First-Principles Calculation of the Desolvation Effect of Functionalized Carbon Nanotubes
Abstract
1. Introduction
2. Calculation Method
3. Results and Discussion
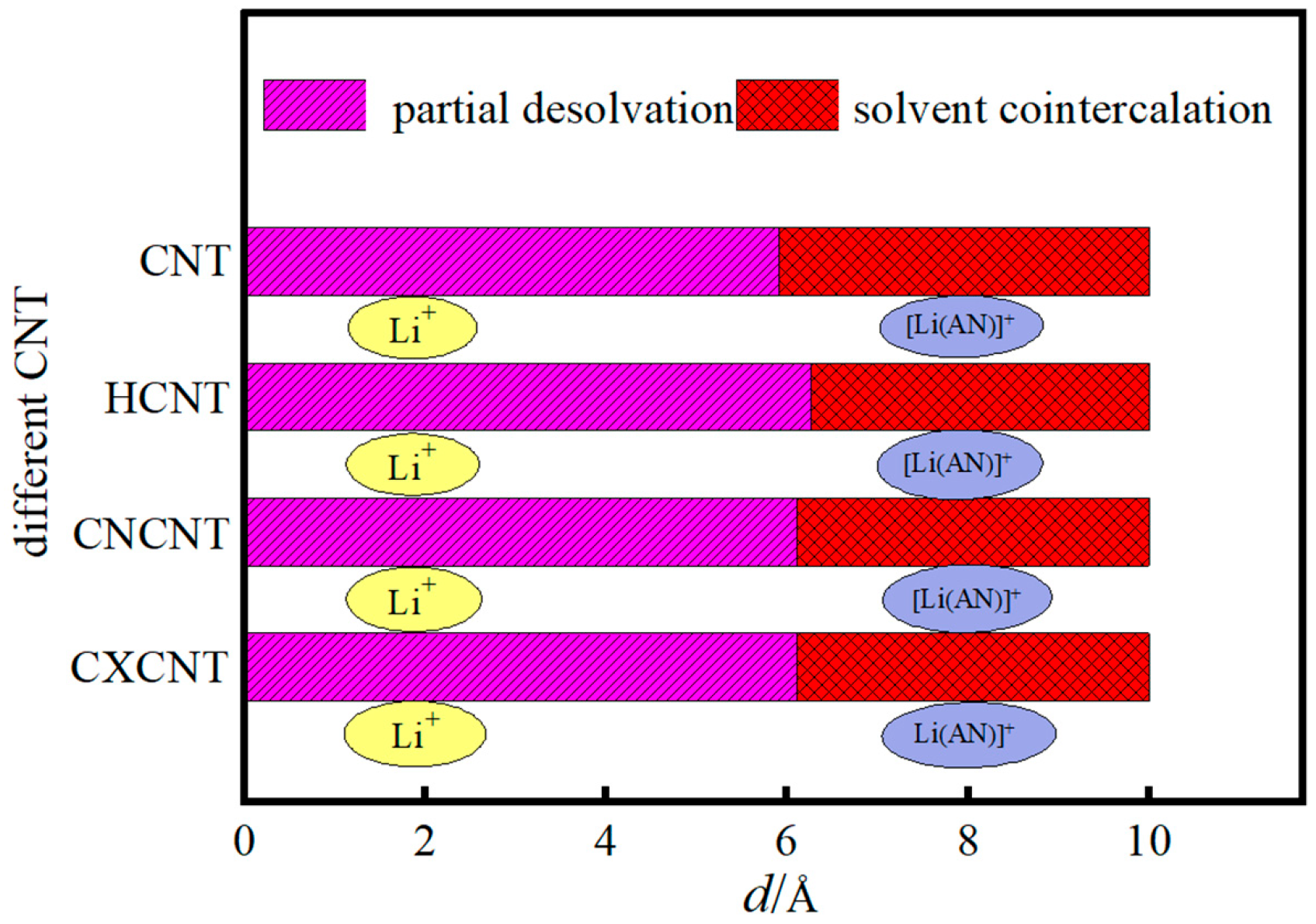
3.1. Reaction Principle
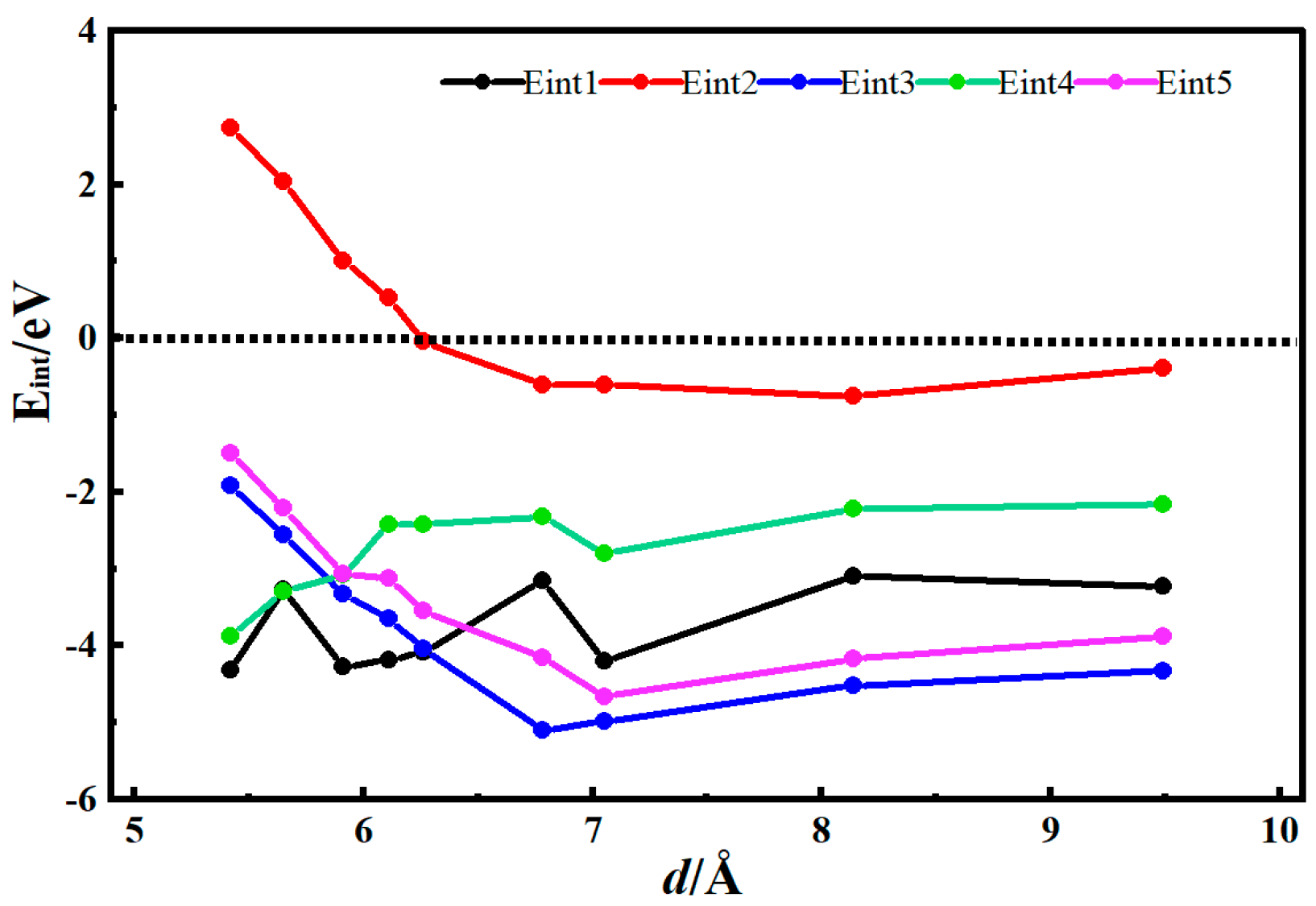
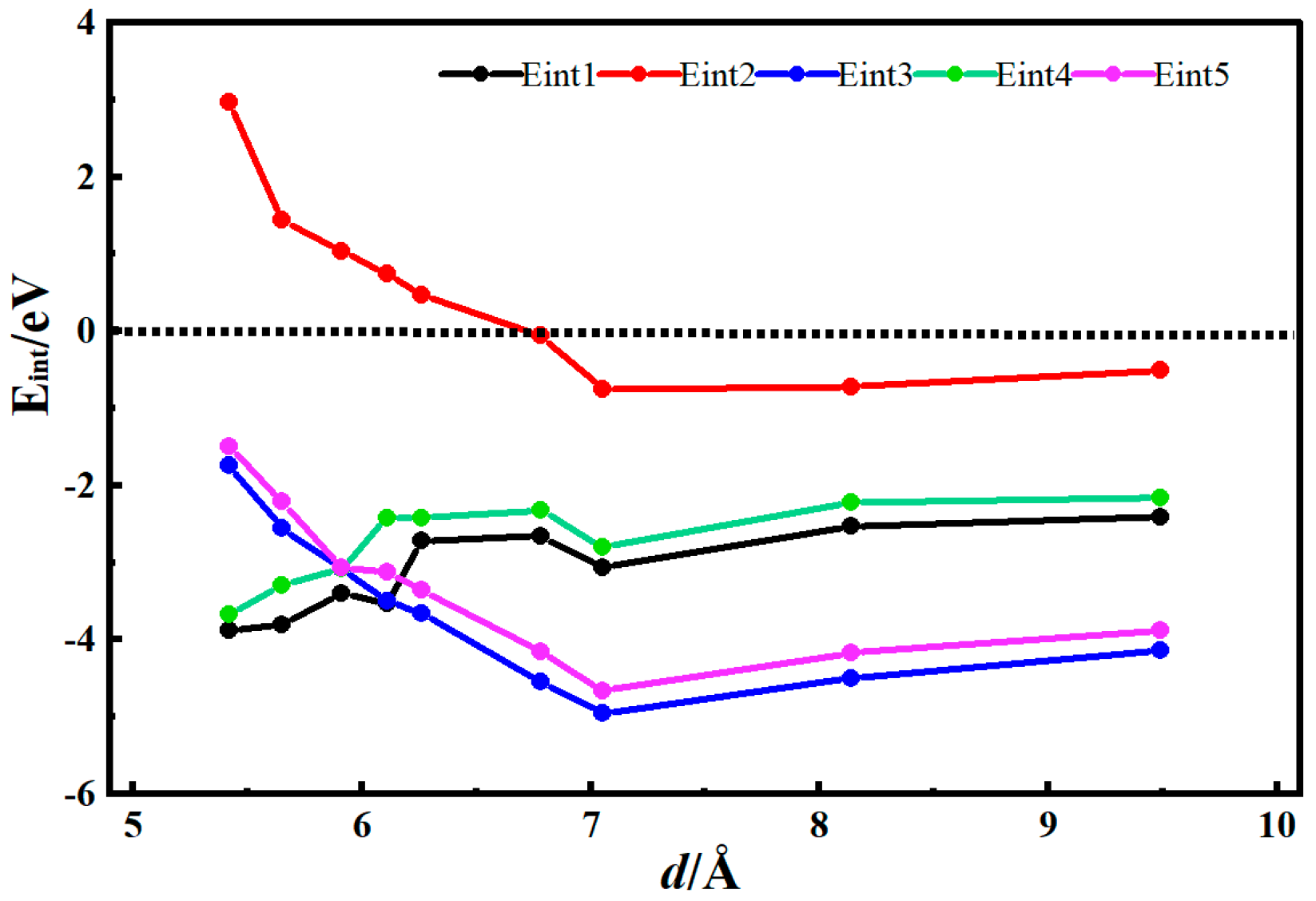
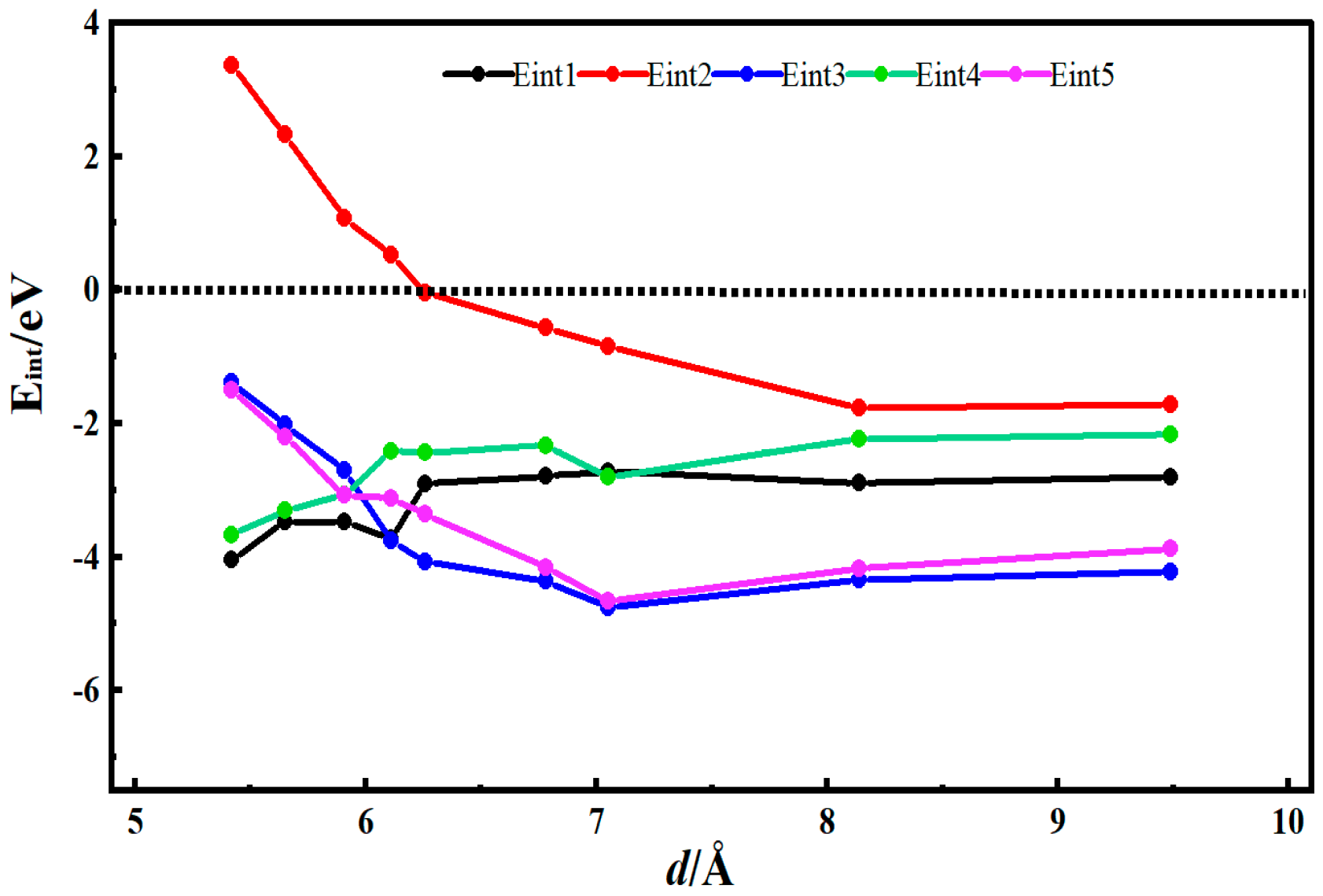
3.2. Desolvation of Li+ Complexes
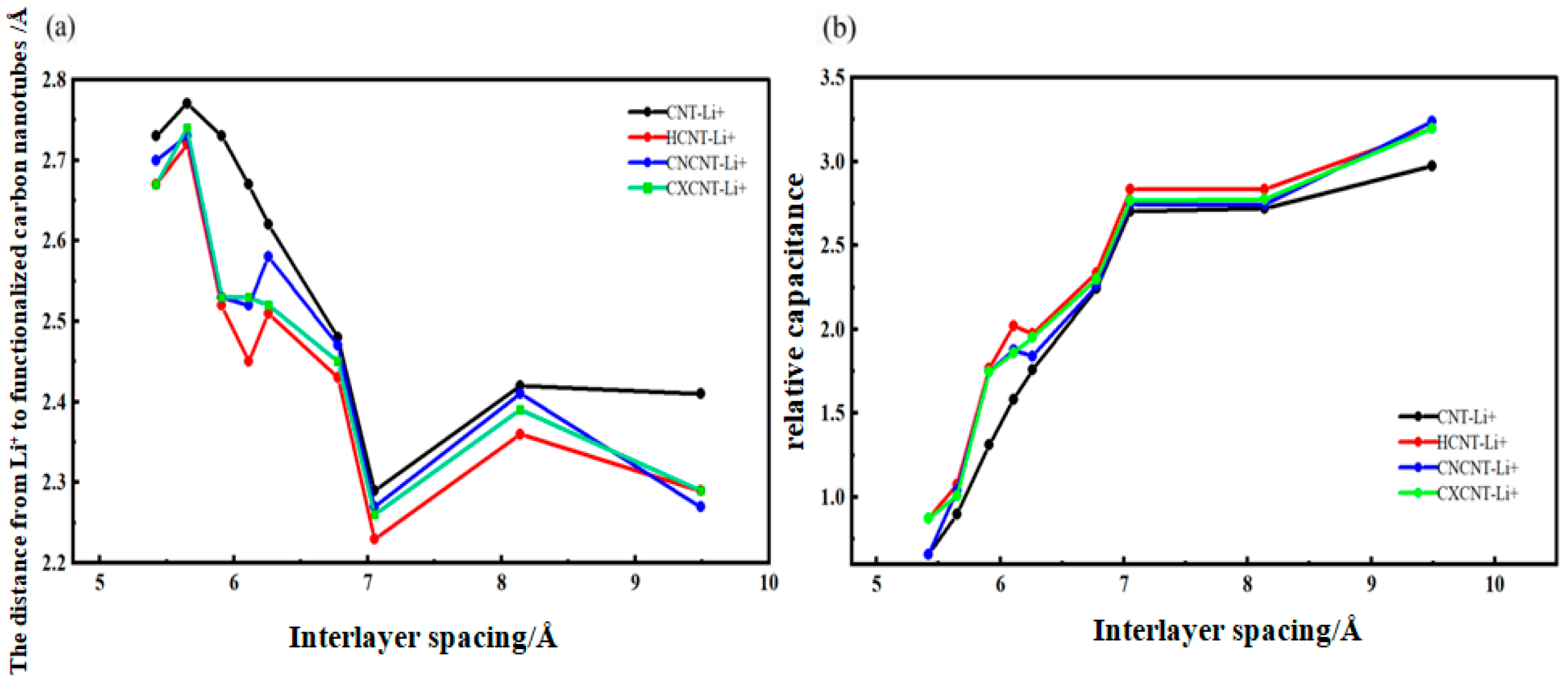
3.3. Analysis of the Effect of Carbon Nanotubes Functionalized with Different Functional Groups on the Desolvation Size of Li+
