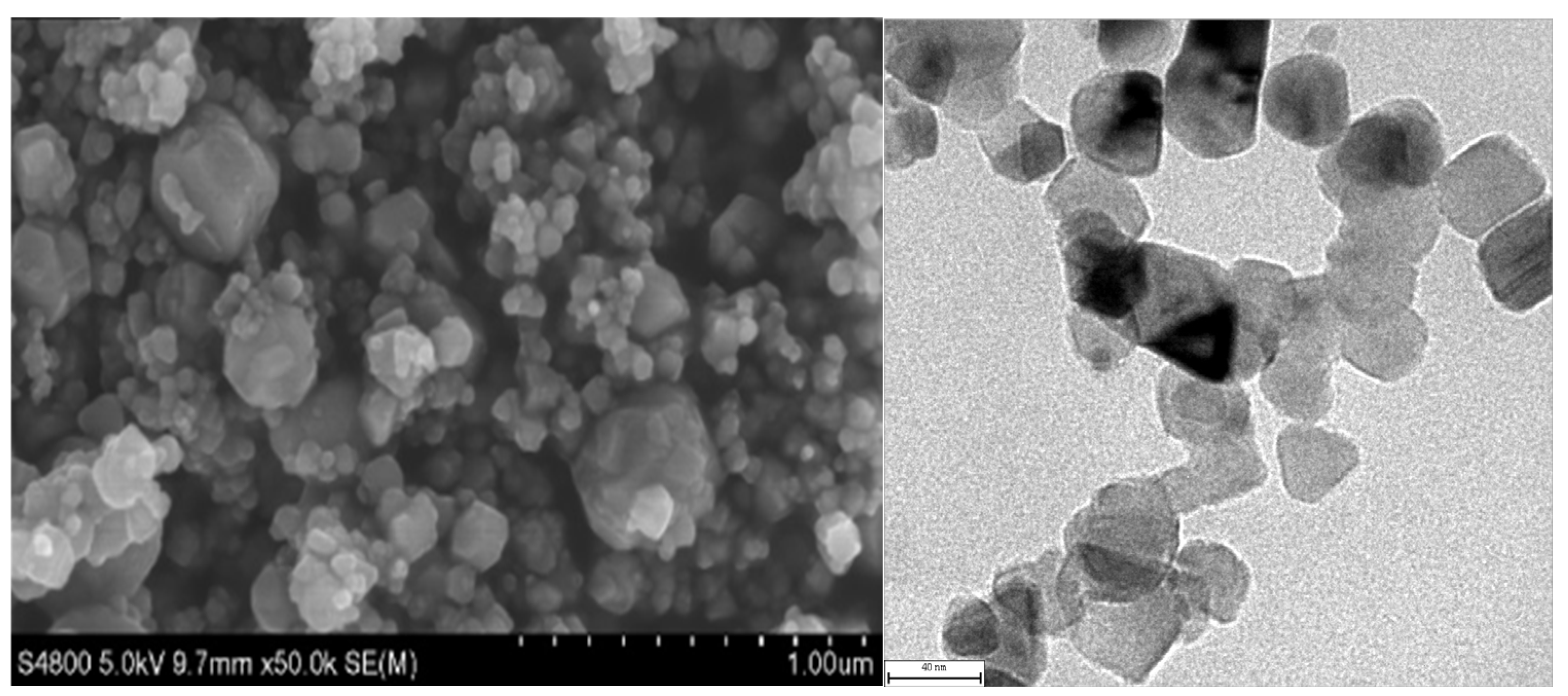
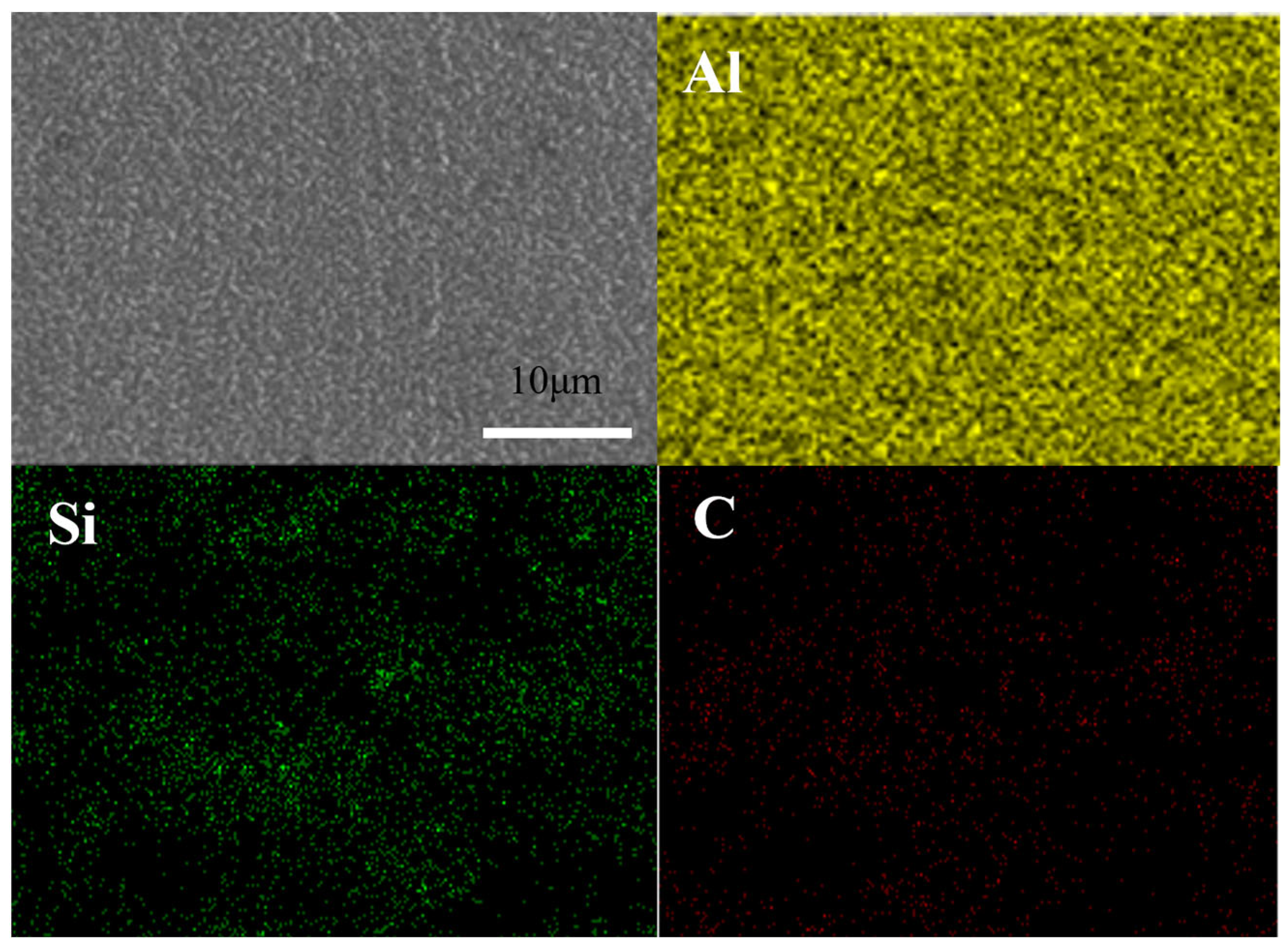
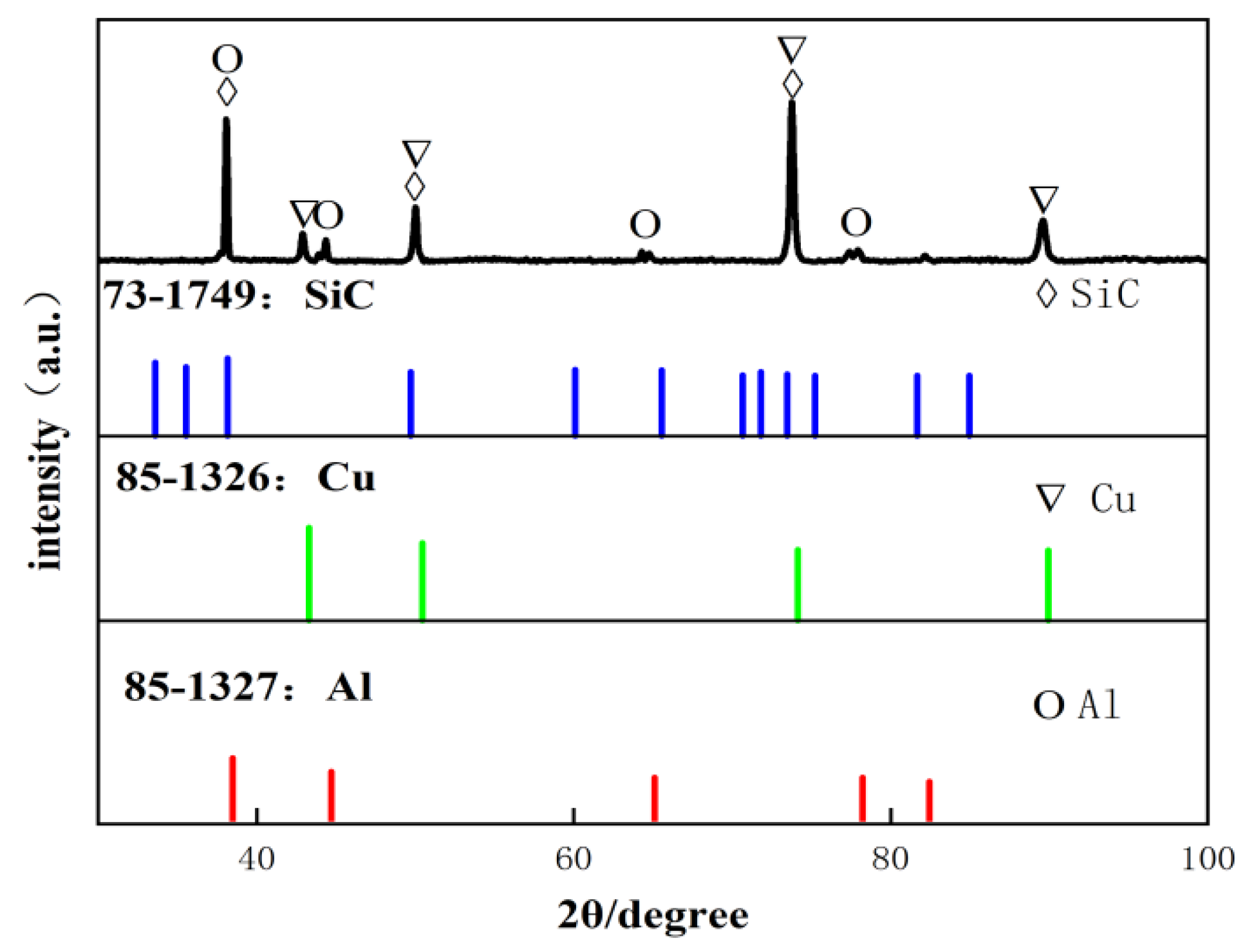
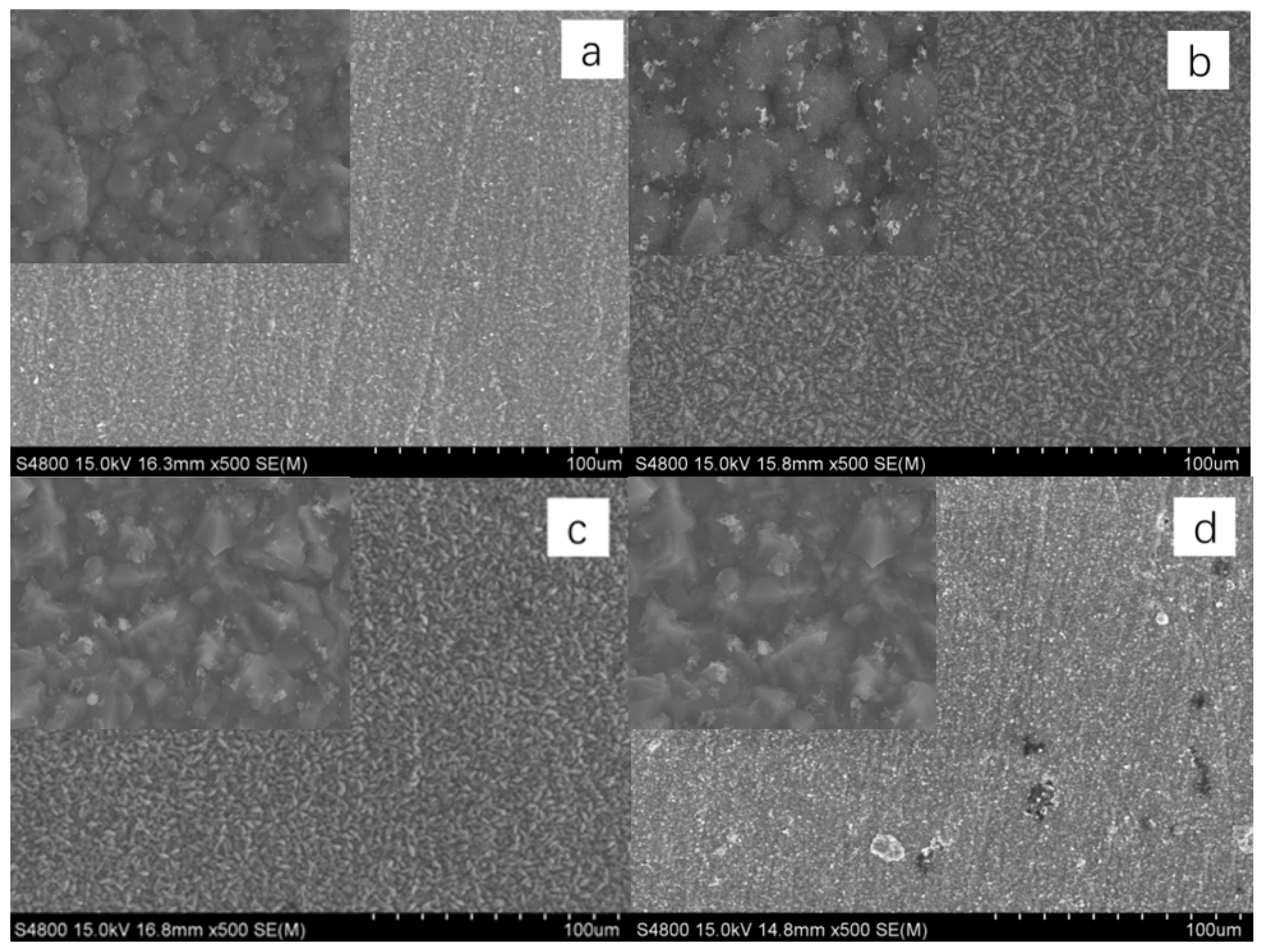
During the electrochemical corrosion process of the Al-SiC composite coating prepared in the AlCl3-LiAlH4-benzene-THF system, corrosion initially commences at the microscopic defects (pores, micro-cracks) of the coating and the interface between the Al matrix and SiC. Aggressive ions such as Cl− preferentially attack these areas, and the micro-galvanic coupling at the interface due to thermal stress generated during the preparation process becomes the sensitive point for corrosion initiation. Subsequently, the pitting