1,4-Bis(trimethylsilyl)piperazine—Thermal Properties and Application as CVD Precursor
Abstract
1. Introduction
- (a)
- It is possible to change both the elemental composition with a wide range and the local environment of atoms of silicon carbonitrides by varying experimental conditions, including the gas-phase activation method (thermal, plasma, laser); the substrate material; the nature of the precursors, additional gases, and gas activator; the substrate temperature; the composition of the reaction gas mixture; the total pressure in the reactor chamber; as well as the parameters of the plasma generator (in the case of PECVD processes), the laser wavelength and power (in the case of LCVD processes), and other deposition parameters appropriate to a particular method. The desired properties of SiCxNy films are achieved by the optimization of the deposition process, including the choice of the proper precursor and deposition parameters.
- (b)
- Given that the properties of the films, in addition to the above parameters, are affected by the geometry of the reactor, it is interesting to identify the influence of the design of the precursors in a series of works in which the processes using various organosilicon compounds were studied with the same experimental setup [15,16,17,18,19,20,21,22].
- (c)
- Films obtained at low synthesis temperatures (below 300 °C) and a low input power of plasma are a polymer-like hydrogenated material. They contain organic functionalities, such as Si–CH3, CHx, NHx and Si–H bonds. The compositions of these films are described by the general formula of SiCxNy(Oz):H. The high input power or the high substrate temperature contribute to the destruction of organic groups and hydrogen-containing bonds and to the formation of a silicon carbonitride network, leading to the growth in the density of SiCxNy films [15].