3.4. Analysis of Relative Capacitance for Li+ Intercalation into Carbon Nanotubes with Different Functionalizations
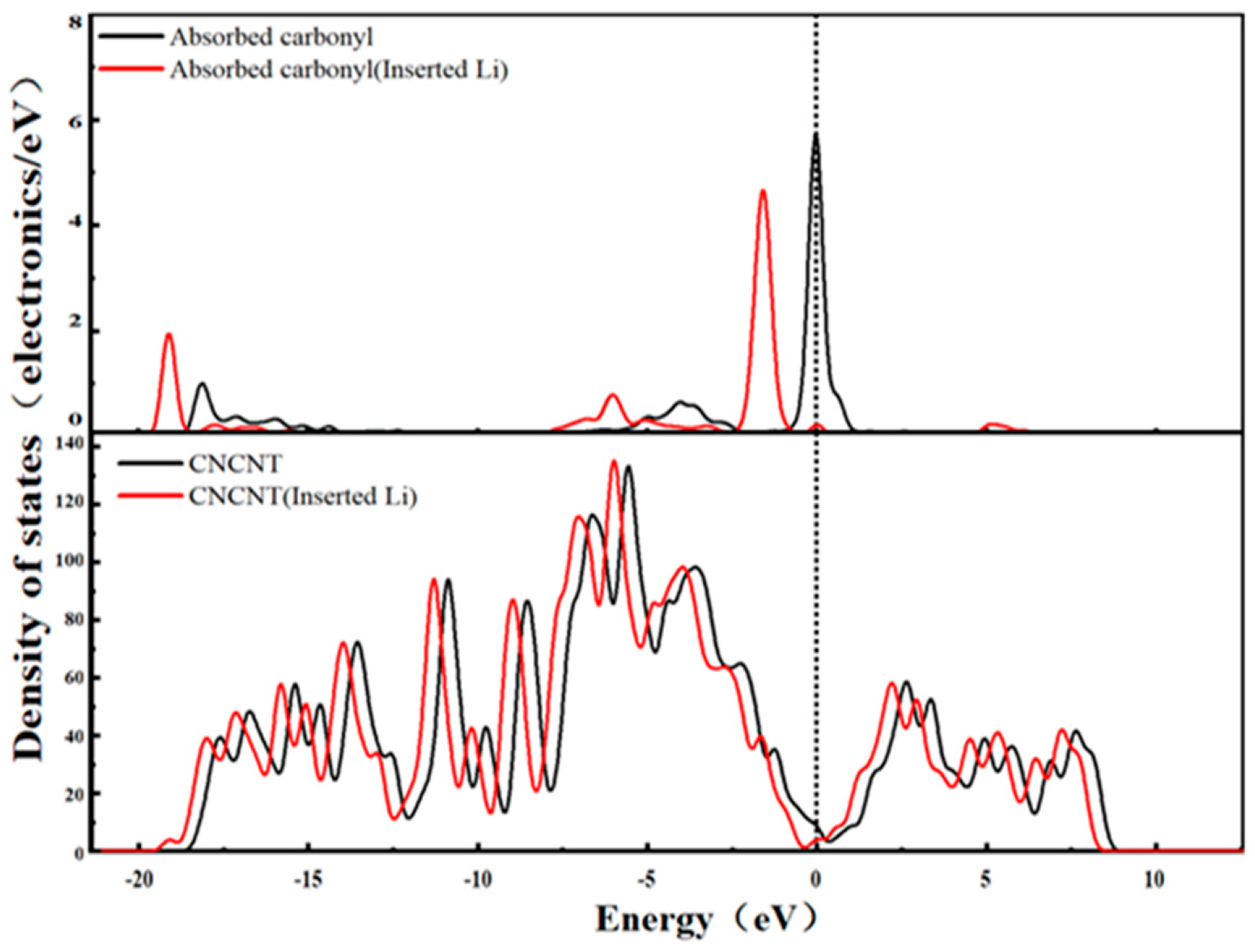
3.5. Density of States Analysis of Li+ After Desolvation in Functionalized Carbon Nanotubes
3.5.1. DOS Characteristics of the Hydroxylated Carbon Nanotube (HCNT)-Li+ System
3.5.2. DOS Characteristics of the Carbonylated Carbon Nanotube (CNCNT)-Li+ System
3.5.3. DOS Characteristics of the Carboxylated Carbon Nanotube (CXCNT)-Li+ System
4. Conclusions
- •
- Functionalization of CNTs exerts a significant regulatory effect on the complete desolvation size of [Li(AN)]+ complexes. Among the systems studied, hydroxyl-modified CNTs (HCNT) exhibit the largest desolvation size, followed by carbonyl-modified CNTs (CNCNT) and carboxyl-modified CNTs (CXCNT) (which show identical desolvation sizes), while pristine CNTs present the smallest size. This result confirms that the introduction of oxygen-containing functional groups modifies the pore microenvironment of CNTs, thereby directly regulating the desolvation process of Li+ complexes.
- •
- All three types of oxygen-containing functional groups contribute to the enhancement of CNTs’ relative capacitance, with hydroxyl modification yielding the most notable improvement (a maximum enhancement of ~1.4-fold compared to pristine CNTs), and carbonyl/carboxyl modifications achieving a maximum enhancement of ~1.3-fold. The underlying mechanism for this capacitance improvement lies in the fact that functionalization strengthens the Li+ storage capacity of the cylindrical pores in CNTs, which in turn enhances the charge storage capability of the electrode material.
