Abstract
Reactive co-sputtering was applied to deposit TaN-(Ag,Cu) nanocomposite films on Si and tool steels. Prior to post-deposition annealing, the films were deposited with TaN cap (diffusion barrier) layers in various thicknesses in order to slow down the nucleation and growth of emerging Ag and Cu particles. The thickness of the cap layers was set at 5, 10, 20, or 50 nm. The films were then annealed using Rapid Thermal Annealing (RTA) at 400 °C to induce the nucleation and growth of Ag and Cu nanoparticles. These films’ surface morphologies and structures were examined. The samples were tested for their anti-wear and antibacterial behaviors against Gram-positive S. aureus and Gram-negative E. coli, with a variation in cap layer thickness. It is found that, through the application of TaN cap layers, the out-diffusion of Ag and Cu atoms may be slowed down. The surface concentrations of Ag and Cu might decrease from 35 at.% and 17 at.% to 18 at.% and 6 at.%, respectively, when the cap layer thickness increases to 50 nm (after being annealed for 12 min). The diffusion mechanism is proposed to explain the formation of nanoparticles on the surface through boundary diffusion. Antibacterial behaviors against both bacteria, as well as tribological properties, could still be effective but become less significant with an increase in the cap layer thickness. The antibacterial efficiency after 3 h testing decreased from 99% to 5% and 8% against E. coli and S. aureus, respectively. At 12 h, all the samples reached >99% antibacterial efficiency, despite the variation in cap thickness. For sliding wear, the wear rate was doubled when the cap thickness increased to 50 nm (when the normal load was 1 N). On the other hand, the difference was minor when the normal load was changed to 5 N. The sliding lifetime of the samples was studied using a tribometer. The total lifetime may increase with an increase in the cap thickness. The wear is found to be due to the oxidation of Ag and Cu nanoparticles, which results in the loss of low coefficient behaviors.
1. Introduction
Various lubricious organic and inorganic materials are currently applied for the self-lubrication of coatings, such as graphite, MoS2, and soft metals. Ag and Cu are widely applied in the fabrication of self-lubricating coatings due to their high thermal ductility and thermal conductivity [1,2,3,4,5]. Furthermore, many studies on high-entropy alloy thin films also found that increasing Cu or Ag contents could gradually improve the friction behaviors and enhance the mechanical properties and wear resistance of the coatings. At the same time, the antibacterial behavior was greatly improved [6,7].
Recently, anti-wear and antibacterial TaN-Ag, TaN-Cu, and TaN-(Ag,Cu) nanocomposite films were deposited by a combinative process involving reactive co-sputtering and post-deposition RTA (rapid thermal annealing) [8,9,10]. Ta metal and alloys are known to have excellent mechanical properties and biocompatibility. This makes TaN a suitable protective coating in bio-related applications [11]. Ag and Cu were proven to be immiscible in TaN, which explains the possible synthesis of TaN-Ag, TaN-Cu, or TaN-(Ag,Cu) nanocomposite thin films [10,12]. The exposed Cu and Ag particles on the surfaces provide these films with excellent bactericidal effects. Therefore, by combining co-sputtering in argon and nitrogen plasma, followed by RTA, the preparation of lubricious and antibacterial TaN-(soft metals) films has become feasible. Generally speaking, the addition of both Ag-Cu simultaneously in TaN has some benefits over TaN-Cu or TaN-Ag. These benefits include (a) similar tribological behaviors, (b) a broader range of bactericides (covers more types of bacteria), (c) increased dissolution rates of critical metal ions, and (d) lower processing temperature [13,14]. However, there may be some disadvantages that include (a) a short lifetime due to a large volume of Ag and Cu atoms diffused out on the surface, and (b) difficulty in controlling the adaptive characteristics in Ag-rich or Cu-rich films. To solve the first problem, a nitride cap (diffusion barrier) layer may be applied to slow down the out-diffusion of Ag/Cu atoms [15,16,17].
Transition-metal nitrides are known as effective diffusion barrier layers for some critical metals [18,19]. It is normally assumed that nitride barrier layers with a random array of columnar boundaries could be covered with an adaptive coating layer to slow down soft and conductive metals from transporting to the surface, even though the boundary path is the major route. In this case, the depletion rate of soft metal near the surface of the coating can be reduced. This may prolong the lifetime of the coating in some applications, including sliding wear at various temperatures or loads, and bactericidal activity in a contaminated environment [20,21,22,23]. The applications of thin nitride layers as diffusion-control layers have been studied by Muratore et al. [20] and Papi et al. [21]. A coating serving this purpose may be a nitride, or possibly various polymer thin coatings deposited by plasma polymerization.
In this study, TaN barrier layers with various thicknesses (0 ~ 50 nm) were deposited onto TaN-(Ag,Cu) nanocomposite thin film in order to control the size and density of emerging Cu and Ag particles on the surface. Hopefully, the effectiveness of these films against wear and bacteria can be prolonged. Among all the related studies, one paper [20] described using TiN as a diffusion barrier layer to control high-temperature tribological behaviors. Another paper [15] applied CrN as the diffusion barrier layer to control sliding wear occurring on CrN-Ag nanocomposite coatings within high-temperature ranges. In the present study, TaN barrier layers with various thicknesses were applied onto the films in order to study tribological properties as well as antibacterial behaviors at room temperature, as a function of barrier thickness. No other publication has reported a similar subject so far. In addition, the mechanisms of sliding wear against the composite films were studied. Diffusion processes of Ag/Cu through the barrier layer and the formation of Ag/Cu particles were also proposed.
2. Materials and Methods
2.1. Film Deposition
TaN-(Ag,Cu) thin coatings were deposited onto Si in the [001] direction and tool steel (M2) substrates using reactive co-sputtering with Ag, Ta, and Cu targets. All targets had the same diameter of 50 mm and were inclined by 30°. The distances of target-to-substrate and target-to-target were 100 mm and 150 mm, respectively. For deposition, the sputtering system was first pumped down to 7 × 10−4 Pa. Following this, argon flow rate at 35 sccm was let in to fill up the chamber to 0.65 Pa. During deposition, the powers of Ta, Ag, and Cu targets were set at 170 W, 44 and 20 W (RF), respectively, in order to prepare films with 11 at.% soft metal (atomic ratio of Ag to Cu was 7/4). For the preparation of TaN, 4.5 sccm of N2 was added to the flow rate. During deposition, a bias power of 40 W (RF) was applied. No additional heating was applied. The substrate temperature was not higher than 100 °C during the deposition. The thickness of these thin coatings was around 1 μm. Overall, the deposition rate was 12.5 nm/min. Some of the films were then deposited with a TaN cap layer with a thickness varied at 5, 10, 20, or 50 nm. These films were annealed for 4, 8, and 12 min at 400 °C by RTA (SJ, ARTS-150, Hsinchu, Taiwan). Ramping rate was at 100 °C/s [8,24]. The flow rate of Ar was 2000 sccm during RTA. Further detailed procedures can be found in the studies [8,24].
