Abstract
Multifunctional coatings integrating high transparency, thermal insulation, and self-cleaning properties are critically needed for optical devices and energy-saving applications, yet simultaneously optimizing these functions remains challenging due to material and structural limitations. This study designed a superhydrophobic transparent thermal insulation coating via synergistic co-construction of micro–nano structures using antimony-doped tin oxide (ATO) and SiO2 nanoparticles dispersed in an epoxy resin matrix, with surface modification by perfluorodecyltriethoxysilane (PFDTES) and γ-glycidyl ether oxypropyltrimethoxysilane (KH560). The optimal superhydrophobic transparent thermal insulating (SHTTI) coating, prepared with 0.6 g SiO2 and 0.8 g ATO (SHTTI-0.6-0.8), achieved a water contact angle (WCA) of 162.4°, sliding angle (SA) of 3°, and visible light transmittance of 72% at 520 nm. Under simulated solar irradiation, it reduced interior temperature by 7.3 °C compared to blank glass. The SHTTI-0.6-0.8 coating demonstrated robust mechanical durability by maintaining superhydrophobicity through 40 abrasion cycles, 30 tape-peel tests, and sand impacts, combined with chemical stability, effective self-cleaning capability, and exceptional anti-icing performance that prolonged freezing time to 562 s versus 87 s for blank glass. This work provides a viable strategy for high-performance multifunctional coatings through rational component ratio optimization.
1. Introduction
In recent years, the widespread application of functional coatings in fields such as optical devices, electronic packaging, energy systems, transportation, and architecture has led to increasingly stringent demands for comprehensive performance [1,2,3]. Single-function coatings can no longer meet the demands of complex and variable service environments, making multifunctional composite coatings a significant research focus [4,5]. Systems such as transparent optical devices [6], solar modules [7], display encapsulation [8], automotive windows [9], and architectural glass curtain walls [10], require high visible light transmittance to satisfy daylighting needs, efficient near-infrared (NIR) shielding to reduce heat transfer and energy consumption, and self-cleaning properties to minimize maintenance costs [11,12,13,14,15].
Inspired by natural plants like lotus leaves, superhydrophobic surfaces have remarkable water repellency [16], low adhesion [17], and self-cleaning capabilities [18,19]. Typical superhydrophobic surfaces are characterized by a water contact angle (WCA) > 150° and a slide angle (SA) < 10° [20,21]. Its wetting behavior mainly originates from the synergistic interaction between surface micro- and nanostructures and low surface energy materials [22]. Significant progress has been made in highly transparent superhydrophobic coatings. Chang et al. fabricated a transparent, self-cleaning coating via one-step spraying of hydrophobic fumed SiO2, silicone resin, and epoxy resin, achieving a WCA of 160°, SA of 1°, and 3% higher transmittance than bare glass, along with good durability [23]. Sun et al. synthesized a transparent, flexible PDMS-EP/SiO2 superhydrophobic coating using spray coating, demonstrating excellent mechanical durability, chemical stability, high transparency, flexibility, and self-cleaning performance [24]. Li et al. constructed a honeycomb-structured epoxy coating via PDMS template replication followed by hydrophobic SiO2 nanoparticle spraying. This coating exhibited high transparency (average transmittance: 90.0%), abrasion resistance, and superhydrophobicity, maintaining its properties under harsh mechanical wear and climatic conditions [25].
Beyond surface functionality, infrared management is critical for energy efficiency. Wide-bandgap n-type semiconductor materials, including indium tin oxide (ITO) [26], antimony-doped tin oxide (ATO) [27], aluminum/gallium-doped zinc oxide (AZO/GZO) [28], and cesium tungsten bronze (CWO) [29], exhibit strong absorption in the UV-NIR spectrum while maintaining high transparency in the visible region. These properties make them ideal for energy-saving transparent conductive materials [30,31,32]. Tan et al. developed a superhydrophobic, transparent, and insulating film using Rb+/Cs+ co-doped tungsten bronze filler combined with PVA, PDMS, and SiO2. The film achieved 55.6% visible light transmittance, 94.6% NIR shielding efficiency, a WCA of 163.3°, and a maximum temperature difference of 8.9 °C compared to uncoated glass [33]. Ke et al. prepared a composite coating on glass by combining ATO nanopowder with LMPGP, optimizing the ATO content (4 g), applying rapid thermal treatment at 690 °C, and modifying the surface with PFOTS. The coating exhibited superhydrophobicity (WCA = 154°), high transparency (visible transmittance of 86.3%), and effective thermal insulation (13.3% reduction in NIR transmittance, 4.7 °C temperature difference) [34].
Despite these advances, significant challenges remain, including the difficulty in simultaneously optimizing thermal insulation, self-cleaning, and high transparency; insufficient mechanical stability; and high production costs. ATO is particularly promising for building glass and energy-saving coatings due to its excellent NIR blocking capability, high thermal stability, and low cost [35]. Although ATO is generally considered a low-toxicity conductive oxide, excessive release of antimony compounds may raise environmental concerns. In this work, the ATO nanoparticles are firmly immobilized within the epoxy matrix, which effectively prevents their release and ensures negligible toxicity risk in practical applications.” Meanwhile, SiO2, with its controllable particle size and ease of surface modification, is widely used to construct micro–nano composite rough structures [36]. Epoxy resin (EP) serves as the matrix material, forming a stable three-dimensional network through crosslinking curing. It strengthens the adhesive properties of the coating and protects the structural stability and long-term durability [37]. This synergistic design enables the development of coating materials with concurrent optimization of superhydrophobicity, high transparency, and infrared shielding, along with robust mechanical properties.
