Polypyrrole-Modified Molybdenum Disulfide Nanocomposite Epoxy Coating Inhibits Corrosion of Mild Steel
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Method
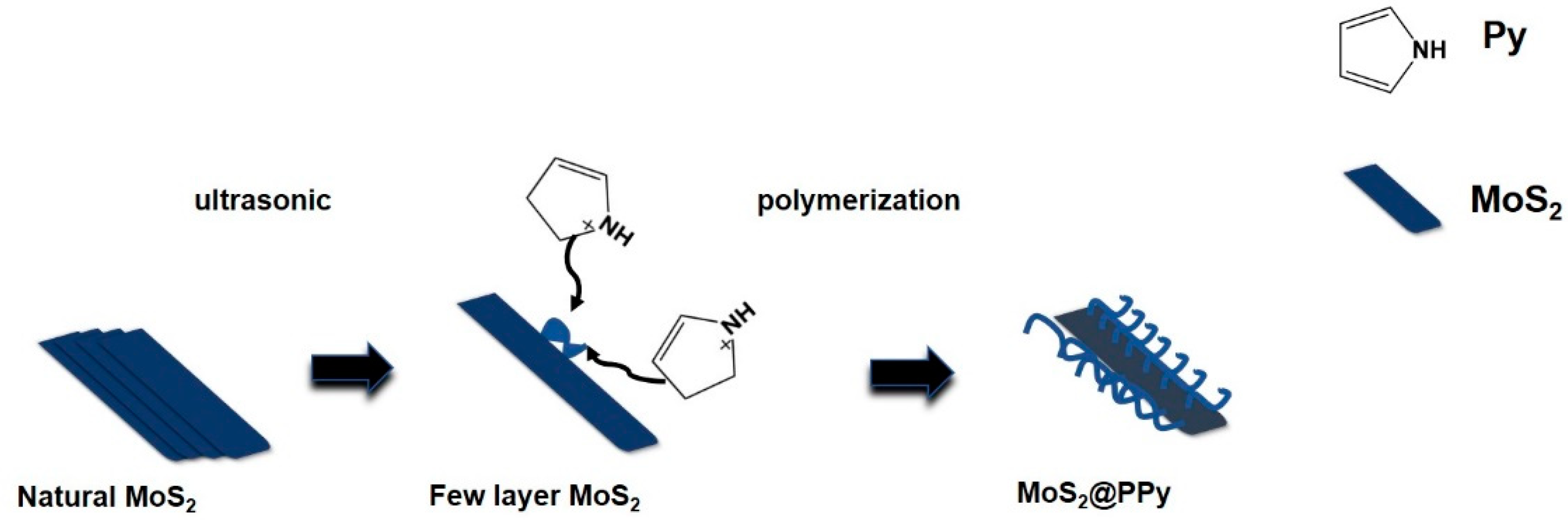
2.2.1. Synthesis of MoS2 Nanosheets
2.2.2. Synthesis of PPy
2.2.3. Synthesis of MoS2@PPy Nanomaterials
2.2.4. Preparation of MoS2/EP Coating and MoS2@PPy EP Coating
2.3. Characterization
2.3.1. Characterizations of MoS2 and MoS2@PPy
2.3.2. Electrochemical Testing
2.3.3. Salt Spray Test
3. Results and Discussion
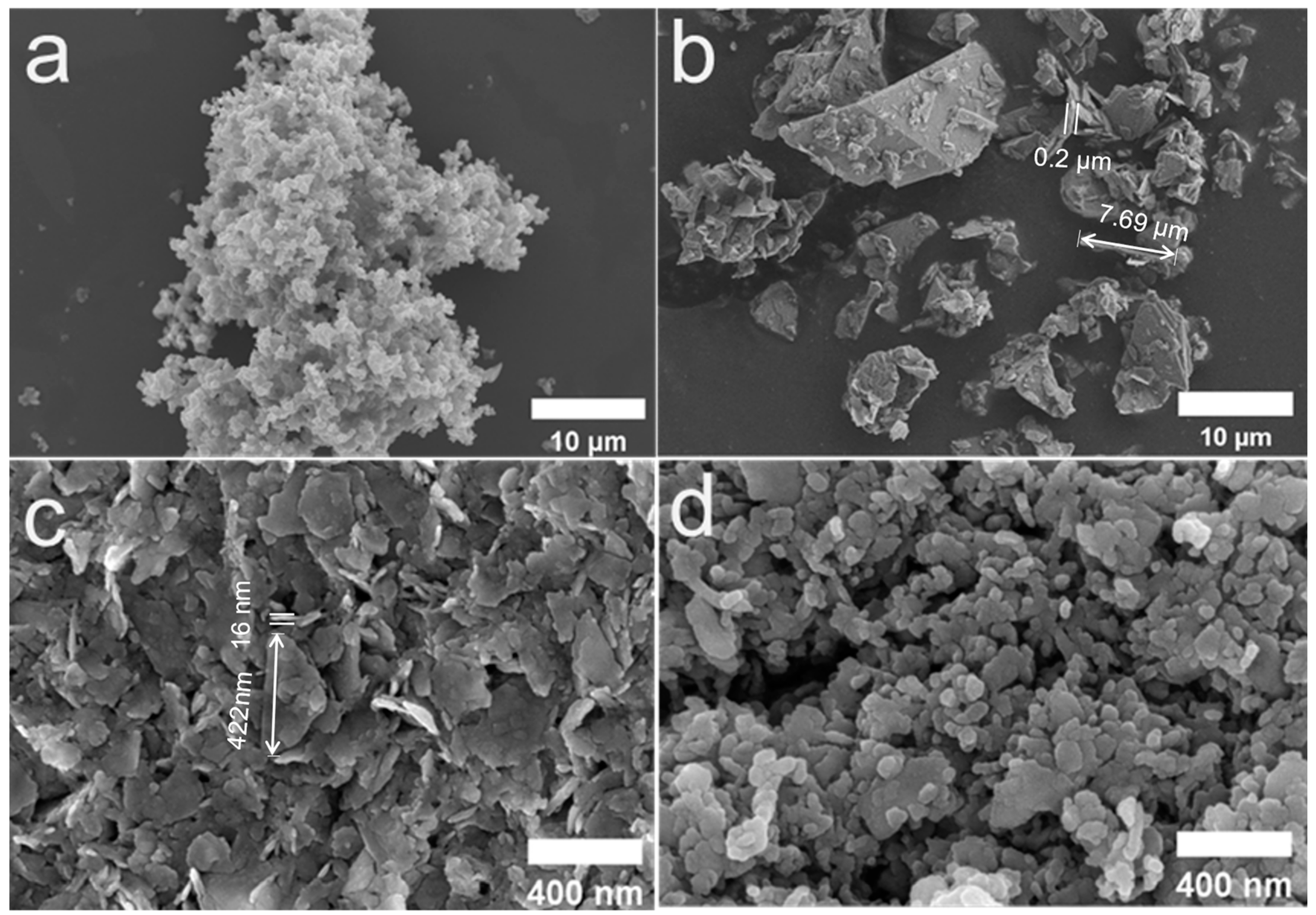
3.1. Microstructure Characterization of the MoS2 and the MoS2@PPy
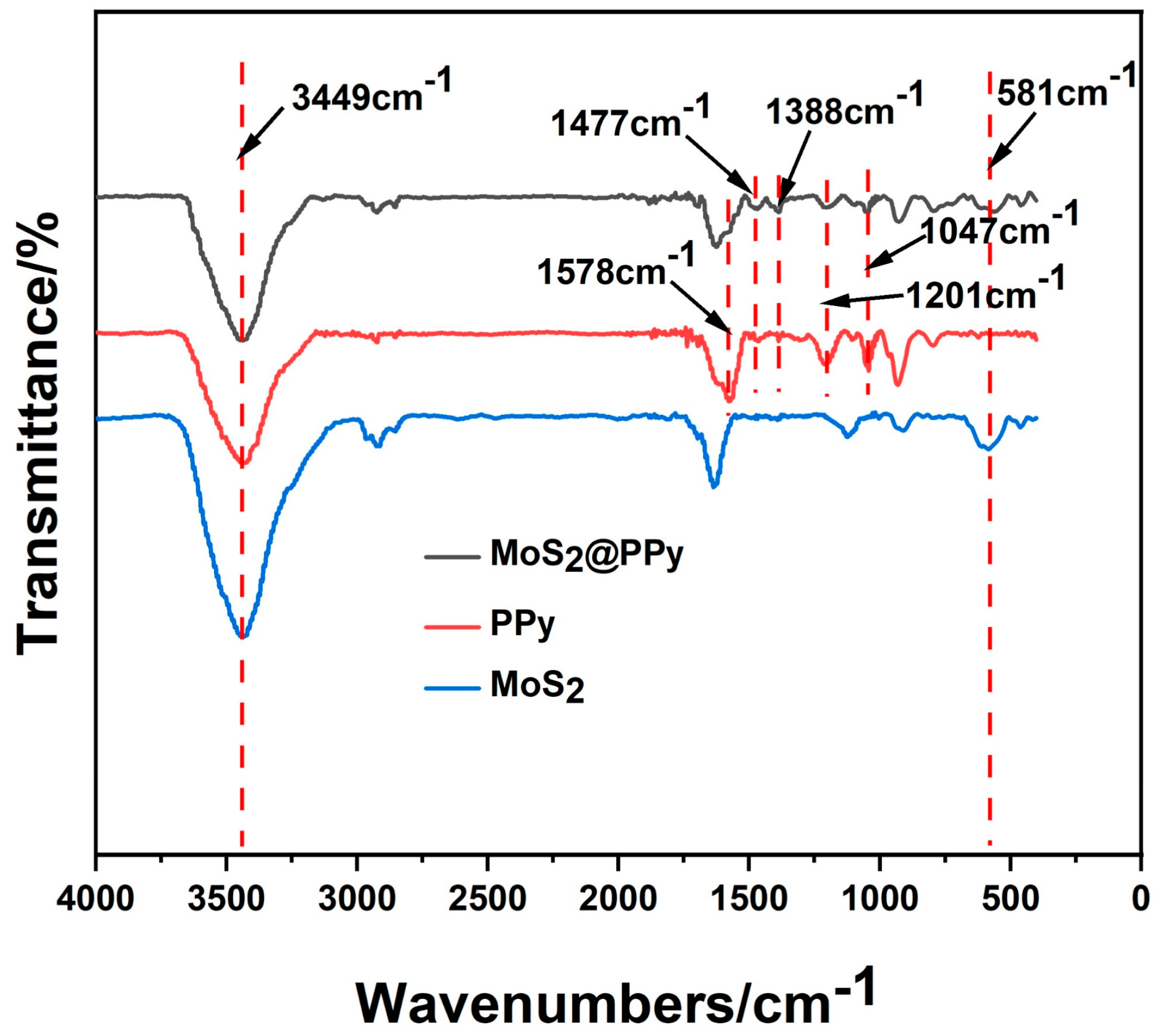
3.2. Chemical Structure and Composition of MoS2 and MoS2@PPy
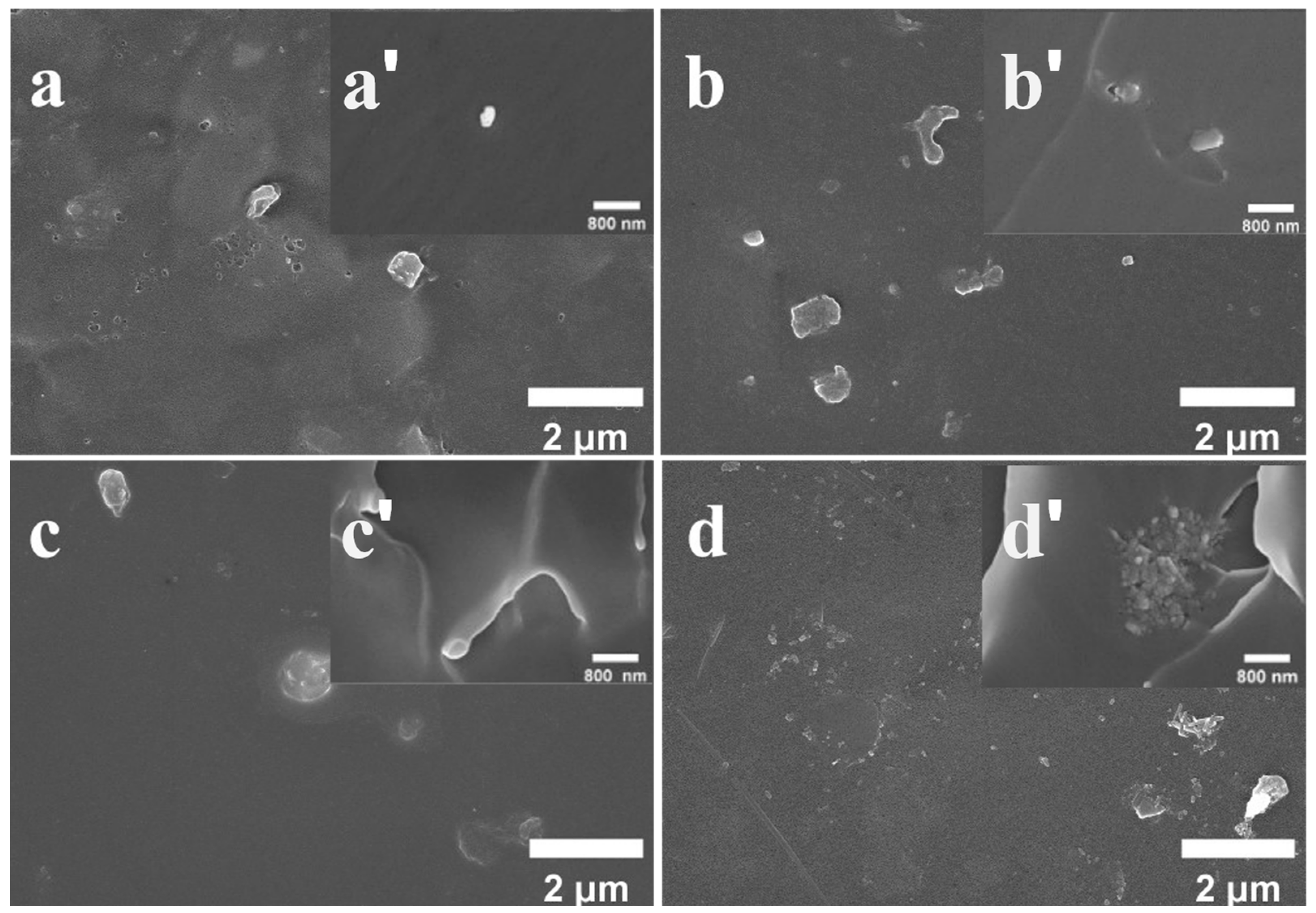
3.3. Surface Morphology of Different Composite Coatings
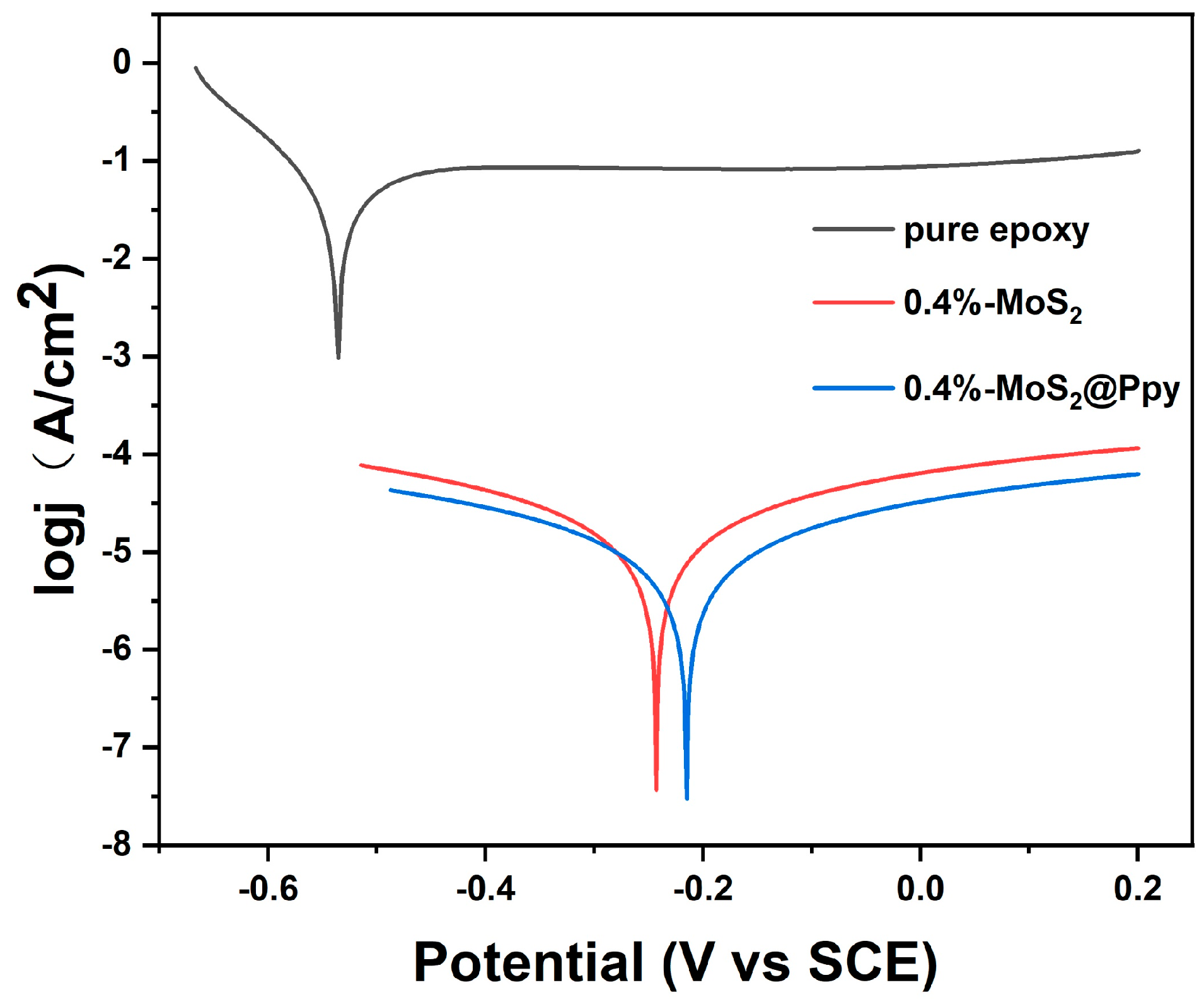
3.4. Polarization Curves for Different Coatings
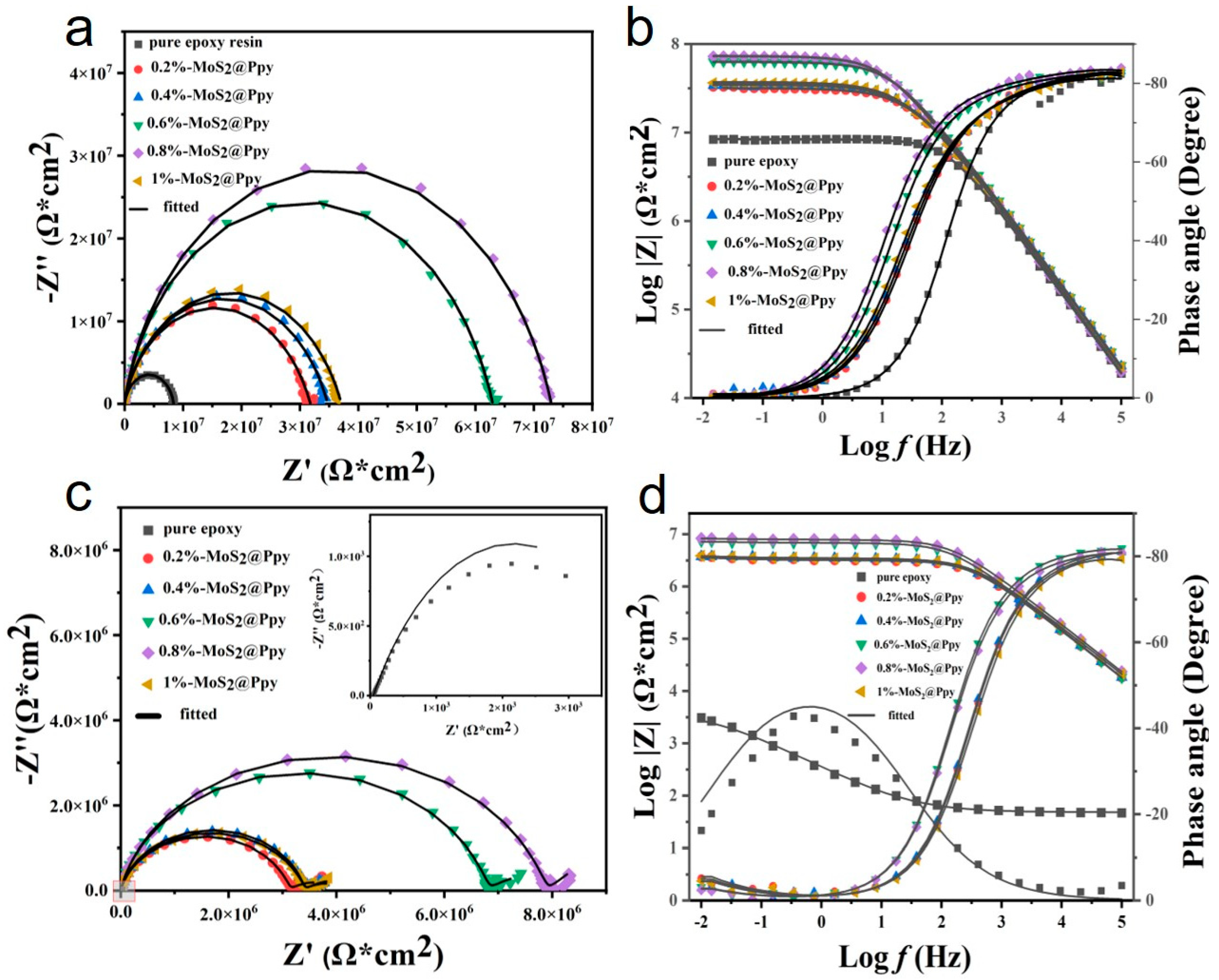
3.5. Electrochemical Impedance Test
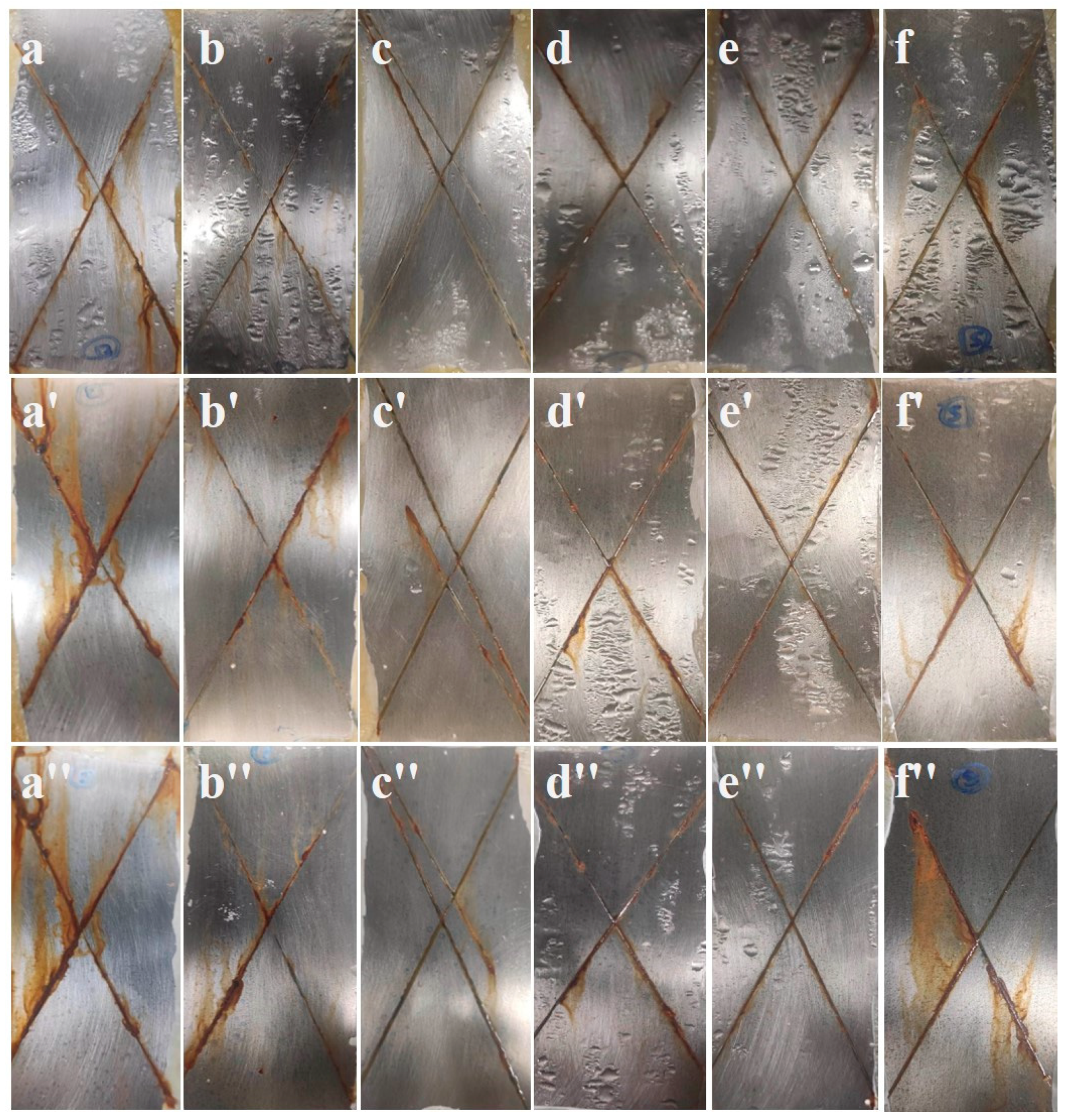
3.6. Salt Spray Test
3.7. Anti-Corrosion Mechanism
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Morin, J.; Brochard, G.; Bergé, V.; Rosset, A.; Artous, S.; Clavaguera, S.; Strekowski, R.S.; Wortham, H. Uptake of m-xylene and VOC emissions by mineral photocatalytic paints of indoor air building interest. Environ. Sci. Nano 2023, in press. [CrossRef]
- Massou, M.; Babu, N.; Xian, G. Experimental study on the mechanical properties of CFRP/epoxy composite plates under seawater immersion. Structures 2023, 54, 48–57. [Google Scholar] [CrossRef]