- (d)
- High decomposition temperatures (≥500 °C) of the precursor promote the appearance of an additional phase of amorphous graphite-like carbon in the films.
- (e)
- The structure of the precursor has a strong influence on the composition and chemical structure of the films. The silicon-to-nitrogen ratio of the precursor molecule is also an important factor. For example, some studies were carried out with dimethylaminosilanes [HxMeySi(NMe2)4–x–y]. The authors [22] reported that in the case of using a precursor with a ratio of Si:N = 1:1, films close to SiC were obtained, and with a ratio of 1:3, SiNx-like films were produced.
- (f)
- The addition of N2 or NH3 to organosilicon compounds in the initial gas mixture was used in order to increase the [N]:[Si] ratio and to control the carbon content in the films.
- (g)
- The silicon carbonitride films contained up to 20 at.% of oxygen, which is believed to be due to the plasma etching of the walls of the quartz reactor, as well as the moisture or oxygen uptake during the CVD process and/or after exposure to air. An increase in the synthesis temperature contributes to a decrease in the oxygen content in the films.
- (h)
- Low-temperature SiCxNy(Oz):H films have a low dielectric constant, high transmittance (IR and visible regions of the spectrum) and a large band gap. High-temperature SiCxNy films possess high values for their hardness and Young’s modulus. A smooth change in the element composition of the films makes tuning the functional performance possible, such as the refractive index and band gap.
- (i)
- (j)