In this work, we designed a transparent, multifunctional superhydrophobic coating by synergistically utilizing nano-sized ATO and SiO2 to construct a micro–nano surface structure. This imparts the coating with excellent superhydrophobicity (WCA = 162.4°, SA = 3°) while preserving a high transmittance of about 72% in the visible band. A systematic investigation was conducted on the influence of nanoparticle ratios on the coating’s wettability, optical properties, and thermal insulation performance, elucidating the mechanistic role of each functional component. The ATO imparts excellent thermal insulation properties to the coating. In thermal insulation experiments under simulated sunlight irradiation, the SHTTI-0.6-0.8 coating reduced the indoor temperature by approximately 7.3 °C compared to blank glass during the same period. Contaminant self-cleaning experiments and a series of environmental tolerance tests (mechanical robustness, weather resistance, anti-icing performance) further confirmed its superior durability. Comprehensive performance analysis indicates that the SHTTI-0.6-0.8 coating achieves an optimal balance between thermal insulation and superhydrophobicity and has high transparency, providing a novel research concept and experimental foundation for developing high-performance multifunctional transparent coatings.
2. Experimental Section
2.1. Materials
First, 7–40 nm vapor-phase SiO2, ethyl acetate, anhydrous ethanol, ammonia, perfluorodecyltriethoxysilane (PFDTES), γ-glycidyl ether oxypropyltrimethoxysilane (KH560), and epoxy resin E51 (bisphenol-A based liquid epoxy, CAS 25068-38-6; epoxy value 0.48–0.54 mol/100 g, epoxy equivalent weight (EEW) ≈ 185–208 g·eq−1) were purchased from Shanghai McLean Biochemical Technology Co., Ltd. (Shanghai, China), and 10–20 nm ATO powder was purchased from Yantai Jialong Nano Industry Co., Ltd (Yantai, China). The modified alicyclic amine epoxy resin curing agent (model 5618; mainly isophorone diamine, IPDA, CAS 2855-13-2) was purchased from Jinan Bajin Chemical Technology Co., Ltd. (Jinan, China). ATO and curing agent were chemically pure reagents, and the remaining components were analytically pure reagents. All reagents were used as received without further purification. Deionized water was homemade in the laboratory. A total of 7101 slides were purchased from Haimen Changlong Instrument Co., Ltd. (Nantong, China).
2.2. Preparation of the Composite Coating
The composite coatings were prepared as illustrated in Figure 1. First, the slides were ultrasonically cleaned using anhydrous ethanol and deionized water, followed by blow-drying for surface hydrophilic treatment and set aside. 0.6 g of gas-phase SiO2 was dispersed in 20 mL of ethyl acetate and stirred at room temperature for 1 h. Then 0.5 mL of ammonia, 0.2 mL of deionized water, and 0.3 mL of PFDTES were added, and stirring was continued at 40 °C for 4 h to obtain dispersion A. The main body of the dispersion A was uniformly dispersed and surface-modified SiO2 nanoparticles. Subsequently, 0.8 g of ATO was dispersed in 20 mL of anhydrous ethanol, ultrasonically treated in a laboratory bath (220 W, 40 kHz, continuous mode) for 30 min, and then stirred for 1 h. Then, 1 mL of KH560 was added and stirred for 2 h to obtain Dispersion B. The purpose of Dispersion B was to homogeneously disperse ATO while modifying its surface as much as possible. Dispersions A and B were mixed together, and 4 g of epoxy resin (epoxy resin E51 mixed with 5618 curing agent at a mass ratio of 1:1) was added and stirred for 2 h at room temperature to obtain the final desired composite coating. The coating mixture was applied onto the pretreated glass substrates using a high-atomization spray gun (0.8 mm nozzle diameter, spray pressure of 25 psi, spraying distance 20 cm). To ensure uniform coverage and reproducibility, the coating was applied in 15 uniform passes (30 strokes) at a constant spraying speed. The samples were then cured at 80 °C for 2 h to produce the target superhydrophobic transparent thermal insulating (SHTTI) coatings.
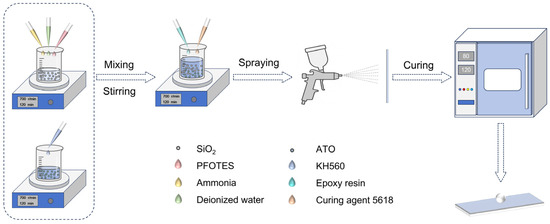
Figure 1.
Schematic illustration of the preparation process of composite coating.
To investigate the influence of the SiO2-to-ATO ratio on the coating performance, a series of formulations was designed as summarized in Table 1. The samples were denoted as SHTTI-x-y, where x and y represent the mass (g) of SiO2 and ATO used, respectively.

Table 1.
The different formulas of main components in the coatings.
2.3. Characterization
The surface morphology of the coatings was investigated using a scanning electron microscope (SEM, Hitachi S-3400N, Kyowa Institute, Tokyo, Japan). The chemical composition of the samples was analyzed using an X-ray energy dispersive spectrometer (EDS, Field Emission Scanning Electron Microscope Accessory, Hitachi High-Tech, Tokyo, Japan) and a Fourier transform infrared spectrometer (FTIR, L1600400 Spectrum TWO DTGS, Perkin Elmer, Waltham, MA, USA). The transmittance of the samples in the visible wavelength band was analyzed using an ultraviolet–visible (UV-vis) spectrophotometer (UV-3150, Shimadzu, Kyoto, Japan). Water contact angle (WCA) and sliding angle (SA) at different locations on the samples were measured using a contact angle meter (Data physics OCA 25, Stuttgart, Germany). For the WCA and SA tests, five different test points were selected on different sample surfaces and the average values were calculated. Self-constructed semiconductor refrigeration platform consists of semiconductor cooling chips, cooling water tank, and temperature controller. The device can provide −15 °C low temperature environment stably for a long time.
