SEM/EDS and Roughness Analysis on Current Titanium Implant Decontamination Systems: In Vitro Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Size and Preparation of the Study Samples
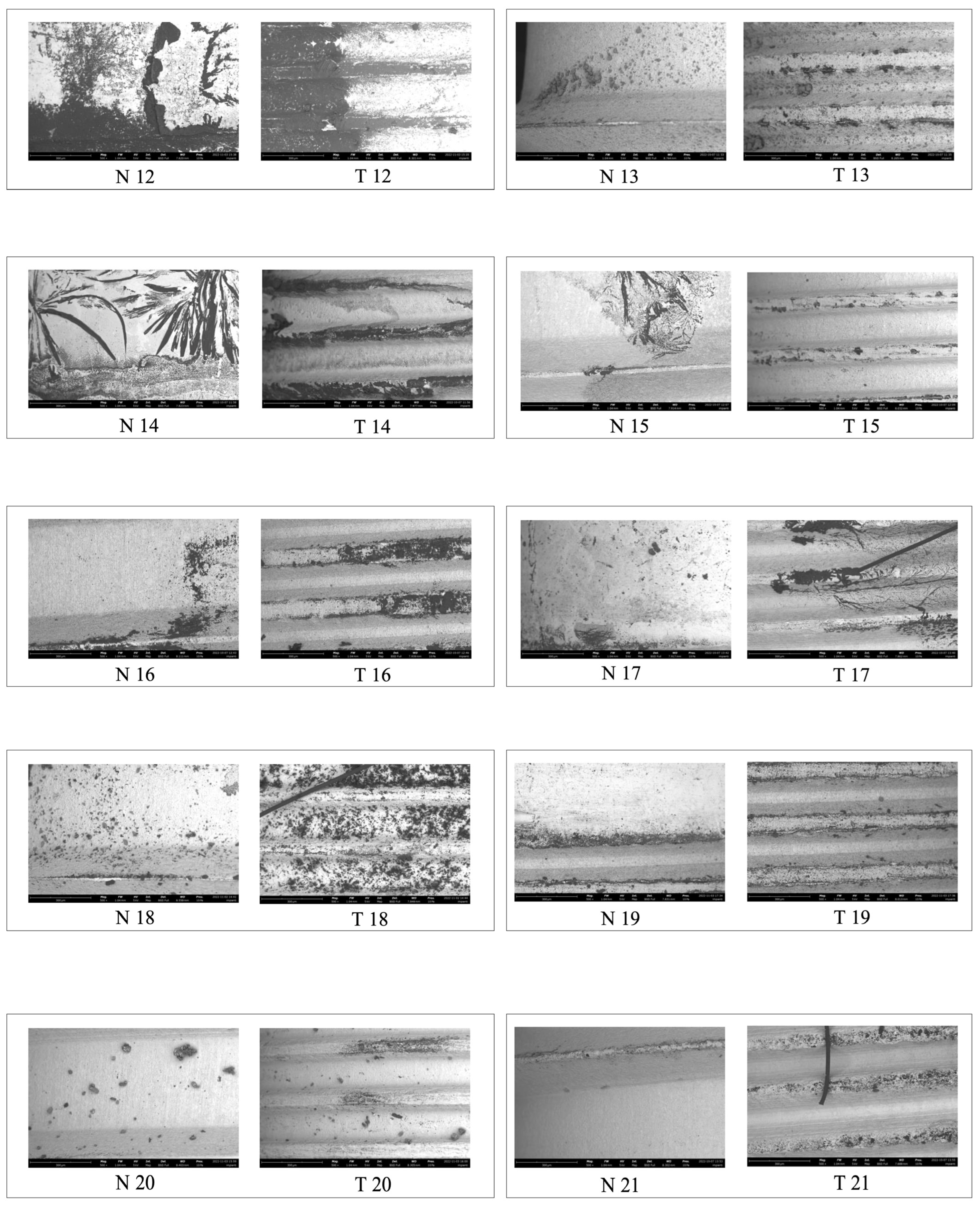
2.2. Microscopic Analysis
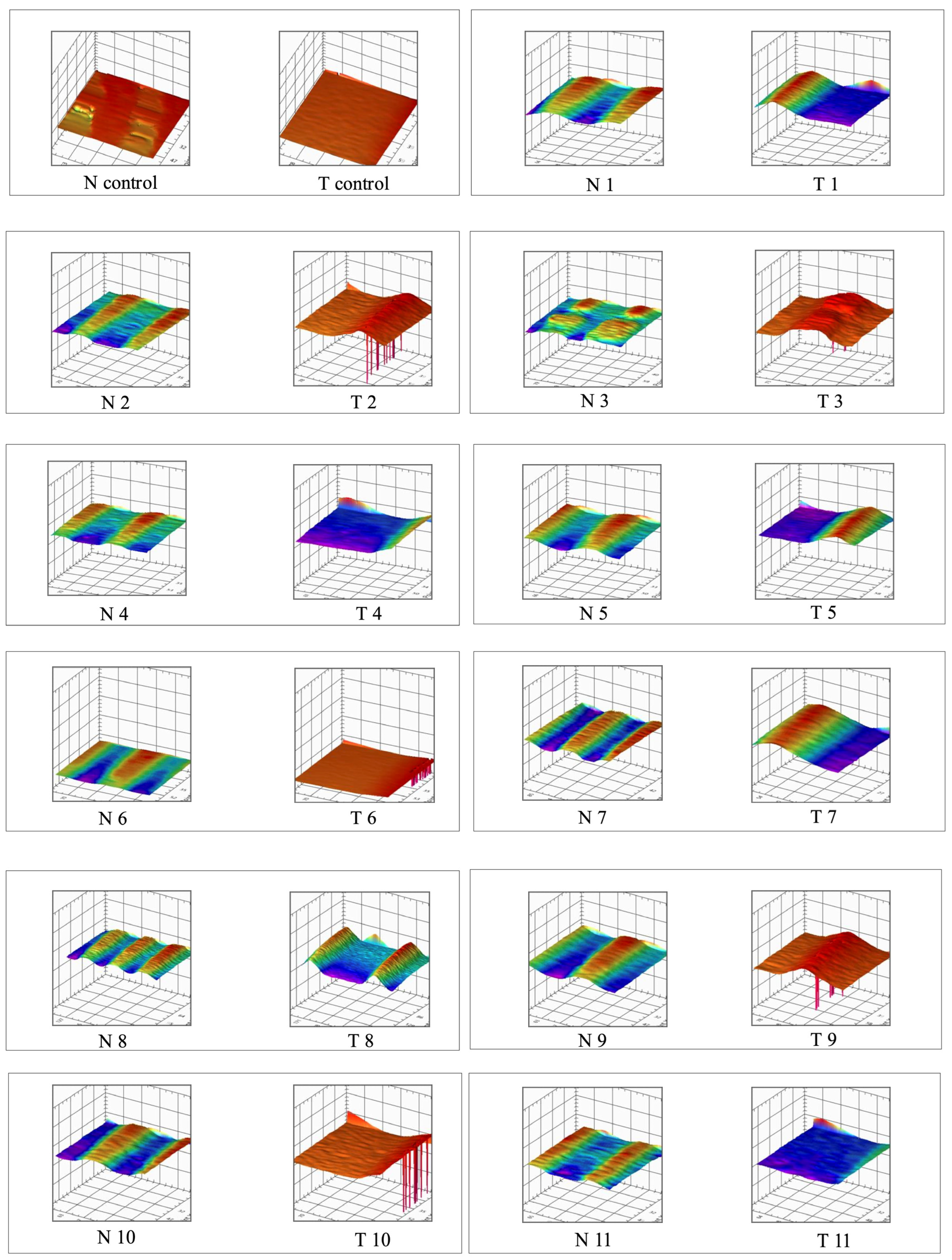
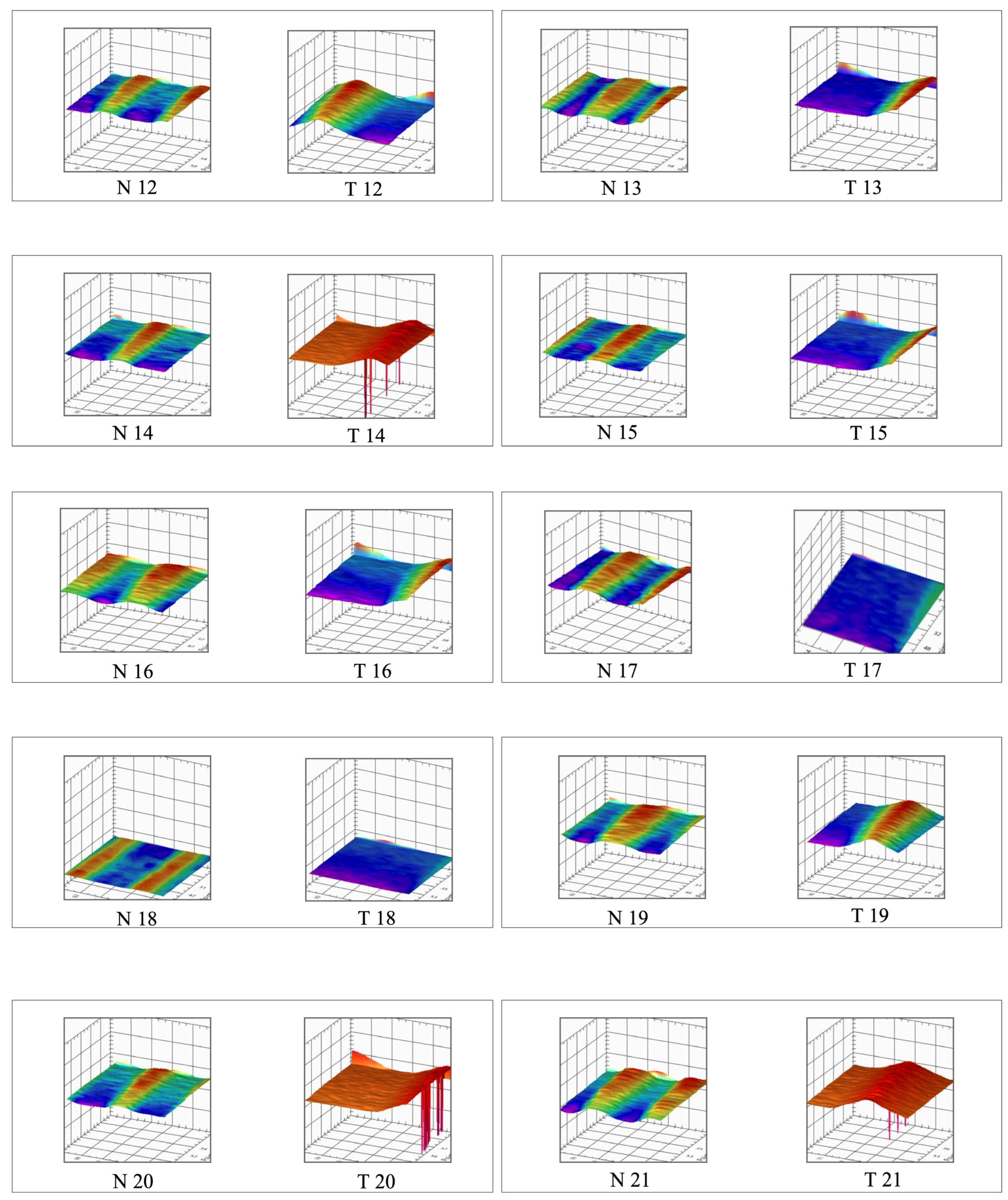
2.3. Roughness (Ra)
2.4. Statistical Analysis
3. Results
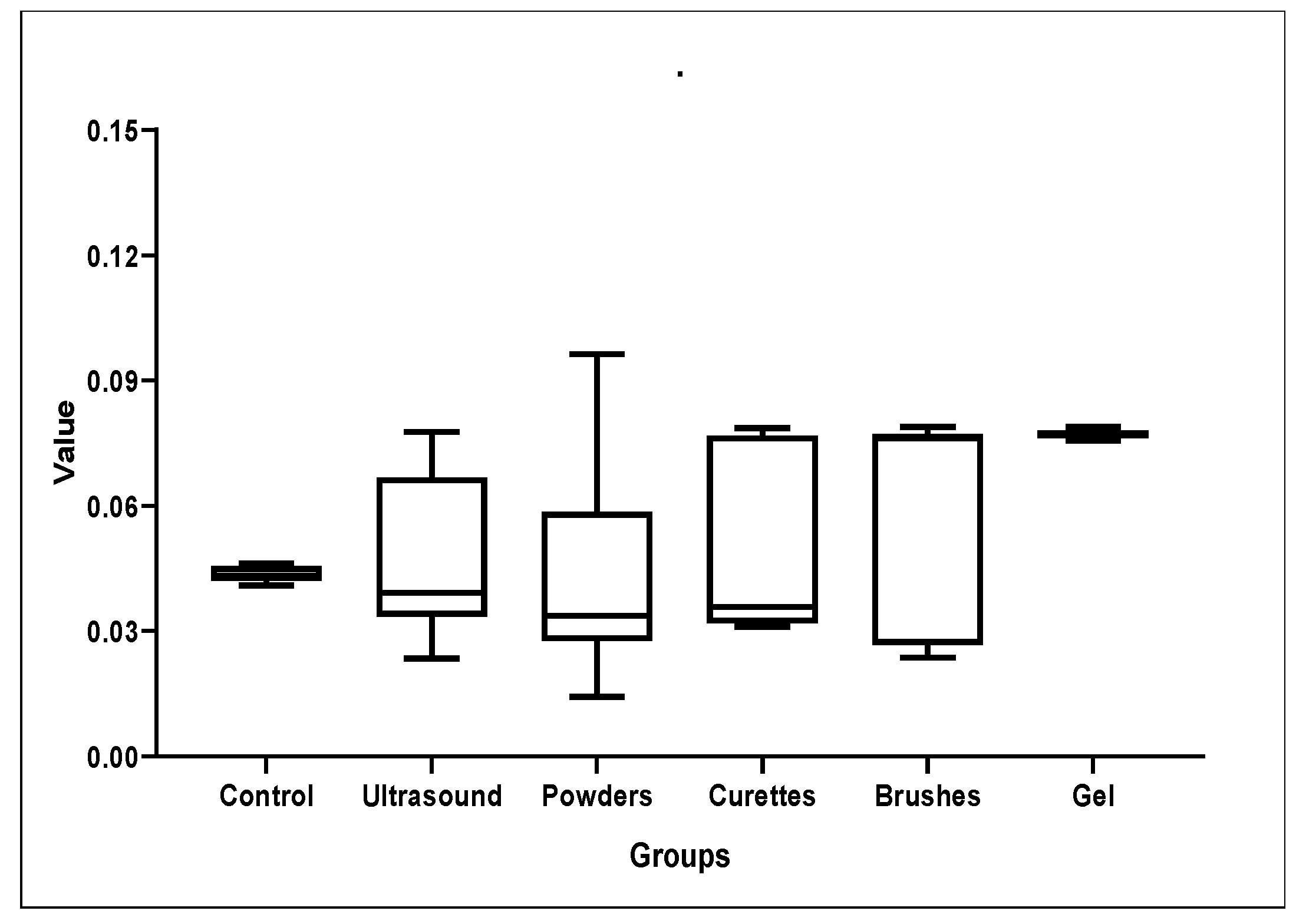
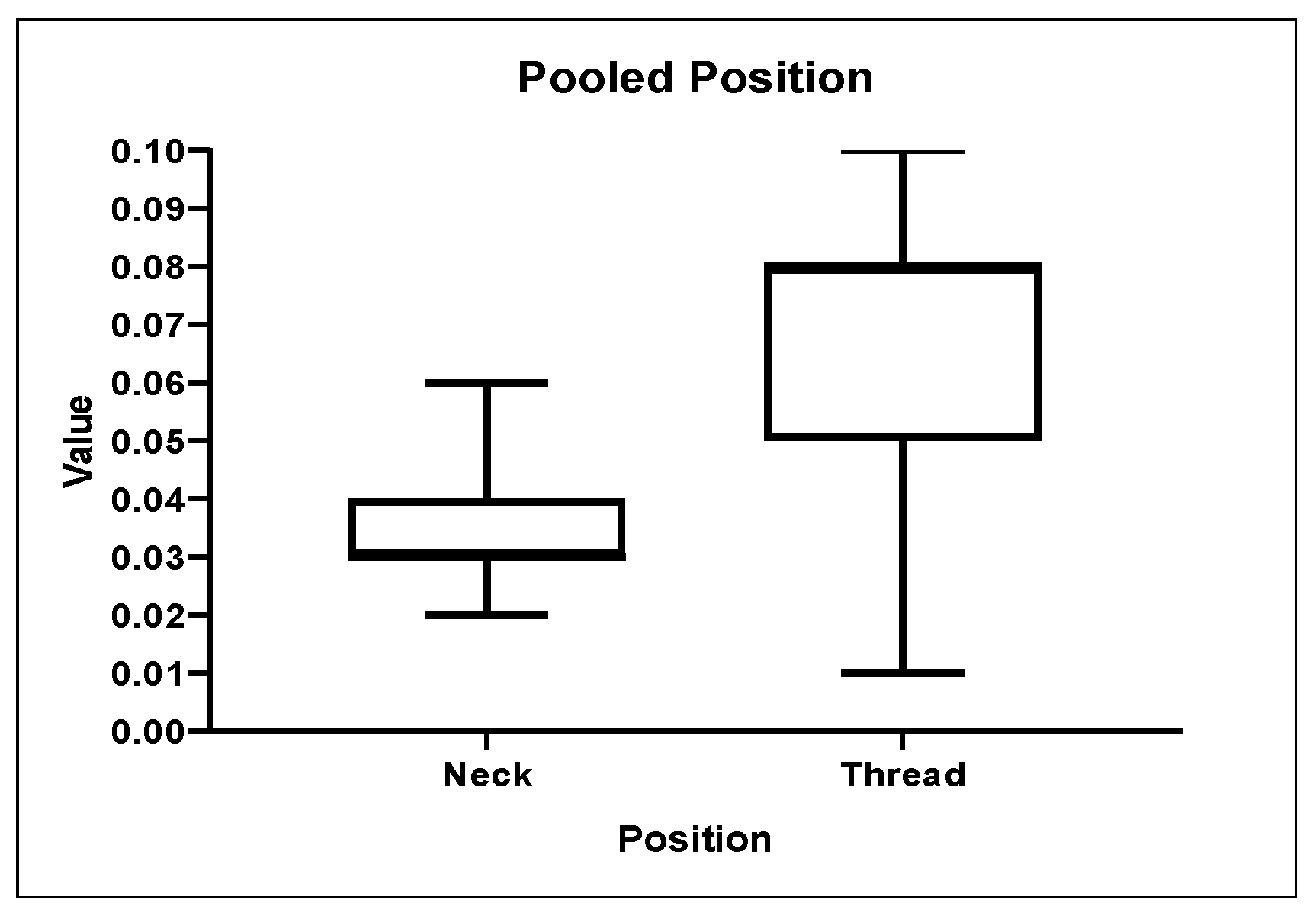
3.1. Roughness Analysis
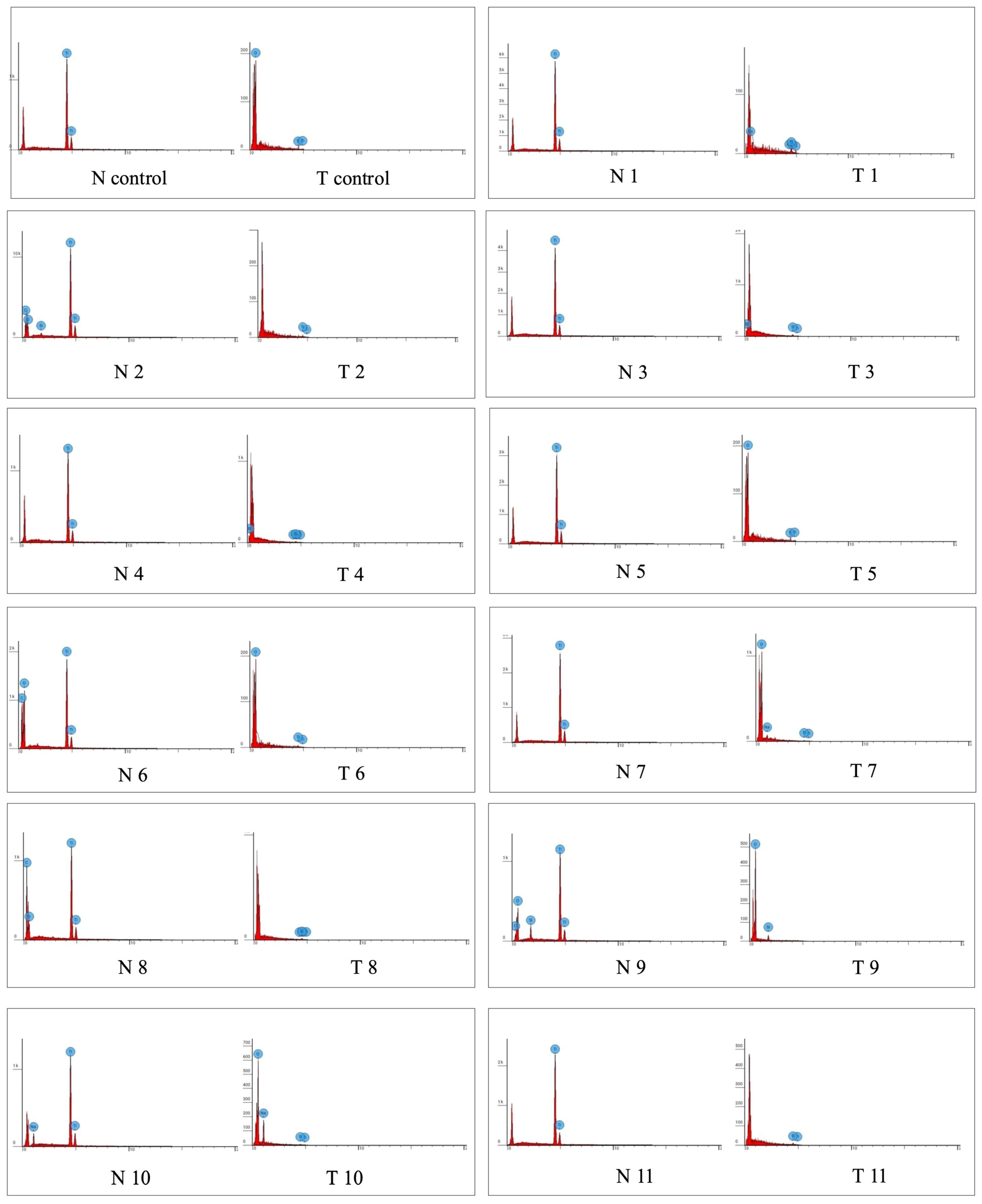
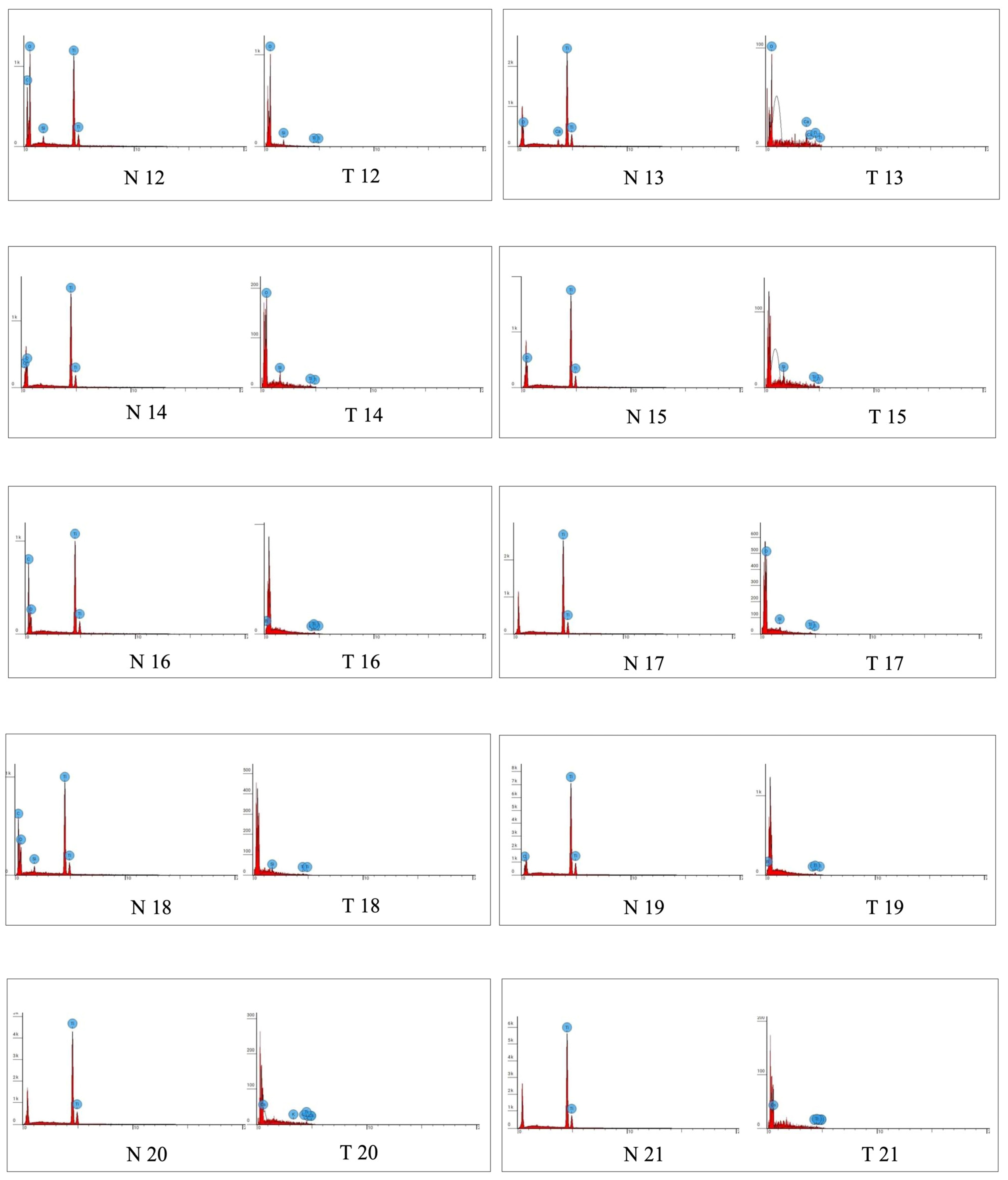
3.2. EDS Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- French, D.; Ofec, R.; Levin, L. Long term clinical performance of 10 871 dental implants with up to 22 years of follow-up: A cohort study in 4247 patients. Clin. Implant. Dent. Relat. Res. 2021, 23, 289–297. [Google Scholar] [CrossRef]
- Ghodrati, H.; Goodarzi, A.; Golrokhian, M.; Fattahi, F.; Anzabi, R.M.; Mohammadikhah, M.; Sadeghi, S.; Mirhadi, S. A narrative review of recent developments in osseointegration and anti-corrosion of titanium dental implants with nano surface. Bone Rep. 2025, 25, 101846. [Google Scholar] [CrossRef]
- Elani, H.W.; Starr, J.R.; Da Silva, J.D.; Gallucci, G.O. Trends in Dental Implant Use in the U.S., 1999–2016, and Projections to 2026. J. Dent. Res. 2018, 97, 1424–1430. [Google Scholar] [CrossRef]
- Berglundh, T.; Armitage, G.; Araujo, M.G.; Avila-Ortiz, G.; Blanco, J.; Camargo, P.M.; Chen, S.; Cochran, D.; Derks, J.; Figuero, E.; et al. Peri-Implant Diseases and Conditions: Consensus Report of Workgroup 4 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J. Clin. Periodontol. 2018, 45 (Suppl. S20), S286–S291. [Google Scholar] [CrossRef]
- Heitz-Mayfield, L.J.A.; Salvi, G.E. Peri-Implant Mucositis. J. Clin. Periodontol. 2018, 45 (Suppl. S20), S237–S245. [Google Scholar] [CrossRef]
- Wada, M.; Mameno, T.; Otsuki, M.; Kani, M.; Tsujioka, Y.; Ikebe, K. Prevalence and Risk Indicators for Peri-Implant Diseases: A Literature Review. Jpn. Dent. Sci. Rev. 2021, 57, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Qin, S.; Lu, B.; Wang, W.; Ma, G. Comparison of the Efficacy of Seven Non-Surgical Methods Combined with Mechanical Debridement in Peri-Implantitis and Peri-Implant Mucositis: A Network Meta-Analysis. PLoS ONE 2024, 19, e0305342. [Google Scholar] [CrossRef] [PubMed]
- Verket, A.; Koldsland, O.C.; Bunaes, D.; Lie, S.A.; Romandini, M. Non-Surgical Therapy of Peri-Implant Mucositis-Mechanical/Physical Approaches: A Systematic Review. J. Clin. Periodontol. 2023, 50 (Suppl. S26), 135–145. [Google Scholar] [CrossRef]
- Scribante, A.; Gallo, S.; Pascadopoli, M.; Soleo, R.; Di Fonso, F.; Politi, L.; Venugopal, A.; Marya, A.; Butera, A. Management of Periodontal Disease with Adjunctive Therapy with Ozone and Photobiomodulation (PBM): A Randomized Clinical Trial. Photonics 2022, 9, 138. [Google Scholar] [CrossRef]