- A limited number of works are devoted to studying the aging and stability of the composition and functional properties of silicon carbonitride films during storage in air and upon annealing.
- (k)
- Very few works have been conducted on the study of the decomposition processes of organosilicon compounds in plasma. The information on mechanisms of monomer transformation in gas phase and film formation is limited. The plasma diagnostic provides the information on the species involved in the PECVD process, which is necessary both for controlling the elemental composition and the chemical bonding structure of films as well as for further improving the film deposition technology.
2. Materials and Methods
2.1. Materials
2.2. Synthesis
2.2.1. 1,4-Bis(trimethylsilyl)piperazine
2.2.2. Thin Film Preparation
2.3. Characterization
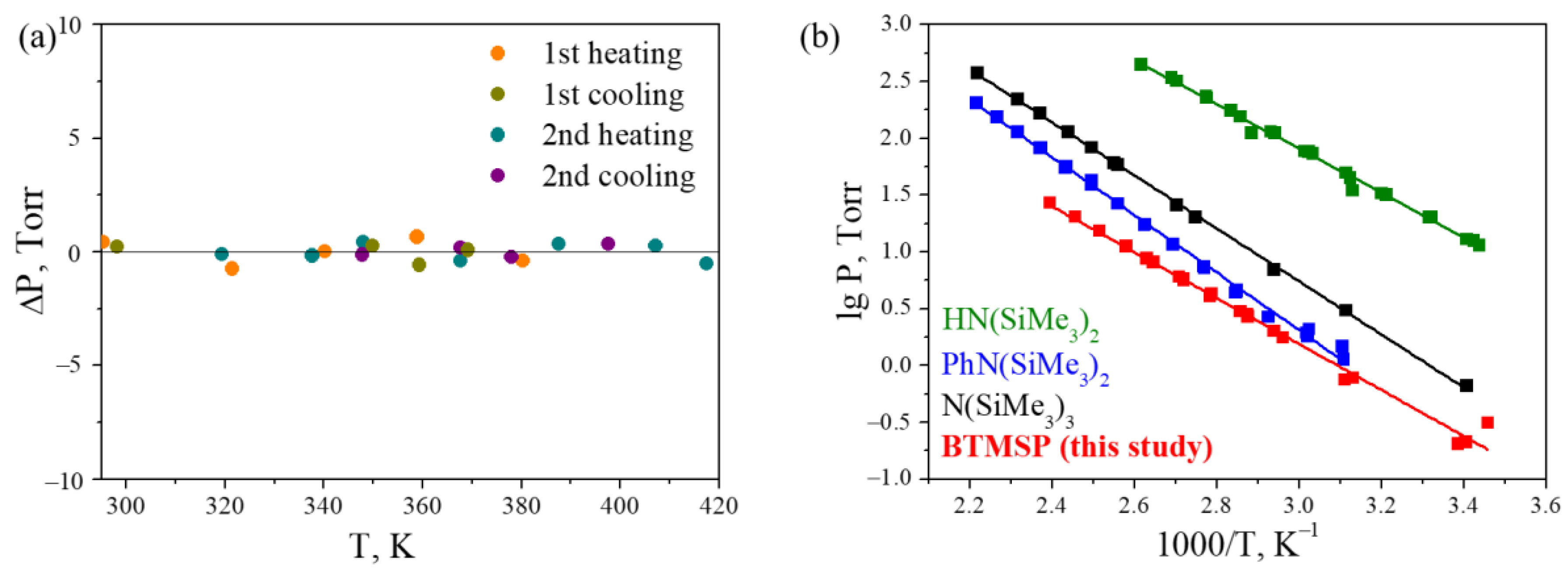
2.3.1. Volatility of BTMSP
2.3.2. Plasma Composition
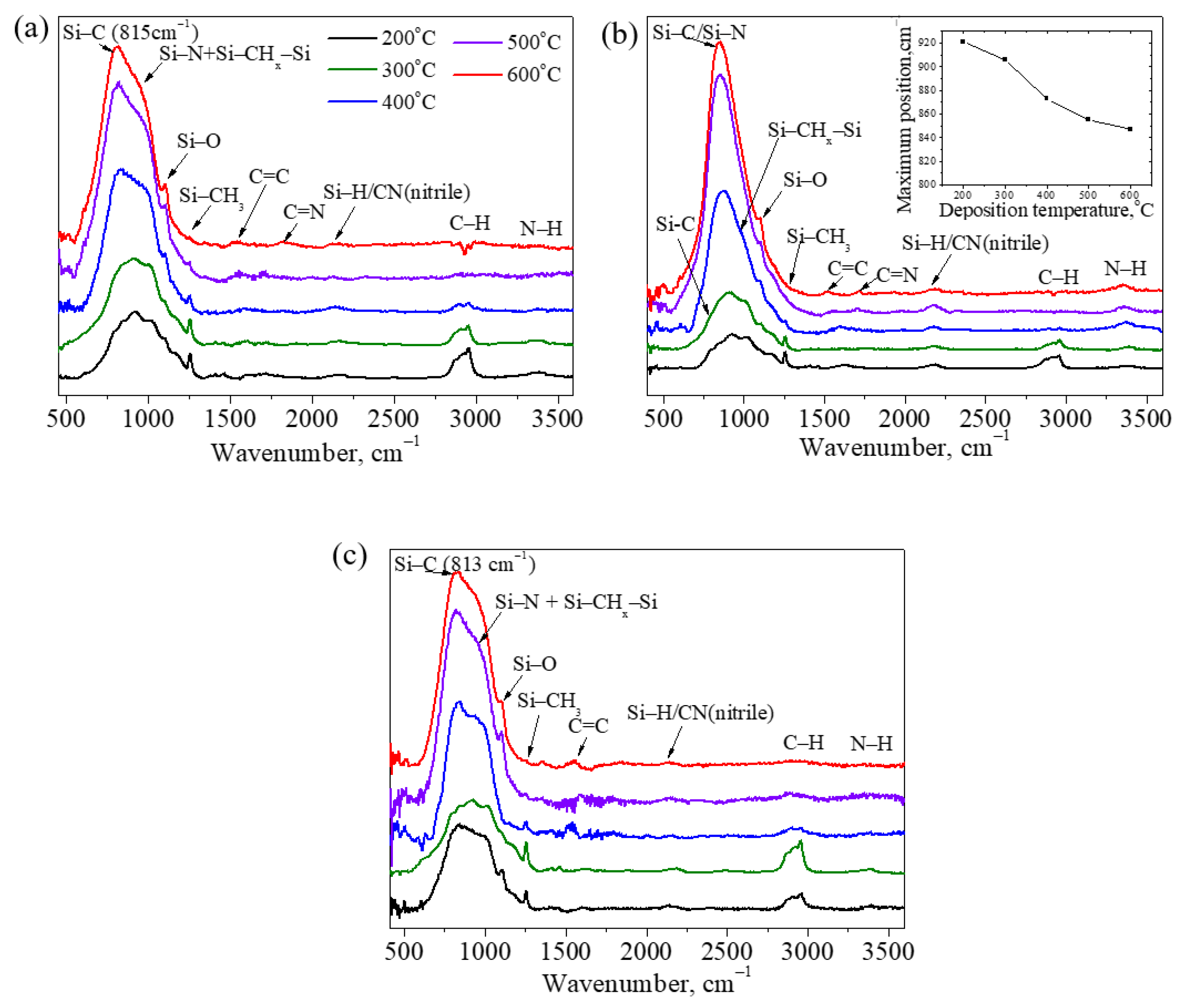
2.3.3. Chemical Bonding Structure and Elemental Composition of the Films
2.3.4. Thickness and Optical Properties
3. Results and Discussion
3.1. Volatility of BTMSP
3.2. Synthesis and Characterization of PECVD SiCN(H) Films
3.2.1. Plasma Characterization
3.2.2. Chemical Bonding Structure and Elemental Composition
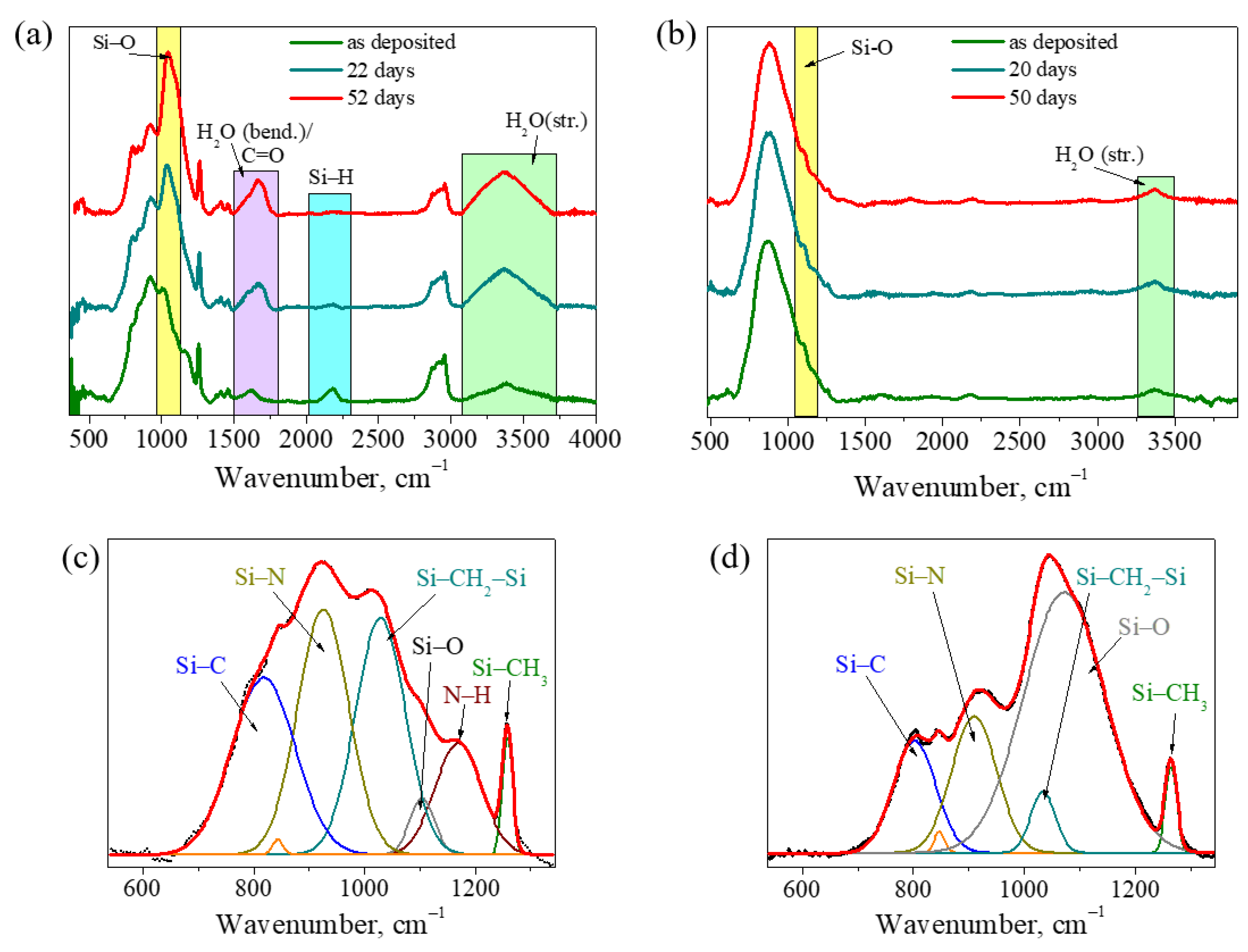
3.2.3. Evolution of Bonding Structure during Storage in Ambient Conditions
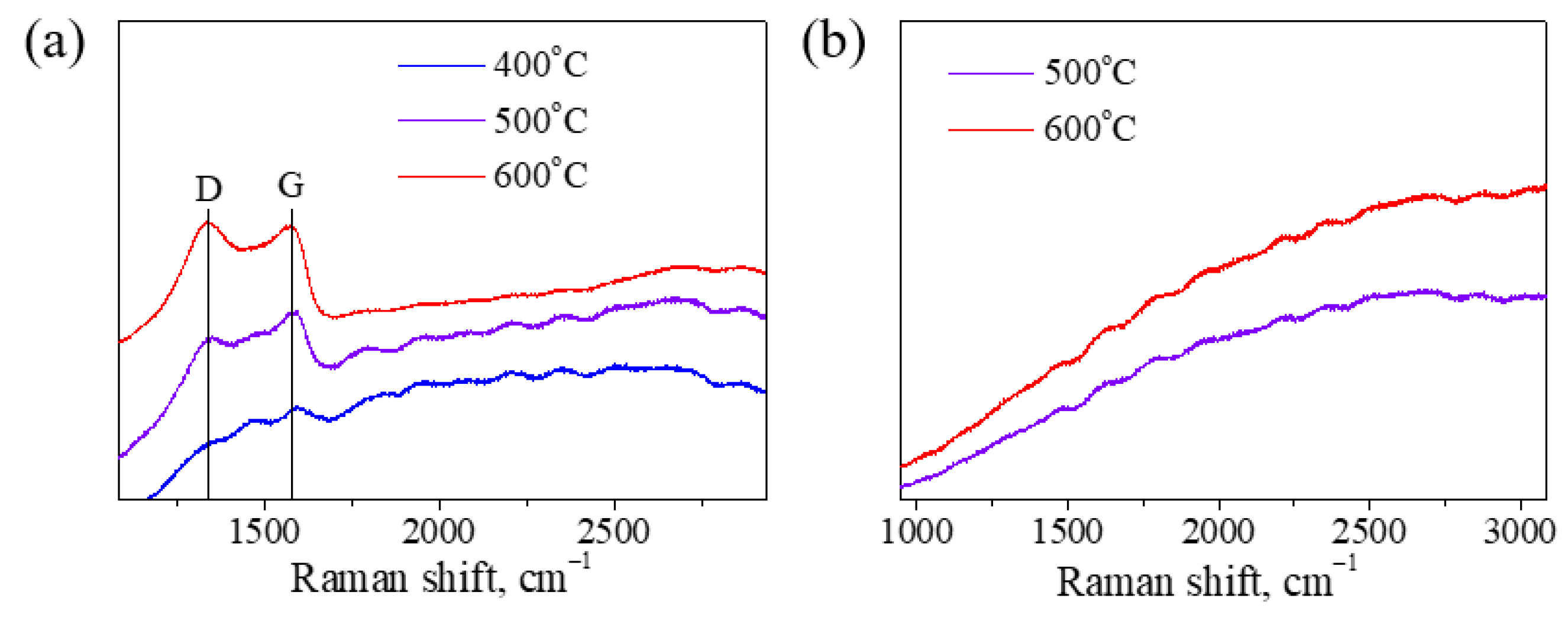
3.2.4. Deposition Rate and Surface Morphology
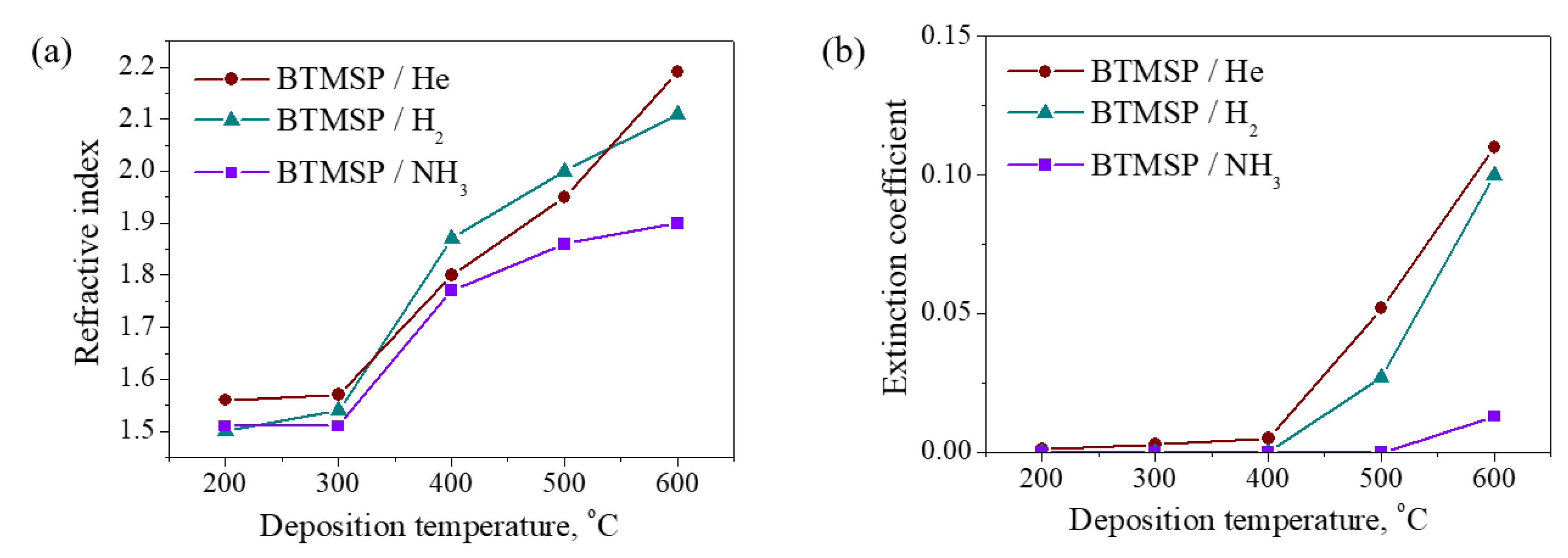
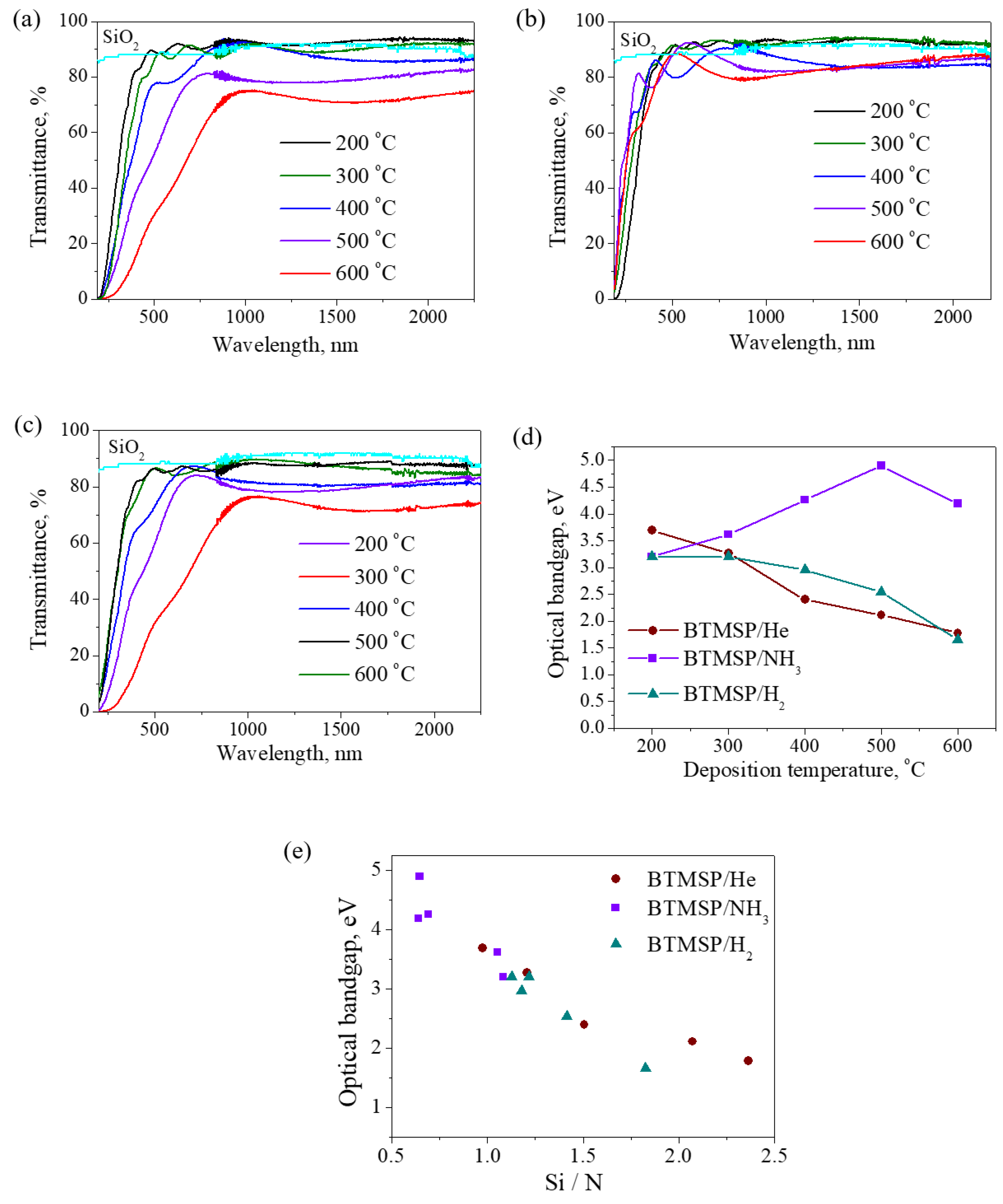
3.2.5. Optical Properties
4. Conclusions
- (i)
- A study of the volatility of the compound by static tensimetry showed that its saturated vapor pressure is comparable with that of similar organosilicon CVD precursors and it is sufficient for its use in film deposition processes.