3. Results and Discussion
3.1. Surface Morphology and Wettability Analysis
The SHTTI-0.6-0.8 coating achieved a multiscale composite surface topography through the synergistic incorporation of ATO and SiO2 nanoparticles. The surface microstructure was characterized by SEM, as shown in Figure 2a–c at various magnifications. At 100× magnification, uniformly distributed microscale protrusions were clearly visible, significantly enhancing surface roughness. Magnification to 500× revealed that these protrusions were secondary structures formed by particle agglomeration. Further magnification to 1000× showed numerous voids between nanoparticles, forming air-trapping “micro-cavities”. These features collectively support an air-solid interface during droplet contact, minimize the liquid-solid contact area, and thus contribute to the observed high water contact angles and low sliding angles. Elemental distribution analysis by EDS mapping is presented in Figure 2d–i, demonstrating a uniform distribution of carbon (C), oxygen (O), silicon (Si), antimony (Sb), tin (Sn), and fluorine (F) throughout the coating. To further evaluate the surface composition, semi-quantitative EDS analysis was carried out, and the atomic percentages are summarized in Table 2. The measured ratios (Si/Sb and Sb/Sn) were generally consistent with the expected composition of the starting materials, with only minor deviations due to the surface-sensitive nature of EDS and the influence of organic modifiers. In particular, the detection of fluorine (2%) confirmed the successful incorporation of PFDTES at the coating surface. These results provide complementary evidence for the homogeneous dispersion of SiO2, ATO, and the fluorinated modifier within the epoxy matrix. To further confirm the effectiveness of the nanoparticle surface modification, the sample structure was analyzed by FTIR. As shown in Figure 2j, the SHTTI-0.6-0.8 coating exhibits strong Si-O-Si stretching vibration peaks in the 1000–1200 cm−1 interval, indicating that the SiO2 backbone structure remains intact. A new absorption peak near 1140 cm−1, attributed to the -CF2- group, confirmed the presence of PFDTES moieties within the coating. While the -CF2- peak itself only indicates the presence of PFDTES, the simultaneous significant decrease in the -OH stretching vibration absorption at 3400 cm−1 suggests that condensation reactions occurred between surface hydroxyl groups and silane coupling agents. Therefore, the FTIR results provide indirect evidence that PFDTES molecules were chemically bonded to the SiO2 surface rather than merely physically mixed. In addition, the C-H stretching vibration peak at 2900 cm−1 and the characteristic Si-O-C absorption near 1100 cm−1 together verified that KH560 formed covalent bonding with the surface of ATO particles via hydrolysis–condensation reactions.
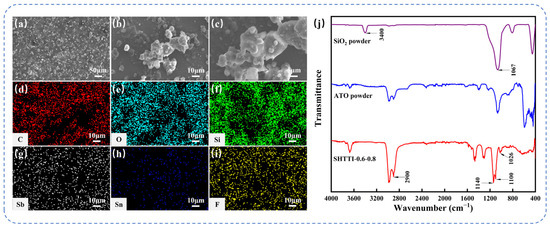
Figure 2.
(a–c) SEM images of SHTTI-0.6-0.8 coating at different magnifications. (d–i) EDS energy spectra of the SHTTI-0.6-0.8 coating. (j) FTIR spectra of fumed SiO2 powders, ATO powders and SHTTI-0.6-0.8 coating.

Table 2.
EDS Elemental analysis of the SHTTI-0.6-0.8 coating surface.
To systematically investigate the functional differences between ATO and SiO2 particles in composite coatings, the WCAs of blank glass, SHTTI-0.6-0, SHTTI-0-0.8, and SHTTI-0.6-0.8 were measured (Figure 3a,b). Compared to blank glass, both SHTTI-0.6-0 and SHTTI-0-0.8 exhibited significantly enhanced contact angles, indicating that both nanoparticles contribute to surface roughness. Notably, SHTTI-0.6-0 achieved a WCA of 151.6°, reaching the superhydrophobic state, while SHTTI-0-0.8 showed a lower WCA of 72.8°. This distinct difference demonstrates that PFDTES-modified SiO2 plays a dominant role in establishing micro–nanostructures and reducing surface energy, whereas ATO alone is unable to form a superhydrophobic interface. Furthermore, the SHTTI-0.6-0.8 coating exhibited a WCA of 162.4°, demonstrating typical superhydrophobic characteristics. Further analysis revealed that when the ATO content was fixed at 0.8 g, increasing the SiO2 content from 0.5 g to 0.6 g significantly enhanced the coating’s superhydrophobicity. However, a further increase to 0.7 g resulted in a negligible improvement in the WCA (Figure 3c). Similarly, with the SiO2 content fixed at 0.6 g, increasing the ATO content from 0.6 g to 1.0 g did not significantly improve superhydrophobicity (Figure 3d).
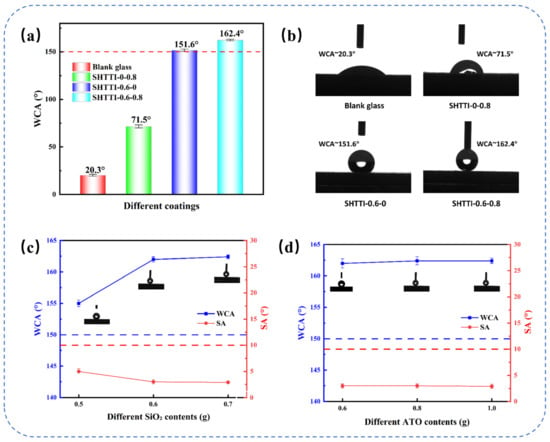
Figure 3.