- Butera, A.; Pascadopoli, M.; Gallo, S.; Pérez-Albacete Martínez, C.; Maté Sánchez de Val, J.E.; Parisi, L.; Gariboldi, A.; Scribante, A. Ozonized Hydrogels vs. 1% Chlorhexidine Gel for the Clinical and Domiciliary Management of Peri-Implant Mucositis: A Randomized Clinical Trial. J. Clin. Med. 2023, 12, 1464. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.; Jo, Y.H.; Luke Yeo, I.S.; Yoon, H.I.; Lee, J.H.; Han, J.S. The effect of surface material, roughness and wettability on the adhesion and proliferation of Streptococcus gordonii, Fusobacterium nucleatum and Porphyromonas gingivalis. J. Dent. Sci. 2023, 18, 517–525. [Google Scholar] [CrossRef] [PubMed]
- Bravo, E.; Serrano, B.; Ribeiro-Vidal, H.; Virto, L.; Sánchez, I.S.; Herrera, D.; Sanz, M. Biofilm formation on dental implants with a hybrid surface microtopography: An in vitro study in a validated multispecies dynamic biofilm model. Clin. Oral Implant. Res. 2023, 34, 475–485. [Google Scholar] [CrossRef]
- Vierling, L.; Liu, C.C.; Wiedemeier, D.; Gubler, A.; Schmidlin, P.R. Assessing the Impact of Various Decontamination Instruments on Titanium and Zirconia Dental Implants: An In Vitro Study. Dent. J. 2024, 12, 136. [Google Scholar] [CrossRef]
- Francis, S.; Caponio, V.C.A.; Spirito, F.; Perrotti, V.; Quaranta, A. Microbiological and Physical Changes Produced by Different Air–Powders on Contaminated Titanium Implant Surfaces: An In Vitro Pilot Study. Appl. Sci. 2023, 13, 1301. [Google Scholar] [CrossRef]
- Yudaev, P.A.; Chistyakov, E.M. Progress in dental materials: Application of natural ingredients. Russ. Chem. Rev. 2024, 93, RCR5108. [Google Scholar] [CrossRef]
- Abdullah, F.M.; Hatim, Q.Y.; Oraibi, A.I.; Alsafar, T.H.; Alsandook, T.A.; Lutfi, W.; Al-Hussaniy, H.A. Antimicrobial Management of Dental Infections: Updated Review. Med. Baltim. 2024, 103, e38630. [Google Scholar] [CrossRef]
- Cafiero, C.; Aglietta, M.; Iorio-Siciliano, V.; Salvi, G.E.; Blasi, A.; Matarasso, S. Implant Surface Roughness Alterations Induced by Different Prophylactic Procedures: An In Vitro Study. Clin. Oral Implant. Res. 2017, 28, e16–e20. [Google Scholar] [CrossRef]
- Oglakci, B.; Kucukyildirim, B.O.; Özduman, Z.C.; Eliguzeloglu Dalkilic, E. The Effect of Different Polishing Systems on the Surface Roughness of Nanocomposites: Contact Profilometry and SEM Analyses. Oper. Dent. 2021, 46, 173–187. [Google Scholar] [CrossRef]
- Butera, A.; Pascadopoli, M.; Gallo, S.; Lelli, M.; Tarterini, F.; Giglia, F.; Scribante, A. SEM/EDS Evaluation of the Mineral Deposition on a Polymeric Composite Resin of a Toothpaste Containing Biomimetic Zn-Carbonate Hydroxyapatite (microRepair®) in Oral Environment: A Randomized Clinical Trial. Polymers 2021, 13, 2740. [Google Scholar] [CrossRef]
- Mombelli, A. Microbiology and Antimicrobial Therapy of Peri-Implantitis. Periodontol 2000 2002, 28, 177–189. [Google Scholar] [CrossRef]