corrosion enters the development and expansion stage. Cl− adsorbs at the defects or interfaces and destroys the passive film on the surface of the Al matrix, triggering local anodic dissolution to form pitting nuclei. The occluded cell effect inside the corrosion pits leads to a decrease in the pH value and an increase in the Cl− concentration within the pits, accelerating the dissolution of the metal inside the pits and promoting the deep-seated development of the pits. As the corrosion progresses, the corrosion mechanism gradually evolves from a mechanism mainly dominated by the charge transfer process in the initial stage to a mixed mechanism jointly controlled by charge transfer and the diffusion of corrosion products within the pits.
3.6.1. The Influence of Current Density on Corrosion Resistance Performance
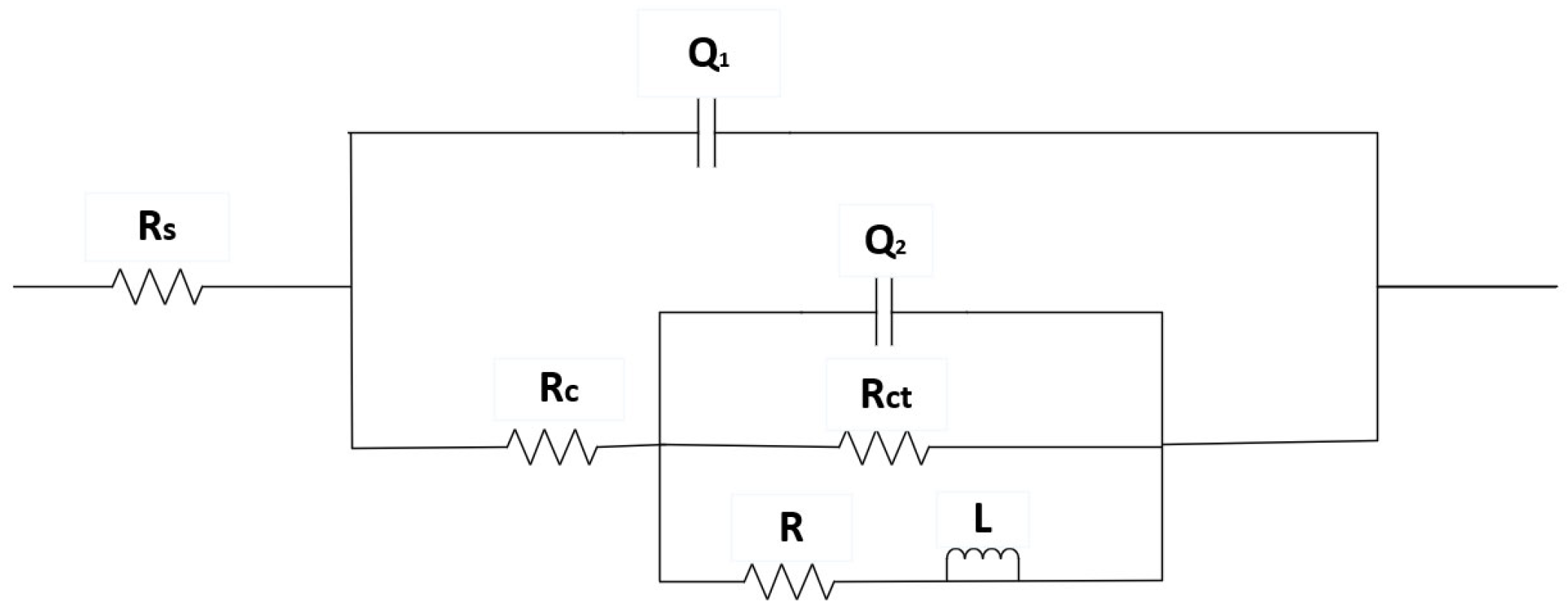
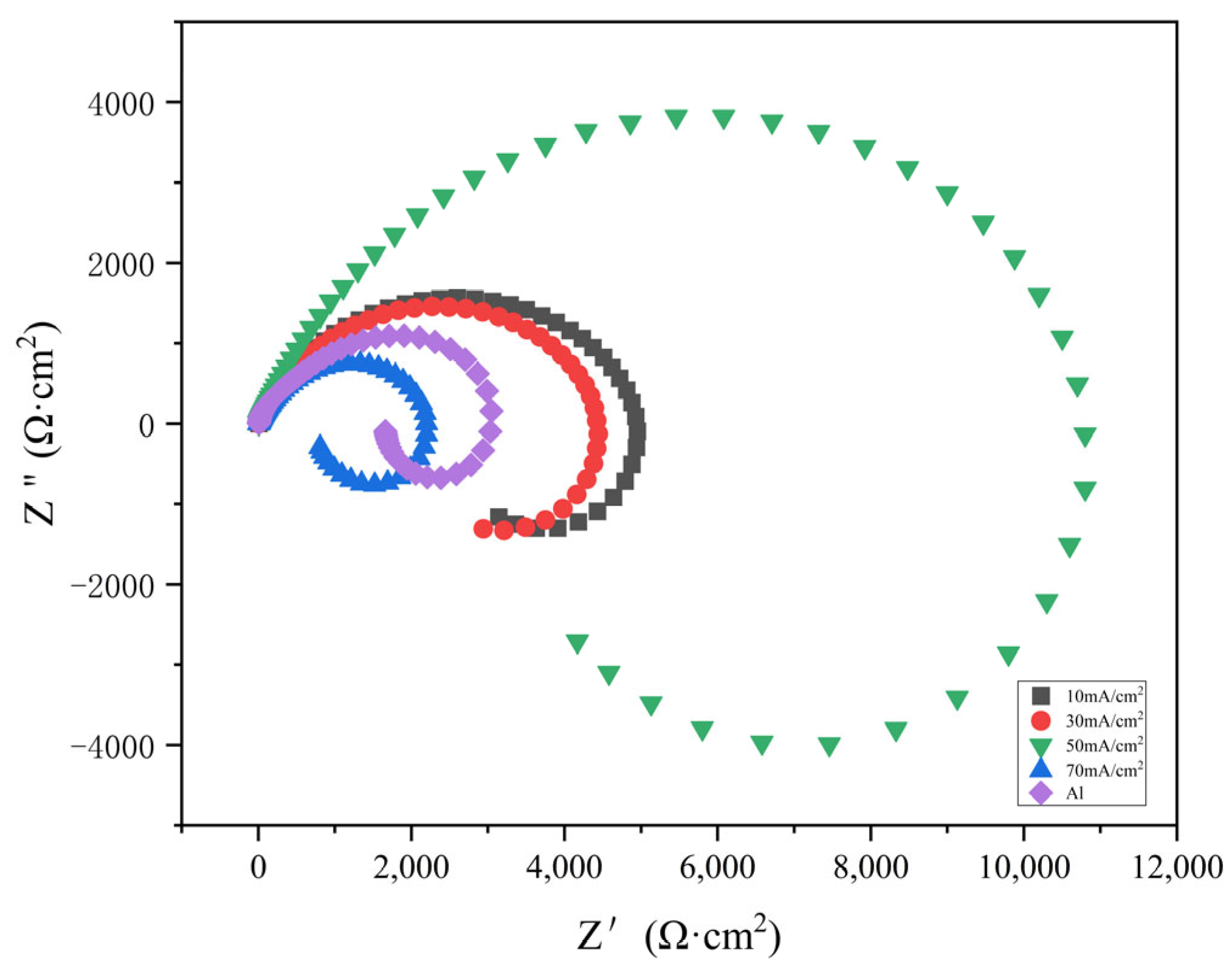
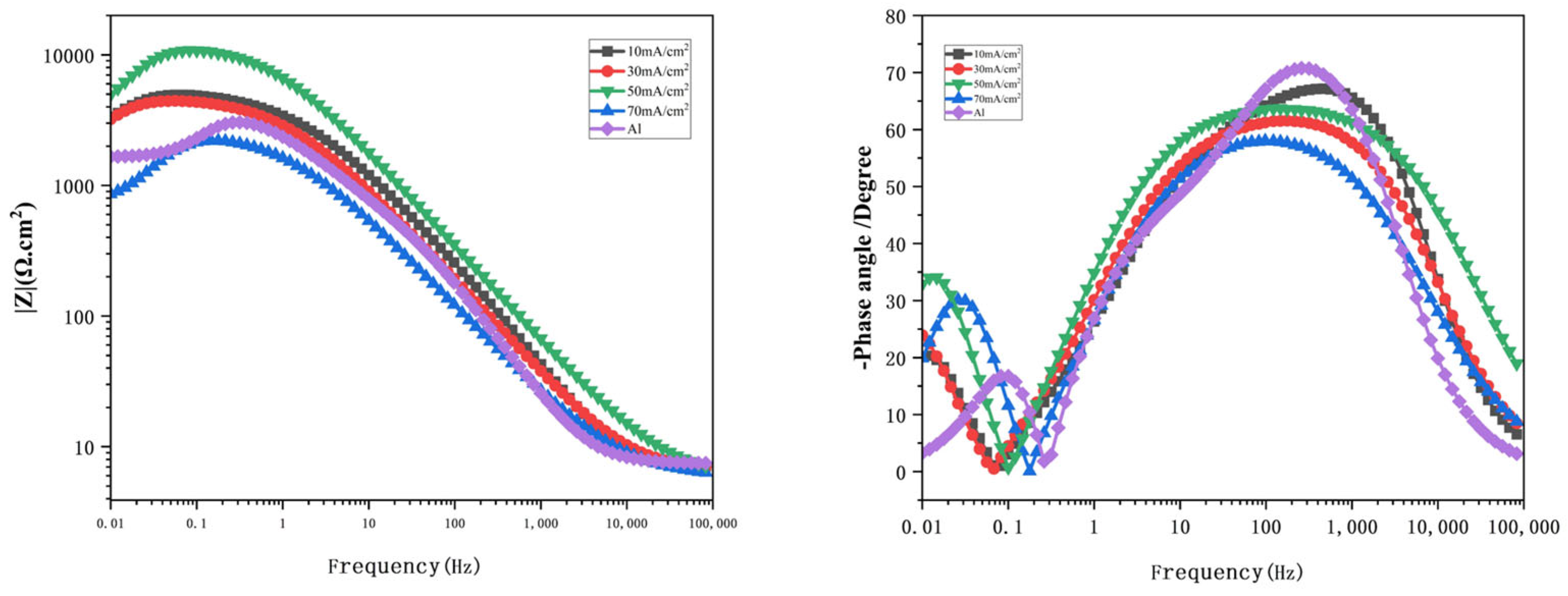
The impedance spectra of Al-SiC composite coatings under different process parameters were explored, and the optimal process parameters were preliminarily determined through fitting analysis. Impedance can to some extent reflect the corrosion resistance of materials. All the tests in this paper were conducted using a 3.5% sodium chloride solution. In the course of this experiment, the Nyquist diagrams for both the composite coating and the pure aluminum coating, assessed at various current densities, were analyzed using equivalent circuits via the ZView software. An equivalent circuit model for the composite, which incorporated solution resistance, charge transfer resistance, constant phase angle components of the double layer, and inductive reactance, was developed. The geometric dimensions of the capacitive reactance arc in the high-frequency region are positively correlated with the corrosion resistance performance. It can be clearly seen from the figure that there is an inductive ring and a capacitive ring. Nyquist diagrams of different current densities show that the loops vary significantly.