(a,b) WCAs for various coatings and the glass slide. (c) WCAs and SAs with different SiO2 contents. (d) WCAs and SAs with different ATO contents. Red and blue dotted lines indicate the superhydrophobic thresholds of 150° and 10°, respectively.
The results indicate that PFDTES-modified SiO2 is the primary contributor to superhydrophobicity in the composite coatings, as it effectively constructs micro–nanostructures and lowers surface energy. In contrast, ATO alone fails to produce a superhydrophobic interface. While the synergistic incorporation of both fillers further enhances WCAs, the effect of increasing either SiO2 or ATO beyond optimal levels is limited.
3.2. Thermal Insulation
To evaluate the thermal insulation performance of the coating, a custom test setup was constructed (Figure 4a). The setup comprised an insulated polystyrene foam box (30 × 18 × 15 cm3), externally wrapped with aluminum foil to minimize heat loss. The box top featured a 10 × 10 cm2 opening for sample placement. A near-infrared (NIR) lamp served as the light source, simulating solar infrared radiation to heat the chamber interior by irradiating the samples. Temperature changes within the chamber were recorded every 5 min using a digital thermometer, with the ambient temperature stabilized at 18.3 °C. To rapidly assess initial insulation capability, a 5 min heating test was performed. The results revealed that the blank glass temperature rose to 29.9 °C, whereas the SHTTI-0.6-0.8 coating temperature reached only 24.4 °C. This significant temperature difference (ΔT = 5.5 °C) demonstrates effective infrared blocking and notable thermal insulation properties for the composite coating (Figure 4b).
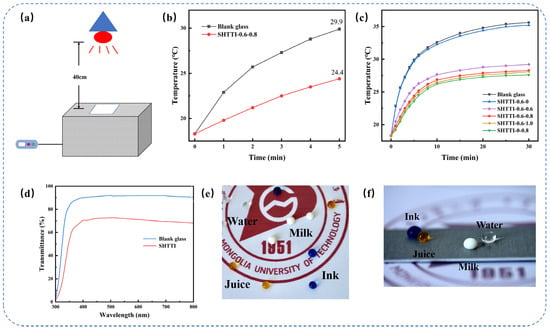
Figure 4.
(a) Schematic photographs of the laboratory’s self-constructed thermal insulation test setup. (b) Blank glass and SHTTI-0.6-0.8 coating 5-Minutes temperature rise curve. (c) Natural warming curves for different coating integrity. (d) Transmittance curves of Blank glass and SHTTI-0.6-0.8 coating. (e) Optical photograph of SHTTI-0.6-0.8 coating. (f) SHTTI-0.6-0.8 coating on the surface of aluminum sheet.
As shown in Figure 4c, a long-duration test was conducted. The results indicated that the temperature of each test sample stabilized and remained essentially unchanged after 30 min, demonstrating significant differences in the thermal insulation performance among the coatings. The blank glass and SHTTI-0.6-0 coatings reached final temperatures of 35.6 °C and 35.3 °C, respectively, indicating negligible insulation effects. In contrast, SHTTI-0-0.8 exhibited the most gradual temperature rise, reaching a final temperature of only 27.6 °C, which confirms its excellent thermal insulation capability. This superior performance is attributed to the more uniform and dense distribution of ATO particles on the surface, resulting in enhanced shielding efficiency against infrared wavelengths.
Comparing ATO composite coatings with varying contents revealed progressively enhanced thermal insulation performance with increasing ATO loading. The coating containing 0.6 g ATO (SHTTI-0.6-0.6) reached a final temperature of 29.2 °C. Further increasing the ATO content to 0.8 g (SHTTI-0.6-0.8) significantly improved insulation, lowering the final temperature to 28.3 °C. However, a further increase to 1.0 g ATO (SHTTI-0.6-1.0) resulted in only a marginal decrease to 28.1 °C, demonstrating negligible improvement over SHTTI-0.6-0.8. Mechanistically, ATO’s thermal insulation primarily stems from free-carrier absorption. As an n-type semiconductor, ATO blocks infrared radiation through two key processes: (1) absorption of infrared light by free carriers generated via antimony doping (dopant-induced free electrons), and (2) localized surface plasmon resonance (LSPR), which selectively reflects near-infrared radiation (780–2500 nm) while maintaining high visible light (380–780 nm) transmittance. Critically, the carrier concentration dictates performance. Insufficient ATO dosage yields low carrier concentration and poor absorption efficiency. Conversely, excessive dosage promotes particle agglomeration, decreasing carrier mobility and limiting further absorption efficiency gains. Importantly, high ATO loadings also significantly reduce visible light transmittance [35]. Therefore, optimizing carrier concentration is essential to balance thermal insulation and optical transparency. Under prolonged irradiation, the SHTTI-0.6-0.8 coating significantly suppressed the temperature rise within the chamber. Compared to the blank glass, the coating achieved a 7.3 °C reduction in chamber temperature after 30 min of irradiation. The heating process also exhibited greater stability. These results further demonstrate the coating’s superior overall performance.
Transmittance curves of SHTTI-0.6-0.8 coating and blank glass in the visible band are shown in Figure 4d. The SHTTI-0.6-0.8 coating achieved a transmittance of approximately 72% at 520 nm, demonstrating favorable optical performance. Furthermore, Figure 4e shows various liquids (water, milk, juice, ink) maintaining near-spherical shapes on the coating surface, confirming excellent repellency towards diverse solutions. The underlying pattern remains clearly visible, indicating good transparency. Finally, Figure 4f demonstrates the coating’s successful application to other flat substrates, such as aluminum. Although the coating thickness was not directly measured in this study, the reproducible optical transmittance (72% at 520 nm) and stable superhydrophobicity (WCA > 160°) obtained across multiple samples confirm the reliability of the preparation process. This indicates that the coating thickness is within an effective and stable range.
