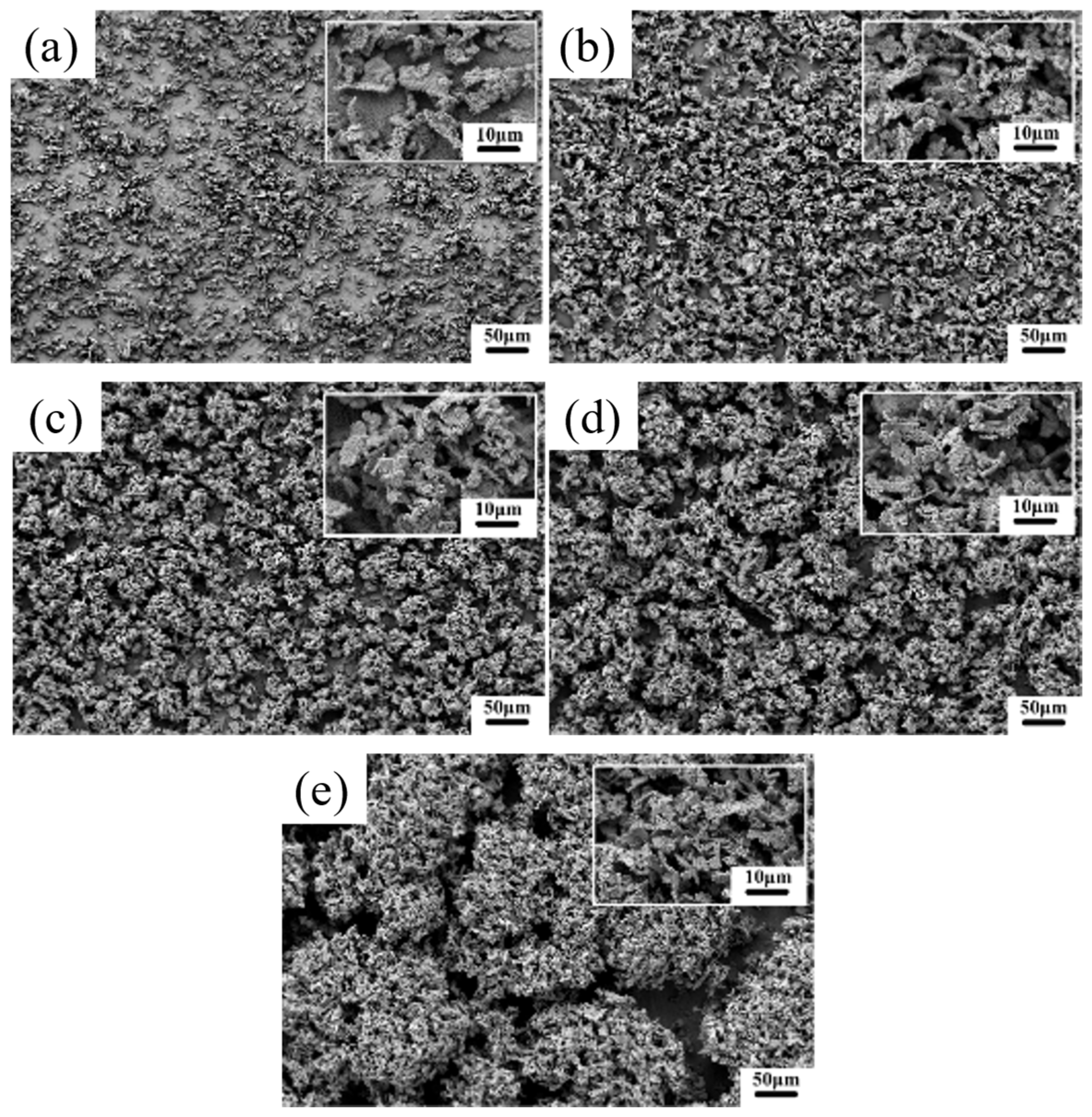
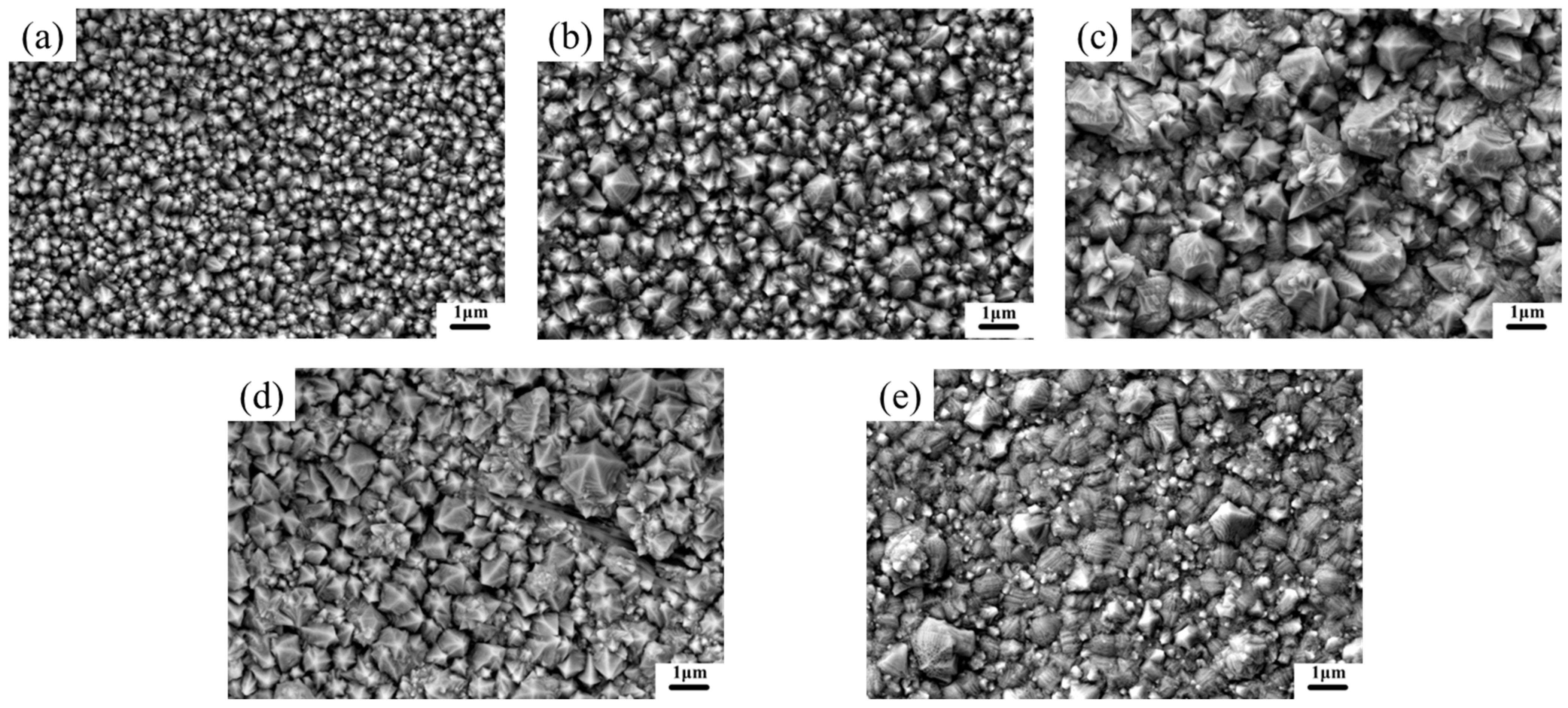
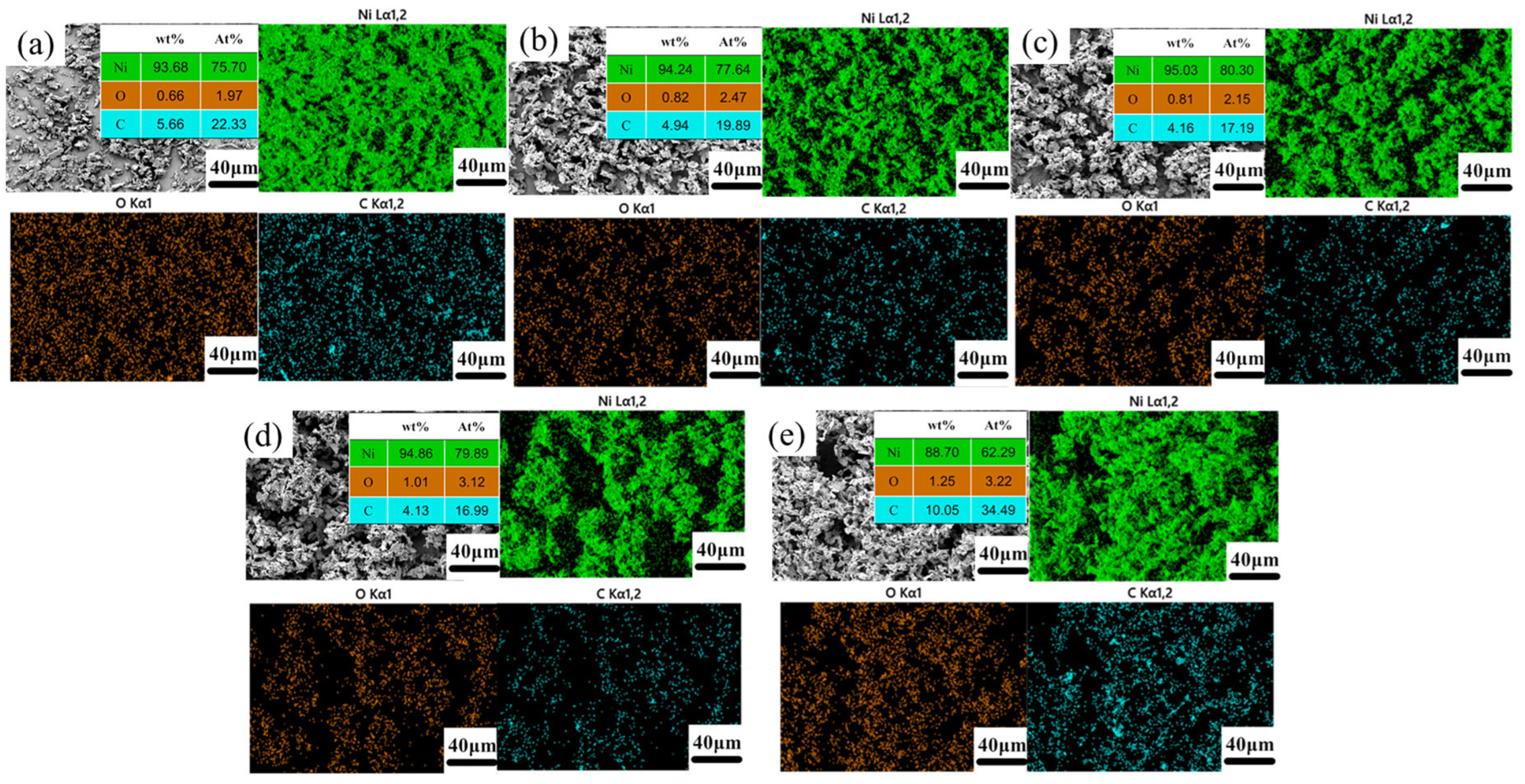
3.1.1. Surface Morphology
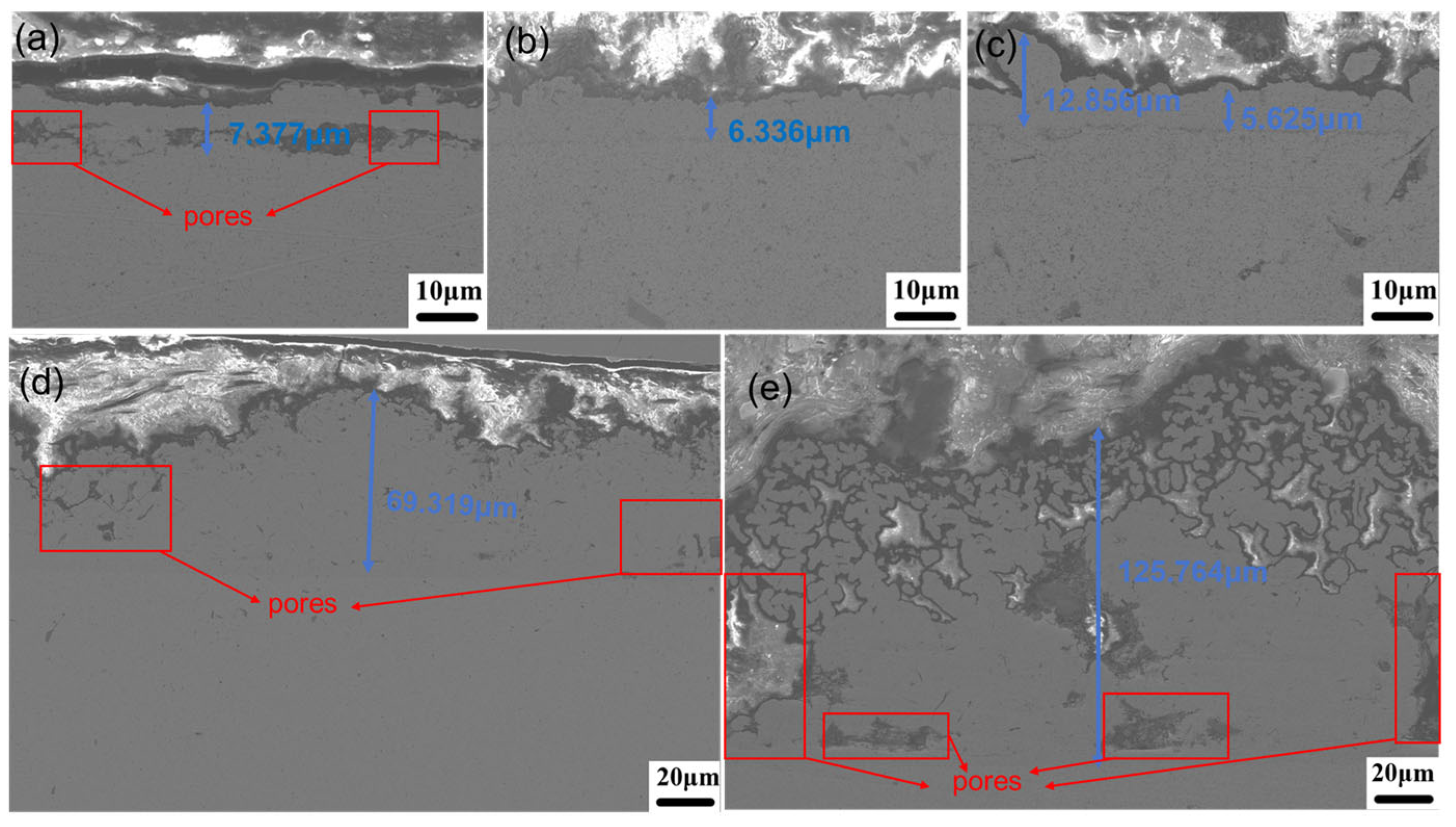
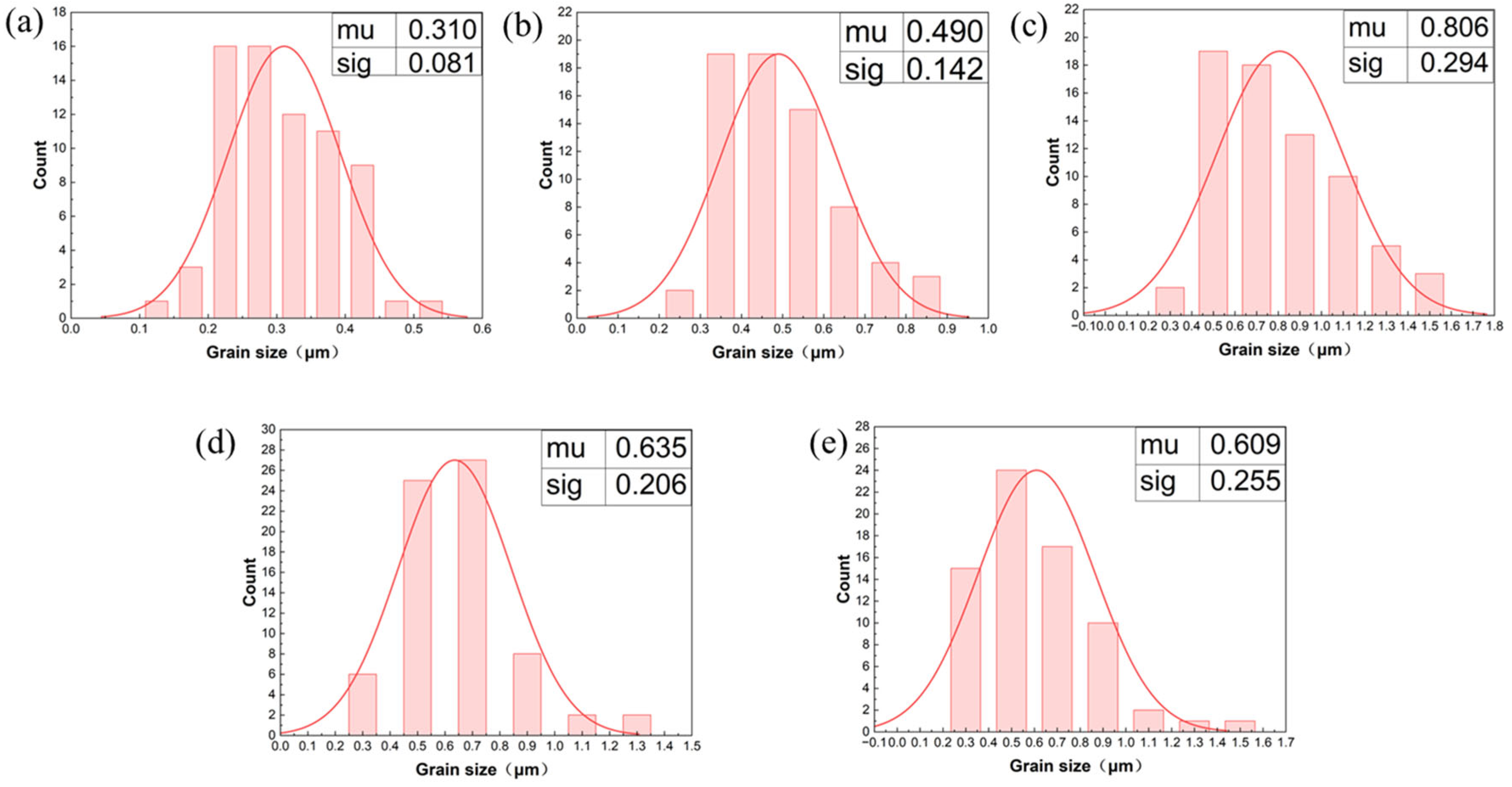
3.1.2. Microstructure
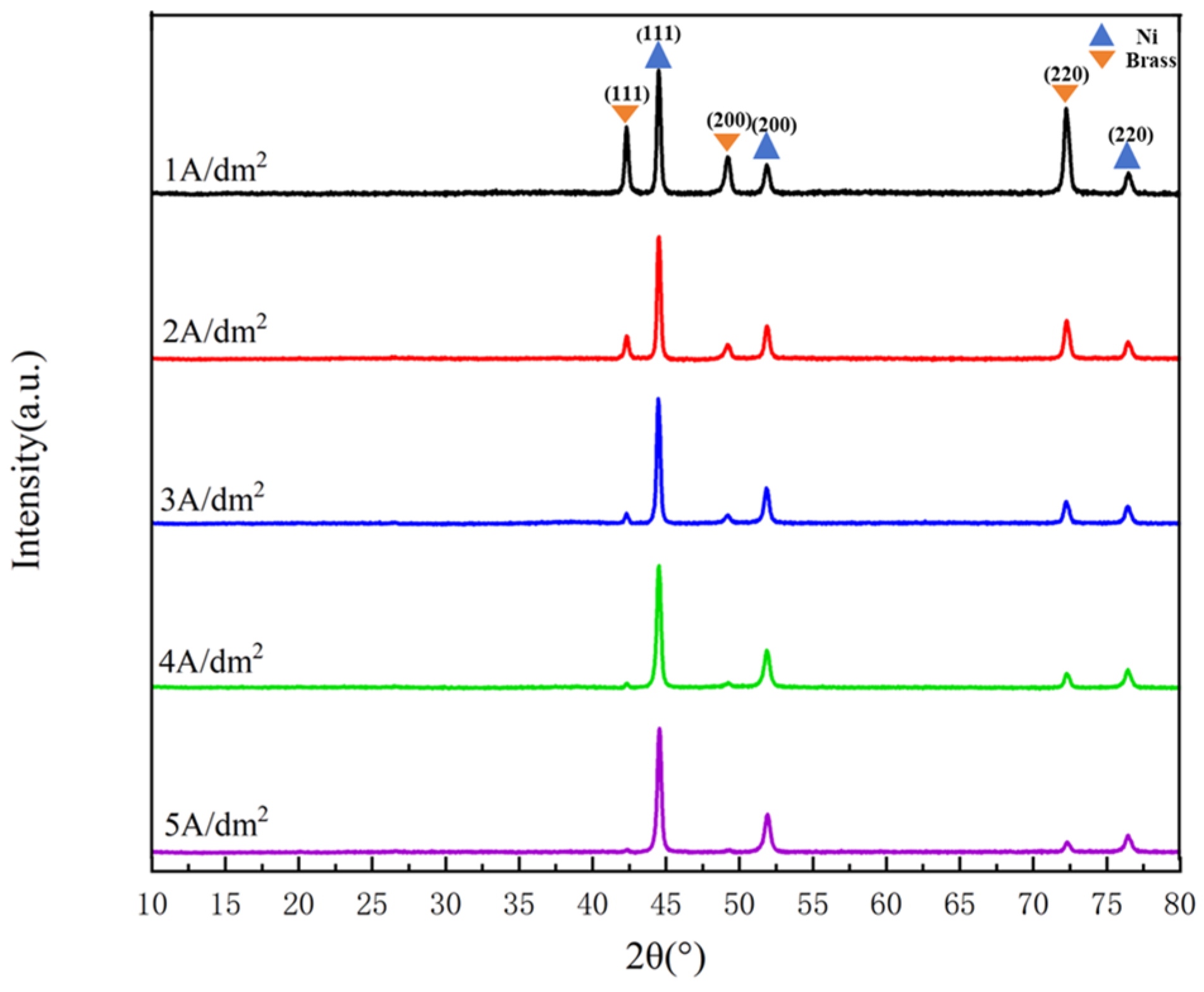
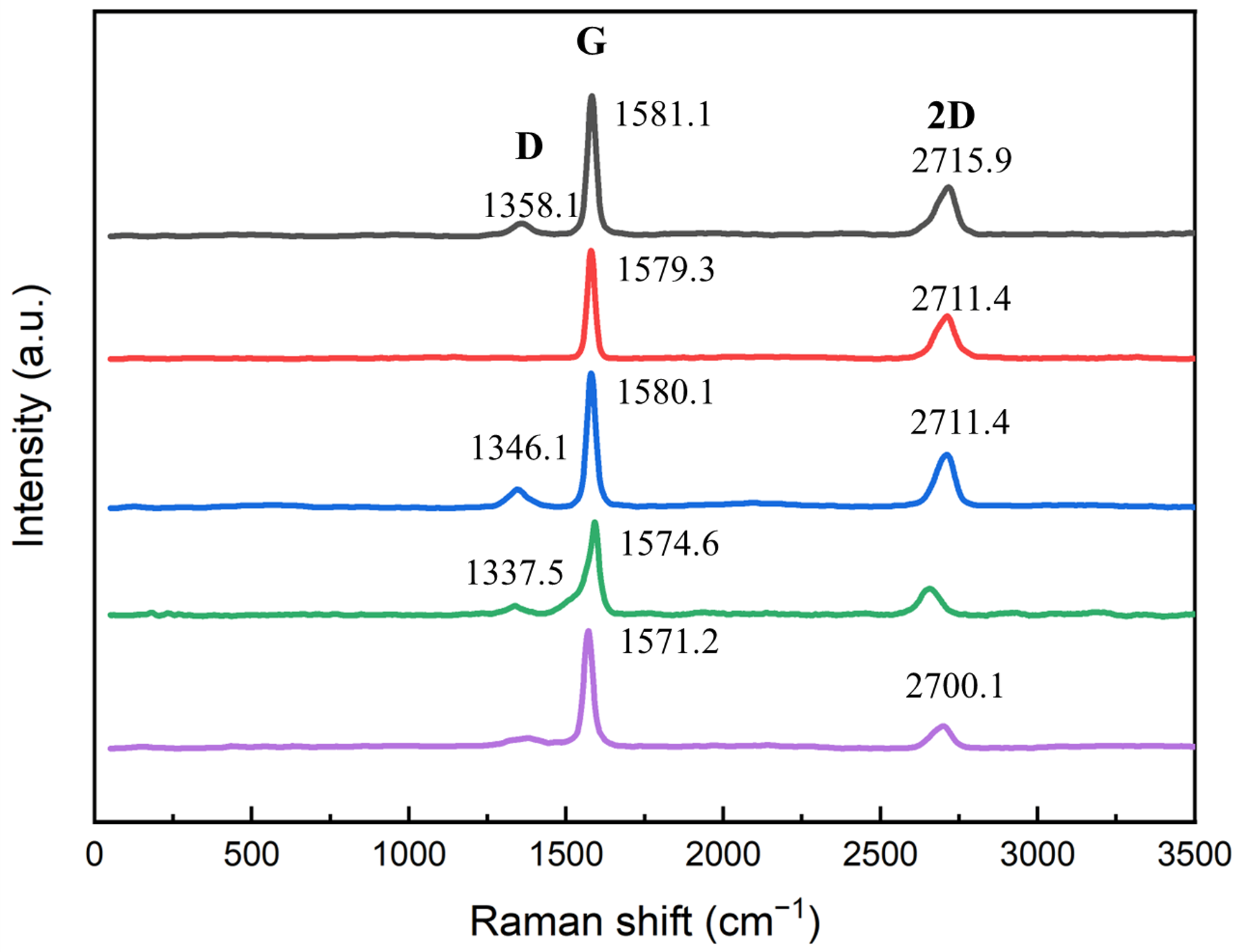
3.1.3. Phase Composition
3.2. Effect of Current Density on Coating Properties
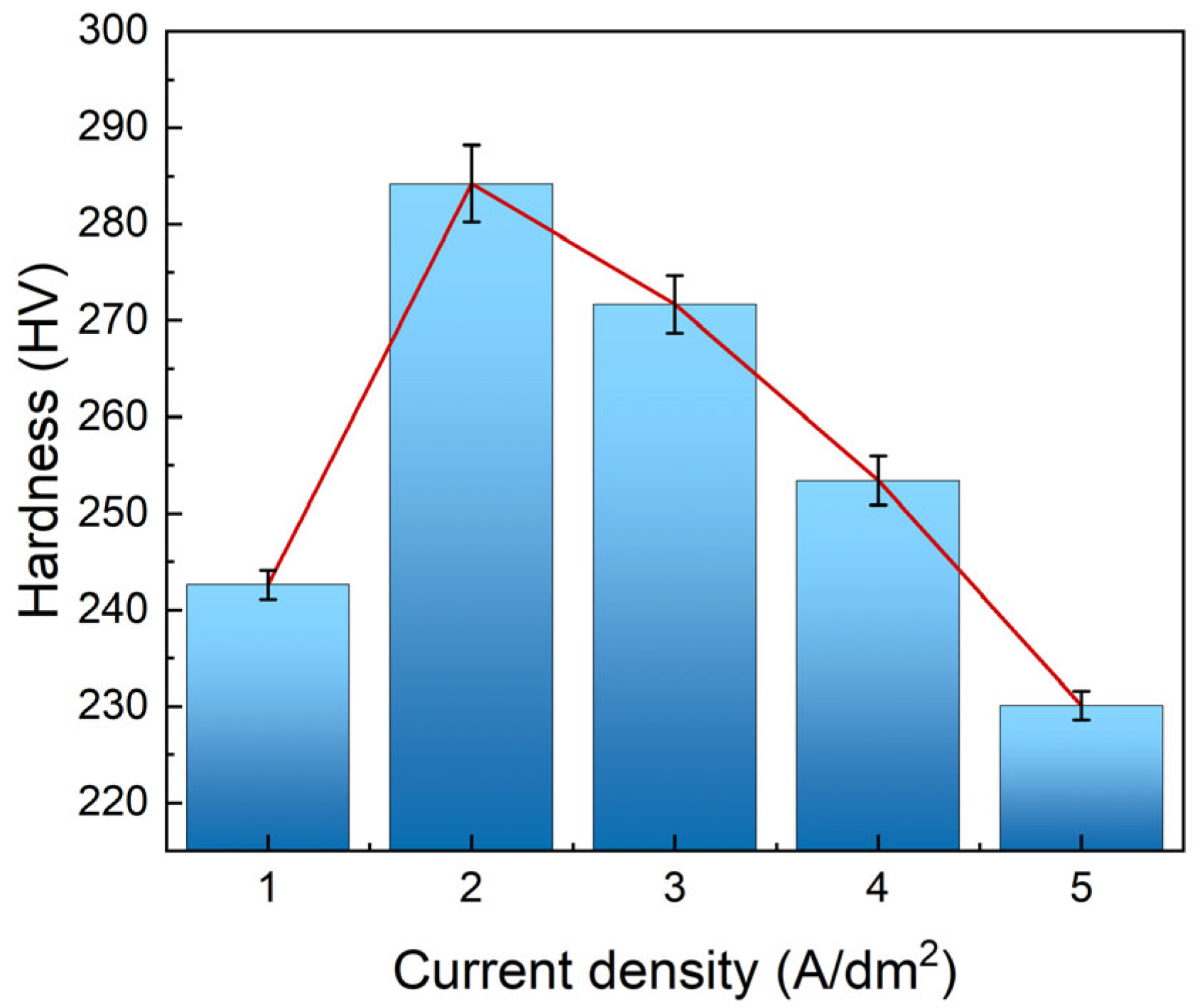
3.2.1. Microhardness
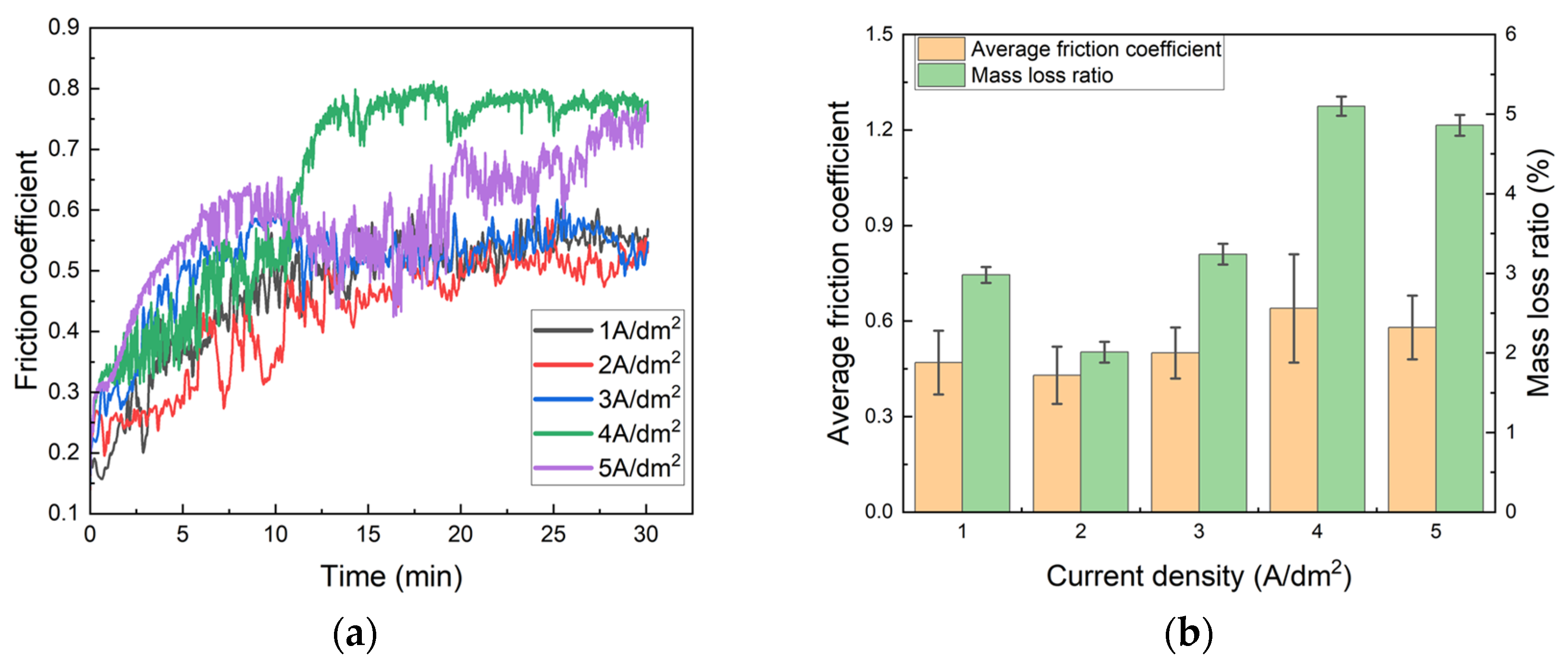
3.2.2. Wear Resistance
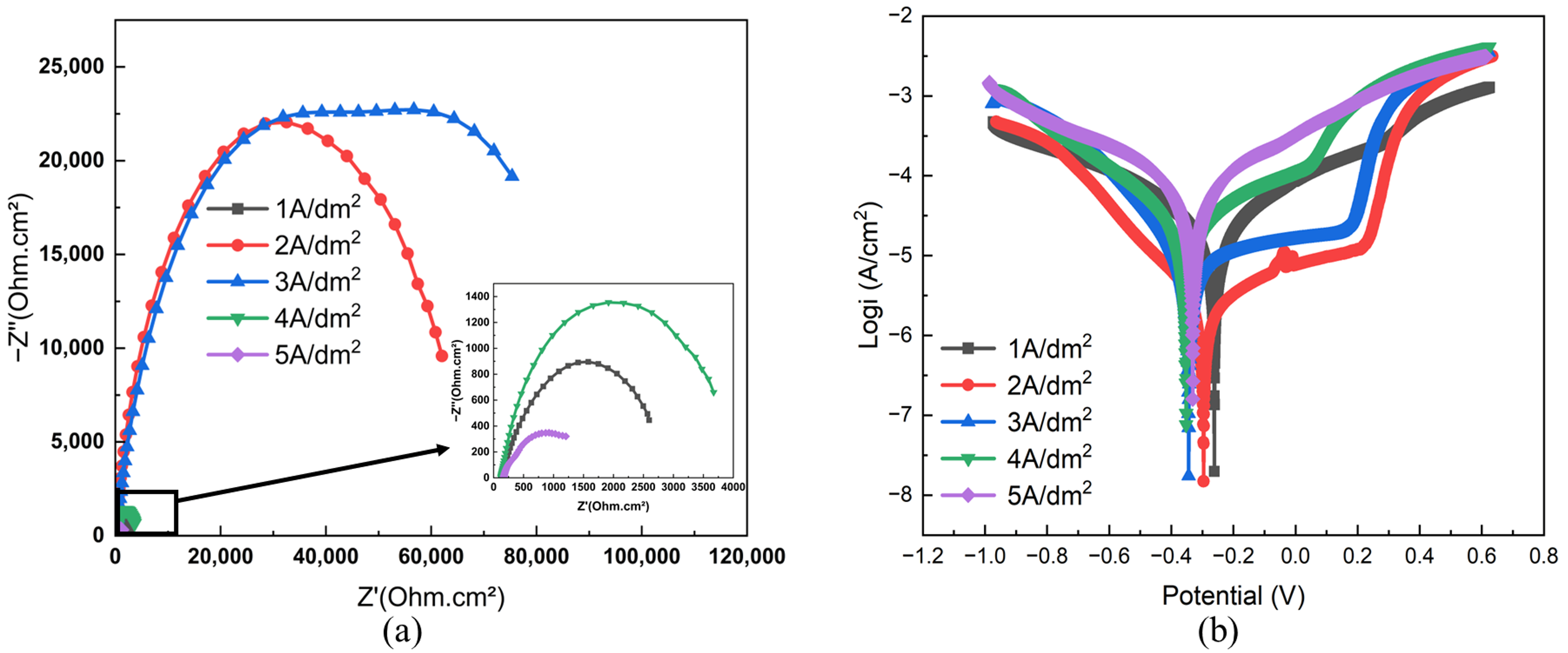
3.2.3. Corrosion Resistance
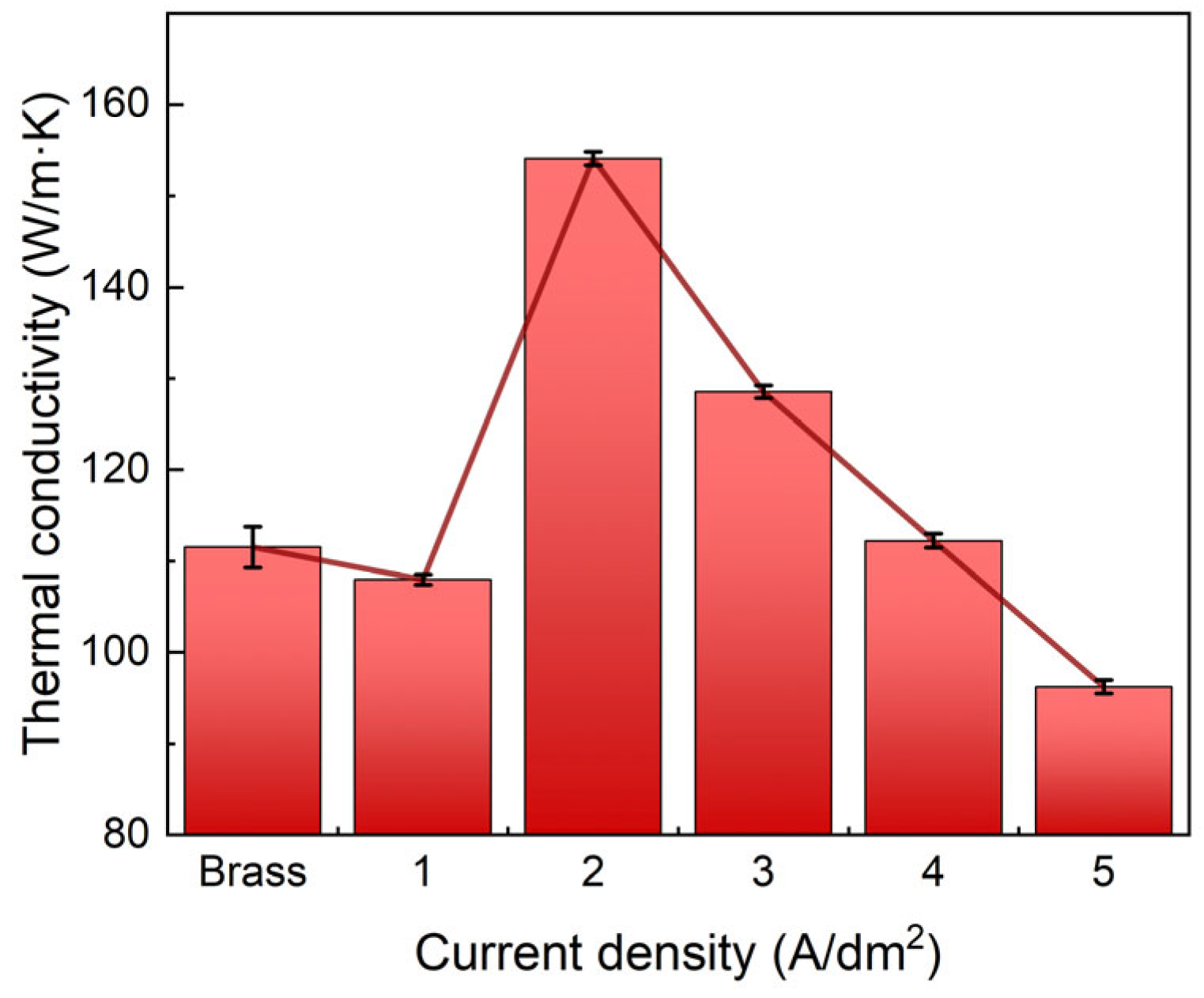
3.2.4. Thermal Conductivity
4. Conclusions
- (1)
- Current density significantly regulates the microstructure of Ni–Gr coatings. At 2 A/dm2, nickel grains are fine and dense, and graphene nanoplatelet disperses uniformly in the nickel matrix, with the nickel phase exhibiting a face-centered cubic (fcc) structure with fine-grained preferred orientation. Deviation from this current density leads to nickel grain coarsening and graphene nanoplatelet dispersion deterioration.
- (2)
- The Ni–Gr coating at 2 A/dm2 achieves optimal comprehensive performance: a peak Vickers hardness of 284 HV, the lowest average friction coefficient (0.43) and wear mass loss rate (2.01%), a minimum self-corrosion current density of 1.8135 × 10−6 A/cm2 in 3.5 wt.% NaCl solution, and a maximum thermal conductivity of 154 W/(m·K) at room temperature, which is 40% higher than that of the brass substrate.
- (3)
- Graphene nanoplatelets are co-deposited with nickel atoms, and their dispersion state and the resultant microstructure (grain size, compactness) dominate the coating’s performance. The fine-grained and dense structure at 2 A/dm2 facilitates the formation of efficient heat conduction channels and physical barriers, while also enhancing mechanical strength and wear resistance.
- (4)