- (ii)
- The BTMSP behavior under conditions of low-temperature plasma (25 W) studied by OES revealed that the monomer undergoes a fragmentation with the formation of H, CH and CN radicals. The decomposition is not complete, as no Si-containing species were detected. An appearance of CN radicals, registered by the OES method, could be regarded as the main way carbon is excluded from the monomer composition.
- (iii)
- SiCN(H) films were produced in the PECVD process using BTMSP as a precursor. It was possible to produce films with a tunable refractive index in the range of 1.5–2.2 and an optical band gap from 1.7 to 4.9 eV by changing the synthesis conditions and thus the composition of the films.
- (iv)
- The aging of the films during storage in ambient conditions was studied. It was shown that the low-temperature film (200 °C) underwent changes. The water adsorption and degradation of the Si–H, N–H and Si–CHx–Si bonds with the formation of the Si–O bond were observed. The film deposited at 400 °C was found to be more stable.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sha, B.; Lukianov, A.N.; Dusheiko, M.G.; Lozinskii, V.B.; Klyui, A.N.; Korbutyak, D.V.; Pritchin, S.E.; Klyui, N.I. Carbon-rich amorphous silicon carbide and silicon carbonitride films for silicon-based photoelectric devices and optical elements: Application from UV to mid-IR spectral range. Opt. Mater. 2020, 106, 109959. [Google Scholar] [CrossRef]
- Khatami, Z.; Bleczewski, L.; Neville, J.J.; Mascher, P. X-ray Absorption Spectroscopy of Silicon Carbide Thin Films Improved by Nitrogen for All-Silicon Solar Cells. ECS J. Solid State Sci. Technol. 2020, 9, 083002. [Google Scholar] [CrossRef]
- Khatami, Z.; Nowikow, C.; Wojcik, J.; Mascher, P. Annealing of silicon carbonitride nanostructured thin films: Interdependency of hydrogen content, optical, and structural properties. J. Mater. Sci. 2018, 53, 1497. [Google Scholar] [CrossRef]
- Chou, T.H.; Kuo, T.W.; Lin, C.Y.; Lai, F.S. A low cost n-SiCN/p-PS/p-Si heterojunction for high temperature ultraviolet detecting applications. Sens. Actuators A Phys. 2018, 279, 462. [Google Scholar] [CrossRef]
- Saloum, S.; Shaker, S.A.; Alkafri, M.N.; Obaid, A.; Hussin, R. Hydrogenated Silicon Carbonitride Thin Film Nanostructuring Using SF6 Plasma: Structural and Optical Analysis. Silicon 2020, 12, 2957. [Google Scholar] [CrossRef]
- Li, Q.; Chen, C.; Wang, M.; Lv, Y.; Mao, Y.; Xu, M.; Wang, Y.; Wang, X.; Zhang, Z.; Wang, S.; et al. Study on photoelectricity properties of SiCN thin films prepared by magnetron sputtering. J. Mater. Res. Technol. 2021, 15, 460. [Google Scholar] [CrossRef]
- Jiang, M.; Xu, K.; Liao, N.; Zheng, B. Effect of sputtering power on piezoresistivity and interfacial strength of SiCN thin films prepared by magnetic sputtering. Ceram. Int. 2022, 48, 2112. [Google Scholar] [CrossRef]
- Tanaka, I.; Matuoka, S.; Harada, Y. Mechanical properties of amorphous SiCN films deposited by ion-beam-assisted deposition. Diamond Relat. Mater. 2022, 121, 108732. [Google Scholar] [CrossRef]
- Li, Q.; Wang, Y.; Shan, X.; Shan, X.; Wang, X.; Zhao, W. Preparation and optical properties of SiCN thin films deposited by reactive magnetron sputtering. J. Mater. Sci.: Mater. Electron. 2017, 28, 6769. [Google Scholar] [CrossRef]
- Lavareda, G.; Vygranenko, Y.; Amaral, A.; Nunes de Carvalho, C.; Barradas, N.P.; Alves, E.; Brogueira, P. Dependence of optical properties on composition of silicon carbonitride thin films deposited at low temperature by PECVD. J. Non-Cryst. Solids 2021, 551, 120434. [Google Scholar] [CrossRef]
- Peter, S.; Ehrler, R.; Seyller, T.; Speck, F. Annealing effects on a-SiC:H and a-SiCN:H films deposited by plasma CVD methods. Vacuum 2020, 178, 109410. [Google Scholar] [CrossRef]
- Cheng, Y.L.; Lin, Y.L. Comparison of SiCxNy barriers using different deposition precursors capped on porous low-dielectric-constant SiOCH dielectric film. Thin Solid Films 2020, 702, 137983. [Google Scholar] [CrossRef]
- Plujat, B.; Glénat, H.; Bousquet, A.; Frézet, L.; Hamon, J.; Goullet, A.; Tomasella, E.; Hernandez, E.; Quoizola, S.; Thomas, L. SiCN:H thin films deposited by MW-PECVD with liquid organosilicon precursor: Gas ratio influence versus properties of the deposits. Plasma Process. Polym. 2020, 17, 1900138. [Google Scholar] [CrossRef]
- Ermakova, E.; Kosinova, M. Organosilicon compounds as single-source precursors for SiCN films production. J. Organomet. Chem. 2022, 958, 122183. [Google Scholar] [CrossRef]
- Wrobel, A.M.; Uznanski, P. Hard silicon carbonitride thin-film coatings produced by remote hydrogen plasma chemical vapor deposition using aminosilane and silazane precursors. 1: Deposition mechanism, chemical structure, and surface morphology. Plasma Process. Polym. 2021, 18, e2000240. [Google Scholar] [CrossRef]
- Wrobel, A.M.; Uznanski, P. Hard silicon carbonitride thin-film coatings by remote hydrogen plasma chemical vapor deposition using aminosilane and silazane precursors. 2: Physical, optical, and mechanical properties of deposited films. Plasma Process. Polym. 2021, 18, 2000241. [Google Scholar] [CrossRef]
