Abstract
NiTi alloys are widely used in biomedical applications due to their shape memory and superelastic properties. However, their surface reactivity requires protective, biofunctional coatings. To enhance NiTi performance, its surface was modified with an Ag-SiO2-TiO2 nanocoating containing small amounts of silica and silver. The coating’s primary phase was rutile with structural defects and a silver solid solution. It showed good adhesion, high scratch resistance, and improved corrosion behavior in Ringer’s solution, as demonstrated by EIS and cyclic polarization. EIS revealed high low-frequency impedance and two time constants, suggesting both barrier protection and slower electrochemical processes. Despite low breakdown and repassivation potentials, the coating effectively limited uniform corrosion. SEM/EDS confirmed localized degradation and partial substrate exposure, while elemental mapping showed well-dispersed silica and silver in a TiO2-rich matrix. The proposed pitting mechanism involves chloride-induced depassivation and galvanic effects. Surface potential mapping indicated electrostatic heterogeneity, mitigated by silica. The coating offers a balanced combination of corrosion protection and biofunctionality, supporting its potential for implant use.
1. Introduction
One of the most effective approaches to enhancing the surface properties of alloys used as biomedical implants is the application of multifunctional coatings. These coatings impart a range of desirable attributes to implant surfaces, including antibacterial activity [1,2,3], improved wear resistance [4], enhanced biocompatibility and bioactivity [5], and increased corrosion resistance [6,7]. The use of nanomaterials for this purpose has garnered significant attention due to their exceptional properties, which arise from their nanoscale dimensions and substantially increased specific surface area compared to conventional materials [8,9,10].
Of particular interest are multifunctional nanocomposite layers that combine the advantages of individual components while providing multiple desirable properties. The combination of ceramic and metallic materials at the nanoscale enables the creation of structures with high biocompatibility and the ability to actively combat bacterial infections. Among these solutions are coatings incorporating metal nanoparticles, including silver [11,12], copper [11,13], or zinc [11,14], which are well known for their potent antimicrobial efficacy in significantly inhibiting the proliferation and colonization of pathogenic microorganisms.
Silver nanoparticles (Ag NPs), recognized for their exceptional and broad-spectrum antibacterial properties, are increasingly used to modify implant surfaces due to their proven efficacy in significantly reducing the risk of bacterial infection and biofilm formation at the implantation site [12]. However, maintaining a low silver content (ca. 1 wt.%) is essential in the design of implant coatings, as higher concentrations have been associated with increased toxicity toward both bacteria and human cells. By contrast, silver levels below 1 wt.% in coatings such as Ag-hydroxyapatite on titanium [15], Ag/SiO2-β-TCP [16], and Ag-TiO2 or Hydroxyapatite-Ag-TiO2 [17] have been reported to stimulate cell proliferation without inducing cytotoxic effects.
AgNPs can be incorporated into ceramic matrices such as hydroxyapatite [18,19], titanium dioxide [20,21,22], or silica [23,24], enabling controlled, gradual release that ensures a sustained antibacterial effect while preserving the biocompatibility and bioactivity of the implant surface.
The presence of a TiO2 layer on the surface of nickel–titanium alloy is widely recognized for its ability to enhance the alloy’s corrosion resistance. TiO2 naturally forms a dense and stable passive oxide layer that acts as a physical barrier against the diffusion of aggressive ions, such as chloride. This protective film significantly reduces the release of nickel ions into the surrounding environment, thereby lowering the corrosion rate of the alloy [25,26]. Moreover, the oxide layer promotes surface passivation, further enhancing the durability of the material in corrosive and biological environments.
In addition to TiO2, SiO2 has been identified as an effective additive in anti-corrosion coatings. SiO2 enhances the mechanical properties of the coating by increasing its hardness and abrasion resistance, thereby contributing to the long-term protection of the metal substrate [27]. Its high chemical stability also provides an additional barrier effect, preventing the penetration of corrosive substances such as water, oxygen, and salts [28,29]. This is particularly beneficial in biomedical applications, where device longevity and structural integrity are critical.
However, despite the many advantages of silica, its use as a coating material may be limited in surface engineering methods involving elevated temperatures, as silica is known to undergo polymorphic transformations under such conditions, resulting in substantial changes in material volume, which in turn can lead to the formation of cracks and the degradation of the coatings [30,31].
The incorporation of Ag NPs into the coating matrix introduces additional functionality. Silver is well known for its antimicrobial activity, but it also contributes to corrosion resistance. When embedded in a TiO2 matrix, Ag NPs can synergistically enhance the protective capabilities of the coating. They promote the formation of a more compact and uniform oxide layer and reduce localized corrosion phenomena, such as pitting, which are commonly observed in physiological environments [32,33,34].
Therefore, the combined use of TiO2, SiO2, and Ag NPs is expected to produce a multifunctional coating with enhanced properties. This nanocomposite layer not only improves the corrosion resistance of the NiTi alloy but also imparts antibacterial and biocompatible characteristics, making it particularly suitable for biomedical applications such as cardiovascular stents, bone implants, and orthodontic devices [25]. The multifunctionality of such coatings addresses multiple clinical requirements simultaneously, namely chemical stability, biological safety, and infection control, and reflects recent advances in materials science aimed at developing next-generation implantable devices.
This article presents the findings of research focused on enhancing the surface properties of NiTi alloy through the application of a multifunctional Ag-SiO2-TiO2 nanocoating. The study evaluates the coating’s adhesive strength, scratch resistance, corrosion resistance, and electronic performance.
2. Materials and Methods
2.1. Materials
The research employed Ag-SiO2-TiO2 nanocomposite coatings fabricated through electrophoretic deposition (EPD) on a passivated NiTi shape memory alloy surface. The coatings were deposited via anaphoresis at 40 V for 3 min, followed by heat treatment at 800 °C for 2 h under vacuum conditions. A detailed description of the Ag-SiO2-TiO2 coating fabrication process and the mechanism of its formation can be found in [22].
2.2. Methods of Testing
2.2.1. Scratch Resistance
The scratch resistance of the coatings was evaluated using a Micro Combi Tester (MCT3) from Anton Paar (Corcelles-Cormondrèche, Switzerland) following the scratch-test method. The testing procedure adhered to the guidelines specified in ISO 19252:2025 [35], ISO 20502:2005 [36], ASTM C1624-22 [37], and ASTM D7027-20 [38]. A Rockwell diamond indenter with a tip diameter of 100 μm was employed for all measurements. Each scratch test was conducted in three distinct stages. In the initial pre-scan phase, the surface profile was scanned under a minimal load of 0.03 N to establish a baseline. The second stage, the scan, involved a progressively increasing load ranging from 0.03 N to 30 N, applied over a scratch length of 6 mm at a constant indenter speed of 12 mm min−1. The final post-scan phase re-scanned the scratched surface using the same 0.03 N load to assess deformation and damage. Key parameters such as friction force (Ft) and acoustic emission (AE) were continuously recorded. The reported results represent the mean values obtained from three independent measurements to ensure repeatability and reliability. Post-test surface analysis was performed using a scanning electron microscopy (SEM) to identify failure mechanisms.
2.2.2. Scanning Electron Microscopy
SEM analysis was performed using a TESCAN Mira 3 LMU microscope (TESCAN, Brno, Czech Republic) at an accelerating voltage of 15 kV, equipped with an Energy-Dispersive Spectrometer (EDS) from Oxford Instruments-Aztek (Abingdon, UK). Imaging was conducted by collecting secondary electrons (SE) and backscattered electrons (BSE). Prior to analysis, the samples were sputtered with a 5 nm chromium layer using a Quorum Q150T ES system (Quorum Technologies Ltd., Laughton, UK). Surface chemical composition was analyzed in three distinct areas of each sample, and the results were averaged to obtain representative data. Additionally, SEM was used to investigate corrosion damage in the NiTi alloy coated with Ag-SiO2-TiO2 after destructive cyclic potentiodynamic polarization measurement.