- The simultaneous improvement in thermal conductivity, wear resistance, and corrosion resistance makes these coatings promising for critical industrial applications, such as heat dissipation components in high-power electronic devices (e.g., 5G base station power amplifiers, server CPUs) and marine electronic equipment, where efficient heat dissipation, surface wear protection, and corrosion resistance are simultaneously required.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Park, S.Y.; Yee, N.Y.; Baek, M.; Kim, H.S.; Lee, K.A. Microstructure heterogeneity, mechanical properties and thermal con-ductivity of pure copper fabricated by metal material extrusion additive manufacturing process. Mater. Sci. Eng. A 2025, 927, 147939. [Google Scholar] [CrossRef]
- Choong, Y.H.; Krishnan, M.; Gupta, M. Recent advances in the 3D printing of pure copper functional structures for thermal management devices. Technologies 2023, 11, 141. [Google Scholar] [CrossRef]
- Salmon, F.; Benisi Ghadim, H.; Godin, A.; Haillot, D.; Veillere, A.; Lacanette, D.; Duquesne, M. Optimizing performance for cooling electronic components using innovative heterogeneous materials. Appl. Energy 2024, 362, 122983. [Google Scholar] [CrossRef]
- Zhang, Y. Strengthening, Corrosion and Protection of High-Temperature Structural Materials. Coatings 2022, 12, 1136. [Google Scholar] [CrossRef]
- Fateh, A.; Aliofkhazraei, M.; Rezvanian, A. Review of corrosive environments for copper and its corrosion inhibitors. Arab. J. Chem. 2020, 13, 481–544. [Google Scholar] [CrossRef]
- Akhtar, S.S. A systematic design to develop high-performance sintered particulate copper-composite as heat spreader material. Eng. Sci. Technol. Int. J. 2022, 27, 101019. [Google Scholar] [CrossRef]
- Mizuuchi, K.; Inoue, K.; Agari, Y. Trend of the development of metal-based heat dissipative materials. Microelectron. Reliab. 2017, 79, 5–19. [Google Scholar] [CrossRef]
