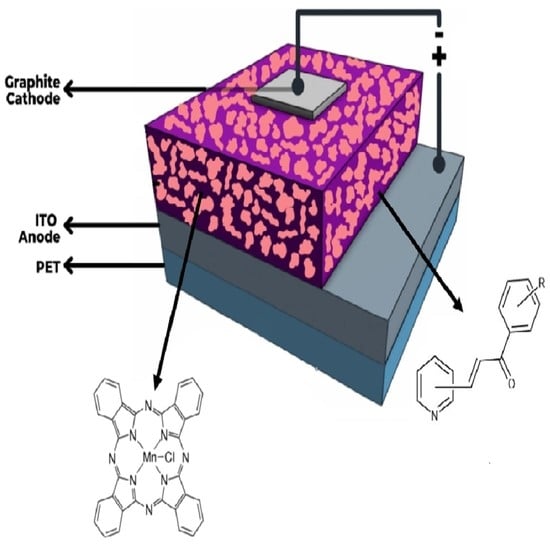
Studies on the Structure, Optical, and Electrical Properties of Doped Manganese (III) Phthalocyanine Chloride Films for Optoelectronic Device Applications
Abstract
1. Introduction
2. Materials and Methods
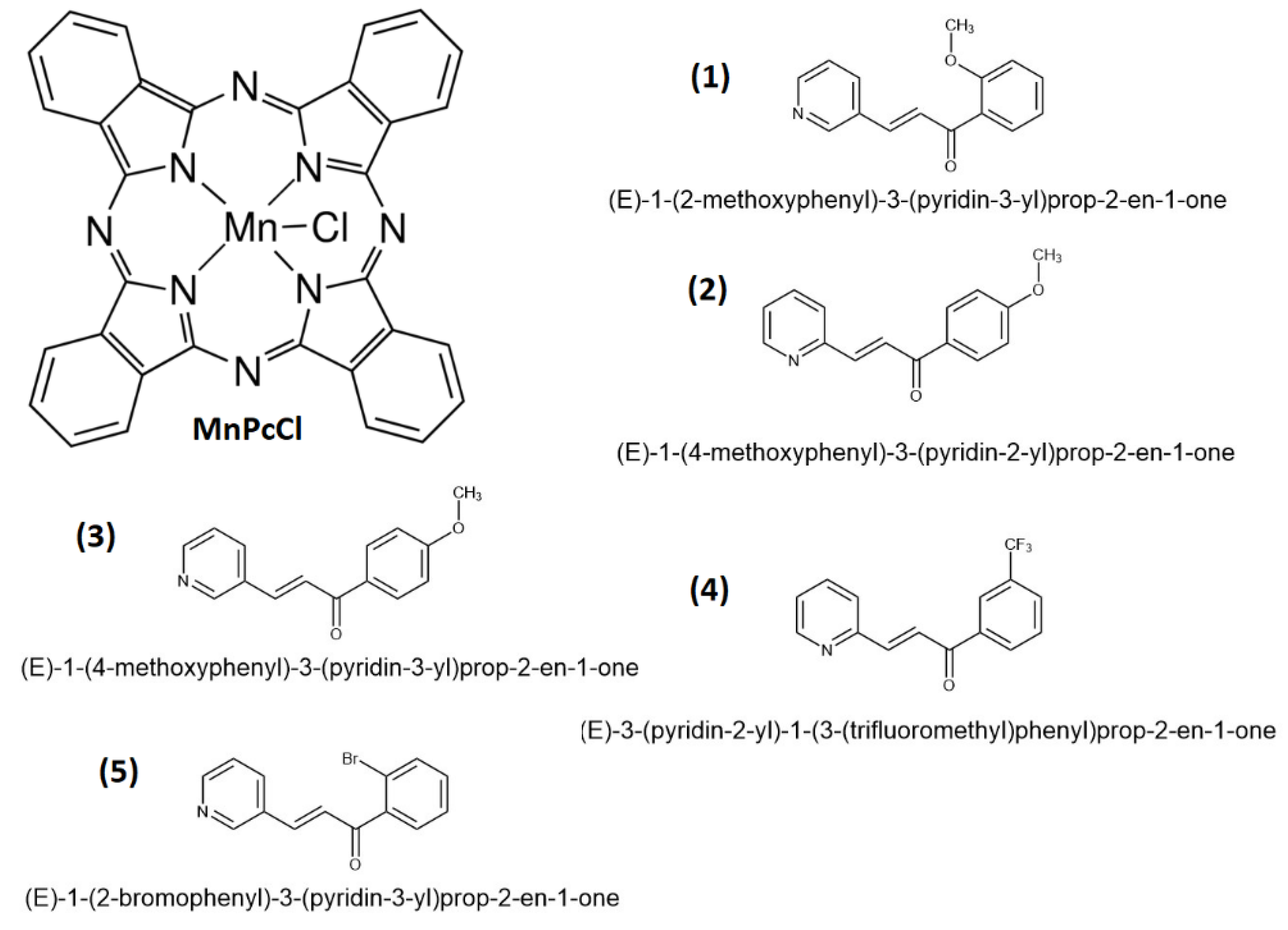
2.1. Doping of Organic Semiconductors (OSC)
2.2. Thin Film Deposition and Characterization
3. Results and Discussion
3.1. Doping, Deposition, and Characterization of the Semiconductor Films
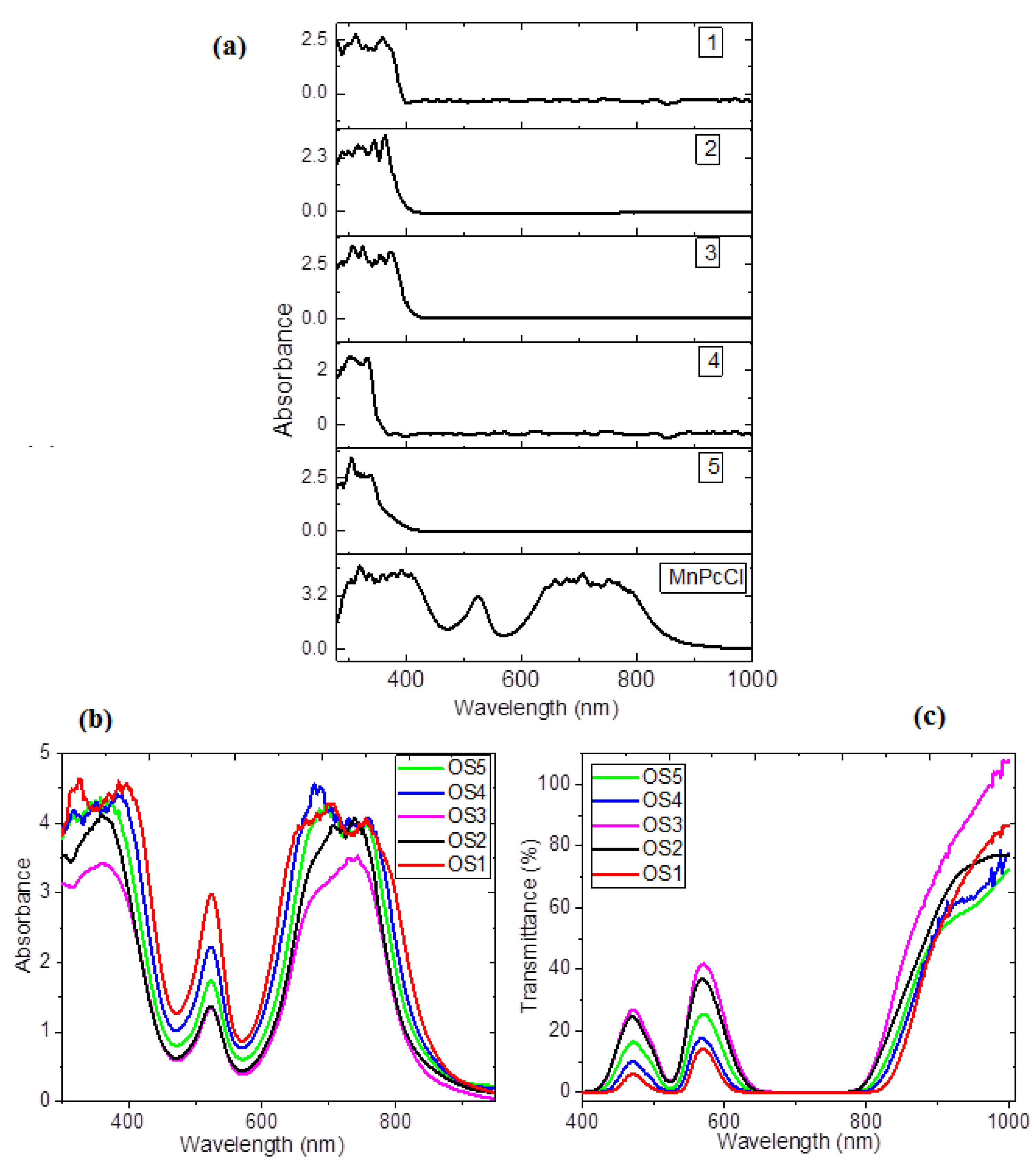
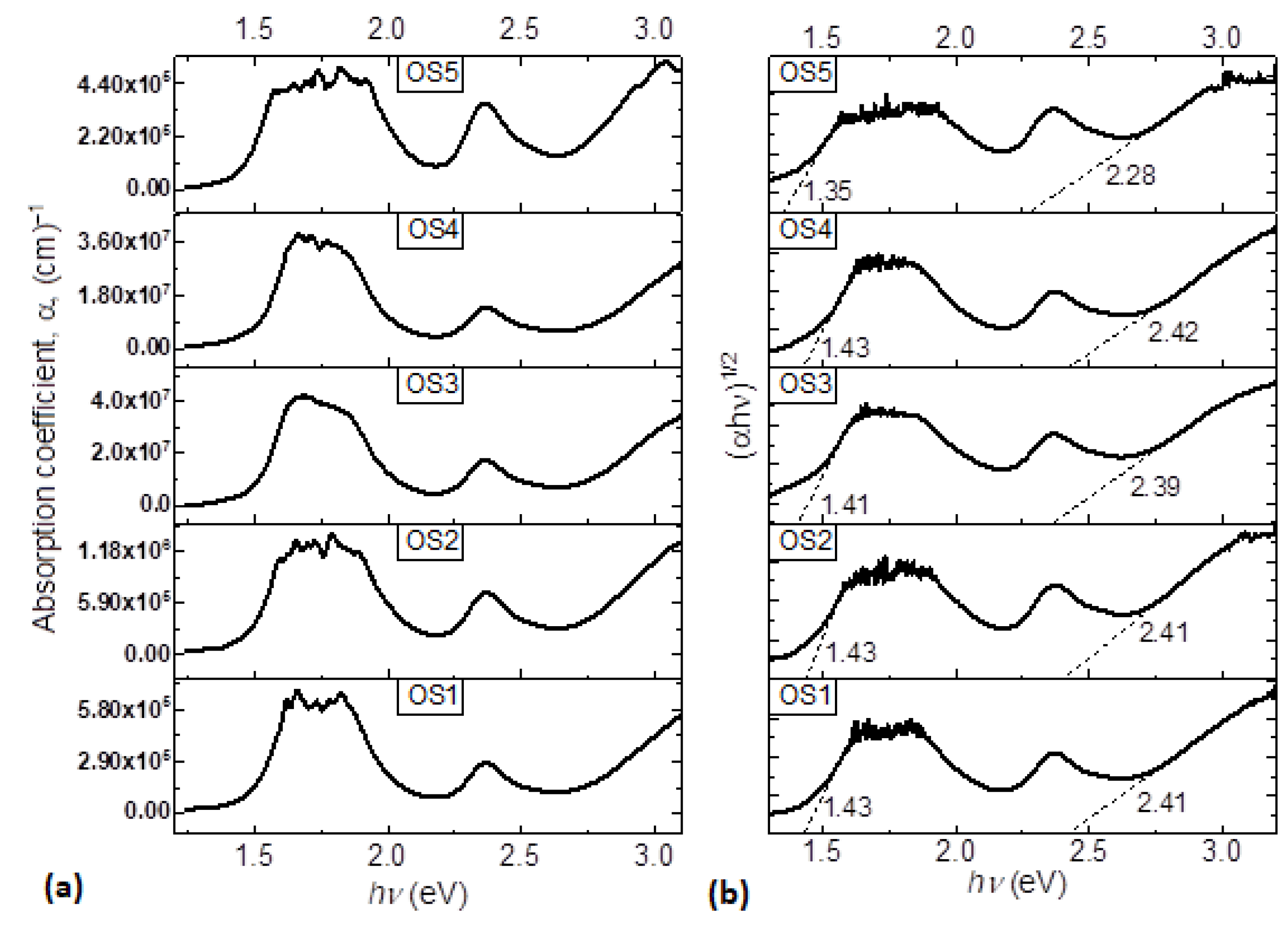
3.2. Optical Behavior Evaluation
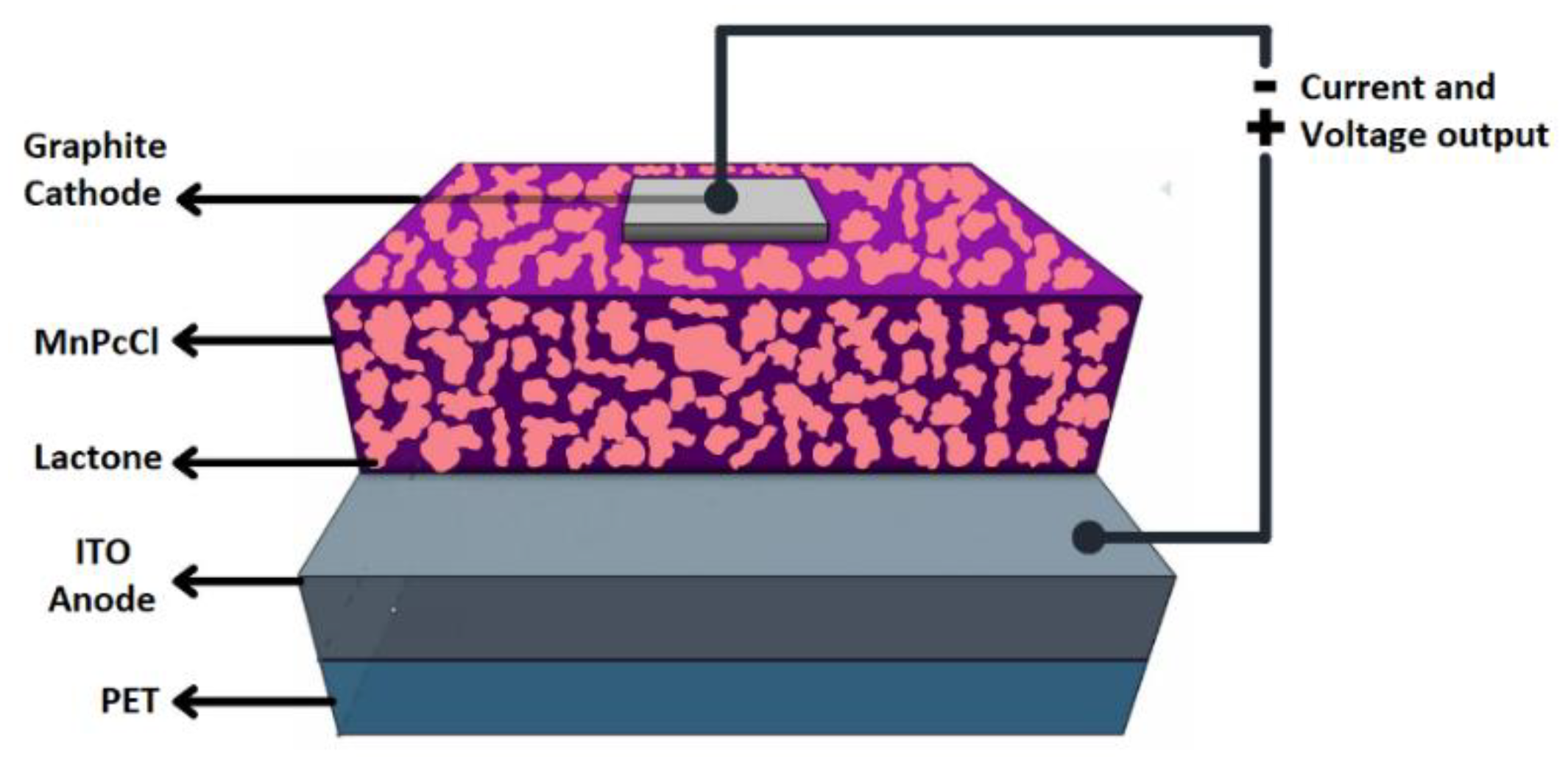
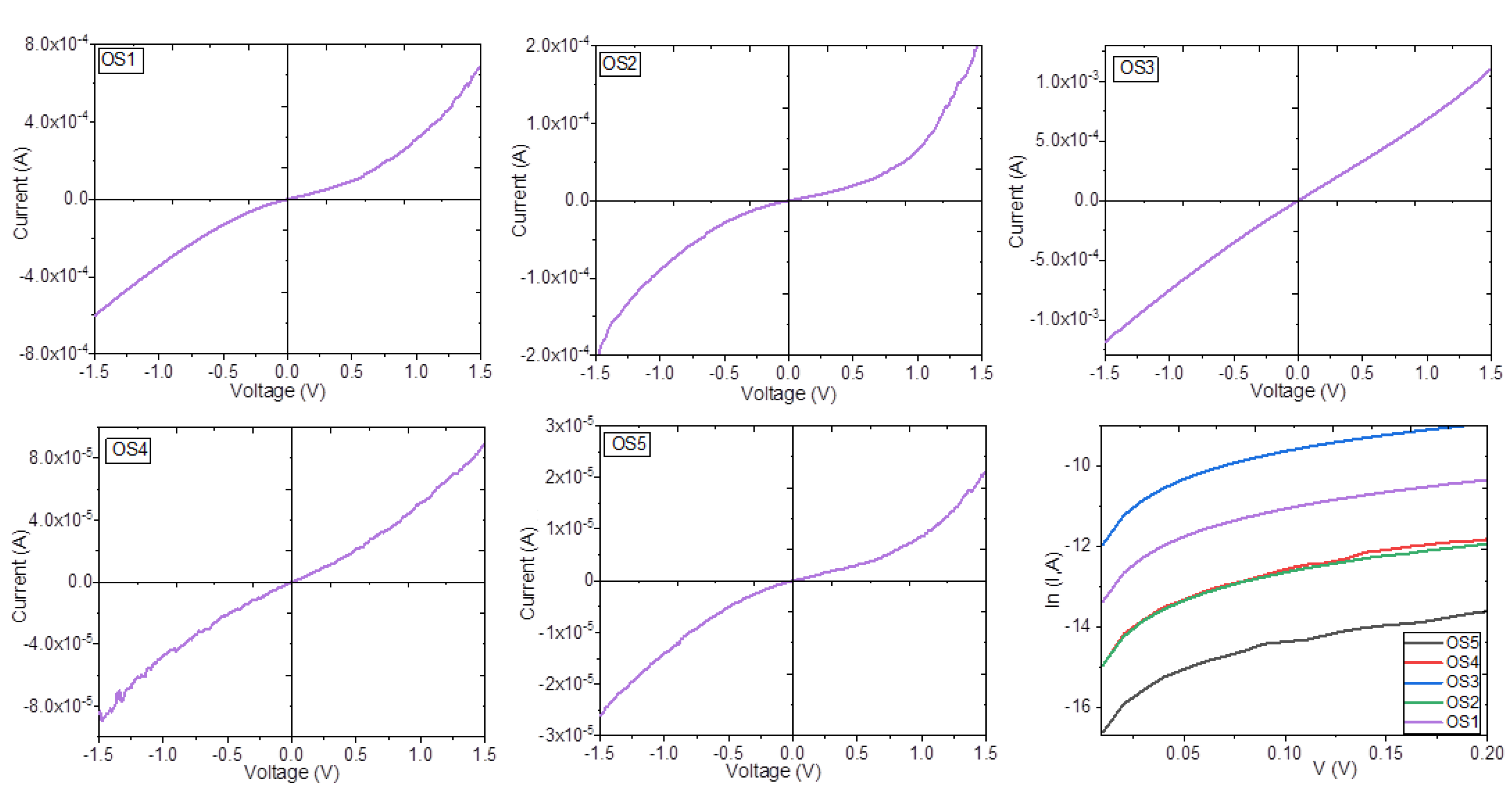
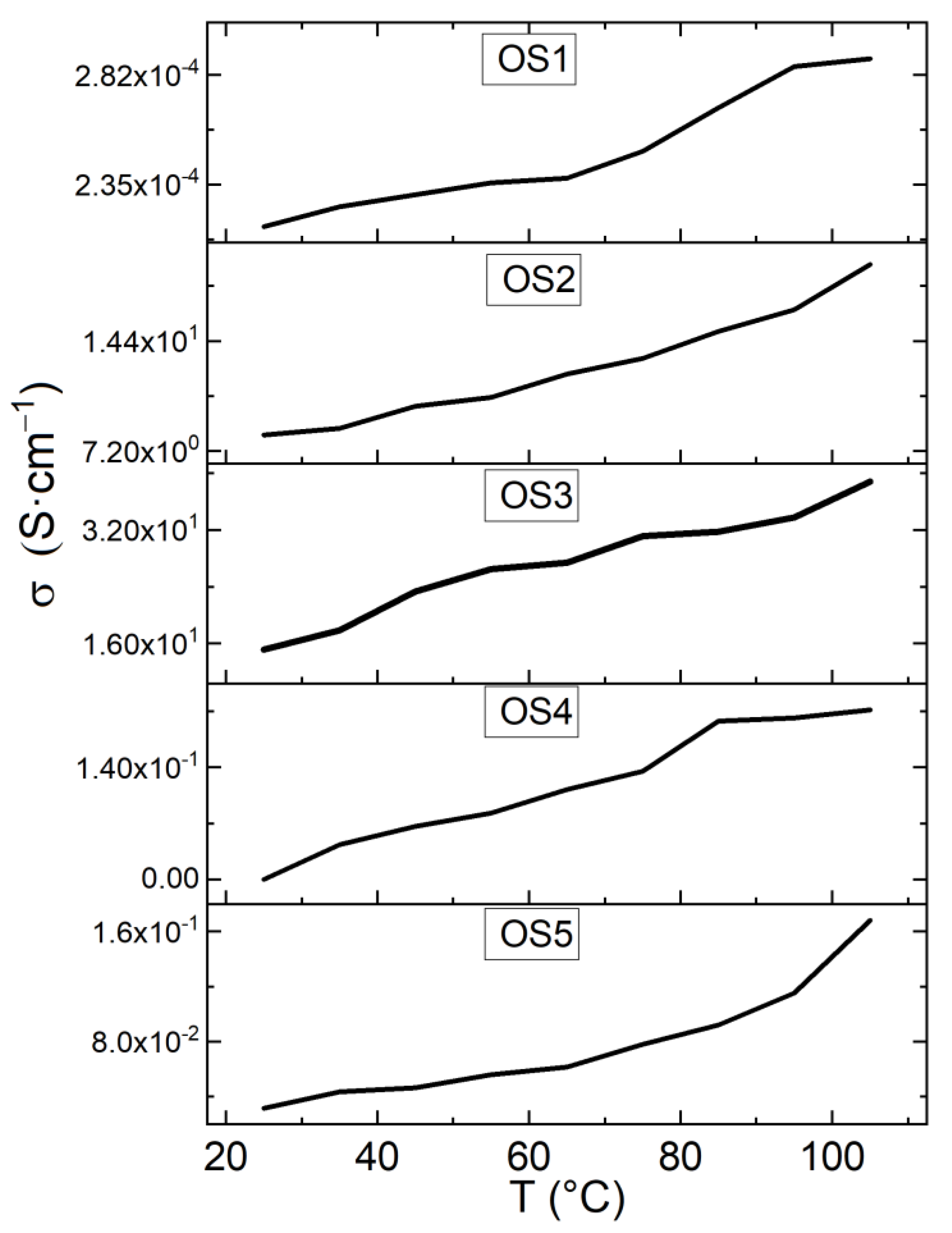
3.3. Manufacture of the Devices and Electrical Characterization
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Shoaee, S.; Sanna, A.L.; Sforazzini, G. Elucidating charge generation in green-solvent processed organic solar cells. Molecules 2021, 26, 7439. [Google Scholar] [CrossRef]
- Moore, K.; Wei, W. Applications of carbon nanomaterials in perovskite solar cells for solar energy conversion. NMS 2021, 3, 276–290. [Google Scholar] [CrossRef]
- Husain, A.A.F.; Hasan, W.Z.W.; Shafie, S.; Hamidon, M.S.; Pandey, S.S. A review of transparent solar photovoltaic technologies. Renew. Sust. Energ. Rev. 2018, 94, 779–791. [Google Scholar] [CrossRef]
- Green, M.; Dunlop, E.; Hohl-Ebinger, J.; Yoshita, M.; Kopidakis, N.; Hao, X. Solar cell efficiency tables (version 57). Prog. Photovolt. Res. Appl. 2021, 29, 3–15. [Google Scholar] [CrossRef]
- Liu, Q.; Jiang, Y.; Jin, K.; Qin, J.; Xu, J.; Li, W.; Xiong, J.; Liu, J.; Xiao, Z.; Sun, K.; et al. 18% Efficiency organic solar cells. Sci. Bull. 2020, 65, 272–275. [Google Scholar] [CrossRef]
- Wu, J.; Gao, M.; Chai, Y.; Liu, P.; Zhang, B.; Liu, J.; Ye, L. Towards a bright future: The versatile applications of organic solar cells. MRE 2021, 1, 100062. [Google Scholar] [CrossRef]
- Shi, D.; Guo, Z.; Bedford, N. 10-Nanoenergy materials. MNT 2015, 1, 255–291. [Google Scholar] [CrossRef]
- Günes, S.; Neugebauer, H.; Sariciftci, N.S. Conjugated polymer-based organic solar cells. Chem. Rev. 2007, 107, 1324–1338. [Google Scholar] [CrossRef]
- Gorduk, S.; Altindal, A. Peripherally tetra-substituted metallophthalocyanines bearing carboxylic acid groups for efficient dye sensitized solar cells. J. Mol. Struct. 2019, 1196, 747–753. [Google Scholar] [CrossRef]
- Sukhikh, A.; Bonegardt, D.; Klyamer, D.; Krasnov, P.; Basova, T. Chlorosubstituted Copper phthalocyanines: Spectral study and structure of thin films. Molecules 2020, 25, 1620. [Google Scholar] [CrossRef]