2.2. Characterization
The films were examined using field-emission scanning electron microscopy (FESEM, JEOL 6700F, Tokyo, Japan) in order to study surface nanoparticles and film morphology. Some cross-sections of the cap layers were examined by transmission electron microscopy (TEM, JEM-2100, JEOL, Tokyo, Japan). The operating parameters for TEM are 200 kV of accelerating voltage and a current of 105 μA. The phases and structures of the composite films were studied by X-ray diffraction (XRD). Through these studies, the nucleation and growth of Ag/Cu phases after annealing could be fully realized. The X-ray diffractometer (XRD, Philips PW 1830, Caerphilly, UK) applied monochromatic intensified Cu Kα radiation (λ = 0.1541 nm). The scanning window was from 25°-2θ to 65°-2θ. The step size was 0.04°, and the measuring time was 1.60 seconds per step. The XRD system was operated using thin film mode with an incident angle of 1.5°.
In order to understand the out-diffusion characteristics of Ag and Cu atoms, with and without a cap layer, EDAX (energy-dispersive analytical X-ray) was used to check the surface concentration of Ag and Cu. The results can be used only for qualitative analysis since the elemental concentrations vary with thickness. In order to obtain the general view of the surface concentrations, low magnification (400×) was selected, and three measurements for each sample were carried out. The elemental mapping on the wear track was also conducted after tribological testing. Hence, the wear mechanisms could be proposed.
The binding status of Ta, N, Ag, and Cu elements on the surface of thin films was examined by X-ray photoelectron spectrometer (XPS, PHI 5000 VersaProbe III, ULVAC-PHI. Inc., Tokyo, Japan). Peak positions were calibrated using the C1s peak (284.6 eV). The pass energy was 15 eV, which had a resolution of 0.9 eV. The status of chemical binding of elements could be determined from the spectra. For depth profiling, the data point was taken from one to five nm, depending on the requirements of the resolution.
In tribological testing, a ball-on-disk setup was applied to evaluate the wear rate and lubricity during sliding. This equipment consists of an alumina ball (6.35 mm in dia.) locked at the pin’s end point. This ball was pressed perpendicularly to the coated substrates. The disk was rotatable against the alumina ball at a speed of 12 cm/s. The load was either 1 or 5 N. The sliding distance for wear testing was set at 500 m. This distance was determined to compare with previous studies. The surface profiler (Surfcoder, ET3000, Kosaka, Tokyo, Japan) was used to scan through the wear tracks in four different directions. The total wear volume (in mm3) after 500 m testing could then be calculated with an average value. The wear rate (in mm3/m) of the tested samples was evaluated by calculating the total wear volume divided by the sliding distance. It was hence an average value over 500 m. After the test, the recorded friction coefficient at the stable (lowest) region was evaluated. The wear rate of the sample was evaluated by calculating the volume of the wear track divided by the sliding distance. One set of the samples was tested to understand the relationship between the friction coefficient and distance (time), from which the lifetime of the solid lubricants could be evaluated. For the evaluation of the lifetime of solid lubricants, the tribometer was set to keep running until the rise in the friction coefficient can be observed. The upper turning point is defined as the lifetime of the solid lubricants. Therefore, wear rate may not be related to the lifetime of the solid lubricants. Figure 1 shows the illustration of a typical example of the friction coefficient vs. sliding distance for rubbing against solid lubricant-covered samples. The wear rate is known as related to the friction coefficient, which depends on the volume of solid lubricants and their characteristics [14,24]. The lifetime of solid lubricants is related to the oxidation rate of Ag and Cu nanoparticles [24].
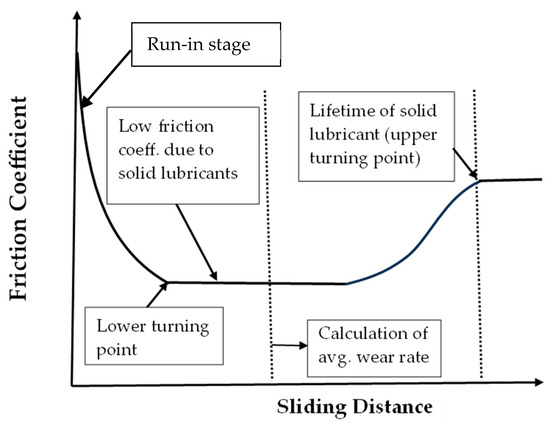
Figure 1.
Illustration of a typical example of the friction coefficient vs. sliding distance for rubbing against solid lubricant-covered samples.
To study the antibacterial testing, the coated and uncoated (controlled) Si substrates were placed on Petri dishes. The dishes were then incubated at 37 °C with a calibrated bacterial suspension, which included E. coli and S. aureus. After incubation for various lengths of time, the antibacterial property (E) could be evaluated according to the following equation:
where A = number of viable bacteria (CFU) on the uncoated sample (control set) in the Petri dish. B = number of viable bacteria (CFU) on the coated sample in the Petri dish.
3. Results and Discussion
3.1. Structural Analysis
Figure 2 shows the XRD patterns of samples without an additional cap layer (denoted as “No cap” in the figure), after being annealed for 4, 8, and 12 min. It can be observed, as expected, that Cu and Ag phases emerged after annealing [25]. Figure 3 presents the diffraction patterns of the samples with 5 nm (Figure 3a) and 50 nm (Figure 3b) TaN cap layers (the diffraction intensity in Figure 2 and Figure 3 was the original value and not normalized). As displayed in Figure 3a, the additional TaN layer would slow down the nucleation and growth of Ag and Cu phases on the films’ surface. When the cap layer thickness increased to 50 nm, the Ag and Cu phases could hardly be observed. These results also imply that there is no accumulation at the interface between TaN-(Ag,Cu) film and the cap layer. As reported by Hong et. al., this may be attributed to fast diffusion paths such as cracks in the grain boundaries [26].
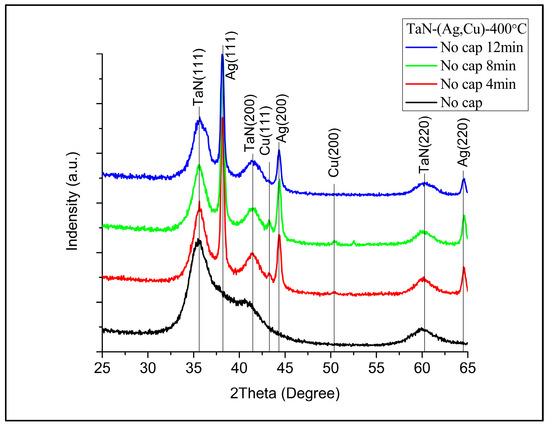
Figure 2.