- Nguyen, D.H.; Ahn, H.S. A comprehensive review on micro/nanoscale surface modification techniques for heat transfer enhancement in heat exchanger. Int. J. Heat Mass Transfer 2021, 178, 121601. [Google Scholar] [CrossRef]
- Wang, T.; Xu, Y.; Liu, Z.; Li, G.; Guo, Y.; Lian, J.; Zhang, Z.; Ren, L. A chitosan/polylactic acid composite coating enhancing the corrosion resistance of the bio-degradable magnesium alloy. Prog. Org. Coat. 2023, 178, 107469. [Google Scholar] [CrossRef]
- Yu, H.; Guo, Q.; Wang, C.; Cao, G.; Liu, Y. Preparation and performance of PANI/CNTs composite coating on 316 stainless steel bipolar plates by pulsed electrodeposition. Prog. Org. Coat. 2023, 182, 107611. [Google Scholar] [CrossRef]
- Liao, F.Q.; Chen, Y.C. Siloxane-based epoxy coatings through cationic photopolymerization for corrosion protection. Prog. Org. Coat. 2022, 174, 107235. [Google Scholar] [CrossRef]
- Abdeen, D.; El Hachach, M.; Koc, M.; Atieh, M. A review on the corrosion behaviour of nanocoatings on metallic substrates. Materials 2019, 12, 210. [Google Scholar] [CrossRef]
- Nguyen-Tri, P.; Nguyen, T.A.; Carriere, P.; Ngo Xuan, C. Nanocomposite coatings: Preparation, characterization, properties, and applications. Int. J. Corros. 2018, 2018, 1–19. [Google Scholar] [CrossRef]
- Yasin, G.; Arif, M.; Mehtab, T.; Shakeel, M.; Khan, M.A.; Khan, W.Q. Metallic nanocomposite coatings. In Corrosion Protection at the Nanoscale, 1st ed.; Rajendran, S., Nguyen, T.A., Kakooei, S., Yeganeh, M., Li, Y., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 245–274. [Google Scholar]
- Gong, B.L.; Ma, X.Q.; Wang, T.G.; Hou, J.L.; Ji, S.X.; Shen, X.X.; Min, Y.L.; Xu, Q.J.; Cao, H.J. Enhanced corrosion resistance of MXene/Zn-Al LDH coating on Al alloy under dry/humid and temperature alternating environments. Corros. Sci. 2025, 255, 113088. [Google Scholar] [CrossRef]