- Madhuri, K.P.; Sagade, A.A.; Santra, P.K.; John, N.S. Templating effect of single-layer graphene supported by an insulating substrate on the molecular orientation of lead phthalocyanine. Beilstein J. Nanotechnol. 2020, 11, 814–820. [Google Scholar] [CrossRef] [PubMed]
- Zanotti, G.; Angelini, N.; Mattioli, G.; Paoletti, A.M.; Pennesi, G.; Caschera, D.; Sobolev, A.P.; Beverina, L.; Calascibetta, A.M.; Sanzone, A.; et al. [1]Benzothieno[3,2-b][1]benzothiophene-phthalocyanine derivatives: A subclass of solution-processable electron-rich hole transport materials. ChemPlusChem 2020, 85, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Cranston, R.R.; Lessard, B.H. Metal phthalocyanines: Thin-film formation, microstructure, and physical properties. RSC Adv. 2021, 11, 21716–21737. [Google Scholar] [CrossRef]
- Williams, G.; Sutty, S.; Klenkler, R.; Aziz, H. Renewed interest in metal phthalocyanine donors for small molecule organic solar cells. Sol. Energy Mater. Sol. Cells. 2014, 124, 217–226. [Google Scholar] [CrossRef]
- Grant, T.M.; Josey, D.S.; Sampson, K.L.; Mudigonda, T.; Bender, T.P.; Lessard, B.H. Boron subphthalocyanines and silicon phthalocyanines for use as active materials in organic photovoltaics. Chem. Rec. 2019, 19, 1093–1112. [Google Scholar] [CrossRef]
- Reddy, G.; Devulapally, K.; Islavath, N.; Giribabu, L. Metallated macrocyclic derivatives as a hole—Transporting materials for perovskite solar cells. Chem Rec. 2019, 19, 2157–2177. [Google Scholar] [CrossRef]
- Urbani, M.; de la Torre, G.; Nazeeruddin, M.K.; Torres, T. Phthalocyanines and porphyrinoid analogues as hole- and electron-transporting materials for perovskite solar cells. Chem. Soc. Rev. 2019, 48, 2738–2766. [Google Scholar] [CrossRef]
- Yu, Z.; Hagfeldt, A.; Sun, L. The application of transition metal complexes in hole-transporting layers for perovskite solar cells: Recent progress and future perspectives. Coord. Chem. Rev. 2020, 406, 213143. [Google Scholar] [CrossRef]
- Ranta, J.; Kaunisto, K.; Niskanen, M.; Efimov, A.; Hukka, T.I.; Lemmetyinen, H. Monoisomeric phthalocyanines and phthalocyanine−Fullerene dyads with polar side chains: Synthesis, modeling, and photovoltage. J. Phys. Chem. 2014, 118, 2754–2765. [Google Scholar] [CrossRef]
- Martínez-Díaz, M.V.; de la Torre, G.; Torres, T. Lightning porphyrins and phthalocyanines for molecular photovoltaics. Chem. Commun. 2010, 46, 7090–7108. [Google Scholar] [CrossRef]
- de la Torre, G.; Claessens, C.G.; Torres, T. Phthalocyanines: Old dyes, new materials. Putting color in nanotechnology. Chem. Commun. 2007, 2000–2015. [Google Scholar] [CrossRef] [PubMed]