2.2.3. Electrochemical Measurements of Corrosion Resistance In Vitro
The corrosion resistance of the NiTi alloy coated with Ag-SiO2-TiO2, deposited at 40 V for 180 s, was investigated in vitro under simulated body fluid (SBF) conditions using Ringer’s solution. The solution contained sodium chloride (8.60 g L−1), potassium chloride (0.30 g L−1), and calcium chloride dihydrate (0.33 g L−1). In accordance with the international standard ISO 10271:2021 [39], the pH of the solution was adjusted to the physiological range of 7.4 ± 0.1 using a 4% sodium hydroxide solution and a 1% lactic acid solution. Ringer’s solution was prepared using analytical-grade reagents (Avantor Performance Materials Poland S.A., Gliwice, Poland) and ultrapure water with a conductivity of 0.055 μS cm−1 at 25 °C, produced by the Milli-Q Advantage A10 water purification system (Millipore SAS, Molsheim, France). Electrochemical measurements were conducted at 37.0 ± 0.1 °C using a thermostat, and the solution was degassed with high-purity (5.0) argon for 30 min prior to testing.
Corrosion resistance measurements were carried out using a three-electrode setup, in which the tested sample served as the working electrode (WE), a platinum foil (8 cm2) was used as the counter electrode (CE), and a saturated calomel electrode (SCE) functioned as the reference electrode (RE). The RE was introduced into Ringer’s solution via a Luggin capillary. The WE had a geometric surface area of 1.0 cm2. To minimize the reintroduction of air into the electrochemical cell, an argon flow was maintained over the surface of the Ringer’s solution throughout the measurements. All electrochemical tests were performed using a modular high-current potentiostat/galvanostat system with a maximum current of 1 A and a compliance voltage of 30 V (Metrohm Autolab B.V., Utrecht, The Netherlands). The open-circuit potential (EOC) was stabilized for 1 h and subsequently considered as an approximate corrosion potential (Ecor) for further analysis.
Electrochemical impedance spectroscopy (EIS) studies were performed using a potentiostat coupled with a frequency response analyzer (FRA) at the EOC. The frequency (f) range spanned from 50 kHz to 1 mHz, with 10 frequencies per decade and a frequency resolution of 0.003%. A small perturbation of 10 mV was applied to the system. The experimental EIS data were analyzed using the concept of an equivalent electrical circuit and fitted using the complex nonlinear least squares (CNLS) method.
In direct current (DC) measurements conducted using the cyclic potentiodynamic polarization method, the potential was scanned at a polarization rate of v = 1 mV s−1, starting from a potential 200 mV more negative than the EOC up to the breakdown potential (Ebd), where corrosion pitting initiated on the electrode surface. After reaching Ebd, the scan direction was reversed toward more negative potentials. The reverse scan continued until the protection potential (Ep) was reached, at which point the pits disappeared and the electrode surface was considered repassivated or protected against further pitting corrosion. The resulting current-potential curves, presented in a semi-logarithmic plot, were analyzed to determine key parameters related to pitting corrosion resistance. To ensure accurate visualization and interpretation of the data, electrochemical noise was minimized using the Savitzky–Golay smoothing algorithm, implemented in the General Purpose Electrochemical System (GPES) software for Windows, version 4.9 [40].
The evaluation of corrosion damage to the NiTi alloy coated with Ag-SiO2-TiO2 was performed after destructive cyclic potentiodynamic polarization measurements, with anodic limits set at 0.5 V, using SEM.
2.2.4. Measurements Using the Kelvin Probe Scanning Method
The electronic properties of the tested materials were evaluated using the Kelvin Probe Scanning (SKP) method in air. The measurements were performed with a scanning electrochemical workstation PAR Model 370 (Princeton Applied Research, Oak Ridge, TN, USA), which included an integrated SKP370 module and an optical video microscope (VCAM3). Ultra-high measurement resolution was achieved through the careful selection of materials and by using the smallest possible diameter for the microsensor tip. A U-SKP-150 tungsten microsensor (Uniscan Instruments, Buxton, UK) was employed, featuring a brass housing and a tungsten wire with a diameter of 150 μm. The microsensor tip was designed to ensure stable and repeatable measurements by maintaining a constant local contact potential difference (CPD) throughout the measurement process. An air gap between the housing and the wire acted as a shield to reduce stray capacitance and improve signal quality.
The microsensor was positioned approximately 100 μm above the surface of the conductive sample. To assess changes in the CPD distribution, a selected area of 2000 × 2000 μm was scanned. The scanning was performed in height-tracking mode, allowing the microsensor to adjust its vertical position within a range of 0.3 to 1.0 mm. Maintaining a constant distance from the sample surface enabled measurements on uneven surfaces. The microsensor moved parallel to the sample surface, with the tip and surface forming a configuration similar to a parallel plate capacitor. This setup allowed measurement of the CPD, representing the voltage difference at the point of contact between the two conductors, providing valuable information about the electronic properties of the tested materials.
Measurement data were collected and processed using Scanning Electrochemical Work Station M370 Version 2.45 software. Surface maps of the CPD distribution were used to generate histograms, which were then fitted with a Gaussian curve. Changes in CPD values on the sample surfaces served as highly sensitive indicators of surface phenomena related to the modification of the NiTi alloy. This sensitivity allowed detection of subtle changes in the electronic properties of the alloy surface, offering valuable insights into the effects of surface modification processes.
3. Results and Discussion
3.1. Morphology, Structure and Chemical Composition of the Coating
The structure of the coating formed on the NiTi alloy surface was characterized using X-ray diffraction (XRD), Raman spectroscopy, and Fourier-transform infrared (FTIR) spectroscopy and comprehensively described in [22]. The nanocomposite coatings exhibited an island-like morphology (Figure 1), comprising a continuous SiO2-TiO2 interlayer uniformly covering the NiTi substrate and small agglomerates [22]. The agglomerates primarily consisted of titanium oxide and its solid solution with silver, which displayed a higher density of defects. Additionally, the presence of silver oxides was identified within the coating. XRD confirmed that the titanium oxide phase in the coating was predominantly in the rutile form. The average coating thickness was measured to be approximately 2.02 ± 0.03 μm [22].
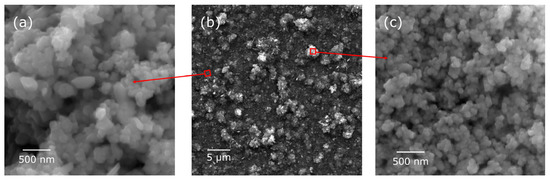
Figure 1.
SE images of the Ag-SiO2-TiO2 coating/NiTi at different magnifications (a–c): (a) enlargement of the area between agglomerates and (c) enlargement of the agglomerate.
The average chemical composition of the Ag-SiO2-TiO2/NiTi coating surface determined by SEM/EDS is summarized in Table 1.

Table 1.
The average chemical composition (wt%) of the Ag-SiO2-TiO2/NiTi coating surface.
The average chemical composition of the Ag-SiO2-TiO2/NiTi coating surface indicates a dominant presence of titanium (53.5 wt%) and oxygen (38.3 wt%), which confirms the formation of oxides, mainly TiO2, responsible for the coating’s high hardness, wear resistance, and excellent corrosion resistance [41]. The presence of nickel (6.8 wt%) likely originates from the NiTi substrate, and its low content on the surface suggests effective limitation of element migration, which is particularly important in terms of biocompatibility, especially in biomedical applications [41]. The content of silicon (0.6 wt%) in the form of SiO2, although small, plays an important role in improving the chemical and thermal stability of the material, and may also enhance its bioactivity, particularly in relation to bonding with bone tissue [42]. Silver (0.8 wt%), introduced into the composition, provides antibacterial properties, significantly enhancing the coating’s functionality, especially in environments prone to infection, while its low concentration prevents negative effects on the material’s mechanical properties [43].