- Zhao, Q.; Lu, Z.; Wu, Y.; Zhao, W. Designing strong interfacial adhesion between carbon fiber and epoxy resin via dopamine towards excellent protection ability under high hydrostatic pressure and severe erosion corrosion condition. Compos. Sci. Technol. 2022, 217, 109090. [Google Scholar] [CrossRef]
- Huang, H.; Sheng, X.; Tian, Y.; Zhang, L.; Chen, Y.; Zhang, X. Two-Dimensional Nanomaterials for Anticorrosive Polymeric Coatings: A Review. Ind. Eng. Chem. Res. 2020, 59, 15424–15446. [Google Scholar] [CrossRef]
- Li, H.; Zhang, Q.-H.; Meng, X.-Z.; Liu, P.; Wu, L.-K.; Cao, F.-H. A novel cerium organic network modified graphene oxide prepared multifunctional waterborne epoxy-based coating with excellent mechanical and passive/active anti-corrosion properties. Chem. Eng. J. 2023, 465, 142997. [Google Scholar] [CrossRef]
- Aleithan, S.H.; Al-Amer, K.; Alabbad, Z.H.; Khalaf, M.M.; Alam, K.; Alhashem, Z.; Abd El-Lateef, H.M. Highly scalable synthesis of MoS2 thin films for carbon steel coatings: Influence of synthetic route on the nanostructure and corrosion performance. J. Mater. Res. Technol. 2023, 23, 1239–1251. [Google Scholar] [CrossRef]
- Wu, J.; Wang, H.; Li, R.; Guo, L. Anticorrosive waterborne polyurethane coating with Gemini surfactant-assisted exfoliation of hexagonal boron nitride nanosheets. J. Appl. Polym. Sci. 2023, e54008. [Google Scholar] [CrossRef]
- Xing, X.; Sui, Y.; Li, Q.; Yuan, M.; Zhao, H.; Zhou, D.; Chu, X.; Liu, S.; Tang, E. Multifunctional ZnAl-MoO4 LDH assembled Ti3C2Tx MXene composite for active/passive corrosion protection behavior of epoxy coatings. Appl. Surf. Sci. 2023, 623, 157092. [Google Scholar] [CrossRef]
- Bai, Z.; Meng, S.; Cui, Y.; Sun, Y.; Pei, L.; Hu, H.; Jiang, Y.; Wang, H. A stable anticorrosion coating with multifunctional linkage against seawater corrosion. Compos. Part B Eng. 2023, 259, 110733. [Google Scholar] [CrossRef]