XRD patterns of TaN-(Ag,Cu)-11% after being annealed at 400 °C for 4, 8, and 12 min (without an additional TaN cap layer).
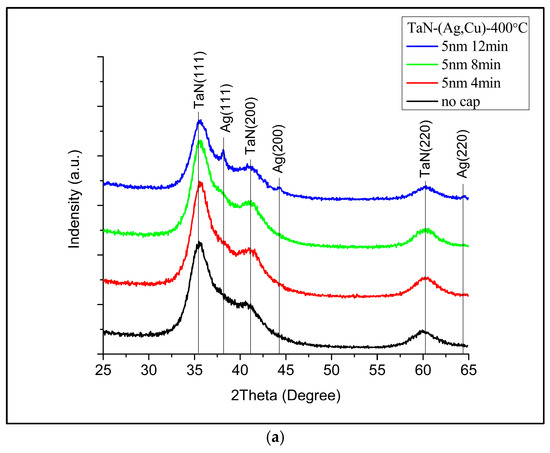
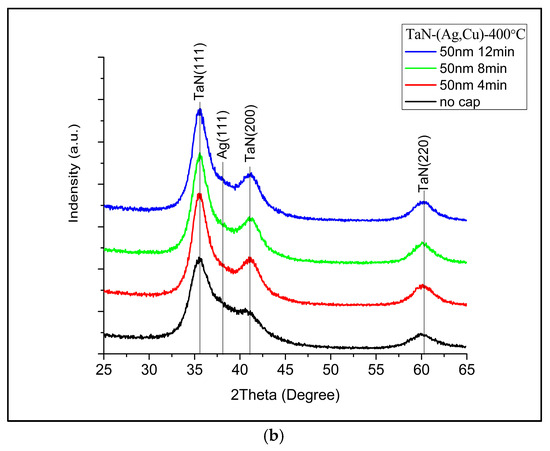
Figure 3.
XRD patterns of TaN-(Ag,Cu)-11% with (a) 5 nm cap layer and (b) 50 nm cap layer, after being annealed at 400 °C for 4, 8, and 12 min.
Figure 4 presents the surface and cross-sectional FESEM micrographs of TaN-(Ag,Cu) films without additional cap layers, after being annealed at 400 °C for various times. By using a 2D image analyzer, the surface coverages of particles are: (a) 43%, (b) 69%, and (c) 72%. It has been reported that Ag and Cu particles are formed separately [27]. This can be roughly observed by the shape of the emerged particles. Ag particles tend to have a rod shape. However, the main problem is the excessively formed surface particles, which might be depleted quickly after tribological or antibacterial testing. This would certainly shorten the lifetime of films in terms of bactericidal and wear resistance. Figure 5 shows the particle size distribution of the analysis was carried out using Image J. With an increase in the annealing time, the particle size increased.
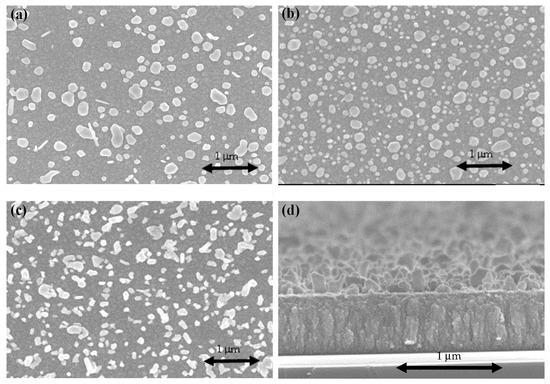
Figure 4.
FESEM micrographs of the surface of TaN-(Ag,Cu)-11% without an additional cap layer: (a) after being annealed for 4 min; (b) after being annealed for 8 min; (c) after being annealed for 12 min; (d) cross-sectional image of the sample shown in (a).
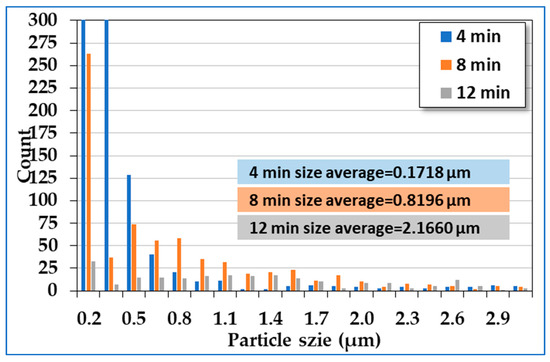
Figure 5.
Particle size distribution from Figure 4a–c with annealing times of 4, 8, and 12 min.
Figure 6 shows the cross-sectional SEM image of TaN-(Ag,Cu) thin film with a 50 nm TaN cap layer. The film has a columnar structure, which can serve as a quick diffusion path for Ag and Cu atoms [28]. Figure 7 shows the surface and cross-sectional SEM micrographs of TaN-(Ag,Cu) films with thickness-controlled cap layers, after being annealed for 4 min at 400 °C.
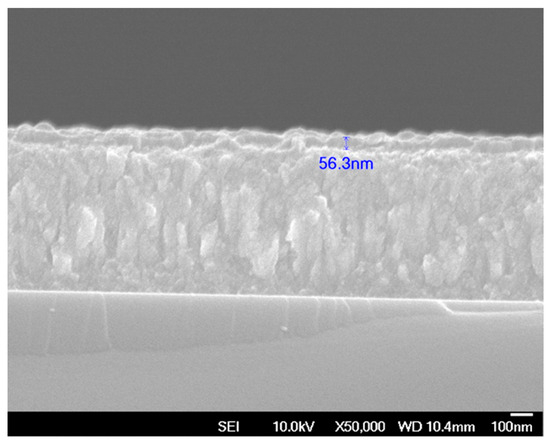
Figure 6.
Cross-sectional FESEM image of TaN-(Ag,Cu) thin film with a 50 nm TaN cap layer.
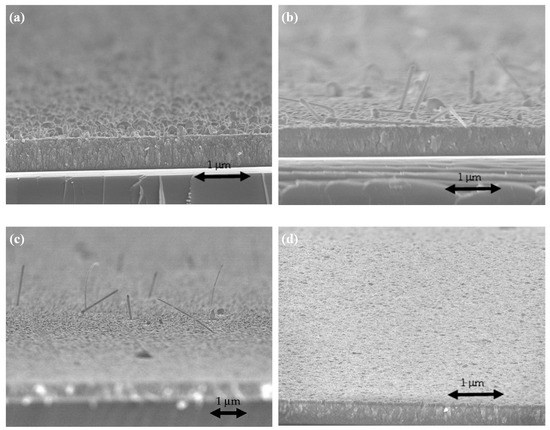
Figure 7.
