Improvement of Water Erosion Resistance of Gypsum Mortars in the Historic Buildings for Conservation Purpose
Abstract
1. Introduction
1.1. Application Background of Gypsum Mortar in Ancient Buildings
1.2. Research Status of Waterproof Materials for Gypsum Mortar
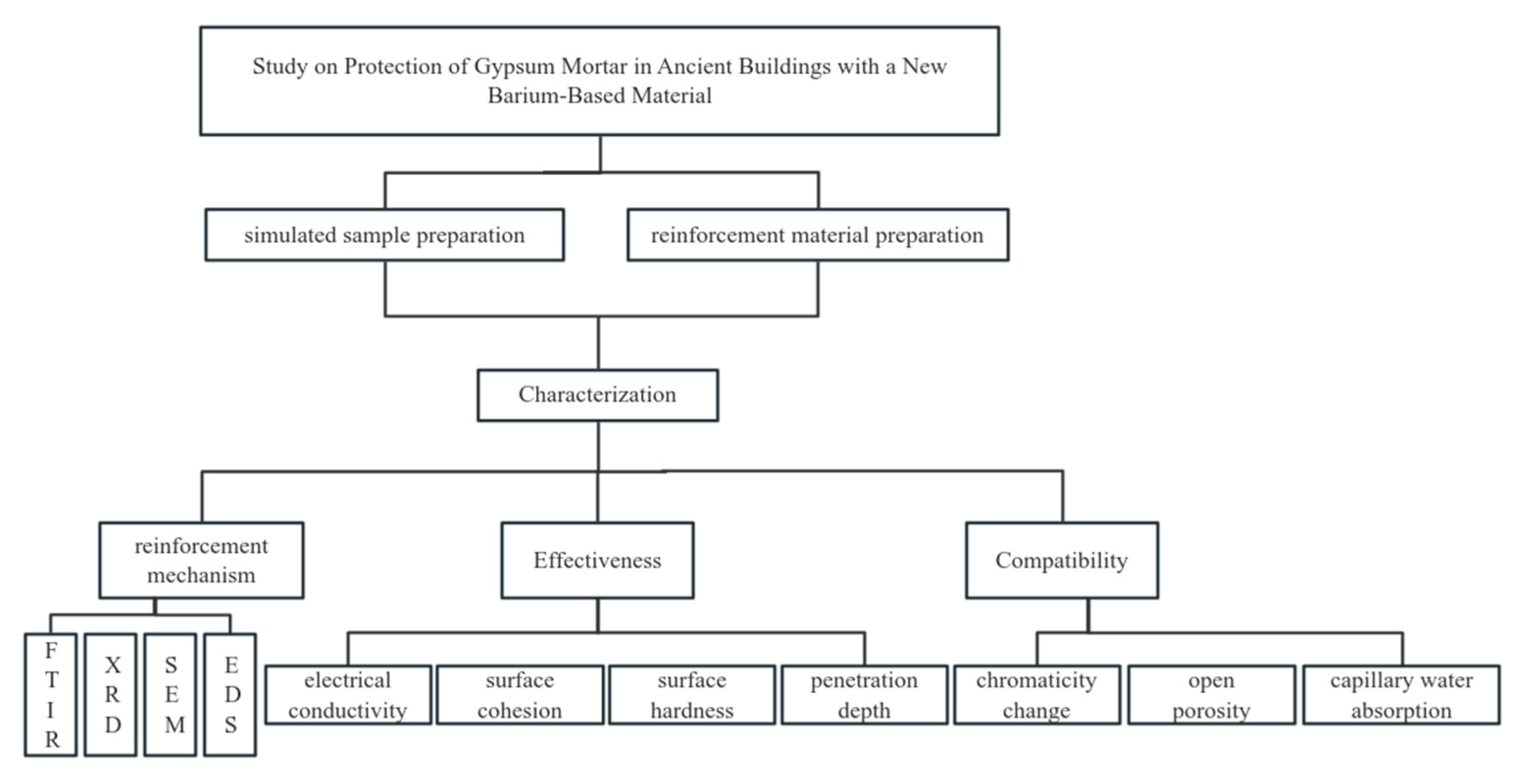
1.3. Research Content
2. Materials and Methods
2.1. Chemicals
2.2. Preparation of Gypsum Mortar Specimens
2.3. Preparation of Barium Hydroxide Solution
2.4. Methods
3. Treatment Methods
3.1. Selection of Treating Agents
3.2. Treatment of the Mortar Specimens
3.3. Equipment Characteristics
4. Results and Discussion
4.1. Action Mechanism
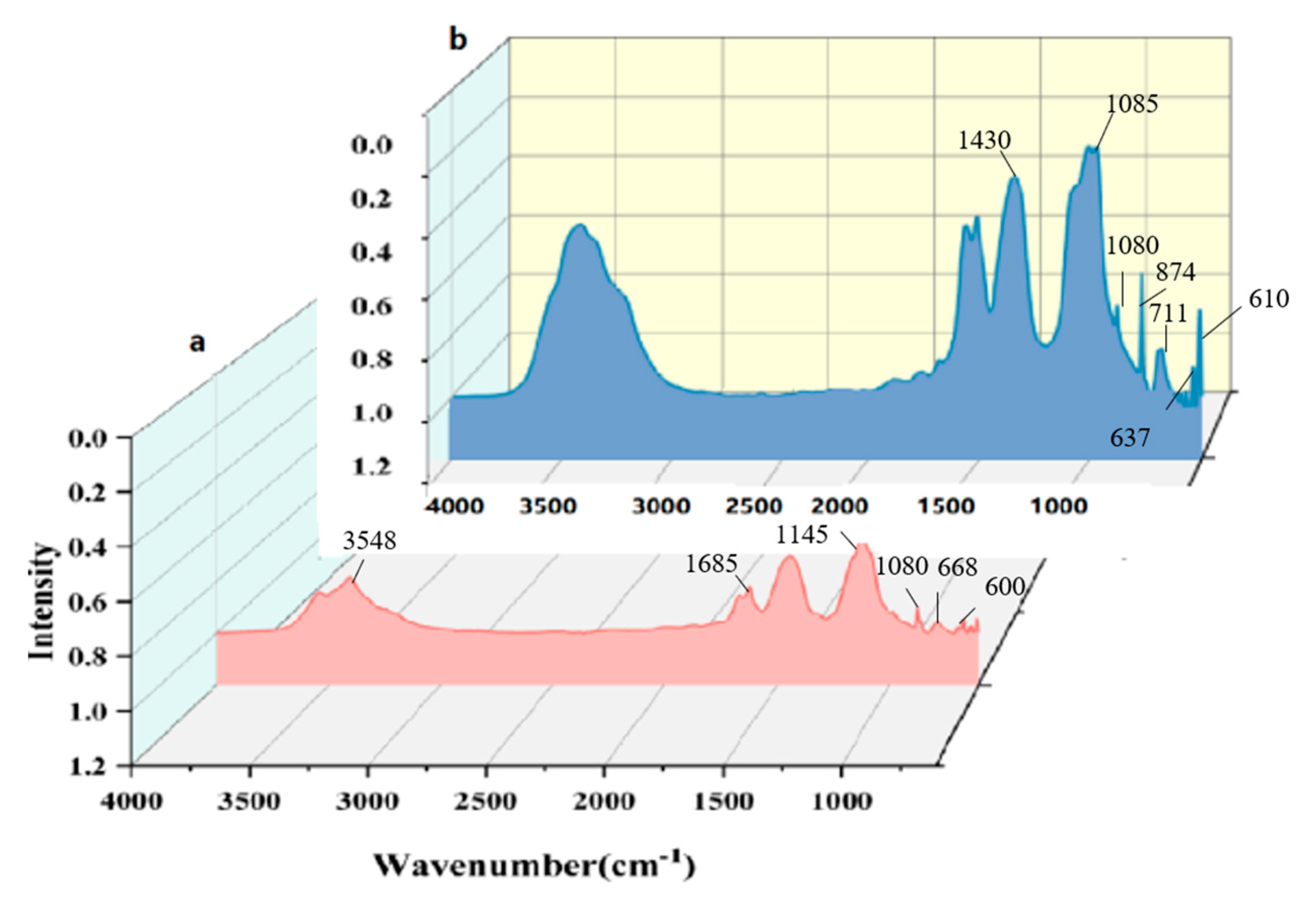
4.1.1. Fourier Infrared Spectrum Analysis
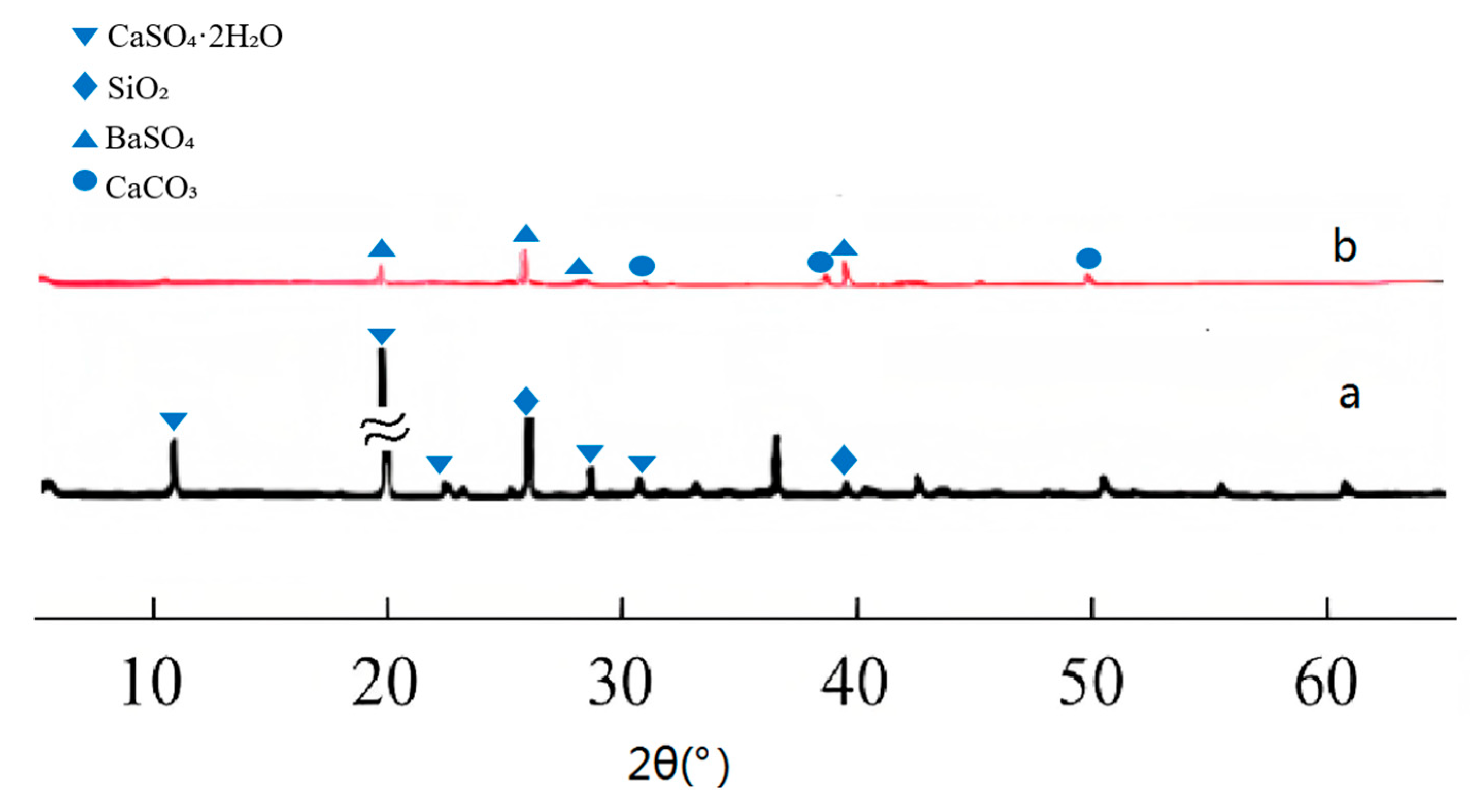
4.1.2. X-Ray Diffraction Analysis
4.1.3. Scanning Electron Microscopy/Energy Dispersive Spectroscopy Analysis
4.2. Performance
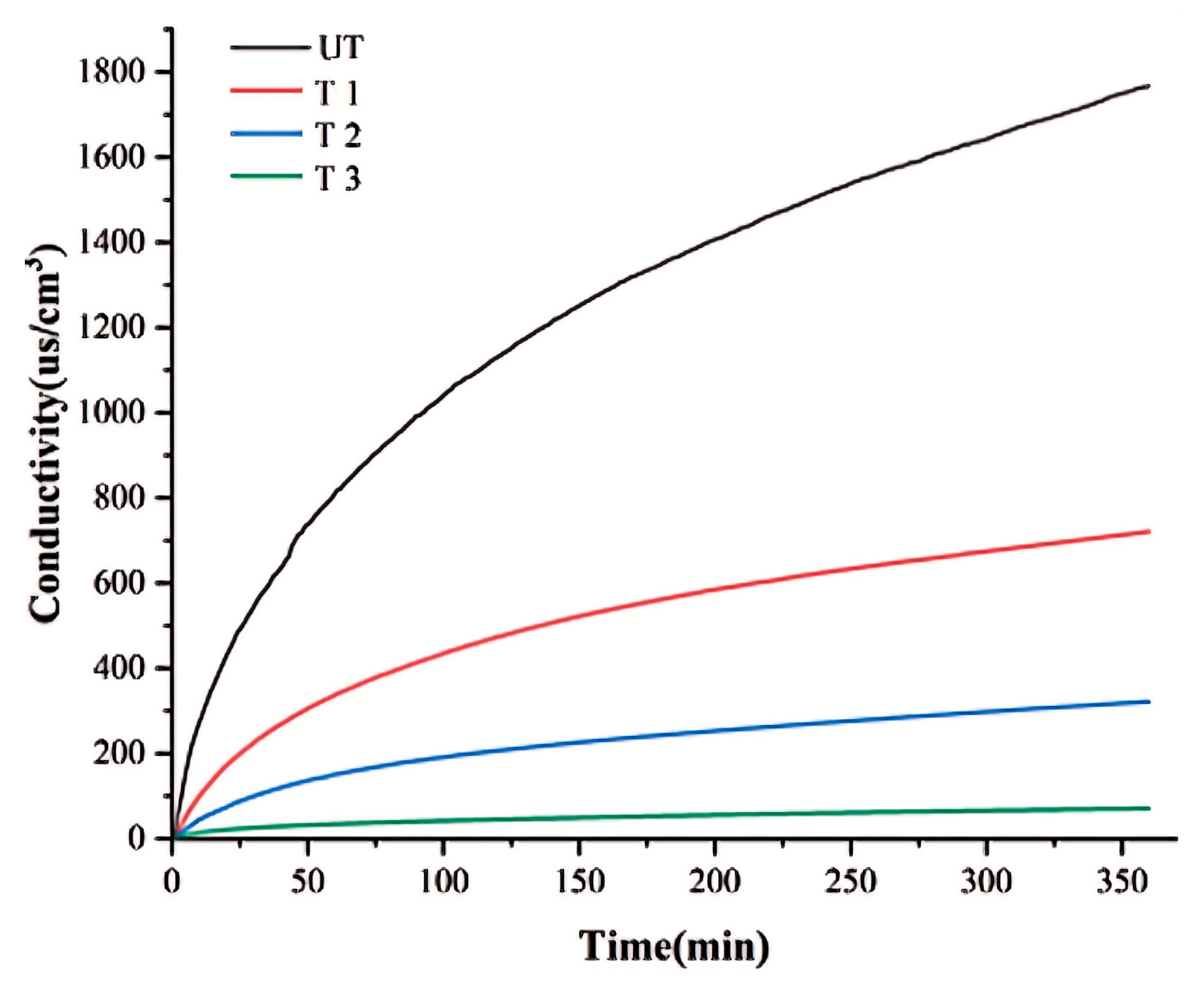
4.2.1. Permeability
4.2.2. Water Erosion Resistance
4.2.3. Mechanical Strength
4.2.4. Compatibility
5. Conclusions