In summary, the ATO content governs the thermal insulation performance of the coating, while SiO2 primarily modulates surface wettability. Therefore, optimizing the ATO/SiO2 ratio is essential to synergistically enhance hydrophobicity, thermal insulation, and transparency. Among the tested formulations, SHTTI-0.6-0.8 coating represents the optimal configuration, delivering balanced multifunctional performance.
3.3. Mechanical Durability and Chemical Stability of Coatings
Mechanical durability is critical to the long-term performance of coatings in complex natural environments, so coatings need to be evaluated for abrasion and impact resistance. For sand impact testing (Figure 5a), the SHTTI-0.6-0.8 coating was positioned at 45° inclination. Sand was released from 40 cm height, impacting the surface under free-fall conditions. Wettability was measured after successive 20 g impact increments. Successive impacts caused partial destruction of the topmost nanoscale asperities; however, the EP matrix absorbed impact energy and preserved the underlying micro–nano hierarchical structures. As a result, the coating maintained superhydrophobicity after 30 impacts (WCA = 150.2°, SA = 8.5°). These results demonstrate exceptional mechanical stability against particulate impact. Complementary water impact tests (Figure 5b) demonstrated sustained superhydrophobicity (WCA > 150°, SA < 10°) after 10 min continuous exposure. This robust performance originates from air-cushion structures that dissipate impact energy, where the entrapped air pockets cushion the droplet and minimize direct liquid-solid contact. Sandpaper abrasion testing (Figure 5c) was performed by pressing the coating face-down against 1000-grit sandpaper under a 100 g load, followed by horizontal movement over 20 cm per cycle. Progressive wear gradually reduced superhydrophobicity, as nanoscale asperities were first removed while the underlying microscale roughness and the strong adhesion of the epoxy matrix maintained functionality. Even after 40 abrasion cycles, the SHTTI-0.6-0.8 coating preserved a WCA of 148.5° and SA of 10.25°, demonstrating robust abrasion resistance.
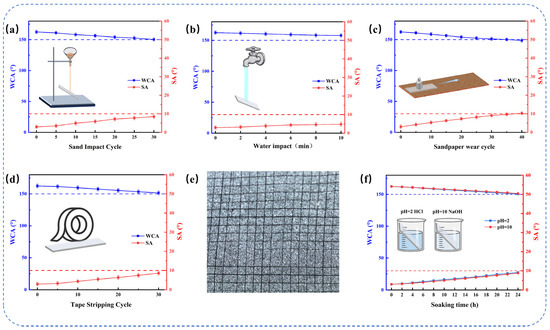
Figure 5.
Schematic diagrams of various tests and wettability change curves of SHTTI-0.6-0.8 coating: (a) Sand impact test. (b) Water impact test. (c) Sandpaper wear test. (d) Tape stripping test. (e) Cross-cut adhesion test results of the SHTTI coating. (f) Acid and alkali solution immersion test. Red and blue dotted lines indicate the superhydrophobic thresholds of 150° and 10°, respectively.
Additionally, adhesion performance was examined by both tape peel and cross-cut adhesion tests. The 3M tape peel test (Figure 5d) confirmed that the coating maintained superhydrophobicity after 30 peel cycles, indicating strong interfacial bonding under repetitive delamination stress. To complement this, a standardized cross-cut adhesion test was performed following ASTM D3359 [38]. The SHTTI-0.6-0.8 coating achieved an adhesion grade of 4B, with slight detachment observed along the lattice cuts and a total flaking area of less than 5% (Figure 5e). This result provides quantitative evidence of robust adhesion between the coating and the glass substrate. The mechanical strength of the above SHTTI-0.6-0.8 coatings is mainly attributed to the synergistic effect of the rough micro- and nano-structures, the adhesion strength of the epoxy resin, and the low surface energy due to the fluorinated components on the surface.
Chemical stability is essential for coatings operating in harsh environments. The SHTTI-0.6-0.8 coating was immersed in HCl solution (pH = 2) and NaOH solution (pH = 10), with wettability monitored (Figure 5f). Even after 24 h of immersion, the coating retained superhydrophobicity, demonstrating strong resistance to chemical weathering. This resilience originates from its hierarchical structure, which traps air to form a protective barrier that effectively isolates corrosive species such as H2O, H+, OH−, Na+, and Cl− [39]. In addition, the inherent stability of the epoxy matrix contributes to preserving the surface architecture under prolonged chemical exposure.
3.4. Self-Cleaning Ability
Superhydrophobic surfaces have attracted much attention due to their excellent self-cleaning property, which is manifested by the fact that contaminants adhering to the surface can be effectively removed by a small amount of water flow. To verify this property, quartz sand was chosen as a simulated contaminant for self-cleaning experiments in this study (Figure 6a,b). It was observed that the droplets rolled down in a spherical shape on the surface of the SHTTI-0.6-0.8 coating placed at an angle. During the rolling down process, the droplets adsorbed the quartz sand particles on the path and realized the effective cleaning of the path. In contrast, the droplets are difficult to move on a normal glass surface and therefore cannot remove contaminants. Figure 6c illustrates the self-cleaning principle. When a droplet touches the surface of a normal slide, it is unable to maintain its spherical shape and spreads out, thus failing to effectively hold and carry away contaminants. However, due to the special wettability of the superhydrophobic surface, the droplet is able to maintain its spherical shape without spreading, and the contaminant can be encapsulated inside the droplet. As the droplet rolls away from the surface, the contaminant is removed and the surface is self-cleaning.