- Preda, C.; Butera, A.; Pelle, S.; Pautasso, E.; Chiesa, A.; Esposito, F.; Oldoini, G.; Scribante, A.; Genovesi, A.M.; Cosola, S. The Efficacy of Powered Oscillating Heads vs. Powered Sonic Action Heads Toothbrushes to Maintain Periodontal and Peri-Implant Health: A Narrative Review. Int. J. Environ. Res. Public Health 2021, 18, 1468. [Google Scholar] [CrossRef]
- Louropoulou, A.; Slot, D.E.; Van der Weijden, F. The Effects of Mechanical Instruments on Contaminated Titanium Dental Implant Surfaces: A Systematic Review. Clin. Oral Implant. Res. 2014, 25, 1149–1160. [Google Scholar] [CrossRef]
- Ramanauskaite, A.; Schwarz, F.; Chea, E.A.C.; Sahrmann, P. Photo-/Mechanical and Physical Implant Surface Decontamination Approaches in Conjunction with Surgical Peri-Implantitis Treatment: A Systematic Review. J. Clin. Periodontol. 2023, 50 (Suppl. S26), 317–335. [Google Scholar] [CrossRef] [PubMed]
- Herrera, D.; Berglundh, T.; Schwarz, F.; Chapple, I.; Jepsen, S.; Sculean, A.; Kebschull, M.; Papapanou, P.N.; Tonetti, M.S.; Sanz, M.; et al. Prevention and Treatment of Peri-Implant Diseases—The EFP S3 Level Clinical Practice Guideline. J. Clin. Periodontol. 2023, 50 (Suppl. S26), 4–76. [Google Scholar] [CrossRef]
- Alovisi, M.; Carossa, M.; Mandras, N.; Roana, J.; Costalonga, M.; Cavallo, L.; Pira, E.; Putzu, M.G.; Bosio, D.; Roato, I.; et al. Disinfection and Biocompatibility of Titanium Surfaces Treated with Glycine Powder Airflow and Triple Antibiotic Mixture: An In Vitro Study. Materials 2022, 15, 4850. [Google Scholar] [CrossRef]
- Ichioka, Y.; Virto, L.; Nuevo, P.; Gamonal, J.D.; Derks, J.; Larsson, L.; Sanz, M.; Berglundh, T. Decontamination of Biofilm-Contaminated Implant Surfaces: An In Vitro Evaluation. Clin. Oral Implant. Res. 2023, 34, 1058–1072. [Google Scholar] [CrossRef]
- Ichioka, Y.; Derks, J.; Larsson, L.; Berglundh, T. Surface Decontamination of Explanted Peri-Implantitis-Affected Implants. J. Clin. Periodontol. 2023, 50, 1113–1122. [Google Scholar] [CrossRef]
- Wilensky, A.; Shapira, L.; Limones, A.; Martin, C. The Efficacy of Implant Surface Decontamination Using Chemicals During Surgical Treatment of Peri-Implantitis: A Systematic Review and Meta-Analysis. J. Clin. Periodontol. 2023, 50 (Suppl. S26), 336–358. [Google Scholar] [CrossRef] [PubMed]
- Kotsakis, G.A.; Black, R.; Kum, J.; Berbel, L.; Sadr, A.; Karoussis, I.; Simopoulou, M.; Daubert, D. Effect of Implant Cleaning on Titanium Particle Dissolution and Cytocompatibility. J. Periodontol. 2021, 92, 580–591. [Google Scholar] [CrossRef] [PubMed]
- Berbel, L.O.; Banczek, E.D.P.; Karoussis, I.K.; Kotsakis, G.A.; Costa, I. Determinants of Corrosion Resistance of Ti-6Al-4V Alloy Dental Implants in an In Vitro Model of Peri-Implant Inflammation. PLoS ONE 2019, 14, e0210530. [Google Scholar]