FESEM micrographs of the annealed surface of TaN-(Ag,Cu)-12% with additional cap layers of various thicknesses: (a) without cap; (b) 5 nm; (c) 10 nm; (d) 50 nm.
Apparently, the surface particles become smaller with the increase in cap layer thickness. When the cap thickness reached 50 nm, the particles could hardly be observed by using FESEM. It is quite interesting to notice that, when the cap thickness was 5 nm, the surface particles became nano-rod shape, most likely caused by the emergence of the Ag phase [29].
3.2. Elemental Analysis
Figure 8a shows the Ag and Cu concentrations obtained from EDS near the surface of TaN-(Ag,Cu) thin coatings after being annealed for various times. Ag first diffused out and then leveled off, while Cu concentration increased with an increase in annealing time. Figure 8b shows the effect of cap layer thickness on the surface concentrations of Ag and Cu after being annealed for four minutes. According to this figure, it is confirmed that the TaN cap layer can effectively slow down the out-diffusion of Ag and Cu atoms [30]. Notably, the cap layer is more effective against Cu atoms under the condition of shorter annealing time.
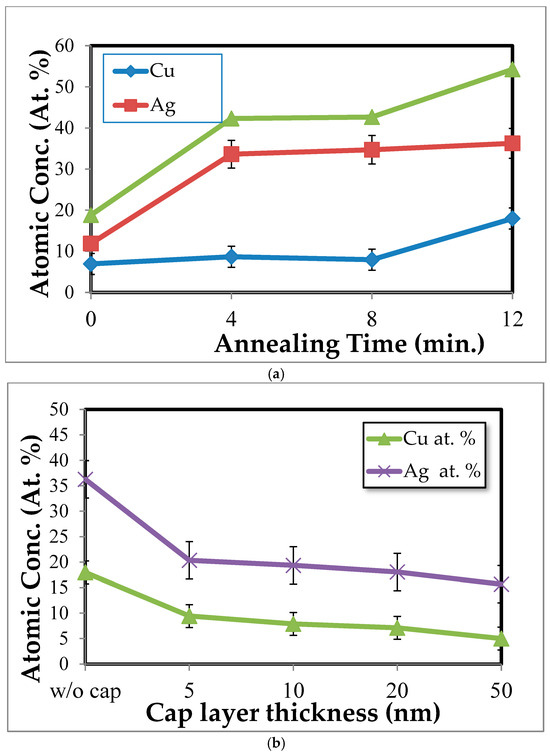
Figure 8.
Surface concentrations of Ag and Cu obtained from (a) TaN-(Ag,Cu) films without a cap layer, with a variation in annealing time, and (b) TaN-(Ag,Cu) films with cap layers of various thicknesses, after being annealed for 12 min.
Figure 9 shows some examples of XPS spectra for Ta 4f, N 1s, Ag 3d, and Cu 2p with a variation in depth within the cap layer of 5 nm. Figure 10a shows the XPS depth profile of a TaN-(Ag,Cu) thin coating with a 5 nm TaN cap layer, annealed at 400 °C for 12 min. Due to the emergence of Ag/Cu particles on the surface, there should be a strong signal of Cu and Ag. The contents of Cu and Ag within the barrier layer of various thicknesses are small and may be less than 1.0 at.%. It can be found that when the etching depth exceeds the thickness of the barrier layer, the concentration of Ag will begin to climb slowly. A similar result is found somewhere else [31]. Figure 10b also shows that the fastest climbing rate at the TaN/TaN-(Ag,Cu) interface was the 5 nm barrier layer, which means that the bulk diffusion rate for Ag and Cu atoms is higher for the composite film with a thicker cap layer. Combining Figure 6, Figure 8 and Figure 10, it is clear that the emergence (agglomeration) of Cu and Ag particles is the result of boundary diffusion, which is the quickest path for atom migration [32]. Figure 10b shows the depth profile near the interface of the cap layer and the TaN-(Ag,Cu) film, with various TaN cap layers of different thicknesses annealed at 400 °C for 12 min. This confirms that bulk diffusion is affected by cap layer thickness [33,34]. Figure 10c shows a hypothetical mechanism for Ag and Cu diffusion. The formation of nanoparticles is dependent on the diffusion rate through the quick diffusion path, i.e., the columnar boundaries.
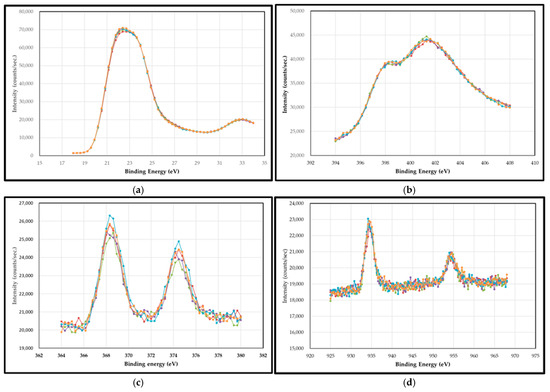
Figure 9.
Some examples of XPS spectra for (a) Ta 4f, (b) N 1s, (c) Ag 3d, and (d) Cu 2p with a variation in depth within the cap layer of 5 nm. (The colors represents various etching depth within the cap layer.)
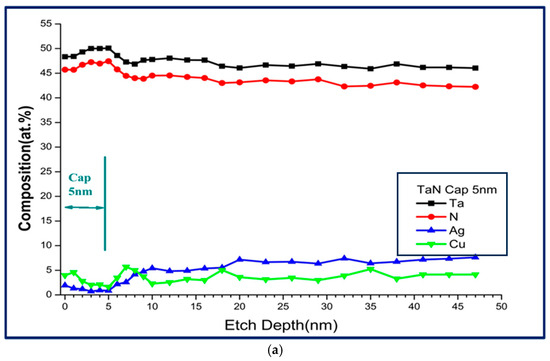
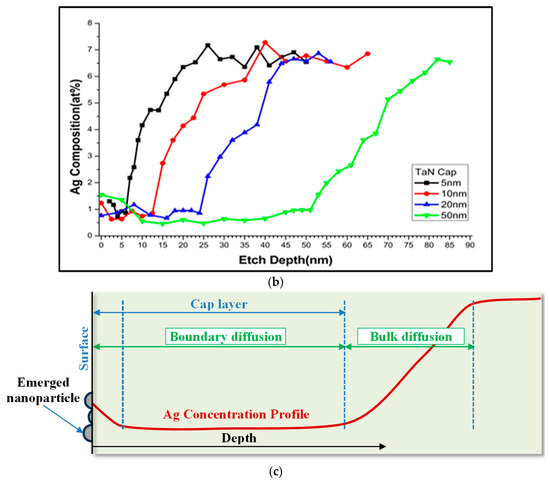
Figure 10.