- Dong, M.; Zhang, H.; Tzounis, L.; Santagiuliana, G.; Bilotti, E.; Papageorgiou, D.G. Multifunctional epoxy nanocomposites reinforced by two-dimensional materials: A review. Carbon 2021, 185, 57–81. [Google Scholar] [CrossRef]
- Quezada-Renteria, J.A.; Chazaro-Ruiz, L.F.; Rangel-Mendez, J.R. Poorly conductive electrochemically reduced graphene oxide films modified with alkyne chains to avoid the corrosion-promoting effect of graphene-based materials on carbon steel. Carbon 2020, 167, 512–522. [Google Scholar] [CrossRef]
- Schriver, M.; Regan, W.; Gannett, W.J.; Zaniewski, A.M.; Crommie, M.F.; Zettl, A. Graphene as a Long-Term Metal Oxidation Barrier: Worse Than Nothing. ACS Nano 2013, 7, 5763–5768. [Google Scholar] [CrossRef] [PubMed]
- Kumari, S.; Chouhan, A.; Sharma, O.P.; Kuriakose, S.; Tawfik, S.A.; Spencer, M.J.S.; Walia, S.; Sugimura, H.; Khatri, O.P. Structural-Defect-Mediated Grafting of Alkylamine on Few-Layer MoS2 and Its Potential for Enhancement of Tribological Properties. ACS Appl. Mater. Interfaces 2020, 12, 30720–30730. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Hu, J.; Li, Y.; Liu, J. Polyaniline/zinc/cerium nitrate pigment for epoxy based anticorrosion coatings. React. Funct. Polym. 2018, 131, 22–28. [Google Scholar] [CrossRef]
- Meng, R.; Liu, L.; Guo, W. Mussel-inspired polydopamine and Al2O3 nanoparticles co-modified MoS2 for reinforcing anticorrosion of epoxy coatings. Colloid Polym. Sci. 2023, 301, 175–187. [Google Scholar] [CrossRef]
- Vandana, S.; Kochat, V.; Lee, J.; Varshney, V.; Yazdi, S.; Shen, J.; Kosolwattana, S.; Vinod, S.; Vajtai, R.; Roy, A.K.; et al. 2D Heterostructure coatings ofhBN-MoS2layers for corrosion resistance. J. Phys. D Appl. Phys. 2017, 50, 045301. [Google Scholar] [CrossRef]