- •
- Post-desolvation DOS analysis reveals distinct variations in the electronic properties of CNT-Li+ systems depending on the type of functional group: the HCNT-Li+ system exhibits simultaneous enhancement in metallicity and conductivity; the CNCNT-Li+ system shows increased metallicity but a reduction in conductivity; and the CXCNT-Li+ system displays decreased metallicity while maintaining nearly unchanged conductivity. These functional group-dependent electronic property differences provide targeted theoretical guidance for selecting functionalized CNTs tailored to the specific electrical performance requirements of supercapacitors.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lawal, T.A. Recent application of carbon nanotubes in energy storage and conversion devices. Carbon Trends 2025, 19, 100470. [Google Scholar] [CrossRef]
- Scarpa, D.; Cirillo, C.; Ponticorvo, E.; Cirillo, C.; Attanasio, C.; Iuliano, M.; Sarno, M. Iron Selenide Particles for High-Performance Supercapacitors. Materials 2023, 16, 5309. [Google Scholar] [CrossRef]
- Liu, F.; Yang, S.; Zhang, X.; Tang, S.; Xia, Y. Insight into the Desolvation of Quaternary Ammonium Cation with Acetonitrile as a Solvent in Hydroxyl-Flat Pores: A First-Principles Calculation. Materials 2023, 16, 3858. [Google Scholar] [CrossRef] [PubMed]
- Simoes, G.R.D.; Pequeno, H.O.D.; Montes, C.I.C. Supercapacitors and triboelectric nanogenerators based on electrodes of greener iron nanoparticles/carbon nanotubes composites. Sci. Rep. 2024, 14, 11555. [Google Scholar] [CrossRef]
- Keller, V.N.; Nikolkin, N.V.; Butakov, S.D. Optimization of the Technology for Manufacturing the Electrodes for Self-Charging Supercapacitors from Carbon Nanotubes. Russ. J. Electrochem. 2024, 60, 526–531. [Google Scholar] [CrossRef]
- An, K.H.; Kim, W.S.; Park, Y.S. Supercapacitor using single-walled carbon nanotubes electrodes. Adv. Mater. 2001, 13, 497–500. [Google Scholar] [CrossRef]
- Chen, Q.L.; Xue, K.H.; Shen, W. Fabrication and electrochemical properties of carbon nanotubes array electrode for supercapacitor. Electrochim. Acta 2004, 49, 4157–4161. [Google Scholar] [CrossRef]
- Chen, J.H.; Li, W.Z.; Wang, D.Z.; Yang, S.X.; Wen, J.G.; Ren, Z.F. Electrochemical characterization of carbon nanotubes as electrode in electrochemical double-layer capacitor. Carbon 2002, 40, 1193–1197. [Google Scholar] [CrossRef]