- Wrobel, A.M.; Walkiewicz-Pietrzykowska, A.; Blaszczyk-Lezak, I. Reactivity of organosilicon precursors in remote hydrogen microwave plasma chemical vapor deposition of silicon carbide and silicon carbonitride thin-film coatings. Appl. Organometal. Chem. 2010, 24, 201. [Google Scholar] [CrossRef]
- Du, L.; Bai, Y.; Chu, W.; Ding, Y. Synthesis of two aminosilanes as CVD precursors of SiCxNy films: Tuning film composition by Molecular Structures. Phosphorus Sulfur Silicon Relat. Elem. 2018, 193, 568. [Google Scholar] [CrossRef]
- Chang, W.Y.; Chang, C.Y.; Leu, J. Optical properties of plasma-enhanced chemical vapor deposited SiCxNy films by using silazane precursors. Thin Solid Films 2017, 636, 671. [Google Scholar] [CrossRef]
- Fainer, N.I.; Nemkova, A.A. Optical Properties of Silicon Carbonitride Films Produced by Plasma-Induced Decomposition of Organic Silicon Compounds. High Energ. Chem. 2015, 49, 273. [Google Scholar] [CrossRef]
- Imada, T.; Nakata, Y.; Ozaki, S.; Kobayashi, Y.; Nakamura, T. Systematic investigation of silylation materials for recovery use of low-k material plasma damage. Jpn. J. Appl. Phys. 2015, 54, 071502. [Google Scholar] [CrossRef]
- Di Mundo, R.; Ricci, M.; d’Agostino, R.; Fracassi, F.; Palumbo, F. PECVD of Low Carbon Content Silicon Nitride-Like Thin Films with Dimethylaminosilanes. Plasma Process. Polym. 2007, 4, S21. [Google Scholar] [CrossRef]
- Di Mundo, R.; Palumbo, F.; Fracassi, F.; d’Agostino, R. Methylaminosilane fed Inductively Coupled Plasmas for Silicon Nitride Deposition. Plasma Process. Polym. 2008, 5, 770. [Google Scholar] [CrossRef]
- Belmahi, M.; Bulou, S.; Thouvenin, A.; de Poucques, L.; Hugon, R.; Brizoual, L.L.; Miska, P.; Geneve, D.; Vasseur, J.-L.; Bougdira, J. Microwave Plasma Process for SiCN:H Thin Films Synthesis with Composition Varying from SiC:H to SiN:H in H2/N2/Ar/Hexamethyldisilazane Gas Mixture. Plasma Process. Polym. 2014, 11, 551. [Google Scholar] [CrossRef]
- Chagin, M.N.; Sulyaeva, V.S.; Shayapov, V.R.; Kolodin, A.N.; Khomyakov, M.N.; Yushina, I.V.; Kosinova, M.L. Synthesis, Properties and Aging of ICP-CVD SiCxNy:H Films Formed from Tetramethyldisilazane. Coatings 2022, 12, 80. [Google Scholar] [CrossRef]
- Wrobel, A.M.; Błaszczyk, I.; Walkiewicz-Pietrzykowska, A.; Tracz, A.; Klemberg-Sapieha, J.E.; Aokic, T.; Hatanaka, Y. Remote hydrogen–nitrogen plasma chemical vapor deposition from a tetramethyldisilazane source. Part 1. Mechanism of the process, structure and surface morphology of deposited amorphous hydrogenated silicon carbonitride films. J. Mater. Chem. 2003, 13, 731. [Google Scholar] [CrossRef]
- Bulou, S.; Le Brizoual, L.; Miska, P.; de Poucques, L.; Bougdira, J.; Belmahi, M. Wide variations of SiCxNy:H thin films optical constants deposited by H2/N2/Ar/hexamethyldisilazane microwave plasma. Surf. Coat. Technol. 2012, 208, 46. [Google Scholar] [CrossRef]
- Di Mundo, R.; Palumbo, F.; Fracassi, F.; d’Agostino, R. Thin Film Deposition in Capacitively Coupled Plasmas Fed with Bis(dimethylamino)-dimethylsilane and Oxygen: An FTIR study. Plasma Process. Polym. 2009, 6, 506. [Google Scholar] [CrossRef]
- Ermakova, E.N.; Sysoev, S.V.; Nikulina, L.D.; Tsyrendorzhieva, I.P.; Rakhlin, V.I.; Kosinova, M.L. Synthesis and characterization of organosilicon compounds as novel precursors for CVD processes. Thermochim. Acta 2015, 622, 2. [Google Scholar] [CrossRef]
- Davydova, E.I.; Sevastianova, T.N.; Suvorov, A.V.; Timoshkin, A.Y. Molecular complexes formed by halides of group 4,5,13–15 elements and the thermodynamic characteristics of their vaporization and dissociation found by the static tensimetric method. Coord. Chem. Rev. 2010, 254, 203. [Google Scholar] [CrossRef]
- Kuznetsov, F.A.; Titov, V.A.; Titov, A.A.; Chernyavskii, L.I. Data bank of properties of microelectronic materials. In Proceedings of the International Symposium on Advanced Materials, Tsukuba, Japan, 24–30 September 1995; pp. 24–30. [Google Scholar]
- Kramida, A.; Ralchenko, Y.; Reader, J.; NIST ASD Team. NIST Atomic Spectra Database (ver. 5.10); National Institute of Standards and Technology, Gaithersburg, MD, USA. 2022. Available online: https://physics.nist.gov/asd (accessed on 15 October 2022).
- Cui, J.H.; Xu, Z.F.; Zhang, J.L.; Nie, Q.Y.; Xu, G.H.; Ren, L.L. Online diagnosis of electron excitation temperature in CH4+H2 discharge plasma at atmospheric pressure by optical emission spectra. Sci. China Ser. G Phys. Mech. Astron 2008, 51, 1892. [Google Scholar] [CrossRef]
- Tauc, J.; Grigorovici, R.; Vancu, A. Optical Properties and Electronic Structure of Amorphous Germanium. Phys. Stat. Sol. B 1966, 15, 627. [Google Scholar] [CrossRef]
- Sysoev, S.V.; Nikulina, L.D.; Kolontaeva, A.O.; Kosinova, M.L.; Titov, A.A.; Rakhlin, V.I.; Tsyrendorzhieva, I.P.; Lis, A.V.; Voronkov, M.G. N-substituted hexamethyldisilazanes as new substances for the synthesis of functional films in the system Si-Ge-C-N-H. Russ. J. Gen. Chem. 2011, 81, 2501. [Google Scholar] [CrossRef]