- Mack, J.; Kobayashi, N. Low symmetry phthlocyanines and their analogues. Chem. Rev. 2011, 111, 281–321. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Díaz, M.V.; Ince, M.; Torres, T. Phthalocyanines: Colorful macroheterocyclic sensitizers for dye-sensitized solar cells. Monatsh. Chem. 2011, 142, 699–707. [Google Scholar] [CrossRef]
- El-Zaidia, E.F.M. Studies structure, surface morphology, linear and nonlinear optical properties of nanocrystalline thin films of manganese (III) phthalocyanine chloride for photodetectors application. Sens. Actuator 2021, 330, 112828. [Google Scholar] [CrossRef]
- Grobosch, M.; Mahns, B.; Loose, C.; Friedrich, R.; Schmidt, C.; Kortus, J.; Knupfer, M. Identification of the electronic states of manganese phthalocyanine close to the Fermi level. Chem. Phys. Lett. 2011, 505, 122–125. [Google Scholar] [CrossRef]
- El-Nahass, M.M.; Soliman, H.S.; Khalifa, B.A.; Soliman, I.M. Structural and optical properties of nanocrystalline aluminum phthalocyanine chloride thin films. Mater. Sci. Semicond. Process. 2015, 38, 177–183. [Google Scholar] [CrossRef]
- El-Zaidia, E.F.M.; Qashou, S.I.; Darwish, A.A.A.; Yahia, I.S. Thermally evaporated of homogeneous nanostructured gallium-phthalocyanine-chloride films: Optical spectroscopy. Opt. Mater. 2020, 109, 110407. [Google Scholar] [CrossRef]
- Darwish, A.A.A.; Hamdalla, T.A.; El-Zaidia, E.F.M.; Hanafy, T.A.; Issa, S.A.M.; Yahia, I.S. Thin films of nanostructured gallium (III) chloride phthalocyanine deposited on FTO: Structural characterization, optical properties, and laser optical limiting. Phys. B Condens. Matter. 2020, 593, 412321. [Google Scholar] [CrossRef]
- Zeyada, H.M.; El-Nahass, M.M.; El-Menyawy, E.M.; El-Sawah, A.S. Electrical and photovoltaic characteristics of indium phthalocyanine chloride/p-Si solar cell. Synth. Met. 2015, 207, 46–53. [Google Scholar] [CrossRef]
- Soliman, I.M.; El-Nahass, M.M.; Khalifa, B.A. Characterization and photovoltaic performance of organic device based on AlPcCl/p-Si heterojunction. Synth. Met. 2015, 209, 55–59. [Google Scholar] [CrossRef]
- Touka, N.; Benelmadjat, H.; Boudine, B.; Halimi, O.; Sebais, M. Copper phthalocyanine nanocrystals embedded into polymer host: Preparation and structural characterization. J. Assoc. Arab Univ. Basic Appl. Sci. 2013, 13, 52–56. [Google Scholar] [CrossRef]
- Chau, L.K.; England, C.D.; Chen, S.; Armstrong, N.R. Visible absorption and photocurrent spectra of epitaxially deposited phthalocyanine thin films: Interpretation of exciton coupling effects. J. Phys. Chem. 1993, 97, 2699–2706. [Google Scholar] [CrossRef]