- Wang, J.F.; Zhang, M.; Dai, R.S.; Shao, L.; Tu, Z.B.; Zhu, D.D.; Xu, Z.Z.; Dai, S.; Zhu, L. Wear and electrochemical corrosion behaviors of Cu matrix WC-Co reinforced composite coating prepared by cold spray. Surf. Coat. Technol. 2024, 488, 131001. [Google Scholar] [CrossRef]
- Xu, L.; Wang, R.; Gen, M.; Lu, L.; Han, G. Preparation and properties of graphene/nickel composite coating based on textured surface of aluminum alloy. Materials 2019, 12, 3240. [Google Scholar] [CrossRef] [PubMed]
- Dhand, D.; Grewal, J.S.; Kumar, P. Study and comparison of wear behaviour of Ni-Al2O3 coatings deposited by hot and cold spray. Surf. Topogr. Metrol. Prop. 2021, 9, 045056. [Google Scholar] [CrossRef]
- Kainz, C.; Schalk, N.; Tkadletz, M.; Saringer, C.; Winkler, M.; Stark, A.; Schell, N.; Julin, J.; Czttl, C. Thermo-physical properties of coatings in the Ti(B,N) system grown by chemical vapor deposition. Surf. Coat. Technol. 2020, 384, 125318. [Google Scholar] [CrossRef]
- Stewart, J.A.; Dingreville, R. Microstructure morphology and concentration modulation of nanocomposite thin-films during simulated physical vapor deposition. Acta Mater. 2020, 188, 181–191. [Google Scholar] [CrossRef]
- Muench, F. Electroless plating of metal nanomaterials. ChemElectroChem 2021, 12, 2993–3012. [Google Scholar] [CrossRef]
- Zhao, Z.; Luo, G.; Cheng, M.; Song, L. Water-Repellent Coatings on Corrosion Resistance by Femtosecond Laser Processing. Coatings 2022, 12, 1736. [Google Scholar] [CrossRef]
- Zhang, F.; Yao, Z.; Moliar, O.; Tao, X.; Yang, C. Nanocrystalline Ni coating prepared by a novel electrodeposition. J. Alloys Compd. 2020, 830, 153785. [Google Scholar] [CrossRef]
- Xia, F.; Li, Q.; Ma, C.; Guo, X. Preparation and characterization of Ni–AlN nanocoatings deposited by magnetic field assisted electrodeposition technique. Ceram. Int. 2020, 46, 2500–2509. [Google Scholar] [CrossRef]
- Walsh, F.C.; Wang, S.; Zhou, N. The electrodeposition of composite coatings: Diversity, applications and challenges. Curr. Opin. Electrochem. 2020, 20, 8–19. [Google Scholar] [CrossRef]