Figure 8 illustrates the Nyquist plots for the Al-SiC composite coating across various current densities. Meanwhile,
Figure 9 presents the Bode diagrams of the Al-SiC composite coating at differing current densities. Nyquist diagrams of different current densities show that the loops vary significantly. Among them, when the current density is 50 mA/cm
2, the arc of the high-frequency capacitor loop of the Al-SiC composite coating is the largest, indicating that its corrosion resistance is relatively good. Upon reaching a current density of 70 mA/cm
2, there is a notable decrease in the corrosion resistance of the coating, falling below that of the pure aluminum coating as illustrated in the figure. This decline occurs because a high current density can adversely affect the surface morphology of the coating, enabling corrosive agents to infiltrate the inner structure of the coating. The corrosion of pure aluminum coatings often begins with the attack of Cl
− toxic ions on surface defects or weaknesses, and is prone to develop into pitting corrosion. In the Al-SiC composite coating, uniformly dispersed SiC nanoparticles/micro-particles play a key role: Firstly, they act as physical barriers, hindering the diffusion of corrosive media and the propagation of corrosion cracks. Second, it refines the aluminum particles to help form a denser and more stable Al
2O
3 passivation film. The third is to fill the pores and reduce the density of coating defects. These effects work together to significantly delay the occurrence and development of pitting.
Based on the equivalent circuit diagram related to the Al-SiC coating presented in
Figure 7, the fitting data are summarized in
Table 3 and
Table 4. The Rs values for each coating are relatively low and show minimal variation, suggesting that the solution resistance remains largely consistent across coatings with varying process parameters, and that the tested medium conditions are fairly similar. At a current density of 50 mA/cm
2, the charge transfer resistance (Rct) attains its highest value of 11,880 Ω·cm
2, signifying that charge transfer poses the greatest challenge. It indicates that charge transfer is the most difficult, and there is a barrier on the surface of the film layer that inhibits charge transfer, which means that the corrosion resistance of the coating is relatively good. The resistance to corrosion exhibited by composite coatings depends on their composition and density. The proportion of particles within the coating influences its corrosion resistance. As the current density ranges from 10 mA/cm
2 to 50 mA/cm
2, the content of SiC rises, the surface matrix grains on the coating diminish, the density improves, and the distribution of SiC becomes increasingly uniform. Consequently, the corrosion resistance of the coating is enhanced. Nevertheless, when the current density escalates to 70 mA/cm
2, the higher current density results in a poorer surface flatness compared to the outcomes observed at 50 mA/cm
2. Furthermore, there is a significant reduction in SiC content and the formation of large agglomerations, which ultimately results in a decline in the corrosion resistance of the coating at 70 mA/cm
2.
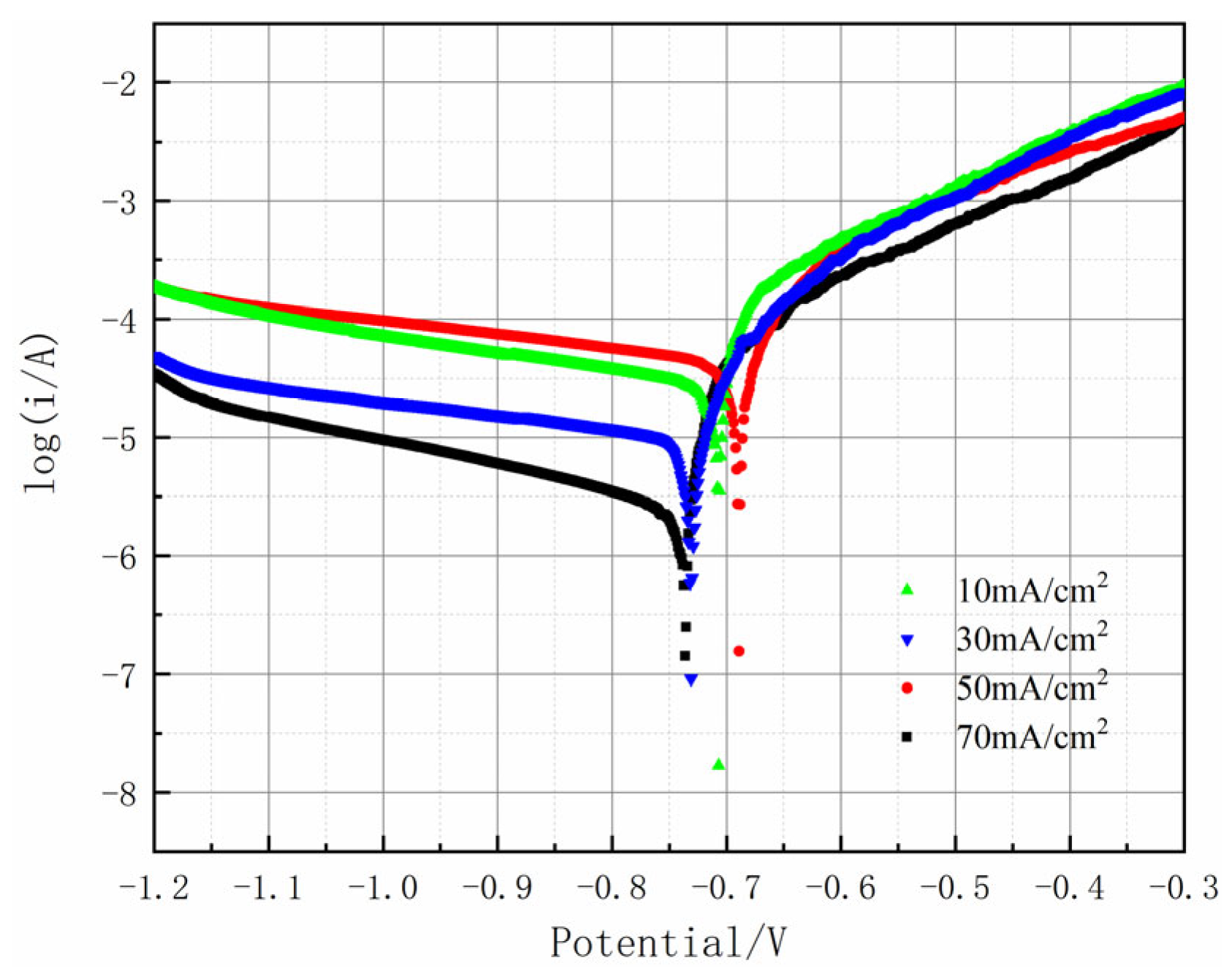
In order to thoroughly investigate how process parameters affect the corrosion resistance of Al-SiC composite coatings, this research conducted a systematic analysis of the electrochemical behavior of samples immersed in a 3.5 wt% NaCl solution, employing the potentiodynamic polarization test method. The potentiodynamic polarization curve serves primarily to evaluate the self-corrosion potential, self-corrosion current density, and the passivation characteristics of the samples. The self-corrosion potential indicates the thermodynamic stability of the material within the solution, while the self-corrosion current density provides insights into the kinetic aspects of the corrosion rate. Furthermore, the passivation behavior reveals whether the material develops a protective passivation film and assesses its stability. This polarization curve is divided into two main sections: the cathodic region and the anodic region. In the cathodic area, reduction reactions take place. As the potential decreases, the driving force for the reduction reaction rises, leading to a surge in cathodic current density and a faster reaction rate. Conversely, in the anodic region, oxidation reactions occur. As the potential increases, the current density progressively elevates, resulting in a higher corrosion rate and observable active dissolution behavior.