- Sánchez-Vergara, M.E.; Rivera, M. Investigation of optical properties of annealed aluminum phthalocyanine derivatives thin films. J. Phys. Chem. Solids 2014, 75, 599–605. [Google Scholar] [CrossRef]
- Soliman, I.M.; El-Nahass, M.M.; Mansour, Y. Electrical, dielectric and electrochemical measurements of bulk aluminum phthalocyanine chloride (AlPcCl). Solid State Commun. 2016, 225, 17–21. [Google Scholar] [CrossRef]
- Rivera, M.; Reyes, B.; Sánchez-Vergara, M.E.; Mendoza-Huizar, L.H. Conductive Behavior and Morphology of Axially Modified Gallium Phthalocyanine Thin Films onto Indium Tin Oxide Substrates. AMPC 2016, 6, 211–219. [Google Scholar] [CrossRef][Green Version]
- Darwish, A.A.A.; Helali, S.; Qashou, S.I.; Yahia, I.S.; El-Zaidia, E.F.M. Studying the surface morphology, linear and nonlinear optical properties of manganese (III) phthalocyanine chloride/FTO films. Phys. B Condens. Matter. 2021, 622, 413355. [Google Scholar] [CrossRef]
- Custodio, J.M.F.; Vaz, W.F.; Faria, E.C.M.; Anjos, M.M.; Campos, C.E.M.; Oliveira, G.R.; Martins, F.T.; da Silva, C.; Valverde, C.; Osório, F.A.P.; et al. On the potential as nonlinear optical material of a new chalcone derivative and its crystal and topological analysis. J. Mol. Struct. 2020, 1201, 127131. [Google Scholar] [CrossRef]
- Ali, M.K.M.; Elzupir, A.O.; Ibrahem, M.A.; Suliman, I.I.; Modwi, A.; Idriss, H.; Ibnaouf, K.H. Characterization of optical and morphological properties of chalcone thin films for optoelectronics applications. Optik 2017, 145, 529–533. [Google Scholar] [CrossRef]
- Vaz, W.; Custodio, J.; Silveira, R.; Castro, A.; Campos, C.; Anjos, M.; Oliveira, G.; Valverde, C.; Baseia, B.; Napolitano, H. Synthesis, Characterization, and third-order nonlinear optical properties of a new neolignane analogue. R. Soc. Chem. 2016, 6, 79215–79227. [Google Scholar] [CrossRef]
- Sai Kiran, M.; Anand, B.; Siva Sankara Sai, S.; Nageswara Rao, G. Second- and third-order nonlinear optical properties of bis-chalcone derivatives. J. Photochem. Photobiol. A Chem. 2014, 290, 38–42. [Google Scholar] [CrossRef]
- Abegão, L.M.G.; Fonseca, R.D.; Santos, F.A.; Souza, G.B.; Barreiros, A.L.B.S.; Barreiros, M.L.; Alencar, M.A.R.C.; Mendonça, C.R.; Silva, D.L.; De Boni, L.; et al. Second- and third-order nonlinear optical properties of unsubstituted and mono-substituted chalcones. Chem. Phys. Lett. 2016, 648, 91–96. [Google Scholar] [CrossRef]
- Krawczyk, P.; Pietrzak, M.; Janek, T.; Jędrzejewska, B.; Cysewski, P. Spectroscopic and nonlinear optical properties of new chalcone fluorescent probes for bioimaging applications: A theoretical and experimental study. J. Mol. Model. 2016, 22, 125. [Google Scholar] [CrossRef] [PubMed]