- Leng, X.Y.; He, S.L.; Wang, W.Q.; Zhang, H.N.; Wang, L.Y.; Song, J.L. Magnetic field-assisted electrodeposition: Mechanisms, materials, and multifunctional applications. J. Sci.-Adv. Mater. Dev. 2025, 10, 100967. [Google Scholar] [CrossRef]
- Chen, Y.; Pang, H.Z.; Liao, J.B.; Shangguan, S.M.; Panov, K.; Huang, Y.Y.; Tian, Z.J.; Gao, W.; Zhao, J.F. Multiphysics simulation and experimental study on uniformity of jet electrodeposited bronze coating in deep small hole. Electrochim. Acta 2025, 536, 146555. [Google Scholar] [CrossRef]
- Zafarghandi, M.S.; Tavandashti, N.P. Electrodeposition of hierarchically structured superhydrophobic Ni-PTFE composite coating with remarkable corrosion resistance, chemical and mechanical stability. Sci. Iran. 2024, 31, 907–919. [Google Scholar] [CrossRef]
- Gao, M.Y.; Liu, L.L.; Huang, Z.X.; Jiang, C.; Wang, X.; Huang, F.; Li, X.X.; Zhang, X.H. Electrodeposition and properties of Ni–W–Ti2AlC composite coatings. Ceram. Int. 2024, 50, 18832–18842. [Google Scholar] [CrossRef]
- Makarava, I.; Esmaeili, M.; Kharytonau, D.S.; Pelcastre, L.; Ryl, J.; Bilesan, M.R.; Vuorinen, E.; Repo, E. Influence of CeO2 and TiO2 Particles on Physicochemical Properties of Composite Nickel Coatings Electrodeposited at Ambient Temperature. Materials 2022, 15, 5550. [Google Scholar] [CrossRef]
- Balandin, A.A.; Ghosh, S.; Bao, W.; Calizo, I.; Teweldebrhan, D.; Miao, F.; Lau, C.N. Superior thermal conductivity of single-layer graphene. Nano Lett. 2008, 8, 902–907. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Calizo, I.; Teweldebrhan, D.; Pokatilov, E.P.; Nika, D.L.; Balandin, A.A.; Bao, W.; Miao, F.; Lau, C.N. Extremely high thermal conductivity of graphene: Prospects for thermal management applications in nanoelectronic circuits. Appl. Phys. Lett. 2008, 92, 151911. [Google Scholar] [CrossRef]