3.2. Adhesion of the Coatings
Strong adhesion between the coating and the metallic substrate is crucial for implant functionality. The combination of strong adhesion and high scratch resistance is essential to ensure the durability and reliable performance of the implant during surgical procedures and under physiological conditions.
A representative SE image of the Ag-SiO2-TiO2 coating/NiTi surface after adhesion testing is presented in Figure 2.
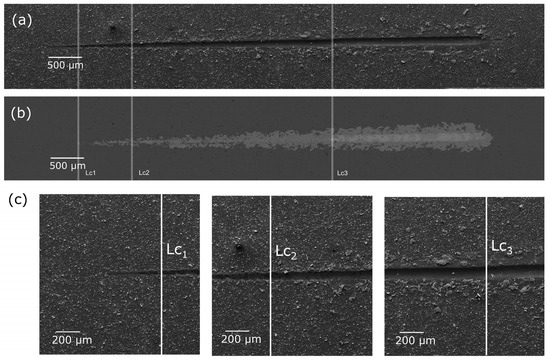
Figure 2.
SE images (a,c) and BSE images (b) of the Ag-SiO2-TiO2/NiTi coating after scratch-test with marked critical load (Lc1–Lc2).
The tests revealed that the produced Ag-SiO2-TiO2 coatings exhibited excellent adhesion to the NiTi substrate and high scratch resistance. Three critical loads were identified, as summarized in Table 2. Lc1 represents the load at which initial damage to the layer is observed, Lc2 corresponds to the load where the first spalling occurs along the scratch edges (both cohesive and interfacial), and Lc3 marks the load at which complete layer failure occurs.

Table 2.
Critical load for Ag-SiO2-TiO2 coatings, where Ft is a friction force and AE an acoustic emission.
A comparison of the test results for the Ag-SiO2-TiO2 coating with those for the Ag-TiO2 coating without silica [17] reveals differences. The incorporation of a small amount of a nanosilica into the system is expected to influence the mechanical properties of the coating, potentially promoting the formation of new phases with a glass-like structure that exhibit strong adhesion to the substrate [44]. For the Ag-SiO2-TiO2 coatings, the development of a continuous SiO2-TiO2 was observed [22]. This interlayer likely contributed to an increase of more than 50% in the Lc1 value compared to the coating without silica. However, the initial spalling of the coating occurred at a lower Lc2 value. Complete failure (Lc3) for both coatings was comparable, with a slightly higher Lc3 value recorded for the silica-containing coating.
3.3. Corrosion Resistance of the Coatings
3.3.1. Evaluation of Corrosion Behavior
EIS was used to determine the impedance of the Ni-Ti electrode|Ag-SiO2-TiO2 coating|Ringer’s solution interface in the electrochemical corrosion process, along with its capacitive characteristics. In vitro studies of corrosion resistance began with stabilization of the EOC for 1 h. Following this, experimental EIS data were recorded at EOC. These data are marked with symbols and presented in Figure 3.
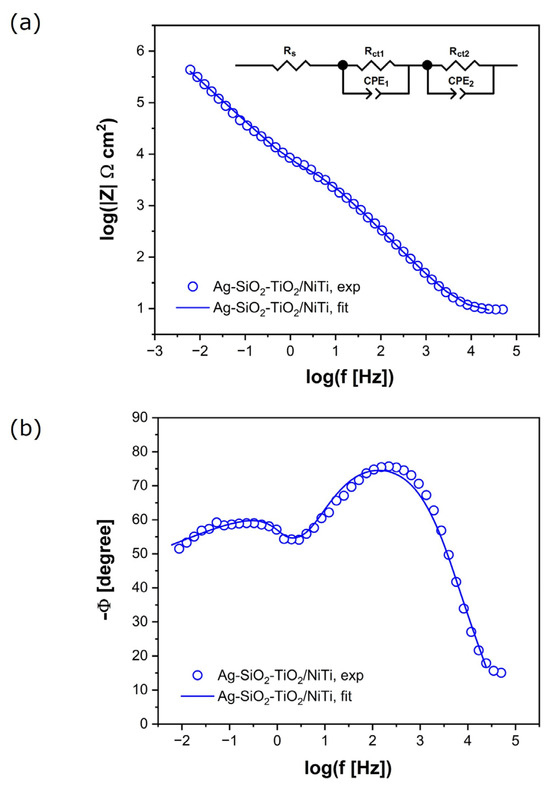
Figure 3.
Experimental (symbols) and simulated (solid lines) EIS spectra on a logarithmic frequency scale for the Ag-SiO2-TiO2 coating on a NiTi substrate in Ringer’s solution at 37 °C: (a) Bode plot of impedance magnitude; (b) Phase angle.
The Bode plot, illustrating the dependence of the logarithm of the impedance magnitude on the logarithm of frequency, shows a slope of |Z| in the medium-frequency range close to −1 (Figure 3a). The maximum values of the phase angle (Φ) are lower than the ideal value of −90° (Figure 3b). Two time constants are observed in the equivalent electrical circuit. The high values of |Z| as f → 0 and the phase angle Φ visible in the impedance characteristics confirm the capacitive behavior of the tested material and its high resistance to electrochemical corrosion in vitro.
To understand the corrosion mechanism and determine the kinetics of the electrochemical corrosion process of the Ag-SiO2-TiO2 coatings on the Ni-Ti substrate in Ringer’s solution, the experimental data were fitted using the electrical equivalent circuit (2CPE model) shown in Figure 3a. Each parameter in the circuit has a defined physicochemical interpretation related to the impedance of the Ni-Ti substrate|Ag-SiO2-TiO2 coating|Ringer’s solution interface. Rs corresponds to the solution resistance. Rct represents the charge transfer resistance at both the Ni-Ti|Ag-SiO2-TiO2 interface and the Ag-SiO2-TiO2|Ringer’s solution interface. CPE denotes the constant phase element, which is used instead of a pure capacitor to account for non-ideal capacitive behavior. This approach is commonly used to improve fitting accuracy for metal materials covered with oxide films, where EIS spectra deviate from the classical Randles equivalent circuit [17,44,45].
The impedance of the CPE is defined by Equation (1):
where T is the CPE capacitive parameter, expressed in F cm−2 sϕ−1, and ϕ is the CPE exponent related to the constant phase angle, α = 90°(1 − ϕ); this is dimensionless and takes values ≤ 1.
The so-called 2CPE model (two constant phase elements), used to study the interfacial properties of electrochemical systems, consists of two modified Randles circuits connected in series, resulting in two semicircles in the Nyquist plot. The semicircle at high frequencies is described by the parameters Rs, T1, ϕ1, and Rct1, while the semicircle at low frequencies is characterized by T2, ϕ2, and Rct2. The resistance values (Rs, Rct1, and Rct2) provide detailed information about the corrosion protection afforded by the coating. In the 2CPE model applied to pitting corrosion, the low-frequency semicircle in the impedance spectrum is associated with faradaic reactions, which are slow and governed by charge transfer at the electrode surface. These reactions typically involve corrosion processes such as metal oxidation and reduction. In contrast, the high-frequency semicircle corresponds to the impedance of pores (pits) in the oxide layer. This impedance is related to fast electrochemical processes, such as electric double-layer formation and surface reactions, which are not limited by reaction kinetics. The fitted EIS data, obtained using the CNLS method and the 2CPE model, are represented by solid lines in Figure 3. A very good agreement with the experimental impedance data was achieved. The parameter values resulting from the fit for the tested electrodes at EOC in Ringer’s solution at 37 °C are summarized in Table 3.

Table 3.
Fitted parameter values (±standard deviation) obtained using the 2CPE model for the EIS data of the Ag-SiO2-TiO2/NiTi electrode, and, for comparison, using the 1CPE model for the Ag–TiO2/NiTi [17], mechanically polished NiTi [44], and steam-passivated NiTi [44] electrodes, all measured at EOC in Ringer’s solution at 37 °C. Rs = 8.42 ± 0.36 Ω cm2.