- Fantz, U. Basics of plasma spectroscopy. Plasma Sources Sci. Technol. 2006, 15, S137. [Google Scholar] [CrossRef]
- Ivashchenko, V.I.; Porada, O.K.; Kozak, A.O.; Manzhara, V.S.; Sinelnichenko, O.K.; Ivashchenko, L.A.; Shevchenko, R.V. An effect of hydrogenation on the photoemission of amorphous SiCN films. Int. J. Hydrogen Energy 2022, 47, 7263. [Google Scholar] [CrossRef]
- Silva, J.A.; Quoizola, S.; Hernandez, E.; Thomas, L.; Massines, F. Silicon carbon nitride films as passivation and antireflective coatings for silicon solar cells. Surf. Coat. Technol. 2014, 242, 157. [Google Scholar] [CrossRef]
- Matsutani, T.; Tai, Y.; Kawasaki, T. Nitrogen ion beam thinning of a-SiCN diaphragm for environmental cell prepared by low-energy ion beam enhanced chemical vapor deposition. Vacuum 2020, 182, 109770. [Google Scholar] [CrossRef]
- Wrobel, A.M.; Walkiewicz-Pietrzykowska, A.; Uznanski, P.; Glebocki, B. a-SiC:H Films by Remote Hydrogen Microwave Plasma CVD From Ethylsilane Precursors. Chem. Vap. Depos. 2013, 19, 242–250. [Google Scholar] [CrossRef]
- Li, Q.; Chen, C.; Xu, M.; Wang, Y.; Wang, X.; Zhang, Z.; Zhao, W.; Stiens, J. Blue-violet emission of silicon carbonitride thin films prepared by sputtering and annealing treatment. Appl. Surf. Sci. 2021, 546, 149121. [Google Scholar] [CrossRef]
- Wrobel, A.M.; Uznanski, P.; Walkiewicz-Pietrzykowska, A.; Glebocki, B.; Bryszewska, E. Silicon Oxycarbide Thin Films by Remote Microwave Hydrogen Plasma CVD Using a Tetramethyldisiloxane Precursor. Chem. Vap. Depos. 2015, 21, 88. [Google Scholar] [CrossRef]
- Zhuang, C.; Schlemper, C.; Fuchs, R.; Zhang, L.; Huang, N.; Vogel, M.; Staedler, T.; Jiang, X. Mechanical behavior related to various bonding states in amorphous Si–C–N hard films. Surf. Coat. Technol. 2014, 258, 353. [Google Scholar] [CrossRef]
- Langford, A.A.; Fleet, M.L.; Nelson, B.P.; Lanford, W.A.; Maley, N. Infrared absorption strength and hydrogen content of hydrogenated amorphous silicon. Phys. Rev. B 1992, 45, 13367. [Google Scholar] [CrossRef]
- Kozak, A.O.; Ivashchenko, V.I.; Porada, O.K.; Ivashchenko, L.A.; Tomila, T.V.; Manjara, V.S.; Klishevych, G.V. Structural, optoelectronic and mechanical properties of PECVD Si-C-N films: An effect of substrate bias. Mater. Sci. Semicon. Proc. 2018, 88, 65. [Google Scholar] [CrossRef]
- Wilamowska, M.; Graczyk-Zajac, M.; Riedel, R. Composite materials based on polymer-derived SiCN ceramic and disordered hard carbons as anodes for lithium-ion batteries. J. Power Sources 2013, 244, 80. [Google Scholar] [CrossRef]
- Bokobza, L.; Bruneel, J.-L.; Couzi, M. Raman Spectra of Carbon-Based Materials (from Graphite to Carbon Black) and of Some Silicone Composites. J. Carbon Res. 2015, 1, 77–94. [Google Scholar] [CrossRef]
- Wrobel, A.M. Aging process in plasma-polymerized organosilicon thin films. J. Macromol. Sci.-Chem. 1985, 22, 1089. [Google Scholar] [CrossRef]
- Huber, C.; Stein, B.; Kalt, H. Plasma-enhanced chemical vapor deposition of amorphous silicon carbonitride: Deposition temperature dependence of bonding structure, refractive index, mechanical stress and their aging under ambient air. Thin Solid Films 2017, 634, 66. [Google Scholar] [CrossRef]
- Jiang, L.; Tian, H.; Li, J.; Xiang, P.; Peng, Y.; Wang, T.; Hou, P.; Xiao, T.; Tan, X. The influence of NH3 flow rate on the microstructure and oxidation properties of a-Si-C-N:H films prepared by PECVD technology. Appl. Surf. Sci. 2020, 513, 145861. [Google Scholar] [CrossRef]
- Rahman, M.A.A.; Goh, B.T.; Chiu, W.S.; Haw, C.Y.; Mahmood, M.R.; Khiew, P.S.; Rahman, S.A. Aging- and thermal-annealing effects on the vibrational- and microstructural-properties of PECVD grown hydrogenated amorphous silicon carbon nitride thin films. Vib. Spectrosc. 2018, 94, 22. [Google Scholar] [CrossRef]
- Riedel, R.; Kleebe, H.J.; Schönfelder, H.; Aldinger, F. A covalent micro/nano-composite resistant to high-temperature oxidation. Nature 1995, 374, 526. [Google Scholar] [CrossRef]
- Vandenbossche, M.; Hegemann, D. Recent approaches to reduce aging phenomena in oxygen- and nitrogen-containing plasma polymer films: An overview. Curr. Opin. Solid St. M. 2018, 22, 26. [Google Scholar] [CrossRef]
- Siow, K.S.; Brichter, L.; Kumar, S.; Griesser, H.J. Plasma methods for the generation of chemically reactive surfaces for biomolecule immobilization and cell colonization—A review. Plasma Process. Polym. 2006, 3, 392. [Google Scholar] [CrossRef]
- Chatelier, R.C.; Xie, X.; Gengenbach, T.R.; Griesser, H.J. Quantitative analysis of polymer surface restructuring. Langmuir 1995, 11, 2576. [Google Scholar] [CrossRef]
- Holländer, A.; Thome, J. Chapter 7—degradation and stability of plasma polymers. In Plasma Polymer Films; Biederman, H., Ed.; Imperial College Press: London, UK, 2004; pp. 247–277. [Google Scholar]