- Xia, Z.; Liu, G.; Dong, Y.; Zhang, Y. Anticorrosive epoxy coatings based on polydopamine modified molybdenum disulfide. Prog. Org. Coat. 2019, 133, 154–160. [Google Scholar] [CrossRef]
- Xia, Y.; He, Y.; Chen, C.; Wu, Y.; Chen, J. MoS2 nanosheets modified SiO2 to enhance the anticorrosive and mechanical performance of epoxy coating. Prog. Org. Coat. 2019, 132, 316–327. [Google Scholar] [CrossRef]
- Ding, J.H.; Zhao, H.R.; Zhao, X.P.; Xu, B.Y.; Yu, H.B. How semiconductor transition metal dichalcogenides replaced graphene for enhancing anticorrosion. J. Mater. Chem. A 2019, 7, 13511–13521. [Google Scholar] [CrossRef]
- Tang, H.; Wang, J.; Yin, H.; Zhao, H.; Wang, D.; Tang, Z. Growth of polypyrrole ultrathin films on MoS2 monolayers as high-performance supercapacitor electrodes. Adv. Mater. 2015, 27, 1117–1123. [Google Scholar] [CrossRef]
- Lu, F.; Liu, C.; Chen, Z.; Veerabagu, U.; Chen, Z.; Liu, M.; Hu, L.; Xia, H.; Cha, L.; Zhang, W. Polypyrrole-functionalized boron nitride nanosheets for high-performance anti-corrosion composite coating. Surf. Coat. Technol. 2021, 420, 127273. [Google Scholar] [CrossRef]
- Halim, U.; Zheng, C.R.; Chen, Y.; Lin, Z.; Jiang, S.; Cheng, R.; Huang, Y.; Duan, X. A rational design of cosolvent exfoliation of layered materials by directly probing liquid-solid interaction. Nat. Commun. 2013, 4, 2213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jing, Y.; Wang, P.; Yang, Q.; Wang, Q.; Bai, Y. MoS2 decorated with ZrO2 nanoparticles through mussel-inspired chemistry of dopamine for reinforcing anticorrosion of epoxy coatings. Colloids Surf. A Physicochem. Eng. Asp. 2021, 608, 125625. [Google Scholar] [CrossRef]