This study analyzed its electrochemical behavior in 3.5 wt% NaCl solution through potentiodynamic polarization testing (
Figure 10 and
Table 5). The results show that with the increase in current density, the corrosion resistance of the coating first enhances and then weakens. 50 mA/cm
2 is the critical value. At this time, the corrosion potential is −0.692 V, the corrosion current density is the lowest (4.762 × 10
−6 A/cm
2), and the corrosion resistance is the preferable. Under this condition, the acceleration of electrochemical reaction kinetics promotes the effective adsorption and co-deposition of SiC particles, increasing the nucleation points of the coating and making the structure denser. The densification and particle synergy inhibit the penetration of corrosive media (the self-corrosion current density is as low as 1.2 × 10
−7 A/cm
2), and when the current density exceeds the critical value, the deposition rate of aluminum ions far exceeds that of SiC. This leads to the appearance of micro-pores and cracks in the coating, and a decline in protective performance.
3.6.2. The Influence of Temperature on Corrosion Resistance Performance
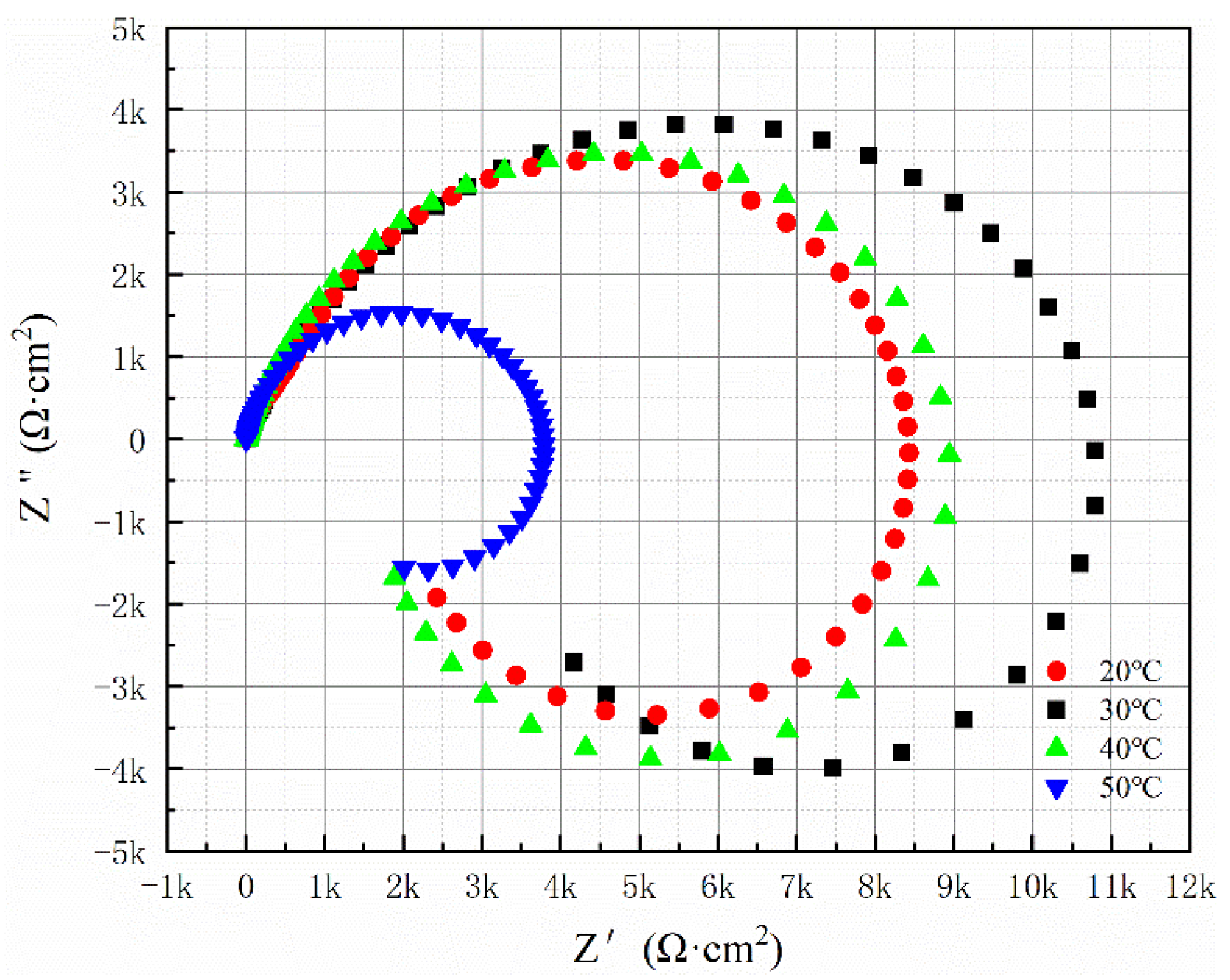
The Nyquist spectrum illustrated in
Figure 11 reveals that with an increase in temperature from 20 °C to 50 °C, the radius of the capacitive reactance arc initially grows before subsequently decreasing. The maximum arc occurs at a temperature of 30 °C. According to
Table 6, the Rct value reaches its peak at this temperature.
Figure 12 presents the Bode diagrams for the Al-SiC composite coating across varying temperatures. The overall findings from the experiments indicate that the Al-SiC composite coating produced at 30 °C exhibits the best corrosion resistance.