- Sarojini, B.; Narayana, B.; Ashalatha, B.; Indira, J.; Lobo, K. Synthesis, crystal growth and studies on non-linear optical property of new chalcones. J. Cryst. Growth. 2006, 295, 54–59. [Google Scholar] [CrossRef]
- Gasull, E.; Blanco, S.; Ferretti, F. A theoretical and experimental study of adsorption from dilute cyclohexane solutions of non-electrolytes: 4-X-chalcones on silica gel. J. Mol. Struct. Teochem. 2002, 579, 121–137. [Google Scholar] [CrossRef]
- Gu, C.L.; Liu, L.; Sui, Y.; Zhao, J.L.; Wang, D.; Chen, Y.J. Highly enantioselective Michael additions of α-cyanoacetate with chalcones catalyzed by bifunctional cinchona-derived thioureaorganocatalyst. Tetrahedron Asymmetry 2007, 18, 455–463. [Google Scholar] [CrossRef]
- Maidur, S.R.; Patil, P.S. Z-scan studies of third-order nonlinear optical and optical limiting properties of chalcones doped Poly(methyl methacrylate) thin films for visible laser protection. Opt. Mater. 2018, 84, 28–37. [Google Scholar] [CrossRef]
- Vidyashree, H.; Bhajantri, R.F.; Jagadish, N.; Sunil, G.R. Thiazole yellow G dyed PVA films for optoelectronics: Microstructural, thermal and photophysical studies. Mater. Res. Express 2016, 3, 075301. [Google Scholar] [CrossRef]
- Sweeting, S.G.; Hall, C.L.; Potticary, J.; Pridmore, N.E.; Warren, S.D.; Cremeens, M.E.; D´Ambruoso, G.D.; Matsumoto, M.; Hall, S.R. The solubility and stability of heterocyclic chalcones compared with trans-chalcones. Acta Cryst. 2020, B76, 13–17. [Google Scholar] [CrossRef]
- Asiri, A.M.; Khan, S.A. Synthesis, characterization and optical properties of mono-and bischalcone. Mater. Lett. 2011, 65, 1749–1752. [Google Scholar] [CrossRef]
- Janardhana, K.; Ravindrachary, V.; Kumar, P.R. Third order nonlinear optical studies of 1-(4- chloro phenyl)-3-(4-dimethylamino phenyl) prop-2-en-1-one. J. Cryst. Growth. 2013, 368, 11–20. [Google Scholar] [CrossRef]
- Devi, J.M.; Tharmaraj, J.; Ramakrishnan, S.; Ramachandran, K. On the thermal properties of metal (II) complexes of chalcone. Mater. Lett. 2008, 62, 852–856. [Google Scholar] [CrossRef]
- Praveen, A.; Jayarama, A. Crucial role of molecular planarity on the second order nonlinear optical property of pyridine based chalcone single crystals. J. Mol. Struct. 2015, 1088, 85–94. [Google Scholar] [CrossRef]
- Carmona-Reyes, G.; Ballinas-Indili, R.; Sánchez-Vergara, M.E.; Toscano, R.A.; Álvarez-Toledano, C. Regiodivergent synthesis of vinyl trifluoromethansulfonates c/d lactones: Via 1,6 addition/intramolecular one-pot annulation of 1,4-dihidropyridines derivated from pyridinyl propenones. Tetrahedron Lett. 2022, 88, 153591. [Google Scholar] [CrossRef]