- Yoon, B.J.; Jeong, S.H.; Lee, K.H.; Kim, H.S.; Park, C.G.; Han, J.H. Electrical properties of electrical doublelayer capacitors with integrated carbon nanotubes electrodes. Chem. Phys. Lett. 2004, 388, 170–174. [Google Scholar] [CrossRef]
- Liu, D.; Shi, L.; Dai, Q.; Lin, X.; Mehmood, R.; Gu, Z.; Dai, L. Functionalization of carbon nanotubes for multifunctional applications. Trends Chem. 2024, 6, 186–210. [Google Scholar] [CrossRef]
- Kim, M.G.; Lee, B.; Li, M.; Noda, S.; Kim, C.; Kim, J.; Song, W.J.; Lee, S.W.; Brand, O. All-Soft Supercapacitors Based on Liquid Metal Electrodes with Integrated Functionalized Carbon Nanotubes. ACS Nano 2020, 14, 5659–5667. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, S.B.; Shan, X.Y. Insights into the effect of the interlayer spacings of bilayer graphene on the desolvation of H+, Li+, Na+, and K+ ions with water as a solvent: A first-principles study. Phys. Chem. 2019, 21, 23697–23704. [Google Scholar] [CrossRef]
- Mattsson, A.E.; Schultz, P.A.; Desjarlais, M.P.; Mattsson, T.R.; Leung, K. Designing meaningful density functional theory calculations in materials science-a primer. Model. Simul. Mater. Sci. Eng. 2004, 13, R1. [Google Scholar] [CrossRef]
- Seifert, G.; Porezag, D.; Frauenheim, T. Calculations of molecules, clusters, and solids with a simplified LCAO-DFT-LDA Scheme. Int. J. Quantum Chem. 1996, 58, 185–192. [Google Scholar] [CrossRef]
- Elstner, M.; Porezag, D.; Jungnickel, G.; Elsner, J.; Haugk, M.T.; Frauenheim, S.; Su, H.; Seifert, G. Self-consistent charge density functional tight-binding method for simulation of complex material properties. Phys. Rev. B 1998, 58, 7260. [Google Scholar] [CrossRef]
- Seifert, G. Tight-binding density functional theory: An approximate Kohn-Sham DFT scheme. J. Phys. Chem. A 2007, 111, 5609–5613. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Perdew, J.P. Rehabilitation of the Perdew-Burke-Ernzerhof generalized gradient approximation for layered materials. Phys. Rev. B 2017, 95, 081105. [Google Scholar] [CrossRef]
- Irelan, R.M.; Henderson, T.M.; Scuseria, G.E. Long-range-corrected hybrids using a range-separated Perdew-Burke-Ernzerhof functional and random phase approximation correlation. J. Chem. Phys. 2011, 135, 94105–94116. [Google Scholar] [CrossRef]
- Segall, D.M.; Lindan, D.J.P.; Probert, J.M.; Pickard, J.C.; Hasnip, J.P.; Clark, J.S.; Payne, C.M. First-principles simulation: Ideas, illustrations and the CASTEP code. J. Phys. Condens. Matter 2002, 14, 2717. [Google Scholar] [CrossRef]