- He, Z.; Lin, H.; Zhang, X.; Chen, Y.; Bai, W.; Lin, Y.; Jian, R.; Xu, Y. Self-healing epoxy composite coating based on polypyrrole@MOF nanoparticles for the long-efficiency corrosion protection on steels. Colloids Surf. A Physicochem. Eng. Asp. 2023, 657, 130601. [Google Scholar] [CrossRef]
- Xiang, L.; Niu, C.-G.; Tang, N.; Lv, X.-X.; Guo, H.; Li, Z.-W.; Liu, H.-Y.; Lin, L.-S.; Yang, Y.-Y.; Liang, C. Polypyrrole coated molybdenum disulfide composites as adsorbent for enhanced removal of Cr(VI) in aqueous solutions by adsorption combined with reduction. Chem. Eng. J. 2021, 408, 127281. [Google Scholar] [CrossRef]
- Yin, X.; Li, Y.; Meng, H.; Wu, W. Surface functionalization of bulk MoS2 sheets for efficient liquid phase exfoliation in polar micromolecular solvents. Appl. Surf. Sci. 2019, 486, 362–370. [Google Scholar] [CrossRef]
- Mak, K.F.; Lee, C.; Hone, J.; Shan, J.; Heinz, T.F. Atomically thin MoS2: A new direct-gap semiconductor. Phys. Rev. Lett. 2010, 105, 136805. [Google Scholar] [CrossRef] [Green Version]
- Su, Q.; Wang, B.; Mu, C.; Zhai, K.; Nie, A.; Xiang, J.; Wen, F. Polypyrrole coated 3D flower MoS2 composites with tunable impedance for excellent microwave absorption performance. J. Alloys Compd. 2021, 888, 161487. [Google Scholar] [CrossRef]
- Yang, J.; Ye, M.; Han, A.; Zhang, Y.; Zhang, K. Preparation and electromagnetic attenuation properties of MoS2–PANI composites: A promising broadband absorbing material. J. Mater. Sci. Mater. Electron. 2019, 30, 292–301. [Google Scholar] [CrossRef]
- Chen, C.; He, Y.; Xiao, G.; Xia, Y.; Li, H.; He, Z. Two-dimensional hybrid materials: MoS2-RGO nanocomposites enhanced the barrier properties of epoxy coating. Appl. Surf. Sci. 2018, 444, 511–521. [Google Scholar] [CrossRef]