- Huang, Z. Study on Preparation and Properties of High Thermal Conductivity Composites Based on Graphene. Master’s Thesis, Anhui University, Hefei, China, 2019. [Google Scholar]
- Yang, L.L.; Zhu, Y.J.; Liu, H.K.; Guo, T.; Tang, H.; Ya, S.D.; Li, Y.; Wang, H.G.; Luo, Y.L.; Ma, J.M.; et al. Co-deposition and thermal conductivity of nickel–graphene composite coatings on copper surface. Appl. Phys. A 2023, 129, 757. [Google Scholar] [CrossRef]
- Li, X.S.; Shen, Q.Q.; Zhang, Y.; Wang, L.L.; Nie, C.Y. Wear behavior of electrodeposited nickel/graphene composite coating. Diam. Relat. Mater. 2021, 119, 108589. [Google Scholar] [CrossRef]
- Zhang, R.Y.; Yang, K.; Zhang, Y.P.; Tao, B.; Wang, S.Q.; Cheng, Q.L. Long-term anticorrosion performance of a modifier-free Ni–Graphene superhydrophobic coating. Mater. Today Commun. 2024, 41, 111053. [Google Scholar] [CrossRef]
- Cheng, D.; Zhang, L.; Zhu, Y.; Xia, H.; Li, N.; Song, W.; Bai, H.; Ma, H. Preparation and Properties of Electrodeposited Ni-B-Graphene Oxide Composite Coatings. Materials 2022, 15, 2287. [Google Scholar] [CrossRef]
- Zhang, W.W.; Li, B.S.; Mei, T.Y.; Li, M.Y.; Hong, M.; Yuan, Z.W.; Chu, H.Q. Effects of graphene oxide and current density on structure and corrosion properties of nanocrystalline nickel coating fabricated by electrodeposition. Colloids Surf. A Physicochem. Eng. Asp. 2022, 648, 129220. [Google Scholar] [CrossRef]
- Yasin, G.; Arif, M.; Shakeel, M.; Dun, Y.C.; Zuo, Y.; Khan, W.Q.; Tang, Y.M.; Khan, A.; Nadeem, M. Exploring the Nickel–Graphene Nanocomposite Coatings for Superior Corrosion Resistance: Manipulating the Effect of Deposition Current Density on its Morphology, Mechanical Properties, and Erosion-Corrosion Performance. Adv. Eng. Mater. 2018, 20, 1701166. [Google Scholar] [CrossRef]
- Wang, J.Q.; Lei, W.N.; Deng, Y.; Xue, Z.M.; Qian, H.F.; Liu, W.Q.; Li, X.P. Effect of current density on microstructure and corrosion resistance of Ni-graphene oxide composite coating electrodeposited under supercritical carbon dioxide. Surf. Coat. Technol. 2019, 358, 765–774. [Google Scholar] [CrossRef]