- Conserva, E.; Generali, L.; Bandieri, A.; Cavani, F.; Borghi, F.; Consolo, U. Plaque Accumulation on Titanium Disks with Different Surface Treatments: An In Vivo Investigation. Odontology 2018, 106, 145–153. [Google Scholar] [CrossRef]
- Esposito, M.; Grusovin, M.G.; Polyzos, I.P.; Felice, P.; Worthington, H.V. Interventions for Replacing Missing Teeth: Dental Implants in Fresh Extraction Sockets (Immediate, Immediate-Delayed and Delayed Implants). Cochrane Database Syst. Rev. 2010, 9, CD005968. [Google Scholar] [CrossRef]
- Ichioka, Y.; Derks, J.; Dahlén, G.; Berglundh, T.; Larsson, L. Mechanical Removal of Biofilm on Titanium Discs: An In Vitro Study. J. Biomed. Mater. Res. B Appl. Biomater. 2022, 110, 1044–1055. [Google Scholar] [CrossRef] [PubMed]
- Safioti, L.M.; Kotsakis, G.A.; Pozhitkov, A.E.; Chung, W.O.; Daubert, D.M. Increased Levels of Dissolved Titanium Are Associated with Peri-Implantitis—A Cross-Sectional Study. J. Periodontol. 2017, 88, 436–442. [Google Scholar] [CrossRef] [PubMed]
- Dukka, H.; Saleh, M.H.A.; Ravidà, A.; Greenwell, H.; Wang, H.L. Is Bleeding on Probing a Reliable Clinical Indicator of Peri-Implant Diseases? J. Periodontol. 2021, 92, 1669–1674. [Google Scholar] [CrossRef]
- Santana, S.I.; Silva, P.H.F.; Salvador, S.L.; Casarin, R.C.V.; Furlaneto, F.A.C.; Messora, M.R. Adjuvant Use of Multispecies Probiotic in the Treatment of Peri-Implant Mucositis: A Randomized Controlled Trial. J. Clin. Periodontol. 2022, 49, 828–839. [Google Scholar] [CrossRef]
- Al Ghazal, L.; O’Sullivan, J.; Claffey, N.; Polyzois, I. Comparison of Two Different Techniques Used for the Maintenance of Peri-Implant Soft Tissue Health: A Pilot Randomized Clinical Trial. Acta Odontol. Scand. 2017, 75, 542–549. [Google Scholar] [CrossRef] [PubMed]
- Tong, Z.; Fu, R.; Zhu, W.; Shi, J.; Yu, M.; Si, M. Changes in the Surface Topography and Element Proportion of Clinically Failed SLA Implants after In Vitro Debridement by Different Methods. Clin. Oral Implant. Res. 2021, 32, 263–273. [Google Scholar] [CrossRef]
- Ungvári, K.; Pelsöczi, I.K.; Kormos, B.; Oszkó, A.; Rakonczay, Z.; Kemény, L.; Radnai, M.; Nagy, K.; Fazekas, A.; Turzó, K. Effects on titanium implant surfaces of chemical agents used for the treatment of peri-implantitis. J. Biomed. Mater. Res. B Appl. Biomater. 2010, 94, 222–229. [Google Scholar] [CrossRef]
- Masa, R.; Pelsőczi-Kovács, I.; Aigner, Z.; Oszkó, A.; Turzó, K.; Ungvári, K. Surface Free Energy and Composition Changes and Ob Cellular Response to CHX-, PVPI-, and ClO2-Treated Titanium Implant Materials. J. Funct. Biomater. 2022, 13, 202. [Google Scholar] [CrossRef]
- Cha, J.K.; Paeng, K.; Jung, U.W.; Choi, S.H.; Sanz, M.; Sanz-Martín, I. The Effect of Five Mechanical Instrumentation Protocols on Implant Surface Topography and Roughness: A Scanning Electron Microscope and Confocal Laser Scanning Microscope Analysis. Clin. Oral Implant. Res. 2019, 30, 578–587. [Google Scholar] [CrossRef] [PubMed]