- Yang, S.B.; Liu, X.L.; Zhang, X.; Tang, S.W. Insights into the effect of hydroxyl-, epoxy-, and carboxyl-pores on the desolvation of K+ with water as a solvent: A first-principles study. J. Phys. Condens. Matter 2021, 33, 445201. [Google Scholar] [CrossRef]
- Wu, S.; Yan, G.; Cheng, X. First-principles investigation of the superconducting properties of thallium sulfide. Phys. C Supercond. Its Appl. 2019, 562, 1–6. [Google Scholar] [CrossRef]
- Schira, R.; Latouche, C. DFT and hybrid-DFT calculations on the electronic Properties of vanadate materials: Theory meets experiments. New J. Chem. 2020, 44, 11602–11607. [Google Scholar] [CrossRef]
- Zhao, P.; Su, Y.; Zhang, Y.; Li, S.J.; Chen, G. CO catalytic oxidation on iron-embedded hexagonal boron nitride sheets. Chem. Phys. Lett. 2011, 515, 159–162. [Google Scholar] [CrossRef]
- Floris, A.; Timrov, I.; Himmetoglu, B.; Marzari, N.; de Gironcoli, S.; Cococcioni, M. Hubbard-corrected density functional perturbation theory with ultrasoft pseudopotentials. Phys. Rev. B 2020, 101, 064305. [Google Scholar] [CrossRef]
- Monkhorst, J.H.; Pack, D.J. Special points for Brillouin-zone integrations. Phys. Rev. B 1976, 13, 5188–5192. [Google Scholar] [CrossRef]
- Tonel, M.Z.; Zanella, I.; Fagan, S.B. Theoretical study of small aromatic molecules adsorbed in pristineand functionalized graphene. J. Mol. Model. 2021, 27, 193. [Google Scholar] [CrossRef]
- Calborean, A.; Buimaga-Iarinca, L.; Graur, F. DFT charge transfer of hybrid molecular ferrocene/Si structures. Phys. Scr. 2015, 90, 055803. [Google Scholar] [CrossRef]
- Rabone, J.; Uffelen, P.V. DFT-based Metadynamics simulation of proton diffusion in tetragonal zirconia at 1500 K. J. Nucl. Mater. 2015, 459, 30–36. [Google Scholar] [CrossRef]
- Janes, A.; Lust, E. Use of organic esters as co-solvents for electrical double layer capacitors with low temperature performance. J. Electroanal. Chem. 2006, 588, 285–295. [Google Scholar] [CrossRef]
- Huang, J.; Sumpter, B.G.; Meunier, V. A Universal Model for Nanoporous Carbon Supercapacitors Applicable to Diverse Pore Regimes, Carbon Materials, and Electrolytes. Chem.-Eur. J. 2008, 14, 6614–6626. [Google Scholar] [CrossRef]
- Huang, J.; Sumpter, B.G.; Meunier, V. Theoretical Model for Nanoporous Carbon Supercapacitors. Angew. Chem. Int. Ed. 2008, 120, 530–534. [Google Scholar] [CrossRef]