- Omastová, M.; Trchová, M.; Pionteck, J.; Prokeš, J.; Stejskal, J. Effect of polymerization conditions on the properties of polypyrrole prepared in the presence of sodium bis(2-ethylhexyl) sulfosuccinate. Synth. Met. 2004, 143, 153–161. [Google Scholar] [CrossRef]
- Wang, T.; Liu, W.; Tian, J.; Shao, X.; Sun, D. Structure characterization and conductive performance of polypyrrol-molybdenum disulfide intercalation materials. Polym. Compos. 2004, 25, 111–117. [Google Scholar] [CrossRef]
- Obaid, A.N.; Al-Bermany, E. Performance of functionalized graphene oxide to improve anti-corrosion of epoxy coating on 2024-T3 aluminium alloy. Mater. Chem. Phys. 2023, 305, 127849. [Google Scholar] [CrossRef]
- Wu, Y.; He, Y.; Chen, C.; Li, H.; Xia, Y.; Zhou, T. MoS2-CNFs composites to enhance the anticorrosive and mechanical performance of epoxy coating. Prog. Org. Coat. 2019, 129, 178–186. [Google Scholar] [CrossRef]
- Jing, Y.; Wang, P.; Yang, Q.; He, Y.; Bai, Y. The effect of a functionalized defect-rich molybdenum disulfide nanosheets on anticorrosion performance of epoxy coating. Mater. Res. Express 2019, 6, 086473. [Google Scholar] [CrossRef]
- Zhou, S.; Wu, Y.; Zhao, W.; Yu, J.; Jiang, F.; Ma, L. Comparative corrosion resistance of graphene sheets with different structures in waterborne epoxy coatings. Colloids Surf. A Physicochem. Eng. Asp. 2018, 556, 273–283. [Google Scholar] [CrossRef]
- Liu, S.; Wang, X.; Yin, Q.; Xiang, X.; Fu, X.-Z.; Wang, X.-Z.; Luo, J.-L. A facile approach to fabricating graphene/waterborne epoxy coatings with dual functionalities of barrier and corrosion inhibitor. J. Mater. Sci. Technol. 2022, 112, 263–276. [Google Scholar] [CrossRef]