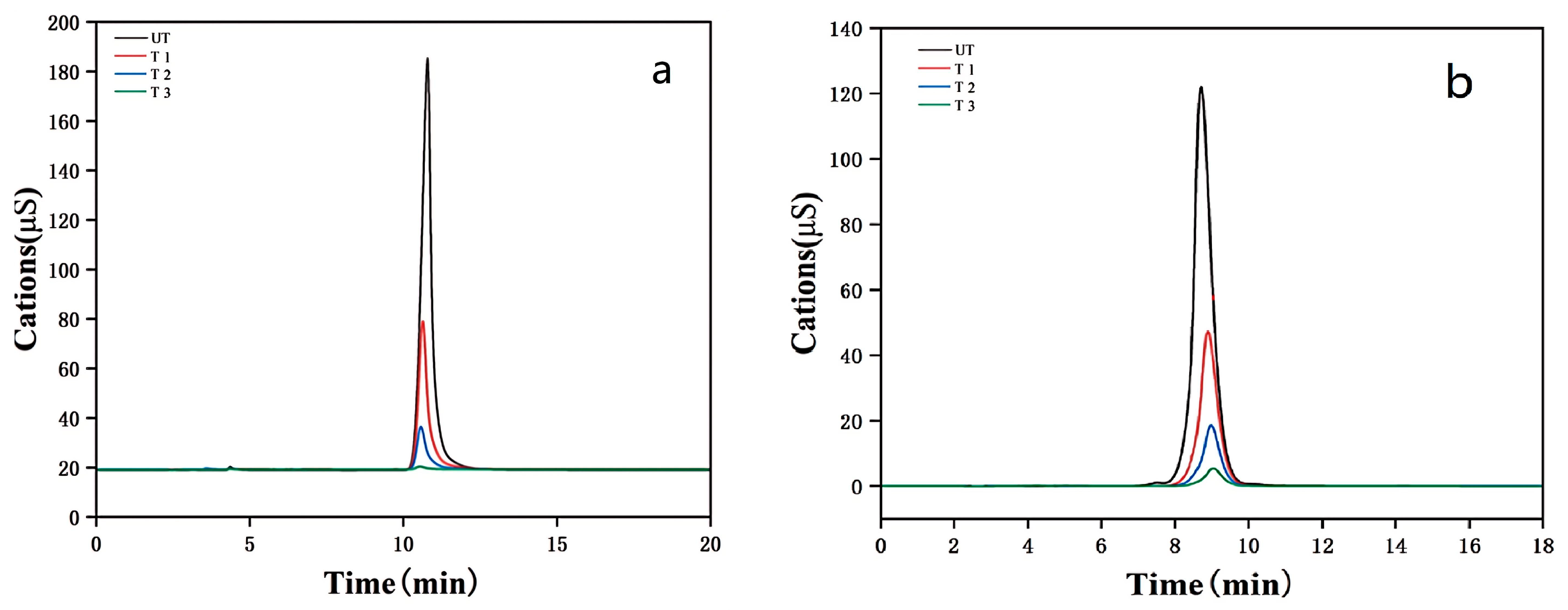
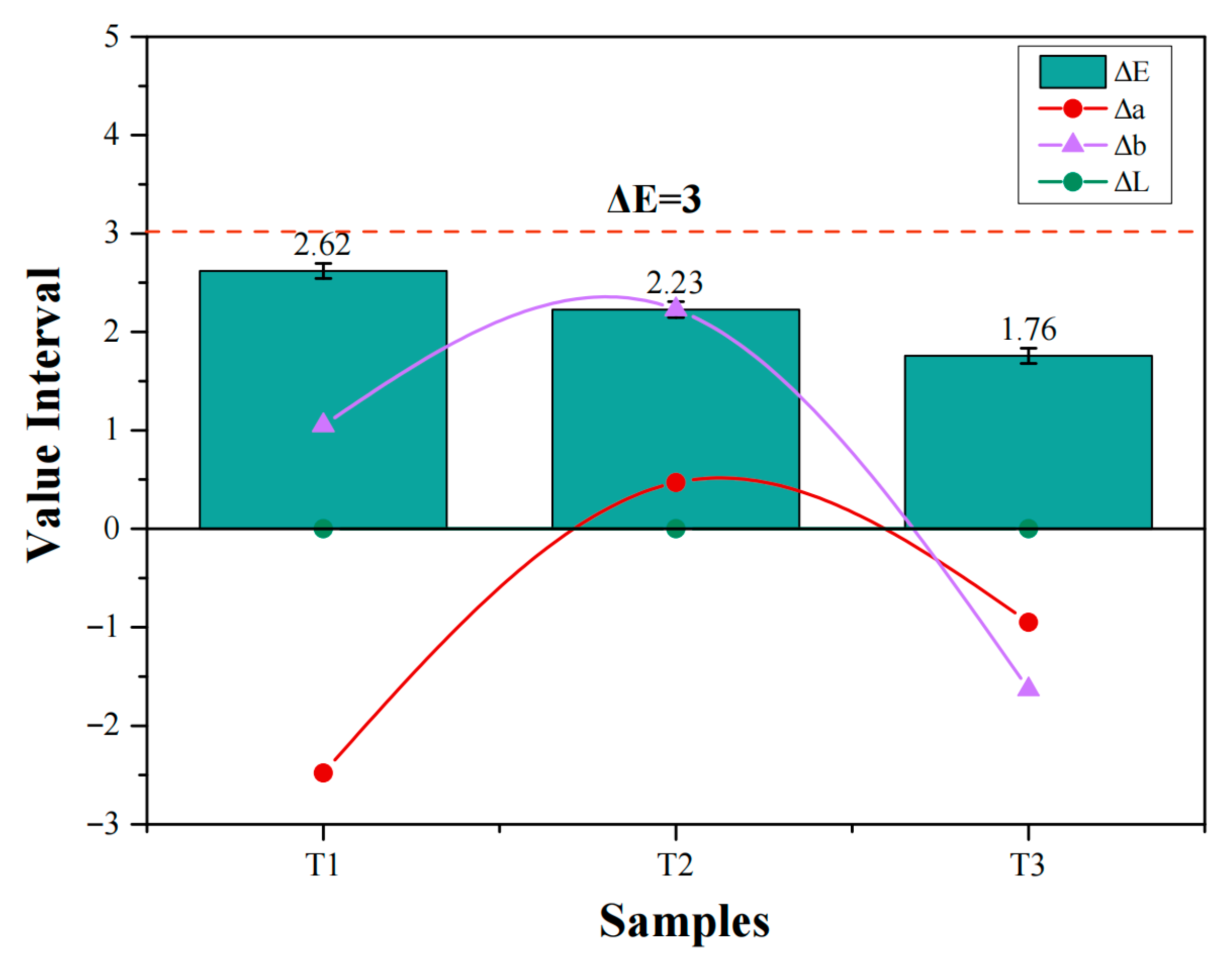
- Owing to its sufficient stability in the open air, the methanol-based barium hydroxide solution exhibits excellent permeability, achieving a penetration depth of 20 mm in gypsum-sand mortar. The total color difference (ΔE*) of the treated specimens is less than 3.0, which is closely associated with the good permeability of the barium hydroxide solution.
- As indicated by the analysis results of X-ray diffraction (XRD), Fourier transform infrared spectroscopy (FTIR) and scanning electron microscopy/energy dispersive spectroscopy (SEM/EDS), calcium sulfate dihydrate (CaSO4·2H2O) in the gypsum-sand mortar is converted into insoluble barium sulfate (BaSO4) and calcium carbonate (CaCO3) after sequential treatment with the methanol-based barium hydroxide solution and water.
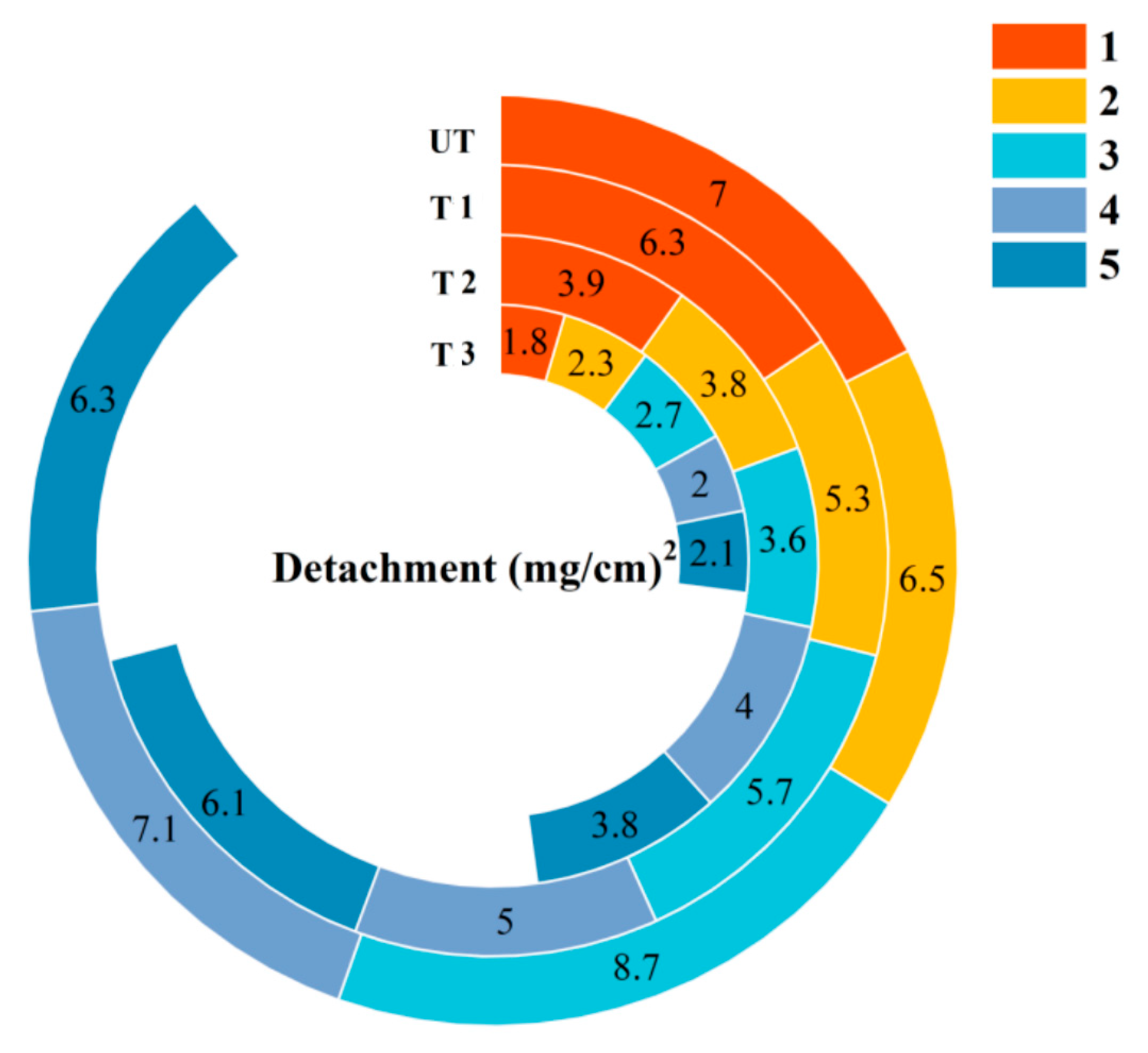
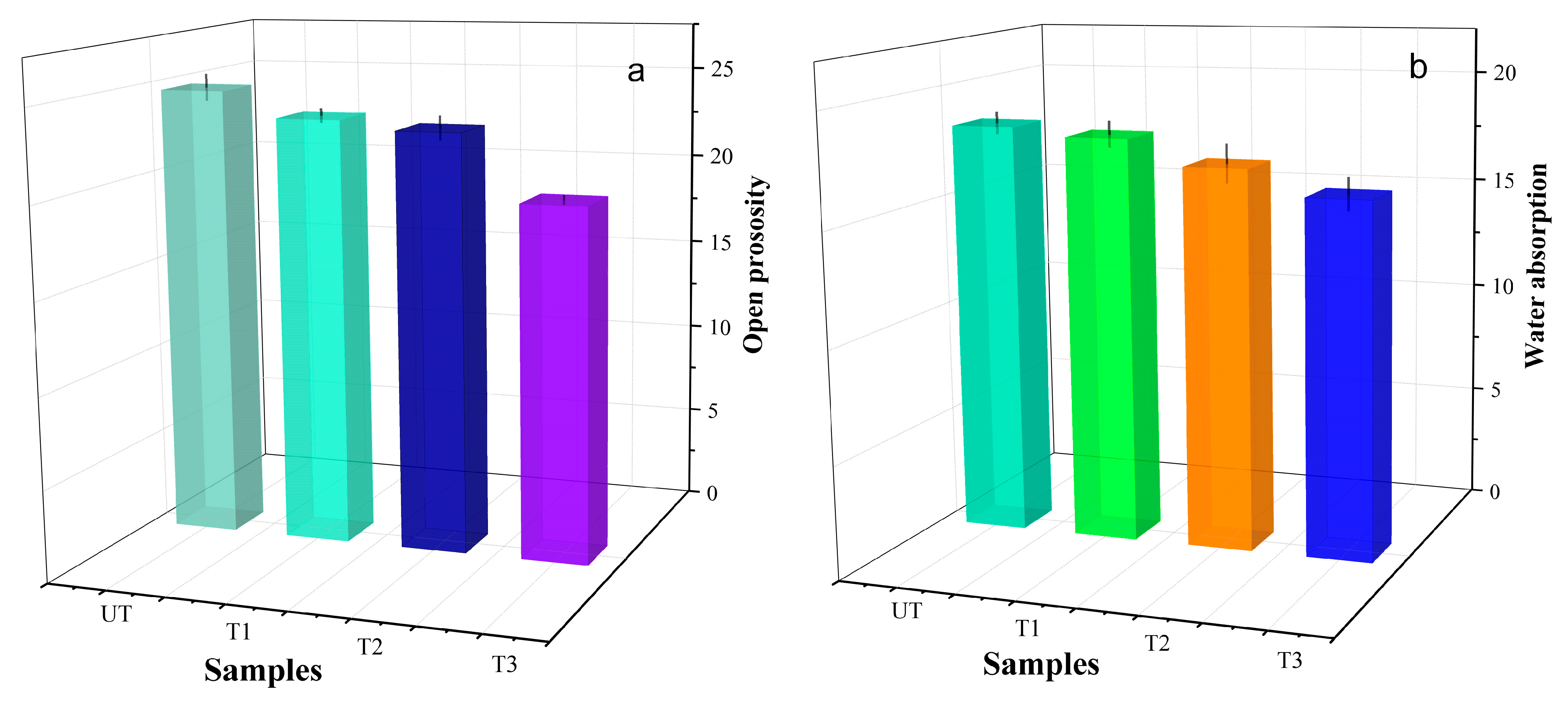