- Korello, K.; Eickholz, P.; Zuhr, O.; Ratka, C.; Petsos, H. In Vitro Efficacy of Non-Surgical and Surgical Implant Surface Decontamination Methods in Three Different Defect Configurations in the Presence or Absence of a Suprastructure. Clin. Implant. Dent. Relat. Res. 2023, 25, 549–563. [Google Scholar] [CrossRef] [PubMed]
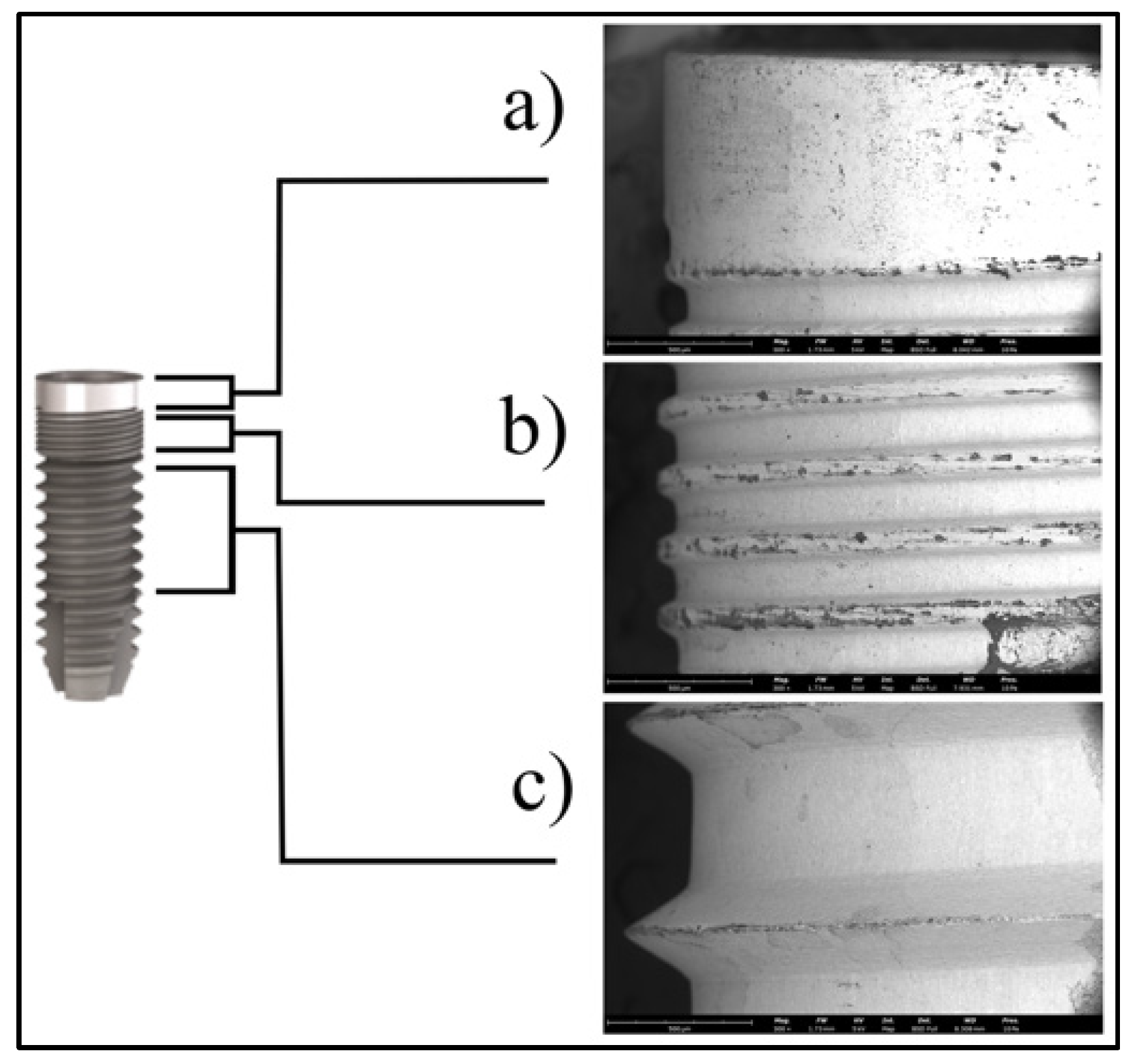

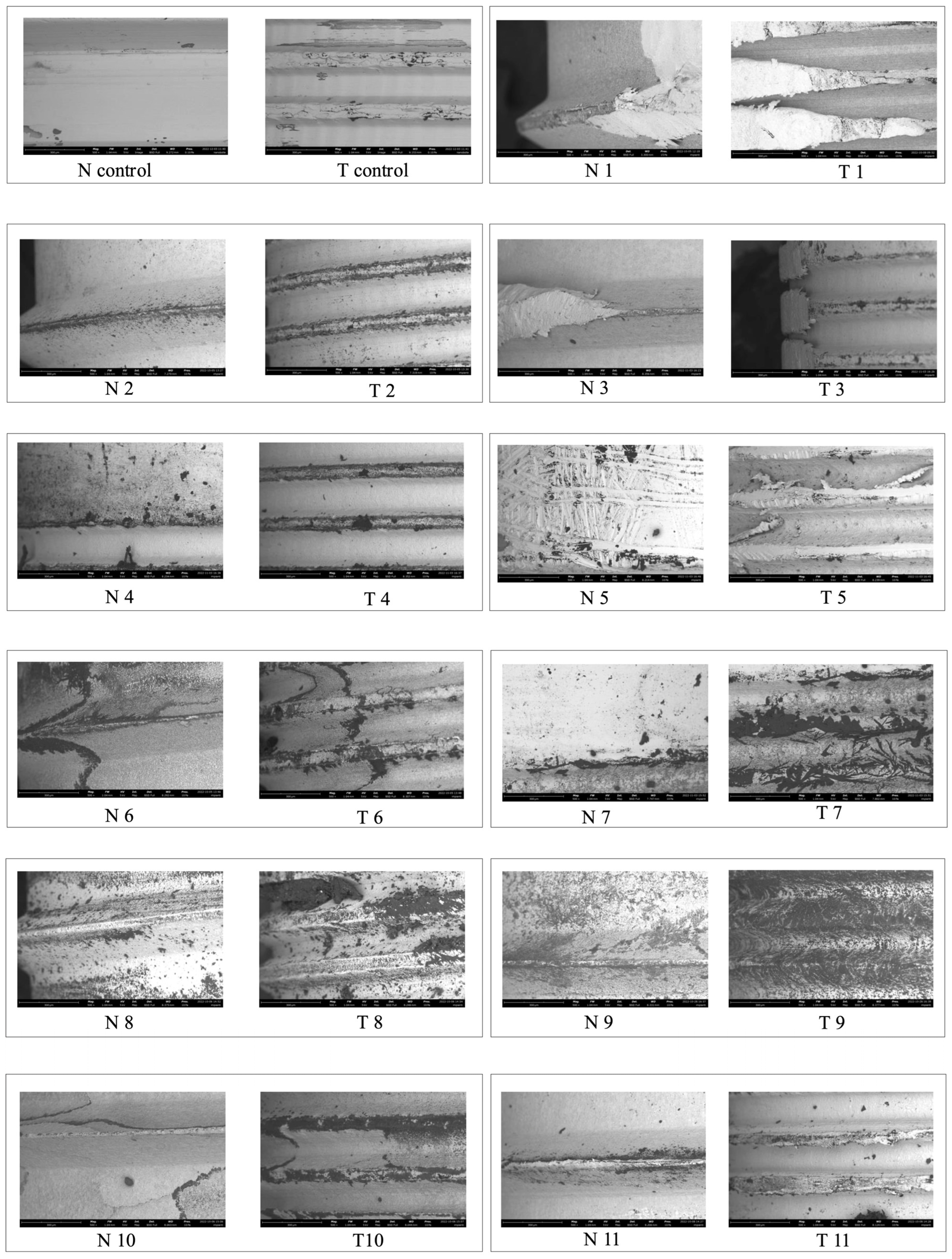
| Treatment | Instrument | Use/Type of Handpiece | Macrogroup | Procedures |
|---|---|---|---|---|
| / | Control | No surface treatment | Control | No procedure |
| 1. | Steel ultrasonic insert | EMS handpiece | Ultrasound | Elliptical movement of the insert between the neck and the implant screw for 60 s |
| 2. | PEEK ultrasonic insert | EMS handpiece | Ultrasound | Elliptical movement of the insert between the neck and the implant screw for 60 s |
| 3. | PEEK sonic insert | KAVO SonicFlex 2003 handpiece | Ultrasound | Elliptical movement of the insert between the neck and the implant screw for 60 s |
| 4. | Steel sonic insert | KAVO SonicFlex 2003 handpiece | Ultrasound | Elliptical movement of the insert between the neck and the implant screw for 60 s |
| 5. | PEEK ultrasonic insert | Mectron handpiece | Curette | Elliptical movement of the curette in a mesio-distal direction, placing the working part between the neck and the waist of the implant for 60 s |
| 6. | Hy-Friedy Steel curette | Manual | Curette | Elliptical movement of the curette in a mesio-distal direction, placing the working part between the neck and the waist of the implant for 60 s |
| 7. | Deppeler Titanium curette | Manual | Curette | Elliptical movement of the curette in a mesio-distal direction, placing the working part between the neck and the waist of the implant for 60 s |
| 8. | KerrHawe Carbon fiber curette | Manual | Curette | Elliptical movement of the curette in a mesio-distal direction, placing the working part between the neck and the waist of the implant for 60 s |
| 9. | Woodpecker insert | Woodpecker handpiece | Ultrasound | Elliptical movement of the insert between the neck and the implant screw for 60 s |
| 10. | Sensitive Mectron Glycine powder (∅ 25 μm) with supragingival insert | Mectron handpiece | Powder | Position the air-polishing handpiece by tilting it at 45° between the neck and the waist of the implant, then spraying the powder for 60 s |
| 11. | Sensitive Mectron Glycine powder (∅ 25 μm) with subgingival insert | Mectron handpiece | Powder | Position the air-polishing handpiece by tilting it at 45° between the neck and the waist of the implant, then spraying the powder for 60 s |
| 12. | Glycine powder with Woodpecker supragingival insert | Periomate NSK handpiece | Powder | Position the air-polishing handpiece by tilting it at 45° between the neck and the waist of the implant, then spraying the powder for 60 s |