- El-Nahass, M.M.; Abd-El-Rahman, K.F.; Al-Ghamdi, A.A.; Asiri, A.M. Optical properties of thermally evaporated tin-phthalocyanine dichloride thin films, SnPcCl2. Phys. B Condens. Matter. 2014, 344, 398–406. [Google Scholar] [CrossRef]
- Hart, M.M. Cationic Exchange Reactions Involving Dilithium Phthalocyanine. Master’s Thesis, Wright State University, Dayton, OH, USA, 2009. [Google Scholar]
- Maidur, S.R.; Patil, P.S. Linear optical and third-order nonlinear optical properties of anthracene chalcone derivatives doped PMMA thin films. Optik 2019, 190, 54–67. [Google Scholar] [CrossRef]
- Sun, J.; Wang, G.; Liu, C.; Shi, Y.; Zhao, M. Synthesis of four pyrene-containing chalcone derivatives: Achieving excellent third-order nonlinear optical properties by optimizing halopyridines. Opt. Laser Technol. 2019, 109, 600–607. [Google Scholar] [CrossRef]
- El-Nahass, M.M.; Farag, A.M.; Abd El-Rahman, K.F.; Darwish, A.A.A. Dispersion studies and electronic transitions in nickel phthalocyanine thin films. Opt. Laser Technol. 2005, 37, 513–523. [Google Scholar] [CrossRef]
- Özçeșmeci, M.; Nar, I.; Hamuryudan, E. Synthesis and electrochemical and spectroelectrochemical characterization of chloromanganese(III) phthalocyanines. Turk. J. Chem. 2014, 38, 1064–1072. [Google Scholar] [CrossRef]
- Maidur, S.R.; Jahagirdar, J.R.; Patil, P.S.; Chia, T.S.; Quah, C.K. Structural characterizations, Hirshfeld surface analyses, and third-order nonlinear optical properties of two novel chalcone derivatives. Opt. Mater. 2018, 75, 580–594. [Google Scholar] [CrossRef]
- Lu, M.; Wang, K.; Han, Y.; Yao, Y.; Gao, P.; Huang, C.; Zhang, W.; Xu, F. Synthesis, structure, and optical properties of manganese phthalocyanine thin films and nanostructures. Prog. Nat. Sci. Mater. Int. 2017, 27, 329–332. [Google Scholar] [CrossRef]
- Mahmoud, A.Z.; Darwish, A.A.A.; Qashou, S.I. Film thickness effects on nanorods organic films of azo quinoline derivatives for optical applications. Prog. Nat. Sci. Mater. Int. 2019, 29, 402–409. [Google Scholar] [CrossRef]
- Elesh, E.; Mohammed, Z. Morphological, linear and nonlinear properties of gallium phthalocyanine chloride annealed thin films. Optik 2020, 219, 165176. [Google Scholar] [CrossRef]
- El-Nahass, M.M.; El-Deeb, A.F.; Metwally, H.S.; Hassanien, A.M. Influence of annealing on the optical properties of 5,10,15,20-tetraphenyl-21H, 23H-porphine iron (III) chloride thin films. Mater. Chem. Phys. 2011, 125, 247–251. [Google Scholar] [CrossRef]
- Bardeen, J.; Blatt, F.J.; Hall, L.H. Energy Dependence of Indirect Optical Absorption in Semiconductors. In Proceedings of the Conference on Photoconductivity; Atlantic: Boston, MA, USA, 4–6 November 1965; p. 149. [Google Scholar]