(a) Depth profile of a TaN-(Ag,Cu) thin film with 5 nm TaN cap barrier layer, annealed at 400 °C for 12 min; (b) depth profile of TaN-(Ag,Cu) thin films with various TaN cap layers of different thicknesses, after being annealed for 12 min at 400 °C; (c) hypothetical mechanism for Ag and Cu diffusion.
Figure 11a shows a TEM brightfield image of a cross-sectional TaN-(Ag,Cu) thin coating with a 50 nm cap layer, annealed for 12 min at 400 °C. The enlarged cross-section image of the cap layer contains Ag and Cu nanoparticles, as proven by the pattern obtained after Fourier transformation. This was also presented in a previous study [35]. Figure 11b shows a diffraction pattern through the 50 nm cap layer, after annealing at 400 °C for 12 min. The diffraction rings further confirm the existence of Cu and Ag phases within the cap layer [36].
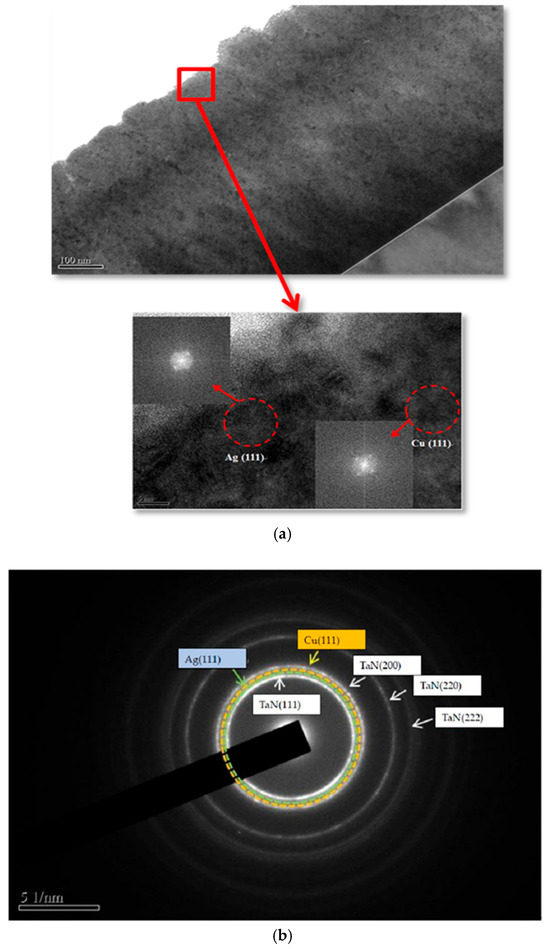
Figure 11.
(a) TEM brightfield image of a cross-sectional TaN-(Ag,Cu) thin film with a TaN 50 nm cap layer annealed at 400 °C for 12 min. The enlarged cross-section image of the cap layer contains Ag and Cu nanoparticles, which were proven by the pattern obtained after Fourier transformation, and (b) the diffraction ring of a cross-sectional image of the 50 nm cap layer, after annealing at 400 °C for 12 min.
3.3. Tribological Testing
Figure 12 presents the results of tribological testing on coated M2 tool steels under a normal load, either at 1 N or 5 N. Under a 1 N normal load and a sliding distance of 500 m, as presented in Figure 12a, it is found that all the annealed samples have a friction coefficient of around 0.4 (down from 1.0 for the as-deposited sample). Among all, the sample without a cap layer has the lowest wear rate. All samples with additional cap layers show similar wear rates. A paper has reviewed similar behaviors [37]. Apparently, under this testing condition, the amount of the surface Ag and Cu does not affect the testing results. Under a 5 N normal load (Figure 12b), the sample with a 5 nm cap layer shows the lowest friction coefficient and, therefore, the wear rate. The friction coefficient and wear rate increased with the increase in cap layer thickness. It has been reported that, under heavier load, the contact temperature would increase, which may cause the oxidation of Cu [24]. As a result, the friction coefficient and wear rate may increase. This can be used to explain why the sample with a 5 nm cap layer showed the lowest wear rate and friction coefficient, since a large portion of rod-shaped Ag could be found on the surface. When the cap layer is thicker than 5 nm, the surface solid lubricant may not be enough to maintain a low friction coefficient, although the values are still much lower than those of the as-deposited samples.
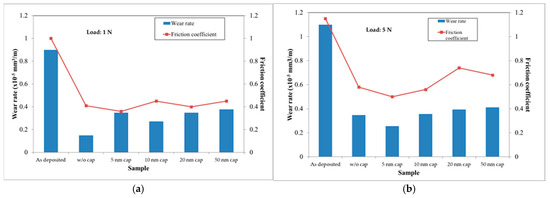
Figure 12.
The tribological testing results obtained from the TaN(Ag,Cu) coated M2 tool steels, with and without a TaN cap layer, under normal loads of (a) 1 N and (b) 5 N (annealing time: 12 min).
The effectiveness of solid lubricants (i.e., Ag and Cu nanoparticles) over time (distance) under constant normal force was evaluated by examining the friction coefficient as a function of time. The results are shown in Figure 13. According to this figure, the low-friction condition may last longer with an increase in the cap layer thickness. This implies that the additional cap layer may prolong the lifetime of TaN-(Ag,Cu) coatings. Without a cap layer, the soft metals rapidly distribute over the entire surface after annealing. During wear testing, the Ag and Cu phases deplete quickly and lose their lubricity [38]. As a result, the lifetime is shortened. Furthermore, the friction coefficients for all the coatings remain within 0.6 to 0.7 after a long sliding time, which is much lower than that of the unannealed sample. Therefore, it is believed that Ag and Cu atoms may still diffuse out due to the high contact temperature during sliding. This theory was proposed and proven in studies [38].
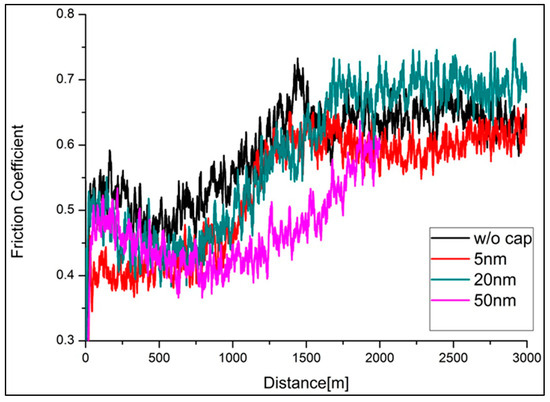
Figure 13.