| 13. | Glycine powder with Woodpecker subgingival insert | Periomate NSK handpiece | Powder | Position the air-polishing handpiece by tilting it at 45° between the neck and the waist of the implant, then spraying the powder for 60 s |
| 14. | Erythritol powder with supragingival insert | EMS handpiece | Powder | Position the air-polishing handpiece by tilting it at 45° between the neck and the waist of the implant, then spraying the powder for 60 s |
| 15. | Erythritol powder with subgingival insert | EMS handpiece | Powder | Position the air-polishing handpiece by tilting it at 45° between the neck and the waist of the implant, then spraying the powder for 60 s |
| 16. | Flash pearls NSK (CaCO3 ∅ 53 μm) with Woodpecker supragingival insert | Periomate NSK handpiece | Powder | Position the air-polishing handpiece by tilting it at 45° between the neck and the waist of the implant, then spraying the powder for 60 s |
| 17. | Kerr Cleanic with perlite (RDA = 27) | Kavo Smartmatic handpiece with Pro-cup insert | Powder | Position the air-polishing handpiece by tilting it at 45° between the neck and the waist of the implant, then spraying the powder for 60 s |
| 18. | Prophylaxis toothbrush (7000 rpm) | Green contra-angle handpiece | Brush | Apply the toothbrush with nylon filaments and prophylaxis paste, on a green handle, placing it half on the neck and half on the screw for 60 s at moderate speed |
| 19. | iDentoflex P13 Silicon pumice cup | Green contra-angle handpiece | Brush | Apply the toothbrush with nylon filaments and prophylaxis paste, on a green handle, placing it half on the neck and half on the screw for 60 s at moderate speed |
| 20. | iDentoflex P13 Silicon cup brown | Green contra-angle handpiece | Brush | Apply the toothbrush with nylon filaments and prophylaxis paste, on a green handle, placing it half on the neck and half on the screw for 60 s at moderate speed |
| 21. | Hybenx gel EPIEN Medical (sulfonate phenolic compounds and sulfuric acid in aqueous solution) | Syringe with 27 G needle | Gel | Activate and apply the pre-dosed gel to the surface of the implant between the neck and the screw; after 60 s, remove it |
| Zone | Mean | Standard Error | Minimum | Median | Maximum | |
|---|---|---|---|---|---|---|
| Control | Neck | 0.06 | 0.00 | 0.06 | 0.06 | 0.06 |
| Control | Thread | 0.04 | 0.00 | 0.04 | 0.04 | 0.05 |
| Treatment 1 | Neck | 0.04 | 0.00 | 0.04 | 0.04 | 0.05 |
| Treatment 1 | Thread | 0.07 | 0.00 | 0.07 | 0.07 | 0.07 |
| Treatment 2 | Neck | 0.03 | 0.00 | 0.03 | 0.03 | 0.03 |
| Treatment 2 | Thread | 0.09 | 0.00 | 0.09 | 0.09 | 0.09 |
| Treatment 3 | Neck | 0.03 | 0.00 | 0.02 | 0.03 | 0.03 |
| Treatment 3 | Thread | 0.23 | 0.00 | 0.08 | 0.08 | 0.80 |
| Treatment 4 | Neck | 0.03 | 0.00 | 0.02 | 0.03 | 0.03 |
| Treatment 4 | Thread | 0.04 | 0.00 | 0.03 | 0.04 | 0.04 |
| Treatment 5 | Neck | 0.04 | 0.00 | 0.04 | 0.04 | 0.04 |