- The mechanical properties, including surface cohesion and hardness, are significantly enhanced. For specimens subjected to 1, 2, and 3 treatment cycles, the surface hardness increases from 20.80 HD to 60.94 HD and the STT shows that the mass loss decreases from 8.7 mg/cm2 to 2.7 mg/cm2.Additionally, its open porosity and capillary water absorption are both reduced. After 1, 2, and 3 treatment cycles, the total open porosity of the specimens decreases from 24.75% to 19.25% and capillary water absorption reduces from 18.37% to 15.75%, improving its water erosion resistance. These changes are attributed to the insoluble barium sulfate (BaSO4) and calcium carbonate (CaCO3) formed after the introduction of the barium-based protectant, which densify the mortar structure and fill weathering-induced cracks and pores.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Elsen, J. Microscopy of historic mortars—A review. Cem. Concr. Res. 2006, 36, 1416–1424. [Google Scholar] [CrossRef]
- Adams, J.; Kneller, W.; Dollimore, D. Thermal analysis (TA) of lime- and gypsum-based medieval mortars. Thermochim. Acta 1992, 211, 93–106. [Google Scholar] [CrossRef]
- Moayedian, S.M.; Hejazi, M. Effect of scale on compressive strength of brick masonry with gypsum mortar. Measurement 2021, 172, 108932. [Google Scholar] [CrossRef]
- Jiang, Q.; Huang, J.; Ma, B.; Yang, Z.; Zhang, T. SiO2/silicone hybrid superhydrophobic coating on gypsum-based materials with self-cleaning and moisture resistance. J. Sol.-Gel. Sci. Technol. 2020, 96, 207–218. [Google Scholar] [CrossRef]
- Artesani, A.; Di Turo, F.; Zucchelli, M.; Traviglia, A. Recent Advances in Protective Coatings for Cultural Heritage—An Overview. Coatings 2020, 10, 217. [Google Scholar] [CrossRef]
- Li, W.; Lin, J.; Zhao, Y.; Pan, Z. The Adverse Effects of TiO2 Photocatalycity on Paraloid B72 Hybrid Stone Relics Protective Coating Aging Behaviors under UV Irradiation. Polymers 2021, 13, 262. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Wang, Y.; Zhang, B. Lifetime prediction of relics conservation materials: An exploratory study in consideration of both chemical and physical factors. Prog. Org. Coat. 2018, 125, 242–248. [Google Scholar] [CrossRef]