- Xing, C.; Wang, W.; Qu, S.; Tang, Y.; Zhao, X.; Zuo, Y. Degradation of zinc-rich epoxy coating in 3.5% NaCl solution and evolution of its EIS parameters. J. Coat. Technol. Res. 2021, 18, 843–860. [Google Scholar] [CrossRef]



| Samples | Content Ratio | ||||||
|---|---|---|---|---|---|---|---|
| Components | 0 | 0.2% | 0.4% | 0.6% | 0.8% | 1% | |
| Composite EP coating | E51 (g) | 10 | 10 | 10 | 10 | 10 | 10 |
| D230 (g) | 3.2 | 3.2 | 3.2 | 3.2 | 3.2 | 3.2 | |
| Fillers (g) | 0 | 0.03 | 0.05 | 0.08 | 0.11 | 0.13 | |
| Samples | Content Ratio | ||||||
|---|---|---|---|---|---|---|---|
| 0 | 0.2% | 0.4% | 0.6% | 0.8% | 1% | ||
| MoS2@PPy | Ecorr (mV) | −539.949 | −238.370 | −243.606 | −223.766 | −219.787 | −249.52 |
| Icorr (µA/cm2) | 19.134 | 0.014 | 0.013 | 0.007 | 0.006 | 0.013 | |
| Rp (Ω) | 594 | 3.51 × 106 | 3.73 × 106 | 7.14 × 106 | 7.95 × 106 | 3.67 × 106 | |
| Corrosion rate (mm/year) | 0.144 | 2.34 × 10−5 | 1.51 × 10−4 | 1.05 × 10−5 | 9.92 × 10−6 | 2.33 × 10−5 | |
| MoS2 | Ecorr (mV) | −539.949 | −422.035 | −259.062 | −575.780 | −524.501 | −251.553 |
| Icorr (µA/cm2) | 19.134 | 0.070 | 0.049 | 12.900 | 9.839 | 0.054 | |
| KH560–MoS2 [35] | Ecorr (mV) | −490.1 | −437.1 | −456.8 | −417.6 | −445.2 | −466.7 |
| Icorr (µA/cm2) | 0.2239 | 0.1549 | 0.1622 | 0.1534 | 0.1413 | 0.1445 | |
| Samples | Content Ratio | ||||||
|---|---|---|---|---|---|---|---|
| 0 | 0.2% | 0.4% | 0.6% | 0.8% | 1% | ||
| 1 d MoS2@PPy | Rs (Ω·cm2) | 4.76 × 103 | 5.09 × 103 | 5.42 × 103 | 5.67 × 103 | 4.10 × 103 | 5.50 × 103 |
| Rc (Ω·cm2) | 8.80 × 106 | 2.97 × 107 | 3.17 × 107 | 5.95 × 107 | 6.89 × 107 | 3.36 × 107 | |
| CPEc (Ω−1·cm−2·sn) | 1.08 × 10−10 | 9.57 × 10−11 | 9.42 × 10−11 | 9.41 × 10−11 | 1.10 × 10−11 | 9.64 × 10−11 | |
| Samples | Content Ratio | ||||||