| Treatment 5 | Thread | 0.08 | 0.00 | 0.07 | 0.08 | 0.08 |
| Treatment 6 | Neck | 0.04 | 0.00 | 0.03 | 0.04 | 0.04 |
| Treatment 6 | Thread | 0.07 | 0.00 | 0.07 | 0.08 | 0.08 |
| Treatment 7 | Neck | 0.11 | 0.00 | 0.04 | 0.04 | 0.40 |
| Treatment 7 | Thread | 0.08 | 0.00 | 0.07 | 0.08 | 0.08 |
| Treatment 8 | Neck | 0.04 | 0.00 | 0.04 | 0.04 | 0.04 |
| Treatment 8 | Thread | 0.36 | 0.00 | 0.08 | 0.08 | 0.79 |
| Treatment 9 | Neck | 0.05 | 0.00 | 0.04 | 0.05 | 0.05 |
| Treatment 9 | Thread | 0.08 | 0.00 | 0.08 | 0.08 | 0.08 |
| Treatment 10 | Neck | 0.04 | 0.00 | 0.04 | 0.04 | 0.04 |
| Treatment 10 | Thread | 0.07 | 0.00 | 0.06 | 0.07 | 0.07 |
| Treatment 11 | Neck | 0.03 | 0.00 | 0.03 | 0.03 | 0.04 |
| Treatment 11 | Thread | 0.04 | 0.00 | 0.03 | 0.04 | 0.04 |
| Treatment 12 | Neck | 0.03 | 0.00 | 0.03 | 0.03 | 0.04 |
| Treatment 12 | Thread | 0.08 | 0.00 | 0.08 | 0.08 | 0.08 |
| Treatment 13 | Neck | 0.03 | 0.00 | 0.03 | 0.03 | 0.03 |
| Treatment 13 | Thread | 0.07 | 0.00 | 0.07 | 0.07 | 0.07 |
| Treatment 14 | Neck | 0.03 | 0.00 | 0.02 | 0.03 | 0.03 |
| Treatment 14 | Thread | 0.09 | 0.00 | 0.09 | 0.09 | 0.10 |
| Treatment 15 | Neck | 0.03 | 0.00 | 0.02 | 0.03 | 0.03 |
| Treatment 15 | Thread | 0.05 | 0.00 | 0.05 | 0.05 | 0.05 |
| Treatment 16 | Neck | 0.03 | 0.00 | 0.03 | 0.03 | 0.04 |
| Treatment 16 | Thread | 0.07 | 0.00 | 0.07 | 0.07 | 0.07 |
| Treatment 17 | Neck | 0.04 | 0.00 | 0.04 | 0.04 | 0.05 |
| Treatment 17 | Thread | 0.01 | 0.00 | 0.01 | 0.01 | 0.02 |
| Treatment 18 | Neck | 0.02 | 0.00 | 0.02 | 0.03 | 0.03 |
| Treatment 18 | Thread | 0.02 | 0.00 | 0.02 | 0.02 | 0.02 |
| Treatment 19 | Neck | 0.03 | 0.00 | 0.03 | 0.03 | 0.03 |
| Treatment 19 | Thread | 0.08 | 0.00 | 0.08 | 0.08 | 0.08 |
| Treatment 20 | Neck | 0.03 | 0.00 | 0.02 | 0.02 | 0.03 |
| Treatment 20 | Thread | 0.08 | 0.00 | 0.08 | 0.08 | 0.08 |
| Treatment 21 | Neck | 0.06 | 0.00 | 0.05 | 0.06 | 0.06 |
| Treatment 21 | Thread | 0.08 | 0.00 | 0.08 | 0.08 | 0.08 |
| Mean | Standard Error | Minimum | Median | Maximum | |
|---|---|---|---|---|---|
| Control | 0.04374 | 0.00218 | 0.0409 | 0.04329 | 0.04633 |
| Ultrasound | 0.04668 | 0.00294 | 0.0235 | 0.03919 | 0.0777 |
| Powders | 0.04406 | 0.00254 | 0.0143 | 0.03367 | 0.09632 |
| Curettes | 0.04833 | 0.00355 | 0.03104 | 0.03581 | 0.07861 |
| Brushes | 0.05974 | 0.00450 | 0.02367 | 0.07622 | 0.07892 |
| Gel | 0.07722 | 0.00353 | 0.07562 | 0.0775 | 0.07891 |
| Mean | Standard Error | Minimum | Median | Maximum | |
|---|---|---|---|---|---|
| Neck | 0.035972 | 000087 | 0.02277 | 0.033995 | 0.05841 |
| Thread | 0.065119 | 0.00204 | 0.0143 | 0.075255 | 0.09632 |
| Dependent Variable (p Value) | |
|---|---|
| Independent Variable | Roughness |
| Implant | 0.119 |
| Instrument | 0.119 |
| Technique | 0.901 |
| Zone | <0.001 * |
| Titanium | Carbon | Calcium | |||||
|---|---|---|---|---|---|---|---|