- Dongol, M.; El-Nahass, M.; El-Denglawey, A.; Elhady, A.; Abuelwafa, A. Optical Properties of Nano 5,10,15,20-Tetraphenyl-21H,23H-Prophyrin Nickel (II) Thin Films. Curr. Appl. Phys. 2012, 12, 1178–1184. [Google Scholar] [CrossRef]
- Alghamdi, S.A.; Darwish, A.A.A.; Yahia, I.S.; El-Zaidia, E.F.M. Structural characterization and optical properties of nanostructured indium (III) phthalocyanine chloride/FTO thin films for photoelectric applications. Optik 2021, 239, 166780. [Google Scholar] [CrossRef]
- Alharbi, S.R.; Darwish, A.A.A.; Al Garni, S.E.; El Saeedy, H.I.; Abd El-Rahman, K.F. Influence of thickness and annealing on linear and nonlinear optical properties of manganese (III) chloride tetraphenyl porphine (MnTPPCl) organic thin films. Infrared Phys. Technol. 2016, 78, 77–83. [Google Scholar] [CrossRef]
- Van Duren, J.; Yang, X.N.; Loos, J.; Bulle-Liewma, C.W.T.; Sieval, A.B.; Hummelen, J.C.; Janssen, R.A.J. Relating the Morphology of Poly(p-phenylene vinylene)/Methanofullerene Blends to Solar-Cell Performance. Adv. Mater. 2004, 14, 425. [Google Scholar] [CrossRef]
- Reyes-Reyes, M.; Kim, K.; Carroll, D.L. High-efficiency photovoltaic devices based on annealed poly(3-hexylthiophene) and 1-(3-methoxycarbonyl)-propyl-1- phenyl-(6,6)C61(6,6)C61 blends. Appl. Phys. Lett. 2005, 87, 083506. [Google Scholar] [CrossRef]
- Peet, J.; Kim, J.Y.; Coates, N.E.; Ma, W.L.; Moses, D.; Heeger, A.J.; Bazán, G.C. Efficiency enhancement in low-bandgap polymer solar cells by processing with alkane dithiols. Nat. Mater. 2007, 6, 497. [Google Scholar] [CrossRef]
- Meijer, E.J.; de Leeuw, D.M.; Setayesh, S.; van Veenendaal, E.; Huisman, B.H.; Blom, P.W.M.; Hummelen, J.C.; Scherf, U.; Klapwijk, T.M. Solution-processed ambipolar organic field-effect transistors and inverters. Nat. Mater. 2003, 2, 678. [Google Scholar] [CrossRef]
- Shafai, T.S.; Anthopoulous, T.D. Junction properties of nickel phthalocyanine thin film devices utilizing indium injecting electrodes. Thin Solid Film. 2001, 398–399, 361–367. [Google Scholar] [CrossRef]
- Ahmad, Z.; Sayyad, M.H.; Karimov, K.S. CuPc based organic-inorganic hetero-junction with Au electrodes. J. Semicond. 2010, 31, 074002-1-4. [Google Scholar] [CrossRef]
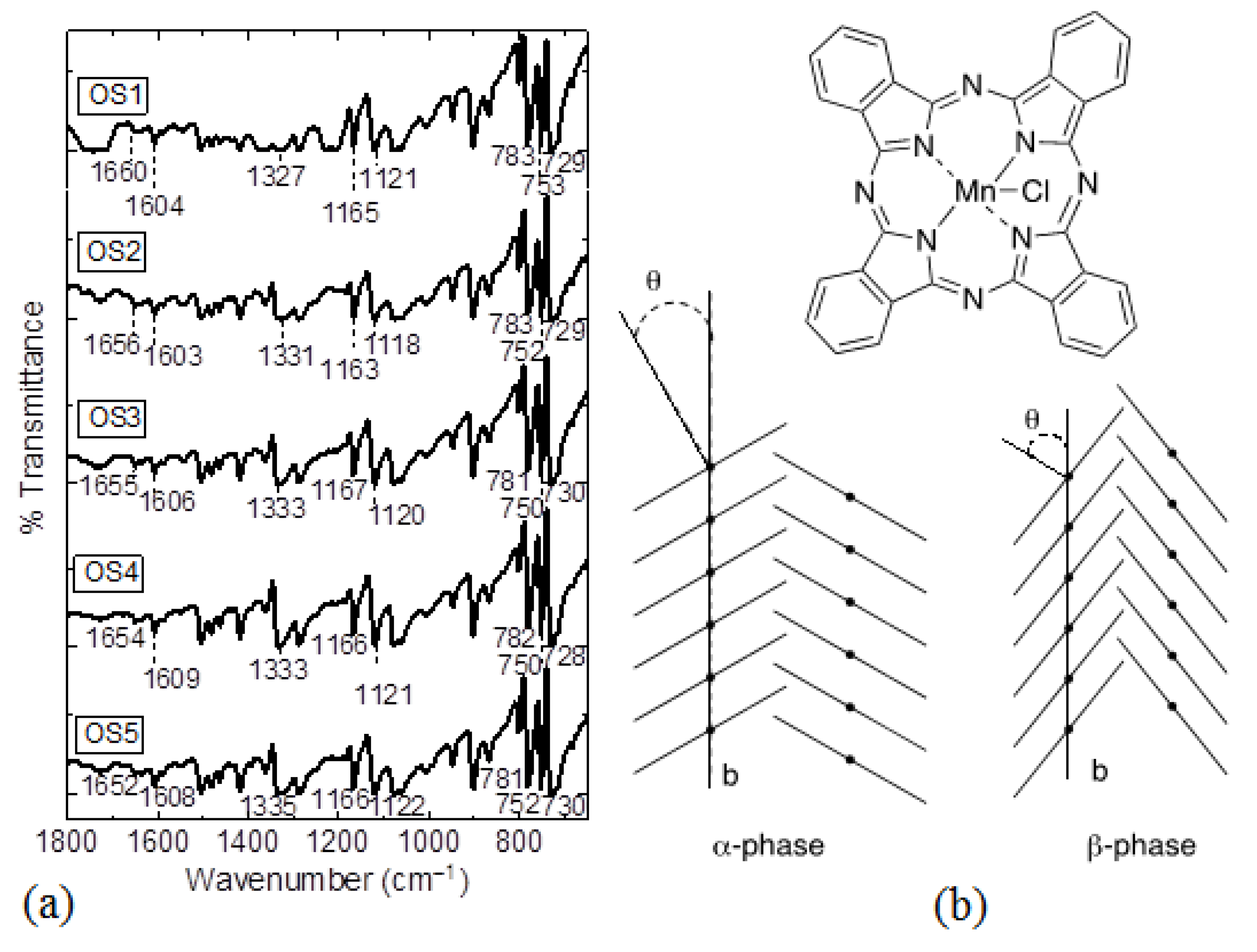
| Assignment | Powder (cm−1) | Film (cm−1) | Powder (cm−1) | Film (cm−1) | Powder (cm−1) | Film (cm−1) | Powder (cm−1) | Film (cm−1) | Powder (cm−1) | Film (cm−1) |
|---|---|---|---|---|---|---|---|---|---|---|
| Sample | OS1 | OS1 | OS2 | OS2 | OS3 | OS3 | OS4 | OS4 | OS5 | OS5 |