Friction coefficient vs. sliding distance for annealed TaN-(Ag,Cu) thin films with and without various cap layers, under a 1 N normal load (annealing time: 12 min).
Overall, the controlled diffusion mechanism may be applied in order to extend the coating service life, and, at the same time, not to incur any toxic response in biological applications [38]. In order to understand the wear mechanisms, Figure 14 shows the elemental mapping by EDS on one of the wear tracks. It can be observed that oxidation wear occurred during sliding. The oxide is mainly composed of Ag, Cu, and O. This results in the loss of low friction coefficients due to the loss of softness and ductility.
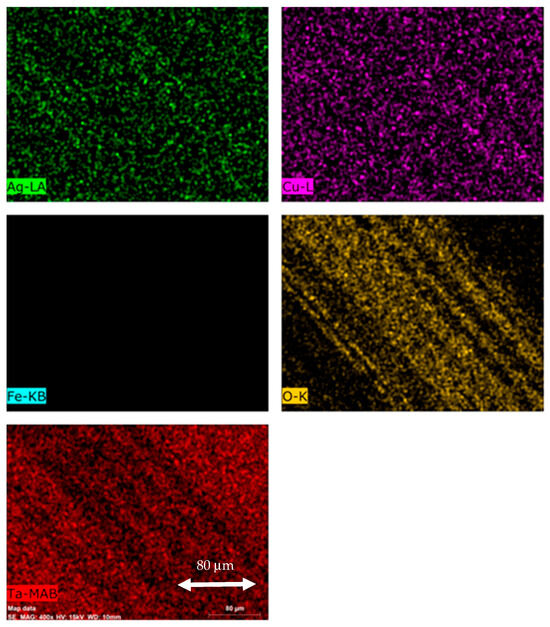
Figure 14.
EDS elemental mapping of the wear track of the TaN-(Ag,Cu) thin film with a 20 nm TaN cap layer (400×).
3.4. Antibacterial Behaviors
Antibacterial efficiency with the variation in cap layer thickness against S. aureus and E. coli is presented in Figure 15a,c. Figure 15b shows the photo images of colony-forming E. coli in Petri dishes after testing. According to the results, the TaN-(Ag,Cu)-coated samples, with and without a cap layer, after being annealed at 400 °C, show significant antibacterial behaviors against E. coli and S. aureus. It has been reported that AgNPs (Ag nanoparticles) or Ag+ can move and attach to the cell wall of bacteria. This action can cause damage to the membrane, as well as the leakage of cellular content through oxidation. Also, the Ag+ in the liquid may interact with enzymes, proteins, DNA, lipids, and other biomolecules or cellular structures. This may also cause an increase in ROS. The increased ROS will cause cell death by an apoptosis-like response, lipid peroxidation, and damage to DNA. In addition, the released Ag+ can move through the bacterial cell wall inward and outward. This enhances interactions with enzymes and proteins. Regarding Ag/Cu nanoparticles (Ag-CuNPs), it is summarized that Ag-CuNPs would have significant synergistic effects against over 160 kinds of bacteria [39].
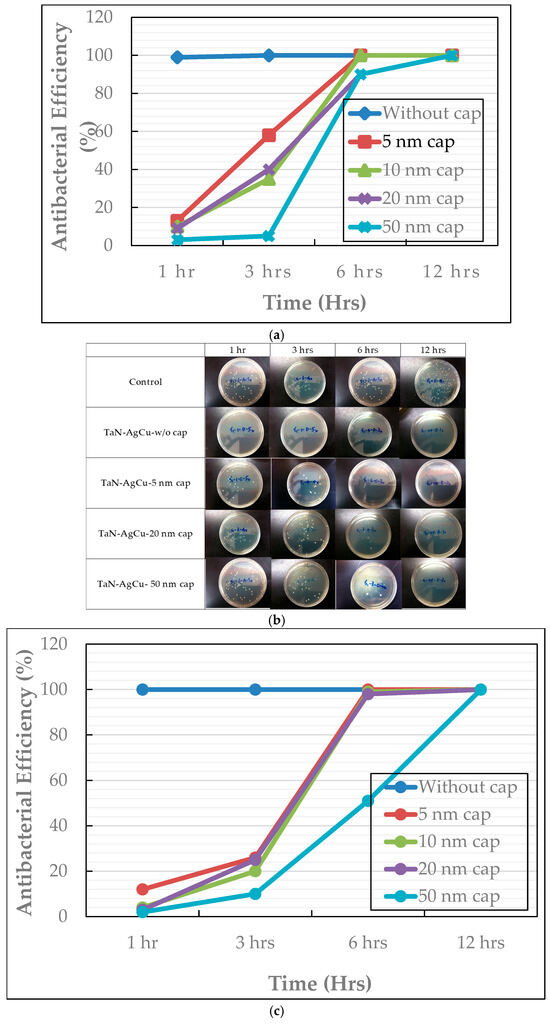
Figure 15.
Antibacterial efficiency of TaN-(Ag,Cu) thin coatings, with and without a cap layer; (a) against E. coli, (b) photos of colony-forming E. coli in Petri dishes, and (c) against S. aureus, with a variation in cap layer thickness.
Although the samples with cap layers were not as effective against E. coli and S. aureus, they all reached >99% efficiency in 12 h. Most importantly, the increase in cap layer thickness may effectively slow down the release of Ag and Cu ions, while maintaining certain antibacterial efficiency [40]. It is well known that the antibacterial efficiency is related to the Ag and Cu ion concentrations. By reducing the size and density of nanoparticles, the lifetime of these thin films, used as antibacterial and wear-resistant coatings, can be improved.
4. Conclusions
TaN-(Ag,Cu) nanocomposite thin films were prepared by reactive co-sputtering on Si and tool steels. Prior to post-deposition annealing, the films were capped with TaN barrier layers in various thicknesses to control the size and density of nucleation and growth of Cu and Ag particles. These films’ structures and surface morphologies were examined. The samples were tested for their anti-wear and antibacterial characteristics against Gram-positive S. aureus and Gram-negative E. coli, with variations in cap layer thickness. It is found that, through the application of TaN cap layers, tribological properties as well as antibacterial efficiency can remain effective. With the addition of cap layers, the film’s instant effectiveness against wear and bacteria may be reduced, but in exchange for a longer lifetime. In detail, the emergence of Ag and Cu particles can be reduced or slowed down depending on the cap layer thickness. With the increase in cap layer thickness, the effect on tribological behavior is not as obvious as that on antibacterial behavior. This could be attributed to the fact that Ag atoms diffuse out faster than Cu atoms. Also, the antibacterial behavior depends on the synergistic effects of Ag and Cu ions.