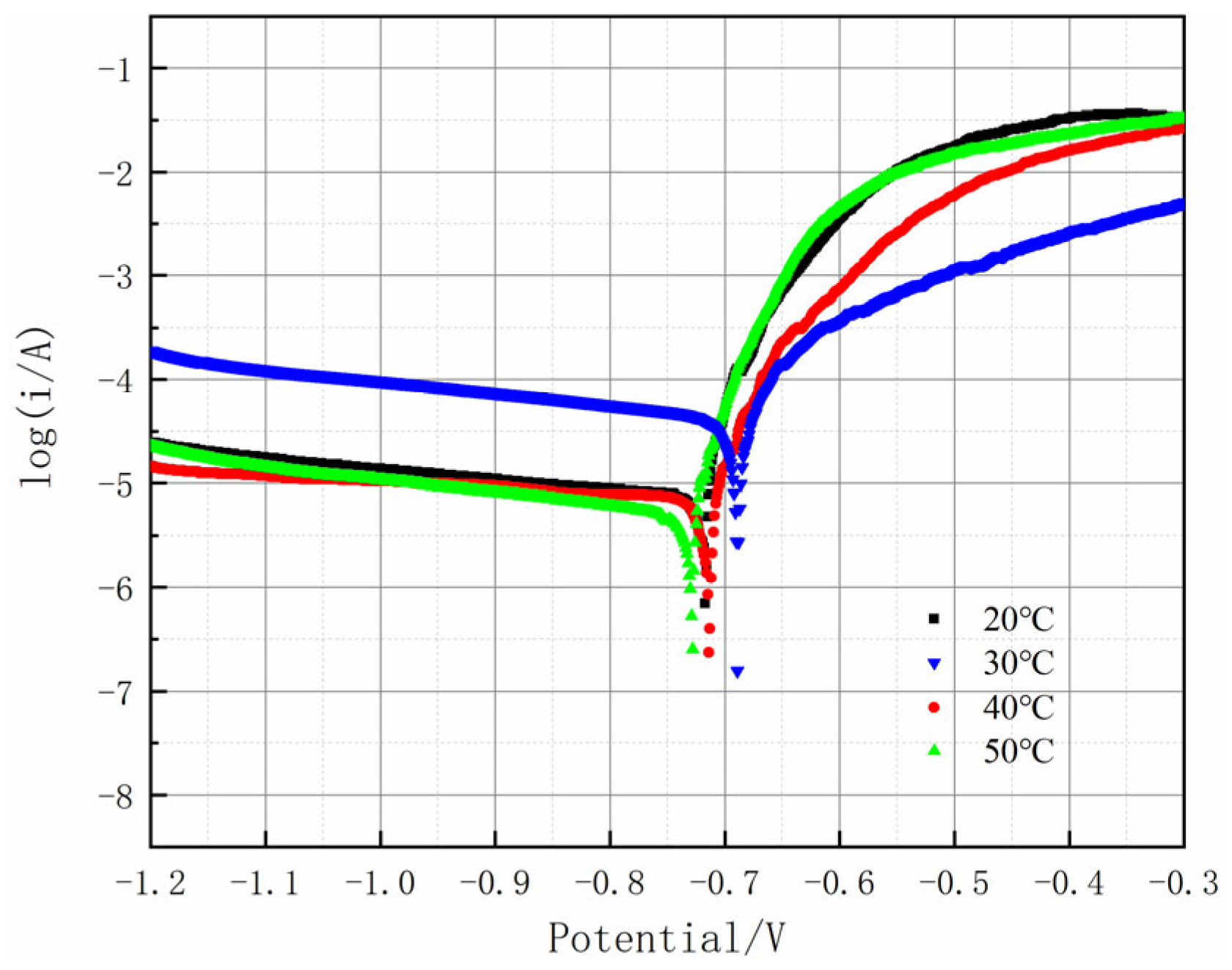
Figure 13 shows the potentiodynamic polarization curves of the Al-SiC composite coating at different temperatures.
Table 7 shows the Tafel extrapolation fitting parameters for the corresponding Al-SiC composite coating. It can be concluded from
Figure 13 and
Table 7 that at 20 °C, the corrosion potential of the Al-SiC composite coating is relatively negative, approximately −0.717 V, and the corrosion current density is relatively high, reaching approximately 1.969 × 10
−5 mA/cm
2. This indicates that the corrosion activity of the coating is strong at this temperature. When the temperature rises to 30 °C, the corrosion potential shifts positively to approximately −0.692 V, and the corrosion current density drops significantly to about 4.762 × 10
−6 mA/cm
2. At this temperature, the density of current in the passivation region is notably minimal, suggesting that a fairly stable passivation layer has developed on the surface, effectively preventing the corrosion process. At A temperature of 50 °C, the corrosion potential (
Ecorr) shifts negatively to approximately −0.728 V, and the corrosion current density rebounds to about 4.66 × 10
−5 A/cm
2. The protective performance of the passivation film has declined, thereby intensifying the corrosion. Therefore, the Al-SiC composite coating prepared under the conditions of a current density of 50 mA/cm
2 and a plating solution temperature of 30 °C has the preferable corrosion resistance.
A suitable rise in temperature will promote the migration rate of ions within the plating solution, facilitating a higher quantity of SiC in the coating. Concurrently, this elevation in temperature will improve the dispersion of SiC particles, which is advantageous for diminishing the occurrence of cracks and defects in the coating. Furthermore, it will enhance the coating’s density, limit the infiltration of corrosive agents, lower the corrosion current density, and advance the corrosion potential. This has notably improved the corrosion resistance of the composite coating. Nevertheless, if the temperature escalates to 40 °C or 50 °C, the excessively high temperature may impair the adhesion of particles within the coating. Consequently, some particles may detach during the deposition process, resulting in a decreased particle content in the coating.
Therefore, the Al-SiC composite coating prepared under the conditions of a current density of 50 mA/cm2 and a plating solution temperature of 30 °C has good corrosion resistance.
3.6.3. The Influence of SiC Addition on Corrosion Resistance Performance
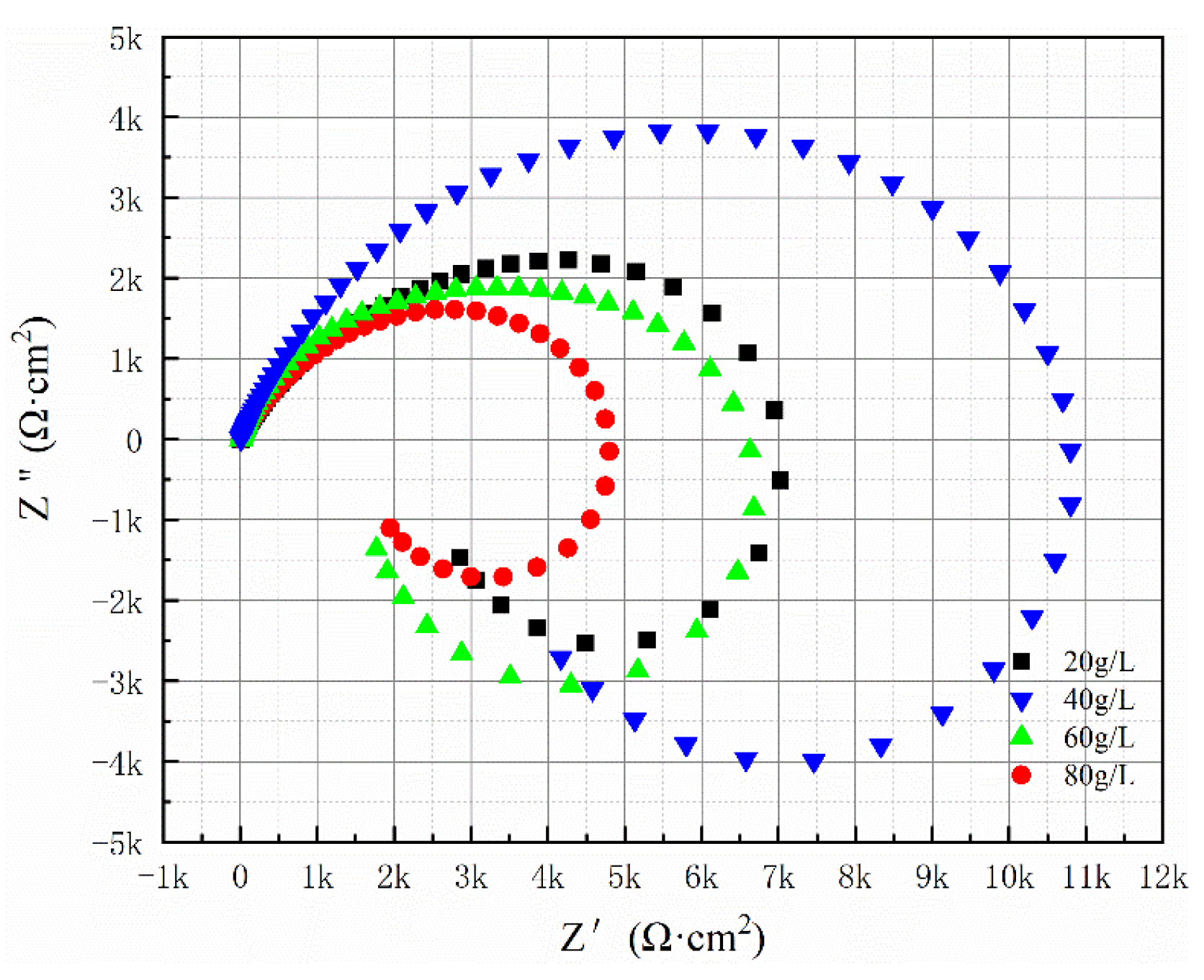
The Nyquist spectrum presented in
Figure 14 demonstrates that increasing the SiC concentration from 20 g/L to 80 g/L initially leads to an expansion of the capacitive reactance arc, followed by a reduction. The maximum capacitance arc is observed when the SiC concentration reaches 40 g/L.