- Ma, C.Y.; He, H.X.; Zhang, H.B.; Xia, F.F.; Li, H.X. Impact of ultrasonic power density on the structure and performance of Ni-W-SiC composite nanocoatings. Surf. Coat. Technol. 2025, 496, 131641. [Google Scholar] [CrossRef]
- Wan, Y.; Chen, L.; Tang, W.C.; Li, J.L. Effect of graphene on tribological properties of Ni based composite coatings prepared by oxidation reduction method. J. Mater. Res. Technol. 2020, 9, 3796–3804. [Google Scholar] [CrossRef]
- Li, H.X.; Ma, C.Y.; Xia, F.F.; Xiao, Z.M. Ultrasonic disorder-induced deposition of TiO2 nanorod arrays and C60 coating on carbon cloth for high-performance supercapacitor electrodes. Ultrason. Sonochem. 2025, 117, 107347. [Google Scholar] [CrossRef]
- Zarei, A.; Jafari, Z.; Dehghanian, C.; Pishbin, F.S. Diopside/graphene-oxide nanocomposite coatings on AZ31 Mg alloy for biomedical applications: Surface characteristics, corrosion, and biocompatibility properties. Surf. Coat. Technol. 2024, 479, 130482. [Google Scholar] [CrossRef]
- Pingale, A.D.; Belgamwar, S.U.; Rathore, J.S. Synthesis and characterization of Cu–Ni/Gr nanocomposite coatings by electro-co-deposition method: Effect of current density. Bull. Mater. Sci. 2020, 43, 66. [Google Scholar] [CrossRef]
- Cao, K.; Yu, M.; Liang, C.M.; Chen, H. Study on thermal conductivity of cold sprayed Cu coating. Surf. Eng. 2020, 36, 1058–1066. [Google Scholar] [CrossRef]
- Kim, Y.; Kim, M.; Seong, H.G.; Jung, J.Y.; Baeck, S.H.; Shim, S.E. Roles of silica-coated layer on graphite for thermal conductivity, heat dissipation, thermal stability, and electrical resistivity of polymer composites. Polymer 2018, 148, 295–302. [Google Scholar] [CrossRef]
- You, Y.X.; Zhang, B.B.; Tao, S.L.; Liang, Z.H.; Tang, B.; Zhou, R.; Yuan, D. Effect of Surface Microstructure on the Heat Dissipation Performance of Heat Sinks Used in Electronic Devices. Micromachines 2021, 12, 265. [Google Scholar] [CrossRef]