| In-plane pyrrole stretching | 1327 | 1335 | 1335 | 1333 | 1332 | 1332 | 1334 | 1331 | 1332 | 1333 |
| C-H bending | 1164, 1119 | 1161, 1119 | 1167, 1119 | 1167, 1121 | 1167, 1119 | 1165, 1121 | 1166, 1120 | 1165, 1119 | 1166, 1119 | 1167, 1119 |
| In-plane C-H deformation | 753 | 752 | 753 | 752 | 753 | 754 | 753 | 754 | 752 | 754 |
| α-form in MnPc | 729 | 726 | 724 | 726 | 729 | 727 | 729 | 726 | 727 | 724 |
| β-form in MnPc | 783 | 782 | 782 | 779 | 782 | 780 | 782 | 778 | 782 | 778 |
| C=O stretching bands of carbonyl | 1660 | 1662 | 1656 | 1665 | 1656 | 1662 | 1652 | 1668 | 1652 | 1667 |
| C=C bending of chalcone | 1607 | 1604 | 1609 | 1609 | 1607 | 1608 | 1607 | 1607 | 1607 | 1603 |
| Device | IS (A) | σ at 0.1 V (S·cm−1) | σ at 0.5 V (S·cm−1) | σ at 1.0 V (S·cm−1) |
|---|---|---|---|---|
| OS1 | 2.24 × 10−8 | 2.92 × 10−2 | 3.16 × 10−2 | 5.56 × 10−2 |
| OS2 | 2.32 × 10−8 | 1.55 × 10−5 | 1.89 × 10−5 | 2.82 × 10−5 |
| OS3 | 1.18 × 10−7 | 1.43 × 10+1 | 1.51 × 10+1 | 3.02 × 10+1 |
| OS4 | 2.73 × 10−9 | 6.27 × 10+0 | 8.26 × 10+0 | 1.45 × 10+1 |
| OS5 | 7.14 × 10−10 | 7.64 × 10−1 | 7.64 × 10−1 | 7.73 × 10−1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez Vergara, M.E.; Canseco Juárez, M.J.; Ballinas Indili, R.; Carmona Reyes, G.; Álvarez Bada, J.R.; Álvarez Toledano, C. Studies on the Structure, Optical, and Electrical Properties of Doped Manganese (III) Phthalocyanine Chloride Films for Optoelectronic Device Applications. Coatings 2022, 12, 246. https://doi.org/10.3390/coatings12020246
Sánchez Vergara ME, Canseco Juárez MJ, Ballinas Indili R, Carmona Reyes G, Álvarez Bada JR, Álvarez Toledano C. Studies on the Structure, Optical, and Electrical Properties of Doped Manganese (III) Phthalocyanine Chloride Films for Optoelectronic Device Applications. Coatings. 2022; 12(2):246. https://doi.org/10.3390/coatings12020246
Chicago/Turabian StyleSánchez Vergara, María Elena, María José Canseco Juárez, Ricardo Ballinas Indili, Genaro Carmona Reyes, José Ramón Álvarez Bada, and Cecilio Álvarez Toledano. 2022. "Studies on the Structure, Optical, and Electrical Properties of Doped Manganese (III) Phthalocyanine Chloride Films for Optoelectronic Device Applications" Coatings 12, no. 2: 246. https://doi.org/10.3390/coatings12020246
APA StyleSánchez Vergara, M. E., Canseco Juárez, M. J., Ballinas Indili, R., Carmona Reyes, G., Álvarez Bada, J. R., & Álvarez Toledano, C. (2022). Studies on the Structure, Optical, and Electrical Properties of Doped Manganese (III) Phthalocyanine Chloride Films for Optoelectronic Device Applications. Coatings, 12(2), 246. https://doi.org/10.3390/coatings12020246