- Girard-Lauriault, P.-L.; Retzko, I.; Swaraj, S.; Matsubayashi, N.; Gross, T.; Mix, R.; Unger, W.E.S. Non-destructive sub-surface chemical characterization of airexposed plasma polymers by energy-resolved XPS. Plasma Process. Polym. 2010, 7, 474. [Google Scholar] [CrossRef]
- Guruvenket, S.; Andrie, S.; Simon, M.; Johnson, K.W.; Sailer, R.A. Atmospheric Pressure Plasma CVD of Amorphous Hydrogenated Silicon Carbonitride (a-SiCN:H) Films Using Triethylsilane and Nitrogen. Plasma Process. Polym. 2011, 8, 1126. [Google Scholar] [CrossRef]
- Sinha, A.K.; Lugujjo, E. Lorentz-Lorenz correlation for reactively plasma deposited Si-N films. Appl. Phys. Lett. 1978, 32, 245. [Google Scholar] [CrossRef]
- Jedrzejowski, P.; Cizek, J.; Amassian, A.; Klemberg-Sapieha, J.E.; Vlcek, J.; Martinu, L. Mechanical and optical properties of hard SiCN coatings prepared by PECVD. Thin Solid Films 2004, 447–448, 201–207. [Google Scholar] [CrossRef]
- Sobczyk-Guzenda, A.; Oleśko, K.; Gazicki-Lipman, M.; Szymański, W.; Balcerzak, J.; Wendler, B.; Szymanowski, H. Chemical structure and optical properties of a-SiNC coatings synthesized from different disilazane precursors with the RF plasma enhanced CVD technique—A comparative study. Mater. Res. Express 2019, 6, 016410. [Google Scholar] [CrossRef]
- Guruvenket, S.; Andrie, S.; Simon, M.; Johnson, K.W.; Sailer, R.A. Atmospheric pressure plasma-enhanced chemical vapor deposition of a-SiCN:H films: Role of precursors on the film growth and properties. Appl. Mater. Interfaces 2012, 4, 5293. [Google Scholar] [CrossRef] [PubMed]
- Ermakova, E.; Mogilnikov, K.; Asanov, I.; Fedorenko, A.; Yushina, I.; Kichay, V.; Maksimovskiy, E.; Kosinova, M. Chemical Structure, Optical and Dielectric Properties of PECVD SiCN Films Obtained from Novel Precursor. Coatings 2022, 12, 1767. [Google Scholar] [CrossRef]
- Ermakova, E.; Kolodin, A.; Fedorenko, A.; Yushina, I.; Shayapov, V.; Maksimovskiy, E.; Kosinova, M. Controlling of Chemical Bonding Structure, Wettability, Optical Characteristics of SiCN:H (SiC:H) Films Produced by PECVD Using Tetramethylsilane and Ammonia Mixture. Coatings 2023, 13, 310. [Google Scholar] [CrossRef]
- Chang, W.Y.; Chen, W.Z.; Lee, H.T.; Leu, J. Embedded carbon bridges in low-k PECVD silicon carbonitride films using silazane precursors. Jpn. J. Appl. Phys. 2019, 58, SHHB01. [Google Scholar] [CrossRef]
- Khatami, Z.; Simpson, P.J.; Mascher, P. Process-dependent mechanical and optical properties of nanostructured silicon carbonitride thin films. Nanotechnology 2019, 30, 14003. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.W.; Huang, C.C.; Lin, Y.Y.; Chen, L.C.; Chen, K.H. The affinity of Si–N and Si–C bonding in amorphous silicon carbon nitride (a-SiCN) thin film. Diamond Relat. Mater. 2005, 14, 1126. [Google Scholar] [CrossRef]
- Peng, X.; Song, L.; Meng, J.; Zhang, Y.; Hu, X. Preparation of silicon carbide nitride thin films by sputtering of silicon nitride target. Appl. Surf. Sci. 2001, 173, 313. [Google Scholar] [CrossRef]
- Malhotra, A.K.; Neudeck, G.W. Effects of hydrogen contamination on the localized states in amorphous silicon. Appl. Phys. Lett. 1976, 28, 47. [Google Scholar] [CrossRef]
- Khatami, Z.; Bosco, G.B.F.; Wojcik, J.; Tesslerand, L.R.; Mascher, P. Influence of Deposition Conditions on the Characteristics of Luminescent Silicon Carbonitride Thin Films. ECS J. Solid State Sci. Technol. 2018, 7, N7. [Google Scholar] [CrossRef]
- Plujat, B.; Glénat, H.; Hamon, J.; Gazal, Y.; Goullet, A.; Hernandez, E.; Quoizola, S.; Thomas, L. Near-field scanning microscopy and physico-chemical analysis versus time of SiCN:H thin films grown in Ar/NH3/TMS gas mixture using MW-Plasma CVD at 400 °C. Plasma Process. Polym. 2018, 15, e1800066. [Google Scholar] [CrossRef]
- Chen, C.W.; Lee, M.-H.; Chen, L.C.; Chen, K.H. Structural and electronic properties of wide band gap silicon carbon nitride materials—A first-principles study. Diamond Relat. Mater. 2004, 13, 1158. [Google Scholar] [CrossRef]
- Zhao, G.L.; Bachlechner, M.E. Electronic structure, charge distribution, and charge transfer in α- and β-Si3N4 and at the Si(111)/Si3N4 interface. Phys Rev. B 1998, 58, 1887. [Google Scholar] [CrossRef]
- Dong, H.; Chen, K.; Yang, H.; Ma, Z.; Xu, J.; Li, W.; Yu, L.; Huang, X. Innovative all-silicon based a-SiNx:O/c-Si heterostructure solar-blind photodetector with both high responsivity and fast response speed. APL Photonics 2022, 7, 026102. [Google Scholar] [CrossRef]
- Nguyen, H.H.; Jayapal, R.; Dang, N.S.; Nguyen, V.D.; Trinh, T.T.; Jang, K.; Yi, J. Investigation of charge storage and retention characteristics of silicon nitride in NVM based on InGaZnO channels for system-on-panel applications. Microelectron. Eng. 2012, 98, 34. [Google Scholar] [CrossRef]
- Fainer, N.I.; Kosinova, M.L.; Rumyantsev, Y.M.; Maximovskii, E.A.; Kuznetsov, F.A. Thin silicon carbonitride films are perspective low-k materials. J. Phys. Chem. Solids 2008, 69, 661. [Google Scholar] [CrossRef]
- Jana, S.; Das, S.; De, D.; Gangopadhyay, U.; Ghosh, P.; Mondal, A. Effect of annealing on structural and optical properties of diamond-like nanocomposite thin films. Appl. Phys. A 2014, 114, 965. [Google Scholar] [CrossRef]