The obtained EIS data indicate the presence of two time constants, confirming the existence of two mechanisms governing the corrosion processes: a faster one dominating the high-frequency range and a slower one associated with faradaic processes in the low-frequency range. The appearance of two semicircular arcs in the Nyquist plot, along with high impedance values at low frequencies (|Z|f→0), demonstrates the coating’s good barrier properties and its effectiveness in providing long-term corrosion protection.
The applied 2CPE model, consisting of two R||CPE branches, provides an accurate fit to the experimental data, confirming that the extracted parameters realistically represent the system’s behavior. The obtained charge transfer resistance values are Rct1 = 7.10 × 103 Ω cm2 in the high-frequency range and Rct2 = 7.76 × 105 Ω cm2 in the low-frequency range. The high Rct2 value indicates effective suppression of redox reactions during prolonged exposure to the electrolyte, which is especially important for biomaterials intended for extended contact with physiological fluids.
In comparison to the results reported for the Ag-TiO2 coating in [17], where only one time constant was identified and Rct1 was approximately 4.40 × 105 Ω cm2, the Ag-SiO2-TiO2 coating demonstrates a more complex protective structure. Although Rct1 in this study is lower, the presence of a second process (Rct2) in the low-frequency range suggests that the coating may offer enhanced resistance to slow degradation mechanisms, such as diffusion through a porous layer or interactions with aggressive ions. Moreover, the lower exponent values ϕ1 and ϕ2 (0.867 and 0.845, respectively), compared to ϕ1 = 0.982 for Ag-TiO2, may indicate greater heterogeneity within the coating. However, this heterogeneity could also reflect improved adaptability to complex environmental conditions, possibly due to the incorporation of SiO2.
When compared to the NiTi surface subjected to mechanical treatment and autoclave passivation, as analyzed in study [44], the Ag-SiO2-TiO2 coating exhibits lower Rct values. For passivated NiTi, Rct1 reached 5.90 × 107 Ω cm2, indicating the highest corrosion resistance among the materials compared. However, it should be noted that despite its excellent barrier performance, this material lacks functional properties such as antibacterial activity and bioactivity. Similarly, for NiTi subjected only to mechanical treatment and natural passivation, the Rct1 value was also high (4.00 × 106 Ω cm2), but again, the absence of synergy between electrochemical protection and biological functionality is evident.
Against this background, the Ag-SiO2-TiO2 coating stands out as a material with balanced properties: it offers reasonable corrosion resistance—particularly in the long term due to its high Rct2—along with the potential for biological functionalization. Ag provides antibacterial effects, SiO2 promotes bioactivity and apatite deposition, while the rutile form of TiO2 enhances surface passivation. The presence of two time constants allows for a more precise analysis of complex corrosion mechanisms and may reflect the simultaneous action of a barrier layer and slow transport through porous or functionalized structures. The Ag-SiO2-TiO2 coating represents a promising solution in the context of modern implant materials. Its complex electrochemical characteristics, synergistic protective and functional properties, and effective corrosion resistance on both micro and macro scales indicate its suitability for applications requiring long-term stability and biologically active surfaces.
3.3.2. Characterization of Pitting Corrosion Susceptibility
The susceptibility of the tested electrodes to pitting corrosion was evaluated using cyclic potentiodynamic polarization measurements. Based on the recorded polarization curves, presented on a semi-logarithmic scale, key potentials characteristic of the initiation and repassivation of pits were determined. The plot showing the change in current density as a function of potential for the Ag-SiO2-TiO2/NiTi electrode in Ringer’s solution at 37 °C is presented in Figure 4.
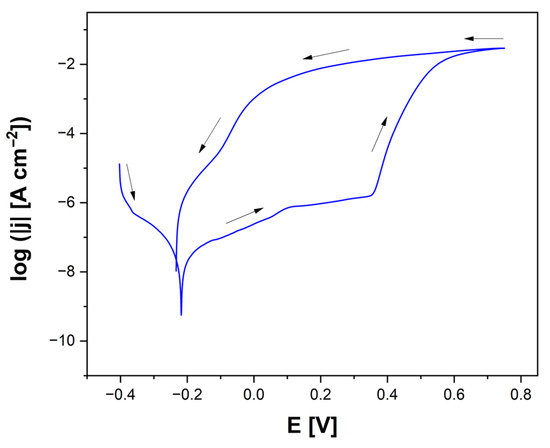
Figure 4.
Cyclic polarization curve for the Ag-SiO2-TiO2/NiTi electrode in Ringer’s solution at 37 °C. Arrows indicate the direction of polarization at a scan rate of v = 1 mV s−1.
It was observed that the reverse (return) branch of the polarization curve did not coincide with the forward (initial) branch over a certain potential range, resulting in a characteristic hysteresis loop. The intersection point of both branches was defined as Ep, below which existing pits could close (repassivate). In contrast, Ebd indicated the threshold potential for the initiation of new pits, which could form only at potentials more positive than Ebd. Within the potential range between Ep and Ebd, no new pits were initiated. However, existing pits could continue to grow. Therefore, the width of the hysteresis loop is a significant indicator of susceptibility to pitting corrosion. The wider the loop, the greater the risk of pitting corrosion progression. A comparison of the electrochemical properties of various NiTi-based electrodes, including Ag-SiO2-TiO2/NiTi, Ag-TiO2/NiTi [17], mechanically polished NiTi [44], and steam-passivated NiTi [36], all tested in Ringer’s solution at 37 °C, is presented in Table 4.

Table 4.
Key electrochemical potentials determined from cyclic polarization curves for Ag-SiO2-TiO2/NiTi, Ag-TiO2/NiTi [17], mechanically polished NiTi [44], and steam-passivated NiTi [44] electrodes tested in Ringer’s solution at 37 °C.
The data clearly highlight differences in Ecor, Ebd, and Ep, as well as in hysteresis widths, providing insight into the relative corrosion resistance of each surface modification. Based on the values of Ecor, Ebd, and Ep, an assessment was conducted regarding both the susceptibility to pitting corrosion and the overall corrosion resistance of the tested surfaces. The Ag-SiO2-TiO2/NiTi electrode exhibits relatively high susceptibility to pitting corrosion, as indicated by its low Ebd value and negative repassivation potential. This suggests a limited ability of the surface to repassivate after pit initiation, which may lead to rapid pit propagation. Nevertheless, the presence of a surface layer containing Ag, SiO2, and TiO2 can still offer long-term protection against both micro- and macroscopic corrosion. This is attributed to the complex surface structure and favorable barrier properties, as evidenced by the presence of two time constants in the 2CPE model (Table 3).
In contrast, the Ag-TiO2/NiTi electrode is characterized by very high Ebd and Ep values, indicating exceptional resistance to the initiation and propagation of pitting corrosion. This type of surface effectively prevents the penetration of the corrosive environment. However, it is worth noting that its electrochemical characterization was simplified to a single arc in the impedance analysis (1CPE model), which may not fully capture the complexity of its protection mechanisms (Table 3). Despite this simplification, the parameters indicate highly effective protective behavior.
The mechanically polished NiTi alloy surface exhibits moderate susceptibility to pitting corrosion. Its Ebd and Ep values are higher than those of the Ag-SiO2-TiO2/NiTi electrode but lower than those of the other tested surfaces. This surface benefits from good natural passivation, resulting from the NiTi alloy’s inherent ability to spontaneously form a thin oxide layer. However, the absence of an additional functional layer containing Ag, SiO2, and TiO2 limits the long-term durability of this protection, especially in more aggressive environments. Mechanically polished NiTi represents the simplest form of protection, effective only to a limited extent.
The best corrosion resistance is demonstrated by the NiTi sample subjected to steam autoclave passivation. Its positive Ecor value clearly indicates high electrochemical stability. The high Ebd and Ep values confirm effective resistance to pitting corrosion and strong repassivation ability following damage to the passive layer. The passive film formed under high-temperature conditions is both durable and stable. However, similar to the mechanically polished NiTi, it lacks additional functional properties, such as antibacterial activity or the ability to support bioactive molecule attachment.