Table 8 displays the equivalent circuit fitting data for the composite coating at various SiC concentrations. Additionally,
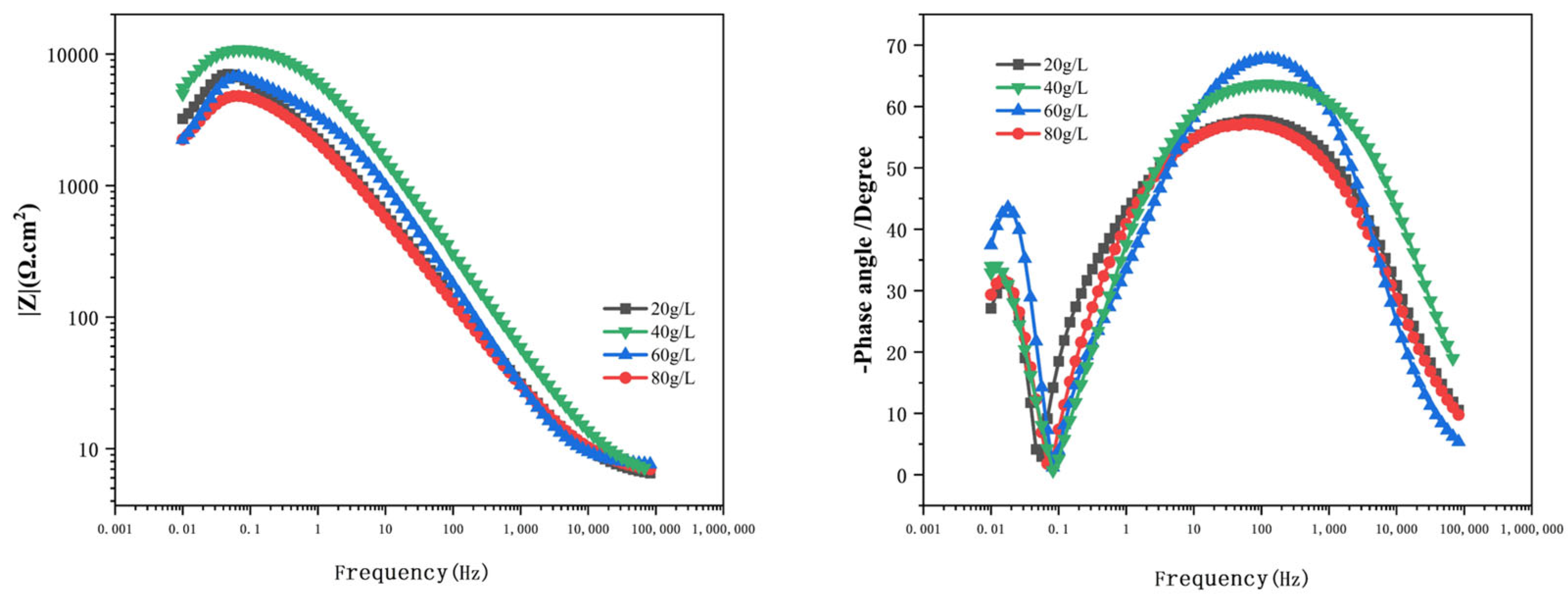
Figure 15 illustrates the Bode plots for the Al-SiC composite coating with different SiC amounts. At 40 g/L of SiC, the Rct value attains its peak. The comprehensive experimental results show that the Al-SiC composite coating obtained under the process condition of 40 g/L has good corrosion resistance.
Figure 16 and
Table 9 show: When the corrosion potential exceeded −0.4 V, the coatings with different SiC concentrations all showed typical passivation characteristics. The anodic dissolution rate decreased significantly with the forward migration of the potential, indicating that a stable protective film was formed on the surface. When the SiC concentration increased from 20 g/L to 40 g/L, the corrosion potential shifted forward to the peak of −0.692 V. The corrosion current density was reduced to the minimum of 4.762 × 10
−6 mA/cm
2, and the coating density was the preferable. Interfacial corrosion was effectively suppressed. As the concentration of SiC surpassed 40 g/L, a noticeable shift in the corrosion potential occurred in the negative direction, accompanied by a significant rise in the corrosion current density with the increase in concentration. This suggests that when the quantity of SiC particles added exceeds a specific threshold, it could compromise the coating’s integrity, thus hastening the localized corrosion process. Increasing the amount of SiC added to the coating can effectively enhance its resistance to corrosion. This enhancement occurs because a higher concentration of SiC promotes the formation of more nucleation sites within the coating, aiding in the development of a denser and more complete composite layer. As the concentration of SiC particles grows from 20 g/L to 40 g/L, there is an increase in SiC content within the coating, leading to better dispersion, uniform distribution of particles across the surface, smaller matrix grains, a more compact arrangement, and an overall denser structure. Consequently, the coating exhibits improved corrosion resistance. However, at SiC concentrations of 60 g/L and 80 g/L, the substrate’s load-bearing capacity becomes constrained. Although the SiC content in the coating shows a slight increase, it is not substantial. At these higher concentrations, SiC tends to agglomerate and become embedded on the coating’s surface, ultimately compromising the corrosion resistance of the composite coating.