|---|---|---|---|---|---|---|---|
| 0 | 0.2% | 0.4% | 0.6% | 0.8% | 1% | ||
| 15 d MoS2@PPy | Rs (Ω·cm2) | 0.53 × 103 | 3.36 × 103 | 4.81 × 103 | 2.43 × 103 | 4.78 × 103 | 4.69 × 103 |
| Rc (Ω·cm2) | 146.6 | 7.66 × 105 | 3.32 × 106 | 1.45 × 106 | 1.58 × 106 | 9.75 × 105 | |
| CPEc (Ω−1·cm−2·sn) | 1.01 × 10−4 | 1.00 × 10−10 | 1.10 × 10−10 | 9.91 × 10−11 | 7.79 × 10−11 | 8.64 × 10−11 | |
| W (Ω·cm2) | 2.15 × 10−3 | 5.43 × 10−6 | 5.86 × 10−6 | 4.29 × 10−6 | 1.94 × 10−6 | 5.81 × 10−6 | |
| CPEdl (Ω−1·cm−2·sn) | 5.72 × 10−4 | 1.19 × 10−10 | 2.26 × 10−8 | 1.08 × 10−10 | 9.09 × 10−11 | 1.23 × 10−10 | |
| Rct (Ω·cm2) | 1.04 × 103 | 2.32 × 106 | 1.51 × 105 | 5.16 × 106 | 5.89 × 106 | 2.36 × 106 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Die, J.; Li, F.; Li, H.; Tu, J.; Zhang, K.; Yu, X. Polypyrrole-Modified Molybdenum Disulfide Nanocomposite Epoxy Coating Inhibits Corrosion of Mild Steel. Coatings 2023, 13, 1046. https://doi.org/10.3390/coatings13061046
Zhang Y, Die J, Li F, Li H, Tu J, Zhang K, Yu X. Polypyrrole-Modified Molybdenum Disulfide Nanocomposite Epoxy Coating Inhibits Corrosion of Mild Steel. Coatings. 2023; 13(6):1046. https://doi.org/10.3390/coatings13061046
Chicago/Turabian StyleZhang, Yafeng, Juncheng Die, Fei Li, Hai Li, Jinchun Tu, Kexi Zhang, and Xiaolong Yu. 2023. "Polypyrrole-Modified Molybdenum Disulfide Nanocomposite Epoxy Coating Inhibits Corrosion of Mild Steel" Coatings 13, no. 6: 1046. https://doi.org/10.3390/coatings13061046
APA StyleZhang, Y., Die, J., Li, F., Li, H., Tu, J., Zhang, K., & Yu, X. (2023). Polypyrrole-Modified Molybdenum Disulfide Nanocomposite Epoxy Coating Inhibits Corrosion of Mild Steel. Coatings, 13(6), 1046. https://doi.org/10.3390/coatings13061046