3.3.3. Assessment of Corrosion Damage After Electrochemical Testing
An assessment of corrosion damage on Ag-SiO2-TiO2 coatings on NiTi substrates was conducted after cyclic potentiodynamic polarization tests in Ringer’s solution at 37 °C, with an anodic limit of 0.5 V. SEM analysis confirmed the susceptibility of the coatings to pitting corrosion, which initiates locally and progresses into the material, as evidenced by the SE images shown in Figure 5. Observations were made at various magnifications (301×–15,000×), enabling a comprehensive evaluation of both the overall surface condition and the microstructural details of individual pits.

Figure 5.
SE images of the Ag-SiO2-TiO2 coating on a NiTi substrate after cyclic potentiodynamic polarization tests in Ringer’s solution at 37 °C, with an anodic limit of 0.5 V. (a,b)—general surface view at lower magnifications; (c–f)—magnified views showing individual pits.
At lower magnifications in Figure 5a,b, numerous corrosion pits with irregular shapes and varying sizes were observed, unevenly distributed across the coating surface. This morphological heterogeneity, characteristic of pitting corrosion, indicates local breakdowns of the passive layer, likely caused by structural defects such as micropores, microcracks, variable coating thickness, and limited adhesion to the substrate, as well as variations in the protective properties of the coating. The surface between the pits exhibits significant roughness, along with small porosities and depressions, suggesting microscopic discontinuities in the coating and the potential accumulation of corrosion products and undissolved components from the Ringer’s solution.
The SE images taken at higher magnifications (Figure 5c–f) reveal the detailed morphology of the pits and the microstructure of the coating surface in their immediate vicinity. Deep, localized material losses with sharp, well-defined edges and irregular interiors are visible. The interiors of the pits exhibit darker contrast and the presence of fibrous, needle-like, or spherical structures, interpreted as corrosion products. Some pits display a morphology resembling cavities with fibrous or dendritic features, which may be associated with the progression of corrosion into the material due to chloride ions, leading to localized depassivation of the surface. This indicates a mechanism typical of pitting corrosion, involving abrupt local breakdown of the passive oxide layer and the development of highly intense electrochemical reactions.
Additionally, in certain areas, the microstructure is characterized by the presence of small, irregularly distributed particles with spherical, flaky, or clumped shapes. Such morphological diversity may indicate the complexity of the corrosion processes, involving both the degradation of ceramic phases (TiO2, SiO2) and the secondary deposition of corrosion products. Grinding lines visible in some images (e.g., Figure 5b) suggest a possible influence of surface preparation on the local initiation of pits. This phenomenon may be related to mechanical stresses and discontinuities within the coating structure, which facilitate the penetration of the electrolyte into the protective layer.
Figure 6 presents an SE image of the surface of the Ag-SiO2-TiO2 coating applied to a NiTi substrate, indicating the presence of a corrosion pit (a), along with the corresponding elemental surface distribution maps for nickel (b), titanium (c), silver (d), silicon (e), and oxygen (f). All elemental distribution maps show a distinctly dark area within the pit, suggesting a reduced concentration of each analyzed element in that microregion.
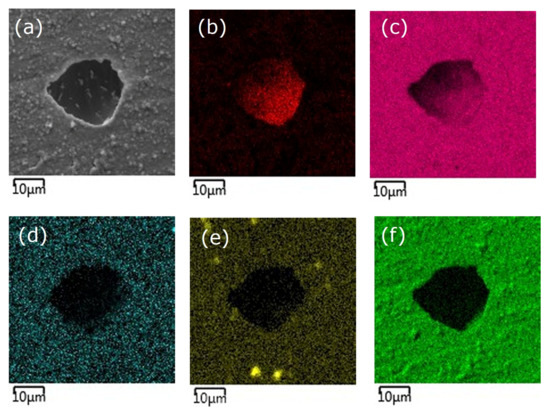
Figure 6.
SE image of the Ag-SiO2-TiO2 coating on a NiTi substrate showing a corrosion pit (a) and elemental surface distribution: Ni (b), Ti (c), Ag (d), Si (e), O (f).
Based on the chemical composition analysis of a corroded micro-area of the Ag-SiO2-TiO2 coating surface, performed using the EDS method at 5000× magnification, the elemental composition in this micro-region was determined to be 40.5 wt% nickel, 29.1 wt% titanium, and 30.4 wt% oxygen (Table 5).

Table 5.
Chemical composition inside the pit on the Ag-SiO2-TiO2 coating surface.
The presence of these elements indicates that the NiTi substrate has been exposed within the corrosion pit, which suggests localized degradation and failure of the protective coating. Silicon and silver were not quantitatively detected in this micro-area, most likely due to their concentrations being below the detection limit of the analytical technique used or their complete removal as a result of the corrosion process.
SEM observations after cyclic polarization confirmed that the Ag–SiO2–TiO2 coatings are susceptible to localized pitting corrosion, manifested as irregularly distributed pits and microstructural heterogeneity. However, despite the presence of such localized defects, the coating still provides long-term protection against generalized (uniform) corrosion. This is evidenced by the high charge transfer resistance Rct2 obtained from EIS fitting, which reflects suppression of slow redox processes during prolonged immersion (Table 3). The presence of SiO2 contributes to maintaining the barrier properties of the coating even after partial damage, as silica nanoparticles hinder the coalescence and growth of corrosion pits. Therefore, the durability of the coating should be considered in terms of dual behavior: while localized degradation can occur at structural defects, the overall barrier effect and functional properties (antibacterial action of Ag, bioactivity of SiO2, passivation by TiO2) remain effective, ensuring satisfactory long-term performance in simulated physiological conditions.
3.3.4. Mechanism of Pitting Corrosion of the Ag-SiO2-TiO2 Coating in Ringer’s Solution
The obtained results confirm that corrosion of the Ag-SiO2-TiO2 coating on the NiTi substrate in Ringer’s solution is a localized process, initiated at sites where the continuity of the protective coating is compromised. These may include microcracks, pores, areas of reduced coating thickness, or technological defects. In such locations, aggressive chloride ions can penetrate and adsorb onto the passive surface layer (mainly TiO2), leading to its local depassivation. Once the continuity of the coating is disrupted and the NiTi substrate is exposed, a micro-galvanic cell is formed. In this cell, the NiTi substrate acts as the anode and undergoes selective dissolution, while the surrounding coating surface functions as the cathode. In the anodic region, local dissolution of the alloy components occurs, according to Reaction (2) for nickel oxidation and Reaction (3) for titanium oxidation, leading to progressive degradation of the substrate:
Ni → Ni2+ + 2e−,
Ti → Ti4+ + 4e−.
As a result of the anodic reactions, the concentration of metal cations (Ni2+, Ti4+) inside the pit increases. These ions undergo hydrolysis, leading to a local decrease in pH (acidification of the pit interior). The hydrolysis of nickel ions is described by Reaction (4):
Ni2+ + H2O → NiOH+ + H+.
The hydrolysis of titanium ions proceeds according to Reaction (5):
Ti4+ + 4H2O → Ti(OH)4 + 4H+.
In the cathodic region, the reduction of oxygen dissolved in the electrolyte takes place. Under neutral or alkaline conditions, Reaction (6) occurs:
O2 + 2H2O + 4e− → 4OH−.
Under acidic conditions present inside the pit, Reaction (7) takes place:
O2 + 4H+ + 4e− → 2H2O.
The acidified and oxygen-depleted environment inside the pit promotes further depassivation and metal dissolution. Limited oxygen diffusion into the pit hinders repassivation, thereby accelerating the corrosion process. Additionally, the presence of silver particles in the coating may catalyze cathodic reactions, increasing the overall corrosion rate.