- Ramamurthi, D.S.; Judes Sujatha, S.; Talluri, R.; Muthuraman, U. Effectiveness of using organic and inorganic consolidants on the properties of friable-porous gypsum mortar. Mater. Today Proc. 2022, 62, 5508–5513. [Google Scholar] [CrossRef]
- Sassoni, E.; Graziani, G.; Franzoni, E.; Scherer, G.W. Conversion of calcium sulfate dihydrate into calcium phosphates as a route for conservation of gypsum stuccoes and sulfated marble. Constr. Build. Mater. 2018, 170, 290–301. [Google Scholar] [CrossRef]
- Caroselli, M.; Ruffolo, S.A.; Piqué, F. Mortars and plasters—How to manage mortars and plasters conservation. Archaeol. Anthropol. Sci. 2021, 13, 188. [Google Scholar] [CrossRef]
- Delgado Rodrigues, J.; Ferreira Pinto, A.P.; Nogueira, R.; Gomes, A. Consolidation of lime mortars with ethyl silicate, nanolime and barium hydroxide. Effectiveness assessment with microdrilling data. J. Cult. Herit. 2018, 29, 43–53. [Google Scholar] [CrossRef]
- Lu, R.; He, L.; Li, T.; Yang, F.; Liu, Y.; Zhang, K.; Chen, X. A Novel Protection Method for Carbonate Stone Artifacts with Gypsum Weathering Crusts. Coatings 2022, 12, 1793. [Google Scholar] [CrossRef]
- Jones, F.; Piana, S.; Gale, J.D. Understanding the Kinetics of Barium Sulfate Precipitation from Water and Water–Methanol Solutions. Cryst. Growth Des. 2008, 8, 817–822. [Google Scholar] [CrossRef]
- Starikova, Z.A.; Kessler, V.G.; Turova, N.Y.; Dantsker, I.A.; Bobyljov, A.P.; Mitiaev, A.S. Interaction of barium oxide and hydroxide with methanol: X-ray single crystal study of Ba(OH)2 methanol solvates. Polyhedron 2006, 25, 2401–2406. [Google Scholar] [CrossRef]
- Zhang, K.; Yan, J.; Huang, Y.; Zhou, W.; Zhang, Y.; Qiang, Z.; Li, X.; Wen, R.; Liu, Y.; Yang, F. Investigation of the Impacts of Nonpolar Amino Acids on Air Lime Mortars. Int. J. Archit. Herit. 2024, 19, 1463–1479. [Google Scholar] [CrossRef]
- ASTM D3359-22; Standard Test Methods for Rating Adhesion by Tape Test. ASTM International: West Conshohocken, PA, USA, 2023; p. 8. [CrossRef]
- Yang, H.; Wang, L.; Wang, Z.; Huang, G.; Xing, Y.; Liu, Y.; Yang, F.; Zhang, K.; Sun, M.; Wang, Z. Conservation of the weathered bricks in ancient constructions using a novel protectant of calcium hydroxy glycolate. Npj Herit. Sci. 2025, 13, 81. [Google Scholar] [CrossRef]
- EN ISO 868:2003; Plastics and Ebonite—Determination of Indentation Hardness by Means of a Durometer (Shore Hardness). International Organization for Standardization: Geneva, Switzerland, 1 March 2003.
- EN 1015-18:2002; Methods of Test for Mortar for Masonry—Part 18: Determination of Water Absorption Coefficient Due to Capillary Action of Hardened Mortar. Comité Européen de Normalisation: Bruxelles, Belgique, 4 December 2002.
- BS EN1936:2006; Natural Stone Test Methods. Determination of Real Density and Apparent Density, and of Total and Open Porosity. British Standards Institution: London, UK, 31 January 2007.
- EN ISO 105-J03:2019; Textiles—Tests for Colour Fastness—Part J03: Calculation of Colour Differences (ISO 105-J03:2009). International Organization for Standardization: Geneva, Switzerland, 3 March 2010.