| Group | Macrogroup | Mean | Standard Error | Mean | Standard Error | Mean | Standard Error |
| Control | Control | 100.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Treatment 1 | Ultrasound | 97.38 | 0.60 | 0.00 | 0.00 | 0.00 | 0.00 |
| Treatment 2 | Ultrasound | 90.14 | 4.88 | 8.52 | 5.16 | 0.00 | 0.00 |
| Treatment 3 | Ultrasound | 100.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Treatment 4 | Ultrasound | 100.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Treatment 5 | Curette | 100.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Treatment 6 | Curette | 89.49 | 7.08 | 10.51 | 7.08 | 0.00 | 0.00 |
| Treatment 7 | Curette | 100.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Treatment 8 | Curette | 54.32 | 8.29 | 45.82 | 8.28 | 0.00 | 0.00 |
| Treatment 9 | Ultrasound | 97.36 | 1.58 | 2.64 | 1.58 | 0.00 | 0.00 |
| Treatment 10 | Powder | 84.95 | 2.25 | 0.00 | 0.00 | 0.00 | 0.00 |
| Treatment 11 | Powder | 100.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Treatment 12 | Powder | 62.85 | 8.23 | 37.15 | 8.23 | 0.00 | 0.00 |
| Treatment 13 | Powder | 70.72 | 5.12 | 0.00 | 0.00 | 29.28 | 5.12 |
| Treatment 14 | Powder | 94.10 | 1.25 | 5.90 | 1.25 | 0.00 | 0.00 |
| Treatment 15 | Powder | 100.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Treatment 16 | Powder | 87.05 | 6.32 | 12.95 | 6.32 | 0.00 | 0.00 |
| Treatment 17 | Powder | 100.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Treatment 18 | Brush | 100.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Treatment 19 | Brush | 80.72 | 3.91 | 19.28 | 3.91 | 0.00 | 0.00 |
| Treatment 20 | Brush | 100.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Treatment 21 | Gel | 100.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lattari, M.; Butera, A.; Roatti, S.; Pascadopoli, M.; Alberti, B.; Cosola, S.; Alovisi, M.; Scribante, A. SEM/EDS and Roughness Analysis on Current Titanium Implant Decontamination Systems: In Vitro Study. Coatings 2025, 15, 1114. https://doi.org/10.3390/coatings15101114
Lattari M, Butera A, Roatti S, Pascadopoli M, Alberti B, Cosola S, Alovisi M, Scribante A. SEM/EDS and Roughness Analysis on Current Titanium Implant Decontamination Systems: In Vitro Study. Coatings. 2025; 15(10):1114. https://doi.org/10.3390/coatings15101114
Chicago/Turabian StyleLattari, Marco, Andrea Butera, Simone Roatti, Maurizio Pascadopoli, Beatrice Alberti, Saverio Cosola, Mario Alovisi, and Andrea Scribante. 2025. "SEM/EDS and Roughness Analysis on Current Titanium Implant Decontamination Systems: In Vitro Study" Coatings 15, no. 10: 1114. https://doi.org/10.3390/coatings15101114
APA StyleLattari, M., Butera, A., Roatti, S., Pascadopoli, M., Alberti, B., Cosola, S., Alovisi, M., & Scribante, A. (2025). SEM/EDS and Roughness Analysis on Current Titanium Implant Decontamination Systems: In Vitro Study. Coatings, 15(10), 1114. https://doi.org/10.3390/coatings15101114