Author Contributions
Conceptualization, J.H.H. and C.L.; methodology, J.H.H. and C.L.; validation, J.H.H. and C.L.; investigation, A.D. and Y.J.C.; writing—original draft preparation, J.H.H., Y.J.C. and A.D.; writing—review and editing, C.L.; visualization, A.D., Y.J.C., J.H.H. and C.L.; supervision, J.H.H.; project administration, J.H.H.; funding acquisition, J.H.H. All authors have read and agreed to the published version of the manuscript.
Funding
National Science Council of the Republic of China (Taiwan): NSC 103-2221-E-131-003-MY2.
Institutional Review Board Statement
The study did not require ethical approval.
Informed Consent Statement
Not applicable.
Data Availability Statement
Available on request due to the restriction form funding agent.
Conflicts of Interest
Author You Jen Cho was employed by the company Jin Hou Electronic Material Co., Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Rajput, S.S.; Gangopadhyay, S.; Cavaleiro, A.; AL-Rjoub, A.; Kumar, C.S.; Fernandes, F. Influence of Ag Additions on the Structure, Mechanical Properties and Oxidation Behaviour of CrAlNAg Coatings Deposited by Sputtering. Surf. Coat. Technol. 2021, 426, 127767. [Google Scholar] [CrossRef]
- Ji, F.; Li, X.; Zhang, S.; Pang, M. Influence of Cu Content Variation on the Tribological Properties of Ni60CuMo with Sandwich-Structured Composite Coatings by Laser Cladding. Micromachines 2024, 15, 1429. [Google Scholar] [CrossRef]
- Zheng, C.; Huang, K.; Mi, T.; Li, M.; Yi, X. Laser Cladding Ni60 @ WC/Cu Encapsulated Rough MoS2 Self-Lubricating Wear Resistant Composite Coating and Ultrasound-Assisted Optimization. Ceram. Int. 2024, 50, 36555–36569. [Google Scholar] [CrossRef]
- Cavaleiro, D.; Carvalho, S.; Cavaleiro, A.; Fernandes, F. TiSiN(Ag) Films Deposited by HiPIMS Working in DOMS Mode: Effect of Ag Content on Structure, Mechanical Properties and Thermal Stability. Appl. Surf. Sci. 2019, 478, 426–434. [Google Scholar] [CrossRef]
- Chauhan, B.; Nadakuduru, V.N.; Mundotiya, B.M. Effect of Ag Content on the Friction and Wear Properties of Electrodeposited Self-Lubricating Ni–Ag Coating. Tribol. Mater. Surf. Interfaces 2024, 18, 124–132. [Google Scholar] [CrossRef]
- Ozdemir, H.C.; Yagci, M.B.; Bedir, E.; Yilmaz, R.; Canadinc, D. Optimizing Mechanical Properties and Ag Ion Release Rate of Silver Coatings Deposited on Ti-Based High Entropy Alloys. Surf. Coat. Technol. 2023, 455, 129221. [Google Scholar] [CrossRef]
- Yang, C.M.; Liu, X.B.; Liu, Y.F.; Zhu, Z.X.; Meng, Y.; Zhou, H.B.; Zhang, S.H. Effect of Cu-Doping on Tribological Properties of Laser-Cladded FeCoCrNiCux High-Entropy Alloy Coatings. Tribol. Int. 2023, 188, 108868. [Google Scholar] [CrossRef]
- Hsieh, J.H.; Liu, P.C.; Li, C.; Cheng, M.K.; Chang, S.Y. Mechanical Properties of TaN-Cu Nanocomposite Thin Films. Surf. Coat. Technol. 2008, 202, 5530–5534. [Google Scholar] [CrossRef]
- Tseng, C.C.; Hsieh, J.H.; Jang, S.C.; Chang, Y.Y.; Wu, W. Microstructural Analysis and Mechanical Properties of TaN-Ag Nanocomposite Thin Films. Thin Solid. Films 2009, 517, 4970–4974. [Google Scholar] [CrossRef]
- Hsieh, J.H.; Yeh, T.H.; Li, C.; Chiu, C.H.; Huang, C.T. Antibacterial properties of TaN–(Ag,Cu) nanocomposite thin films. Vacuum 2013, 87, 160–163. [Google Scholar] [CrossRef]
- Ratner, D.; Hoffman, A.S.; Schoen, F.J.; Lemons, J.E. Biomaterials Science, 2nd ed.; Elsevier: Amsterdam, The Netherlands; Academic Press: Cambridge, MA, USA, 2004. [Google Scholar]
- Laurila, T.; Zeng, K.; Kivilahti, J.K.; Molarius, J.; Riekkinen, T.; Suni, I. Tantalum Carbide and Nitride Diffusion Barriers for Cu Metallisation. Microelectron. Eng. 2002, 60, 71–80. [Google Scholar] [CrossRef]
- Mejía, V.H.D.; Perea, D.; Bejarano, G.G. Development and Characterization of TiAlN (Ag, Cu) Nanocomposite Coatings Deposited by DC Magnetron Sputtering for Tribological Applications. Surf. Coat. Technol. 2020, 381, 125095. [Google Scholar] [CrossRef]
- Hsieh, J.H.; Yeh, T.H.; Li, C.; Chang, S.Y.; Chiu, C.H.; Huang, C.T. Mechanical Properties and Antibacterial Behaviors of TaN-(Ag,Cu) Nanocomposite Thin Films after Annealing. Surf. Coat. Technol. 2013, 228, S116–S119. [Google Scholar] [CrossRef]
- Mulligan, C.P.; Blanchet, T.A.; Gall, D. Control of Lubricant Transport by a CrN Diffusion Barrier Layer during High-Temperature Sliding of a CrN-Ag Composite Coating. Surf. Coat. Technol. 2010, 205, 1350–1355. [Google Scholar] [CrossRef]
- Voevodin, A.A.; Muratore, C.; Aouadi, S.M. Hard Coatings with High Temperature Adaptive Lubrication and Contact Thermal Management: Review. Surf. Coat. Technol. 2014, 257, 247–265. [Google Scholar] [CrossRef]
- Luo, D.; Zhou, Q.; Ye, W.; Ren, Y.; Greiner, C.; He, Y.; Wang, H. Design and Characterization of Self-Lubricating Refractory High Entropy Alloy-Based Multilayered Films. ACS Appl. Mater. Interfaces 2021, 13, 55712–55725. [Google Scholar] [CrossRef]
- Yang, Z.G.; Xu, H.M.; Shuai, T.Y.; Zhan, Q.N.; Zhang, Z.J.; Huang, K.; Dai, C.; Li, G.R. Recent Progress in the Synthesis of Transition Metal Nitride Catalysts and Their Applications in Electrocatalysis. Nanoscale 2023, 15, 11777–11800. [Google Scholar] [CrossRef]