As corrosion progresses, the components of the coating may undergo partial dissolution or detachment. Although silver is a noble metal, it can be one of the first components lost from the coating, especially when present as fine, dispersed particles. In this form, it has a high specific surface area, which facilitates its oxidation and dissolution in environments containing chloride ions. SiO2, although chemically resistant, can also undergo gradual dissolution in alkaline environments or under high anodic potential. Due to its small amount, its removal may occur after the dissolution of Ag or simultaneously with it, but at a much slower rate. The incorporation of SiO2 nanoparticles into the Ag–SiO2–TiO2 coating significantly affects the kinetics of corrosion processes. SiO2 reduces porosity and increases the tortuosity of diffusion paths, thereby slowing the transport of aggressive Cl− ions and corrosion products through the coating. This barrier effect decreases the rate of anodic and cathodic reactions, which is reflected in the high charge transfer resistance observed in the low-frequency region (Rct2, Table 3). In addition, amorphous SiO2 acts as a chemically inert filler, stabilizing the passive TiO2 layer by sealing microcracks and pores and thus lowering the number of active initiation sites for pitting. Although the presence of SiO2 introduces some heterogeneity (as indicated by the reduced CPE exponents ϕ1 and ϕ2, Table 3), this heterogeneity may favor long-term stability by suppressing fast localized corrosion events. Therefore, SiO2 modifies the corrosion kinetics not by changing the fundamental mechanism of localized depassivation, but by retarding ion transport and pit propagation, leading to enhanced long-term corrosion resistance. TiO2 is one of the most chemically and electrochemically resistant components of the coating. However, it can also dissolve under very aggressive conditions, such as strongly acidic or alkaline pH, or in the presence of complexing ions.
As dissolution progresses, the integrity of the coating is further compromised, leading to the exposure of the NiTi substrate, which becomes the primary site of corrosion reactions. The resulting pits, acting as microcapillaries, limit mass exchange with the surrounding environment, creating a confined corrosive environment rich in metal ions, chloride ions, and characterized by low pH. This localized environment is maintained within the developing pit and further hinders the regeneration of the passive layer. Pitting corrosion thus becomes a self-sustaining process, which can lead to deep and difficult-to-detect damage to the material. Eventually, the pit may stabilize due to repassivation of the surface or continue to grow if the environmental conditions remain favorable to corrosion. The entire process leads to localized damage of the coating and increases the risk of mechanical and electrochemical degradation of the material under operating conditions.
3.4. Electronic Behavior and Surface Potential
The CPD distribution map on the surface of the Ag-SiO2-TiO2 coating obtained by the EPD method on the NiTi substrate is shown in Figure 7. The non-uniform CPD distribution on the surface of the Ag-SiO2-TiO2 coating indicates a heterogeneous microstructure and local variations in surface potential, most likely resulting from the irregular distribution of the coating components, particularly silver nanoparticles. The CPD topography analysis reveals the presence of numerous grain-like structures and distinct potential differences on the micrometer scale, which may be associated with local changes in chemical composition, coating thickness, or the presence of oxide phases with varying conductivity. The applied electrochemical deposition parameters (40 V, 180 s) lead to the formation of a surface with high electrostatic activity, which may positively influence the functional properties of the coating, such as antibacterial activity or ion adsorption capacity. The obtained results confirm that CPD analysis is a sensitive tool for evaluating the uniformity and electrical properties of composite layers and serves as an important complement to morphological and chemical studies in the design of advanced functional coatings on NiTi alloys.
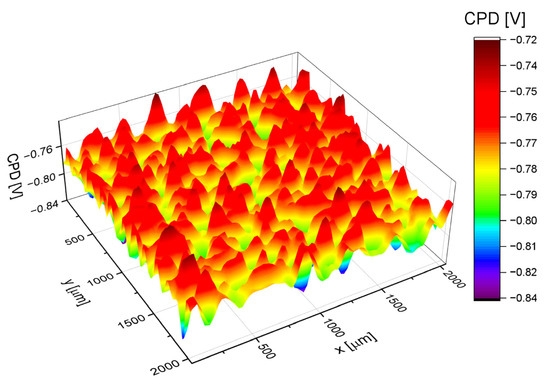
Figure 7.
The CPD distribution map on the surface of the Ag-SiO2-TiO2 coating deposited on a NiTi alloy substrate at 40 V for 180 s.
Based on the CPD distribution map on the surface of the Ag-SiO2-TiO2 coating in Figure 7, where CPD is the variable z, a CPD distribution histogram was obtained, as shown in Figure 8.
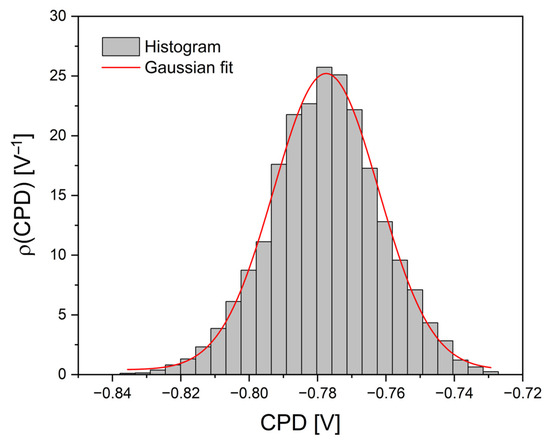
Figure 8.
CPD distribution histogram with a Gaussian fitting curve for the Ag-SiO2-TiO2 coating, obtained based on Figure 7.
The histogram shows the frequency of occurrence of individual CPD values. The peak of the distribution is located around −0.778 V. The distribution is symmetrical, which suggests the absence of significant structural disturbances in the sample and indicates its electrostatic homogeneity. The data were fitted using a Gaussian function (red line), resulting in a very good agreement between the model and the experimental data. The width of the distribution is 0.031 V, corresponding to the standard deviation. The coefficient of determination R2 reached a value of 0.995, and the adjusted R2 was also 0.995, confirming the high quality of the fit and indicating that the CPD distribution of the analyzed coating is well described by a normal distribution.
Table 6 presents the values of statistical parameters such as the arithmetic mean of the CPD height (CPDav), the root mean square of the CPD height (CPDq), skewness (CPDsk), and excess kurtosis (CPDku), which were determined based on the CPD distribution map (Figure 7) and the CPD distribution histogram (Figure 8). For comparison, the corresponding statistical parameters for the surface of the Ag–TiO2/NiTi coating [40] are also included. These parameters characterize the surface condition of the analyzed coatings, and their analysis enabled the evaluation of the statistical properties of the CPD distribution. The calculated values describe the electronic state of the surface, including the uniformity of the potential distribution, providing important insights into the electrostatic properties of the investigated coatings.

The CPDav value for both coatings is similar, amounting to −0.775 V for the Ag-SiO2-TiO2/NiTi sample and −0.770 V for Ag-TiO2/NiTi, indicating that the addition of SiO2 does not significantly affect the electron work function level but may influence the local properties of the potential distribution. In contrast, the CPDq for the Ag-SiO2-TiO2/NiTi coating is lower (16.0 mV) than for Ag-TiO2/NiTi (22.2 mV), indicating a more uniform charge distribution on the surface in the presence of SiO2. The CPDsk is slightly negative for both samples, meaning that CPD values below the average are more prevalent, with the SiO2-containing sample showing a more symmetrical distribution (−0.075). The CPDku for Ag-SiO2-TiO2/NiTi is 0.060, suggesting a slightly peaked distribution, while the negative value for Ag-TiO2/NiTi (−0.240) indicates a flatter-than-normal distribution. Thus, the addition of SiO2 to the Ag-TiO2 system results in improved electronic surface homogeneity, which may positively impact the stability of the Ag-SiO2-TiO2/NiTi coating’s electrical and chemical properties. Such a structure can be advantageous in applications where surface potential uniformity is required, such as in bioactive materials.
4. Conclusions
To functionalize the surface of the NiTi alloy, a multifunctional continuous layer composed of an Ag-SiO2-TiO2 nanocomposite was fabricated. The produced coating exhibited good adhesion to the NiTi substrate and demonstrated high scratch resistance. Complete failure of the coating occurred at an Lc3 value exceeding 20 N.