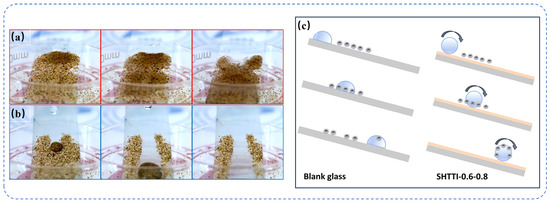
Figure 6.
Schematic diagram of self-cleaning effect of (a) SHTTT-0.6-0.8 coating, (b) blank glass. (c) Self-cleaning principle schematic.
3.5. Anti-Icing Performance
The anti-icing properties of SHTTI-0.6-0.8 coatings were characterized using a self-constructed semiconductor refrigeration platform (Figure 7a) to simulate a low-temperature environment. The test samples were placed on the surface of the refrigeration platform and the working surface temperature was cooled down to −15 °C for the experiment. A 10 μL water droplets were placed on the surface of different sample coatings and their freezing times were recorded. The droplets on the blank glass surface froze rapidly within 87 s (Figure 7b). In contrast, the initial morphology of the water droplets on the SHTTI-0.6-0.8-coated surface was nearly hemispherical (Figure 7c), and its freezing process was significantly delayed. At 163 s, only a thin layer of ice was formed on the surface of the droplet, and the interior began to freeze slowly; at 419 s, the droplet began to form ice crystal nuclei from the bottom to the top and from the outside to the inside; at 500 s, the ice crystal nuclei were basically formed completely; and finally, the droplet was completely frozen at 562 s, and the top of the droplet froze to form a milky protuberance, presenting the appearance of a homogeneous, structurally stable ice sphere morphology. The mechanism of this phenomenon is that the smooth surface of the slide makes the contact area between the water droplet and the coating larger, which is conducive to the rapid transfer of cold conducted by the refrigeration plate to the water droplet and accelerates its freezing. The SHTTI-0.6-0.8 coating, however, has a rough micro- and nanostructured surface, which can trap a large amount of air to form air pockets, effectively reducing the actual contact area between the water droplet and the solid surface, blocking the efficient transfer path of the cold energy, and thus prolonging the freezing time from 87 s to 562 s. The stability of the coating’s anti-freezing performance is crucial in practical applications. For this reason, we conducted several freeze–thaw cycle tests on the coating and measured the freezing time of the surface water droplets after the cycles. As shown in Figure 7d, after 30 freeze–thaw cycles, the icing time of water droplets on the surface of the coating shows only a slight decrease. This demonstrates the excellent stability of the SHTTI-0.6-0.8 coating against ice.
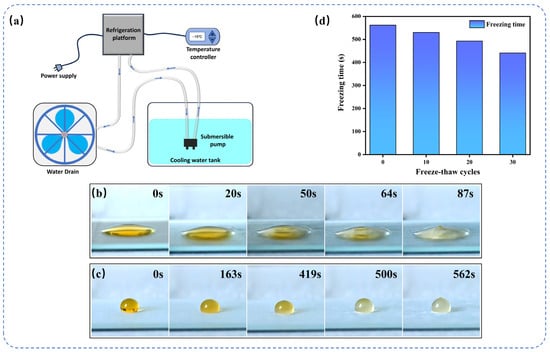
Figure 7.
(a) Schematic diagram of the custom-built semiconductor refrigeration platform. Freezing process of the water droplet on (b) blank glass, (c) SHTTI-0.6-0.8 coating. (d) Freeze–thaw cycle test for anti-icing.
4. Conclusions
A multifunctional superhydrophobic transparent thermal-insulating (SHTTI) coating was fabricated by synergistically integrating ATO and SiO2 nanoparticles into an epoxy matrix via spray coating. The optimized composition (0.6 g SiO2:0.8 g ATO) enabled the formation of a hierarchical micro–nano structure, yielding a water contact angle of 162.4°, sliding angle of 3°, and visible-light transmittance of 72% at 520 nm. Significant thermal insulation was achieved, with a 7.3 °C reduction in chamber temperature under simulated solar irradiation compared with blank glass. The coating further exhibited robust durability, maintaining WCA = 148.5° after 40 abrasion cycles and strong adhesion after 30 tape-peel tests, along with particulate impact resistance. Chemical stability under acidic (pH = 2) and alkaline (pH = 10) environments, outstanding anti-icing capability (freezing delay extended to 562 s versus 87 s for glass), and reliable self-cleaning against particulate contamination confirmed its environmental tolerance. This study demonstrates a rational design strategy for constructing multifunctional coatings by nanomaterial hybridization, providing potential applications in energy-saving architectural glazing and optical devices. Future work will focus on quantitatively analyzing the effect of coating thickness, extending the strategy to flexible substrates, and evaluating long-term outdoor durability, thereby advancing the practical implementation of transparent thermal-insulating coatings.
Author Contributions
Conceptualization, G.Q.; methodology, G.Q. and Q.A.; writing—original draft preparation, G.Q.; formal analysis, G.Q., L.L. and Q.A.; writing—review and editing, G.Q. and Q.A.; supervision: Q.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Basic Scientific Research in Universities of Inner Mongolia (No. RZ2200002188), the Center for Applied Mathematics of Inner Mongolia (No. ZZYJZD2022002), and the University Talent Introduction Project/PhD Start-up Grant (No. DC2100000905).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest. We certify that we have participated sufficiently in the work to take public responsibility for the appropriateness of the experimental design and the collection, analysis, and interpretation of the data. We have reviewed the final version of the manuscript and approve it for publication.