| Composition | Concentration (g/L) |
|---|---|
| NiSO4·6H2O | 240 |
| NiCl2·6H2O | 45 |
| H3BO3 | 40 |
| C12H25SO4Na | 0.1 |
| C7H5NO3S | 1 |
| Graphene Nanoplatelets | 0.5 |
| Parameter | Value |
|---|---|
| Current density (J) | 1–5 A/dm2 |
| Bath temperature (T) | 50 °C |
| pH | 3.5 |
| Stirring rate v | 200 r/min |
| Plating time ttotal | 30 min |
| Current Density (A/dm2) | Ecorr (V) | Icorr (A/cm2) |
|---|---|---|
| 1 | −0.26143 | 9.9066 × 10−6 |
| 2 | −0.29582 | 1.8135 × 10−6 |
| 3 | −0.34395 | 5.6102 × 10−6 |
| 4 | −0.35248 | 1.1148 × 10−5 |
| 5 | −0.33097 | 2.6735 × 10−5 |
| α (mm2/s) | ρ (g/cm3) | Cp (J/(g·K)) | |
|---|---|---|---|
| 1 A/dm2 | 34.183 | 8.1 | 0.39 |
| 2 A/dm2 | 33.808 | 8.1 | 0.562 |
| 3 A/dm2 | 35.238 | 8.1 | 0.454 |
| 4 A/dm2 | 36.52 | 8.1 | 0.379 |
| 5 A/dm2 | 31.246 | 8.1 | 0.38 |
| Brass | 34.416 | 8.1 | 0.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.; Wang, H.; Ma, W.; Ma, Y. Electrodeposition of Copper-Based Nickel–Graphene Coatings: Effect of Current Density on Microstructure and Properties. Coatings 2025, 15, 1360. https://doi.org/10.3390/coatings15121360
Zhang Z, Wang H, Ma W, Ma Y. Electrodeposition of Copper-Based Nickel–Graphene Coatings: Effect of Current Density on Microstructure and Properties. Coatings. 2025; 15(12):1360. https://doi.org/10.3390/coatings15121360
Chicago/Turabian StyleZhang, Zhongke, Haonan Wang, Wenhao Ma, and Yingbo Ma. 2025. "Electrodeposition of Copper-Based Nickel–Graphene Coatings: Effect of Current Density on Microstructure and Properties" Coatings 15, no. 12: 1360. https://doi.org/10.3390/coatings15121360
APA StyleZhang, Z., Wang, H., Ma, W., & Ma, Y. (2025). Electrodeposition of Copper-Based Nickel–Graphene Coatings: Effect of Current Density on Microstructure and Properties. Coatings, 15(12), 1360. https://doi.org/10.3390/coatings15121360