Analysis of the corrosion resistance of the Ag-SiO2-TiO2 (rutile) coating in Ringer’s solution using EIS confirmed that the examined coating exhibits high impedance at low frequencies and the presence of two time constants, indicating a complex corrosion protection mechanism involving both rapid barrier-level system responses and slower processes related to ion transport and redox reactions. The applied equivalent electrical circuit model of the 2CPE type allowed precise fitting of experimental data and obtaining electrical parameter values that indicate high resistance of the coating to long-term electrochemical corrosion. Potentiodynamic tests indicated low breakdown and repassivation potentials, suggesting vulnerability to pitting initiation, yet the multicomponent coating provides effective barrier protection against uniform corrosion in vitro. SEM analysis confirmed localized pit formation and coating degradation exposing the substrate. EDS revealed dominant TiO2 with minor Ag and Si, while elemental mapping inside pits showed substrate exposure due to coating breakdown. A pitting corrosion mechanism was proposed involving chloride adsorption, TiO2 depassivation, galvanic microcells, and local acidification. Contact potential difference mapping indicated surface electrostatic heterogeneity but overall stability, with silica improving charge distribution uniformity. The developed Ag-SiO2-TiO2 nanocomposite coating offers balanced corrosion protection and biological functionality, making it suitable for implant applications requiring durability and biointegration.
Author Contributions
Conceptualization, B.Ł. and K.D.; methodology, B.Ł., K.D., J.K. and A.B.; validation, B.Ł., K.D., J.K. and A.B.; investigation, B.Ł., K.D., J.K. and A.B.; resources, B.Ł., K.D., J.K. and A.B.; data curation, K.D.; writing—original draft preparation, B.Ł. and K.D.; writing—review and editing, B.Ł. and K.D.; visualization, B.Ł., K.D. and J.K.; supervision, B.Ł. and K.D.; project administration, B.Ł. and K.D.; funding acquisition, K.D. Author A.B. passed away prior to the publication of this manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Science Center in Poland (NCN), grant number 2020/39/D/ST5/01531.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Akay, S.; Yaghmur, A. Recent Advances in Antibacterial Coatings to Combat Orthopedic Implant-Associated Infections. Molecules 2024, 29, 1172. [Google Scholar] [CrossRef]
- Akshaya, S.; Rowlo, P.K.; Dukle, A.; Nathanael, A.J. Antibacterial Coatings for Titanium Implants: Recent Trends and Future Perspectives. Antibiotics 2022, 11, 1719. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhou, J.; Qian, Y.; Zhao, L. Antibacterial Coatings on Orthopedic Implants. Mater. Today Bio 2023, 19, 100586. [Google Scholar] [CrossRef] [PubMed]
- Skjöldebrand, C.; Tipper, J.L.; Hatto, P.; Bryant, M.; Hall, R.M.; Persson, C. Current Status and Future Potential of Wear-Resistant Coatings and Articulating Surfaces for Hip and Knee Implants. Mater. Today Bio 2022, 15, 100270. [Google Scholar] [CrossRef]
- Neto, J.V.C.; Teixeira, A.B.V.; Cândido dos Reis, A. Hydroxyapatite Coatings versus Osseointegration in Dental Implants: A Systematic Review. J. Prosthet. Dent. 2023, 134, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Baakili, S.E.; Semlali, A.; Issa, H.; Bricha, M.; Mabrouk, K.E. Tailored Electrophoretic Coatings for Enhanced Corrosion Resistance of 316L Stainless Steel Implants Using Bioactive Glasses. New J. Chem. 2024, 48, 5696–5709. [Google Scholar] [CrossRef]
- Guo, T.; Scimeca, J.-C.; Ivanovski, S.; Verron, E.; Gulati, K. Enhanced Corrosion Resistance and Local Therapy from Nano-Engineered Titanium Dental Implants. Pharmaceutics 2023, 15, 315. [Google Scholar] [CrossRef]
- Gautam, S.; Bhatnagar, D.; Bansal, D.; Batra, H.; Goyal, N. Recent Advancements in Nanomaterials for Biomedical Implants. Biomed. Eng. Adv. 2022, 3, 100029. [Google Scholar] [CrossRef]
- Marasli, C.; Katifelis, H.; Gazouli, M.; Lagopati, N. Nano-Based Approaches in Surface Modifications of Dental Implants: A Literature Review. Molecules 2024, 29, 3061. [Google Scholar] [CrossRef]
- Smołka, A.; Rodak, K.; Dercz, G.; Dudek, K.; Łosiewicz, B. Electrochemical Formation of Self-Organized Nanotubular Oxide Layers on Ti13Zr13Nb Alloy for Biomedical Applications. Acta Phys. Pol. A 2014, 125, 932–935. [Google Scholar] [CrossRef]
- Sahoo, J.; Sarkhel, S.; Mukherjee, N.; Jaiswal, A. Nanomaterial-Based Antimicrobial Coating for Biomedical Implants: New Age Solution for Biofilm-Associated Infections. ACS Omega 2022, 7, 45962–45980. [Google Scholar] [CrossRef]
- Burdușel, A.-C.; Gherasim, O.; Grumezescu, A.M.; Mogoantă, L.; Ficai, A.; Andronescu, E. Biomedical Applications of Silver Nanoparticles: An Up-to-Date Overview. Nanomaterials 2018, 8, 681. [Google Scholar] [CrossRef]
- Astasov-Frauenhoffer, M.; Koegel, S.; Waltimo, T.; Zimmermann, A.; Walker, C.; Hauser-Gerspach, I.; Jung, C. Antimicrobial Efficacy of Copper-Doped Titanium Surfaces for Dental Implants. J. Mater. Sci. Mater. Med. 2019, 30, 84. [Google Scholar] [CrossRef]
- Memarzadeh, K.; Sharili, A.S.; Huang, J.; Rawlinson, S.C.F.; Allaker, R.P. Nanoparticulate Zinc Oxide as a Coating Material for Orthopedic and Dental Implants. J. Biomed. Mater. Res. A 2015, 103, 981–989. [Google Scholar] [CrossRef]
- Morozova, O.V.; Klinov, D.V.; Morozova, O.V.; Klinov, D.V. Nanosilver in Biomedicine: Advantages and Restrictions. In Silver Micro-Nanoparticles-Properties, Synthesis, Characterization, and Applications; IntechOpen: London, UK, 2021; ISBN 978-1-83968-660-3. [Google Scholar]
- Dulski, M.; Dudek, K.; Chalon, D.; Kubacki, J.; Sulowicz, S.; Piotrowska-Seget, Z.; Mrozek-Wilczkiewicz, A.; Gawecki, R.; Nowak, A. Toward the Development of an Innovative Implant: NiTi Alloy Functionalized by Multifunctional β-TCP+Ag/SiO2 Coatings. ACS Appl. Bio Mater. 2019, 2, 987–998. [Google Scholar] [CrossRef] [PubMed]
- Dudek, K.; Strach, A.; Wasilkowski, D.; Łosiewicz, B.; Kubisztal, J.; Mrozek-Wilczkiewicz, A.; Zioła, P.; Barylski, A. Comparison of Key Properties of Ag-TiO2 and Hydroxyapatite-Ag-TiO2 Coatings on NiTi SMA. J. Funct. Biomater. 2024, 15, 264. [Google Scholar] [CrossRef] [PubMed]