- Tsetseris, L.; Logothetidis, S.; Pantelides, S.T. Atomic-Scale Mechanisms for Diffusion of Impurities in Transition-Metal Nitrides. Surf. Coat. Technol. 2010, 204, 2089–2094. [Google Scholar] [CrossRef]
- Muratore, C.; Hu, J.J.; Voevodin, A.A. Adaptive Nanocomposite Coatings with a Titanium Nitride Diffusion Barrier Mask for High-Temperature Tribological Applications. Thin Solid Film. 2007, 515, 3638–3643. [Google Scholar] [CrossRef]
- Papi, P.A.; Mulligan, C.P.; Gall, D. CrN-Ag Nanocomposite Coatings: Control of Lubricant Transport by Diffusion Barriers. Thin Solid Film. 2012, 524, 211–217. [Google Scholar] [CrossRef]
- Fenker, M.; Balzer, M.; Kappl, H. Corrosion Protection with Hard Coatings on Steel: Past Approaches and Current Research Efforts. Surf. Coat. Technol. 2014, 257, 182–205. [Google Scholar] [CrossRef]
- Wen, L.; Zhou, M.; Wang, C.; Mi, Y.; Lei, Y. Nanoengineering Energy Conversion and Storage Devices via Atomic Layer Deposition. Adv. Energy Mater. 2016, 6, 1600468. [Google Scholar] [CrossRef]
- Hsieh, J.H.; Chiu, C.H.; Li, C.; Wu, W.; Chang, S.Y. Development of Anti-Wear and Anti-Bacteria TaN-(Ag,Cu) Thin Films—A Review. Surf. Coat. Technol. 2013, 233, 159–168. [Google Scholar] [CrossRef]
- Echavarría1, A.M.; Bejarano, G.G.; Meza, J.M. Mechanical and tribological features of TaN(Ag-Cu) duplex nanocomposite coatings: Their response to heat treatment, Ingeniare. Rev. Chil. Ing. 2017, 25, 662–673. [Google Scholar] [CrossRef]
- Hong, C.; He, P.; Tian, J.; Chang, F.; Wu, J.; Zhang, P.; Dai, P. On the Microstructure and Mechanical Properties of CrNx/Ag Multilayer Films Prepared by Magnetron Sputtering. Materials 2020, 13, 1316. [Google Scholar] [CrossRef]
- Im, H.S.; Sim, K.B.; Seong, T.Y. Thermally Stable AgCu Alloy Disc Array for near Infrared Filters. Curr. Appl. Phys. 2020, 20, 1321–1327. [Google Scholar] [CrossRef]
- Elofsson, V.; Almyras, G.A.; Lü, B.; Boyd, R.D.; Sarakinos, K. Atomic Arrangement in Immiscible Ag–Cu Alloys Synthesized Far-from-Equilibrium. Acta Mater. 2016, 110, 114–121. [Google Scholar] [CrossRef]
- Li, S.; Hu, S.; Xu, K.; Jiang, W.; Liu, Y.; Leng, Z.; Liu, J. Construction of Fiber-Shaped Silver Oxide/Tantalum Nitride p-n Heterojunctions as Highly Efficient Visible-Light-Driven Photocatalysts. J. Colloid. Interface Sci. 2017, 504, 561–569. [Google Scholar] [CrossRef]
- Baek, W.-C.; Zhou, J.P.; Im, J.; Ho, P.S.; Lee, J.G.; Hwang, S.B.; Choi, K.-K.; Kyun Park, S.; Jung, O.-J.; Smith, L.; et al. Oxidation of the Ta Diffusion Barrier and Its Effect On The Reliability of Cu Interconnects. In Proceedings of the 2006 IEEE International Reliability Physics Symposium Proceedings, San Jose, CA, USA, 26–30 March 2006. [Google Scholar]
- Lee, H.W.; Bien, D.C.S.; Badaruddin, S.A.M.; Teh, A.S. Silver (Ag) as a Novel Masking Material in Glass Etching for Microfluidics Applications. Microsyst. Technol. 2013, 19, 253–259. [Google Scholar] [CrossRef]
- Herzig, C.; Divinski, S. V Grain Boundary Diffusion in Metals: Recent Developments. Mat. Trans. 2003, 44, 14–27. [Google Scholar] [CrossRef]
- Ou, K.-L.; Wu, W.-F.; Chou, C.-P.; Chiou, S.-Y.; Wu, C.-C. Improved TaN Barrier Layer against Cu Diffusion by Formation of an Amorphous Layer Using Plasma Treatment. J. Vac. Sci. Technol. B Microelectron. Nanometer Struct. Process. Meas. Phenom. 2002, 20, 2154–2161. [Google Scholar] [CrossRef]
- Yan, H.; Wu, J.; Xie, H.; Thomas, H.R.; Feng, S. An Analytical Model for Chemical Diffusion in Layered Contaminated Sediment Systems with Bioreactive Caps. Int. J. Numer. Anal. Methods Geomech. 2019, 43, 2471–2490. [Google Scholar] [CrossRef]
- Chee, S.S.; Lee, J.H. Preparation and Oxidation Behavior of Ag-Coated Cu Nanoparticles Less than 20 Nm in Size. J. Mater. Chem. C Mater. 2014, 2, 5372–5381. [Google Scholar] [CrossRef]
- Vanegas Parra, H.S.; Calderón Velasco, S.; Alfonso Orjuela, J.E.; Olaya Florez, J.J.; Carvalho, S. Influence of Ag Doping on the Microstructural, Optical, and Electrical Properties of ZrSiN Coatings Deposited through Pulsed-DC Reactive Magnetron Sputtering. Coatings 2023, 13, 1154. [Google Scholar] [CrossRef]
- Wu, X.; Jiang, Y.; Wu, T.; Zuo, B.; Bian, S.; Lu, K.; Zhao, L.; Yu, L.; Xu, J. Insight into the Mechanisms of Nitride Films with Excellent Hardness and Lubricating Performance: A Review. Nanomaterials 2023, 13, 2205. [Google Scholar] [CrossRef]
- Mulligan, C.P.; Gall, D. CrN-Ag Self-Lubricating Hard Coatings. Surf. Coat. Technol. 2005, 200, 1495–1500. [Google Scholar] [CrossRef]
- Tan, G.; Xu, J.; Chirume, W.M.; Zhang, J.; Zhang, H.; Hu, X. Antibacterial and Anti-Inflammatory Coating Materials for Orthopedic Implants: A Review. Coatings 2021, 11, 1401. [Google Scholar] [CrossRef]
- Ali, A.; Petrů, M.; Azeem, M.; Noman, T.; Masin, I.; Amor, N.; Militky, J.; Tomková, B. A Comparative Performance of Antibacterial Effectiveness of Copper and Silver Coated Textiles. J. Ind. Text. 2022, 53, 15280837221134990. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).