- De Falco, G.; Commodo, M.; Bonavolontà, C.; Pepe, G.P.; Minutolo, P.; D’Anna, A. Optical and electrical characterization of carbon nanoparticles produced in laminar premixed flames. Combust. Flame 2014, 161, 3201. [Google Scholar] [CrossRef]
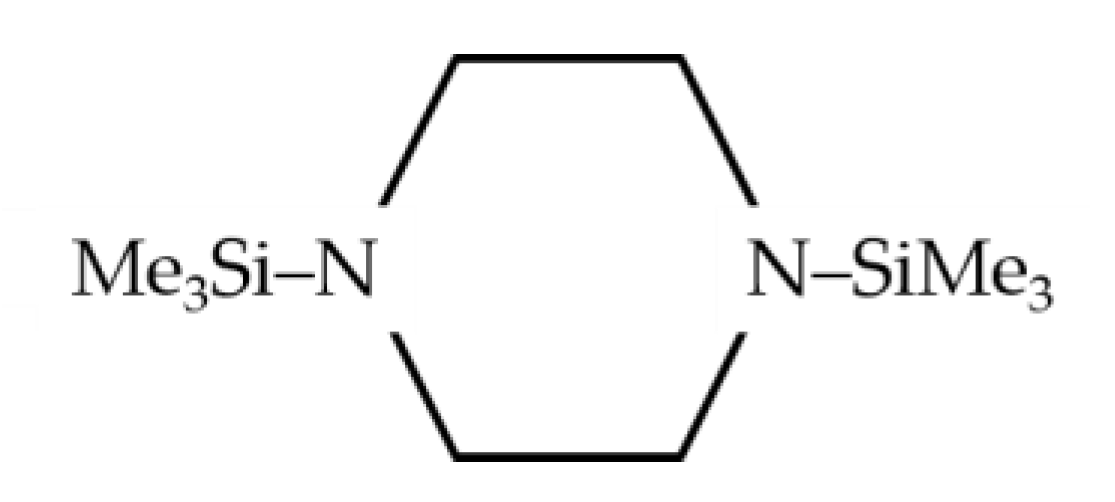
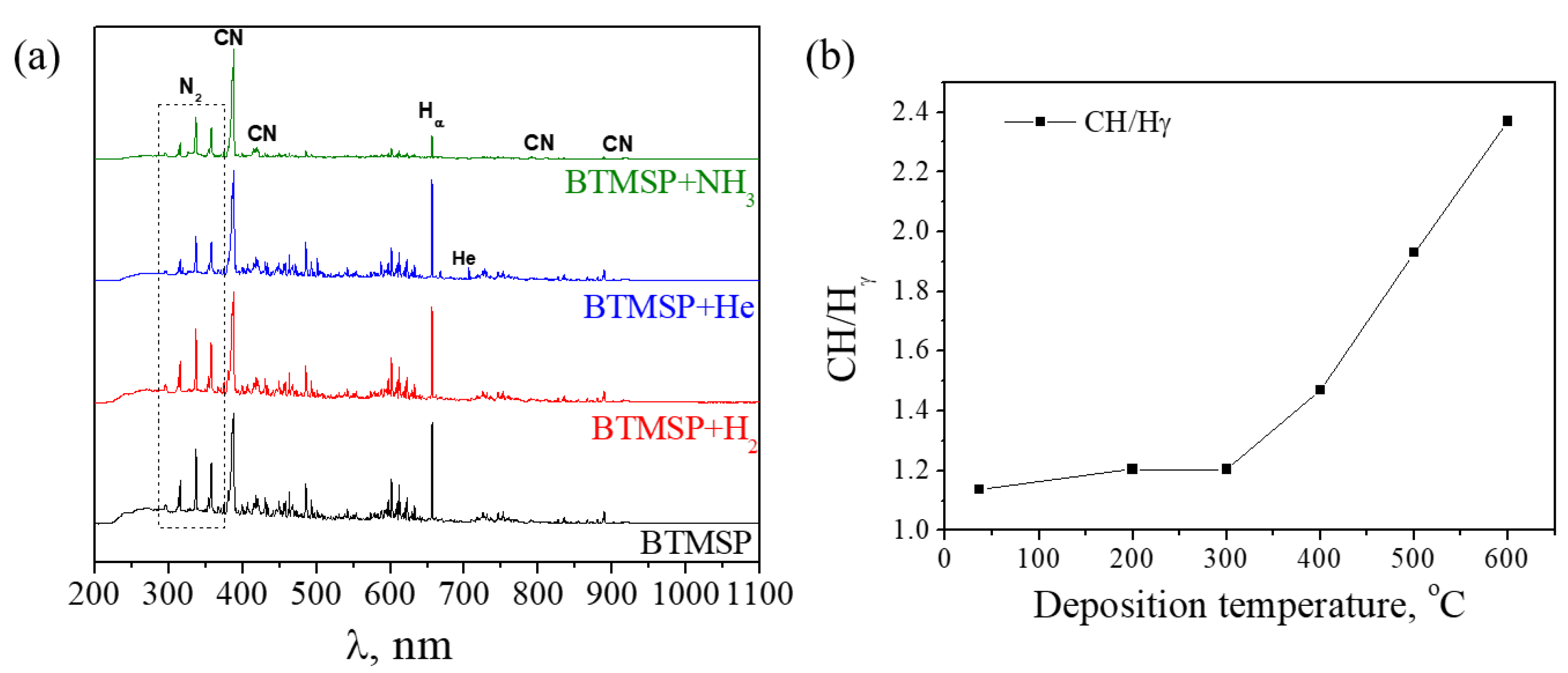


| Precursors | Additional Gases | CVD Process | Plasma Parameters. Frequency, Power | Plasma-Phase Composition | Ref. | |
|---|---|---|---|---|---|---|
| IR Spectroscopy | OES | |||||
| 1,1,1,3,3,3-Hexamethyl-disilazane (Me3Si)2NH | H2 + N2 + Ar | MW CVD | 2.45 GHz 600 W | CH4, C2H2, HCN | CN, NH | [24,27] |
| 1,1,3,3-Tetramethyldisilazane (Me2HSi)2NH | N2 + Ar | ICP CVD | 13.56 MHz 50–400 W | - | CN, NH, H, N2, Si, Ar | [25] |
| Bis(dimethylamino)- dimethylsilane Me2Si(NMe2)2 | - | ICP CVD | 13.56 MHz 700 W | CH4, HCN, C2H2, CO2 | H, CH, H2, CN, NH, N2 | [23] |
| Bis(dimethylamino)- dimethylsilane Me2Si(NMe2)2 | Ar | ICP CVD | 13.56 MHz 700 W | - | H, CH, H2, CN, NH, N2, Si | [23] |
| Bis(dimethylamino)- dimethylsilane Me2Si(NMe2)2 | Ar | ICP CVD | 13.56 MHz 500 W | ≈25% monomer (fragments containing Si–N, Si–Me and N–Me), CH4, C2H2, HCN, (NH) | - | [28] |
| Bis(dimethylamino)- dimethylsilane Me2Si(NMe2)2 | Ar (NH3) | ICP CVD | 13.56 MHz | - | CN, NH, CO, OH, O, N2, H2 | [22] |
| Bis(dimethylamino)- Piperazine Me3SiN(CH2CH2)2NSiMe3 | He (NH3) | RF PECVD | 40.68 MHz 25 W | - | CN, H, H2, CH, N2 | This study |
| Series | Temperature, K | Saturated Vapor Pressure, Pa |
|---|---|---|
| First heating | 295.4 | 155 |
| 321.5 | 567 | |
| 340.3 | 1521 | |
| 358.9 | 3242 | |
| 380.3 | 6645 | |
| First cooling | 369.2 | 4592 |
| 359.3 | 3126 | |
| 349.9 | 2272 | |
| 289.2 | 237 | |
| Second heating | 319.4 | 589 |
| 337.7 | 1341 | |
| 348.0 | 2132 | |
| 367.7 | 4293 | |
| 387.6 | 8541 | |
| 407.2 | 15,450 | |
| 417.6 | 20,603 | |
| Second cooling | 397.6 | 11,641 |
| 378.0 | 6170 | |
| 367.7 | 4368 | |
| 347.8 | 2042 | |
| 293.7 | 161 |
| Additional Gas | T | Deposition Rate, nm/min | Optical Properties | Elemental Composition, at.% | |||||
|---|---|---|---|---|---|---|---|---|---|
| n | k | Eg | Si | C | N | O | |||
| He | 200 | 28 | 1.56 | 0.001 | 3.7 | 14 | 61 | 14 | 11 |
| 300 | 21 | 1.57 | 0.003 | 3.3 | 18 | 57 | 16 | 9 | |
| 400 | 9 | 1.80 | 0.005 | 2.4 | 24 | 55 | 16 | 5 | |
| 500 | 6 | 1.95 | 0.052 | 2.1 | 25 | 61 | 12 | 3 | |
| 600 | 6 | 2.19 | 0.11 | 1.8 | 24 | 64 | 10 | 2 | |
| NH3 | 200 | 17 | 1.51 | 0 | 3.2 | 13 | 54 | 12 | 21 |
| 300 | 16 | 1.51 | 0 | 3.6 | 20 | 40 | 19 | 21 | |
| 400 | 11 | 1.77 | 0 | 4.3 | 29 | 19 | 43 | 9 | |
| 500 | 5 | 1.86 | 0 | 4.9 | 33 | 13 | 51 | 3 | |
| 600 | 4 | 1.9 | 0.013 | 4.2 | 32 | 15 | 50 | 3 | |
| H2 | 200 | 22 | 1.5 | 0 | 3.2 | 14 | 59 | 13 | 14 |
| 300 | 11 | 1.54 | 0 | 3.2 | 16 | 54 | 16 | 14 | |
| 400 | 4 | 1.87 | 0 | 1.5 | 24 | 50 | 20 | 6 | |
| 500 | 5 | 2 | 0.027 | 3.0 | 24 | 55 | 17 | 4 | |
| 600 | 5 | 2.11 | 0.1 | 1.7 | 24 | 58 | 14 | 4 | |
| Vibration | Band Position (This Study), cm−1 | Band Position (Literature Data), cm−1 | Ref. |
|---|---|---|---|
| Si–C stretching | 816 | 796–805 | [3,37] |
| Si–N stretching Si–N in SiN4 configuration | 926 850 | 911–940 850 | [3,37] [38] |
| Si–CHx–Si wagging | 1020 | 990–1020 | [39,40] |
| Si–O stretching | 1105 | 1070 | [28] |
| N–H bending | 1170 | 1156–1185 | [15,41] |
| Si–CH3 symmetric bending | 1255 | 1260–1264 | [38,42] |
| C=C/C=N stretching | 1600 | 1500 (C=C) 1600 (C=N) | [43] |
| Si–H stretching | 2110 | 2000–2100 | [44] |
| C≡N | 2180 | 2200 | [39] |
| C–H stretching | 2800–3000 | 2865–2953 | [37,45] |
| N–H stretching | 3200–3400 | 3370–3385 | [37,45] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ermakova, E.; Sysoev, S.; Tsyrendorzhieva, I.; Mareev, A.; Maslova, O.; Shayapov, V.; Maksimovskiy, E.; Yushina, I.; Kosinova, M. 1,4-Bis(trimethylsilyl)piperazine—Thermal Properties and Application as CVD Precursor. Coatings 2023, 13, 1045. https://doi.org/10.3390/coatings13061045
Ermakova E, Sysoev S, Tsyrendorzhieva I, Mareev A, Maslova O, Shayapov V, Maksimovskiy E, Yushina I, Kosinova M. 1,4-Bis(trimethylsilyl)piperazine—Thermal Properties and Application as CVD Precursor. Coatings. 2023; 13(6):1045. https://doi.org/10.3390/coatings13061045
Chicago/Turabian StyleErmakova, Evgeniya, Sergey Sysoev, Irina Tsyrendorzhieva, Alexander Mareev, Olga Maslova, Vladimir Shayapov, Eugene Maksimovskiy, Irina Yushina, and Marina Kosinova. 2023. "1,4-Bis(trimethylsilyl)piperazine—Thermal Properties and Application as CVD Precursor" Coatings 13, no. 6: 1045. https://doi.org/10.3390/coatings13061045
APA StyleErmakova, E., Sysoev, S., Tsyrendorzhieva, I., Mareev, A., Maslova, O., Shayapov, V., Maksimovskiy, E., Yushina, I., & Kosinova, M. (2023). 1,4-Bis(trimethylsilyl)piperazine—Thermal Properties and Application as CVD Precursor. Coatings, 13(6), 1045. https://doi.org/10.3390/coatings13061045