- Pajor, K.; Michalicha, A.; Belcarz, A.; Pajchel, L.; Zgadzaj, A.; Wojas, F.; Kolmas, J. Antibacterial and Cytotoxicity Evaluation of New Hydroxyapatite-Based Granules Containing Silver or Gallium Ions with Potential Use as Bone Substitutes. Int. J. Mol. Sci. 2022, 23, 7102. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, T.; Hirata, H.; Eto, S.; Hashimoto, A.; Kii, S.; Kobayashi, T.; Tsukamoto, M.; Yoshihara, T.; Toda, Y.; Mawatari, M. Development of Silver-Containing Hydroxyapatite-Coated Antimicrobial Implants for Orthopaedic and Spinal Surgery. Medicina 2022, 58, 519. [Google Scholar] [CrossRef]
- Zhang, L.; Jin, Z. Antibacterial Activities of Titanium Dioxide (TiO2) Nanotube with Planar Titanium Silver (TiAg) to Prevent Orthopedic Implant Infection. J. Orthop. Surg. Res. 2024, 19, 144. [Google Scholar] [CrossRef]
- Dudek, K.; Dulski, M.; Podwórny, J.; Kujawa, M.; Rawicka, P. Optimization of the Electrophoretic Deposition Parameters and Mechanism of Formation of Ag-TiO2 Nanocoatings on a NiTi Shape Memory Alloy: Part I. Coatings 2024, 14, 44. [Google Scholar] [CrossRef]
- Dudek, K.; Dulski, M.; Podwórny, J.; Kujawa, M.; Rawicka, P. Mechanism of Ag-SiO2-TiO2 Nanocomposite Coating Formation on NiTi Substrate for Enhanced Functionalization. Coatings 2024, 14, 1055. [Google Scholar] [CrossRef]
- Dulski, M.; Peszke, J.; Włodarczyk, J.; Sułowicz, S.; Piotrowska-Seget, Z.; Dudek, K.; Podwórny, J.; Malarz, K.; Mrozek-Wilczkiewicz, A.; Zubko, M.; et al. Physicochemical and Structural Features of Heat Treated Silver-Silica Nanocomposite and Their Impact on Biological Properties. Mater. Sci. Eng. C 2019, 103, 109790. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, Y.; Katakami, H.; Mine, E.; Nagao, D.; Konno, M.; Liz-Marzán, L.M. Silica Coating of Silver Nanoparticles Using a Modified Stöber Method. J. Colloid. Interface Sci. 2005, 283, 392–396. [Google Scholar] [CrossRef]
- Amirtharaj Mosas, K.K.; Chandrasekar, A.R.; Dasan, A.; Pakseresht, A.; Galusek, D. Recent Advancements in Materials and Coatings for Biomedical Implants. Gels 2022, 8, 323. [Google Scholar] [CrossRef] [PubMed]
- Nasakina, E.O.; Sudarchikova, M.A.; Sergienko, K.V.; Konushkin, S.V.; Sevost’yanov, M.A. Ion Release and Surface Characterization of Nanostructured Nitinol during Long-Term Testing. Nanomaterials 2019, 9, 1569. [Google Scholar] [CrossRef]
- Xijing, L.; Yong, C. Effect of SiO2 Nanoparticles on the Hardness and Corrosion Resistance of NiW/SiO2 Nano Composite Coating Prepared by Electrodeposition. Int. J. Electrochem. Sci. 2023, 18, 100138. [Google Scholar] [CrossRef]
- Basiaga, M.; Walke, W.; Paszenda, Z.; Karasiński, P.; Szewczenko, J. The Effects of a SiO2 Coating on the Corrosion Parameters cpTi and Ti-6Al-7Nb Alloy. Biomatter 2014, 4, e28535. [Google Scholar] [CrossRef] [PubMed]
- Stambolova, I.; Yordanov, S.; Lakov, L.; Vassilev, S.; Blaskov, V.; Jivov, B. Preparation of Sol-Gel SiO2 Coatings on Steel and Their Corrosion Resistance. MATEC Web Conf. 2018, 145, 05011. [Google Scholar] [CrossRef]
- Nadachowski, F. Zarys Technologii Materiałów Ogniotrwałych; Śląskie Wydawnicwto Techniczne: Katowice, Poland, 1995. [Google Scholar]
- Svidró, J.; Diószegi, A.; Svidró, J.T. The Origin of Thermal Expansion Differences in Various Size Fractions of Silica Sand. Int. J. Cast. Met. Res. 2020, 33, 242–249. [Google Scholar] [CrossRef]
- Dhiflaoui, H.; Zayani, W.; Dabaki, Y.; Hajjaji, M.A.; Bessadok-Jemai, A.; Khezami, L.; Karrech, A.; Gaidi, M.; Amlouk, M.; Larbi, A.B.C.; et al. Corrosion Study of TiO2 Nanotubes Decorated with Ag Silver Nanoparticles Prepared by Photoreduction Process. J. Mater. Eng. Perform. 2025, 34, 16230–16243. [Google Scholar] [CrossRef]
- Yetim, T. An Investigation of the Corrosion Properties of Ag-Doped TiO2-Coated Commercially Pure Titanium in Different Biological Environments. Surf. Coat. Technol. 2017, 309, 790–794. [Google Scholar] [CrossRef]
- Yetim, T. Corrosion Behavior of Ag-Doped TiO2 Coatings on Commercially Pure Titanium in Simulated Body Fluid Solution. J. Bionic Eng. 2016, 13, 397–405. [Google Scholar] [CrossRef]
- ISO 19252:2025(En); Plastics—Determination of Scratch Properties. ISO: Geneva, Switzerland, 2025.
- ISO 20502:2005; Fine Ceramics (Advanced Ceramics, Advanced Technical Ceramics)-Determination of Adhesion of Ceramic Coatings by Scratch Testing. ISO: Geneva, Switzerland, 2005.
- ASTM C1624-22(2022); Standard Test Method for Adhesion Strength and Mechanical Failure Modes of Ceramic Coatings by Quantitative Single Point Scratch Testing. ASTM International: West Conshohocken, PA, USA, 2022.
- ASTM D7027-20(2020); Standard Test Method for Evaluation of Scratch Resistance of Polymeric Coatings and Plastics Using an Instrumented Scratch Machine. ASTM International: West Conshohocken, PA, USA, 2020.
- ISO 10271:2021; Dentistry-Corrosion Test Methods for Metallic Materials. ISO: Geneva, Switzerland, 2021.
- AUTOLAB. Electrochemical Instruments, Description of the Instrument; Eco Chemie B.V., Ed.; Kanaalweg: Utrecht, The Netherlands, 1998. [Google Scholar]
- Zhao, Q.; Wu, J.; Li, Y.; Xu, R.; Zhu, X.; Jiao, Y.; Luo, R.; Ni, X. Promotion of Bone Formation and Antibacterial Properties of Titanium Coated with Porous Si/Ag-Doped Titanium Dioxide. Front. Bioeng. Biotechnol. 2022, 10, 1001514. [Google Scholar] [CrossRef]
- Buzaev, A.A.; Lyutova, E.S.; Tkachuk, V.A.; Borilo, L.P.; Chen, Y.-W. Synthesis of TiO2–SiO2–Ag/Fiberglass with Antibacterial Properties and Its Application for Air Cleaning. ACS Omega 2023, 8, 23521–23527. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Polepalli, L.; Chowdhury, S.; Carr, M.A.; Janorkar, A.V.; Marquart, M.E.; Griggs, J.A.; Bumgardner, J.D.; Roach, M.D. Silver-Doped Titanium Oxide Layers for Improved Photocatalytic Activity and Antibacterial Properties of Titanium Implants. J. Funct. Biomater. 2024, 15, 163. [Google Scholar] [CrossRef]
- Dudek, K.; Dulski, M.; Łosiewicz, B. Functionalization of the NiTi Shape Memory Alloy Surface by HAp/SiO2/Ag Hybrid Coatings Formed on SiO2-TiO2 Glass Interlayer. Materials 2020, 13, 1648. [Google Scholar] [CrossRef]
- Kowalski, K.; Łosiewicz, B.; Budniok, A.; Kupka, M. Effect of Alloying on Corrosion Resistance of B2 FeAl Alloy in Aqueous Solution of Sulfuric Acid. Mater. Chem. Phys. 2011, 126, 314–318. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).