- Chen, Z.Z.; Pei, X.X.; Song, J.L.; Song, W.Y.; Shi, Z.F.; Onditi, K.O.; Li, Q.; Jiang, X.L. Systematics and evolutionary history of the genus Micromys (Mammalia: Rodentia: Muridae). Mamm. Biol. 2023, 103, 389–403. [Google Scholar] [CrossRef]
- Yang, S.B.; Shan, X.Y.; Li, S.N. Insight into the Adsorption and Diffusion Behaviors of Na on XC3 (X = B, N and P) Doping Graphene Surfaces: A First Principle Study. Mater. Rep. B Res. Pap. 2019, 33, 1640–1645. [Google Scholar] [CrossRef]
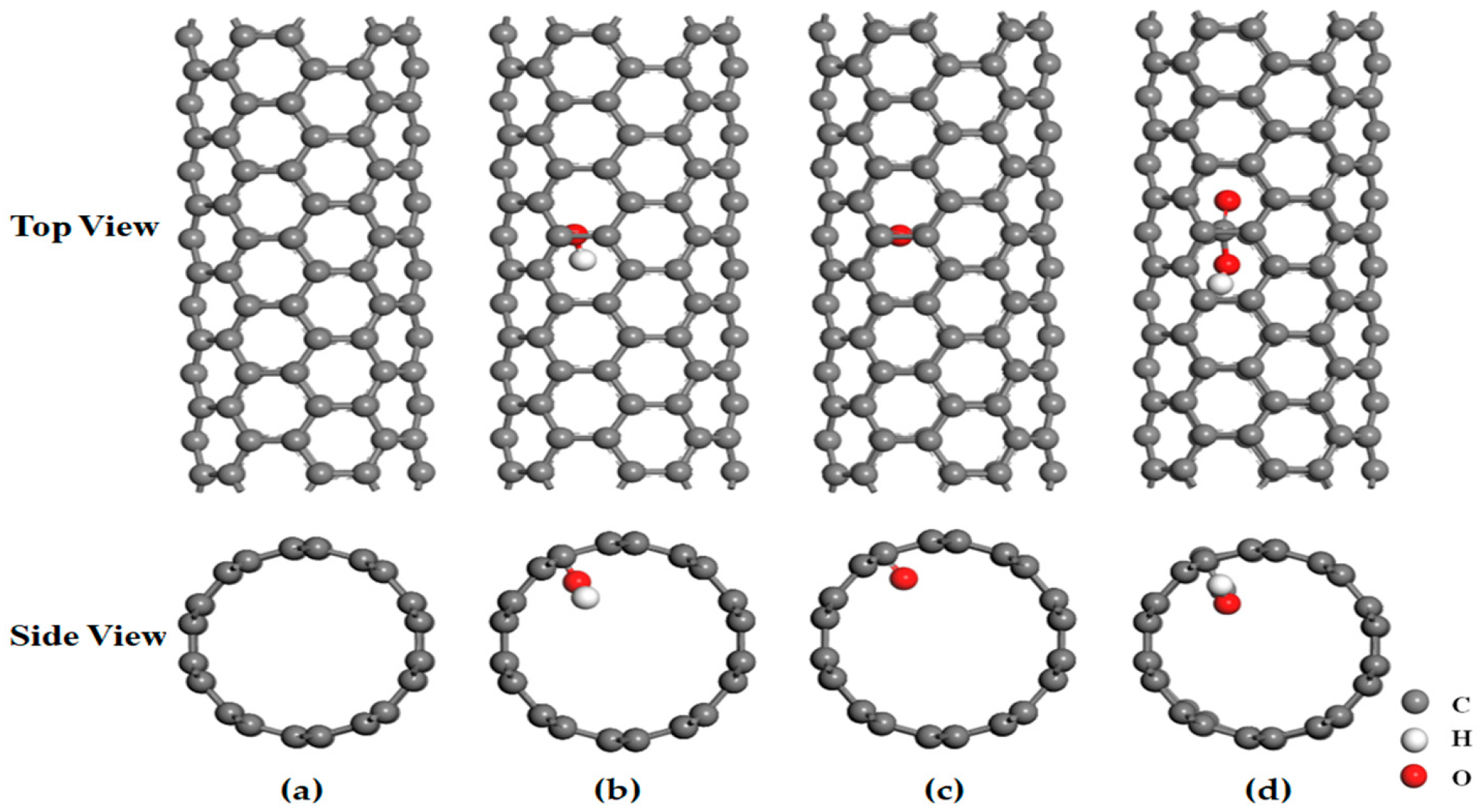
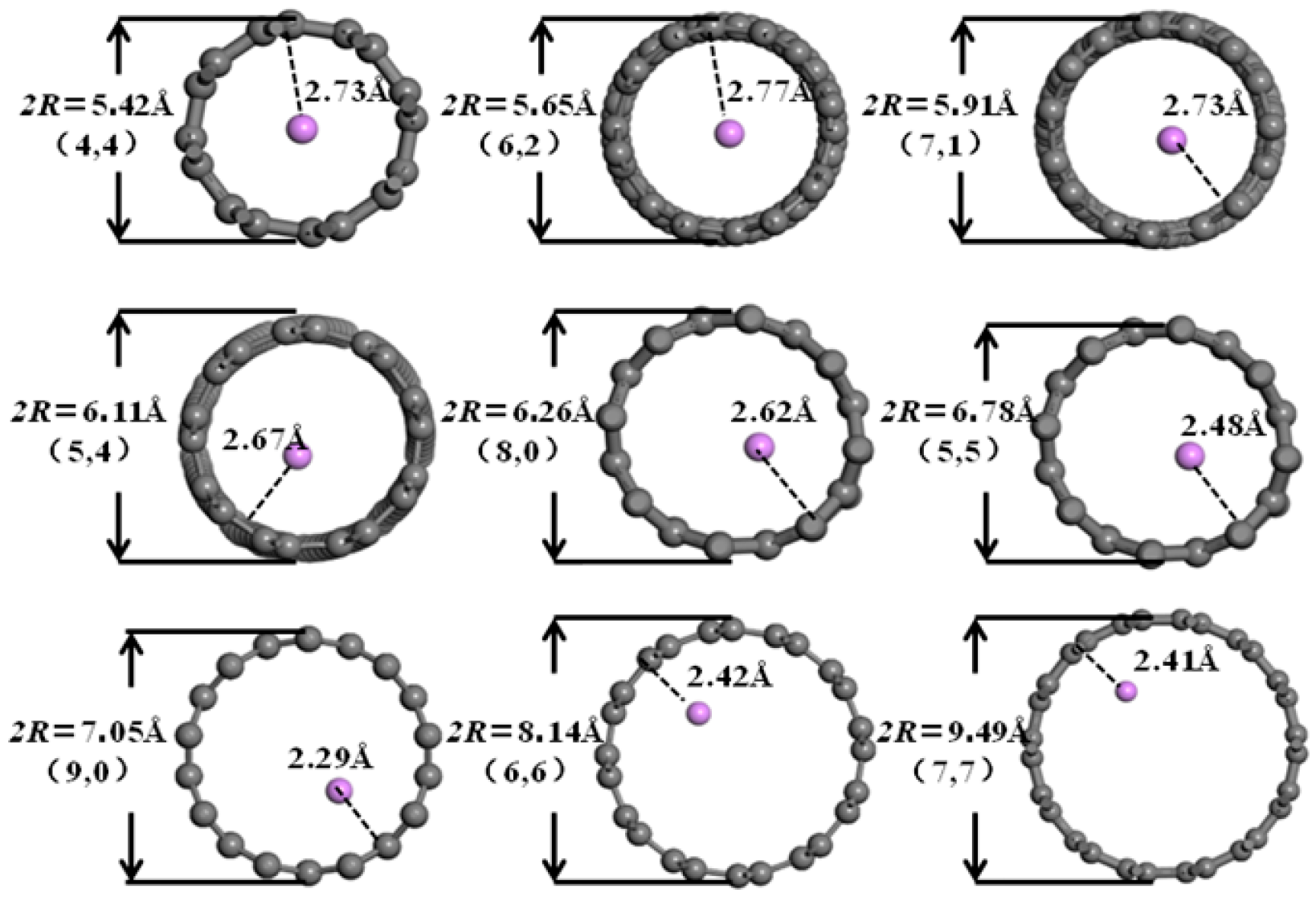

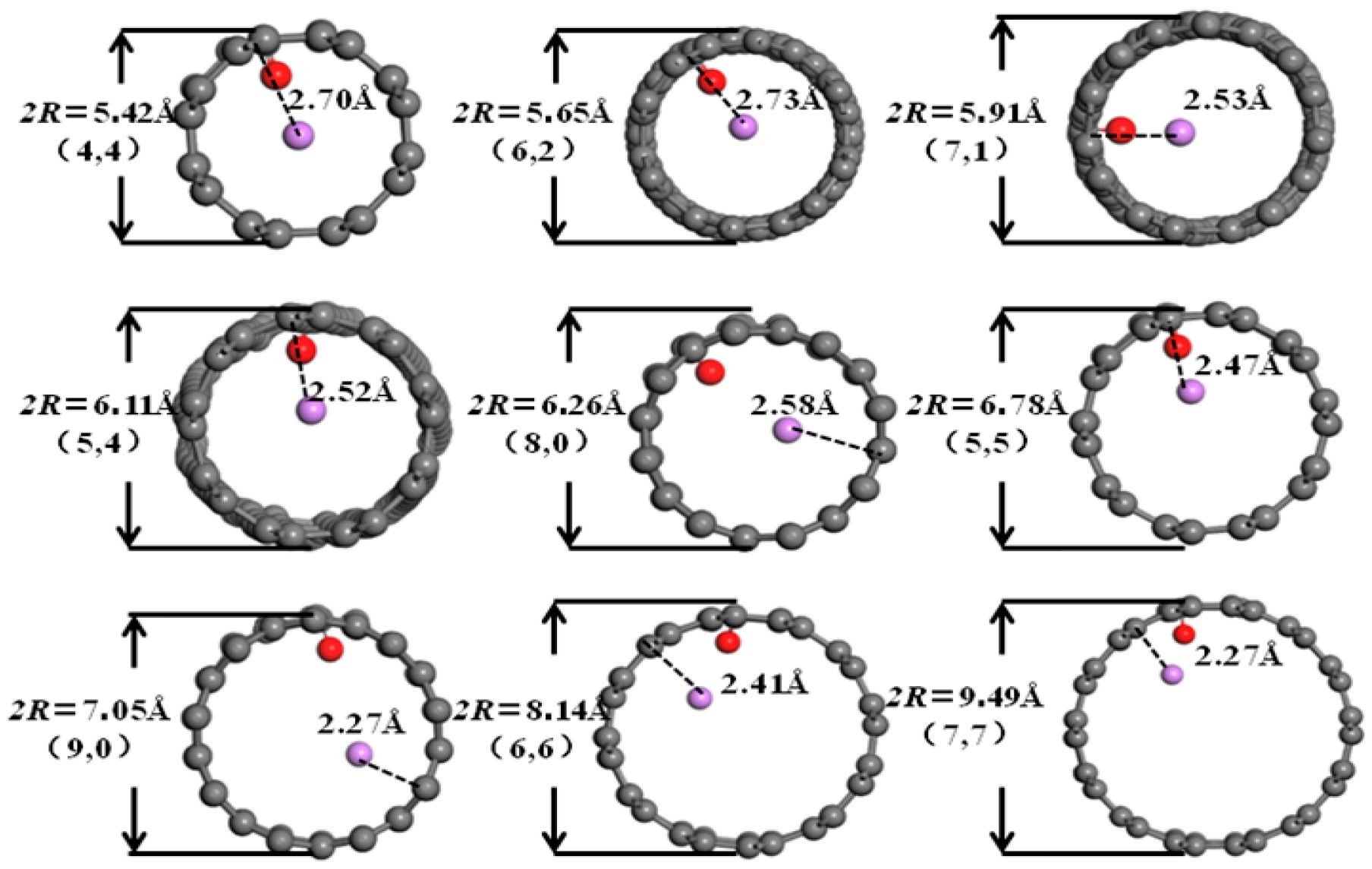
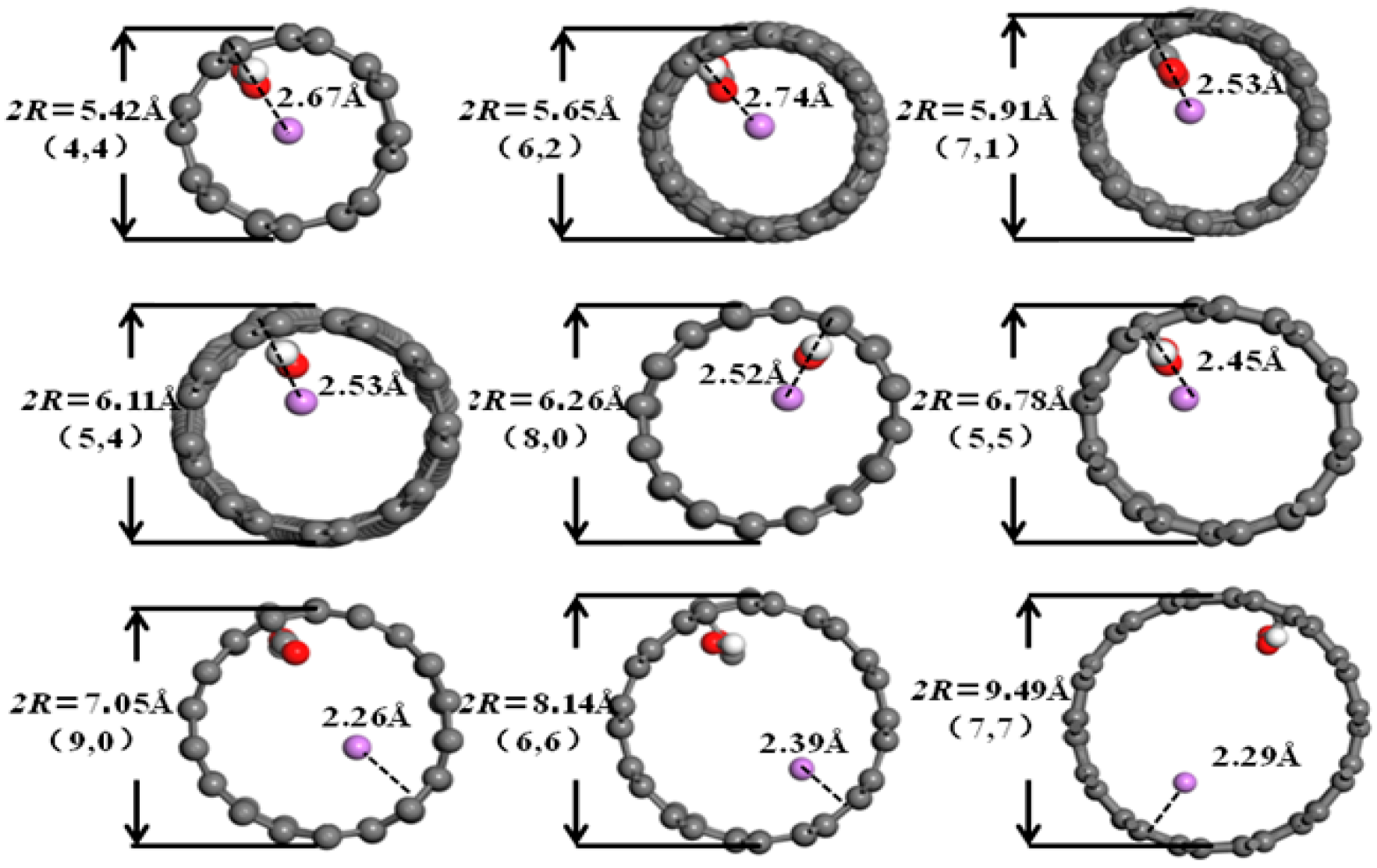


| Structure Form | Armchair | Chiral | Chiral | Chiral | Zigzag | Armchair | Zigzag | Armchair | Armchair |
|---|---|---|---|---|---|---|---|---|---|
| Chiral index | 4:4 | 6:2 | 7:1 | 5:4 | 8:0 | 5:5 | 9:0 | 6:6 | 7:7 |
| Diameter/Å | 5.42 | 5.65 | 5.91 | 6.11 | 6.26 | 6.78 | 7.05 | 8.14 | 9.49 |
| Structure | CNT | HCNT | CNCNT | CXCNT |
|---|---|---|---|---|
| CNT-5.42@ Li+ | 0.66 | 0.88 | 0.66 | 0.88 |
| CNT-5.65@ Li+ | 0.89 | 1.08 | 1.04 | 1.01 |
| CNT-5.91@ Li+ | 1.31 | 1.77 | 1.75 | 1.75 |
| CNT-6.11@ Li+ | 1.58 | 2.02 | 1.88 | 1.86 |
| CNT-6.26@ Li+ | 1.76 | 1.97 | 1.84 | 1.95 |
| CNT-6.78@ Li+ | 2.24 | 2.34 | 2.26 | 2.29 |
| CNT-7.05@ Li+ | 2.70 | 2.83 | 2.75 | 2.77 |
| CNT-8.14@ Li+ | 2.72 | 2.83 | 2.74 | 2.78 |
| CNT-9.49@ Li+ | 2.97 | 3.19 | 3.24 | 3.19 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, F.; Li, S.; Zhu, W.; Zhao, M.; Liu, B. First-Principles Calculation of the Desolvation Effect of Functionalized Carbon Nanotubes. Coatings 2025, 15, 1190. https://doi.org/10.3390/coatings15101190
Liu F, Li S, Zhu W, Zhao M, Liu B. First-Principles Calculation of the Desolvation Effect of Functionalized Carbon Nanotubes. Coatings. 2025; 15(10):1190. https://doi.org/10.3390/coatings15101190
Chicago/Turabian StyleLiu, Fudong, Sinan Li, Wanjun Zhu, Miaomiao Zhao, and Bing Liu. 2025. "First-Principles Calculation of the Desolvation Effect of Functionalized Carbon Nanotubes" Coatings 15, no. 10: 1190. https://doi.org/10.3390/coatings15101190
APA StyleLiu, F., Li, S., Zhu, W., Zhao, M., & Liu, B. (2025). First-Principles Calculation of the Desolvation Effect of Functionalized Carbon Nanotubes. Coatings, 15(10), 1190. https://doi.org/10.3390/coatings15101190