References
- Bai, Y.; Zhang, H.; Shao, Y.; Sun, J.; Liu, S. Recent progresses of superhydrophobic coatings in different application fields: An overview. Coatings 2021, 11, 116. [Google Scholar] [CrossRef]
- Ebert, D.; Bhushan, B. Transparent, superhydrophobic, and wear-resistant coatings on glass and polymer substrates using SiO2, ZnO, and ITO nanoparticles. Langmuir 2012, 28, 11391–11399. [Google Scholar] [CrossRef]
- Parent, O.; Ilinca, A. Anti-icing and de-icing techniques for wind turbines: Critical review. Cold Reg. Sci. Technol. 2011, 65, 88–96. [Google Scholar] [CrossRef]
- Yong, H.; Li, Z.; Huang, X.; Zhang, L.; Wang, J. Superhydrophobic materials: Versatility and translational applications. Adv. Mater. Interfaces 2022, 9, 2200435. [Google Scholar] [CrossRef]
- Tong, W.; Wu, Z.; Xiong, D.X. Transparent superhydrophobic coating based on a carbonaceous soot framework for the self-cleaning of glass. Sustain. Mater. Technol. 2023, 37, e00693. [Google Scholar] [CrossRef]
- Borrebaek, P.O.A.; Jelle, B.P.; Zhang, Z. Avoiding snow and ice accretion on building integrated photovoltaics–challenges, strategies, and opportunities. Sol. Energy Mater. Sol. Cells 2020, 206, 110306. [Google Scholar] [CrossRef]
- Nomeir, B.; Lakhouil, S.; Boukheir, S.; Ait Ali, M.; Naamane, S. Recent progress on transparent and self-cleaning surfaces by superhydrophobic coatings deposition to optimize the cleaning process of solar panels. Sol. Energy Mater. Sol. Cells 2023, 257, 112347. [Google Scholar] [CrossRef]
- Gao, Y.; Gereige, I.; El Labban, A.; Cha, D.; Isimjan, T.T.; Beaujuge, P.M. Highly transparent and UV-resistant superhydrophobic SiO2-coated ZnO nanorod arrays. ACS Appl. Mater. Interfaces 2014, 6, 2219–2223. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Tian, N.; Wang, W.; Zhang, X.; Chen, Y. Highly transparent superhydrophobic coatings for prevention of raindrop adhesion on rearview mirrors. Langmuir 2025, 41, 2916–2923. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Yan, S.; Ming, T.; Zhao, X.; Zhang, N. Analysis and modeling of dust accumulation-composed spherical and cubic particles on PV module relative transmittance. Sustain. Energy Technol. Assess. 2021, 44, 101015. [Google Scholar] [CrossRef]
- Niu, H.; Yao, X.; Luo, S.; Zhang, Q.; Liu, Y. Composite superhydrophobic coating with transparency and thermal insulation for glass curtain walls. ACS Appl. Mater. Interfaces 2024, 16, 48374–48385. [Google Scholar] [CrossRef]
- Lu, X.; Yu, G.; Tan, Q.; Hu, B.; Zhang, J.; Dong, Q. Preparation and characterization of transparent fluorocarbon emulsion doped with antimony tin oxide and TiO2 as thermal-insulating and self-cleaning coating. J. Coat. Technol. Res. 2014, 11, 567–574. [Google Scholar] [CrossRef]
- Nomeir, B.; Lakhouil, S.; Boukheir, S.; Abdallah, N.; Naamane, S.; Ait Ali, M. Synthesis of a novel high-performance siloxene-based 2D material for durable and transparent superhydrophobic coatings with self-cleaning and anti-icing properties. Ind. Eng. Chem. Res. 2025, 64, 6460–6474. [Google Scholar] [CrossRef]
- Seo, T.W.; Jeon, Y.; Park, C.; Kim, J. Survey on glass and façade-cleaning robots: Climbing mechanisms, cleaning methods, and applications. Int. J. Precis. Eng. Manuf.-Green Technol. 2019, 6, 367–376. [Google Scholar] [CrossRef]
- Dalawai, S.P.; Aly, M.A.S.; Latthe, S.S.; Xing, R.; Sutar, R.S.; Nagappan, S.; Ha, C.-S.; Sadasivuni, K.K.; Liu, S. Recent advances in durability of superhydrophobic self-cleaning technology: A critical review. Prog. Org. Coat. 2020, 138, 105381. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, Y.; Du, F.; Zhang, S. Rational design of self-cleaning superhydrophobic coating with outstanding abrasion resistance and weatherability: Towards highly efficient oil–water separation and anti-corrosion application. Prog. Org. Coat. 2023, 179, 107439. [Google Scholar] [CrossRef]
- Zhu, Y.; Li, H.; Huang, W.; Lai, X.; Zeng, X. Facile fabrication of superhydrophobic wood aerogel by vapor deposition method for oil–water separation. Surf. Interfaces 2023, 37, 102746. [Google Scholar] [CrossRef]
- Liu, Y.; Han, X.; Chen, C.; Huang, C.; Long, L.; He, Y.; Yang, G.; Shen, F.; Zhang, X.; Zhang, Y. A fluorine-free and nanoparticle-free superhydrophobic coating: A mechanism and self-cleaning application investigation. Appl. Surf. Sci. 2023, 608, 155103. [Google Scholar] [CrossRef]
- Mendoza, A.I.; Larroche, P.; Nilsson, F.; Hedenqvist, M.; Strömberg, E.; Hillborg, H.; Morian, R. Image analysis of PDMS/ZnO nanocomposite surfaces for optimized superhydrophobic and self-cleaning surface design. Surf. Interfaces 2023, 37, 102733. [Google Scholar] [CrossRef]