- Xu, Y.; Liao, Y.; Lin, Z.; Lin, J.; Li, Q.; Lin, J.; Jin, Z. Precipitation of calcium sulfate dihydrate in the presence of fulvic acid and magnesium ion. Chem. Eng. J. 2019, 361, 1078–1088. [Google Scholar] [CrossRef]
- Musić, S.; Filipović-Vinceković, N.; Sekovanić, L. Precipitation of amorphous SiO2 particles and their properties. Braz. J. Chem. Eng. 2011, 28, 89–94. [Google Scholar] [CrossRef]
- Wang, L.; Yang, H.; Chen, W.; Yang, F.; Liu, Y.; Zhang, K.; Wang, X.; Guo, S.; Chen, X. Preparation of infiltrating calcium carbonate layer in gypsum substrate using novel calcium precursor: The implication for the protection of the surface weathered carbonate heritages from water erosion damage. Surf. Interfaces 2024, 44, 103685. [Google Scholar] [CrossRef]
- Kader, M.S.; Weyer, C.; Avila, A.; Stealey, S.; Sell, S.; Zustiak, S.P.; Buckner, S.; McBride-Gagyi, S.; Jelliss, P.A. Synthesis and Characterization of BaSO4–CaCO3–Alginate Nanocomposite Materials as Contrast Agents for Fine Vascular Imaging. ACS Mater. Au 2022, 2, 260–268. [Google Scholar] [CrossRef]
- Lei, Q.; Elele, E.; Shen, Y.; Tang, J.; Guerra, K.L.; Leitz, F.; Khusid, B. Evaluating the Efficiency of Magnetic Treatment for Feed Water in Reverse Osmosis Processes. Membranes 2023, 13, 641. [Google Scholar] [CrossRef]
- Yin, X.; Yao, J.; Gong, X.; Yin, W.; Yu, J.; Wang, X. Role of pH value and inhibitor in the simultaneous separation of dolomite and quartz from magnesite. Colloids Surf. A Physicochem. Eng. Asp. 2025, 726, 137905. [Google Scholar] [CrossRef]
- Leamkaew, V.; Crespy, D. Preparation of barium sulfate/polymer hybrid nanoparticles from Bunte salt precursors. Chem. Commun. 2025, 61, 4499–4502. [Google Scholar] [CrossRef]
- Larissa Siqueira Matos-Pimentel, H.; Maria Alves de Oliveira, C.; Gomes de Souza, F.; Gasparotto, G.; Leite, B.; Freitas Carvalho, J. Exploring calcite Coprecipitation: Synthesis, Crystal Growth, and pH effect. J. Cryst. Growth 2024, 643, 127808. [Google Scholar] [CrossRef]
- Lu, R.; Wang, L.; Liu, Y.; Yang, F.; Yang, L.; Wang, L.; Gao, X. Conservation of surface gypsification stone relics using methanol solution of barium hydroxide as a novel treating agent. Appl. Phys. A 2021, 128, 37. [Google Scholar] [CrossRef]
- Rouwendaal, S.E.; Birgel, D.; Grossi, V.; Aloisi, G.; Guibourdenche, L.; Labrado, A.L.; Brunner, B.; Rouchy, J.-M.; Peckmann, J. Two modes of gypsum replacement by carbonate and native sulfur in the Lorca Basin, SE Spain. Front. Earth Sci. 2023, 11, 1153415. [Google Scholar] [CrossRef]
- Yan, J.; Huang, G.; Li, X.; Liu, Q.; Liu, Y.; Yang, F.; Zhang, K.; Sun, Y. A comparative study between aqueous and methanol solutions of barium hydroxide: Implications for applying barium protectants in gypsification calcareous relics. Herit. Sci. 2024, 12, 203. [Google Scholar] [CrossRef]
- López-Martínez, T.; Otero, J. Preventing the Undesired Surface Veiling after Nanolime Treatments on Wall Paintings: Preliminary Investigations. Coatings 2021, 11, 1083. [Google Scholar] [CrossRef]
- Rodrigues, J.D.; Grossi, A. Indicators and ratings for the compatibility assessment of conservation actions. J. Cult. Herit. 2007, 8, 32–43. [Google Scholar] [CrossRef]
- Hou, P.; Zhang, R.; Cai, Y.; Cheng, X.; Shah, S.P. In situ Ca(OH)2 consumption of TEOS on the surface of hardened cement-based materials and its improving effects on the Ca-leaching and sulfate-attack resistivity. Constr. Build. Mater. 2016, 113, 890–896. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Y.; Li, T.; Dong, J.; Liu, Y.; Yan, X.; Ling, Y.; Huang, G.; Yang, F. Improvement of Water Erosion Resistance of Gypsum Mortars in the Historic Buildings for Conservation Purpose. Coatings 2025, 15, 1165. https://doi.org/10.3390/coatings15101165
Sun Y, Li T, Dong J, Liu Y, Yan X, Ling Y, Huang G, Yang F. Improvement of Water Erosion Resistance of Gypsum Mortars in the Historic Buildings for Conservation Purpose. Coatings. 2025; 15(10):1165. https://doi.org/10.3390/coatings15101165
Chicago/Turabian StyleSun, Yichen, Ting Li, Jianing Dong, Yan Liu, Xiaoqin Yan, Yong Ling, Guang Huang, and Fuwei Yang. 2025. "Improvement of Water Erosion Resistance of Gypsum Mortars in the Historic Buildings for Conservation Purpose" Coatings 15, no. 10: 1165. https://doi.org/10.3390/coatings15101165
APA StyleSun, Y., Li, T., Dong, J., Liu, Y., Yan, X., Ling, Y., Huang, G., & Yang, F. (2025). Improvement of Water Erosion Resistance of Gypsum Mortars in the Historic Buildings for Conservation Purpose. Coatings, 15(10), 1165. https://doi.org/10.3390/coatings15101165