- Li, X.M.; Reinhoudt, D.; Crego-Calama, M. What do we need for a superhydrophobic surface? A review on the recent progress in the preparation of superhydrophobic surfaces. Chem. Soc. Rev. 2007, 36, 1350–1368. [Google Scholar]
- Ma, M.; Hill, R.M. Superhydrophobic surfaces. Curr. Opin. Colloid Interface Sci. 2006, 11, 193–202. [Google Scholar] [CrossRef]
- Ke, C.; Fang, Y.; Zhou, Z.; Wang, G.; Liu, Y.; Wu, W.; Xiao, L.; Zhang, M.; Hu, H.; Liu, J. Superhydrophobic composite coating with excellent mechanical durability. Coatings 2022, 12, 185. [Google Scholar] [CrossRef]
- Chang, G.; Zhou, B.; Li, C.; Wu, S.; Yang, T.; Tang, J.; Liu, L.; Wang, J.; Yang, Y. Highly transparent and superhydrophobic SiO2 coating with nanoscale structures by one-step spraying technology for applications as self-cleaning coatings. ACS Appl. Nano Mater. 2024, 7, 24592–24602. [Google Scholar] [CrossRef]
- Sun, J.; Chen, Y.; Li, J.; Su, J.; Huang, S.; Zhang, Y.; Su, F. Synthesis of transparent and flexible superhydrophobic PDMS-EP/SiO2 coating with excellent mechanical and chemical durability. Appl. Surf. Sci. 2025, 707, 163586. [Google Scholar] [CrossRef]
- Li, H.; Tu, S.; Tu, H.; Chen, M.; Zhou, S.; Wu, L. Construction of transparent, robust and haze-selectable superhydrophobic coatings with honeycomb structure. Chem. Eng. J. 2024, 483, 149319. [Google Scholar] [CrossRef]
- Shih, G.H.; Allen, C.G.; Potter, B.G., Jr. RF-sputtered Ge–ITO nanocomposite thin films for photovoltaic applications. Sol. Energy Mater. Sol. Cells 2010, 94, 797–802. [Google Scholar] [CrossRef]
- Sun, H.; Liu, X.; Liu, B.; Yin, Z. Preparation and properties of antimony doped tin oxide nanopowders and their conductivity. Mater. Res. Bull. 2016, 83, 354–359. [Google Scholar] [CrossRef]
- Zhang, D.; Tang, Y.; Jiang, F.; Han, Z.; Chen, J. Electrodeposition of silver nanoparticle arrays on transparent conductive oxides. Appl. Surf. Sci. 2016, 369, 178–182. [Google Scholar] [CrossRef]
- Qi, S.; Xiao, X.; Lu, Y.; Cong, S.; Huan, C.; Liu, H.; Xu, G.; Iqbal, J. Preparation and energy consumption evaluation of bifunctional energy-efficient glass with superior superhydrophobic and heat shielding properties. Energy Build. 2020, 215, 109913. [Google Scholar] [CrossRef]
- Gao, W.; Ma, F.; Yin, Y.; Li, J. Robust and durable transparent superhydrophobic F-TNTs/TiN coating fabricated by structure tuning on surface of TiN hard coating. Appl. Surf. Sci. 2023, 613, 155967. [Google Scholar] [CrossRef]
- Li, Q.; Liang, H.; Song, J.; Guo, C.; Tang, J. Preparation of transparent sandwich-like superhydrophobic coating on glass with high stability and self-cleaning properties. Coatings 2022, 12, 228. [Google Scholar] [CrossRef]
- Wang, Y.; Yan, Z.; Zhang, M.; Zhang, Z.; Li, T.; Chen, M.; Dong, W. Flexible core–shell CsₓWO3-based films with high UV/NIR filtration efficiency and stability. Nanoscale Adv. 2021, 3, 3177–3183. [Google Scholar] [CrossRef]
- Tan, Y.; Lyu, J.; Zhang, D.; Duan, X. RbxCsyWO3 based superhydrophobic transparent thermal insulation film for energy saving. Colloids Surf. A Physicochem. Eng. Asp. 2024, 692, 133994. [Google Scholar] [CrossRef]
- Ke, C.; Zhang, C.; Pan, L.; Jiang, Y. Transparent superhydrophobic and thermal insulating dual-functional coatings fabricated by a rapid thermal process. Int. J. Appl. Ceram. Technol. 2025, 22, e15121. [Google Scholar] [CrossRef]
- Han, T.; Yan, Y.; Wang, Y.; Yang, P.; Li, X.; Zhang, R.; Zu, C. Enhanced and tunable visible-light and near-infrared transmittance of VO2/ATO composite coatings for smart windows. J. Wuhan Univ. Technol. Mater. Sci. Ed. 2025, 40, 627–634. [Google Scholar] [CrossRef]
- Yu, L.; Ma, K.; Yin, H.; Zhou, C.; He, W.; Yu, G.; Zhang, Q.; Liu, Q.; Zhao, Y. Superhydrophobic coating based on nano-silica modification for antifog application of partition glass. Coatings 2024, 14, 1375. [Google Scholar] [CrossRef]
- Jing, C.; Wang, T.; Zhang, J.; Ma, J.; Chen, J. Superhydrophobic, superoleophobic, robust and sprayable radiative cooling nanocomposites via multifluorination and multilevel interfacial regulation strategy. Adv. Funct. Mater. 2025, 35, 2422260. [Google Scholar] [CrossRef]
- Aldwais, S.; Al-Muntaser, A.A.; Chen, C.; Robles, J.; Pal, A.; Abiade, J.T. Enhancing the Adhesion of Polyaniline on Steel Substrates without a Binding Agent: Evaluated by ASTM D3359 Tape Test and Sodium Chloride (NaCl) Exposure. Polymers 2025, 17, 1082. [Google Scholar] [CrossRef] [PubMed]
- Fu, P.; Ou, J.; He, Y.; Hu, Y.; Wang, F.; Fang, X.; Li, W.; Amirfazli, A. Robust superhydrophobic coating with carbon nanotubes and silica nanoparticles in the matrix of fluorinated polyurethane. Surf. Interfaces 2024, 45, 103890. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).